Abstract
The present study examined the effect of dietary genistein, a soy isoflavone, on breast cancer patients who take tamoxifen, an antiestrogen treatment, using a preclinical model. The interaction of various doses of genistein with tamoxifen on the growth of estrogen receptor-positive breast cancer MCF-7 cells was investigated by subcutaneously injecting MCF-7 cells into the flank of ovariectomized athymic mice. Animals were randomized into eight experimental groups with 10–13 mice per group: control (C), estrogen (E) (0.08 mg E implant), tamoxifen (T) (3 mg T implant), estrogen + tamoxifen (E + T), tamoxifen + 500 p.p.m. genistein (T + G500), estrogen + tamoxifen + 250 p.p.m. genistein (E + T + G250), estrogen + tamoxifen + 500 p.p.m. genistein (E + T + G500) and estrogen + tamoxifen + 1000 p.p.m. genistein (E + T + G1000). Treatment of tamoxifen significantly reduced the estrogen-induced MCF-7 tumor prevalence and tumor size. This inhibitory effect of tamoxifen was significantly negated by the low doses of dietary genistein (250 and 500 p.p.m.), whereas the 1000 p.p.m. genistein did not have the same effect. Cells harvested from tamoxifen-treated tumors retained estrogen responsiveness of their progenitor MCF-7 cells, indicating that the abrogating effect of genistein on tamoxifen-treated tumor growth was not caused by a diminished tamoxifen response but directly by genistein. The low doses of dietary genistein abrogated the inhibitory effect of tamoxifen potentially by acting on the tumor cell proliferation/apoptosis ratio and the messenger RNA (mRNA) expression of cyclin D1 in addition to regulating the mRNA expression of progesterone receptor. Therefore, data from the current study suggest that caution is warranted regarding the consumption of dietary genistein by breast cancer patients while on tamoxifen therapy.
Introduction
Breast cancer is the most common type of cancer among women in the USA and accounts for one in four newly diagnosed female cancer cases (1). More than 75% of breast cancer cases occur in postmenopausal women (2). In addition, women diagnosed with breast cancer usually experience more menopausal symptoms than healthy women (3). Menopausal symptoms that are caused by reduced hormonal levels, primarily estrogen and progesterone, can be relieved with hormone replacement therapy (HRT). However, long-term use of HRT is associated with the increased risk of developing breast cancer (4), and further work has shown that estrogen receptor-positive (ER+) breast cancer incidence decreased following the drop in HRT use that began in 2002 (5). The decrease was particularly pronounced among women aged 50–69 years, a group in which HRT use was the most common. Given these reports, in an effort to avoid the risk of recurrence, women with breast cancer may seek alternative options to relieve menopausal symptoms, which could include dietary supplements commonly available on the market.
Isoflavones, with soybeans as the most significant source, are a group of plant-derived compounds that have similar chemical structures to the female hormone, 17β-estradiol (estrogen). Due to this structural similarity, isoflavones can bind to estrogen receptors (ERs) and exhibit estrogen-like properties (6). High dietary intake of soybean foods has been associated with lower risk of breast cancer among Asian women, particularly for prepubertal exposures (7), which has helped to promote the perception that consuming soy isoflavones is safe or even beneficial for breast cancer prevention and treatment. This assumption may not be correct. Since ∼75% of breast cancers are ER+ (8), patients are likely to be treated with endocrine therapy using either tamoxifen or aromatase inhibitors (9,10). Although aromatase inhibitors show advantages over tamoxifen in treating breast cancer in postmenopausal women, tamoxifen is still being widely used as an antiestrogen drug throughout the world (11). We have previously examined the interaction of a soy isoflavone, genistein, with one of the aromatase inhibitors, letrozole, on the growth of aromatase-expressing MCF-7Ca tumor cells, and the result indicated that genistein negated the inhibitory effect of letrozole (12). In the present study, we examined the combined effect of tamoxifen and genistein on human breast MCF-7 tumor growth in mice. Tamoxifen acts by competing with estrogen for ER binding and then inhibiting the actions of estrogen on ER-mediated gene transcription, DNA synthesis and cancer cell growth (13). Since isoflavones can act as estrogen agonists at physiologically relevant doses (14), the clinical response to tamoxifen therapy may be affected if coadministered with dietary isoflavones, thereby decreasing its efficacy.
Genistein, the major isoflavone present in soybeans, at physiological doses stimulates the growth of ER+ breast cancer cells (14–17), thus having the potential to negate the efficacy of tamoxifen. Several animal studies have investigated the combined effect of isoflavones and tamoxifen on mammary tumor growth. Ju et al. (18) demonstrated that dietary genistein at 1000 p.p.m. abrogated the inhibitory effect of tamoxifen on ER+ human breast cancer MCF-7 cells in ovariectomized athymic mice. Liu et al. (19) reported that a diet supplemented with a low dose of total isoflavones (211 p.p.m.) abrogated the effect of tamoxifen in preventing cancer in ErbB2/neu transgenic mice, whereas a diet with a high dose of total isoflavones (491 p.p.m.) did not have the same effect. A similar response was observed by Constantinou et al. (20), showing that genistein (140 p.p.m.) counteracted the effect of tamoxifen on tumor incidence, tumor latency and survival rate in rats. These reports indicated that soy isoflavone-containing diets fed to rodents increased tumor growth in the presence of tamoxifen, suggesting that the combination of genistein and tamoxifen may negatively affect breast cancer development. An opposite effect, however, was also observed in other studies, providing evidence that genistein given with tamoxifen elicited synergistic effects on the inhibition of tumor growth, both in vitro and in vivo (21–23). Considering this conflicting evidence, the concurrent use of dietary genistein with tamoxifen remains a safety concern and needs further investigation.
We have previously demonstrated that a diet containing 1000 p.p.m. genistein abrogated the inhibitory effect of tamoxifen (2.5–5.0 mg) on MCF-7 cells in the presence of a 0.25 mg estrogen implant in ovariectomized athymic mice (18). In the current study, to better represent plasma estrogen levels in postmenopausal women, we decreased the amount of estrogen in the implant to 0.08 mg. Furthermore, considering the differential effects of genistein on cancer cell growth, we conducted a comprehensive analysis to investigate the interaction of genistein at several dietary levels (250, 500 and 1000 p.p.m.) with tamoxifen, using the previously utilized model to determine whether dietary genistein could negate the beneficial effect of tamoxifen at different dietary levels.
Materials and methods
Materials
Minimal essential medium (MEM) and phenol red-free MEM were purchased from the Media Facility at the University of Illinois at Urbana-Champaign. Bovine calf serum (BCS) was purchased from Hyclone (Logan, UT). Matrigel™ matrix was obtained from BD Biosciences (San Jose, CA). The AIN-93G semi-purified diet was purchased from Research Diets (Brunswick, NJ). Estrogen, tamoxifen, cholesterol and ICI 182 780 were purchased from Sigma–Aldrich (St Louis, MO). Genistein was purchased from Indofine Chemical Company (Hillsborough, NJ).
Cell culture
MCF-7 cells were maintained in MEM supplemented with 5% BCS, 100 U/ml penicillin, 100 μg/ml streptomycin and 1 nM estrogen at 37°C in a humidified 5% CO2 incubator. One week prior to cell inoculation, the media was switched to phenol red-free MEM containing 5% charcoal dextran-stripped BCS. The cells were collected at 70% confluence using trypsin/ethylenediaminetetraacetic acid, counted and suspended in Matrigel™ matrix for injection.
Silastic implants
Three types of silastic implants (cholesterol, estrogen and tamoxifen) were prepared as described (18). The cholesterol implant (Chol) contained 3 mg cholesterol. The estrogen implant (E) contained 0.08 mg estrogen and 3.76 mg cholesterol (1:47 estrogen:cholesterol). The tamoxifen implant (T) contained 3 mg tamoxifen and 9 mg cholesterol. Both ends of the silastic tubing were sealed with medical silicone adhesive. The implants were sterilized with 70% ethanol and conditioned with PBS before implantation.
Animals and diets
Female athymic nude mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were ovariectomized at 21 days of age by the vendor and were allowed 7 days for acclimation after arrival. Each animal was housed separately with free access to food and water. Artificial light was provided with a 12 h light/dark cycle. The AIN-93G semi-purified diet was selected as a base diet as it has been established to meet all nutritional requirements of mice (24). Instead of soybean oil, corn oil was used to prepare the diet to eliminate any additional components of soy being added into the diet. Animal care was conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana-Champaign.
Study design
Mice were randomly divided into eight groups with 10–13 animals per group: control (C), estrogen (E), tamoxifen (T), estrogen + tamoxifen (E + T), tamoxifen + 500 p.p.m. genistein (T + G500), estrogen + tamoxifen + 250 p.p.m. genistein (E + T + G250), estrogen + tamoxifen + 500 p.p.m. genistein (E + T + G500) and estrogen + tamoxifen + 1000 p.p.m. genistein (E + T + G1000). Animals in the control and estrogen groups received a cholesterol or an estrogen implant in the inter-scapular region, respectively. Animals in the T and T + G500 groups received a tamoxifen implant and animals in the E + T, E + T + G250, E + T + G500, E + T + G1000 groups received both estrogen and tamoxifen implants. Two days after the implantation, MCF-7 cells were injected subcutaneously at 1 × 105 cells in 40 μl Matrigel™ per site into four sites on the flank of each mouse and dietary treatment was initiated. The animals in the T + G500, E + T + G250, E + T + G500, E + T + G1000 received the AIN-93G diet supplemented with either 500, 250, 500 or 1000 p.p.m. genistein, respectively. The remaining animals received the control AIN-93G diet. Food intake and body weight were measured regularly throughout the study.
Tumor growth monitoring
MCF-7 tumors were measured weekly using a caliper from 4 weeks post-cell inoculation. Tumor size was determined by calculating the cross-sectional area using the formula length/2 × width/2 × 3.14 and each tumor was considered separately when calculating the mean tumor size for individual treatment groups. Total of estimated tumors for each group was calculated as animal number multiplied by 4, as MCF-7 cells were injected into four sites on the flank of each mouse. Tumor-free number for each group was calculated as total of estimated tumor subtracted by total of palpable tumor. Tumor prevalence was calculated as the percentage of total of palpable tumors relative to total of estimated tumors for each group at the end of the study.
Tumor cell characterization
Tumors from the E + T + G250, E + T + G500 and E + T + G1000 groups were harvested for primary cell culture. The tumors were minced and transferred to cell culture plates. The harvested cells were maintained in MEM supplemented with 5% BCS and 1 nM estrogen. One week prior to treatment, the media was switched to phenol red-free MEM containing 5% charcoal dextran-stripped BCS. MCF-7 cells and the tumor cells were seeded in 24-well plates at 5000 cells per well and treated with estrogen (1 nM), estrogen (1 nM) + tamoxifen (1 μM) or estrogen (1 nM) + ICI 182 780 (1 μM) for 6 days. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was used to determine cell viability. Optical density at 570 nm was measured and normalized against the control. The experiment was repeated three times.
Analysis of proliferation and apoptosis in tumors
Harvested tumor samples were fixed in 10% formalin and embedded in paraffin blocks, cut into 5 μm sections and placed on microscopic slides. Cell proliferation in tumors was determined using immunohistochemical staining for the cell proliferation marker, Ki-67, as described (18). In brief, the slides were incubated with a 1:3000 primary anti-human Ki-67 antibody (Pharmingen, San Diego, CA) at 24°C in a humidified chamber for 2 h and then incubated with a biotinylated anti-mouse secondary antibody (VECTASTAIN Elite ABS reagent; Vector Laboratories, Burlingame, CA) for 30 min at room temperature. Positive cells were stained brown with 3,3′-diaminobenzidine tetrahydrachloride, and background cells were stained blue with hematoxylin. Cell apoptosis in tumors was analyzed using In situ cell death detection kit (Roche Diagnostics GmbH, Mennheim, Germany) and terminal deoxynucleotidyl transferase biotin-deoxyuridine triphosphate nick end labeling (TUNEL) staining was performed following the manufacture's procedure. Positive cells were green fluorescent and background cells were blue fluorescent. Both the positive and the background cells in Ki-67 or TUNEL staining were counted in a given field of tissue under an AxioSkop 40 microscope (Carl Zeiss, Thornwood, NY). Six tumors within the range of mean ± standard error from individual treatment groups were selected for proliferation and apoptosis molecular analyses. A total of 30 fields from the six tumors per experimental group were randomly selected for evaluation. Data are presented either as proliferative index (PI) or apoptotic index (AI) as the percentage of proliferative or apoptotic cells in a given area of tumor tissue.
Analysis of gene expression in tumors
Harvested tumors were immediately frozen in liquid N2, and RNA was extracted as described (18). Complementary DNA was generated using SuperScript First-Strand Synthesis System for RT–PCR (Invitrogen, Carlsbad, CA) following the company's protocol. The messenger RNA (mRNA) expression of an estrogen-responsive gene progesterone receptor (PR) and a cell cycle-regulated gene cyclin D1 was analyzed using ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). 36B4 housekeeping gene was used as internal control and the results were normalized to the value of 36B4 gene using the ΔΔCT method (25). The primers were ordered from Integrated DNA Technologies (Coralville, IA) and the sequences for each gene were: PR (forward: 5′-GAACCAGATGTGATCTATGCAGGA-3′; reverse: 5′-CGAAAACCTGGCAATGATTTAGAC-3′); cyclin D1 (forward: 5′-AGAGGCGGAGAACAAAC-3′; reverse: 5′-GGCACAAGAGGCAACGAA-3′) and 36B4 (forward: 5′-CGACCTGGAAGTCCAACTAC-3′; reverse: 5′-ATCTGCTGCATCTGCTTG-3′).
Analysis of plasma estrogen concentrations
Blood samples were collected by cardiac puncture at the time of sacrifice and centrifuged at 2500 g for 30 min at 4°C. Plasma estrogen levels were measured using Estradiol ELISA kit following the company's protocol (Alpha Diagnostic International, San Antonio, TX). Briefly, 50 μl of each plasma sample was transferred into an estrogen antibody-coated plate in duplicate. After 1 h of incubation at 24°C, the plate was read at 450 nm using a μQuant plate reader (Bio-Tek Instruments, Winooski, VT). The limit of detection was 10 pg/ml and the intra-assay coefficient of variation was <3%.
Statistics
Tumor prevalence was analyzed using Fisher's exact test. The remaining data were analyzed with one-way analysis of variance with Fisher's LSD post hoc analysis using the SPSS statistical software (SPSS, Chicago, IL). Error bars in all graphs represent standard error of the mean. P < 0.05 is considered statistically significant.
Results
Low-dose dietary genistein negated the inhibitory effect of tamoxifen on human breast cancer MCF-7 tumor growth and tumor prevalence in mice
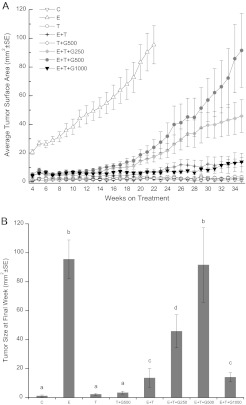
To examine the interaction of genistein at several dietary levels with tamoxifen on tumor growth, 1 × 105 human breast cancer MCF-7 cells were subcutaneously injected into mice. Silastic tubings of estrogen (E) and/or tamoxifen (T) were implanted and genistein was fed as a dietary supplement as described in Materials and methods. The E group was terminated at week 22 due to tumor burden and the remaining experimental groups were continued until 35 weeks post-cell inoculation (Figure 1A). Figure 1B displays the average cross-sectional tumor area for each group at the end of the treatment. No significant differences were observed in the average tumor size between the C (1.0 ± 0.7 mm2), T (2.2 ± 0.8 mm2) and T + G500 groups (3.3 ± 1.1 mm2), indicating that tamoxifen alone or tamoxifen plus 500 p.p.m. genistein did not affect tumor growth in the absence of estrogen. Exogenous estrogen significantly increased tumor size in the E group to 95.5 ± 13.2 mm2 when compared with the C group (P < 0.05), and tamoxifen significantly inhibited the increase in tumor size induced by estrogen. The tumor size in the E + T group was reduced to 13.4 ± 6.5 mm2 (P < 0.05 when compared with the E group). The inhibitory effect of tamoxifen on tumor growth was significantly abrogated by low doses of dietary genistein in a dose-dependent manner (E + T + G250, 45.8 ± 12.7 mm2, P < 0.05; E + T + G500, 91.6 ± 25.4 mm2, P < 0.05), whereas the high dose of genistein (E + T + G1000, 13.9 ± 3.1mm2) did not negate the inhibitory effect of tamoxifen (E + T, 13.4 ± 6.5 mm2) on tumor growth.
Fig. 1.
Low-dose dietary genistein negated the inhibitory effect of tamoxifen on human breast cancer MCF-7 tumor growth in athymic nude mice. (A) Tumor growth curves over 35 weeks. (B) Average tumor size at termination. Female ovariectomized athymic nude mice received subcutaneous injection of 1 × 105 MCF-7 cells and were randomly assigned into eight groups: (i) control (C), (ii) estrogen (E), (iii) tamoxifen (T), (iv) estrogen + tamoxifen (E + T), (v) tamoxifen + 500 p.p.m. genistein (T + G500), (vi) estrogen + tamoxifen + 250 p.p.m. genistein (E + T + G250), (vii) estrogen + tamoxifen + 500 p.p.m. genistein (E + T + G500) and (viii) estrogen + tamoxifen + 1000 p.p.m. genistein (E + T + G1000). Estrogen, tamoxifen and cholesterol implants were subcutaneously implanted accordingly, and the T + G500, E + T + G250, E + T + G500 and E + T + G1000 group animals received diets supplemented with 500, 250, 500 and 1000 p.p.m. genistein, respectively. Data are presented as mean ± standard error (SE) and analyzed using one-way analysis of variance. Bars with different letters indicate significant differences at the level of 0.05.
Tumor prevalence for each group observed at termination (Table I) was consistent with the result of tumor size analysis. Tumor prevalence in the C, T and T + G500 groups was <20% with no significant differences among these three groups. The E group showed the highest prevalence of 95.8% when compared with the C group (P < 0.001), whereas tamoxifen reduced the prevalence to 30.0% when compared with the E group (P < 0.001). The low doses of dietary genistein significantly negated the inhibitory effect of tamoxifen on tumor prevalence (E + T + G250, 65.4%, P < 0.01; E + T + G500, 65.0%, P < 0.01), whereas tumor prevalence in the high dose of genistein group (E + T + G1000, 46.2%, P = 0.135) was not significantly different from the E + T group.
Table I.
Low-dose dietary genistein negated the inhibitory effect of tamoxifen on tumor prevalence in mice
| Total estimated tumors | Palpable tumors | Tumor free | Tumor prevalence | |
| C | 48 | 3 | 45 | 6.3% |
| Ea | 48 | 46 | 2 | 95.8% |
| T | 48 | 9 | 39 | 18.8% |
| E + Tab | 40 | 12 | 28 | 30.0% |
| T + G500 | 44 | 9 | 35 | 20.5% |
| E + T + G250abc | 52 | 34 | 18 | 65.4% |
| E + T + G500abc | 40 | 26 | 14 | 65.0% |
| E + T + G1000ab | 52 | 24 | 28 | 46.2% |
Total of estimated tumors for each group was calculated as animal number for each group multiplied by 4, as MCF-7 cells were injected into four sites on the flank of each mouse. Tumor-free number for each group was calculated as total of estimated tumor subtracted by total of palpable tumor. Tumor prevalence for each group was calculated as total of palpable tumor divided by total of estimated tumor number. Fisher's exact test was used to calculate the P value between two experimental groups. No significant difference was observed between the C, T and T + G500 groups. The differences between the T, T + G500 groups and the E, E + T and E + T + G-treated groups were not analyzed.
Significantly different with the C group, P < 0.001.
Significantly different with the E group, P < 0.001.
Significantly different with the E + T group, P < 0.01.
Tumor cells harvested from E + T + G-treated mice retained estrogen responsiveness
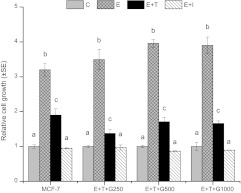
To confirm that tumor cells retained their estrogen responsiveness, tumor cells harvested from the E + T + G250, E + T + G500 and E + T + G1000 animals, along with parental MCF-7 cells, were cultured in vitro. The estrogen-dependent nature of MCF-7 cells was confirmed, as 1 nM estrogen induced cell growth by 3.2-fold of control, while 1 μM tamoxifen and 1 μM ICI 182 780 reduced the stimulatory effect of estrogen to <2-fold of control (P < 0.05) (Figure 2). Under the same condition, the growth of the harvested tumor cells from the E + T + G250, E + T + G500 and E + T + G1000 animals was similar to that seen with MCF-7 cells, in that, estrogen stimulated cell growth by 3.5- to 3.9-fold and tamoxifen and ICI 182 780 inhibited cell growth to <1.8-fold of control (P < 0.05) (Figure 2). Although individual differences among tumor cells existed, the overall responsiveness to estrogen and antiestrogens demonstrated that these tumors after 35 weeks treatment retained their original estrogen-dependent phenotype.
Fig. 2.
Tumor cells harvested from the E + T + G-treated mice retained the estrogen-dependent phenotype as MCF-7 cells. Tumors harvested from the E + T + G250, E + T + G500 and E + T + G1000 animals were processed and the harvested tumor cells were cultured in vitro to evaluate the responsiveness to estrogen. The tumor cells were treated with estrogen (1 nM), estrogen (1 nM) + tamoxifen (T, 1 μM) or estrogen (1 nM) + ICI 182 780 (I, 1 μM) for 6 days, and cell viability was determined using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Data are the average of four independent experiments and presented as mean ± standard error (SE). Data are analyzed using one-way analysis of variance. Bars with different letters indicate significant differences at the level of 0.05.
Low-dose dietary genistein abrogated the inhibitory effect of tamoxifen on the ratio of proliferation to apoptosis in tumor cells
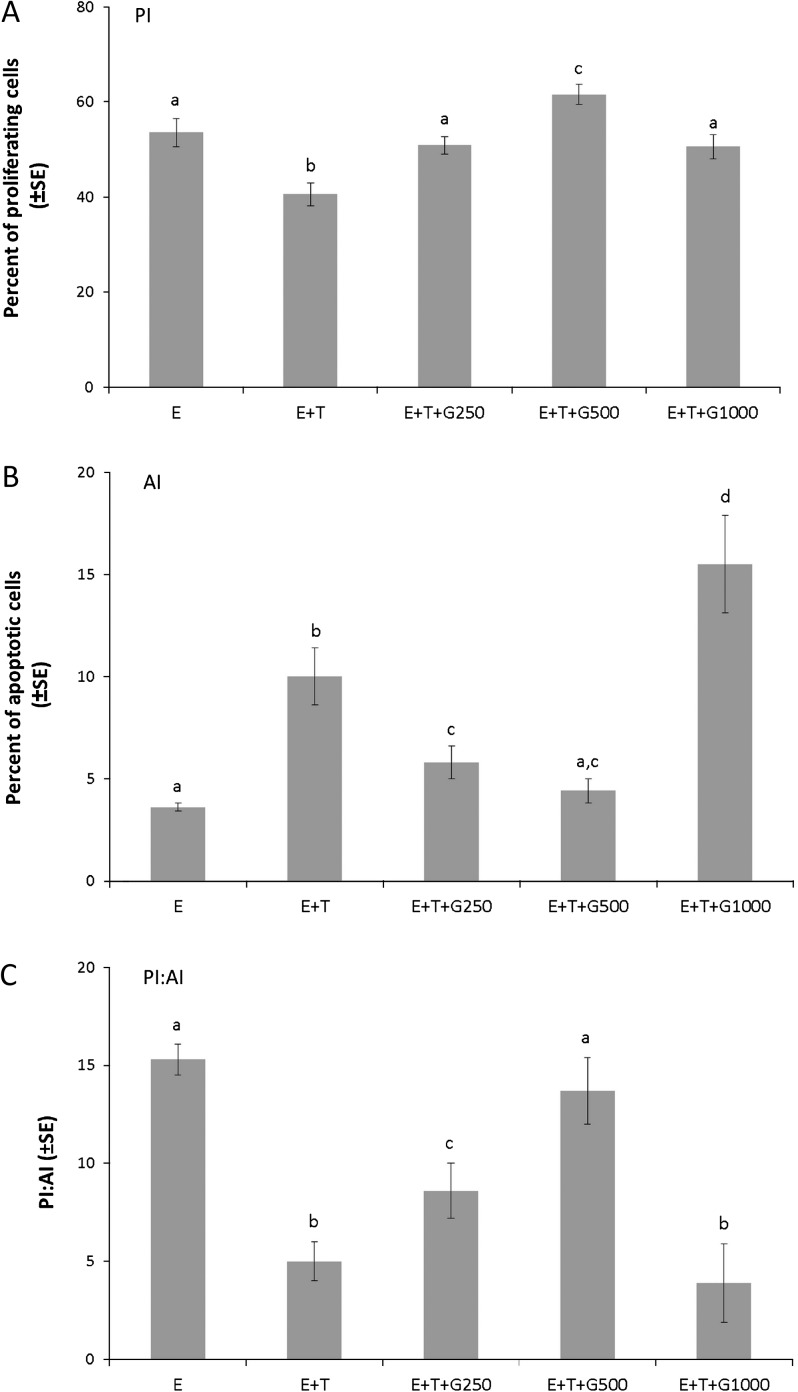
To test the interaction of dietary genistein with tamoxifen on tumor cell proliferation and apoptosis, tumors from the five groups (E, E + T, E + T + G250, E + T + G500 and E + T + G1000) were collected. Tumors from the remaining groups were excluded from evaluation due to size limitation. The PIs and AIs of the experimental groups are displayed in Figure 3A and B. Tamoxifen treatment significantly reduced the PI to 40.6 ± 2.4% when compared with the E group (53.6 ± 3.0%) (P < 0.05). This inhibitory effect of tamoxifen on cell proliferation was abrogated by low doses of dietary genistein in a dose-dependent manner (E + T + G250, 50.9 ± 1.8%, P < 0.05; E + T + G500, 61.6 ± 2.1%, P < 0.05). The high dose of genistein (E + T + G1000, 50.6 ± 2.5%) also significantly negated the inhibitory effect of tamoxifen on cell proliferation (P < 0.05). The significant difference (P < 0.05) in the AI between the E (3.6 ± 0.2%) and E + T groups (10 ± 1.4%) indicated that tamoxifen reversed the effect of estrogen on cell apoptosis. The low doses of dietary genistein (E + T + G250, 5.8 ± 0.8%, P < 0.05; E + T + G500, 4.4 ± 0.6%, P < 0.05) significantly abrogated the inducing effect of tamoxifen on cell apoptosis, whereas the high dose of dietary genistein (E + T + G1000, 15.5 ± 2.4%) exhibited a significant synergistic effect with tamoxifen (P < 0.01). The PI:AI ratio was also examined, as it has been reported to be more informative than either index alone as an indication of net cell production (26). As shown in Figure 3C, tamoxifen significantly decreased the PI:AI ratio when compared with the E group (P < 0.05). The low doses of dietary genistein abrogated this inhibitory effect of tamoxifen on PI:AI ratio in a dose-dependent manner (P < 0.05), whereas the high dose of dietary genistein did not affect the PI:AI ratio when compared with the E + T group.
Fig. 3.
Low-dose dietary genistein negated the effect of tamoxifen on cell proliferation and apoptosis in human breast cancer MCF-7 tumors. Paraffin tumor sections from five treatment groups (E, E + T, E + T + G250, E + T + G500 and E + T + G1000) were analyzed using Ki-67 immunohistochemical staining (cell proliferation) and TUNEL assay (cell apoptosis). (A) PI: average percentage of proliferating cells in a given field. (B) AI: average percentage of apoptotic cells in a given field. (C) PI:AI: the ratio of PI to AI. Data are presented as mean ± standard error (SE) and analyzed using one-way analysis of variance. Bars with different letters indicate significant differences at the level of 0.05.
Dietary genistein and tamoxifen interacted to regulate cyclin D1 and PR mRNA levels in the presence of estrogen
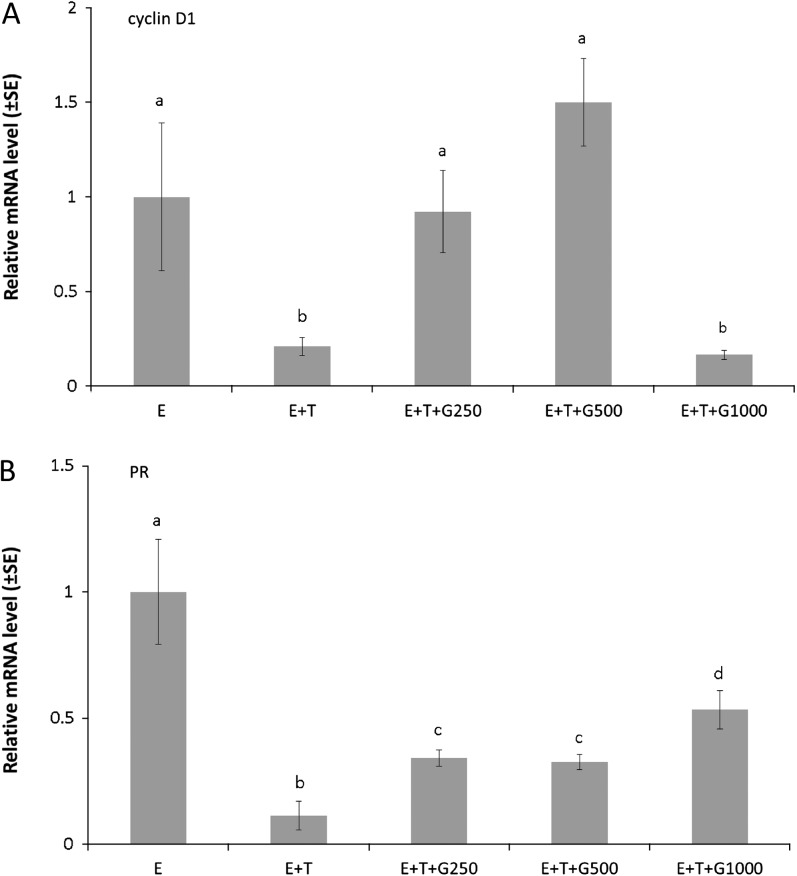
Tumors from the E, E + T, E + T + G250, E + T + G500 and E + T + G1000 groups were collected for mRNA analysis. Tumors from the remaining groups were excluded from evaluation due to size limitation. The cell cycle-regulated gene marker cyclin D1 and the estrogen-responsive gene marker PR were evaluated to examine the interaction of dietary genistein with tamoxifen on their gene expression in the presence of estrogen .The relative mRNA expression of cyclin D1 was normalized to the E group and is displayed in Figure 4A. A significant decrease in the E + T group (0.21-fold over the E group) was observed when compared with the E group (P < 0.05). The low doses of dietary genistein (E + T + G250, 0.92-fold over the E group; E + T + G500, 1.50-fold over the E group) significantly abrogated the inhibitory effect of tamoxifen on cyclin D1 gene expression (P < 0.05), whereas the high dose of dietary genistein did not alter the effect of tamoxifen on cyclin D1 gene expression. The relative mRNA expression of PR, normalized to the E group, is displayed in Figure 4B. Tamoxifen significantly reduced the relative PR gene expression to 0.11-fold over the E group (P < 0.05). Dietary genistein significantly counteracted the inhibitory effect of tamoxifen on PR gene expression at the three dietary levels (P < 0.05).
Fig. 4.
Dietary genistein interacted with tamoxifen to regulate cyclin D1 and PR mRNA levels in human breast cancer MCF-7 tumors. Paraffin tumor sections from five treatment groups (E, E + T, E + T + G250, E + T + G500 and E + T + G1000) were collected for gene expression analysis using real-time PCR. Data were expressed as the relative mRNA levels to the E group. (A) cyclin D1. (B) PR. Data are presented as mean ± standard error (SE) and analyzed using one-way analysis of variance. Bars with different letters indicate significant differences at the level of 0.05.
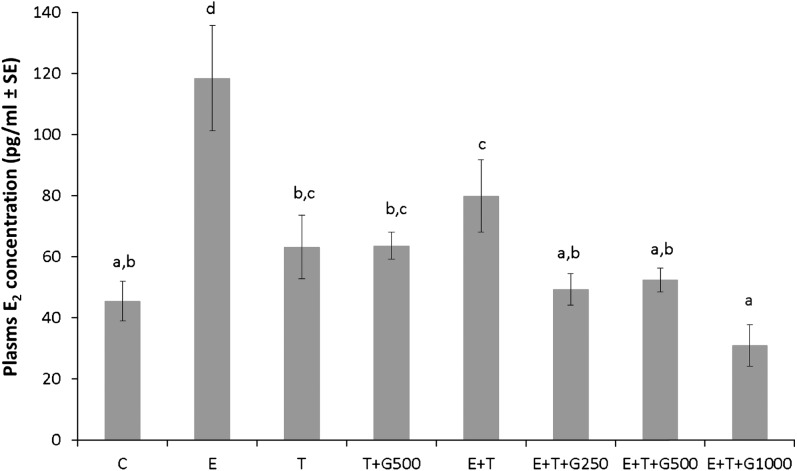
Genistein modulated plasma estrogen levels in the presence of exogenous estrogen and tamoxifen
Plasma estrogen concentrations for each treatment group are displayed in Figure 5. The plasma estrogen level in the C group was 45 ± 6 pg/ml, and no significant differences in plasma estrogen levels were observed between the C, T and T + G500 groups, indicating that tamoxifen alone or tamoxifen plus genistein did not affect plasma estrogen concentration in the absence of exogenous estrogen. Silastic E tubings significantly elevated the plasma estrogen level to 119 ± 16 pg/ml when compared with the C group (P < 0.05), and tamoxifen reduced the elevated plasma estrogen concentration to 80 ± 11 pg/ml (P < 0.05). All three genistein-supplemented diets, E + T + G250, E + T + G500 and E + T + G1000, further significantly reduced plasma estrogen concentration to 50 ± 5 pg/ml, 52 ± 4 pg/ml and 31 ± 6 pg/ml (P < 0.05) when compared with the E + T group, whereas no significant differences were observed among the three levels of dietary genistein-treated groups. No significant differences were observed between the C group and the three levels of dietary genistein-treated groups as well.
Fig. 5.
Dietary genistein interacted with tamoxifen to regulate plasma estrogen levels in mice bearing human breast cancer MCF-7 tumors. Data are presented as mean ± standard error (SE) and analyzed using one-way analysis of variance. Bars with different letters indicate significant differences at the level of 0.05.
Discussion
Currently, tamoxifen is the standard endocrine therapy for breast cancer in premenopausal women (27–29) and also being widely used to treat breast cancer in postmenopausal women, either as monotherapy or combined with aromatase inhibitors (11). Due to their reduced estrogen levels, menopausal women experience symptoms such as hot flashes, sleeping disorders and mood swings, which are exacerbated by antiestrogen treatment. Traditionally, HRT has been used to relieve these symptoms, but many oncologists do not recommend HRT to breast cancer patients due to the concern that it may increase the risk of tumor recurrence, which, directly or indirectly, results in 28–91% of breast cancer patients using one or more alternative therapies without the knowledge of their physicians (30). This indicates that many breast cancer patients, possibly also being treated with tamoxifen, self-medicate with complementary treatments, including soy isoflavones, without proper guidance. Genistein, the major soy isoflavone, has been reported to bind to ER and exhibit weak but substantial estrogenic effects (6,15,31). Thus, if genistein negates the effect of tamoxifen, the simultaneous consumption of genistein and tamoxifen may lead to an undesirable outcome for breast cancer patients.
We have demonstrated that estrogen silastic tubing implanted in athymic mice was able to produce blood estrogen at similar levels with those observed in postmenopausal women and thus is an appropriate model for postmenopausal breast cancer (32). Using this postmenopausal animal model, we reported that 1000 p.p.m. genistein negated the inhibitory effect of tamoxifen on MCF-7 breast tumor growth in athymic mice implanted with a 0.25 mg E silastic tubing (18). In the present study, we evaluated the effect of the interaction between tamoxifen and various doses of dietary genistein on mammary tumor growth using the previously utilized model but with a lower dose of estrogen silastic implant (0.08 mg) to better represent blood levels of estrogen in postmenopausal women. Our results revealed that the 0.08 mg E implant produced a circulating estrogen level of 119 ± 16 pg/ml (0.4 nM), whereas the implantation of a 1–2 mg estrogen pellet generated circulating estrogen levels of 2–3 nM in mice that were similar to levels observed in premenopausal women (32). The low dose of estrogen presented here was sufficient to stimulate MCF-7 tumor growth and this stimulation effect was significantly inhibited by tamoxifen, indicating a successful establishment of a postmenopausal ER+ breast cancer model.
Previous reports have demonstrated that genistein can act as an estrogen agonist and stimulate ER+ cancer cell growth both in vitro and in vivo (14,15,17,33). Our laboratory has reported that 250, 500 and 1000 p.p.m. genistein increased MCF-7 tumor growth in a dose-dependent manner in the absence of exogenous estrogen and tamoxifen (14). These doses of dietary genistein produced plasma levels of total genistein in the range of 1.4–3.3 μM (14), which were similar to those in humans consuming high soy isoflavones-containing diets (34–36). Thus, to evaluate the interaction between genistein and tamoxifen, the same doses of genistein were utilized and we hypothesized that genistein would abrogate the inhibitory effect of tamoxifen on tumor growth induced by estrogen in a dose-dependent manner. In the present study, we observed that the two lower doses of genistein (250 and 500 p.p.m.) abrogated the inhibitory effect of tamoxifen on tumor prevalence and tumor size of MCF-7 tumors in a dose-dependent manner, whereas the high dose of genistein (1000 p.p.m.) did not interfere with the antitumor action of tamoxifen. Our results were similar to an earlier study conducted by Liu et al. (19) showing that a diet containing 211 μg/g total isoflavone abrogated the preventative effect of tamoxifen on MCF-7 tumor growth in MMTV-wt-erbB-2/neu transgenic mice. The percentage of tumor-free mice in this group was reduced to 46.8% when compared with 87.5% in the tamoxifen-treated group, whereas a diet containing 491 μg/g total isoflavone had no impact. These results suggested that low doses of dietary genistein may interfere with tamoxifen, thereby decreasing its efficacy on breast cancer treatment. However, there were some reports showing that genistein synergistically enhanced the preventive effect of tamoxifen on the growth of MCF-7 tumors in mice (22). This inconsistency to our result was probably caused by the interaction of genistein with different estrogen and tamoxifen levels. In the present study, we used a lower E silastic implant that caused a more relevant tumor growth rate. We also used lower tamoxifen levels (3 mg in a slow releasing silastic implant), whereas Mai et al. (22) used 25 mg in a more rapidly releasing pellet. Thus, the interaction of genistein with lower E2 and tamoxifen levels was the most likely reason for differences in the two studies.
The differential effect of dietary genistein at low lose or high dose observed here on tumor growth in mice bearing MCF-7 tumors in the presence of both estrogen and tamoxifen was probably due to the ability of genistein to exhibit an estrogenic effect at low doses through estrogen receptor-mediated pathways (15,37,38) and an inhibitory effect at high doses via ER-independent mechanisms, such as induction of cell apoptosis and cell cycle arrest, protein tyrosine kinase inhibition and topo-isomerase II inhibition (39–43). Although no apparent interaction with tamoxifen was observed on tumor growth in the current study, we previously showed that 1000 p.p.m. genistein counteracted the effect of tamoxifen (18). In the previous study, we used a 0.25 mg estrogen implant with a 2.5 mg (E:T = 1:10) or a 5.0 mg (E:T = 1:20) tamoxifen implant. Here, to better represent plasma estrogen level in postmenopausal women, we reduced the amount of estrogen in the implant to 0.08 mg and used a 3 mg tamoxifen silastic tubing (E:T = 1:37.5) to block the action of exogenous estrogen. Thus, the 1000 p.p.m. genistein diet acted differently in the presence of high or low ratio of E to T implants, suggesting that the interaction of dietary genistein with tamoxifen was possibly affected by the ratio of E to T implants, thus, by the plasma estrogen and tamoxifen levels.
Previous analyses of plasma estrogens in postmenopausal breast cancer patients treated with tamoxifen have provided conflicting results (44,45). In animal models, a trend of decrease in plasma estrogen levels was observed in the tamoxifen-treated mice, although it did not reach a level of statistical significance (18,46). Results from the present study indicated that with the lower ratio of E to T implants, tamoxifen significantly reduced plasma estrogen level increased by E implants. Dietary genistein further reduced plasma estrogen levels, which was consistent with what was shown previously (18). These results suggested that genistein can, in an undefined manner, reduce circulating estrogen levels, which may be an important factor in determining the overall effect of genistein on tumor growth.
Acquired resistance of tumor cells to tamoxifen treatment has been reported both in vitro and in vivo (47,48). Tumor cells may not react to tamoxifen if they progress from the estrogen-dependent stage to the estrogen-independent stage as observed in tamoxifen-resistant breast cancer (49,50). Recently, tamoxifen downregulation of miR-451 and consequent elevation of the key survival factor 14-3-3zeta have been identified as a mechanistic basis of tamoxifen-associated development of endocrine resistance (51), whereas genistein (25–50 μM) has been shown to inhibit the expression of 14-3-3 protein in head and neck cancer cells (52). Although there is no report on the association of low doses of dietary genistein and the expression of 14-3-3 protein, based on what we have seen, it is possible that low doses of dietary genistein increases 14-3-3 expression in MCF-7 tumors and then elicits an additive effect with tamoxifen on endocrine resistance. Thus, to confirm that the abrogating effect of dietary genistein on tamoxifen-treated tumor growth was not due to tamoxifen resistance, we evaluated estrogen responsiveness of the tumors excised from mice treated with tamoxifen and genistein. The result indicated that these tumors retained estrogen responsiveness of the progenitor MCF-7 cells as the estrogen antagonists, tamoxifen and ICI 182 780, significantly inhibited cell proliferation induced by estrogen. This suggested that the growth of tumors in genistein-treated animals was most probably due to the effect from dietary genistein rather than a diminished response to tamoxifen.
Genistein has been reported to regulate MCF-7 cell growth at the transcriptional level. Genistein (1–10 μM) increased progesterone receptor levels in MCF-7 cells in a dose-dependent manner (39) and increased cyclin D1 synthesis and then stimulated MCF-7 cells to enter the cell cycle in a manner similar to estrogen (53–55). Thus, we further investigated the effect of genistein on the expression of PR and cyclin D1 genes in the tumor cells in order to determine the possible underlying mechanisms by which genistein negated the inhibitory effect of tamoxifen on MCF-7 tumor growth. Our observation revealed that the interaction between dietary genistein and tamoxifen on gene expression, in addition to tumor growth, was also complicated. Tamoxifen treatment decreased the expression of PR and cyclin D1 genes, while the effect of genistein on the inhibitory effect of tamoxifen on the gene expression was not consistent. Genistein at 1000 p.p.m. reversed the inhibitory effect of tamoxifen on PR expression. Low doses of genistein negated the effect of tamoxifen on cyclin D1 expression in a dose-dependent manner, whereas 1000 p.p.m. genistein did not have the same effect. The observation on the gene expression of cyclin D1 was consistent with that observed on the PI:AI ratio, tumor prevalence and tumor growth, suggesting that genistein modulated the effect of tamoxifen on estrogen-induced tumor growth, potentially through the regulation of the cell cycle, in addition to acting through estrogen signaling.
In summary, the current study presents evidence that the low doses of dietary genistein negate the inhibitory effect of tamoxifen, in the presence of low blood estrogen levels, on ER+ MCF-7 tumor growth in athymic nude mice by regulating cell proliferation and apoptosis. This effect is also probably, at least in part, due to the regulation of the ER-dependent signaling pathway. Although the high dose of dietary genistein does not negate the inhibitory effect of tamoxifen on gross tumor size, it still results in high tumor prevalence. Therefore, the results from this study raise safety concerns of consuming dietary isoflavone supplements across a broad range of internal exposures in breast cancer patients, especially postmenopausal women, while on tamoxifen treatment.
Funding
National Cancer Institute (NCI) (CA77355 to W.G.H.); National Institute on Aging; National Institute for Complementary and Alternative Medicine (NCCAM); Office of Dietary Supplements (ODS) and Women’s Health Initiative (P01 AG024387 to W.G.H. and Y.H.J.); and NCCAM, ODS and NCI (P50AT006268 to W.G.H.). Its contents are sorely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ODS, NCI or the National Institutes of Health.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AI
apoptotic index
- BCS
Bovine calf serum
- ER
estrogen receptor
- ER+
estrogen receptor positive
- HRT
hormone replacement therapy
- MEM
Minimal essential medium
- mRNA
messenger RNA
- PI
proliferative index
- PR
progesterone receptor
References
- 1.American Cancer Society. Breast Cancer Facts & Figures 2007–2008. Atlanta, GA.: American Cancer Society, Inc; [Google Scholar]
- 2.Harlan LC, et al. Adjuvant therapy for breast cancer: practice patterns of community physicians. J. Clin. Oncol. 2002;20:1809–1817. doi: 10.1200/JCO.2002.07.052. [DOI] [PubMed] [Google Scholar]
- 3.Ganz PA, et al. Life after breast cancer: understanding women's health-related quality of life and sexual functioning. J. Clin. Oncol. 1998;16:501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 4.Rossouw JE, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Ravdin PM, et al. The decrease in breast-cancer incidence in 2003 in the United States. N. Engl. J. Med. 2007;356:1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 6.Setchell KD. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 1998;68:1333S–1346S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- 7.Pisani P, et al. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int. J. Cancer. 2002;97:72–81. doi: 10.1002/ijc.1571. [DOI] [PubMed] [Google Scholar]
- 8.Onitilo AA, et al. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin. Med. Res. 2009;7:4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Early Breast Cancer Trialists' Collaborative Group (EBCTCG). (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 10.Morandi P, et al. The role of aromatase inhibitors in the adjuvant treatment of breast carcinoma: the M. D. Anderson Cancer Center evidence-based approach. Cancer. 2004;101:1482–1489. doi: 10.1002/cncr.20522. [DOI] [PubMed] [Google Scholar]
- 11.Burstein HJ, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J. Clin. Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ju YH, et al. Dietary genistein negates the inhibitory effect of letrozole on the growth of aromatase-expressing estrogen-dependent human breast cancer cells (MCF-7Ca) in vivo. Carcinogenesis. 2008;29:2162–2168. doi: 10.1093/carcin/bgn161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osborne CK. Tamoxifen in the treatment of breast cancer. N. Engl. J. Med. 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 14.Ju YH, et al. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J. Nutr. 2001;131:2957–2962. doi: 10.1093/jn/131.11.2957. [DOI] [PubMed] [Google Scholar]
- 15.Wang TT, et al. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17:271–275. doi: 10.1093/carcin/17.2.271. [DOI] [PubMed] [Google Scholar]
- 16.Zava DT, et al. Estrogenic and antiproliferative properties of genistein and other flavonoids in human breast cancer cells in vitro. Nutr. Cancer. 1997;27:31–40. doi: 10.1080/01635589709514498. [DOI] [PubMed] [Google Scholar]
- 17.Allred CD, et al. Soy processing influences growth of estrogen-dependent breast cancer tumors. Carcinogenesis. 2004;25:1649–1657. doi: 10.1093/carcin/bgh178. [DOI] [PubMed] [Google Scholar]
- 18.Ju YH, et al. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Res. 2002;62:2474–2477. [PubMed] [Google Scholar]
- 19.Liu B, et al. Low-dose dietary phytoestrogen abrogates tamoxifen-associated mammary tumor prevention. Cancer Res. 2005;65:879–886. [PubMed] [Google Scholar]
- 20.Constantinou AI, et al. The soy isoflavone daidzein improves the capacity of tamoxifen to prevent mammary tumours. Eur. J. Cancer. 2005;41:647–654. doi: 10.1016/j.ejca.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Tanos V, et al. Synergistic inhibitory effects of genistein and tamoxifen on human dysplastic and malignant epithelial breast cells in vitro. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002;102:188–194. doi: 10.1016/s0301-2115(01)00582-6. [DOI] [PubMed] [Google Scholar]
- 22.Mai Z, et al. Soy phytochemicals synergistically enhance the preventive effect of tamoxifen on the growth of estrogen-dependent human breast carcinoma in mice. Carcinogenesis. 2007;28:1217–1223. doi: 10.1093/carcin/bgm004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mai Z, et al. Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells. Mol. Carcinog. 2007;46:534–542. doi: 10.1002/mc.20300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reeves PG, et al. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, et al. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Michael-Robinson JM, et al. Proliferation, apoptosis, and survival in high-level microsatellite instability sporadic colorectal cancer. Clin. Cancer Res. 2001;7:2347–2356. [PubMed] [Google Scholar]
- 27.Gonzalez Martin A, et al. Adjuvant endocrine therapy in premenopausal women with breast cancer. Breast Cancer Res. Treat. 2010;123(suppl. 1):43–47. doi: 10.1007/s10549-010-1045-2. [DOI] [PubMed] [Google Scholar]
- 28.Bao T, et al. Adjuvant endocrine therapy for premenopausal women with early breast cancer. Breast Cancer Res. 2007;9:115. doi: 10.1186/bcr1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pritchard K. Endocrinology and hormone therapy in breast cancer: endocrine therapy in premenopausal women. Breast Cancer Res. 2005;7:70–76. doi: 10.1186/bcr1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.VandeCreek L, et al. Use of alternative therapies among breast cancer outpatients compared with the general population. Altern. Ther. Health Med. 1999;5:71–76. [PubMed] [Google Scholar]
- 31.Katzenellenbogen BS, et al. Therapeutic targeting in the estrogen receptor hormonal pathway. Semin. Oncol. 2004;31:28–38. doi: 10.1053/j.seminoncol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Ju YH, et al. Genistein stimulates growth of human breast cancer cells in a novel, postmenopausal animal model, with low plasma estradiol concentrations. Carcinogenesis. 2006;27:1292–1299. doi: 10.1093/carcin/bgi370. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh CY, et al. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998;58:3833–3838. [PubMed] [Google Scholar]
- 34.Gardner CD, et al. Effects of isoflavone supplements vs. soy foods on blood concentrations of genistein and daidzein in adults. J. Nutr. Biochem. 2009;20:227–234. doi: 10.1016/j.jnutbio.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atteritano M, et al. Effects of phytoestrogen genistein on cytogenetic biomarkers in postmenopausal women: 1 year randomized, placebo-controlled study. Eur. J. Pharmacol. 2008;589:22–26. doi: 10.1016/j.ejphar.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 36.Setchell KD, et al. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350:23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 37.Makela S, et al. Dietary estrogens act through estrogen receptor-mediated processes and show no antiestrogenicity in cultured breast cancer cells. Environ. Health Perspect. 1994;102:572–578. doi: 10.1289/ehp.94102572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miodini P, et al. The two phyto-oestrogens genistein and quercetin exert different effects on oestrogen receptor function. Br. J. Cancer. 1999;80:1150–1155. doi: 10.1038/sj.bjc.6690479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fioravanti L, et al. Genistein in the control of breast cancer cell growth: insights into the mechanism of action in vitro. Cancer Lett. 1998;130:143–152. doi: 10.1016/s0304-3835(98)00130-x. [DOI] [PubMed] [Google Scholar]
- 40.Pagliacci MC, et al. Growth-inhibitory effects of the natural phyto-oestrogen genistein in MCF-7 human breast cancer cells. Eur. J. Cancer. 1994;30A:1675–1682. doi: 10.1016/0959-8049(94)00262-4. [DOI] [PubMed] [Google Scholar]
- 41.Constantinou AI, et al. Genistein inactivates bcl-2, delays the G2/M phase of the cell cycle, and induces apoptosis of human breast adenocarcinoma MCF-7 cells. Eur. J. Cancer. 1998;34:1927–1934. doi: 10.1016/s0959-8049(98)00198-1. [DOI] [PubMed] [Google Scholar]
- 42.Leung LK, et al. Bcl-2 is not reduced in the death of MCF-7 cells at low genistein concentration. J. Nutr. 2000;130:2922–2926. doi: 10.1093/jn/130.12.2922. [DOI] [PubMed] [Google Scholar]
- 43.Magee PJ, et al. Phyto-oestrogens, their mechanism of action: current evidence for a role in breast and prostate cancer. Br. J. Nutr. 2004;91:513–531. doi: 10.1079/BJN20031075. [DOI] [PubMed] [Google Scholar]
- 44.Lonning PE, et al. Influence of tamoxifen on sex hormones, gonadotrophins and sex hormone binding globulin in postmenopausal breast cancer patients. J. Steroid Biochem. Mol. Biol. 1995;52:491–496. doi: 10.1016/0960-0760(94)00189-s. [DOI] [PubMed] [Google Scholar]
- 45.Lum SS, et al. Changes in serum estrogen levels in women during tamoxifen therapy. Am. J. Surg. 1997;173:399–402. doi: 10.1016/S0002-9610(97)00072-X. [DOI] [PubMed] [Google Scholar]
- 46.Iino Y, et al. Reversible control of oestradiol-stimulated growth of MCF-7 tumours by tamoxifen in the athymic mouse. Br. J. Cancer. 1991;64:1019–1024. doi: 10.1038/bjc.1991.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howell A, et al. Response to a specific antioestrogen (ICI 182780) in tamoxifen-resistant breast cancer. Lancet. 1995;345:29–30. doi: 10.1016/s0140-6736(95)91156-1. [DOI] [PubMed] [Google Scholar]
- 48.Connor CE, et al. Circumventing tamoxifen resistance in breast cancers using antiestrogens that induce unique conformational changes in the estrogen receptor. Cancer Res. 2001;61:2917–2922. [PubMed] [Google Scholar]
- 49.Clarke R, et al. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003;22:7316–7339. doi: 10.1038/sj.onc.1206937. [DOI] [PubMed] [Google Scholar]
- 50.Lykkesfeldt AE, et al. Effects of the antioestrogen tamoxifen on the cell cycle kinetics of the human breast cancer cell line, MCF-7. Br. J. Cancer. 1984;49:717–722. doi: 10.1038/bjc.1984.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergamaschi A, et al. Tamoxifen downregulation of miR-451 increases 14-3-3zeta and promotes breast cancer cell survival and endocrine resistance. Oncogene. 2012;31:39–47. doi: 10.1038/onc.2011.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alhasan SA, et al. Genistein elicits pleiotropic molecular effects on head and neck cancer cells. Clin. Cancer Res. 2001;7:4174–4181. [PubMed] [Google Scholar]
- 53.Dees C, et al. Dietary estrogens stimulate human breast cells to enter the cell cycle. Environ. Health Perspect. 1997;105(suppl. 3):633–636. doi: 10.1289/ehp.97105s3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foster JS, et al. Estrogen regulates activity of cyclin-dependent kinases and retinoblastoma protein phosphorylation in breast cancer cells. Mol. Endocrinol. 1996;10:488–498. doi: 10.1210/mend.10.5.8732680. [DOI] [PubMed] [Google Scholar]
- 55.Sakamoto T, et al. Effects of diverse dietary phytoestrogens on cell growth, cell cycle and apoptosis in estrogen-receptor-positive breast cancer cells. J. Nutr. Biochem. 2010;21:856–864. doi: 10.1016/j.jnutbio.2009.06.010. [DOI] [PubMed] [Google Scholar]