Abstract
Curcumin can induce p53-independent apoptosis. However, the underlying mechanism remains to be defined. Here, we show that curcumin-induced apoptosis in a panel of tumor cells with mutant p53. Curcumin rapidly induced activation of the mitogen-activated protein kinases (MAPKs) including extracellular signal-regulated kinase 1/2 (Erk1/2) and c-Jun N-terminal kinase (JNK). Inhibition of JNK (with SP600125) or Erk1/2 (with U0126) partially prevented curcumin-induced cell death in the cells. Similarly, expression of dominant negative c-Jun or downregulation of Erk1/2 in part attenuated curcumin-induced cell death. It appears that curcumin-induced activation of MAPKs and apoptosis was due to induction of reactive oxygen species (ROS), as pretreatment with N-acetyl-L-cysteine, a ROS scavenger, blocked these events. Furthermore, we found that curcumin-induced activation of MAPK pathways was related to inhibition of the serine/threonine protein phosphatases 2A (PP2A) and 5 (PP5). Overexpression of PP2A or PP5 partially prevented curcumin-induced activation of JNK and Erk1/2 phosphorylation as well as cell death. The results suggest that curcumin induction of ROS activates MAPKs, at least partially by inhibiting PP2A and PP5, thereby leading to p53-independent apoptosis in tumor cells.
Introduction
Curcumin (diferuloylmethane) is a polyphenol natural product isolated from turmeric, a powder produced from the rhizome of the plant Curcuma longa (1,2). Curcumin has been shown to produce a wide range of medicinal benefits in the human body and has proven to be a powerful therapeutic agent against many human disease processes (1,2). In recent years, curcumin has been shown to be a potential anticancer agent against various human tumors in both in vitro and in vivo models as well as in clinical trials (1,2). Preclinical studies have revealed that curcumin can affect and disrupt virtually every major stage of carcinogenesis, including cell proliferation, survival, angiogenesis and metastasis (1,2). The mechanisms underlying the anticancer activities of curcumin have been extensively studied. Multiple signaling molecules have been identified to be targeted by curcumin, including ornithine decarboxylase (3), p53 (4,5), c-myc (4,5), cyclin D1 (6,7), nuclear factor-κB (3,5,8), activator protein-1 (5,9), protein tyrosine kinases (10,11), Akt (12,13), protein kinase C (14) and mammalian target of rapamycin (15–18).
Curcumin can induce p53-dependent and -independent apoptosis in a variety of tumor cell lines including those derived from breast carcinoma, prostate carcinoma, colon carcinoma, kidney cancer, hepatocellular carcinoma, leukemia, lymphoma, basal cell carcinoma, melanoma and rhabdomyosarcoma (1,2). It has been described that curcumin can induce p53-dependent apoptosis by induction of p53, p21Cip1 and Bax expression in certain tumor cell lines, such as hepatoblastoma (4), neuroblastoma (19), glioma (20), melanoma (21) and breast carcinoma cells (3). On the other hand, it has also been found that curcumin can induce p53-dependent apoptosis by downregulation of p53 in breast carcinoma (5) and B lymphoma cells (22). For example, in B cell lymphoma (BKS-2) cells, curcumin decreased expression of p53 and pro-survival proteins, including Egr-1, c-myc and Bcl-XL (22). In colon cancer (RKO) cells, curcumin disrupted the conformation of the p53 protein required for its tumor suppression functions, including serine phosphorylation, binding to DNA, transactivation of p53-responsive genes and p53-mediated cell cycle arrest (23). The finding of reduced p53 activity by curcumin was also observed in normal thymocytes and myeloid leukemic cells, where curcumin induced p53 degradation. Mechanistically, curcumin promoted the dissociation of NAD(P)H:quinone oxidoreductase 1 (NQO1)-p53 complexes by suppressing the activity of NQO1, a flavoenzyme that binds and stabilizes wild-type p53 (24). Therefore, the molecular mechanism of curcumin induction of p53-dependent apoptosis appears to be ‘cell-type dependent.’ Despite these findings, the molecular mechanism by which curcumin induces p53-independent apoptosis is not well understood.
Increasing evidence has implicated that members of the mitogen-activated protein kinase (MAPK) family play a critical role in p53-independent apoptosis (25–27). In mammalian cells, there exist at least three distinct groups of MAPKs, including extracellular signal-regulated protein kinase 1/2 (Erk1/2), c-Jun N-terminal kinase (JNK) and p38 MAPK (28,29). Erk1/2 is primarily activated by growth factors and controls cellular proliferation, differentiation and development, whereas JNK and p38 are preferentially activated by environmental stress and inflammatory cytokines and regulates survival/apoptosis (28,29). In response to oxidative stress or reactive oxygen species (ROS), all three MAPK family members could be activated, leading to apoptosis (30–32). Phosphorylation of MAPKs is balanced by specific MAPK kinases and phosphatases (28,29). Studies have shown that cross-talk between parallel pathways of the MAPK cascade is dependent on increases in activity and expression of protein phosphatases, such as MAPK phosphatase 1 (MKP-1), serine/threonine protein phosphatase 2A (PP2A), and protein phosphatase 5 (PP5), which have been identified to directly dephosphorylate and thereby inactivate JNK, Erk1/2 or p38 (27,31–35). Accumulating data have demonstrated that MKP-1 and PP2A are the major phosphatases that negatively regulate phosphorylation of Erk1/2, JNK and/or p38, whereas PP5 negatively regulates JNK/p38 pathway, involved in stress response (27,31–35).
Here, we show that curcumin induces p53-independent apoptosis in a panel of tumor cell lines, including human rhabdomyosarcoma cells (Rh30), Ewing sarcoma (Rh1), colon adenocarcinoma (HT29) and cervical cancer (HeLa) cells, through induction of ROS. Furthermore, we identified that curcumin induction of ROS activates phosphorylation of Erk1/2 and JNK pathways, partially by inhibiting PP2A and PP5, thereby leading to p53-independent apoptosis of tumor cells.
Materials and methods
Materials
Curcumin (Sigma, St. Louis MO) was dissolved in 100% ethanol to prepare a stock solution (10 mM), aliquoted and stored at −20°C. RPMI 1640 and Dulbecco’s Modified Eagle Medium were purchased from Mediatech (Herndon, VA). Fetal bovine serum (FBS) was from Hyclone (Logan, UT), whereas 0.05% Trypsin-EDTA was from Invitrogen (Grand Island, NY). Enhanced chemiluminescence solution was from Perkin–Elmer Life Science (Boston, MA). CellTiter 96® AQueous One Solution Cell Proliferation Assay kit was from Promega (Madison, WI). Annexin V-FITC Apoptosis Detection Kit I was purchased from BD Biosciences (San Jose, CA). U0126 and SP600125 were obtained from LC Laboratories (Woburn, MA), and 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) was from Invitrogen (Carlsbad, CA). Dihydroethidium was purchased from AnaSpec (Fremont, CA).
Cell lines and culture
Human rhabdomyosarcoma (Rh30) (p53 mutant, R273C) and Ewing sarcoma (Rh1) (p53 mutant, Y220C) cells (26) were generously provided by Dr Peter J. Houghton (Nationwide Children’s Hospital, Columbus, OH) and were grown in antibiotic-free RPMI 1640 medium supplemented with 10% FBS at 37°C and 5% CO2. Human colon adenocarcinoma (HT29) (p53 mutant, R273H) (36) and cervical carcinoma (HeLa) cells (expressing wild-type p53, but the protein product is inactivated by human papillomavirus type 16 E6) (37) (American Type Culture Collection, Manassas, VA) were grown in antibiotic-free Dulbecco’s Modified Eagle Medium supplemented with 10% FBS at 37°C and 5% CO2. Human embryonic kidney 293A and 293TD cells (Invitrogen) were grown in antibiotic-free Dulbecco’s Modified Eagle Medium supplemented with 10% heat-inactivated FBS and non-essential amino acids at 37°C and 5% CO2.
Recombinant adenoviral constructs and infection
The recombinant adenoviruses encoding hemagglutinin (HA)-tagged wild-type (wt) human PP5 (Ad-PP5), FLAG-tagged wt rat PP2ACα (Ad-PP2A), FLAG-tagged dominant negative c-Jun (FLAG-Δ169) (Ad-dn-c-Jun), GFP (Ad-GFP) and β-galactosidase (Ad-LacZ) were described previously (26,27,32). For experiments, cells were grown in the growth medium and infected with the individual adenovirus for 24 h at 5 of multiplicity of infection (MOI = 5). Subsequently, cells were used for experiments. Ad-GFP or Ad-LacZ served as a control. Expression of FLAG-tagged dn-c-Jun or PP2A and HA-tagged PP5 was determined by western blotting with antibodies to FLAG and HA, respectively.
Lentiviral shRNA cloning, production and infection
Lentiviral shRNAs to Erk1/2 and GFP were generated as described previously (31). For experiments, cells, when grown to ∼70% confluence, were infected with lentiviral shRNA to Erk1/2 or GFP (control) in the presence of 8 μg/ml polybrene and then exposed to 2 μg/ml puromycin after 24 h of infection. In 5 days, cells were used for experiments.
Cell viability assay
Cell viability was evaluated using CellTiter 96® AQueous One Solution Cell Proliferation Assay kit (Promega), which is a colorimetric method to determine the number of viable cells in proliferation or the cytotoxicity of a compound. Briefly, cells suspended in the growth medium were seeded in a 96-well plate at a density of 1 × 104 cells/well (in six replicates) and were grown overnight at 37°C in a humidified incubator with 5% CO2. Next day, cells were treated with curcumin (0–40 μM) for 48 h or with/without curcumin (10 and 20 μM) following preincubation with 20 μM JNK inhibitor SP600125 or 5 μM MEK1/2 (upstream of Erk1/2) inhibitor U0126, respectively or were treated with/without curcumin (10 and 20 μM) following 1 h of N-acetyl-l-cysteine (NAC) (5 mM) preincubation. Additionally, cells, infected with Ad-c-Jun, Ad-GFP, Ad-PP2A, Ad-PP5 and Ad-LacZ, or Erk1/2 shRNA, and GFP shRNA, respectively, were exposed to curcumin (0–20 μM). After incubation for 48 h, each well was added 20 μl of one solution reagent and incubated for 3 h. Cell viability was determined by measuring the optical density at 490 nm using a Wallac 1420 Multilabel Counter (PerkinElmer Life Sciences, Wellesley, MA).
Apoptosis assay
Rh30 or HT29 cells were seeded in 100-mm dishes at a density of 1 × 106 cells/dish in the growth medium and were grown overnight at 37°C in a humidified incubator with 5% CO2. Cells were treated with 20 μM curcumin for 72 h, followed by apoptosis assay using the Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences), as described previously (15).
Cell morphological analysis
Cells were seeded at a density of 1 × 106 cells/well in a 6-well plate. Next day, cells were treated with curcumin (0–40 μM), following preincubation with/without SP600125 (40 μM), U0126 (5 μM) or NAC (5 mM) for 1 h. Additionally, cells, which were infected with Ad-PP2A, Ad-PP5 and Ad-GFP, respectively, were exposed to curcumin (0–40 μM). After incubation for 24 h, images were taken with an Olympus inverted phase-contrast microscope (Olympus Optical Co., Melville, NY) (×200) equipped with the Quick Imaging system.
ROS assay
The ROS level was measured by using CM-H2DCFDA (31). Cells were seeded at a density of 1 × 104 cells/well in 96-well plates. Next day, cells were loaded with 10 μM CM-H2DCFDA following the manufacturer’s protocol, incubated in the presence of curcumin (0–20 μM) for 0–24 h with six replicates of each treatment. In some cases, cells were treated curcumin (0–20 μM) for 24 h following 1 h of NAC (5 mM) preincubation and then loaded with 10 μM CM-H2DCFDA for 3 h. Fluorescent intensity was recorded by excitation at 485 nm and emission at 535 nm using a Wallac 1420 Multilabel Counter (PerkinElmer Life Sciences).
O2−· detection
Dihydroethidium was used to detect O2−· level produced in intact cells, as described (38). Briefly, cells (4 × 104/well) were seeded onto a sterilized glass coverslip in the bottom of a 6-well plate. Next day, the cells were treated with curcumin (0–20 μM) for 24 h, followed by incubation with dihydroethidium (40 μM) for 30 min. The cells were then rinsed with PBS and fixed with 4% paraformaldehyde for 2 h at 4°C. The coverslip was mounted on a microscope slide and visualized and photographed with a Nikon Eclipse TE300 fluorescence microscope (Nikon Instruments Inc., Melville, NY) (×200) equipped with a digital camera.
In vitro Ser/Thr phosphatase assay
Cells were lysed in 50 mM Tris–HCl buffer, pH 7.0, containing 1% Nonidet P-40, 2 mM EDTA and protease inhibitor cocktail (Sigma, 1:1000). PP2Ac or PP5 was immunoprecipitated with antibodies to PP2Ac (Millipore, Temecula, CA) or PP5 (BD Biosciences) and protein A/G-agarose (Santa Cruz Biotechnology, Santa Cruz, CA). Subsequently, the beads were washed three times with the above lysis buffer and twice with the phosphatase assay buffer (50 mM Tris–HCl, pH 7.0, 0.1 mM CaCl2). The phosphatase activity of immunoprecipitated PP2A or PP5 was assayed with a Ser/Thr Phosphatase Assay kit 1 using KRpTIRR as the substrate peptide (Millipore) following the manufacturer’s instructions. Absorbance was measured at 595 nm using a Wallac 1420 Multilabel Counter (PerkinElmer Life Sciences). Because the optical density values varied from experiment to experiment, PP2A or PP5 activity was calculated using the fold change (arbitrary units) in each experiment. Finally, all data (from different batches of experiments) were pooled for statistical analysis.
Western blot analysis
Western blotting was performed as described previously (15). The following antibodies were used: phospho-Erk1/2 (Thr202/Tyr204), phospho-p38 (Thr180/Tyr182) (Cell Signaling Technology, Beverly, MA), PP2ACα, PP5 (BD Biosciences), PP2A-A subunit, PP2A-B subunit, (Millipore, Billerica, MA), phospho-PP2A (Tyr307) (Epitomics, Burlingame, CA), JNK1, phospho-JNK (Thr183/Tyr185), c-Jun, phospho-c-Jun (Ser63), Erk2, p38, demethylated-PP2A, HA (Santa Cruz Biotechnology), FLAG, β-tubulin (Sigma), goat anti-mouse IgG-horseradish peroxidase and goat anti-rabbit IgG-horseradish peroxidase (Pierce, Rockland, IL).
Statistical analysis
Results were expressed as mean values ± standard error. The data were analyzed by one-way analysis of variance followed by post hoc Dunnett’s t-test for multiple comparisons. A level of P < 0.05 was considered to be statistically significant.
Results
Curcumin-induced apoptosis is associated with induction of ROS
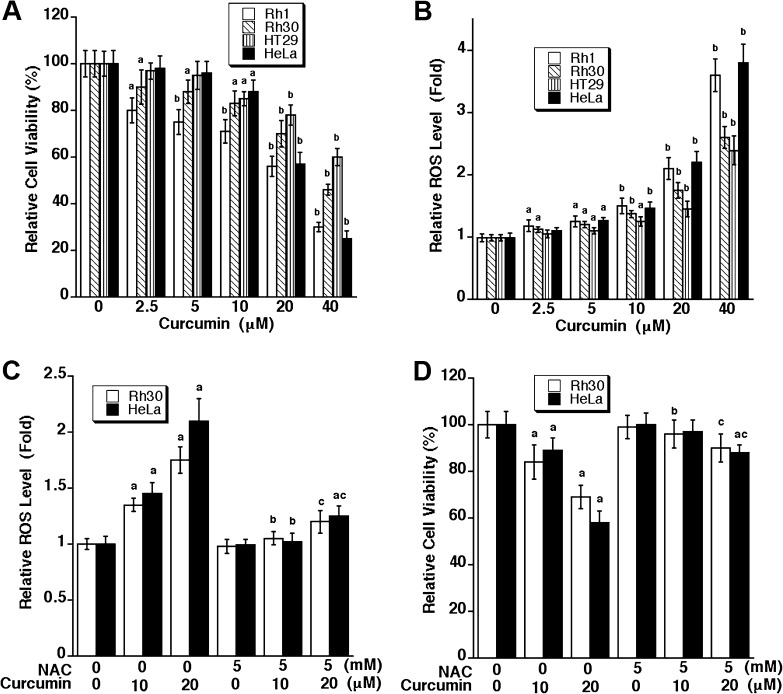
Recently, we have shown that curcumin induces apoptosis in rhabdomyosarcoma (Rh30) (p53 mutant, R273C) and Ewing sarcoma (Rh1) (p53 mutant, Y220C) cells in a p53-independent manner (15). To substantiate the notion that curcumin is able to induce p53-independent cell death, human colon adenocarcinoma (HT29) (p53 mutant, R273H) (36) and cervical carcinoma (HeLa) cells (expressing wild-type p53, but the protein product is inactivated by human papillomavirus type 16 E6) (37) were also employed in this study. In agreement with our previous finding (15), curcumin reduced cell viability in Rh1 and Rh30 cells in a concentration-dependent manner (Figure 1A). Similar results were also observed in HT29 and HeLa cells (Figure 1A). It appeared that treatment with 10–20 μM curcumin for 48 h was able to reduce the viability significantly in all cell lines tested. This is consistent with our morphological analysis. Under a phase-contrast microscope, more round or shrunken cells were observed, when exposed to curcumin (10–20 μM) for 48 h (data not shown), suggesting induction of cell death.
Fig. 1.
Curcumin induction of ROS triggers cell death in tumor cells. Rh30, Rh1, HeLa and HT29 cells were exposed to curcumin at indicated concentrations for 24 h (for ROS detection) and 48 h (for cell viability assay), respectively, followed by cell viability assay using one solution reagent (A) and ROS detection using CM-H2DCFDA (B). Results (A and B) are presented as mean ± SE (n = 6). aP < 0.05, bP < 0.01, difference versus control group. Rh30 and HeLa cells were pretreated with/without NAC (5 mM) for 1 h and then exposed to curcumin (10 and 20 μM) for 24 h (for ROS detection) and 48 h (for cell viability assay), respectively, followed by ROS detection using CM-H2DCFDA (C), and cell viability assay using one solution reagent (D). Results (C and D) are presented as mean ± SE (n = 6). aP < 0.05, difference versus control group; bP < 0.05, difference versus 10 μM curcumin group; cP < 0.05, difference versus 20 μM curcumin group.
As curcumin is an antioxidant and also an oxidant (1,2), we wondered whether curcumin-induced cell death is associated with induction of ROS. As shown in Figure 1B, treatment with curcumin (0–40 μM) for 24 h resulted in a concentration-dependent increase of ROS level in Rh1, Rh30, HeLa and HT29 cells, which is in agreement with a decreased cell viability shown in Figure 1A. Furthermore, we observed that curcumin elevated ROS levels in the cells rapidly. Consistent with a previous report (39), we found that 10 μM curcumin was able to elevate ROS level in Rh30 cells by ∼1.5 fold within 1–2 h treatment (Supplementary Figure 1S, available at Carcinogenesis Online), which was sustained by ∼24 h (Figure 1B). Similar results were observed in Rh30 cells treated with 20 μM curcumin (data not shown). Among the ROS, such as superoxide anion radical (O2−·), hydrogen peroxide (H2O2) and hydroxyl radical (·OH), treatment with curcumin (20 μM) for 24 h was able to obviously induce O2−· in Rh30 and HeLa cells, as detected by dihydroethidium staining (Supplementary Figure 2S, available at Carcinogenesis Online). Although we cannot rule out the possibility that curcumin may also induce H2O2 and ·OH, our current data at least suggest that curcumin-induced ROS may contribute to cell death of the tumor cells.
To confirm whether curcumin-induced cell death is indeed due to ROS induction, Rh30 and HeLa cells were pretreated for 1 h with NAC (5 mM), an antioxidant and ROS scavenger, and then exposed to curcumin (10 and 20 μM) for 24 h. As expected, NAC strongly blocked curcumin induction of ROS in the cells (Figure 1C). Also, NAC potently suppressed curcumin-induced loss of cell viability in the cells (Figure 1D). Similarly, morphological analysis revealed that NAC itself did not alter cell shape but obviously prevented 10 and 20 μM curcumin-induced rounding and shrinkage of Rh30, Rh1, HeLa and HT29 cells (data not shown). Taken together, the findings indicate that curcumin-induced cell death through induction of ROS in the tumor cells.
Curcumin-induced ROS activate JNK and Erk1/2, leading to apoptosis
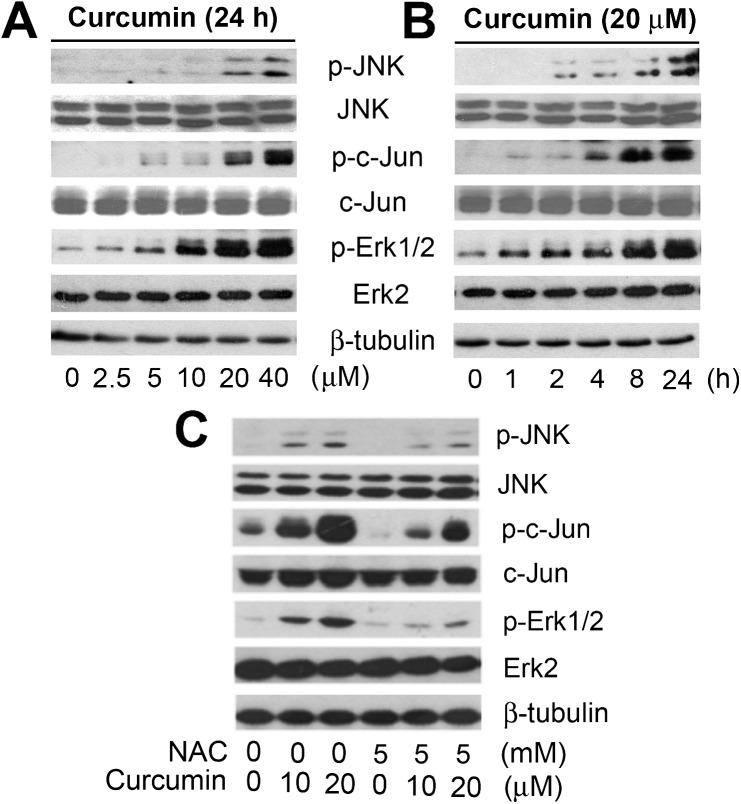
Previous studies have demonstrated that oxidative stress may trigger apoptosis by activation of MAPK pathways (30–32). We reasoned that curcumin-induced cell death is through ROS activation of MAPK cascade. To this end, Rh30 cells were treated with curcumin (0–20 μM) for 24 h, followed by western blot analysis. We found that curcumin treatment induced phosphorylation of JNK and Erk1/2 in a concentration-dependent manner (Figure 2A), although there was no obvious effect on phosphorylation of p38 MAPK (data not shown). Noticeably, curcumin activation of JNK also resulted in a robust phosphorylation of c-Jun, a substrate of JNK (Figure 2A). We also found that curcumin activated MAPKs in a time-dependent manner. Within 2–4 h, curcumin obviously increased phosphorylation of JNK and Erk1/2, and such phosphorylation was sustained for ∼48 h (Figure 2B and Supplementary Figure 3S, available at Carcinogenesis Online). Consistently, a high level of phospho-c-Jun was induced. Furthermore, pretreatment with NAC (5 mM) for 1 h dramatically inhibited curcumin-induced phosphorylation of Erk1/2 and JNK in the cells (Figure 2C). Similar results were observed in Rh1 cells (data not shown). Therefore, the findings suggest that curcumin induction of ROS activates JNK and Erk1/2.
Fig. 2.
Curcumin induction of ROS activates Erk1/2 and JNK in tumor cells. (A) Rh30 cells were treated with 0–40 μM curcumin for 24 h, (B) with 20 μM curcumin for 0–24 h or (C) pretreated with NAC (5 mM) for 1 h and then exposed to curcumin (0–20 μM) for 24 h. The lysates were subjected to western blot analysis using indicated antibodies. The blots were probed for β-tubulin for a loading control.
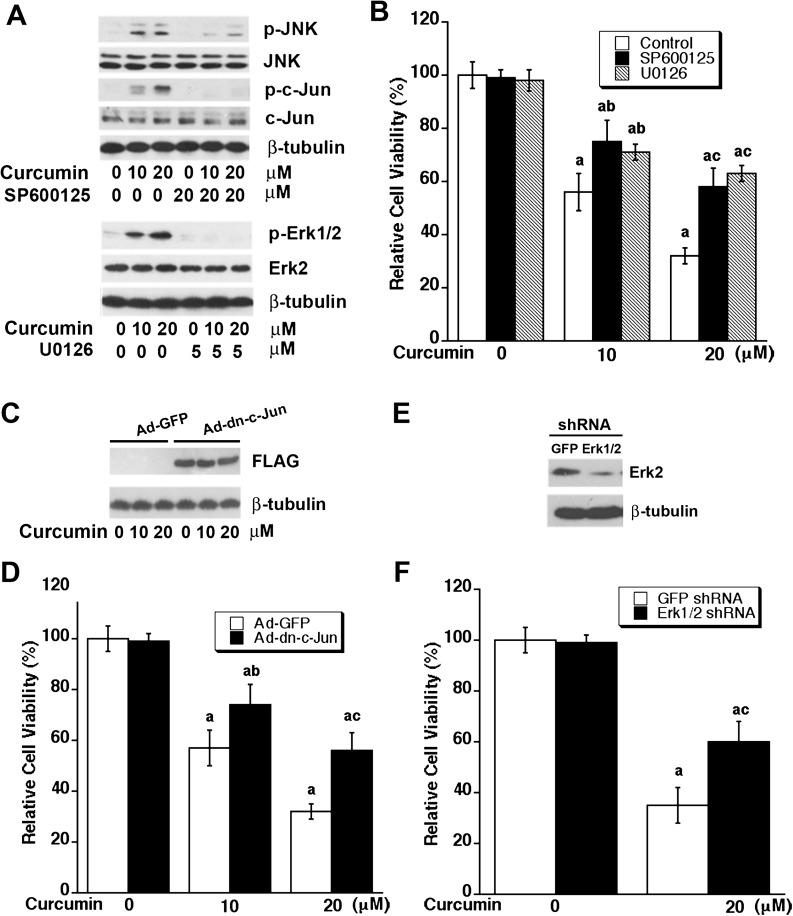
To determine the roles of Erk1/2 and JNK in curcumin-induced apoptosis in tumor cells, Rh30 cells were exposed to curcumin (0–20 μM) for 24 h after pretreatment of MEK1/2 (upstream of Erk1/2) inhibitor U0126 or JNK inhibitor SP600125 for 30 min, respectively. As shown in Figure 3A, curcumin-induced phosphorylation of c-Jun, as the readout of JNK activity, was dramatically blocked by SP600125 (20 μM). Similarly, U0126 (5 μM) blocked curcumin-induced phosphorylation of Erk1/2. As these inhibitors were able to inhibit JNK and Erk1/2 phosphorylation induced by curcumin at 10 and 20 μM, we next investigated whether the individual inhibitor could attenuate curcumin-induced death of tumor cells. Rh30 cells were pretreated with each inhibitor for 30 min, followed by exposure of curcumin (10 or 20 μM) for 48 h. As shown in Figure 3B, SP600125 or U0125 alone did not obviously alter the cell viability, but both attenuated curcumin-reduced cell viability. Similar results were also seen in Rh1, HeLa and HT29 cells (data not shown). In addition, we further confirmed the above finding by genetic manipulation. Infection of Rh30 with Ad-dn-c-Jun resulted in expression of a FALG-tagged dominant negative c-Jun as detected by western blotting with antibodies to FLAG (Figure 3C). Expression of the dominant negative c-Jun attenuated curcumin-reduced cell viability in Rh30 cells (Figure 3D, and Supplementary Figure 4SA, available at Carcinogenesis Online). In addition, infection of Rh30 with lentiviral shRNA to Erk1/2 downregulated expression of the proteins by ∼90% (Figure 3E). Silencing Erk1/2 also partially reduced curcumin-induced cell death in Rh30 cells (Figure 3F and Supplementary Figure 4SB, available at Carcinogenesis Online). Similar results were observed in HT29 cells (data not shown). The data indicate that curcumin-induced apoptosis of tumor cells is at least in part by activation of JNK and Erk1/2 pathways.
Fig. 3.
Inhibition of JNK or Erk1/2 attenuates curcumin-induced cell death. (A and B) Inhibition of JNK or Erk1/2 with selective inhibitors attenuates curcumin-induced cell death. Rh30 cells were pretreated with SP600125 (20 μM) or U0126 (5 μM) for 30 min and then exposed to curcumin (0–20 μM) for 24 h (for western blotting) or 48 h (for cell viability assay). (A) Western blot analysis was performed with indicated antibodies. (B) Cell viability was evaluated using one solution reagent. Results are presented as mean ± SE (n = 6). aP < 0.05, difference versus control group; bP < 0.05, difference versus 10 μM curcumin group; cP < 0.05, difference versus 20 μM curcumin group. (C–F) Overexpression of dominant negative c-Jun or downregulation of Erk1/2 attenuates curcumin-induced cell death. Rh30 cells, infected with recombinant adenovirus encoding FLAG-tagged dominant negative c-Jun (Ad-dn-c-Jun) or GFP (control) for 24 h, were exposed to curcumin (0–20 μM) for 24 h (for western blotting) or 48 h (for cell viability assay). (C) Western blot analysis was performed with indicated antibodies. (D) Cell viability was evaluated using one solution reagent. Results are presented as mean ± SE (n = 6). aP < 0.05, difference versus control group; bP < 0.05, difference versus 10 μM curcumin group with Ad-GFP infection; cP < 0.05, difference versus 20 μM curcumin group with Ad-GFP infection. Rh30 cells were infected with lentiviral shRNA to Erk1/2 or GFP for 5 days, followed by western blotting with indicated antibodies (E) or further exposed to curcumin (0–20 μM) for 48 h, followed by cell viability assay using one solution reagent (F). Results are presented as mean ± SE (n = 6). aP < 0.05, difference versus control group; bP < 0.05, difference with 20 μM curcumin group with shRNA-GFP infection.
Curcumin-induced ROS downregulate protein phosphatase activity
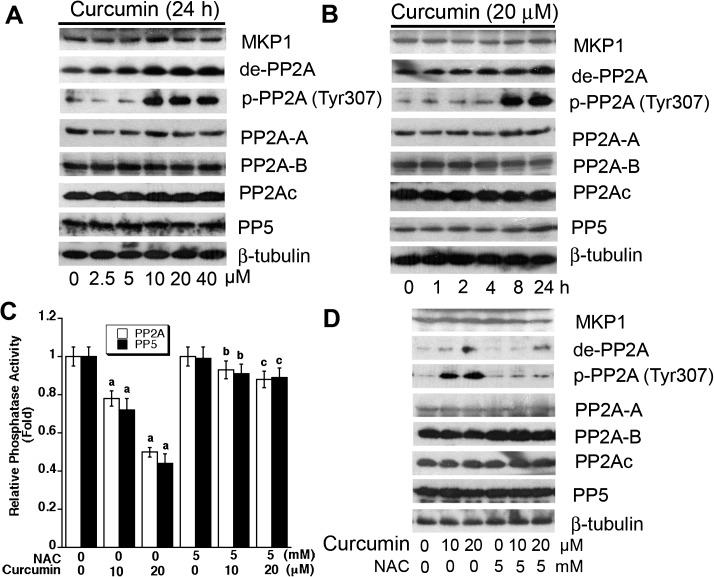
Studies have demonstrated that MKP-1 and PP2A negatively regulate phosphorylation of Erk1/2, JNK and/or p38, and PP5 negatively regulates JNK/p38 cascade, involved in oxidative stress (27,31–35). Therefore, we hypothesized that curcumin may activate JNK and Erk1/2 pathways by downregulation of MKP-1, PP2A and/or PP5 protein levels or activities. To test this hypothesis, Rh30 cells were exposed to 0–40 μM curcumin for 24 h or to 20 μM curcumin for 0–24 h, followed by western blotting. As shown in Figure 4A and B, curcumin did not apparently alter cellular protein level of MKP-1, PP2Ac or PP5, but increased expression of demethylated- and phospho-PP2A, two events that are related to decreased activity of PP2A (40), in a dose- and time-dependent manner. PP2A is a heterotrimeric holoenzyme composed of a catalytic subunit (PP2Ac), an A subunit (also termed PR65), and members of the B subunit families, such as B (PR55), B′ (PR61), B′′ (PR72) and B′′′ (PR93/PR110) (40). As the phosphatase activity of PP2Ac or PP5 is modulated by the association with PP2A-A and PP2A-B regulatory subunits (27), we also examined whether curcumin affects expression of PP2A-A or PP2A-B. It turned out that curcumin did not alter cellular protein levels of PP2A-A or PP2A-B (Figure 4A and B). However, using Ser/Thr phosphatase assay, we found that curcumin inhibited PP2A and PP5 activities (Figure 4C). Similar data were also observed in HT29 cells (data not shown). The results suggest that curcumin inhibits PP2A and PP5 activities.
Fig. 4.
Curcumin induction of ROS downregulates protein phosphatase activities of PP2A and PP5. (A) Rh30 cells were treated with 0–40 μM curcumin for 24 h or (B) treated with 20 μM curcumin for 0–24 h and (C and D) Rh30 cells were pretreated with/without NAC (5 mM) for 1 h and then exposed to curcumin (0–20 μM) for 24 h, followed by western blot analysis with indicated antibodies (A, B, and D) or in vitro Ser/Thr phosphatase assay (C). Results are presented as mean ± SE (n = 6). aP < 0.05, difference versus control group; bP < 0.05, difference versus 10 μM curcumin group; cP < 0.05, difference versus 20 μM curcumin group.
Since curcumin-induced ROS activate MAPK pathway in cancer cells (Figure 2C), we next asked whether curcumin inhibition of protein phosphatase activities is related to ROS induction. By western blot analysis, we found that NAC potently blocked curcumin-induced demethylated- and phospho-PP2A expression in Rh30 cells (Figure 4D). Similarly, NAC prevented curcumin from inhibition of PP2A and PP5 activities (Figure 4C), as determined by the in vitro Ser/Thr phosphatase assay. Our findings imply that curcumin-induced ROS may inhibit PP2A and PP5, resulting in activation of JNK and Erk1/2.
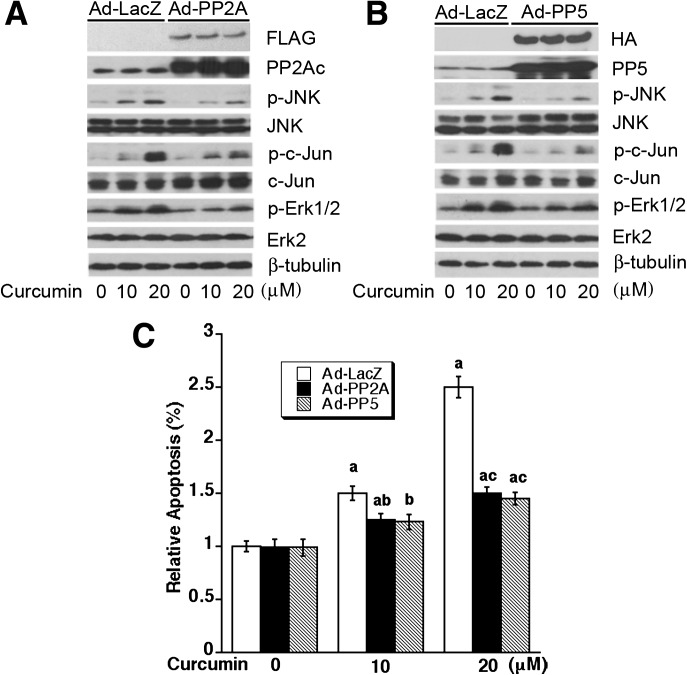
Overexpression of PP2A and PP5 partially prevents curcumin-induced activation of JNK and Erk1/2, as well as tumor cell death
To further verify the roles of PP2A and PP5 in curcumin-induced activation of MAPKs and tumor cell apoptosis, we next studied whether overexpression of PP2Ac or PP5 affects curcumin activation of JNK and Erk1/2, as well as cell death. Rh30 cells, infected with Ad-PP2A, Ad-PP5 and Ad-LacZ (as control), were exposed to curcumin (10 and 20 μM) for 4 h (for western blotting) or 48–72 h (for cell viability assay, apoptosis assay or morphological analysis). We observed that overexpression of PP2A partially prevented curcumin-induced activation of JNK and Erk1/2 (Figure 5A), as well as cell death (Figure 5C and Supplementary Figure 5SA and B, available at Carcinogenesis Online). Similarly, overexpression of PP5 in part rescued curcumin-induced cell death by inactivation of JNK and Erk1/2 (Figure 5B and C and Supplementary Figure 5SA and B, available at Carcinogenesis Online). Together, our data indicate that curcumin-induced apoptosis of tumor cells is at least in part by inhibiting protein phosphatase activities of PP2A and PP5, leading to activation of JNK and Erk1/2.
Fig. 5.
Overexpression of PP2A or PP5 partially prevents curcumin-induced activation of JNK and Erk1/2, as well as cell death. Rh30 cells, infected with Ad-PP2A, Ad-PP5 or Ad-LacZ (as control), were exposed with curcumin (0–20 μM) for 4 h (for western blotting) or 72 h (for apoptosis assay), followed by western blot analysis using indicated antibodies (A and B) or apoptosis assay using Annexin V-FITC Apoptosis Detection Kit I (C). Results (C) are presented as mean ± SE (n = 3). aP < 0.05, difference versus control group; bP < 0.05, difference with 10 μM curcumin group with Ad-LacZ infection; cP < 0.05, difference with 20 μM curcumin group with Ad-LacZ infection.
Discussion
Curcumin can induce p53-dependent apoptosis (1,2). Recently, we have shown that curcumin can induce p53-independent apoptosis in human rhabdomyosarcoma (Rh30, p53 mutant, R273C) and Ewing sarcoma (Rh1, p53 mutant, Y220C) cells (15). Here, we further found that curcumin was able to induce p53-independent cell death in more cell lines, including human colon adenocarcinoma (HT29) (p53 mutant, R273H) (36) and cervical carcinoma (HeLa) cells (expressing wild-type p53, but inactivated by human papillomavirus type 16 E6) (37). Y220C, the most common mutation outside the DNA-binding core domain of p53, restricts p53 predominantly in the cytoplasm (41) and destabilizes the protein (42), whereas R273C and R273H mutations make p53 protein defective in sequence-specific DNA binding to p53 responsive elements in the p53 target genes (42). All these mutations result in loss of p53 function (41,42). Among the tumor cell lines tested, Rh1 cells were the most sensitive to curcumin (Figure 1A). Whether this is related to the specific mutation at Y220C remains to be defined. Our results are in agreement with the previous observations in human melanoma cells (43), lung cancer cells (44) and ovarian carcinoma cells (45). The ability of curcumin to induce apoptosis regardless of p53 status strongly support the idea that curcumin is able to induce not only p53-dependent but also p53-independent apoptosis in tumor cells.
In this study, we noticed that curcumin reduced the cell viability in a concentration-dependent manner. Exposure to curcumin at 2.5 μM for 48 h was able to reduce cell viability significantly in Ewing sarcoma (Rh1) and rhabdomyosarcoma (Rh30) cells (Figure 1A). Phase I clinical trials have demonstrated that oral administration of curcumin at a dose of 8 g/day did not show deleterious side effects, and the average peak serum concentration of curcumin was 1.77 ± 1.87 μM (46). Addition of a low dose of piperine (from black pepper) can increase the uptake of curcumin by 2000% in humans (47). If a nanoparticle-based delivery system is utilized, the pharmacologic properties, such as the bioavailability and the half-life of curcumin could be much improved (47,48). Our finding suggests that curcumin can induce tumor cell death at pharmacologically relevant concentrations and may be explored for cancer prevention and treatment.
To gain insight into the mechanism by which curcumin induces p53-independent apoptosis in tumor cells, at the very beginning, we examined whether curcumin affects expression of pro-apoptotic (e.g. BAX, BAK, BAD and BIM) or anti-apoptotic proteins (e.g. Bcl-2, Bcl-XL and Mcl-1). However, to our surprise, curcumin failed to alter expression of those proteins in Rh1 and Rh30 cells (data not shown). Since activation of MAPK cascade also contributes to p53-indepenednt apoptosis (25–27), we further studied whether curcumin-induced apoptosis links to activation of MAPK pathways. Here, we provide evidence that curcumin-induced p53-independent apoptosis is indeed attributed to activation of JNK and Erk1/2 in Rh1 and Rh30 cells. This is evidenced by the findings that curcumin activated JNK and Erk1/2, resulting in cell death, which could be partially attenuated by SP600125 (JNK inhibitor) and U0126 (inhibitor of MEK1/2, upstream kinases of Erk1/2) (Figure 3B), by expression of dominant negative c-Jun (Figure 3D) or by downregulation of Erk1/2 in Rh30 cells (Figure 3F). The result is, to some degree, in agreement with the findings in other cells (49,50). For instance, in human colon cancer cells (HCT116, p53 status not mentioned), curcumin-induced sustained phosphorylation and activation of JNK and p38 MAPK, but only activation of JNK was associated with curcumin-induced cell death (49). In human leukemia cells (HL-60, p53-null), tetrahydrocurcumin, a major metabolite of curcumin, induced a transient phosphorylation of Erk1/2 and JNK within 1–3 h, but inhibited phosphoryaltion of p38 MAPK (51). In murine splenic lymphocytes, curcumin and dimethoxycurcumin (a synthetic curcumin analog) stimulated the basal phosphorylation of JNK, Erk1/2 and p38 MAPK, although they inhibited Con A-induced phosphorylation of the MAPKs (52). From the above findings, we have noticed that there exist discrepant effects of curcumin on Erk1/2, JNK and p38 MAPK. Likely, this is related to different phenotype each cell line has or different experimental conditions used.
In this study, we further identified that curcumin-induced activation of MAPK cascade is due to induction of ROS, leading to inhibition of PP2A and PP5, two serine/threonine protein phosphatases that negatively regulate MAPKs. This is supported by the following findings. First, curcumin-induced ROS and cell death in a spectrum of tumor cells (Rh1, Rh30, HT29 and HeLa), which was almost completely blocked by NAC (Figure 1), a ROS scavenger. Secondly, curcumin-induced activation of the MAPK signaling could be remarkably inhibited by NAC (Figure 2C). Finally, curcumin inhibited the activities of PP2A and PP5 (Figure 4), and overexpression of PP2A or PP5 partially prevented curcumin-induced activation of JNK and Erk1/2, as well as cell death (Figure 5). Recent studies have shown that curcumin-induced apoptosis via induction of ROS in small cell lung cancer cells (NCI-H446) (53), mantle cell lymphoma cells (Granta and NCEB) (54), and nasopharyngeal carcinoma cells (NPC-TW 076) (55). To our knowledge, this is the first report showing that curcumin-induced ROS inhibit PP2A and PP5, leading to MAPKs activation and cell death.
We observed that curcumin inhibits PP2A or PP5 not through altering cellular protein expression of the catalytic subunit (PP2Ac or PP5) and the regulatory proteins (PP2A-A and PP2A-B) (Figure 4A and B). Current data indicate that curcumin inhibits the phosphatase activity of PP2A at least by enhancing demethylation and phosphorylation of PP2Ac (Tyr307) (Figure 4A and B), two events responsible for PP2A inactivation (40). It is unclear how curcumin inhibits PP5. Rapamycin inhibits PP5 by dissociating PP2A-B from PP5 (27). Whether curcumin inhibits PP5 by dissociating PP2A-A or PP2A-B from PP5 remains to be determined. It should be mentioned that in the present study, we did not notice an apparent effect of curcumin on the cellular protein level of MKP-1 (Figure 4A and B), a phosphatase that dephosphorylates Erk1/2, JNK and p38 MAPK. However, we could not exclude the possibility that curcumin might actually inhibit the phosphatase activity of MKP-1, by unknown mechanism.
In conclusion, we have shown that curcumin activates MAPK cascade by induction of ROS, and inhibition of PP2A and PP5 activity, leading to p53-independent apoptosis in tumor cells. Our findings reveal a novel antitumor mechanism of curcumin.
Supplementary material
Supplementary Figures 1S–5S can be found at http://carcin.oxfordjournals.org/.
Funding
This work was supported in part by National Institutes of Health (CA115414; S.H.), American Cancer Society (RSG-08-135-01-CNE; S.H.), and the Chinese Scholarship Council (X.H.).
Supplementary Material
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- FBS
fetal bovine serum
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- NAC
N-acetyl-L-cysteine
- ROS
reactive oxygen species
References
- 1.Goel A, et al. Curcumin as “Curecumin”: from kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Zhou H, et al. The targets of curcumin. Curr. Drug Targets. 2011;12:332–347. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta K, et al. Antiproliferative effect of curcumin (diferuloylmethane) against human breast tumor cell lines. Anticancer Drugs. 1997;8:470–481. doi: 10.1097/00001813-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Jiang MC, et al. Differential regulation of p53, c-Myc, Bcl-2 and Bax protein expression during apoptosis induced by widely divergent stimuli in human hepatoblastoma cells. Oncogene. 1996;13:609–616. [PubMed] [Google Scholar]
- 5.Han SS, et al. Curcumin causes the growth arrest and apoptosis of B cell lymphoma by downregulation of egr-1, c-myc, bcl-XL, NF-κB, and p53. Clin. Immunol. 1999;93:152–161. doi: 10.1006/clim.1999.4769. [DOI] [PubMed] [Google Scholar]
- 6.Mukhopadhyay A, et al. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene. 2002;21:8852–8861. doi: 10.1038/sj.onc.1206048. [DOI] [PubMed] [Google Scholar]
- 7.Chen A, et al. Activation of PPARγ by curcumin inhibits Moser cell growth and mediates suppression of gene expression of cyclin D1 and EGFR. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G447–G456. doi: 10.1152/ajpgi.00209.2004. [DOI] [PubMed] [Google Scholar]
- 8.Singh S, et al. Activation of transcription factor NF-κB is suppressed by curcumin (diferuloylmethane) J. Biol. Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 9.Huang TS, et al. Suppression of c-Jun/AP-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proc. Natl Acad. Sci. USA. 1991;88:5292–5296. doi: 10.1073/pnas.88.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korutla L, et al. Inhibitory effect of curcumin on epidermal growth factor receptor kinase activity in A431 cells. Biochim. Biophys. Acta. 1994;1224:597–600. doi: 10.1016/0167-4889(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 11.Hong RL, et al. Curcumin inhibits tyrosine kinase activity of p185neu and also depletes p185neu. Clin. Cancer Res. 1999;5:1884–1891. [PubMed] [Google Scholar]
- 12.Chaudhary LR, et al. Inhibition of cell survival signal protein kinase B/Akt by curcumin in human prostate cancer cells. J. Cell. Biochem. 2003;89:1–5. doi: 10.1002/jcb.10495. [DOI] [PubMed] [Google Scholar]
- 13.Woo JH, et al. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24:1199–1208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 14.Liu JY, et al. Inhibitory effects of curcumin on protein kinase C activity induced by 12-O-tetradecanoyl-phorbol-13-acetate in NIH 3T3 cells. Carcinogenesis. 1993;14:857–861. doi: 10.1093/carcin/14.5.857. [DOI] [PubMed] [Google Scholar]
- 15.Beevers CS, et al. Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. Int. J. Cancer. 2006;119:757–764. doi: 10.1002/ijc.21932. [DOI] [PubMed] [Google Scholar]
- 16.Li M, et al. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–1996. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 17.Yu S, et al. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol. Cancer Ther. 2008;7:2609–2620. doi: 10.1158/1535-7163.MCT-07-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beevers CS, et al. Curcumin disrupts the Mammalian target of rapamycin-raptor complex. Cancer Res. 2009;69:1000–1008. doi: 10.1158/0008-5472.CAN-08-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liontas A, et al. Curcumin and resveratrol induce apoptosis and nuclear translocation and activation of p53 in human neuroblastoma. Anticancer Res. 2004;24:987–998. [PubMed] [Google Scholar]
- 20.Choi BH, et al. p21 Waf1/Cip1 expression by curcumin in U-87MG human glioma cells: role of early growth response-1 expression. Cancer Res. 2008;68:1369–1377. doi: 10.1158/0008-5472.CAN-07-5222. [DOI] [PubMed] [Google Scholar]
- 21.Zheng M, et al. Inhibition of nuclear factor-kappaB and nitric oxide by curcumin induces G2/M cell cycle arrest and apoptosis in human melanoma cells. Melanoma Res. 2004;14:165–171. doi: 10.1097/01.cmr.0000129374.76399.19. [DOI] [PubMed] [Google Scholar]
- 22.Ramachandran C, et al. Differential sensitivity of human mammary epithelial and breast carcinoma cell lines to curcumin. Breast Cancer Res. Treat. 1999;54:269–278. doi: 10.1023/a:1006170224414. [DOI] [PubMed] [Google Scholar]
- 23.Moos PJ, et al. Curcumin impairs tumor suppressor p53 function in colon cancer cells. Carcinogenesis. 2004;25:1611–1617. doi: 10.1093/carcin/bgh163. [DOI] [PubMed] [Google Scholar]
- 24.Tsvetkov P, et al. Inhibition of NAD(P)H:quinone oxidoreductase 1 activity and induction of p53 degradation by the natural phenolic compound curcumin. Proc. Natl Acad. Sci. USA. 2005;102:5535–5540. doi: 10.1073/pnas.0501828102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, et al. Arsenic induces apoptosis through a c-Jun NH2-terminal kinase-dependent, p53-independent pathway. Cancer Res. 1999;59:3053–3058. [PubMed] [Google Scholar]
- 26.Huang S, et al. Sustained activation of the JNK cascade and rapamycin-induced apoptosis are suppressed by p53/p21Cip1. Mol. Cell. 2003;11:1491–1501. doi: 10.1016/s1097-2765(03)00180-1. [DOI] [PubMed] [Google Scholar]
- 27.Huang S, et al. Inhibition of mammalian target of rapamycin activates apoptosis signal-regulating kinase 1 signaling by suppressing protein phosphatase 5 activity. J. Biol. Chem. 2004;279:36490–36496. doi: 10.1074/jbc.M401208200. [DOI] [PubMed] [Google Scholar]
- 28.Kyriakis JM, et al. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 29.Kim EK, et al. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Baldys A, et al. Apoptosis induced by crocidolite asbestos in human lung epithelial cells involves inactivation of Akt and MAPK pathways. Apoptosis. 2007;12:433–447. doi: 10.1007/s10495-006-0577-8. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, et al. Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic. Biol. Med. 2008;45:1035–1044. doi: 10.1016/j.freeradbiomed.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, et al. Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int. J. Biochem. Cell Biol. 2009;41:1284–1295. doi: 10.1016/j.biocel.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 33.Franklin CC, et al. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J. Biol. Chem. 1997;272:16917–16923. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- 34.Morita K, et al. Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. EMBO J. 2001;20:6028–6036. doi: 10.1093/emboj/20.21.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao WQ, et al. Impairment of phosphatase 2A contributes to the prolonged MAP kinase phosphorylation in Alzheimer's disease fibroblasts. Neurobiol. Dis. 2003;14:458–469. doi: 10.1016/s0969-9961(03)00124-4. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues NR, et al. p53 mutations in colorectal cancer. Proc. Natl Acad. Sci. USA. 1990;87:7555–7559. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoppe-Seyler F, et al. Repression of endogenous p53 transactivation function in HeLa cervical carcinoma cells by human papillomavirus type 16 E6, human mdm-2, and mutant p53. J. Virol. 1993;67:3111–3117. doi: 10.1128/jvi.67.6.3111-3117.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ke Q, et al. Essential role of ROS-mediated NFAT activation in TNF-α induction by crystalline silica exposure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L257–L264. doi: 10.1152/ajplung.00007.2006. [DOI] [PubMed] [Google Scholar]
- 39.Atsumi T, et al. Comparative cytotoxicity and ROS generation by curcumin and tetrahydrocurcumin following visible-light irradiation or treatment with horseradish peroxidase. Anticancer Res. 2007;27:363–371. [PubMed] [Google Scholar]
- 40.Janssens V, et al. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dearth LR, et al. Inactive full-length p53 mutants lacking dominant wild-type p53 inhibition highlight loss of heterozygosity as an important aspect of p53 status in human cancers. Carcinogenesis. 2007;28:289–298. doi: 10.1093/carcin/bgl132. [DOI] [PubMed] [Google Scholar]
- 42.Joerger AC, et al. Structural basis for understanding oncogenic p53 mutations and designing rescue drugs. Proc. Natl Acad. Sci. USA. 2006;103:15056–15061. doi: 10.1073/pnas.0607286103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bush JA, et al. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp. Cell Res. 2001;271:305–314. doi: 10.1006/excr.2001.5381. [DOI] [PubMed] [Google Scholar]
- 44.Radhakrishna Pillai G, et al. Induction of apoptosis in human lung cancer cells by curcumin. Cancer Lett. 2004;208:163–170. doi: 10.1016/j.canlet.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Watson JL, et al. Curcumin-induced apoptosis in ovarian carcinoma cells is p53-independent and involves p38 mitogen-activated protein kinase activation and downregulation of Bcl-2 and survivin expression and Akt signaling. Mol. Carcinog. 2010;49:13–24. doi: 10.1002/mc.20571. [DOI] [PubMed] [Google Scholar]
- 46.Cheng AL, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 47.Singh S. From exotic spice to modern drug? Cell. 2007;130:765–768. doi: 10.1016/j.cell.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 48.Li N, et al. Nanoparticle multilayers: surface modification of photosensitive drug microparticles for increased stability and in vitro bioavailability. J. Nanosci. Nanotechnol. 2006;6:3252–3260. doi: 10.1166/jnn.2006.421. [DOI] [PubMed] [Google Scholar]
- 49.Collett GP, et al. Curcumin induces c-jun N-terminal kinase-dependent apoptosis in HCT116 human colon cancer cells. Carcinogenesis. 2004;25:2183–2189. doi: 10.1093/carcin/bgh233. [DOI] [PubMed] [Google Scholar]
- 50.Patwardhan RS, et al. Dimethoxycurcumin, a metabolically stable analogue of curcumin, exhibits anti-inflammatory activities in murine and human lymphocytes. Biochem. Pharmacol. 2011;82:642–657. doi: 10.1016/j.bcp.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 51.Wu JC, et al. Tetrahydrocurcumin, a major metabolite of curcumin, induced autophagic cell death through coordinative modulation of PI3K/Akt-mTOR and MAPK signaling pathways in human leukemia HL-60 cells. Mol. Nutr. Food Res. 2011;55:1646–1654. doi: 10.1002/mnfr.201100454. [DOI] [PubMed] [Google Scholar]
- 52.Lee JY, et al. Curcumin induces EGFR degradation in lung adenocarcinoma and modulates p38 activation in intestine: the versatile adjuvant for gefitinib therapy. PLoS One. 2011;6:e23756. doi: 10.1371/journal.pone.0023756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang CL, et al. Curcumin induces small cell lung cancer NCI-H446 cell apoptosis via the reactive oxygen species-mediated mitochondrial pathway and not the cell death receptor pathway. DNA Cell Biol. 2012;31:139–150. doi: 10.1089/dna.2011.1300. [DOI] [PubMed] [Google Scholar]
- 54.Singh AT, et al. Curcumin nanodisk-induced apoptosis in mantle cell lymphoma. Leuk. Lymphoma. 2011;52:1537–1543. doi: 10.3109/10428194.2011.584253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuo CL, et al. Apoptotic death in curcumin-treated NPC-TW 076 human nasopharyngeal carcinoma cells is mediated through the ROS, mitochondrial depolarization and caspase-3-dependent signaling responses. Int. J. Oncol. 2011;39:319–328. doi: 10.3892/ijo.2011.1057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.