Abstract
The results of most case-control studies have suggested a positive association between eating frequency and colorectal cancer risk. Because no prospective cohort studies have done so to date, the authors prospectively examined this association. In 1992, eating frequency was assessed in a cohort of 34,968 US men in the Health Professionals Follow-up Study. Cox proportional hazards regression models were used to estimate relative risks and 95% confidence intervals for various levels of eating frequency. Effect modifications by overall dietary quality (assessed using the Diet Approaches to Stop Hypertension score) and by factors that influence insulin resistance were further assessed. Between 1992 and 2006, a total of 583 cases of colorectal cancer were diagnosed. When comparing the highest eating frequency category (5–8 times/day) with the reference category (3 times/day), the authors found no evidence of an increased risk of colorectal cancer (multivariate relative risk = 0.88, 95% confidence interval: 0.62, 1.26) or colon cancer (multivariate relative risk = 0.78, 95% confidence interval: 0.49, 1.25). There was an implied inverse association with eating frequency among participants who had healthier diets (high Diet Approaches to Stop Hypertension score; P for interaction = 0.01), especially among men in the high-insulin-sensitivity group (body mass index (weight (kg)/height (m)2) <25, ≥2 cups of coffee/day, and more physical activity; P for interaction < 0.01, P for trend = 0.01). There was an implied protective association between increased eating frequency of healthy meals and colorectal cancer risk and in men with factors associated with higher insulin sensitivity.
Keywords: colorectal neoplasms, diet, food, nutritional status
Colorectal cancer (CRC) is the third leading cause of cancer death in both the United States (1) and worldwide (2). Findings from most case-control studies have suggested that CRC risk increases with higher eating frequency (meals and/or snacks) (3–8), although the results of 2 of these studies did not reach statistical significance (3, 4). Conversely, in 1 case-control study, de Verdier and Longnecker (9) found a null association between the risk of colon cancer and meal frequency but a positive association between the risk of colon cancer and snack frequency. In another case-control study, investigators found no association between CRC risk and snack frequency or eating frequency, but they did find a positive association with meal frequency (10). On the basis of inconsistent results across studies and the case-control study design that may be subject to recall bias, no firm conclusion could be made regarding the influence of meal frequency on CRC risk. Examining this association between meal frequency and CRC risk prospectively is important to exclude potential reporting biases.
Further, if eating frequency is associated with CRC risk, it is reasonable to hypothesize that the association might be modified by dietary quality. The Dietary Approaches to Stop Hypertension (DASH) diet entails high intakes of fruits, vegetables, and legumes and nuts, moderate intakes of low-fat dairy products, and low intakes of animal protein and sweets, as well as reduced sodium intake (11). Although the DASH diet was originally designed to aid in blood pressure reduction, adherence to this diet has previously been shown to be associated with a reduced risk of prevalent colorectal adenomas (12) and CRC (13). Glycemic load, another variable that represents both the quality and the quantity of the carbohydrates consumed in a diet, also reflects dietary quality and has previously been associated with colon cancer risk in some studies (14). Moreover, increased meal frequency could have a complex association with insulin secretion (15); both high insulin secretion and insulin resistance increase the risk of CRC (14). We prospectively examined data from the Health Professionals Follow-Up Study to determine whether meal frequency was associated with CRC risk and whether any association was modified by indicators of dietary quality (i.e., DASH score and glycemic load) and the major factors that influence insulin resistance, including coffee intake (16), physical activity level (17), and body mass index (BMI, measured as weight (kg)/height (m)2).
MATERIALS AND METHODS
Study population
The Health Professionals Follow-up Study is an ongoing prospective study of 51,529 male health professionals, including dentists, veterinarians, pharmacists, optometrists, osteopaths, and podiatrists, who were 40–75 years of age upon enrollment in 1986. Participants have been followed through questionnaires mailed biennially that asked about their medical history, lifestyle, and health-related behaviors. This study was approved by the institutional review board of the Harvard School of Public Health, Boston, Massachusetts.
Dietary assessment
Diet over the previous year was first assessed in 1986 using a 131-item food frequency questionnaire (FFQ). Dietary information was updated with subsequent similar FFQs mailed every 4 years thereafter (in 1990, 1994, 1998, 2002, and 2004). Follow-up was complete for over 90% of participants in each 2-year cycle. Nine mutually exclusive response categories were provided for the frequencies of intakes of various foods and beverages. Nutrient intakes were calculated by converting the frequency responses to daily intakes for each food or beverage, multiplying the daily intakes of each food and beverage by the corresponding nutrient contents, and summing the contributions of all items. The validity and reproducibility of the FFQ have been reported elsewhere (18, 19). In brief, the deattenuated correlation coefficient between FFQ and diet records ranged from 0.32 for energy-adjusted protein to 0.81 for energy-adjusted vitamin B6.
In 1992, subjects were asked to report the times of the day at which they usually ate. The questionnaire ascertained the number of times per day food was eaten before breakfast, at breakfast, between breakfast and lunch, at lunch, between lunch and dinner, at dinner, between dinner and bed time, and after going to bed. In the present article, breakfast, lunch, and dinner were considered to be meals, whereas eating at other times was considered snacking. Hence, participants could report eating from 1 to 8 times/day. Because the questionnaire focused on eating, it is most likely that drinking coffee, tea, or soda was not considered snacking in this study. However, we accounted for coffee intake in our models.
Exclusion of participants at baseline
Because eating frequency was first assessed in 1992, we used data from 1992 as the baseline for the present analysis. Participants who did not complete the original 1986 FFQ, had implausible energy intakes (>4,200 or <800 kcal/day), had skipped 70 or more questions on various food items, or did not answer the meal frequency questions in 1992 were excluded from the study. We also excluded participants with a history of ulcerative colitis or cancer (except for nonmelanoma skin cancer) between 1986 and 1992. Thus, 34,968 men remained for follow-up from 1992 to 2006.
Other covariates
We previously examined various composite dietary scores (e.g., Mediterranean diet score) in relation to CRC risk in the Health Professionals Follow-up Study cohort, and the DASH score was the one most strongly associated with CRC risk (13). The DASH score was constructed based on foods and nutrients emphasized or minimized in the DASH diet (20), focusing on 8 components: high intakes of fruits, vegetables, nuts and legumes, low-fat dairy products, and whole grains and low intakes of sodium, sweetened beverages, and red and processed meats. We calculated a DASH score for each FFQ starting in 1986 (21). For each of the components, we classified men into quintiles according to their intake ranking such that a higher quintile ranking reflected a better-quality diet. For instance, for fruits, vegetables, nuts and legumes, low-fat dairy products, and whole grains, the highest quintile was assigned a score of 5 points and the lowest quintile was assigned 1 point. For sodium, sweetened beverages, and red and processed meats, the lowest quintile was assigned a score of 5 points and the highest quintile was assigned 1 point. We then summed up the component scores to obtain overall DASH scores that ranged from 8 to 40.
Glycemic load was calculated by multiplying the carbohydrate content of each food by its glycemic index, a measure of the relative postprandial blood glucose response per gram of carbohydrate, multiplying that value by the frequency of consumption, and then summing these values for all foods. Hence, glycemic load represented the quality and the quantity of the carbohydrates consumed. Participants were asked to report their average intakes of coffee with and without caffeine (in cups) over the preceding year. We then converted the responses for individual beverages into an average daily intake for each participant. Physical activity level was expressed as hours per week and converted to metabolic equivalent hours/week.
Case ascertainment
On each biennial questionnaire, participants were asked whether they had been diagnosed with cancer of the colon or rectum during the previous 2 years. If a participant reported a diagnosis of colon or rectal cancer, we requested an authorization to obtain his or her medical records. Information on the histologic type, anatomic location, and stage of the cancer was then extracted from medical records by study researchers blinded to exposure status. Between 1992 and 2006, a total of 583 CRC cases (375 colon cancers, 133 rectal cancers, and 75 cancers of unknown site within the colorectum) were diagnosed among eligible men. Patients with carcinoid cancer or nonepithelial CRC were excluded from our analyses. Cases whose specific tumor site within the colorectum was unknown were included in the analyses of total CRC risk but were excluded from separate analysis of the colon only.
Statistical analysis
Participants were followed from the date they returned their baseline questionnaire (in 1992) to the date of diagnosis of CRC, death, or end of the study period (January 31, 2006), whichever came first. To examine associations between eating frequency and CRC risk, we estimated relative risks and 95% confidence intervals using the Cox proportional hazards regression model with age in months as the time scale and calendar year as a stratification variable. We analyzed eating frequency (meals and snacks), snacking frequency (snacks only), and breakfast consumption patterns (with other meals and snacks) modeled in 3 or 4 groups. We also analyzed colon cancer separately. Although there is some evidence that risk factors for colon and rectal cancers may differ (22), we did not present separate results for rectal cancer because of the small number of rectal cancer cases.
In the basic multivariate models, in addition to stratifying by age and time period, we adjusted for known and suspected risk factors of CRC, including race (white, nonwhite, or missing), family history of CRC (yes, no, or missing), pack years of smoking before 30 years of age (continuous), BMI (<24, 24–26.0, 26.1–29, or >29), physical activity level (quintiles of metabolic equivalent hours/week), aspirin use (≥2 times per week, <2 times/week, or missing), use of supplements containing antioxidants (ever, never, or missing), history of previous endoscopy (screening in the last 2 years, never, or missing), energy intake (kilocalories/day, continuous), alcohol intake (0, 0.1–4.9, 5–14.9, or ≥15 g/day), red meat consumption (quintiles), total calcium intake (quintiles), dietary folate intake (quintiles), and dietary vitamin D intake (quintiles). To control for diet quality, we also controlled for DASH score (quintiles). Because nutrients are correlated with total energy intake, energy-adjusted nutrient intakes were calculated using the residual method (23). For continuous variables, such as physical activity level and BMI, outliers (i.e., values greater than mean + 4 × SD or less than mean – 4 × SD) were included in the missing category. We additionally adjusted for coffee intake (continuous) and glycemic load (continuous) in separate models. The time-varying covariates were cumulatively averaged (BMI, physical activity level, energy intake, alcohol intake, red meat consumption, total calcium intake, dietary folate intake, dietary vitamin D intake, coffee intake, and glycemic load) or updated using the information collected every 2 years (all other covariates). These biennially updated covariates were carried forward from previous years if data were missing in subsequent rounds and were coded as missing if absent at baseline. Trend tests were calculated by including the median eating frequency in each category as a continuous variable in the model.
We showed the joint association between eating frequency (categorical) and diet quality, as assessed by the cumulative-averaged biennially updated variables, such as the DASH score (below the median value vs. the median value or higher) and glycemic load (below the median value vs. the median value or higher). We also conducted joint analysis with variables associated with insulin sensitivity, including the 3 items most strongly associated with C-peptide levels: coffee intake (0–1 vs. ≥2 servings/day) (16), physical activity level (below the median value vs. the median value or higher in metabolic equivalent hours/week) (17), and BMI (<25 vs. ≥25). Moreover, these 3 risk factors for insulin sensitivity were summed into 1 score ranging from 0 to 3 such that a person with high insulin sensitivity would get 1 point for a coffee intake of 2 or more cups/day, 1 point for a high physical activity level, and 1 point for a BMI <25. Thus, a relatively insulin-sensitive individual would get the highest score of 3 and an insulin-resistant person would get the worst score of 0. The insulin sensitivity score was then categorized into 2 groups (0–1 vs. 2–3) for joint-analysis purposes. To test the hypothesis that there was no association modification between each factor and eating frequency with regard to risk of colorectal or colon cancer, we used the likelihood ratio test to compare the model that included the different combinations of eating frequency (continuous term) and the potential effect modifier (e.g., DASH score (continuous) and glycemic load, coffee intake, physical activity, and BMI (median of deciles, continuous)) with a model that included only eating frequency and the potential effect modifier as separate variables. All tests were 2-sided.
RESULTS
In this cohort of 34,968 men, the majority ate 3 times/day (47.8%) or 4 times/day (33.8%), and fewer ate more frequently (5–8 meals/day; 7.9%) or less frequently (1–2 times/day; 10.5%). Men who ate less frequently mostly skipped breakfast (71%) or lunch (51%), ate more at dinner time (89%), smoked more, took fewer aspirin, multivitamins, and antioxidant supplements, drank more alcohol, consumed fewer calories, and had a lower intake of all measured nutrients and foods in general (Table 1). The positive association observed between DASH score and eating frequency (r = 0.14) was mainly driven by the positive association between eating frequency and intakes of whole grains (r = 0.14), low-fat dairy (r = 0.11), and fruits (r = 0.10), which were also positively associated with DASH score.
Table 1.
Age-standardized Baseline Participant Characteristics, by Eating Frequency per Day, Among 34,968 US Men From the Health Professional Follow-up Study, 1992–2006
| Eating Frequency, times/day |
||||||||
| 1–2 (n = 3,682; 10.5%) |
3 (n = 16,709; 47.8%) |
4 (n = 11,830; 33.8%) |
5–8 (n = 2,747; 7.9%) |
|||||
| Mean | % | Mean | % | Mean | % | Mean | % | |
| Age, years | 57 | 59.7 | 59 | 58 | ||||
| Body mass index in 1992a | 25.8 | 25.5 | 25.9 | 25.8 | ||||
| Pack-years of smoking before 30 years of age | 6.2 | 4.9 | 5.1 | 5.3 | ||||
| Current smoking | 14 | 7 | 5 | 4 | ||||
| Physical activity level, metabolic equivalent hours/week | 30.2 | 30.8 | 29.2 | 31.0 | ||||
| Family history of colorectal cancer | 8 | 8 | 8 | 8 | ||||
| Previous endoscopy | 24 | 29 | 31 | 33 | ||||
| Aspirin use (≥2 times/week) | 27 | 28 | 29 | 33 | ||||
| Multivitamin or antioxidant supplement useb | 48 | 53 | 52 | 56 | ||||
| Energy intake, kcal/day | 1,778 | 1,849 | 1,973 | 2,077 | ||||
| Alcohol intake, g/day | 15.1 | 11.2 | 8.2 | 7.3 | ||||
| Red meat intake, servings/day | 0.7 | 0.8 | 0.8 | 0.8 | ||||
| Dietary vitamin D intake, IU/dayc | 264 | 294 | 304 | 308 | ||||
| Dietary folate intake, μg/dayc | 345 | 376 | 375 | 371 | ||||
| Total calcium intake, mg/dayc,d | 848 | 904 | 925 | 973 | ||||
| DASH score | 22.2 | 24.0 | 24.6 | 25.3 | ||||
| Dietary glycemic load | 108 | 122 | 130 | 140 | ||||
| Coffee intake (includes decaffeinated coffee), servings/day | 2 | 1.8 | 1.8 | 1.9 | ||||
| Eating before breakfast | 1 | 1 | 1 | 3 | ||||
| Eating breakfast | 29 | 85 | 97 | 97 | ||||
| Eating between breakfast and lunch (brunch) | 9 | 3 | 7 | 56 | ||||
| Eating lunch | 49 | 93 | 98 | 99 | ||||
| Eating between lunch and dinner | 4 | 3 | 12 | 81 | ||||
| Eating dinner | 89 | 98 | 100 | 100 | ||||
| Eating before going to bed | 11 | 17 | 84 | 88 | ||||
| Eating after going to bed | 0 | 0 | 2 | 6 | ||||
| Snacking | 21 | 21 | 100 | 100 | ||||
Abbreviation: DASH, Dietary Approaches to Stop Hypertension.
Weight (kg)/height (m)2.
Any multivitamin supplements or any antioxidant supplements (e.g., vitamin E, vitamin A, vitamin C, selenium, beta carotene, coenzyme Q10, and lycopene).
Nutrient intakes are energy-adjusted.
Includes the use of supplements.
No statistically significant association was found between increased eating frequency, increased snack frequency, or breakfast pattern and the incidence of CRC or colon cancer. After adjusting for the known risk factors for CRC, results were close to the age-adjusted values, and the observed 12% lower risk of CRC and the 22% lower risk of colon cancer were not statistically significant when comparing persons in the highest eating frequency category (5–8 times/day) with those in the reference category (3 times/day) (Table 2). Further, no association was observed when comparing persons in the highest snack frequency category (2–4 snacks/day) with those in the lowest (0 snacks/day) for CRC and colon cancer risk (Table 3). Similarly, no statistically significant association was found with CRC and colon cancer risk when comparing participants who skipped breakfast and ate 1–7 times/day with persons who regularly had breakfast and ate 1–3 or 4–8 other times per day (Table 4). The results remained unchanged when participants who did not consume breakfast and ate 1–7 times per day were further broken down into 3 groups: persons who ate 1–2 times/day, persons who ate 3 times/day, and persons who ate 4–7 times/day (data not shown). All of these associations remained unchanged after further adjustment for coffee intake and glycemic load (data not shown).
Table 2.
Relative Risk of Colorectal Cancer and Colon Cancer for 4 Categories of Eating (Meals and Snacks) Frequency Among 34,968 US Men From the Health Professional Follow-up Studya, 1992–2006
| Eating Frequency, times/day | No. of Cases | Person-Years | Age Adjusted |
Multivariate Adjustedb |
||
| RR | 95% CI | RR | 95% CI | |||
| Colorectal cancer (n=583) | ||||||
| 1–2 | 59 | 47,956 | 1.13 | 0.85, 1.51 | 1.01 | 0.75, 1.34 |
| 3 | 281 | 214,772 | 1.00 | Referent | 1.00 | Referent |
| 4 | 207 | 153,777 | 1.07 | 0.89, 1.29 | 1.10 | 0.91, 1.32 |
| 5–8 | 36 | 35,898 | 0.85 | 0.60, 1.21 | 0.88 | 0.62, 1.26 |
| P for trend | 0.35 | 0.84 | ||||
| Colon cancer (n=375) | ||||||
| 1–2 | 36 | 47,976 | 1.11 | 0.77, 1.59 | 1.00 | 0.75, 1.34 |
| 3 | 175 | 214,874 | 1.00 | Referent | 1.00 | Referent |
| 4 | 144 | 153,839 | 1.21 | 0.96, 1.51 | 1.23 | 0.91, 1.32 |
| 5–8 | 20 | 35,912 | 0.77 | 0.48, 1.22 | 0.78 | 0.62, 1.26 |
| P for trend | 0.57 | 0.89 | ||||
Abbreviations: CI, confidence interval; RR, relative risk.
Values are from Cox proportional hazards models. All tests were 2-sided.
Multivariate models were adjusted for age (in months), aspirin use (≥2 times per week, <2 times per week, or missing), family history of colorectal cancer (yes, no, or missing), previous endoscopy (screening in the last 2 years, never, or missing), use of supplements containing antioxidants (ever, never, or missing), body mass index (weight (kg)/height (m)2; 24, 24–26.0, 26.1–29, or >29), energy intake (kilocalories/day, continuous), alcohol intake (0, 0.1–4.9, 5–14.9, or ≥15 g/day), physical activity level (quintile of metabolic equivalent hours/week), red meat consumption (quintile of servings/day), total calcium intake (quintile of mg/day), dietary folate intake (quintile of μg/day), dietary vitamin D intake (quintile of IU/day); pack years of smoking before 30 years of age (continuous or missing indicator), race (white, nonwhite, or missing), and Dietary Approaches to Stop Hypertension score (quintile).
Table 3.
Relative Risk of Colorectal Cancer and Colon Cancer for 3 Categories of Snacking Frequency Among 34,968 US Men From the Health Professional Follow-up Studya, 1992–2006
| Snacking Frequency, times/day | No. of Cases | Person-Years | Age Adjusted |
Multivariate Adjustedb |
||
| RR | 95% CI | RR | 95% CI | |||
| Colorectal cancer (n=583) | ||||||
| 0 | 279 | 208,934 | 1.00 | Referent | 1.00 | Referent |
| 1 | 248 | 194,161 | 1.07 | 0.89, 1.27 | 1.06 | 0.89, 1.26 |
| 2–4 | 56 | 49,309 | 0.99 | 0.74, 1.32 | 0.99 | 0.74, 1.34 |
| P for trend | 0.92 | 0.90 | ||||
| Colon cancer (n=375) | ||||||
| 0 | 170 | 209,038 | 1.00 | Referent | 1.00 | Referent |
| 1 | 172 | 194,234 | 1.23 | 0.99, 1.53 | 1.22 | 0.98, 1.52 |
| 2–4 | 33 | 49,329 | 0.97 | 0.66, 1.41 | 0.96 | 0.66, 1.41 |
| P for trend | 0.69 | 0.77 | ||||
Abbreviations: CI, confidence interval; RR, relative risk.
Values are from Cox proportional hazards models. All tests were 2-sided.
Multivariate models were adjusted for age (in months), aspirin use (≥2 times per week, <2 times per week, or missing), family history of colorectal cancer (yes, no, or missing), previous endoscopy (screening in the last 2 years, never, or missing), use of supplements containing antioxidants (ever, never, or missing), body mass index (weight (kg)/height (m)2; 24, 24–26.0, 26.1–29, or >29), energy intake (kilocalories/day, continuous), alcohol intake (0, 0.1–4.9, 5–14.9, or ≥15 g/day), physical activity level (quintile of metabolic equivalent hours/week), red meat consumption (quintile of servings/day), total calcium intake (quintile of mg/day), dietary folate intake (quintile of μg/day), dietary vitamin D intake (quintile of IU/day); pack years of smoking before 30 years of age (continuous or missing indicator), race (white, nonwhite, or missing), and Dietary Approaches to Stop Hypertension score (quintile).
Table 4.
Relative Risk of Colorectal Cancer and Colon Cancer for 3 Categories of Eating and Breakfast Frequency Among 34,968 US Men From the Health Professional Follow-up Studya, 1992–2006
| Breakfast and Eating Frequency, times/day | No. of Cases | Person-Years | Age Adjusted |
Multivariate Adjustedb |
||
| 95% CI | RR | 95% CI | RR | |||
| Colorectal cancer (n=583) | ||||||
| No and 1–7 times/day | 75 | 75,151 | 1.04 | 0.79, 1.35 | 0.87 | 0.66, 1.14 |
| Yes and 1–3 times/day | 273 | 194,423 | 1.00 | Referent | 1.00 | Referent |
| Yes and 4–7 times/day | 235 | 182,829 | 1.00 | 0.84, 1.20 | 1.02 | 0.85, 1.22 |
| P for trend | 0.98 | 0.65 | ||||
| Colon cancer (n=375) | ||||||
| No and 1–7 times/day | 49 | 75,172 | 1.13 | 0.81, 1.57 | 0.97 | 0.69, 1.36 |
| Yes and 1–3 times/day | 167 | 194,527 | 1.00 | Referent | 1.00 | Referent |
| Yes and 4–7 times/day | 159 | 182,903 | 1.12 | 0.90, 1.40 | 1.13 | 0.90, 1.42 |
| P for trend | 0.37 | 0.24 | ||||
Abbreviations: CI, confidence interval; RR, relative risk.
Values are from Cox proportional hazards models. All tests were 2-sided.
Multivariate models were adjusted for age (in months), aspirin use (≥2 times per week, <2 times per week, or missing), family history of colorectal cancer (yes, no, or missing), previous endoscopy (screening in the last 2 years, never, or missing), use of supplements containing antioxidants (ever, never, or missing), body mass index (weight (kg)/height (m)2; 24, 24–26.0, 26.1–29, or >29), energy intake (kilocalories/day, continuous), alcohol intake (0, 0.1–4.9, 5–14.9, or ≥15 g/day), physical activity level (quintile of metabolic equivalent hours/week), red meat consumption (quintile of servings/day), total calcium intake (quintile of mg/day), dietary folate intake (quintile of μg/day), dietary vitamin D intake (quintile of IU/day); pack years of smoking before 30 years of age (continuous or missing indicator), race (white, nonwhite, or missing), and Dietary Approaches to Stop Hypertension score (quintile).
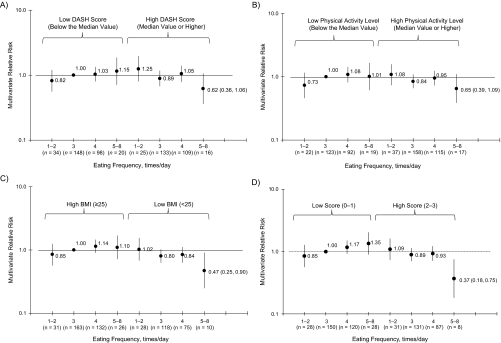
We then examined interactions with dietary patterns. An inverse association between meal frequency and CRC risk was suggested primarily in persons with a high DASH score (P for trend = 0.14; P for interaction = 0.01) (Figure 1A) and in persons with a low-glycemic-load diet (all relative risks <1 for the low glycemic load stratum), although the interaction was not significant (P for trend = 0.94, P for interaction = 0.99; data not shown). As for the factors associated with insulin sensitivity, the inverse association between increased eating frequency and CRC risk was stronger in participants who were highly insulin sensitive (i.e., coffee intake ≥2 cups/day) (P for trend = 0.25, P for interaction = 0.20; data not shown), had a physical activity level higher than the median value (P for trend = 0.13, P for interaction = 0.04; Figure 1B), and had a BMI <25 (P for trend = 0.04, P for interaction = 0.07; Figure 1C). Moreover, when these 3 insulin-sensitivity variables were combined, the inverse association was observed only in the high-insulin-sensitivity group (P for trend = 0.01, P for interaction < 0.01; Figure 1D).
Figure 1.
Associations between colorectal cancer and other variables in 34,968 US men from the Health Professional Follow-up Study, 1992–2006. Values are hazard ratios from Cox proportional hazards models. All tests were 2-sided. A) Joint association between eating frequency and Dietary Approaches to Stop Hypertension (DASH) score and colorectal cancer risk (P for interaction = 0.01). Multivariate models were adjusted for age (in months), aspirin use (≥2 times per week, <2 times per week, or missing), family history of colorectal cancer (yes, no, or missing), previous endoscopy (screening in the past 2 years, never, or missing), use of supplements containing antioxidants (ever, never, or missing), body mass index (BMI, measured as weight (kg)/height (m)2; 24, 24–26.0, 26.1–29, or >29), energy intake (kilocalories/day, continuous), alcohol intake (0, 0.1–4.9, 5–14.9, or ≥15 g/day), physical activity level (quintile of metabolic equivalent hours/week), red meat consumption (quintile of servings/day), total calcium intake (quintile of mg/day), dietary folate intake (quintile of μg/day), dietary vitamin D intake (quintile of IU/day), pack years of smoking before 30 years of age (continuous or missing indicator), race (white, nonwhite, or missing), and DASH score (continuous) together with eating frequency (continuous). B) Joint association between eating frequency and physical activity level and colorectal cancer risk (P for interaction = 0.04). The same adjustments were made to the model as in A, except that physical activity level (median of deciles) was combined with eating frequency (continuous). C) Joint association between eating frequency and BMI with colorectal cancer risk (P for interaction = 0.07). The same adjustments were made to the model as in A, except that BMI (median of deciles) was combined with eating frequency (continuous). D) Joint association between eating frequency and insulin resistance score and colorectal cancer risk (P for interaction < 0.01). The same adjustments were made to the model as in A, except that physical activity level and BMI were omitted. The insulin sensitivity score consisted of the 3 combined variables (coffee intake, physical activity level, and BMI) and was categorized into 2 groups (0–1 vs. 2–3), such that a higher score denoted less insulin sensitivity (i.e., a relatively insulin-sensitive individual would get the highest score of 3 and an insulin-resistant person would get the worst score of 0). Bars and numbers in parentheses, 95% confidence intervals.
Additionally, the implied decreased association between increased snack frequency (2–4 snacks/day) and the risk of CRC or colon cancer was only observed among participants whose diets fell in the higher DASH score category (data not shown), who drank 2 or more cups of coffee per day (data not shown), who were more physically active (data not shown), or who had a lower BMI (data not shown). The only exception was glycemic load. As for breakfast consumption pattern, the joint analysis revealed no particular benefit for participants who consumed breakfast and who were either in the high DASH score category, the low glycemic load category, the coffee drinkers category, the more physically active group, or the lower BMI category when compared with persons who skipped breakfast and fell within a category opposite of one of those mentioned (data not shown). For the above multivariate relative risks derived from the basic multivariate model that included the standard risk factor for CRC and DASH score, further adjustment for coffee intake and glycemic load did not modify the results; therefore, the results are not shown.
DISCUSSION
In contrast to previous studies, we did not observe a positive association between frequencies of eating or snacking and CRC or colon cancer risk in the present prospective study. A higher diet quality as reflected by a higher DASH score showed a suggestive, yet not statistically significant, inverse association between increased meal or snack frequency—but not breakfast consumption—and CRC or colon cancer risk. Additionally, an inverse association between eating frequency and CRC or colon cancer was observed only in participants who had high insulin sensitivity (persons who drank >2 servings/day of coffee), were physically active, and were lean (BMI <25)).
Few studies have assessed the relation between meal frequency and quality and CRC or colon cancer risk. Most of these studies were case-control studies that showed a direct association between the frequency of eating (meals and snacks) and colon cancer risk (3–5) or CRC risk (6–8), even after adjustment for potential confounders, including total energy intake (3, 7). However, 2 studies did not reach statistical significance (3, 4). Conversely, in 1 case-control study, Shoff et al. (10) found that although the frequency of snacking or eating (meals and snacks) was not associated with CRC, meal frequency was positively associated with CRC risk in women. The authors suggested that the nutrient composition of the meals and snacks (e.g., high fat vs. low fat) might be more relevant than the frequency of meals per se. In another case-control study, de Verdier and Longnecker (9) found that although frequency of snacking was directly associated with colon cancer risk, the frequency of eating meals (breakfast, lunch, and dinner) was not. Presumably, these snacks could be high in sucrose, which has been hypothesized to be positively associated with CRC risk (24).
From a mechanistic perspective, arguments can be made as to why increased meal frequency can decrease or increase CRC risk. Increased meal frequency could be deleterious by influencing the concentration of bile acids subsequent to food intake (9). Bile acids are secreted from the gallbladder into the bowel upon each food intake. A small amount of bile acid is absorbed into the colon to be transformed into secondary bile acids by bacterial activity (25). Secondary bile acids may damage DNA and promote cellular proliferation in the epithelium of the colon (26). Their role in colorectal carcinogenesis has been suggested in animal and human studies (26, 27). A decreased serum cholesterol level due to increased meal frequency (15) could potentially reflect increased excretion of bile acids into the intestinal lumen. A second potential mechanism is the rise in serum glucose and insulin levels that occurs after every meal (14), which could promote colon tumor growth.
On the other hand, infrequent eating might have deleterious effects. Men who ate infrequently had higher rates of obesity, hypercholesterolemia, impaired glucose tolerance, and ischemic heart disease than did men who ate 5 or more meals/day (28), possibly because of the lower insulin levels (15) and decreased adipocyte enzyme activity (29). Although insulin secretion occurs after any food consumption, the magnitude of the rise in insulin is contingent on the glycemic load of the meal consumed and the degree of insulin insensitivity (14). Our results suggested an inverse association between eating frequency or snack frequency and CRC risk only among participants who had factors consistent with improved insulin sensitivity, such as a high coffee intake, a high physical activity level, and a low BMI. Because detailed information on the types of snacks consumed was not available, we used the DASH score and glycemic load to reflect the quality of these snacks. One would expect that participants who had the highest DASH scores or were in the lowest glycemic load category and who ate more frequently would be at a decreased risk of CRC as opposed to persons who had the lowest DASH scores or were in the highest glycemic load category and who ate less frequently. This pattern was clearly suggested in CRC and colon cancer risk in the joint analysis of eating frequency and DASH score and less clearly in the joint analysis of eating frequency and glycemic load. It is possible that frequent meals with low glycemic loads in insulin-sensitive individuals may keep the insulin concentration at a low flat level rather than at the high spikes that result from consumption of fewer meals with higher glycemic load.
There are several limitations to the present study. First, nondifferential measurement error in our assessment of eating frequency in 1992 may have biased our results toward the null (30). Although another question about eating frequency was asked in 2004, the question did not differentiate between meals and snacks. In addition, the association between the total number of eating times in 1992 and 2004 was modest (Spearman correlation coefficient = 0.33). Hence, repeated assessment of the meal frequency question over a 12-year period would have reduced random within-person measurement error (23). Second, there was no information about the nutrient composition of the snacks. However, the DASH score was used to assess diet quality. Third, we had limited power to evaluate associations between eating frequency and rectal cancer risk. Fourth, we had limited power to divide participants who skipped breakfast into 2 groups (i.e., no breakfast and 1–3 meals/day vs. no breakfast and 4–8 meals/day) to clearly remove the potential effect of eating frequency from skipping breakfast.
Despite these limitations, the present study has several strengths. First, it included a large sample of men who were followed for 14 years. Second, to our knowledge, this is the first prospective study design used to assess the relation between eating frequency and CRC risk. Third, information on a wide variety of potential confounding variables was collected repeatedly during follow-up, which allowed for more complete control of known potential confounders.
In conclusion, our findings do not support a direct association between eating frequency and the risk of CRC and colon cancer. Contrarily, there was an implied protective association with increased eating frequency of healthy meals and CRC risk, especially in individuals with presumably high insulin sensitivity. Further studies are needed to elucidate this association in women and in other ethnic and racial groups and to confirm factors that might modify this association.
Acknowledgments
Author affiliations: Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts (Rania A. Mekary, Frank B. Hu, Walter C. Willett, Stephanie Chiuve, Kana Wu, Teresa T. Fung, Edward Giovannucci); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Frank B. Hu, Walter C. Willett, Edward Giovannucci); Department of Nutrition, Simmons College, Boston, Massachusetts (Teresa T. Fung); Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, Massachusetts (Frank B. Hu, Walter C. Willett, Stephanie Chiuve, Edward Giovannucci); and Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts (Charles Fuchs).
This work was supported by National Institutes of Health grants CA55075 and CA95589.
W. C. W. obtained study funding and was an investigator in the Nurses’ Health Study. R. A. M., F. B. H., W. C. W., S. C., K. W., C. F., T. T. F., and E. G. collected data and had the idea for the current analysis. R. A. M., F. B. H., W. C. W., S. C., C. F., T. T. F., and E. G. provided statistical expertise. R. A. M., T. T. F., and E. G. analyzed the data. R. A. M. wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript.
The funding sources were not involved in data collection, data analysis, manuscript writing, or publication.
Conflict of interest: none declared.
Glossary
Abbreviations
- BMI
body mass index
- CRC
colorectal cancer
- DASH
Dietary Approaches to Stop Hypertension
- FFQ
food frequency questionnaire
References
- 1.American Cancer Society. What Are the Key Statistics for Colorectal Cancer? Atlanta, GA: American Cancer Society; 2008. ( http://www.cancer.org/Cancer/ColonandRectumCancer/DetailedGuide/colorectal-cancer-key-statistics). (Accessed May 9, 2011) [Google Scholar]
- 2.International Agency for Research on Cancer. Cancer Incidence in Five Continents. Lyon, France: IARC; 2005. [Google Scholar]
- 3.Potter JD, McMichael AJ. Diet and cancer of the colon and rectum: a case-control study. J Natl Cancer Inst. 1986;76(4):557–569. doi: 10.1093/jnci/76.4.557. [DOI] [PubMed] [Google Scholar]
- 4.Young TB, Wolf DA. Case-control study of proximal and distal colon cancer and diet in Wisconsin. Int J Cancer. 1988;42(2):167–175. doi: 10.1002/ijc.2910420205. [DOI] [PubMed] [Google Scholar]
- 5.Favero A, Franceschi S, La Vecchia C, et al. Meal frequency and coffee intake in colon cancer. Nutr Cancer. 1998;30(3):182–185. doi: 10.1080/01635589809514661. [DOI] [PubMed] [Google Scholar]
- 6.Benito E, Obrador A, Stiggelbout A, et al. A population-based case-control study of colorectal cancer in Majorca. I: Dietary factors. Int J Cancer. 1990;45(1):69–76. doi: 10.1002/ijc.2910450114. [DOI] [PubMed] [Google Scholar]
- 7.Franceschi S, La Vecchia C, Bidoli E, et al. Meal frequency and risk of colorectal cancer. Cancer Res. 1992;52(13):3589–3592. [PubMed] [Google Scholar]
- 8.La Vecchia C, Ferraroni M, Mezzetti M, et al. Attributable risks for colorectal cancer in northern Italy. Int J Cancer. 1996;66(1):60–64. doi: 10.1002/(SICI)1097-0215(19960328)66:1<60::AID-IJC11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.de Verdier MG, Longnecker MP. Eating frequency—a neglected risk factor for colon cancer? Cancer Causes Control. 1992;3(1):77–81. doi: 10.1007/BF00051916. [DOI] [PubMed] [Google Scholar]
- 10.Shoff SM, Newcomb PA, Longnecker MP. Frequency of eating and risk of colorectal cancer in women. Nutr Cancer. 1997;27(1):22–25. doi: 10.1080/01635589709514496. [DOI] [PubMed] [Google Scholar]
- 11.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 12.Dixon LB, Subar AF, Peters U, et al. Adherence to the USDA Food Guide, DASH Eating Plan, and Mediterranean dietary pattern reduces risk of colorectal adenoma. J Nutr. 2007;137(11):2443–2450. doi: 10.1093/jn/137.11.2443. [DOI] [PubMed] [Google Scholar]
- 13.Fung TT, Hu FB, Wu K, et al. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr. 2010;92(6):1429–1435. doi: 10.3945/ajcn.2010.29242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6(2):164–179. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins DJ, Wolever TM, Vuksan V, et al. Nibbling versus gorging: metabolic advantages of increased meal frequency. N Engl J Med. 1989;321(14):929–934. doi: 10.1056/NEJM198910053211403. [DOI] [PubMed] [Google Scholar]
- 16.Wu T, Willett WC, Hankinson SE, et al. Caffeinated coffee, decaffeinated coffee, and caffeine in relation to plasma C-peptide levels, a marker of insulin secretion, in U.S. women. Diabetes Care. 2005;28(6):1390–1396. doi: 10.2337/diacare.28.6.1390. [DOI] [PubMed] [Google Scholar]
- 17.Qi L, Hu FB, Hu G. Genes, environment, and interactions in prevention of type 2 diabetes: a focus on physical activity and lifestyle changes. Curr Mol Med. 2008;8(6):519–532. doi: 10.2174/156652408785747915. [DOI] [PubMed] [Google Scholar]
- 18.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 19.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 20.National Heart, Lung, and Blood Institute. Your Guide to Lowering Your Blood Pressure With DASH. Bethesda, MD: National Institutes of Health; 2006. ( http://www.nhlbi.nih.gov/health/public/heart/hbp/dash/). (Accessed May 9, 2011) [Google Scholar]
- 21.Fung TT, Chiuve SE, McCullough ML, et al. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 22.Wei EK, Giovannucci E, Wu K, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108(3):433–442. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willett WC. Nutritional Epidemiology. 2nd ed. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 24.Franceschi S, Favero A, La Vecchia C, et al. Food groups and risk of colorectal cancer in Italy. Int J Cancer. 1997;72(1):56–61. doi: 10.1002/(sici)1097-0215(19970703)72:1<56::aid-ijc8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman A. The enterohepatic circulation of bile acids in health and disease. In: Sleisenger MH, Fordtran JS, editors. Gastrointestinal Disease. Philadelphia, PA: W. B. Saunders Company; 1989. pp. 144–161. [Google Scholar]
- 26.Cheah PY. Hypotheses for the etiology of colorectal cancer—an overview. Nutr Cancer. 1990;14(1):5–13. doi: 10.1080/01635589009514073. [DOI] [PubMed] [Google Scholar]
- 27.Hill MJ. Aetiology of colorectal cancer: current concepts. Baillieres Clin Gastroenterol. 1989;3(3):567–592. doi: 10.1016/0950-3528(89)90018-3. [DOI] [PubMed] [Google Scholar]
- 28.Fábry P, Tepperman J. Meal frequency—a possible factor in human pathology. Am J Clin Nutr. 1970;23(8):1059–1068. doi: 10.1093/ajcn/23.8.1059. [DOI] [PubMed] [Google Scholar]
- 29.Bray GA. Lipogenesis in human adipose tissue: some effects of nibbling and gorging. J Clin Invest. 1972;51(3):537–548. doi: 10.1172/JCI106842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barron BA. The effects of misclassification on the estimation of relative risk. Biometrics. 1977;33(2):414–418. [PubMed] [Google Scholar]