Abstract
Celastrol (CSL) is a naturally occurring triterpenoid acid that exhibits anticancer activity, and in KU7 and 253JB-V bladder cells, CSL induced apoptosis, inhibited growth, colony formation and migration and CSL decreased bladder tumor growth in vivo. CSL also decreased expression of specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 and several Sp-regulated genes/proteins including vascular endothelial growth factor, survivin and cyclin D1 and fibroblast growth factor receptor-3, a potential drug target for bladder cancer therapy, has now been characterized as an Sp-regulated gene downregulated by CSL. The mechanism of Sp downregulation by CSL was cell context-dependent due to activation of proteosome-dependent (KU7) and -independent (253JB-V) pathways. In 253JB-V cells, CSL induced reactive oxygen species (ROS) and inhibitors of ROS blocked CSL-induced growth inhibition and repression of Sp1, Sp3 and Sp4. This response was due to induction of the Sp repressors ZBTB10 and ZBTB4 and downregulation of miR-27a and miR-20a/17-5p, respectively, which regulate expression of these transcriptional repressors. Thus, the anticancer activity of CSL in 253JB-V cells is due to induction of ROS and ROS-mediated induction of Sp repressors (ZBTB4/ZBTB10) through downregulation of miR-27a and miR-20a/17-5p.
Introduction
Bladder cancer is the ninth most common cancer worldwide and ranks 13th as the cause of cancer deaths (1). It was estimated that 70 530 new cases were diagnosed in 2010 and 14 680 deaths occurred from this disease in the USA. In spite of the initial favorable response to intra-vesicular bacillus Calmette-Guerin administration, bladder tumors eventually recur to form an invasive phenotype, which often lead to surgical removal of the bladder (2,3). MVAC (methotrexate, vinblastine, adriamycin and cisplatin) chemotherapy has been extensively used for treatment of advanced bladder cancer and is accompanied by toxic side effects and, thus, it is important to develop less toxic alternate chemotherapeutic therapies and dietary management strategies for prevention of this disease (4,5).
New mechanism-based drugs for bladder cancer chemotherapy have been reported and these include tyrosine kinase inhibitors against the epidermal growth factor receptor and antiangiogenic drugs or antibodies that block vascular endothelial growth factor (VEGF) and activation of VEGF receptors (6). Diindolylmethane derivatives developed in this laboratory inhibit bladder cancer cell and tumor growth through both orphan nuclear receptor-dependent and -independent pathways (7). In addition, curcumin, the active component of turmeric, also exhibits anticancer activity in vitro and in vivo against bladder cancer and studies in this laboratory suggest that the mechanism of action of curcumin is due, in part, to downregulation of specificity protein (Sp) transcription factors and Sp-regulated gene products (8,9).
In this study, we have investigated the activity of the triterpenoid celastrol (CSL) as an inhibitor of bladder cancer cell and tumor growth. CSL is a quinone methide triterpenoid acid extracted from the root of Tripterygium wilfordii (also known as Thunder of God Vine), and this compound has been used in traditional Chinese medicine to treat immune-inflammatory diseases such as rheumatoid arthritis, chronic nephritis, chronic hepatitis and lupus erythematous. CSL acts as a cytokine release inhibitor and is antiallergic (10) and, currently, CSL is in clinical trials for rheumatoid arthritis (11). CSL also inhibits cancer cell growth, survival, angiogenesis and inflammation (10) and these responses are accompanied by downregulation of multiple gene products including cyclin D1 (proliferation), bcl-2 and survivin (survival), VEGF and its receptors (VEGFR1 and 2) (angiogenesis) and NFκBp65/p50 (inflammation) (2,12–23).
Previous studies in this laboratory show that Sp transcription factors Sp1, Sp3 and Sp4 are overexpressed in bladder and other cancer cell lines (8,9,24–29), and the anticancer activity of curcumin and arsenic trioxide in bladder cancer cells is due, in part, to activation of proteasomes or induction of reactive oxygen species (ROS) and ROS-dependent downregulation Sp1, Sp3 and Sp4 and Sp-regulated genes (8,29). Moreover, RNA interference studies (Sp knockdown) in bladder or pancreatic cancer show that expression of many of the gene products decreased by CSL are regulated by Sp1, Sp3 and Sp4 transcription factors (8,9). Therefore, we hypothesized that one of the underlying mechanisms of action of CSL as an anticancer agent was due to targeting Sp transcription factors. Results of this study confirm this hypothesis and also demonstrate the role of ROS and ROS-dependent disruption of microRNA-27a (miR-27a):ZBTB10 and miR-20a/17-5p:ZBTB4, resulting in the induction of the Sp transcriptional repressors ZBTB10 and ZBTB4.
Materials and methods
Cell lines, reagents, proliferation, ROS and MMP assays
KU7 and 253JB-V human bladder cancer cells were provided by Dr A.Kamat (University of Texas M. D. Anderson Cancer Center, Houston, TX), and non-transformed SVHUC-1 bladder cells were kindly provided by Dr Y.Luo (University of Iowa, Iowa City, IA). 253JB-V and KU7 cell lines and reagents and their sources were used or purchased as described previously (8,9,29). Mitochondrial membrane potentials (MMPs) (JC-1 dye) and ROS (CM-H2DCFDA dye) were also determined as described (27,29). 253JB-V cells are a metastatic variant of 253J-P cells; both cell lines are derived from transitional cell carcinomas and they overexpress Sp1, Sp3 and Sp4 proteins (4,7–9). CSL was purchased from Calbiochem/EMD Chemicals (La Jolla, CA).
Soft agar colony, scratch and apoptosis assays
The colony forming assay was performed by seeding 4 × 103 253JB-V and KU7 cells per 35-mm dish and cultured in 0.35% soft agar in Dulbecco's modified Eagle's medium plus 2.5% fetal bovine serum at 37°C for 7 days in triplicate. Colonies >60 μM in diameter were counted at the end of day 10 using light microscopy. 253JB-V and KU7 cells were seeded at a density of 8 × 105 cells per well in a 6-well microplate. Day 1 was the reference control. For the migration (scratch) assay, 12 h after seeding, cells were wounded by manually scratching with a pipette tip, washed twice with phosphate-buffered saline and incubated at 37°C with or without CSL (1 μM) for 48 h. Images of wound gap were taken at 0 and 48 h by an EVOS microscope. These experiments were repeated at least three times. The terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay was carried out using the cell death detection POD kit as described (29) and Annexin V was determined using the Vybrant apoptosis kit (Molecular, Grand Island, NY) as described (25).
Western blot, RNA interference and transfection assays
Bladder cancer cells were seeded in Dulbecco's modified Eagle's medium:Ham's F-12 medium containing 2.5% charcoal-stripped fetal bovine serum. After 24 h, cells were treated with either vehicle (dimethyl sulfoxide) or the indicated compounds for 24 h and lysates were analyzed by western blots as described (8). The triple Sp small inhibitory RNA knockdown (iSp1, iSp3, iSp4) complex (iSp) along with iLamin were prepared by Sigma (St Louis, MO) and used as described previously (8). The YH633p(-220/-27)FR3-luc(C4) plasmid was a kind gift from Dr Young-Kwon Hong (University of Southern California, Los Angeles, CA) and Dr David Ornitz (Washington University School of Medicine, St Louis, MO). The miR-27a-luc construct was provided by Dr V.N.Kim (Seoul National University, Korea) and the miR-27-92(pro1353)-luc plasmid was provided by Dr S.M.Hammond (University of North Carolina, Chapel Hill, NC). The fibroblast growth factor receptor (FGFR)-3 construct containing minimal Sp binding sites on FGFR3 YH633p(-220/-27)FR3-luc(C4) and other Sp-regulated promoter constructs (pVEGF, pSurvivin, pSp1For4 and pSp3For5) were transfected in the two bladder cancer cell lines and luciferase activity (normalized to β-galactosidase) was determined essentially as described (8).
Xenograft study
KU7 cells (1 × 106 cells) in 1:1 ratio of Matrigel (BD Biosciences, San Jose, CA) were injected into the either side of flank area of nude mice. Female athymic nude mice, age 4–6 weeks, were purchased from Harlan (Indianapolis, CA). When the tumors were palpable, mice were divided into two groups of 10 animals each. The first group received 100 μl vehicle (corn oil) by intraperitoneal route, and the second group of animals received 4 mg/kg/day dose of CSL in corn oil every second day for 22 days (11 doses). The dose selected for this study was based on results of previous studies (30). After 23 days, mice were killed and the tumors in control and treated animals were homogenized and probed for Sp protein levels using western blotting.
Statistical analysis
Statistical significance of differences was determined by an analysis of variance and student t-test, and the levels of probability were noted.
Results
CSL inhibits bladder cancer cell and tumor growth and cell migration and induces apoptosis
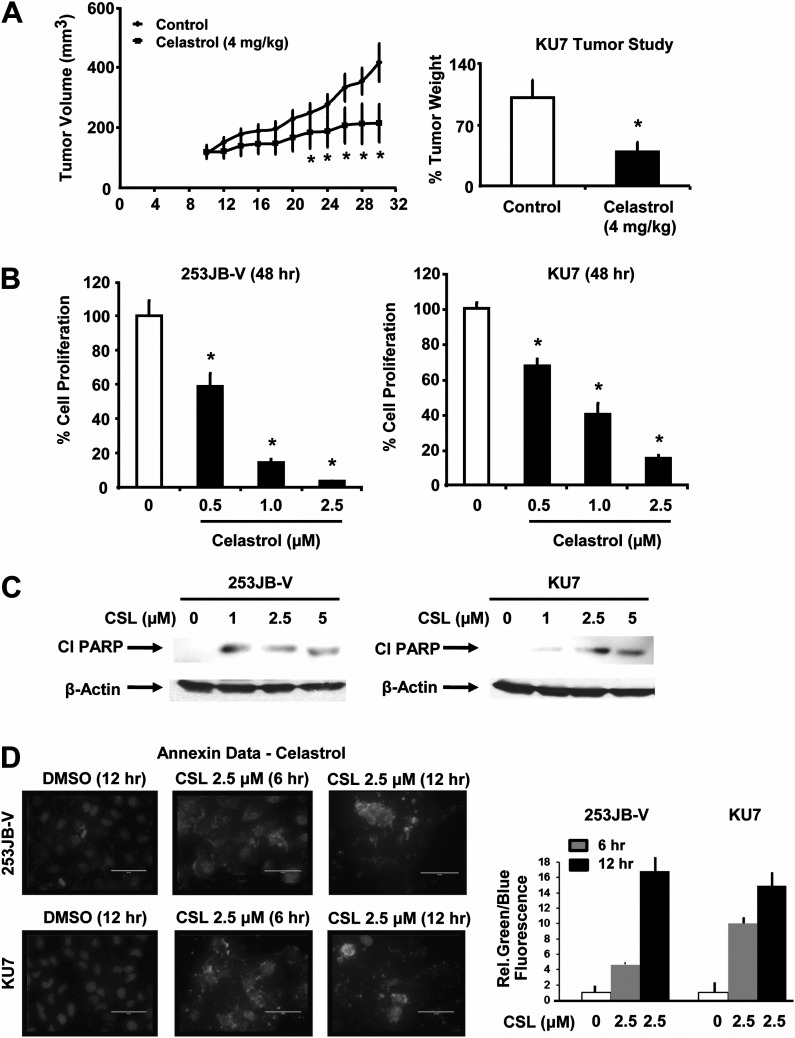
The in vivo antitumor activity of CSL (4 mg/kg/day) was investigated using female athymic nude mice bearing KU7 bladder cancer cells as xenografts and CSL significantly decreased tumor volumes and tumor weights (Figure 1A). These results are comparable with previous studies in several different tumor models where tumor growth inhibition by CSL was generally observed at doses of 1–4 mg/kg/day (30). CSL (0.5–2.5 μM) also inhibited growth of 253JB-V and KU7 bladder cancer cells (Figure 1B) and growth inhibitory IC50 values for 253JB-V and KU7 cells were 0.93 and 1.13 μM, respectively, after treatment for 48 h. Non-transformed SVHUC-1 cell growth was also inhibited by CSL (Supplementary Figure 1A is available at Carcinogenesis Online); however, the IC50 value was higher than observed in the cancer cells. CSL also significantly induced poly (ADP ribose) polymerase cleavage, a signature protein for apoptosis in both the cell lines (Figure 1C). 23JB-V cells were more sensitive than KU7 cells to CSL-induced poly (ADP ribose) polymerase cleavage and the decreased poly (ADP ribose) polymerase cleavage at the higher concentration was due to toxicity. CSL (3 μM) significantly inhibited anchorage-independent growth in both cell lines over a period of 10 days (Supplementary Figure 1B and C is available at Carcinogenesis Online) and cell migration in a scratch assay (48 h) was also inhibited (Supplementary Figure 1D and E is available at Carcinogenesis Online). CSL also significantly increased Annexin V staining in 253JB-V and KU7 cells 6 and 12 h after treatment (Figure 1D), and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling-positive staining was also increased (Supplementary Figure 2 is available at Carcinogenesis Online), confirming that CSL induced apoptosis in both cell lines.
Fig. 1.
CSL inhibits growth of bladder tumor xenografts, cell proliferation and induces apoptosis. (A) Mice were injected and treated and analyzed as described in the Materials and methods. (B) Cells were treated with dimethyl sulfoxide (DMSO) (solvent control); 0.5, 1.0 or 2.5 μM/l CSL and the effects of cell growth were determined after treatment for 48 h as described in Materials and methods. (C) 253JB-V and KU7 cells were treated with DMSO (0) and 1.0, 2.5 or 5.0 μM/l CSL for 24 h and induction of cleaved poly (ADP ribose) polymerase (PARP) protein was determined as described in Materials and methods. (D) 253JB-V and KU7 cells were treated with DMSO (0) or 2.5 μM/l CSL for 6 and 12 h and an increase in Annexin V positive cells was determined as described in Materials and methods. Results in (A), (B) and (D) are expressed as means ± SE for three or more replicate determinations for each treatment group, and significant (P < 0.05) CSL-induced inhibition (*) or increases (**) compared with the control are indicated.
CSL inhibits expression of VEGF, cyclin D1, survivin and NFκB expression in bladder cells
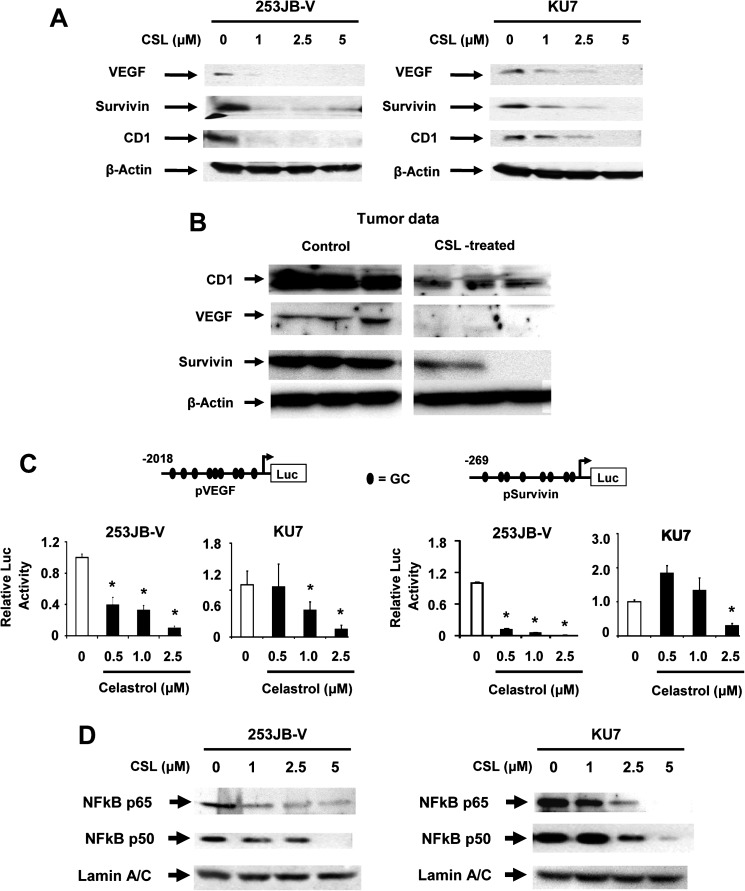
Figure 2A illustrates that CSL decreased expression of survivin, VEGF and cyclin D1 proteins in both 253JB-V and KU7 bladder cancer cells. We observed that concentrations of CSL ≤1.0 μM decreased expression of all three proteins in 253JB-V cells, whereas higher concentrations (≥1.0 μM) were required to induce similar effects in KU7 cells. Figure 2B also shows that CSL decreased cyclin D1, VEGF and survivin proteins in tumors compared with controls. CSL also decreased luciferase activity in 253JB-V and KU7 bladder cancer cells transfected with pVEGF and pSurvivin constructs containing GC-rich −2019 to +50 and −269 to +49 promoter inserts from the VEGF and survivin gene promoters, respectively (Figure 2C). The 253JB-V cells were also more responsive to the effects of CSL than KU7 cells in the transfection assays and this was similar to that observed for protein downregulation shown in Figure 2A.
Fig. 2.
Effects of CSL on angiogenic, survival and cell cycle proteins. (A) Bladder cancer cells were treated with dimethyl sulfoxide (DMSO) and 0.5, 1.0, 2.5 or 5.0 μM/l CSL for 24 h and whole-cell lysates were analyzed as indicated in Materials and methods. (B) Lysates from control (corn oil) and CSL-treated tumors were analyzed by western blots as described in the Materials and methods. (C) Bladder cancer cells were treated with DMSO and 0.5, 1.0, 2.5 or 5.0 μM/l CSL and luciferase promoter activities were analyzed as indicated in Materials and methods. (D) Cells were treated with DMSO and 0.5, 1.0, 2.5 or 5.0 μM/l CSL and nuclear extracts were analyzed by western blot analysis as described in Materials and methods. β-Actin served as a loading control and similar results were observed in duplicate experiments. Results in (C) are expressed as means ± SE for three replicate determinations for each treatment group, and significant (P < 0.05) CSL-induced decreases (*) compared with the solvent (DMSO, set at 1.0) are indicated.
Since CSL has been used in clinical trials to treat inflammatory conditions (22), the effects of CSL on the expression of the p65 and p50 subunits of NFκB in nuclear extracts of 253JB-V and KU7 bladder cancer cells were determined (Figure 2D). CSL significantly decreased p65 and p50 protein subunits of NFκB and these results are consistent with previous findings that CSL inhibits NFκB activation in multiple cell lines (4,12,19).
CSL decreases expression of Sp1, Sp3 and Sp4 transcription factors in bladder cancer cells and tumors
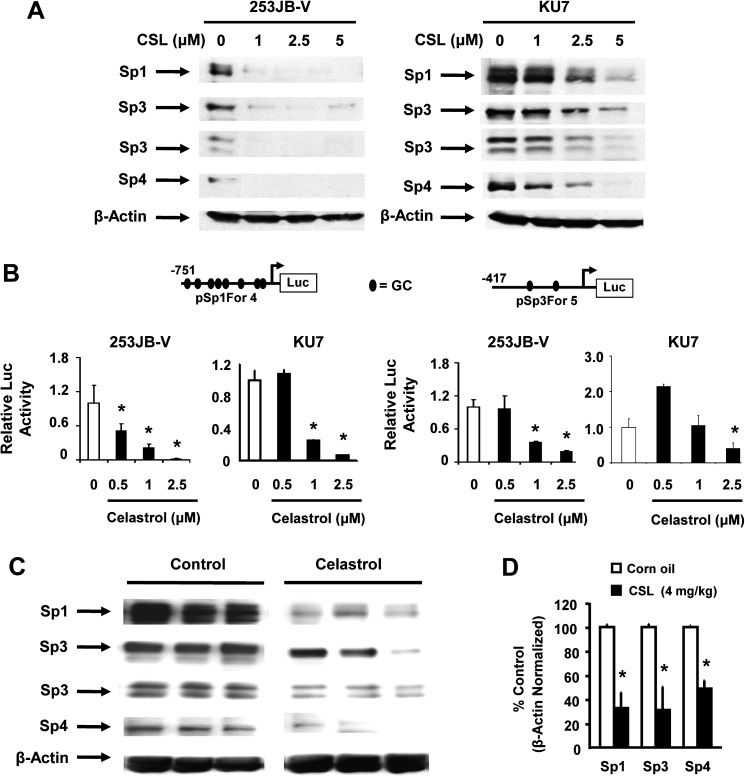
VEGF, survivin, p65 and cyclin D1 are Sp-regulated genes in bladder and other cancer cell lines, and results in Figure 3A show that CSL induced a concentration-dependent decrease in levels of Sp1, Sp3 and Sp4 proteins in both the cell lines. 253JB-V cells were more sensitive than KU7 cells to this effect as observed for other responses. Results in Figure 3B show that there was a concentration-dependent decrease in luciferase activity in KU7 and 253JB-V cells transfected with pSp1 (Sp1For4) and pSp3 (Sp3For5) constructs which contain −751 to −20 and −417 to −38 regions of Sp1 and Sp3 gene promoters, respectively, suggesting that CSL also affects transcription of Sp1 and Sp3 (note: Sp4 promoter constructs are not yet available). The in vitro effects of CSL were also observed in the in vivo study where CSL (4 mg/kg/day) also significantly decreased Sp1, Sp3 and Sp4 protein levels in tumors compared with corn oil-treated controls (Figure 3C and D). In contrast, expression of Sp1, Sp3 and Sp4 proteins in non-tumor tissue was minimal (data not shown).
Fig. 3.
In vitro and in vivo effects of CSL on Sp1, Sp3 and Sp4 transcription factors. (A) Cells were treated with dimethyl sulfoxide (DMSO) and 1.0, 2.5 or 5.0 μM/l CSL for 24 h and whole-cell lysates were analyzed by western blot analysis as described in Materials and methods. β-Actin served as a loading control and similar results were observed in duplicate experiments. (B) Bladder cancer cells were transfected with the indicated constructs, treated with DMSO and 0.5, 1.0 or 2.5 μM/l CSL for 24 h and luciferase activity was determined as described in Materials and methods. Results are expressed as means ± SE for three replicate determinations for each treatment group, and significant (P < 0.05) CSL-induced decreases (*) compared with the solvent (DMSO, set at 1.0) are indicated. (C) Tumor lysates from three representative mice in the treated and control groups were analyzed using western blotting as described in Materials and methods. (D) Sp protein band intensities from control and treated groups were determined (relative to β-actin) as described in Materials and methods; significant (P < 0.05) decreases compared with controls are indicated (*). There are four Sp3 isoforms that vary only in the N-terminus and include two overlapping high molecular weight bands (>100 kDa) and two low molecular weight bands (∼72 kDa) and these are observed in most cancer cell lines (8,24–29).
FGFR3 is an Sp-regulated that is decreased by CSL in bladder cancer cells and tumors
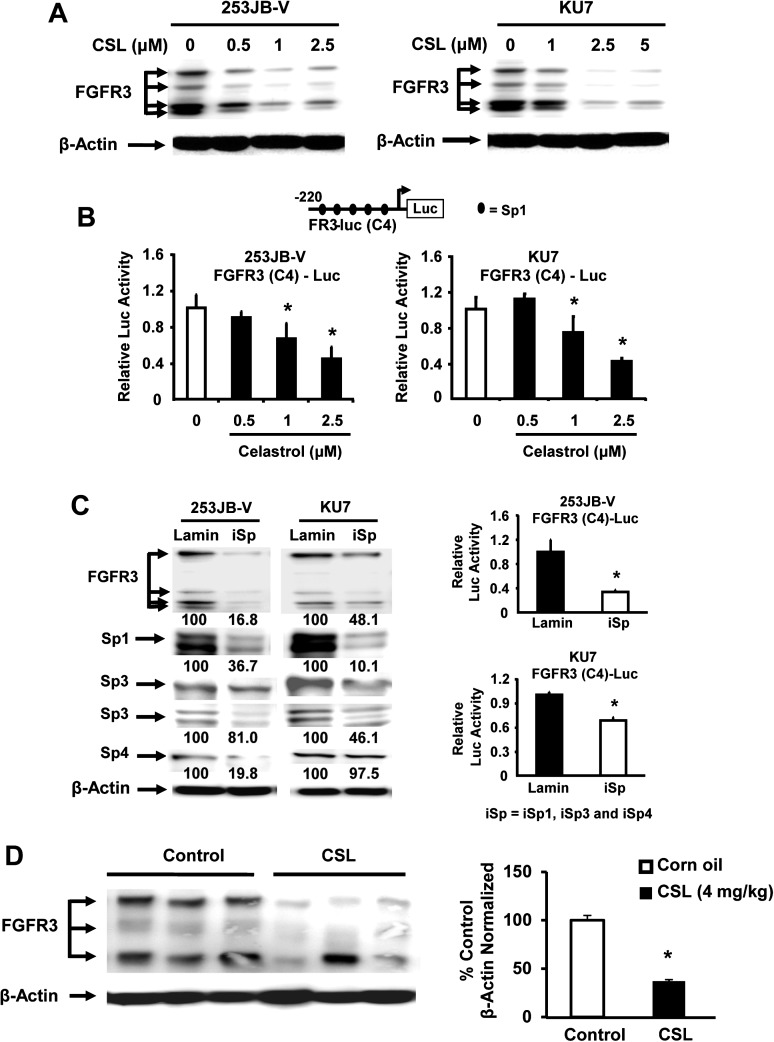
FGFR3 is overexpressed and/or activated by mutations in bladder cancer, and recent reports show that FGFR3 knockdown attenuated tumor progression in a mouse xenograft study indicating that FGFR3 is a potential drug target in bladder cancer (23). Since the FGFR3 promoter also contains GC-rich Sp binding sites, we investigated the effects of CSL on FGFR3 expression and the role of Sp transcription factors in mediating these effects. Treatment of 253JB-V and KU7 cells with CSL significantly decreased FGFR3 protein levels in both bladder cancer cell lines in a dose-dependent manner (Figure 4A). The western blot illustrates multiple FGFR3 bands (including the non-glycosylated, precursor and glycosylsated forms), and CSL decreased expression of all forms of FGFR3 in both cell lines. Moreover, CSL also decreased luciferase activity in bladder cancer cells transfected with a construct containing the minimal −220 to −27 GC-rich region of the FGFR3 gene promoter (Figure 4B). The role of Sp proteins in mediating the expression of FGFR3 was further investigated using a small inhibitory RNA cocktail (iSp) containing small inhibitory RNAs targeted to Sp1 (iSp1), Sp3 (iSp3) and Sp4 (iSp4) as described previously (9). Cells were transfected with iSp and analysis of whole-cell lysates by western blots shows that Sp1, Sp3 and Sp4 were decreased with variable overall efficiency in both cell lines (Figure 4C). We also observed that transfection of iSp decreased FGFR3 protein levels by 83.2 and 51.9% in 253JB-V and KU7 cells, respectively (Figure 4C). We also investigated the effects of iSp (iSp1, iSp3 and iSp4) on FGFR3 promoter activity and transfection with the iSp cocktail significantly decreased the FGFR3 promoter activity (Figure 4C). Figure 4D shows that CSL also downregulated expression of FGFR3 protein in tumors compared with tumors from control (corn oil-treated) mice and these results were similar to those observed for decreased Sp1, Sp3 and Sp4 expression in these tumors (Figure 2C), confirming a role for Sp transcription factors in regulating FGFR3 expression in bladder cancer. Previous reports showed that CSL is a heat shock protein 90 (Hsp90) inhibitor (31–33), and Hsp90 also regulates FGFR3 (34). Results illustrated in Supplementary Figure 3, available at Carcinogenesis Online show that CSL decreased expression of Hsp90 in KU7 and 253JB-V cells and this was blocked by the proteasome inhibitor MG132, suggesting that at least at the 5 μM concentration, CSL-mediated downregulation of Hsp90 may contribute to decreased FGFR3 expression.
Fig. 4.
FGFR3 expression in bladder cancer cells is regulated by Sp transcription factors. (A) 253JB-V and KU7 cells were treated with DMSO alone and 0.5, 1.0, 2.5 or 5.0 μM/l CSL and whole-cell lysates were analyzed by western blots as described in Materials and methods. (B) Bladder cancer cells were transfected with FGFR3 (C4)-luciferase promoter and then treated with DMSO and 0.5, 1.0 or 2.5 μM/l CSL for 24 h and the luciferase activity was determined as described in Materials and methods. (C) 253JB-V and KU7 cells were transfected with iSp (a cocktail of iSp1, iSp3 and iSp4) or iLamin, and whole-cell lysates were analyzed for the efficiency of Sp knockdown and the effects on FGFR3 protein by western blots and FGFR3 (C4)-luciferase promoter activity were determined as described in Materials and methods. (D) Western blot analysis of tumor lysates from three randomly selected mice in the treated and control groups was also determined as described in Materials and methods. Relative FGFR3 protein band intensities in tumors from control and CSL-treated mice were determined as described in the Materials and methods and significant (P < 0.05) CSL-induced decreases (*) in band intensities relative to the control (set at 100%) are indicated. Results in B, C and D are mean ± SE for at least three replicate determinations and significant (P < 0.05) decreases are indicated (*).
Mechanisms associated with CSL-induced downregulation of Sp transcription factors
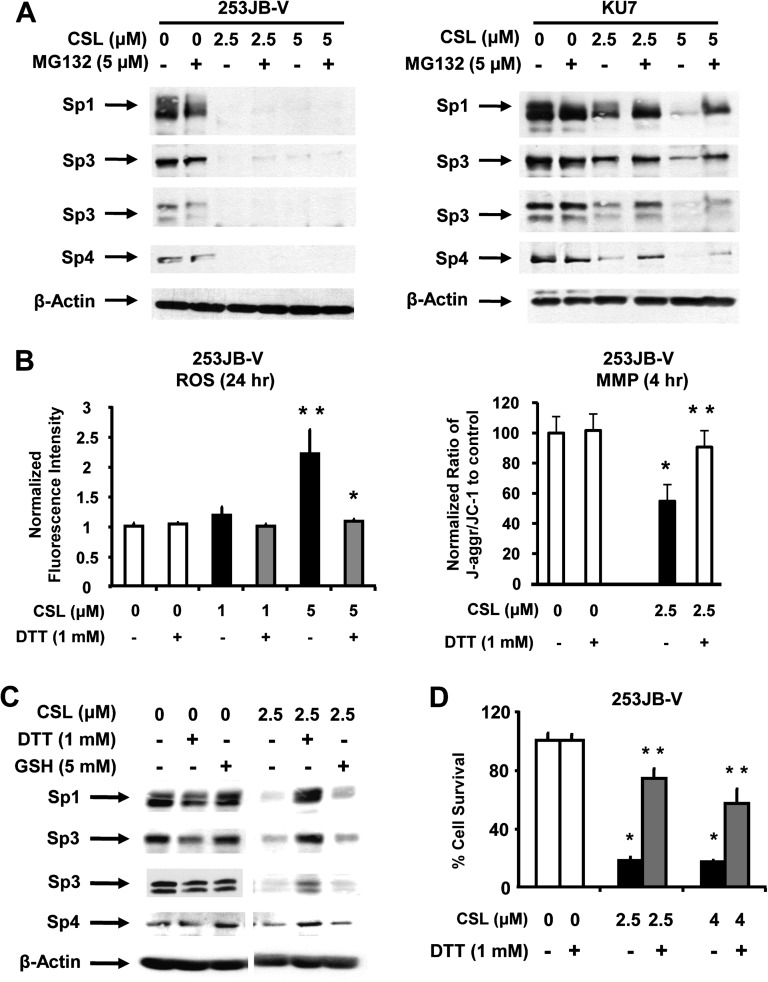
Results summarized in Figure 5A show that the proteosome inhibitor MG132 partially inhibited CSL-induced Sp degradation in KU7 cells as previously reported for curcumin in this cell line (8). However, MG132 did not inhibit the CSL-induced downregulation of Sp proteins in 253JB-V cells, demonstrating that CSL-mediated proteasome-dependent degradation of Sp1, Sp3 and Sp4 was cell context-dependent. Drug-induced decreases in MMP and of ROS have also been linked to downregulation of Sp1, Sp3 and Sp4 in bladder cancer cells (9,26) and the role of this pathway was further investigated in 253JB-V cells. Results summarized in Figure 5B illustrate that treatment of 253JB-V cells with CSL-decreased MMP and this was accompanied by induction of ROS using JC-1 and the cell-permeant dye CM-H2DCFDA, respectively.
Fig. 5.
CSL induces proteasome- and ROS-dependent Sp protein degradation, cell growth inhibition and also induces ROS and decreases MMP. (A) Bladder cancer cells were treated with dimethyl sulfoxide (DMSO) alone and 2.5 or 5.0 μM/l CSL in the presence or absence of 5 μM/l MG132 and whole-cell lysates were analyzed by western blots as described in Materials and methods. (B) Induction of ROS and loss of MMP by CSL. 253JB-V cells were treated with DMSO and 1.0 or 2.5 μM/l CSL for 4 h or 24 h, in the presence or absence of the antioxidant DTT, and ROS and MMP were determined as described under Materials and methods. (C) 253JB-V cells were treated with DMSO or 2.5 μM/l CSL for 24 h, in the presence or absence of antioxidants DTT or glutathione (GSH) and the protein lysates were subjected to western blotting as described in Materials and methods. β-Actin served as a loading control. (D) 253JB-V cells were treated with DMSO and 2.5 or 5 μM/l CSL for 24 h, in the presence or absence of the antioxidant DTT and the cells were counted as described in Materials and methods. Results are expressed as means ± SE for three replicate determinations for each treatment group, and significant (P < 0.05) CSL-induced increases (**) or decreases (*) compared with the solvent (DMSO) control are indicated.
Moreover, induction of ROS and decreased MMP after treatment of 253JB-V cells with CSL were inhibited after cotreatment with antioxidants [dithiothreitol (DTT) or glutathione] (Figure 5B). CSL-induced downregulation of Sp1, Sp3 and Sp4 was also inhibited after cotreatment with DTT, whereas the inhibitory effects of glutathione were less than observed for DTT (Figure 5C). The contributions of ROS to growth inhibition were also investigated in 253JB-V cells where treatment with 2.5 or 4.0 μM CSL inhibited cell proliferation and cotreatment with 1 mM DTT significantly reversed the growth inhibitory effects of CSL (Figure 5D). DTT and glutathione also blocked the growth inhibitory and morphological changes induced by CSL in 253JB-V bladder cancer cells as determined by differential interference contrast microscopy (Supplementary Figure 2 is available at Carcinogenesis Online).
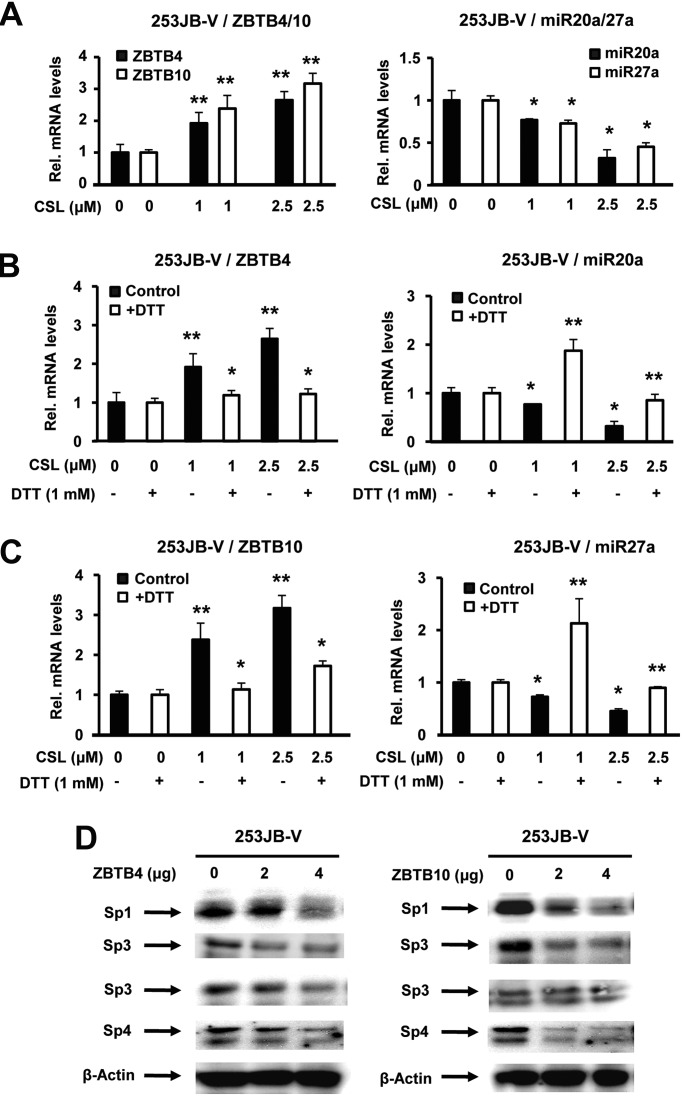
CSL-induced ROS disrupts miR-27a:ZBTB10 and miR-20a:ZBTB4
GT-094 and CDDO-Me induce Sp downregulation through ROS-dependent disruption of miR-27a:ZBTB10 resulting in the induction of the Sp repressor in colon and pancreatic cancer cells, respectively (9,26). Results in Figure 6A show that CSL also induced ZBTB10 and downregulated miR-27a in 253JB-V cells. A recent study reported that miR-20a inhibited expression of ZBTB4, another Sp repressor in breast cancer cells (27) and CSL also decreased miR-20a and induced ZBTB4 in 253JB-V cells (Figure 6A). Moreover, CSL also decrease luciferase activity in both cell lines after transfection with miR-27a (miR-27a-luc) and miR-20 [miR-17-92(pro1353)-luc] constructs containing miR promoter inserts (Supplementary Figure 4 is available at Carcinogenesis Online) (35,36). The role of CSL-induced ROS in mediating disruption of miR-20a:ZBTB4 (Figure 6B) and miR-27a:ZBTB10 (Figure 6B) was also investigated in 253JB-V cells treated with CSL alone or in combination with DTT, and CSL-dependent disruption of both miR-20a:ZBTB4 and miR-27a/ZBTB10 was inhibited after cotreatment with the antioxidant (Figure 6B and C). We also observed that overexpression of ZBTB10 and ZBTB4 decreased levels of Sp1, Sp3 and Sp4 proteins in 253JB-V cells and these observations were comparable with results obtained in other cancer cell lines showing that both transcriptional repressors decreased Sp protein expression (27,28). This demonstrates that the mechanism of action of CSL in 253JB-V cells is due to induction of ROS and the downstream effects of ROS on miR-27a:ZBTB10 and miR-20a:ZBTB4 circuits.
Fig. 6.
Effect of CSL on miR-20a:ZBTB4 and miR-27a:ZBTB10 and ZBTB4/ZBTB10-dependent repression of Sp1, Sp3 and Sp4. (A) 253JB-V cells were treated with dimethyl sulfoxide (DMSO) and 1.0 or 2.5 μM/l CSL for 24 h and ZBTB4, ZBTB10 messenger RNA (mRNA) and miR-20a and miR-27a mRNA levels were analyzed by real-time PCR as described in Materials and methods. Effects of DTT on CSL induced ZBTB4/miR-20a (B) and ZBTB10/miR-27a (C). 253JB-V cells were treated with DMSO and 1.0 or 2.5 μM/l CSL in the presence or absence of DTT and effects on ZBTB4, ZBTB10, miR-20a and miR-27a levels were analyzed by real-time PCR as described in Materials and methods. (D) Effect of ZBTB4 and ZBTB10 overexpression on Sp proteins. 253JB-V cells were transfected with empty vector (pCMV6-XL4) or 4 μg per well ZBTB4 or ZBTB10 expression plasmid pCMV6-XL4 vector, and whole-cell lysates were analyzed by western blots as described under Materials and methods. Results in (A) and (C) are expressed as means ± SE for three replicate determinations for each treatment group, and significant (P < 0.05) CSL-induced increases (**) or decreases by DTT (*) compared with the solvent (DMSO) are indicated; significance (P < 0.05).
Discussion
The anticancer activity of CSL has been linked to inhibition of proteasome activity, HSP90 and topoisomerase II, and suppression of genes required for cell growth, survival, angiogenesis and inflammation (10,12,18,37,38). Many genes suppressed by CSL have previously been identified by RNA interference (Sp knockdown) as Sp-regulated genes in bladder and other cancer cell lines and these include survivin, VEGF, cyclin D1 and p65NFκB (8,9,23,24,26,29). Therefore, we hypothesized that CSL also decreased Sp proteins in bladder cancer cells and this was confirmed in KU7 and 253JB-V cell lines and KU7 tumors in a xenograft experiment, indicating that CSL, like curcumin, betulinic acid and arsenic trioxide, targets Sp1, Sp3 and Sp4 transcription factors (Figure 3A and C) and Sp-regulated genes in bladder cancer cells and tumors (Figure 2A and B). Similar responses have been observed for a number of other anticancer agents in multiple cancer cell lines (9,23,24,26).
Several studies show that a high percentage of bladder cancers overexpress the FGFR3 gene (39–41) and somatic mutations in the FGFR3 gene have been identified in 60–70% papillary and 16–20% of muscle-invasive bladder cancers (39–42). FGFR3 is important for bladder tumor growth and is an important target for bladder cancer therapy (23). This study demonstrates for the first time that FGFR3 is also an Sp-regulated gene in bladder cancer cells (Figure 4) and this observation correlates with previous reports showing that knockdown of Sp proteins results in growth inhibition and induction of apoptosis in bladder cancer cells (8,9,29). We also showed that 5 μM CSL induces proteasome-dependent degradation of Hsp90 (Supplementary Figure 3 is available at Carcinogenesis Online), which also regulates FGFR3 (34) and this pathway may also contribute to CSL-induced repression of FGFR3.
Tolfenamic acid, betulinic acid and curcumin induce proteasome-dependent degradation of Sp proteins in Panc1 (pancreatic), LNCaP (prostate) and 253JB-V/KU7 cells, respectively (8,24,43), and CSL also induced proteasome-dependent downregulation of Sp1, Sp3 and Sp4 in KU7 but not 253JB-V cells (Figure 5A). Downregulation of Sp1, Sp3 and Sp4 by anticancer agents such as arsenic trioxide, curcumin and synthetic triterpenoids in different cancer cell lines has also been linked to decreased MMP and induction of ROS, and similar results were observed in 253JB-V cells treated with CSL (Figure 5B). Moreover, antioxidants such as DTT inhibited CSL-induced effects on MMP and ROS but also blocked CSL-induced downregulation of Sp1, Sp3 and Sp4 and growth inhibition. Thus, induction of ROS by CSL was a key event in targeting Sp transcription factors in 253JB-V cells, and this is consistent with results of previous studies with other cancer agents (9,25,26).
The synthetic triterpenoid methyl 2-cyano-3,12-dioxooleana-1,9-dien-20-oate (CDDO-Me) and the NO-NSAID (GT-094) decreased expression of Sp transcription factors in pancreatic and colon cancer cells by induction of the transcriptional repressor ZBTB10 (26,44), which competitively displaces Sp1, Sp3 and Sp4 from GC-rich binding sites (44). ZBTB10 is repressed in cancer cells through interaction with miR-27a; however, both CDDO-Me and GT-094 induced an ROS-dependent decrease in miR-27a and similar results were observed for CSL in 253JB-V cells (Figure 6). ZBTB4 has recently been identified as second Sp repressor and miR-20a and related paralogs (27) that are overexpressed in many tumors interact with and repress ZBTB4 expression in breast cancer cells (27). Results in Figure 6 show that CSL also decreased miR-20a and induced ZBTB4 in 253JB-V cells and these effects were blocked by antioxidants, demonstrating that CSL-induced ROS disrupts both miR-27a:ZBTB10 and miR-20a:ZBTB4 circuitry in bladder cancer cells. CSL also decreased luciferase activity in cells transfected with miR-27a and miR-17-92 promoter constructs (Supplementary Figure 4 is available at Carcinogenesis Online) and the mechanisms of CSL (ROS)-mediated regulation of these miRs are currently being investigated.
In summary, this study reports for the first time that CSL decreased expression of Sp1, Sp3 and Sp4 transcription factors and Sp-regulated genes which include FGFR3, a clinically important therapeutic target for bladder cancer treatment. CSL decreased MMP and induced ROS that triggered disruption of miR-27a- and miR-20a-dependent repression of ZBTB10 and ZBTB4. Activation of both transcriptional repressors resulted in downregulation of Sp transcription factors as previously reported (27,28) and these responses were all inhibited by antioxidants. The induction of ROS by CSL and the importance of ROS as a chemotherapeutic pathway have been previously reported (19,37). Results of this study with CSL and previous reports with GT-094 and CDDO-Me demonstrate the role of ROS induction as part of their anticancer activity since ROS downregulates Sp1, Sp3, Sp4 and several Sp-regulated genes such as epidermal growth factor receptor and FGFR3 that are themselves important individual targets for bladder cancer chemotherapy.
Supplementary material
Supplementary Figures 1–4 can be found at http://carcin.oxfordjournals.org/.
Funding
National Institutes of Health (R01-CA136571) and Texas A&M AgriLife.
Supplementary Material
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CSL
celastrol
- DTT
dithiothreitol
- FGFR
fibroblast growth factor receptor
- Hsp90
heat shock protein 90
- MMP
mitochondrial membrane potential
- ROS
reactive oxygen species
- Sp
specificity protein
- VEGF
vascular endothelial growth factor
References
- 1.Jemal A, et al. Cancer statistics, 2010. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Kompier LC, et al. The development of multiple bladder tumour recurrences in relation to the FGFR3 mutation status of the primary tumour. J. Pathol. 2009;218:104–112. doi: 10.1002/path.2507. [DOI] [PubMed] [Google Scholar]
- 3.Dreicer R. Locally advanced and metastatic bladder cancer. Curr. Treat. Options Oncol. 2001;2:431–436. doi: 10.1007/s11864-001-0048-y. [DOI] [PubMed] [Google Scholar]
- 4.Kamat AM, et al. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-kappaB and nuclear factor-kappaB-regulated gene products in IFN-alpha-sensitive and IFN-alpha-resistant human bladder cancer cells. Mol. Cancer Ther. 2007;6:1022–1030. doi: 10.1158/1535-7163.MCT-06-0545. [DOI] [PubMed] [Google Scholar]
- 5.Paneau C, et al. [Epidemiology of bladder cancer] Ann. Urol. (Paris) 1992;26:281–293. [PubMed] [Google Scholar]
- 6.Dinney CP, et al. Focus on bladder cancer. Cancer Cell. 2004;6:111–116. doi: 10.1016/j.ccr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Kassouf W, et al. Inhibition of bladder tumor growth by 1,1-bis(3'-indolyl)-1-(p-substitutedphenyl)methanes: a new class of peroxisome proliferator-activated receptor gamma agonists. Cancer Res. 2006;66:412–418. doi: 10.1158/0008-5472.CAN-05-2755. [DOI] [PubMed] [Google Scholar]
- 8.Chadalapaka G, et al. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008;68:5345–5354. doi: 10.1158/0008-5472.CAN-07-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chadalapaka G, et al. Drugs that target specificity proteins downregulate epidermal growth factor receptor in bladder cancer cells. Mol. Cancer Res. 2010;8:739–750. doi: 10.1158/1541-7786.MCR-09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kannaiyan R, et al. Celastrol inhibits proliferation and induces chemosensitization through downregulation of NF-kappaB and STAT3 regulated gene products in multiple myeloma cells. Br. J. Pharmacol. 2011;164:1506–1521. doi: 10.1111/j.1476-5381.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corson TW, et al. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell. 2007;130:769–774. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai Y, et al. Natural proteasome inhibitor celastrol suppresses androgen-independent prostate cancer progression by modulating apoptotic proteins and NF-kappaB. PLoS One. 2010;5:e14153. doi: 10.1371/journal.pone.0014153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwen DG, et al. Regulation of the fibroblast growth factor receptor 3 promoter and intron I enhancer by Sp1 family transcription factors. J. Biol. Chem. 1998;273:5349–5357. doi: 10.1074/jbc.273.9.5349. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, et al. Celastrol inhibits the growth of human glioma xenografts in nude mice through suppressing VEGFR expression. Cancer Lett. 2008;264:101–106. doi: 10.1016/j.canlet.2008.01.043. [DOI] [PubMed] [Google Scholar]
- 15.Jang SY, et al. Celastrol inhibits the growth of estrogen positive human breast cancer cells through modulation of estrogen receptor alpha. Cancer Lett. 2011;300:57–65. doi: 10.1016/j.canlet.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Abbas S, et al. Preclinical studies of celastrol and acetyl isogambogic acid in melanoma. Clin. Cancer Res. 2007;13:6769–6778. doi: 10.1158/1078-0432.CCR-07-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang X, et al. Celastrol suppresses angiogenesis-mediated tumor growth through inhibition of AKT/mammalian target of rapamycin pathway. Cancer Res. 2010;70:1951–1959. doi: 10.1158/0008-5472.CAN-09-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng B, et al. HSP90 inhibitor, celastrol, arrests human monocytic leukemia cell U937 at G0/G1 in thiol-containing agents reversible way. Mol. Cancer. 2010;9:79. doi: 10.1186/1476-4598-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G, et al. Celastrol targets mitochondrial respiratory chain complex I to induce reactive oxygen species-dependent cytotoxicity in tumor cells. BMC Cancer. 2011;11:170. doi: 10.1186/1471-2407-11-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang H, et al. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66:4758–4765. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, et al. Celastrol acts as a potent antimetastatic agent targeting beta1 integrin and inhibiting cell-extracellular matrix adhesion, in part via the p38 mitogen-activated protein kinase pathway. J. Pharmacol. Exp. Ther. 2010;334:489–499. doi: 10.1124/jpet.110.165654. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y, et al. Celastrol binds to ERK and inhibits FcepsilonRI signaling to exert an anti-allergic effect. Eur. J. Pharmacol. 2009;612:131–142. doi: 10.1016/j.ejphar.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 23.Qing J, et al. Antibody-based targeting of FGFR3 in bladder carcinoma and t(4;14)-positive multiple myeloma in mice. J. Clin. Invest. 2009;119:1216–1229. doi: 10.1172/JCI38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdelrahim M, et al. Tolfenamic acid and pancreatic cancer growth, angiogenesis, and Sp protein degradation. J. Natl. Cancer Inst. 2006;98:855–868. doi: 10.1093/jnci/djj232. [DOI] [PubMed] [Google Scholar]
- 25.Jutooru I, et al. Methyl 2-cyano-3,12-dioxooleana-1,9-dien-28-oate decreases specificity protein transcription factors and inhibits pancreatic tumor growth: role of microRNA-27a. Mol. Pharmacol. 2010;78:226–236. doi: 10.1124/mol.110.064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pathi SS, et al. GT-094, a NO-NSAID, inhibits colon cancer cell growth by activation of a reactive oxygen species-microRNA-27a: ZBTB10-specificity protein pathway. Mol. Cancer Res. 2011;9:195–202. doi: 10.1158/1541-7786.MCR-10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim K, et al. Identification of oncogenic microRNA-17-92/ZBTB4/specificity protein axis in breast cancer. Oncogene. 2012;31:1034–1044. doi: 10.1038/onc.2011.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mertens-Talcott SU, et al. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 29.Jutooru I, et al. Arsenic trioxide downregulates specificity protein (Sp) transcription factors and inhibits bladder cancer cell and tumor growth. Exp. Cell Res. 2010;316:2174–2188. doi: 10.1016/j.yexcr.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kannaiyan R, et al. Molecular targets of celastrol derived from Thunder of God Vine: potential role in the treatment of inflammatory disorders and cancer. Cancer Lett. 2011;303:9–20. doi: 10.1016/j.canlet.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 31.Hieronymus H, et al. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell. 2006;10:321–330. doi: 10.1016/j.ccr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Zhang T, et al. A novel Hsp90 inhibitor to disrupt Hsp90/Cdc37 complex against pancreatic cancer cells. Mol. Cancer Ther. 2008;7:162–170. doi: 10.1158/1535-7163.MCT-07-0484. [DOI] [PubMed] [Google Scholar]
- 33.Zhang T, et al. Characterization of celastrol to inhibit hsp90 and cdc37 interaction. J. Biol. Chem. 2009;284:35381–35389. doi: 10.1074/jbc.M109.051532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laederich MB, et al. Fibroblast growth factor receptor 3 (FGFR3) is a strong heat shock protein 90 (Hsp90) client: implications for therapeutic manipulation. J. Biol. Chem. 2011;286:19597–19604. doi: 10.1074/jbc.M110.206151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woods K, et al. Direct regulation of an oncogenic micro-RNA cluster by E2F transcription factors. J. Biol. Chem. 2007;282:2130–2134. doi: 10.1074/jbc.C600252200. [DOI] [PubMed] [Google Scholar]
- 37.Trachootham D, et al. Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood. 2008;112:1912–1922. doi: 10.1182/blood-2008-04-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagase M, et al. Apoptosis induction in HL-60 cells and inhibition of topoisomerase II by triterpene celastrol. Biosci. Biotechnol. Biochem. 2003;67:1883–1887. doi: 10.1271/bbb.67.1883. [DOI] [PubMed] [Google Scholar]
- 39.Gomez-Roman JJ, et al. Fibroblast growth factor receptor 3 is overexpressed in urinary tract carcinomas and modulates the neoplastic cell growth. Clin. Cancer Res. 2005;11:459–465. [PubMed] [Google Scholar]
- 40.Tomlinson DC, et al. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J. Pathol. 2007;213:91–98. doi: 10.1002/path.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Rhijn BW, et al. The fibroblast growth factor receptor 3 (FGFR3) mutation is a strong indicator of superficial bladder cancer with low recurrence rate. Cancer Res. 2001;61:1265–1268. [PubMed] [Google Scholar]
- 42.van Rhijn BW, et al. Frequent FGFR3 mutations in urothelial papilloma. J. Pathol. 2002;198:245–251. doi: 10.1002/path.1202. [DOI] [PubMed] [Google Scholar]
- 43.Chintharlapalli S, et al. Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res. 2007;67:2816–2823. doi: 10.1158/0008-5472.CAN-06-3735. [DOI] [PubMed] [Google Scholar]
- 44.Tillotson LG. RIN ZF, a novel zinc finger gene, encodes proteins that bind to the CACC element of the gastrin promoter. J. Biol. Chem. 1999;274:8123–8128. doi: 10.1074/jbc.274.12.8123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.