Abstract
Our present understanding of ocean acidification (OA) impacts on marine organisms caused by rapidly rising atmospheric carbon dioxide (CO2) concentration is almost entirely limited to single species responses. OA consequences for food web interactions are, however, still unknown. Indirect OA effects can be expected for consumers by changing the nutritional quality of their prey. We used a laboratory experiment to test potential OA effects on algal fatty acid (FA) composition and resulting copepod growth. We show that elevated CO2 significantly changed the FA concentration and composition of the diatom Thalassiosira pseudonana, which constrained growth and reproduction of the copepod Acartia tonsa. A significant decline in both total FAs (28.1 to 17.4 fg cell−1) and the ratio of long-chain polyunsaturated to saturated fatty acids (PUFA:SFA) of food algae cultured under elevated (750 µatm) compared to present day (380 µatm) pCO2 was directly translated to copepods. The proportion of total essential FAs declined almost tenfold in copepods and the contribution of saturated fatty acids (SFAs) tripled at high CO2. This rapid and reversible CO2-dependent shift in FA concentration and composition caused a decrease in both copepod somatic growth and egg production from 34 to 5 eggs female−1 day−1. Because the diatom-copepod link supports some of the most productive ecosystems in the world, our study demonstrates that OA can have far-reaching consequences for ocean food webs by changing the nutritional quality of essential macromolecules in primary producers that cascade up the food web.
Introduction
Anthropogenic emissions of carbon dioxide (CO2) and its uptake by the surface ocean cause profound changes in marine carbonate chemistry, including seawater acidification and lowering of the calcium carbonate saturation state [1], [2]. Contemporary surface ocean pH has decreased on average by 0.1 units due to CO2 invasion since preindustrial times. According to IPCC projections atmospheric partial pressure of CO2 (pCO2) is expected to further increase from current ∼390 µatm to ∼760 µatm, corresponding to a drop in mean oceanic surface pH by 0.3 to 0.4 units until the end of the 21st century (‘business-as-usual scenario’ [3], [4]). This change in carbonate chemistry, termed ocean acidification (OA), is thought to primarily affect calcifying organisms building their shells and skeletons of calcium carbonate [5], [6], [7]. Biological effects of OA on non-calcifying organisms are diverse and often highly species-specific [8].
Our present understanding of potential OA impacts is almost entirely limited to single species responses, while OA consequences for food web interactions remain poorly understood. Indirect impacts through trophic interactions are expected because OA may change the biochemical composition of primary producers that affects nutritional food quality for consumers. Increased CO2 can stimulate carbon fixation by photosynthetic organisms and thereby reduce the nutrient content relative to carbon [9], [10], [11], which determines the food quality for herbivores [12]. Enhanced carbon consumption relative to nutrients under elevated CO2 conditions [13], [14] can cause an imbalance between phytoplankton stoichiometric composition and consumer nutrient demand for somatic growth [11]. Besides elemental stoichiometry, fatty acid (FA) associated food quality is a critical factor that regulates the energy transfer between primary producers and consumers [15], [16], because essential FAs cannot be synthesized de novo by heterotrophic organisms and have to be acquired through the diet. In particular long-chain polyunsaturated FAs (PUFAs) such as docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and arachidonic acid (ARA) play an important role in growth, development and reproduction success in heterotrophs [17], [15]. OA may impact phytoplankton FA synthesis because extracellular pH is known to affect various intracellular physiological parameters [18] that influences enzyme activity.
The classic diatom-copepod-fish link in the ocean supports some of the most productive ecosystems in the world and is an important source of highly nutritious food for upper trophic levels. Experimental studies indicate a weak sensitivity of primary production to CO2 [14] and no direct effects on copepod growth and hatching success at CO2 levels within the range expected by the end of this century [19], [20]. However, CO2 may indirectly affect zooplankton growth through its potential impact on the nutritional quality of phytoplankton, their major food source. To test this hypothesis, we independently manipulated CO2 concentration in both diatoms used as food algae and copepod cultures, and investigated dietary OA effects on copepod growth and reproduction. The experiment consisted of a two-by-two factorial design crossing two CO2 levels in food algae media and seawater used for copepod growth. The cryptophyte Rhodomonas sp. and diatom Thalassiosira pseudonana were used as food source and the copepod Acartia tonsa as consumer. We determined resulting FA composition of both alga and copepod as well as copepod development and reproduction. Our experiment showed that elevated CO2 affected biochemical composition of the diatom that constrained copepod growth performance.
Methods
CO2 manipulation and experimental design
The target values for experimental CO2 manipulation were 380 µatm for the low (L) and 740 µatm for the high (H) CO2 treatment. Phytoplankton (P) was grown at both L and H pCO2 concentrations and fed to copepod zooplankton (Z) grown in seawater at the same L and H target levels in a crossed design. It is important to note that biological activity, such as photosynthesis and respiration, alter the carbonate system. In addition, water exchange and combining treatments with low and high CO2, as was done in the copepod growth experiment, can result in deviations from the target CO2 levels. Nevertheless, pCO2 levels of L and H treatments were maintained close to target values and differences among treatments persisted throughout the experiment (Figure S1).
Rhodomonas sp. and T. pseudonana were cultured as food sources in artificial seawater at pCO2 of ∼495±100 SD (L) and ∼760±110 (H) for Rhodomonas sp. and ∼365±120 (L) and ∼915±270 (H) µatm for T. pseudonana, respectively. Juvenile copepods were fed with Rhodomonas to ensure optimal growth of the first developmental stages and T. pseudonana was used as food source after copepodite stage 1. The carbonate system of T. pseudonana cultures was manipulated by combined additions of sodium carbonate (Na2CO3) and hydrogen chloride (HCl) at constant alkalinity; the two CO2 treatments for Rhodomonas cultures were continuously aerated with CO2-enriched air. Algae were grown in laboratory batch cultures on a 18∶6 light∶dark cycle with replete nutrients. To investigate the response time of algae fatty acid composition alterations to changing pCO2, T. pseudonana was grown at high (∼1120 µatm) pCO2 for five days and then transferred to a low (∼380 µatm) pCO2 media. FA concentration was measured every five hours over a 30 h time period.
Acartia tonsa eggs were hatched in seawater under pCO2 conditions of ∼380 µatm. After the nauplii reached developmental stage 2, they were transferred into 2-L NALGENE bottles (1000 individuals L−1) filled with seawater (salinity 18.2) from a tank that was aerated continuously with appropriately CO2-enriched air of ∼495±100 (L) and ∼760±110 (H) µatm pCO2, respectively. Copepod zooplankton (Z) were fed with CO2 preconditioned phytoplankton (P) at about 1000 µg C L−1 in a factorial design with four treatment combinations: PL/ZL, PL/ZH, PH/ZL and PH/ZH, each with three replicates. Water and food during the copepod growth experiment were replaced every other day. All replicates were randomly placed in a temperature-controlled culture room at 18°C and 14∶10h light∶dark cycle until the copepods reached adult stage. Over the course of the experiment, developmental stages were identified and at the end of the growth experiment egg production of females measured over 24 h and hatching success of eggs and nauplii morphological formation observed for two days. Species involved for this experiment were lab cultures and thus no specific permits were required for the sample collection.
Dissolved inorganic carbon (DIC) was measured after every water exchange and pH was recorded daily during the copepod growth experiment. For DIC the water was smoothly filtered via syringe and a 0.2 µm pre-filter and stored in 4 ml borosilicate flasks at 4°C. The sample flasks were closed with a plastic screw cap and a Teflon septum. DIC was determined photometrically with an auto-analyzer (QUAATRO, Bran & Lübbe) at a precision of ±20 µmol kg−1 [21], [22]. DIC and pH were used for seawater carbonate system calculations (Text S1). During the copepod growth experiment measured mean (±SD) pH values were 8.14±0.12 and 7.94±0.08, and for DIC 480±110 and 725±140 µatm CO2 in the L and H treatment, respectively (Figure S1). DIC values in the crossed treatments were 485±80 (PH/ZL) and 745±80 (PL/ZH) µatm CO2. Due to the fact that NBS based pH measurements are rather weak for reliable carbonate chemistry calculations, total alkalinity (TA) measurements (Text S1) were taken two times per week for crosscheck calculations. Values for pCO2 calculated from pH and DIC differed from pCO2 calculations using DIC and TA on average ∼110 and ∼210 µatm at the low and high CO2 treatment level, respectively, over the duration of the experiment. These uncertainties are probably higher than the real error since they were caused by outliers in TA and DIC measurements to which the carbonate system is relative insensitive when pH is involved in the calculations.
FA composition of T. pseudonana was analyzed from the stock culture during exponential growth phase and of copepod females at the end of the experiment. FAs were measured as fatty acid methyl esters (FAMEs) with a Thermo GC Ultra gas chromatograph equipped with a nonpolar column (RXI1-SIL-MS 0.32 µm, 30 m) using a flame ionization detector (FID). A complete method description is provided in Text S1.
Statistical analysis
Algal responses to experimental conditions were assessed using two-tailed t-tests. Differences in copepod FA classes and egg production between treatments were tested using analysis of variance (ANOVA). A Tukey HSD post hoc test was used to assess differences among treatments in egg production. Generalized linear models (GLM) were used to examine the effect of the seawater pCO2 used for algal and copepod cultures on the relative proportion of FA classes in copepods. Principal component analysis (PCA) was used to assess the difference in individual FA composition of the diet algae and copepods across the treatment combinations. For algal food, log-transformed FA concentration per cell and for copepods arcsine-square root transformed percentage of total FA was used since the proportion of FA classes varies between T. pseudonana and A. tonsa (see Figure 1). Each FA was standardized by subtracting its mean and dividing by its standard deviation, assembling the resulting standardized series into a 15-FA by 20-treatment combination data matrix. The PCA used a covariance matrix and Varimax rotation. This analysis identified FA that explained most to the observed variance. Statistical analyses were performed using Statistica and the R software environment 2.14.1 [23].
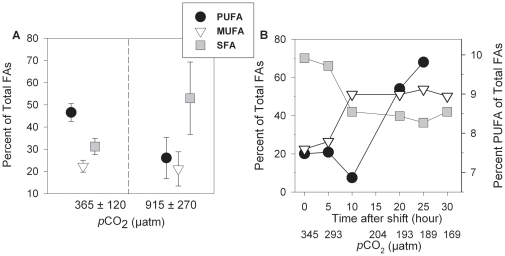
Figure 1. Fatty acid composition and concentration of Thalassiorira pseudonana cultured at different CO2 treatments.
A) Percentage of polyunsaturated (PUFA), monounsaturated (MUFA), and saturated (SFA) fatty acids relative to total fatty acids during the exponential growth phase cultured at low (realized value of 365 µatm pCO2, n = 5) and high (realized value of 915 µatm pCO2, n = 3) CO2 treatments used as copepod food source. B) Change in the fatty acid composition in T. pseudonana after a shift from high to low pCO2 conditions (n = 1 per treatment level). Time 0 are measured values before the culture media shift. Error bars indicate standard errors.
Results and Discussion
Our experiment showed that CO2 concentration significantly changed FA concentration and composition in the diatom T. pseudonana used for copepod diet. The relative amount of PUFAs was significantly lower (t = 4.48, p = 0.004) and the amount of SFAs higher (t = −3.37, p = 0.015) at high pCO2 compared to the low pCO2 treatment (Figure 1A). Essential PUFA concentrations were significantly reduced at high pCO2 (Table S1), specifically DHA (22:6n-3; t = 2.81, p = 0.03) and the group ARA-EPA (20:4n-6, 20:5n-3; t = 6.63, p<0.001). A shift in FA composition at projected future CO2 levels is consistent with observations in the coccolithophorid Emiliania huxleyi [24] and with green algae and prymnesiophyte experiments conducted at extreme CO2 changes [25], [26], [27].
A separate experiment confirmed that the shift in FA occurred rapidly in response to changing pCO2 in the diatom T. pseudonana. When transferred from high to low CO2, FA composition was already significantly different from its initial composition after 15 h (Figure 1B) and FA components changed in the same direction as observed at constant high and low pCO2 treatments. Similarly, a rapid transition in FA composition can be expected when algae are transferred from low to high pCO2, which was, however, not tested in our experiment. Though, a rapid reversible FA response to changing pCO2 concentration has been reported in green algae [26]. The higher unsaturation levels of FAs in algae cells cultured at low pCO2 compared to cells at high pCO2 has been suggested to be partially a consequence of repressed FA synthesis, which promotes the desaturation of pre-existing SFAs [26]. Recently it has been proposed that pH might act as a regulation signal for the formation of cell membranes, which are mainly composed of fatty acids, by controlling the production of its synthesizing enzymes [28]. A high environmental pCO2 (low pH) can decrease the internal cell-pH [29]. Therefore the increased amount of SFAs could be a mechanism to control the internal cell-pH, as a membrane built of short-chain FAs is less fluid and permeable to CO2. However, the cellular processes involved in FA synthesis under changing pH or pCO2 levels are not fully understood.
Similar to FA modification in algal food, FA concentration and composition of adult copepods varied significantly between CO2 treatments. The mean ±SD total amount of FAs in A. tonsa was significantly different across treatments (F (3, 8) = 5.15, p = 0.028) and higher when raised and fed with algae cultured at low pCO2, with 8.9±5.6 ng ind.−1 compared to 0.8±0.2 ng ind.−1 when both copepods and algal diet were cultured at high pCO2 and to 2.3±0.5 ng ind.−1 in the crossed treatment combinations (Table S1). Copepods raised and fed with algae at low pCO2 contained high proportions of PUFAs relative to total FAs that are in the same range with reports in marine calanoids [30]. The PUFA fraction in copepods decreased from more than 30% at low pCO2 to less than 5% at high pCO2 (F (3, 8) = 54.51, p<0.001) (Figure 2A). The long-chain highly unsaturated FAs DHA and ARA-EPA, which are important components for growth and reproduction of consumers [31], decreased from 15% in copepods raised at low pCO2 below detection limit in those at high pCO2 (Table S1). Similarly, the proportion of MUFAs (monounsaturated fatty acids) varied significantly across treatments (F (3, 8) = 8.2, p = 0.008) and decreased from around 20% at low pCO2 to less than 10% at high pCO2. On the other hand, the relative amount of SFAs tripled in copepods at high pCO2 (Figure 2A) and FA compositions were different between treatments (F (3, 8) = 26.22, p = <0.001).
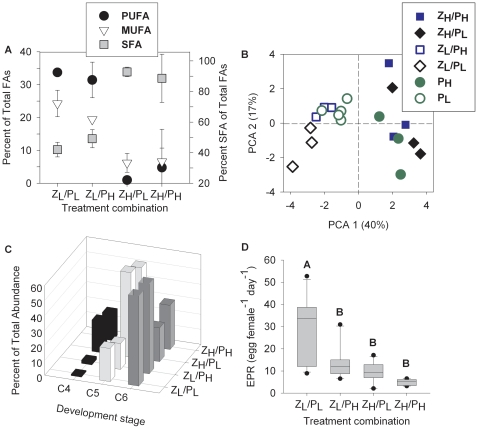
Figure 2. Fatty acid composition, somatic growth and reproduction of Acartia tonsa across CO2 treatment combinations.
A) Percentage of polyunsaturated (PUFA), monounsaturated (MUFA), and saturated (SFA) fatty acids relative to total fatty acids in female copepods. B) Principal component analysis (PCA) of fatty acid composition for the dietary algae Thalassiorira pseudonana and A. tonsa of the different treatment combinations. PCA scores 1 explained 40% of the variability (see x-axis of c) and was highly negatively correlated with 22:6n-3 (r2 = 0.73), 20:4n-6+20:5n-3 (r2 = 0.85), 18:3n-6 (r2 = 0.73) and 16:1 (r2 = 0.79), and positively with 22:1n-9 (r2 = 0.25) and 18:1n-9t (r2 = 0.57). PCA score 2 explained 17% of the overall variability (see y-axis of c) and was strongest positively correlated with 24:0 (r2 = 0.84). Loadings of the PC scores are shown in Figure S2). C) Stage distribution of A. tonsa individuals at day 10. C4, C5, C6 = copepodite stage 4, 5, and adult, respectively. D) Egg production rate (EPR) of incubated females (n = 12 per treatment level). EPR was significantly different between treatments (F(3, 44) = 18.02, p<0.001). Different letters above bars represent significant differences from a Tukey HSD test. The bars represent the 25th, 50th and 75th percentiles, whiskers stand for the 10th and the 90th percentiles and black points show outliers. Legend refers to treatment combinations of copepod zooplankton (Z) and phytoplankton food source (P) at low (L) and high (H) pCO2.
Contrary to our expectation, FA composition in copepods differed between individuals raised at low and high seawater pCO2, irrespective of the CO2 level of their algal diet (Figure 2A). Because consumers are unable to synthesize PUFAs we expected that copepod FA composition in the crossed pCO2 treatments of copepod culture and food algae (PL/ZH, PH/ZL) would reflect changes in FA of their diet. Principal component analysis (PCA) of individual FAs in diet algae and copepods also showed distinctive clustering of the copepod groups raised at low and high pCO2 treatments, irrespective of the CO2 conditions of their diet algal culture (Figure 2B), which was mainly explained by PUFAs and SFAs (Figure S2). A GLM model supported that CO2 concentration of the seawater used to raise copepods significantly negatively affected the relative proportion of PUFAs (p<0.001) and positively affected the proportion of SFAs in copepods (p<0.001), which was consistent across combinations and not dependent on the pCO2 level of the algal culture.
These findings suggests that the FA composition of algae changed rapidly when transferred from low pCO2 culture media to high pCO2 seawater used to raise copepods and vice versa. Since consumers are unable to synthesize PUFAs [30] and previous experiments showed that copepod growth is rather insensitive to CO2 levels within OA predictions [19], [20], direct CO2 effects on copepod FA synthesis seem unlikely. In our experiment, water and food was exchanged every second day and algae were in their exponential growth. Thus, we rather expect that high turnover rates and the ability of T. pseudonana to rapidly change the FA composition in a variable pCO2 environment (Figure 1B) are responsible for an adjustment in FA composition in the crossed treatments within the first day. Rapid modification in algae FA and the fact that A. tonsa has no lipid reserves [32] likely explains the absence of the influence from the algae culture media pCO2 on copepod FA composition within both crossed treatment combinations.
The CO2-dependent dietary shift in FAs had a significant effect on A. tonsa growth and development. Copepods of the same age (10 d) showed a delay in stage development of 1 to 2 days at high pCO2 (Figure 2C). Egg production decreased from a median of 34 eggs female−1 d−1 at low water and food pCO2 to less than 12 eggs female−1 d−1 in all other treatments, with the lowest production (5 eggs female−1 d−1) at high water and food pCO2 (Figure 2D). The egg production rate was significantly related to the ratio of PUFA:SFA and the content of DHA and ARA-EPA within the female copepods (Table 1), consistent with other observations in zooplankton [33]. Copepod egg production raised at low pCO2 and fed with algae grown at high pCO2 produced significantly less eggs compared to copepods in the low pCO2 treatment combination (Figure 2D). This significant decline is most likely a result of the overall lower copepod FA quantity when fed with algae cultured at high CO2 compared to food at low CO2 (Table S1). Given that adult A. tonsa females invest the majority of their lipids into reproduction [34], the significant decrease of essential PUFAs due to low quality food algae is most likely the reason for the considerable decline in egg production observed in the high pCO2 treatment combinations (Figure 2).
Table 1. Regression statistics of Acartia tonsa egg production as a linear function of fatty acid composition.
| Fatty acid | Slope | Y-intercept | r2 | p-value |
| PUFA (%) | 0.03 | 1.9 | 0.52 | 0.013 |
| MUFA (%) | 0.07 | 1.6 | 0.73 | <0.001 |
| SFA (%) | −0.02 | 3.8 | 0.60 | 0.005 |
| PUFA:SFA | 1.3 | 1.9 | 0.59 | 0.006 |
| ARA-EPA (ng cop−1) | 0.68 | 2.06 | 0.67 | 0.002 |
| DHA (ng cop−1) | 1.23 | 1.92 | 0.77 | <0.001 |
Bonferroni-corrected significance levels for multiple fatty acid comparisons were α = 0.008 (0.05/6). Significant correlations are highlighted in bold; n = 11. PUFA = polyunsaturated fatty acid; MUFA = monounsaturated fatty acid; SFA = saturated fatty acid; ARA-EPA = 20:5n3; DHA = docosahexaenoic acid (22:6n3).
Some studies showed that the disruption of diatom cells induced by feeding triggered the transformation of unsaturated FAs into aldehydes causing adverse effects on copepod development, egg production and hatching success [35]. Even though several diatom species are known to possess these deleterious effects, T. pseudonana has recently been reported not to produce aldehydes [36]. We also could not find any malformations of the hatched nauplii by microscopic observations (data not shown), suggesting that negative aldehyde effects were not present.
Our study suggests that OA can have important consequences for consumer growth and production by affecting the nutritional quality of primary producers that translates to higher trophic levels. These results are consistent with experiments on freshwater cladocerans, fed with algae from an acidic lake [37], suggesting that our results are not restricted to monospecific laboratory cultures and may be expected at community level. However, future experimental manipulations are required to clarify the widespread response of phytoplankton biochemical composition to ocean acidification at relevant pCO2 levels in other taxonomic groups and natural communities. It can be expected that trophic upgrading and differential algae sensitivity to pCO2 at the community and ecosystem level may compensate for low food quality observed at the single species level. Moreover, the tolerance to pCO2 and pH might be lower for monocultures compared to natural populations, which have high ecophysiological variability [38] and genetic diversity, important for adaption to various environmental factors [39]. Nonetheless, shifts in FA composition as a response to changing CO2 have been documented in other phytoplankton species [26], [40], and FA-responses in phytoplankton as observed here might be important during bloom periods if CO2 sensitive organisms dominate.
The effect of OA on nutritional quality in the diatom-copepod food chain relationship observed in our study may have far reaching consequences for food webs since FAs originating in phytoplankton are sequentially incorporated into the total lipid fraction of zooplankton and triacylglycerol of larval fish [41]. Given that fish is a critical natural resource [42], acidification-driven food quality deterioration may impair fish production by changing the biochemical composition of food algae and its transfer to higher trophic levels [43], [44]. While it is difficult to extrapolate from monocultures to community level, these results point to the likelihood that OA consequences go beyond direct physiological impacts and that indirect effects through trophic interactions need to be considered.
Supporting Information
Carbonate system over the course of the copepod growth experiment.
(DOCX)
Loadings for Principal Component Analysis (PCA) of fatty acids for Thalassiosira pseudonana and Acartia tonsa.
(DOCX)
Full material and methods description.
(DOCX)
Amount of fatty acids of the food algae Thalassiosira pseudonana and the copepod consumer Acartia tonsa at different CO2 treatment combinations.
(DOCX)
Acknowledgments
We thank Thomas Hansen, Andrea Ludwig, and Maike Blankartz for laboratory and technical assistance. Valuable comments provided from anonymous reviewers are appreciated.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work is a contribution to the “European Project on OCean Acidification” (EPOCA), which received funding from the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 211384. In addition, financial support from the German Research Foundation (DFG) as part of the priority program 1162 AQUASHIFT and the Marie Curie IRG grant n° 276917 is gratefully acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Doney SC. The growing human footprint on coastal and open-ocean biogeochemistry. Science. 2010;328:1512–1516. doi: 10.1126/science.1185198. [DOI] [PubMed] [Google Scholar]
- 2.Riebesell U, Kortzinger A, Oschlies A. Sensitivities of marine carbon fluxes to ocean change. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20602–20609. doi: 10.1073/pnas.0813291106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon S, Qin D, Manning M, Chen Z, Marquis M, et al., editors. IPCC. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge and New York: Cambridge University Press; 2007. 996 [Google Scholar]
- 4.Gosling SN, Warren R, Arnell NW, Good P, Caesar J, et al. A review of recent developments in climate change science. Part II: The global-scale impacts of climate change. Progress in Physical Geography. 2011;35:443–464. [Google Scholar]
- 5.Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 6.Beaufort L, Probert I, de Garidel-Thoron T, Bendif EM, Ruiz-Pino D, et al. Sensitivity of coccolithophores to carbonate chemistry and ocean acidification. Nature. 2011;476:80–83. doi: 10.1038/nature10295. [DOI] [PubMed] [Google Scholar]
- 7.Lischka S, Budenbender J, Boxhammer T, Riebesell U. Impact of ocean acidification and elevated temperatures on early juveniles of the polar shelled pteropod Limacina helicina: mortality, shell degradation, and shell growth. Biogeosciences. 2011;8:919–932. [Google Scholar]
- 8.Nielsen LT, Jakobsen HH, Hansen PJ. High resilience of two coastal plankton communities to twenty-first century seawater acidification: evidence from microcosm studies. Marine Biology Research. 2010;6:542–555. [Google Scholar]
- 9.Engel A, Schulz KG, Riebesell U, Bellerby R, Delille B, et al. Effects of CO2 on particle size distribution and phytoplankton abundance during a mesocosm bloom experiment (PeECE II). Biogeosciences. 2008;5:509–521. [Google Scholar]
- 10.Bellerby RGJ, Schulz KG, Riebesell U, Neill C, Nondal G, et al. Marine ecosystem community carbon and nutrient uptake stoichiometry under varying ocean acidification during the PeECE III experiment. Biogeosciences. 2008;5:1517–1527. [Google Scholar]
- 11.Urabe J, Togari J, Elser JJ. Stoichiometric impacts of increased carbon dioxide on a planktonic herbivore. Global Change Biology. 2003;9:818–825. [Google Scholar]
- 12.Sterner RW, Elser JJ. Ecological stoichiometry. In: Levin SA, Carpenter SR, Godfray HCJ, Kinzig AP, Loreau M, et al., editors. The Princeton Guide to Ecology. Princeton Univeristy Press; 2009. pp. 376–385. [Google Scholar]
- 13.Riebesell U, Schulz KG, Bellerby RGJ, Botros M, Fritsche P, et al. Enhanced biological carbon consumption in a high CO2 ocean. Nature. 2007;450:545-U510. doi: 10.1038/nature06267. [DOI] [PubMed] [Google Scholar]
- 14.Riebesell U, Tortell PD. Effects of Ocean Acidification on Pelagic Organisms and Ecosystems. In: Gattuso J, Hansson L, editors. Ocean Acidification. Oxford University Press; 2011. pp. 99–121. [Google Scholar]
- 16.Brett MT, Mueller-Navarra DC. The role of highly unsaturated fatty acids in aquatic food web processes. Freshwater Biology. 1997;38:483–499. [Google Scholar]
- 17.Glencross B. Exploring the nutritional demand for essential fatty acids by aquaculture species. Reviews in Aquaculture. 2009;1:71–124. [Google Scholar]
- 18.Suffrian K, Schulz K, Gutowska M, Riebesell U, Bleich M. BCECF measurements in Emiliania huxleyi reveal dominant membrane proton permeability. New Phytologist. 2011;190:595–608. doi: 10.1111/j.1469-8137.2010.03633.x. [DOI] [PubMed] [Google Scholar]
- 19.Kurihara H, Ishimatsu A. Effects of high CO2 seawater on the copepod (Acartia tsuensis) through all life stages and subsequent generations. Marine Pollution Bulletin. 2008;56:1086–1090. doi: 10.1016/j.marpolbul.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Mayor DJ, Matthews C, Cook K, Zuur AF, Hay S. CO2-induced acidification affects hatching success in Calanus finmarchicus. Marine Ecological Progress Series. 2007;350:91–97. [Google Scholar]
- 21.Stoll MHC, Bakker K, Nobbe GH, Haese RR. Continuous-flow analysis of dissolved inorganic carbon content in seawater. Analytical Chemistry. 2001;73:4111–4116. doi: 10.1021/ac010303r. [DOI] [PubMed] [Google Scholar]
- 22.Dickson A, Afghan J, Anderson G. Reference materials for oceanic CO2 analysis: a method for the certification of total alkalinity. Marine Chemistry. 2003;80:185–197. [Google Scholar]
- 23.R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2009. ISBN 3-900051-07-0 http://wwwr-projectorg/. Accessed 2012 Mar 12. [Google Scholar]
- 24.Riebesell U, Revill A, Holdsworth D, Volkman J. The effects of varying CO2 concentration on lipid composition and carbon isotope fractionation in Emiliania huxleyi. Geochimica et Cosmochimica Acta. 2000;64:4179–4192. [Google Scholar]
- 25.Tsuzuki M, Ohnuma E, Sato N, Takaku T, Kawaguchi A. Effects of CO2 concentration during growth on fatty acid composition in microalgae. Plant Physiology. 1990;93:851–856. doi: 10.1104/pp.93.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato N, Tsuzuki M, Kawaguchi A. Glycerolipid synthesis in Chlorella kessleri 11h - II. Effect of the CO2 concentration during growth. Iochimica et Biophysica Acta-molecular and Cell Biology of Lipids. 2003;1633:35–42. doi: 10.1016/s1388-1981(03)00070-2. [DOI] [PubMed] [Google Scholar]
- 27.Carvalho A, Malcata F. Optimization of ω-3 fatty acid production by microalgae: crossover effects of CO2 and light intensity under batch and continuous cultivation modes. Journal of Marine Biotechnology. 2005;7:381–388. doi: 10.1007/s10126-004-4047-4. [DOI] [PubMed] [Google Scholar]
- 28.Young BP, Shin JJH, Orij R, Chao JT, Li SC, et al. Phosphatidic acid is a pH biosensor that links membrane biogenesis to metabolism. Science. 2010;329:1085–1088. doi: 10.1126/science.1191026. [DOI] [PubMed] [Google Scholar]
- 29.Lane AE, Burris JE. Effects of environmental pH on the internal pH of Chlorella pyrenoidosa, Scenedesmus quadricauda, and Euglena mutabilis. Plant Physiology. 1981;68:439–442. doi: 10.1104/pp.68.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brett MT, Müller-Navarra DC, Persson J. Crustacean zooplankton fatty acid composition. In: Arts MT, Brett MT, Kainz M, editors. Lipids in aquatic ecosystems. New York, USA: Springer; 2009. pp. 115–146. [Google Scholar]
- 31.Jónasdóttir S, Visser A, Jespersen C. Assessing the role of food quality in the production and hatching of Temora longicornis eggs. Marine Ecology-Progress Series. 2009;382:139–150. [Google Scholar]
- 32.Kiorboe T, Mohlenberg F, Hamburger K. Bioenergetics fo the planktonic copepod Acartia tonsa relation between feeding, egg-production and respriation, and compositon of specific dynamic action. Marine Ecology-Progress Series. 1985;26:85–97. [Google Scholar]
- 34.Hazzard S, Kleppel G. Egg production of the copepod Acartia tonsa in Florida Bay: role of fatty acids in the nutritional composition of the food environment. Marine Ecology-Progress Series. 2003;252:199–206. [Google Scholar]
- 35.Pohnert G. Phospholipase A(2) activity triggers the wound-activated chemical defense in the diatom Thalassiosira rotula. Plant Physiology. 2002;129:103–111. doi: 10.1104/pp.010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wichard T, Gerecht A, Boersma M, Poulet S, Wiltshire K, et al. Lipid and fatty acid composition of diatoms revisited: rapid wound-activated change of food quality parameters influences herbivorous copepod reproductive success. Chembiochem. 2007;8:1146–1153. doi: 10.1002/cbic.200700053. [DOI] [PubMed] [Google Scholar]
- 37.Locke A, Sprules WG. Effects of acidic pH and phytoplankton on survival and condition of Bosmina longirostris and Daphnia pulex. Hydrobiologia. 2000:187–196. [Google Scholar]
- 38.Paasche E. A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation, and calcificationphotosynthesis interactions. Phycologia. 2010;40:503–5029. [Google Scholar]
- 39.Sunday J, Crim R, Harley C, Hart M. Quantifying rates of evolutionary adaptation in response to acean acidification. PLoS One. 2011;6:e22881. doi: 10.1371/journal.pone.0022881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riebesell U, Zondervan I, Rost B, Tortell PD, Zeebe RE, et al. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature. 2000;407:364–367. doi: 10.1038/35030078. [DOI] [PubMed] [Google Scholar]
- 41.Fraser A, Sargent J, Gamble J, Seaton D. Formation and transfer of fatty acids in an enclosed marine food chain comprising phytoplankton, zooplankton and herring (Clupea harengus L.) larvae. Marine Chemistry. 1989;27:1–18. [Google Scholar]
- 42.Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2010. Rome: FAO; 2010. 197 [Google Scholar]
- 43.Kang JX. Omega-3: A link between global climate change and human health. Biotechnology Advances. 2011;29:388–390. doi: 10.1016/j.biotechadv.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fabry VJ, Seibel BA, Feely RA, Orr JC. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci. 2008;65:414–432. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Carbonate system over the course of the copepod growth experiment.
(DOCX)
Loadings for Principal Component Analysis (PCA) of fatty acids for Thalassiosira pseudonana and Acartia tonsa.
(DOCX)
Full material and methods description.
(DOCX)
Amount of fatty acids of the food algae Thalassiosira pseudonana and the copepod consumer Acartia tonsa at different CO2 treatment combinations.
(DOCX)