Abstract
The Tibetan Plateau is an essential area to study the potential feedback effects of soils to climate change due to the rapid rise in its air temperature in the past several decades and the large amounts of soil organic carbon (SOC) stocks, particularly in the permafrost. Yet it is one of the most under-investigated regions in soil respiration (Rs) studies. Here, Rs rates were measured at 42 sites in alpine grasslands (including alpine steppes and meadows) along a transect across the Tibetan Plateau during the peak growing season of 2006 and 2007 in order to test whether: (1) belowground biomass (BGB) is most closely related to spatial variation in Rs due to high root biomass density, and (2) soil temperature significantly influences spatial pattern of Rs owing to metabolic limitation from the low temperature in cold, high-altitude ecosystems. The average daily mean Rs of the alpine grasslands at peak growing season was 3.92 µmol CO2 m−2 s−1, ranging from 0.39 to 12.88 µmol CO2 m−2 s−1, with average daily mean Rs of 2.01 and 5.49 µmol CO2 m−2 s−1 for steppes and meadows, respectively. By regression tree analysis, BGB, aboveground biomass (AGB), SOC, soil moisture (SM), and vegetation type were selected out of 15 variables examined, as the factors influencing large-scale variation in Rs. With a structural equation modelling approach, we found only BGB and SM had direct effects on Rs, while other factors indirectly affecting Rs through BGB or SM. Most (80%) of the variation in Rs could be attributed to the difference in BGB among sites. BGB and SM together accounted for the majority (82%) of spatial patterns of Rs. Our results only support the first hypothesis, suggesting that models incorporating BGB and SM can improve Rs estimation at regional scale.
Introduction
Soil respiration (Rs) is the major pathway for carbon (C) exiting terrestrial ecosystems and plays a central role in global carbon cycles [1]–[3]. Because soil is the largest carbon pool in terrestrial ecosystems, containing more than 1500 Pg C (1 PG = 1015 g) [4]–[6], small change in the rate of Rs may have a profound impact on atmospheric CO2 concentration, exerting positive feedbacks to global warming [2], [7]–[9]. Therefore, it is important to understand and be able to predict how Rs responds to environmental variation and climate change.
Rs has been a major research theme, particularly since the beginning of 1990s [2], [6], [10]–[16]. Many studies in a variety of ecosystems have been devoted to evaluation of various influencing factors, including microbial activity [17]–[19], C allocation [20], [21], root dynamics [22], and regulators such as temperature, soil moisture, soil texture and other climatic and soil variables [23], [24]. Nevertheless, synthetic analyses of existing data show a substantially huge heterogeneity in Rs, for which reason we require comprehensive datasets before being able to discuss the uncertainties that may arise owing to differences in intensity of sampling in different ecosystems [25].
It has been well documented that Rs varies greatly with time and space [25]. With the advanced equipment for high-frequency records of Rs, temperature, moisture and other variables (e.g. [26]), within-site temporal patterns of Rs can be relatively easily obtained. However, to address patterns of ecosystem C cycling at regional scale, to predict responses of Rs to future climate change based on mechanistic data, and to scale-up from specific sites to vegetation biomes, studies on Rs need to move beyond within-site variations in soil temperature and soil moisture and to incorporate differences among broad ecosystem types [6], [27], [28]. At regional scale, patterns of biogeochemical cycling of different ecosystem types are governed by at least five independent controls or so-called state factors, i.e. climate, parent material, topography, biota, and time [3], [29]. Hence, factors closely associated with Rs within-ecosystem and among-ecosystems are not identical. However, compared with the plenty of studies on temporal variations, relatively fewer publications have explored in-depth the regional patterns of Rs and the factors revolving around Rs process (but see [30]).
The Tibetan Plateau is one of the most under-studied regions for Rs research, despite its essential role in the global C cycles. Due to rough natural conditions, only a few studies have measured Rs. Some in alpine steppe [31], some in alpine meadow [32]–[35], and others in cropland [36]. Alpine grassland accounts for 62% of the total area of the plateau, out of which 32% is alpine steppe, and 30% alpine meadow [37]. Alpine grassland is of special interest because of the high C density [38], [39] and potential feedbacks to climate warming [40]. We previously estimated that SOC storage in the top one meter in these alpine grasslands was 7.4 Pg C, with an average density of 6.5 kg m−2 [39]. Moreover, the Tibetan Plateau is the largest high-altitude and low-latitude permafrost area on the earth, with over 50% of its total surface in permafrost [41], [42]. The observed rapid rises in air temperature [43], degradation of the permafrost and the associated changes in soil hydrology in the last several decades [42], [44], [45] will seriously impact the C cycles [34], [46]. The high-altitude ecosystems, low-latitude permafrost, unique vegetation composition and physiological adaptation to the extreme environments, as well as the relatively low intensity of human disturbance motivated us to focus on carbon cycle and the effects of global climate change on natural ecosystems of the Tibetan Plateau.
The primary objective of this study is to investigate large-scale spatial patterns of Rs and to examine their responses to naturally occurring environmental gradients in order to identify factors most closely associated with Rs in such extreme environments. We hypothesized that:
Belowground biomass is most related to large-scale variations in Rs, because alpine grasslands have a high root biomass density [47]. As a result autotrophs will contribute a large proportion of the total respiratory CO2 efflux.
Soil temperature is another important influential factor for alpine grassland Rs. This is because low growing-season temperature is a limiting factor for physiological processes in high-altitude grassland ecosystems [48], [49]. Therefore, it is predicted that Rs increases with increasing soil temperature.
These two hypothesis were tested in a transect study across alpine grasslands on the Tibetan Plateau. The measurement of Rs in this vast, remote, high-altitude area complements the existing data and help to estimate the global C flux from soils.
Materials and Methods
Ethics Statement
No specific permits were required for the described field studies in the Tibetan Plateau. The research sites are not privately-owned or protected in any way and field studies did not involve endangered or protected species.
Study sites
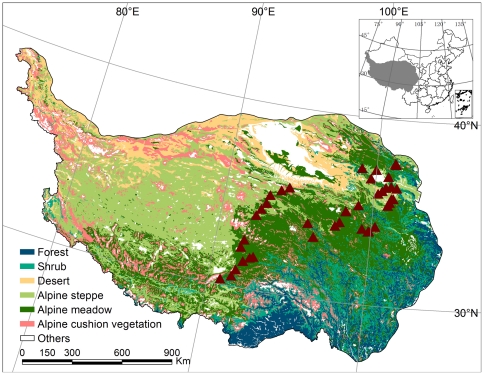
This study was conducted during two expeditions in late July and early August of 2006 and 2007, in collaboration with University of Tuebingen, Germany. Out of the 51 sites, 42 were selected for soil respiration measurements along a transect which stretches from latitudes of 30.31 to 37.69°N and longitudes of 90.80 to 101.48°E, and elevations from 2925 to 5105 m a.s.l. (Table 1, Fig. 1). Mean annual air temperature (MAT) and mean annual precipitation (MAP) range from −5.75 to 2.57°C and 218 to 604 mm yr−1, respectively. The vegetation represents alpine grassland, including the two main ecosystem types, alpine meadow and alpine steppe [49], [50]. Out of the 42 sites, 23 were alpine meadows and 19 alpine steppes. Alpine meadows are dominated by perennial tussock grasses such as Kobresia pygmaea and K. tibetica, while alpine steppes are dominated by short and dense tussock grasses such as Stipa purpurea; both ecosystem types have extensive distributions. The sites were selected by visual inspection of the vegetation, aiming to sample sites subject to minimal grazing and other anthropogenic disturbances.
Table 1. Description of 42 sites where soil respiration measurements were taken.
| Site | Latitude | Longitude | Altitude (m) | MAT (°C) | GST (°C) | MAP (mm yr−1) | GSP (mm yr−1) | Rs (µmol m−2 s−1) | Vegetation |
| QZ01 | 36.37 | 101.48 | 3454 | −1.83 | 7.33 | 466 | 326 | 4.35 | Meadow |
| QZ02 | 35.80 | 101.30 | 3302 | 0.03 | 8.98 | 475 | 328 | 5.27 | Meadow |
| QZ03 | 35.78 | 101.17 | 3263 | 0.39 | 9.37 | 466 | 322 | 3.26 | Steppe |
| QZ04 | 35.58 | 101.08 | 3416 | −0.37 | 8.50 | 488 | 336 | 2.87 | Steppe |
| QZ06 | 35.41 | 100.97 | 3517 | −0.79 | 7.99 | 501 | 346 | 4.07 | Meadow |
| QZ07 | 34.24 | 100.25 | 4282 | −4.23 | 3.96 | 604 | 414 | 5.09 | Meadow |
| QZ08 | 33.96 | 99.88 | 4053 | −2.11 | 6.06 | 580 | 395 | 4.08 | Meadow |
| QZ11 | 33.94 | 99.83 | 4156 | −2.77 | 5.38 | 589 | 402 | 5.15 | Meadow |
| QZ13 | 34.06 | 99.40 | 4231 | −3.22 | 5.05 | 568 | 389 | 12.9 | Meadow |
| QZ14 | 34.92 | 98.21 | 4267 | −3.96 | 4.96 | 464 | 326 | 5.06 | Meadow |
| QZ15 | 34.89 | 98.23 | 4224 | −3.63 | 5.27 | 462 | 325 | 2.14 | Steppe |
| QZ17 | 34.28 | 97.88 | 4667 | −5.74 | 2.84 | 522 | 364 | 9.32 | Meadow |
| QZ18 | 33.32 | 96.28 | 4506 | −2.89 | 5.48 | 482 | 333 | 6.00 | Meadow |
| QZ19 | 34.01 | 95.80 | 4201 | −1.60 | 7.15 | 390 | 274 | 3.58 | Steppe |
| QZ22 | 34.06 | 97.60 | 4700 | −5.55 | 2.97 | 523 | 365 | 2.46 | Meadow |
| QZ23 | 35.29 | 99.01 | 4217 | −4.48 | 4.47 | 478 | 336 | 1.19 | Steppe |
| QZ24 | 36.01 | 100.25 | 3109 | 1.63 | 10.92 | 393 | 274 | 1.59 | Steppe |
| QZ25 | 36.17 | 100.51 | 2925 | 2.57 | 11.93 | 380 | 264 | 0.89 | Steppe |
| QZ26 | 36.36 | 100.74 | 3233 | 0.08 | 9.43 | 409 | 287 | 2.19 | Steppe |
| QZ27 | 36.44 | 101.09 | 3486 | −1.94 | 7.32 | 446 | 314 | 5.36 | Meadow |
| QZ28 | 36.95 | 100.86 | 3130 | −0.01 | 9.62 | 372 | 265 | 2.52 | Steppe |
| QZ29 | 37.26 | 99.98 | 3215 | −0.55 | 9.39 | 319 | 233 | 1.78 | Steppe |
| QZ30 | 37.28 | 98.99 | 3437 | −1.61 | 8.48 | 290 | 216 | 4.04 | Steppe |
| QZ31 | 35.74 | 94.25 | 4222 | −3.14 | 6.70 | 218 | 170 | 2.17 | Steppe |
| QZ32 | 35.52 | 93.74 | 4564 | −5.01 | 4.80 | 238 | 185 | 0.39 | Steppe |
| QZ33 | 35.17 | 93.04 | 4682 | −5.41 | 4.31 | 234 | 182 | 0.75 | Steppe |
| QZ34 | 34.72 | 92.89 | 4801 | −5.75 | 3.76 | 348 | 249 | 4.23 | Meadow |
| QZ35 | 33.99 | 92.35 | 4654 | −4.22 | 4.94 | 336 | 248 | 1.24 | Steppe |
| QZ36 | 32.18 | 91.72 | 4903 | −4.18 | 4.12 | 473 | 327 | 2.79 | Meadow |
| QZ38 | 31.45 | 92.02 | 4494 | −0.25 | 7.94 | 480 | 341 | 1.12 | Meadow |
| QZ40 | 31.77 | 92.62 | 4605 | −2.05 | 5.89 | 523 | 361 | 3.03 | Meadow |
| QZ41 | 31.69 | 92.41 | 4596 | −1.92 | 6.00 | 511 | 355 | 3.99 | Meadow |
| QZ42 | 30.94 | 91.66 | 4756 | −2.76 | 5.45 | 539 | 371 | 1.90 | Steppe |
| QZ43 | 30.56 | 91.45 | 4506 | −0.53 | 7.32 | 507 | 359 | 5.94 | Meadow |
| QZ44 | 30.31 | 90.80 | 4324 | 1.23 | 8.81 | 442 | 326 | 1.99 | Steppe |
| QZ45 | 32.58 | 91.86 | 5105 | −5.75 | 2.77 | 488 | 331 | 4.59 | Meadow |
| QZ46 | 34.37 | 92.61 | 4656 | −4.56 | 4.78 | 327 | 241 | 1.78 | Steppe |
| QZ47 | 36.78 | 99.67 | 3391 | −1.00 | 8.72 | 348 | 251 | 8.25 | Meadow |
| QZ48 | 37.61 | 101.31 | 3196 | −1.53 | 7.74 | 363 | 309 | 7.26 | Meadow |
| QZ49 | 37.61 | 101.31 | 3196 | −2.12 | 7.19 | 364 | 311 | 10.6 | Meadow |
| QZ50 | 37.69 | 101.28 | 3268 | −1.89 | 7.67 | 313 | 270 | 5.32 | Meadow |
| QZ51 | 37.28 | 98.99 | 3437 | −1.61 | 8.48 | 290 | 216 | 1.99 | Steppe |
MAT, Mean annual temperature; GST, growing season temperature; MAP, mean annual precipitation; GSP, growing season precipitation; Rs, daily mean soil respiration rate.
Figure 1. Vegetation map of the sampling sites, selected from the Vegetation Map of China [80].
Triangles represent sampling sites.
Field measurements
At each site, we conducted (1) measurement of plant biomass after surveying the entire plant community, (2) collections of soil samples at three depths (0–5, 5–10, and 10–20 cm) using soil corer, followed by volumetric samples at equal depths for bulk density and gravimetric water content determinations, (3) on-site extraction of soil mineralized N (Nmin) consisting of nitrate (NO3-N) and ammonium (NH4-N), and (4) measurement of soil respiration rates.
Plant biomass measurement
We harvested aboveground biomass (AGB) in three plots (1×1 m2) and belowground biomass (BGB) in three soil pits (0.5×0.5 m2) described in Yang et al. [47]. Biomass samples were dried using a custom-built portable oven in the field, and oven-dried at 60°C to a constant weight, and weighed to the nearest 0.01 g upon returning to the laboratory.
Soil property measurement
Soil sampling procedures, soil bulk density (SBD), soil total N (STN) and SOC measurements have been detailed elsewhere [39]. On-site extraction of Nmin was carried out using a custom-designed equipment which could perform on-site extraction without any disturbances. In brief, 10 g of homogenized soil was extracted with 50 ml 1 mol KCl for 60 minutes immediately after sampling, filtered through Whatman No. 42 cellulose filter paper into 100 ml PE-vials, and conserved by acidification with 3 ml hydrochloric acid (HCl, 30%) [38].
Soil respiration measurement
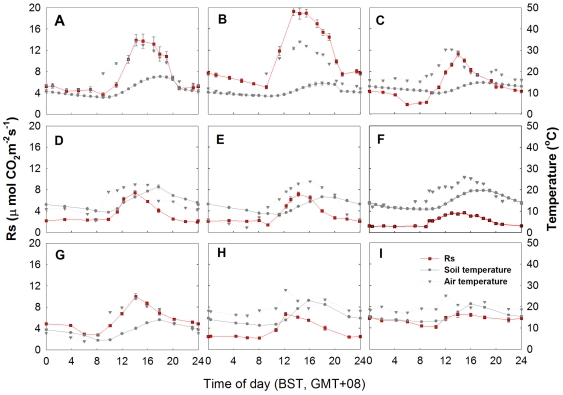
At each site, seven PVC soil collars (10 cm inside diameter and 5 cm in height) were installed 2–3 cm into the soil along a straight line at one-meter intervals. Rs (CO2 efflux) was measured with a Li-6400 infrared gas analyzer equipped with the 6400-09 soil flux chamber (Li-Cor Inc, Lincoln, NE, USA). The protocol recommended by LiCor (LI-6400-09 manual) was changed to five observations of 10 µmol mol−1 (for steppes) and 30 µmol mol−1 (for meadows) per measurement. Typically, soil respiration rates were measured 3–4 times during 4–5 hours from 10:00 to 16:00 (Beijing Standard Time) when soil respiration peaked. To obtain the diurnal pattern, we also measured the complete diurnal variation of soil respiration at nine sites (Fig. 2). We then calculated the ratios of instant Rs from 10:00 to 16:00 to the daily mean Rs for the nine sites. Using these ratios, we calculated daily mean Rs of non-diurnal sites according to similarity in community composition and closeness in distance. On average, diurnal courses of soil respiration were measured every four to five sites. Soil temperature at 10 cm was monitored simultaneously with soil respiration measurement using the attached soil temperature probe. Air temperature was measured with the temperature probe of Li-6400 infrared gas analyzer.
Figure 2. Diurnal changes of soil respiration rate, soil temperature and air temperature.
Complete diurnal courses of soil respiration were measured for seven alpine meadows and two alpine steppes on the Tibetan Plateau. Vertical bars indicate the standard error of the measurement mean (n = 5–7) for each time. (A), Haibei, Kobresia and Festuca mixed meadow; (B), Haibei, Kobresia tibetica meadow; (C), Haibei, Kobresia pygmaea meadow; (D) Naqu, Kobresia pygmaea meadow; (E) Naqu, Kobresia tibetica meadow; (F) Tianjun, Stipa purpurea steppe; (G) Fenghuoshan, Kobresia pygmaea meadow; (H) Qumalai, Kobresia pygmaea meadow; (I) Qumalai, Festuca steppe.
Laboratory analysis
Dried soil samples were grounded using a ball mill (NM200, Retsch, Germany). Total C and N concentrations were determined on 5–6 mg aliquot of the homogenously grounded material for each sample using an elemental analyzer (2400 II CHNS/O Elemental Analyzer, Perkin-Elmer, Boston, MA, USA) with a combustion temperature of 950°C and a reduction temperature of 640°C. Soil inorganic carbon (SIC) was measured volumetrically using an inorganic carbon analyzer (Calcimeter 08.53, Eijkelkamp, Netherland). Thus SOC was calculated as the difference between STC and SIC. Soil pH was determined in both 0.01 M CaCl2 and bi-distilled H2O potentiometrically, but only those of water solution were used in the current study. The KCl-extractions for Nmin-analysis were measured photometrically using a Continuous Flow Analyzer (SAN Plus, Skalar, Netherlands). Soil moisture (SM) was determined gravimetrically by taking the skeleton content into account.
Climate data and statistical analysis
At each site, we installed temperature data loggers (Hobo U12, Onset Computer Corporation, Pocasset, MA) in July 2006 to measure soil temperature (−10 cm) at 1 h interval. We revisited these sites in July or August in 2007, 2008 and 2009 to download the recorded temperature data. Based on those measurements mean annual soil temperature (MAST) of each site was calculated. The climate data used in this study were calculated based on linear models using latitude, longitude, and altitude as variables from 55-year averaged temperature and precipitation records (1951–2005) at 680 well-distributed climate stations across China [48], [51], [52]
The variables to explain the spatial variation of soil respiration consist of (1) soil properties, measured by SOC, SM, MAST, soil C/N ratio, SBD, soil acidity (pH), soil texture (sand content, clay content), and Nmin, (2) average climate, encompassing growing season temperature (GST), growing season precipitation (GSP), and (3) plant community characteristics, including vegetation type (VT, meadow or steppe), AGB and BGB (Table 2). We used regression tree analysis [53], as implemented in the SAS statistical software package version 8.01 [54], to screen important variables influencing soil respiration, as tree-based modeling is an exploratory data analytic technique for summarizing multivariable and uncovering its structure in large datasets [55]. We selected F test's p-value as splitting criterion, and set observations required for a split search at 5. Our sample size (42 sites) doesn't allow us to do cross validation, but when we set the F test significant level at 0.20, the tree developed was adequate in complexity (depth) and explanation (R 2). From the relative importance in the regression tree which was calculated as the cumulative variance reduction at each split for a particular independent variable, five variables with the importance values greater than 0.4 were screened out, i.e. AGB, BGB, VT, SOC, and SWC (Table 2).
Table 2. Variables included in the regression tree analysis and their importance value.
| Variable | n | Mean | SD | Range | Importance in regression tree |
| Soil organic carbon (SOC, %) | 42 | 5.25 | 4.79 | 0.339–19.4 | 1.0000 |
| Aboveground biomass (AGB, g m−2) | 42 | 119 | 100 | 29.9–530 | 0.8997 |
| Belowground biomass (BGB, g m−2) | 42 | 1816 | 1957 | 202–9393 | 0.8889 |
| Vegetation type (VT) | 42 | - | - | - | 0.4577 |
| Soil moisture (SM, v/v, %) | 42 | 38.3 | 50.2 | 0.44–220 | 0.4383 |
| Growing season temperature (GST, °C) | 42 | 6.67 | 2.25 | 2.77–11.93 | 0.1719 |
| Mean annual soil temperature (MAST, °C) | 42 | 17.0 | 5.53 | −1.12–8.14 | 0.1621 |
| Growing season precipitation (GSP, mm yr−1) | 42 | 306 | 61.5 | 170–414 | 0.0000 |
| Soil temperature (ST, °C) | 42 | 17.0 | 5.53 | 6.30–31.55 | 0.0000 |
| Soil C/N ratio (C/N, g g−1) | 39 | 12.1 | 2.85 | 7.97–20.1 | 0.0000 |
| Soil bulk density (SBD, g cm−3) | 38 | 0.94 | 0.32 | 0.31–1.65 | 0.0000 |
| pH | 38 | 7.3 | 0.52 | 6.0–8.1 | 0.0000 |
| Sand content (%) | 37 | 42.3 | 18.4 | 20.0–80.0 | 0.0000 |
| Clay content (%) | 37 | 7.60 | 6.59 | 3.0–24 | 0.0000 |
| Available nitrogen (mmol l−1) | 37 | 0.080 | 0.046 | 0.026–0.218 | 0.0000 |
To address how these variables affect soil respiration both directly and indirectly is challenging because variables measured in field are cross-correlated [11], [14], [28]. Structural equation modelling (SEM) [56]–[58] has been used in recent studies to explicitly evaluate the causal relationships among multiple interacting variables (e.g. [59]–[61]). SEM aims to account for the roles of multiple variables in a single analysis, providing mechanisms behind the overall patterns by partitioning direct from indirect effects that act through other components of the system.We used SEM here to partition the total effect of variables on soil respiration into direct effects and indirect effects. A path model was developed to relate soil respiration to AGB, BGB, VT, SOC, and SWC, based on theoretical knowledge of the major factors associated with soil respiration at ecosystem level [3]. The model was fitted using EQS 6.1 for Windows [62].
As the results of SEM are dependent on correctly specifying theoretical causal relationships between variables prior to analysis [56], [58], the initial theoretical model was modified to improve the fit between model and data. The final model was strong: Bentler's comparative fit index (CFI) = 0.96, Bentler-Bonett normed fit index (NFI) = 0.95. Furthermore, R-squares for Rs, AGB, BGB are very high in the path model.
Results
Overall soil respiration
Across 42 sites, the daily mean Rs of alpine grassland at peak growing season was 3.92 µmol CO2 m−2 s−1, and ranged from 0.39 to 12.88 µmol CO2 m−2 s−1 (Table 1), with a coefficient of variation (CV) of 69.1%. The daily mean Rs of steppes was 2.01 µmol CO2 m−2 s−1 (ranged from 0.39 to 4.04), while Rs of meadows, 5.49 µmol CO2 m−2 s−1 (ranged from 1.12 to 12.88), was approximately two and half times that of the steppes. Although the meadows had a significantly higher Rs than steppes, their CV were similar, being 48.9 and 47.1% for meadow and steppe, respectively.
Large diurnal variations in Rs were observed, although the diurnal patterns were generally similar for meadow and steppe (Fig. 2), both exhibiting the highest Rs during the time from 12:00 to 14:00 BST. Rs and their climatic, community and soil properties for the important ecosystem types, such as Kobresia pygmaea meadow, K. tibetica meadow, species-rich meadow (mixed-species meadow), and Stipa spp. steppe are lised in Table 3. K. tibetica meadow had the highest Rs, while Stipa steppe had the lowest Rs.
Table 3. Soil respiration, community biomass, soil properties, and climatic variables in different ecosystem types.
| Variable | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD |
| Kobresia pygmaea meadow | Kobresia tibetica meadow | Stipa spp. steppe | Mixed-specie meadow | Others | |||||||||||
| Rs (µmol m−2 s−1) | 11 | 4.36 | 1.52 | 4 | 9.34 | 3.49 | 12 | 2.18 | 0.95 | 3 | 6.93 | 1.52 | 12 | 2.68 | 1.49 |
| SOC (%) | 11 | 6.23 | 3.61 | 4 | 11.51 | 2.54 | 12 | 1.96 | 1.07 | 3 | 8.82 | 6.50 | 12 | 4.66 | 5.68 |
| AGB (g m−2) | 11 | 107 | 65 | 4 | 285 | 188 | 12 | 78 | 40 | 3 | 253 | 114 | 12 | 84 | 41 |
| BGB (g m−2) | 11 | 2390 | 1261 | 4 | 5852 | 2682 | 12 | 528 | 272 | 3 | 3299 | 2051 | 12 | 862 | 619 |
| SM (g water g−1 dry soil) | 11 | 32.2 | 20.4 | 4 | 125.1 | 26.3 | 12 | 7.0 | 4.2 | 3 | 45.4 | 37.7 | 12 | 44.6 | 68.3 |
| GST (°C) | 11 | 6.28 | 1.46 | 4 | 4.46 | 2.10 | 12 | 7.61 | 2.20 | 3 | 8.48 | 0.66 | 12 | 6.38 | 2.64 |
| Soil MAT ((°C)) | 11 | 3.37 | 1.61 | 4 | 0.91 | 1.88 | 12 | 3.80 | 2.36 | 3 | 3.66 | 0.31 | 12 | 2.72 | 3.09 |
| GSP (mm yr−1) | 11 | 351 | 42 | 4 | 349 | 35 | 12 | 257 | 55 | 3 | 296 | 40 | 12 | 301 | 59 |
| C/N (g g−1) | 10 | 13.75 | 3.20 | 3 | 15.19 | 0.77 | 12 | 10.36 | 1.16 | 2 | 11.79 | 1.56 | 12 | 11.81 | 3.10 |
| SBD (g cm−3) | 10 | 0.79 | 0.20 | 3 | 0.44 | 0.01 | 12 | 1.14 | 0.24 | 2 | 0.74 | 0.20 | 12 | 1.00 | 0.35 |
| pH | 8 | 6.9 | 0.59 | 3 | 6.8 | 0.32 | 12 | 7. 7 | 0.26 | 3 | 7.4 | 0.09 | 12 | 7.3 | 0.53 |
| Sand content (%) | 10 | 34.6 | 6.0 | 3 | 31.0 | 4.5 | 12 | 55.7 | 23.7 | 2 | 37.5 | 9.2 | 10 | 36.1 | 13.5 |
| Clay content (%) | 10 | 8.2 | 6.0 | 3 | 4.0 | 3.8 | 12 | 5.1 | 3.7 | 2 | 12.5 | 12.0 | 10 | 9.4 | 8.7 |
| Available N (mmol l−1) | 10 | 0.11 | 0.05 | 3 | 0.12 | 0.03 | 11 | 0.05 | 0.02 | 2 | 0.15 | 0.02 | 11 | 0.06 | 0.04 |
Rs, daily mean soil respiration rate; SOC, soil organic carbon content; AGB, above-ground biomass; BGB, below-ground biomass; SM, soil moisture; GST, growing season temperature; MAT, Mean annual temperature; GSP, growing season precipitation; SBD, soil bulk density; n, number of sampling sites; SD, standard deviation.
Factors associated with spatial variations in soil respiration
Based on regression analysis, five variables with an importance value greater than 0.4383 were selected (Table 2), and thus were included in the development of the structural equation models. Other variables had negligible or no impact on soil respiration.
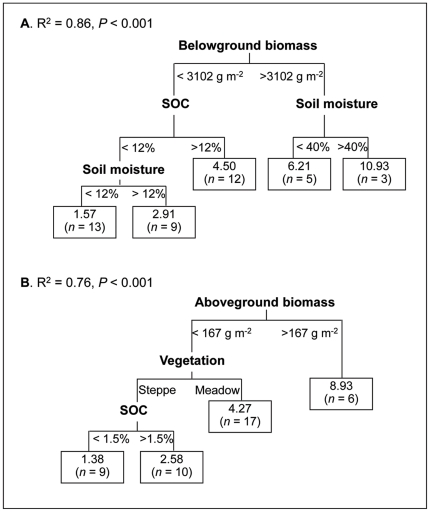
When all five variables were entered into the model, a tree with AGB, vegetation type, and SOC as explanatory variables was developed (Fig. 3B), while BGB and SM were excluded from the model because of the close correlations between BGB and AGB, and SM and vegetation type. When BGB and SM were entered into the model, another tree was developed (Fig. 3A). Both trees are significantly more than a random tree (P<0.001), explaining 86% (Fig. 3A) or 76% (Fig. 3B) of the variance in Rs rate.
Figure 3. Regression tree showing generalized relationships between daily mean soil respiration rate and environmental variables.
Relationships between soil respiration rate and belowground biomass, soil organic carbon content (SOC) and soil moisture (A), aboveground biomass, vegetation type, and SOC (B). Branches are labelled with criteria used to segregate data. Values in terminal nodes represent mean soil respiration rate of sites grouped within the cluster. The tree explained 86% (A) and 76% (B) of the variance in soil respiration rate, which is significantly more than a random tree (P<0.001). n = number of plots in the category.
These analyses indicated that BGB, SOC, SM, AGB, and vegetation types are biotic and abiotic factors that are most closely associated with large-scale variations in soil respiration. For the first tree (Fig. 3A), in the areas with BGB>3102 g m−2, only SM had a statistically significant influence on soil respiration rate; while in the areas with BGB<3102 g m−2, both SOC and SM had a detectable effect. For the second tree (Fig. 3B), when AGB>167 g m−2, soil respiration rate was not significantly affected by vegetation type or SOC; by contrast, when AGB<167 g m−2, soil respiration rate was influenced by both vegetation type and SOC.
Structural equation modelling to explain variations in soil respiration
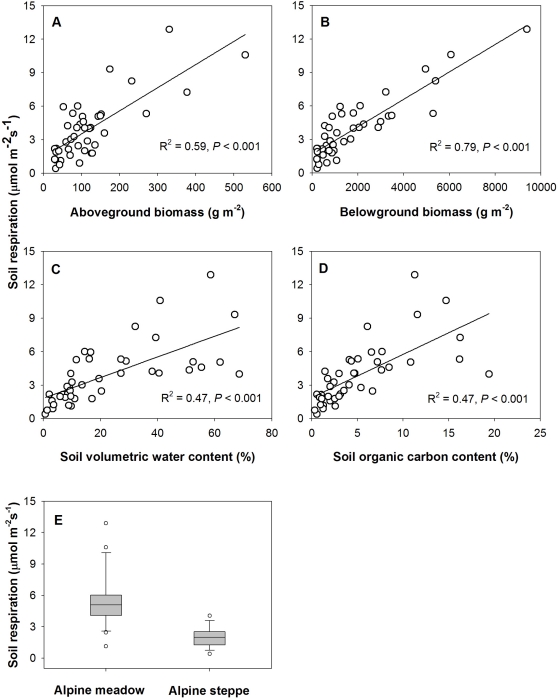
From the scatter plots and the box plot (Fig. 4), each of the selected variables such as AGB, BGB, SM, SOC and vegetation type was closely related to Rs. However, because these five variables were intercorrelated, these apparent relationships combined both direct and indirect correlations. Thus, we further used SEM to explicitly evaluate the causal relationships among these interacting variables.
Figure 4. Scatterplots and box plot for daily mean soil respiration rate versus biotic and abiotic factors.
Relationships between soil respiration rate and aboveground biomass (A), belowground biomass (B), soil moisture (C), soil organic carbon content (D), and vegetation type (E).
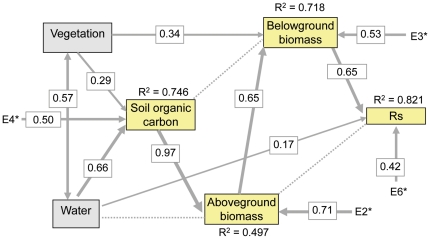
The final SEM explained 82.1% of the variation in Rs (Fig. 5). Direct, indirect and total effects of the variables are summarized in Table 4. Increasing BGB and SM were strongly associated with increases in Rs, indicating that Rs could be well-predicted from these two variables (R 2 = 0.82). Even though there were significant bivariate relationships between AGB, SOC and Rs, they only had strong indirect positive effects on RS. Vegetation type had only an indirect effect on Rs (0.379) through its direct effect on BGB and its indirect effect on SOC and AGB. The rank of total effects, in decreasing order, was: BGB, AGB, SOC, vegetation type, and SM (Table 4).
Figure 5. Final structural equation model for soil respiration.
Non-significant paths are showed in dashed lines. The thickness of the solid arrows reflects the magnitude of the standardized SEM coefficients. Standardized coefficients are listed on each significant path.
Table 4. Total direct and indirect effects in the structural model. Effects were calculated using standardized path coefficients.
| Variable | Direct effect | Indirect effect | Total |
| Rs | |||
| Belowground biomass | 0.654 | - | 0.654 |
| Aboveground biomass | 0.191 ns | 0.427 | 0.618 |
| Soil organic carbon | - | 0.586 | 0.586 |
| Vegetation type | - | 0.397 | 0.397 |
| Soil moisture | 0.165 | 0.175 ns | 0.335 |
| Belowground biomass | |||
| Aboveground biomass | 0.652 | - | 0.652 |
| Soil organic carbon | −0.021 ns | 0.634 | 0.613 |
| Vegetation type | 0.345 | 0.179 | 0.524 |
| Soil moisture | - | 0.175 ns | 0.175 ns |
| Aboveground biomass | |||
| Soil organic carbon | 0.971 | - | 0.971 |
| Vegetation type | - | 0.283 | 0.283 |
| Soil moisture | −0.355 ns | 0.644 | 0.29 |
| Soil organic carbon | |||
| Vegetation type | 0.292 | - | 0.292 |
| Soil moisture | 0.663 | - | 0.663 |
Nonsignificant effects are indicated by “ns”.
It is also evident that, from the SEM (Fig. 5), BGB can well be predicted from vegetation type and AGB, explaining 71.8% of the variation. Moreover, SOC explained about 50% of the variations in AGB.
Discussion
One common feature of natural grasslands is the climate, usually characterized by periodic droughts [63]. For a specific region, it may also be associated with basic parameters such as soil characteristics, frequent fires, grazing pressure and human activities. Chinese grasslands are generally distributed in three different regions: temperate grassland on the Inner Mongolian Plateau, alpine grassland on the Tibetan Plateau, and mountain grassland in the Xinjiang mountain areas [64]. Tibetan alpine grasslands, which are associated with cold climate of the high altitudes [49], differ from tropical and temperate grasslands. Yet, they are poorly documented in C cycles. Our survey on the large-scale patterns of Rs was preliminary, but the trend and relationships were clear.
Magnitude of soil respiration of alpine grasslands
Large differences were observed between Rs from two vegetation types, alpine meadow and alpine steppe, being about two and half times greater in the alpine meadows. The daily mean Rs rates measured in alpine meadows (5.49 µmol CO2 m−2 s−1 by daily average) are similar to previously reported results. For example, Cao et al. [32]reported that during peak growing season (Mid-July or August), daily Rs was 4.4 and 3.2 µmol CO2 m−2 s−1 for light and heavy grazed meadows on the north-eastern edge of the Plateau. Li and Sun [33] reported a range of Rs from 0.93 to 8.02 µmol CO2 m−2 s−1 during growing season in their recently published results. However, the only study from the alpine steppe by Zhang et al. [31], with a daily mean Rs rate of 0.38 µmol CO2 m−2 s−1 at peak growing season, and an annual mean soil respiration rate of 0.248 µmol CO2 m−2 s−1 using a closed static chamber-gas chromatograph method in a Stipa purpurea and Carex moocroftii community, was at the lower end of our measurement. The Rs rates of alpine steppe from this study (2.01 µmol CO2 m−2 s−1 by daily average) are similar to the temperate steppe on the Inner Mongolia Plateau [23], [65]–[69].
Consequently, the question arises: why is there such a difference in Rs rates between the two main grassland types, alpine meadow and alpine steppe? We suggest biological differences in standing biomass and productivity as well as physical differences in soil water availability were the major factors affecting Rs. On average, AGB (proxy of aboveground productivity) and BGB of the typical Kobresia meadows were much greater than the typical Stipa steppe (Table 3). Furthermore, SM of alpine meadow was also much higher than Stipa steppe. These high BGB and SM in alpine meadows significantly increased Rs rate.
The alpine grasslands of the Tibetan Plateau are sometimes called alpine tundra, despite their different species composition and environmental conditions compared to arctic tundra. Nevertheless, Tibetan alpine grassland and arctic tundra share some common features, such as large below ground standing biomass (averaging 1658 g m−2 for arctic tundra in Alaska [70], and 1816 g m−2 on the Tibetan Plateau in current study), relatively large soil C density [39], [40], relatively high soil moisture (particularly in alpine meadow), and influences of permafrost. These characteristics mean that they are more responsive to global warming than other ecosystems, because their soils have the potential to release significant amounts of carbon-based greenhouse gases [46], [71], [72].
Factors associated with the large-scale patterns of soil respiration
Our analysis showed that among biotic and abiotic factors, BGB and SM together well explained the spatial patterns of peak growing-season Rs, accounting for 82% of the variation among 42 sampling sites. The important role of SM for Rs is in good accordance with results from other studies on soil nitrogen and carbon contents across the Tibetan Plateau [39]. Most of the variation in Rs could be attributed to the difference in BGB among sites (80%), with a small proportion further explained by SM (2%, SM entered after BGB in general linear models, because BGB and SM covaried). Thus, the results support our first hypothesis that BGB is most closely associated with the large-scale variations in Rs. This finding implies that autotrophic Rs (including plant roots and closely associated organisms) contributes a large proportion to total Rs, or/and autotrophic Rs is strongly related to heterotrophic Rs in these alpine grassland ecosystems.
A few studies with data compilation have addressed the general patterns of Rs across biomes. For example, on a global scale, Raich & Schlesinger [6] found Rs is positively correlated with MAT and MAP, as well as a close correlation between mean annual net primary productivity (NPP) of different vegetation biomes and their mean annual Rs. Bond-Lamberty & Thomson [25] built a global database of Rs from 3379 records spanning publication years 1963–2008, and found MAT, MAP and leaf area index together explained approximately 41% of the observed variability in annual Rs. Across the northern hemisphere, Hibbard et al. [28] found Rs and soil temperature are closely correlated for the deciduous and mixed forests, but not for non-forest biomes. These across-biome patterns of Rs are generally controlled by climate and NPP. Furthermore, Mahecha et al. [73] approximated the sensitivity of terrestrial ecosystem respiration to MAT across 60 sites worldwide, and offers substantial evidence for a general temperature sensitivities of soil respiration. Within the grassland biome, aboveground net primary productivity (ANPP, approximation to AGB of peak growing season as in this study) was shown to be positively correlated with Rs rate [12]. Craine et al. [27] also reported in Minnesota grasslands that both AGB and BGB are positively correlated with Rs. These previous studies in grasslands are consistent with the current results, since we observed a positive correlation between AGB, BGB and Rs as well. The novel part of our study is that we found only BGB and SM had direct effects on Rs at regional scale, with other factors indirectly affecting Rs through BGB or SM. It is also evident that factors most closely associated with Rs within-biome and across biomes are different.
In contrast, intra-annual variation in Rs at individual sites are mainly explained by soil temperature and soil moisture, but not by ANPP or AGB [74]. Temporal variations of Rs have been well simulated by using the continuous records of temperature and moisture [75]. Our measurements, across altitudes from 2925 to 5105 m and mean soil temperature (−10 cm) of midday (10:00 to 16:00 BST) from 6.3 to 31.6°C (the highest soil temperature of 31.6°C was recorded in an alpine steppe at 2925 m) during the field measurement exhibited that soil temperature did not have a strong effect on Rs across study sites. For example, Kobresia tibetica meadow on permafrost with a soil temperature of 6.3°C still had a daily mean Rs rate as high as 5.1 µmol CO2 m−2 s−1. Our results from the Tibetan grassland do not support the second hypothesis that Rs increases with increasing soil temperature in alpine grassland, but support the argument by Hibbard et al. [28] that within-site robust relationships with temperature and/or moisture are not adequate to characterize soil CO2 effluxes across space, because for regional variation BGB is the most important factor.
Separating direct and indirect factors influencing soil respiration
In the present study, we used regression tree analysis [53] and SEM [56]–[58] as new approaches to conduct variable selection, to identify direct and indirect factors, and to determine the extent to which these factors may constrain Rs. To our knowledge, the efficiency of these approaches has not been evaluated empirically in soil respiration research.
Traditionally, stepwise selection and linear regression are used to identify and rank the limiting factors in Rs studies. However, when performing stepwise selection, closely covariated parameters cannot be selected simultaneously in the final model, because the explanatory power would not increase when a closely related variable is included. In our case, when BGB retain in the model, AGB will not be selected due to their close correlation. As a matter of fact, AGB has a strong indirect effect through BGB on Rs. This problem can be solved by a regression tree analysis which has the advantage to rank the limiting factors based on their importance [55].
Field studies examining ecosystem responses to climatic and other environmental changes typically use naturally occurring climatic gradients. However, some studies have realized the limitations of correlation method in analyzing factors influencing Rs [12], [76]. For example, Rs rates vary significantly among major plant biomes, suggesting that vegetation type influences the rate of soil respiration. Nevertheless, the correlations among climatic factors, vegetation distributions, and Rs make cause-effect arguments difficult [12]. Burke et al. [76] raised the issue that there are inherent problems with utilizing simple statistical relationships of spatial variability as a foundation for understanding ecosystem function, because complex covariance along the gradient occurs across large spatial scales, leading to the problem that actual and apparent controlling factors may be confounded. Without field experiments, which are difficult to conduct across numerous sites, and without simulation of ecological processes, which need to be based on mechanistic data, SEM is one option. The quantitative procedure in the current study showed that the direct factors influencing Rs at large-scale were BGB and SM, AGB, SOC and vegetation type only had indirect influences despite their significant correlations with Rs. This holistic approach is appropriate in across-site comparisons of ecosystem structure and function.
Limitations of the current study
In the present study the soil PVC collars were installed only one hour before measurement due to the low accessibility of most sites, while the placement of collars are at least 24 hour prior to measurement in most Rs studies. Althouth the insertion of collars may cause unrealistic readings of soil CO2 efflux because of the high fluxes after colloar installation, fluxes stabilize after 10–30 min [77], [78]. In addition, our measurement of Rs followed the same procedure throughout our survey. Therefore the error introduced by soil disturbance could be treated as a systematic error which is weak.
Complete diurnal courses were obtained at nine sites, whereas for most of our sites soil respiration were measured 3–4 times during 4–5 hours when Rs peaked. We acknowledge that soil respiration is a dynamic process that may not be well represented by a few replicated measurements during several hours of a day. However, we found average midday Rs rates of the nine sites were well correlated with their daily mean Rs. Furthermore, we calculate daily mean Rs of each site by extrapolating the nine diurnal courses to all 42 sites according to community composition and closeness in distance. This extrapolation might add uncertainty to the estimates of daily mean Rs. Nevertheless, sites of similar vegetation composition and closest in distance generally share comparable features of geology, climate, soil and vegetation, which in combination are the major determinants of soil respiration.
The main objective of this study is to investigate the large-scale regional patterns of Rs in the Tibetan Plateau. Rs of 42 sites were measured during peak growing season of late July and early August. Measurements over a time span of one month may lead to problems as spatial variation of Rs could interfere with temporal changes. However, a four-year observation on soil CO2 efflux in Haibei Alpine Grassland Research Station of Northwest Institute of Plateau Biology, the Chinese Academy of Sciences (3200 m a.s.l.) revealed that Rs values peak and stabilize in late July and early August (unpublished data by YHW and JSH). This phenomenon was observed in north America as well [79]. Therefore, compared with the large variation of Rs across the plateau, the temporal interference should be minor.
Conclusions and implications
Our understanding of the controls and magnitudes of regional Rs is limited by the uncertainties due to spatial heterogeneity of vegetation across regional environmental gradients. In the current study, we moved beyond within-site differences in soil temperature and moisture to incorporate differences among broad ecosystem types (e.g. biomes). We can conclude with certainty that BGB is the factor most closely associated with Rs rate at regional scale for the grassland ecosystems, suggesting that in future we could develop models for Rs from plant standing biomass, which has a much larger database with wider biogeographic coverage, particularly in remote areas, such as the Tibetan Plateau. We acknowledge that only Rs rates during peak growing season were measured in the current study. Therefore, intensive measurements should be taken on a few sites across environmental gradients to develop more precise prediction models for annual Rs. Our results also have the implication that if we take Rs rates at peak growing season as a parameter of ecosystem metabolic activity, then compared with the plant physiology at individual level, ecosystem metabolism is not so much influenced by temperature itself. Furthermore, our results imply that a shift from alpine meadow to steppe due to changes of soil hydrological properties as a consequence of permafrost degradation will significantly alter Rs.
Acknowledgments
The authors are grateful to members of the Peking University expedition team, particularly Tong Shen, Wenhong Ma, Cunzhu Liang, Liang Wang, Yi Wu, Shanmin Mou and Shanxue Qi for assistance with field measurement, to Bernhard Schmid of University of Zurich, Switzerland, for statistical advice, and to Jingyun Fang and Dan Flynn for helpful comments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by the “Program of One Hundred Talented People” of the Chinese Academy of Sciences (Grant No. KSCX2-YW-Z-0806), the National Natural Science Foundation of China (Grant No. 31025005 and 31021001), National Program on Key Basic Research Project (Grant No. 2010CB950602), and the “Strategic Priority Research Program” of the Chinese Academy of Sciences (Grant No. XDA05050304). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schlesinger WH. Biogeochemistry: an analysis of global change. San Diego: Academic Press; 1997. 588 [Google Scholar]
- 2.Schlesinger WH, Andrews JA. Soil respiration and the global carbon cycle. Biogeochemistry. 2000;48:7–20. [Google Scholar]
- 3.Chapin FS, III, Matson PA, Mooney H. Principles of terrestrial ecosystem ecology. New York: Springer-Verlag; 2002. 529 [Google Scholar]
- 4.Amundson R. The carbon budget in soils. Annu Rev Earth Pl Sc. 2001;29:535–562. [Google Scholar]
- 5.Eswaran H, van der Berg E, Reich P. Organic carbon in soils of the world. Soil Sci Soc Am J. 1993;57:192–194. [Google Scholar]
- 6.Raich JW, Schlesinger WH. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B. 1992;44:81–99. [Google Scholar]
- 7.Davidson EA, Janssens IA. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature. 2006;440:165–173. doi: 10.1038/nature04514. [DOI] [PubMed] [Google Scholar]
- 8.Luo YQ. Terrestrial carbon-cycle feedback to climate warming. Annu Rev Ecol Evol S. 2007;38:683–712. [Google Scholar]
- 9.Melillo JM, Steudler PA, Aber JD, Newkirk K, Lux H, et al. Soil warming and carbon-cycle feedbacks to the climate system. Science. 2002;298:2173–2176. doi: 10.1126/science.1074153. [DOI] [PubMed] [Google Scholar]
- 10.Bond-Lamberty B, Thomson A. Temperature-associated increases in the global soil respiration record. Nature. 2010;464:579–582. doi: 10.1038/nature08930. [DOI] [PubMed] [Google Scholar]
- 11.Luo YQ, Zhou XH. Soil respiration and the environment. San Diego: Academic Press; 2006. 319 [Google Scholar]
- 12.Raich JW, Tufekcioglu A. Vegetation and soil respiration: Correlations and controls. Biogeochemistry. 2000;48:71–90. [Google Scholar]
- 13.Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, et al. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia. 2001;126:543–562. doi: 10.1007/s004420000544. [DOI] [PubMed] [Google Scholar]
- 14.Ryan MG, Law BE. Interpreting, measuring, and modeling soil respiration. Biogeochemistry. 2005;73:3–27. [Google Scholar]
- 15.Subke J-A, Inglima I, Cotrufo MF. Trends and methodological impacts in soil CO2 efflux partitioning: A metaanalytical review. Global Change Biol. 2006;12:1–23. [Google Scholar]
- 16.Xu M, Qi Y. Spatial and seasonal variations of Q(10) determined by soil respiration measurements at a Sierra Nevadan forest. Global Biogeochem Cy. 2001;15:687–696. [Google Scholar]
- 17.Allison SD, Czimczik CI, Treseder KK. Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Global Change Biol. 2008;14:1156–1168. [Google Scholar]
- 18.Fierer N, Colman BP, Schimel JP, Jackson RB. Predicting the temperature dependence of microbial respiration in soil: A continental-scale analysis. Global Biogeochem Cy. 2006;20:GB3026. [Google Scholar]
- 19.Kutsch WL, Persson T, Schrumpf M, Moyano FE, Mund M, et al. Heterotrophic soil respiration and soil carbon dynamics in the deciduous Hainich forest obtained by three approaches. Biogeochemistry. 2010;100:167–183. [Google Scholar]
- 20.Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, et al. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature. 2001;411:789–792. doi: 10.1038/35081058. [DOI] [PubMed] [Google Scholar]
- 21.Wan S, Luo Y. Substrate regulation of soil respiration in a tallgrass prairie: Results of a clipping and shading experiment. Global Biogeochem Cy. 2003;17:1054. [Google Scholar]
- 22.Misson L, Gershenson A, Tang J, McKay M, Cheng W, et al. Influences of canopyphotosynthesis and summer rain pulses on root dynamics and soil respiration ina young ponderosa pine forest. Tree Physiol. 2006;26:833–844. doi: 10.1093/treephys/26.7.833. [DOI] [PubMed] [Google Scholar]
- 23.Chen Q, Wang Q, Han X, Wan S, Li L. Temporal and spatial variability and controls of soil respiration in a temperate steppe in northern China. Global Biogeochem Cy. 2010;24:1–10. [Google Scholar]
- 24.Wan S, Norby RJ, Ledford J, Weltzin JF. Responses of soil respiration to elevated CO2, air warming, and changing soil water availability in a model old-field grassland. Global Change Biol. 2007;13:2411–2424. [Google Scholar]
- 25.Bond-Lamberty B, Thomson A. A global database of soil respiration data. Biogeosciences. 2010;7:1915–1926. [Google Scholar]
- 26.Savage K, Davidson EA, Richardson AD. A conceptual and practical approach to data quality and analysis procedures for high-frequency soil respiration measurements. Funct Ecol. 2008;22:1000–1007. [Google Scholar]
- 27.Craine JM, Tilman D, Wedin D, Reich P, Tjoelkker M, et al. Functional traits, productivity and effects on nitrogen cycling of 33 grassland species. Funct Ecol. 2002;16:563–574. [Google Scholar]
- 28.Hibbard KA, Law BE, Reichstein M, Sulzman J. An analysis of soil respiration across northern hemisphere temperate ecosystems. Biogeochemistry. 2005;73:29–70. [Google Scholar]
- 29.Jenny H. Factors of soil formation. New York: McGraw-Hill; 1941. 229 [Google Scholar]
- 30.McCulley RL, Burke IC, Nelson JA, Lauenroth WK, Knapp AK, et al. Regional patterns in carbon cycling across the Great Plains of North America. Ecosystems. 2005;8:106–121. [Google Scholar]
- 31.Zhang XZ, Shi PL, Liu YF, Ouyang H. Experimental study on soil CO2 emission in the alpine grassland ecosystem on Tibetan Plateau. Sci China Ser D. 2005;48:218–224. [Google Scholar]
- 32.Cao G, Tang Y, Mo W, Wang Y, Li Y, et al. Grazing intensity alters soil respiration in an alpine meadow on the Tibetan plateau. Soil Biol Biochem. 2004;36:237–243. [Google Scholar]
- 33.Li G, Sun S. Plant clipping may cause overestimation of soil respiration in a Tibetan alpine meadow, Southwest China. Ecol Res. 2011;26:497–504. [Google Scholar]
- 34.Lin X, Zhang Z, Wang S, Hu Y, Xu G, et al. Response of ecosystem respiration to warming and grazing during the growing seasons in the alpine meadow on the Tibetan Plateau. Agric For Meteor. 2011;151:792–802. [Google Scholar]
- 35.Wang JF, Wang GX, Hu HC, Wu QB. The influence of degradation of the swamp and alpine meadows on CH4 and CO2 fluxes on the Qinghai-Tibetan Plateau. Environ Earth Sci. 2010;60:537–548. [Google Scholar]
- 36.Shi PL, Zhang XZ, Zhong ZM, Ouyang H. Diurnal and seasonal variability of soil CO2 efflux in a cropland ecosystem on the Tibetan Plateau. Agric For Meteor. 2006;137:220–233. [Google Scholar]
- 37.Hou XY. Vegetation map of the People's Republic of China (1:4M) Beijing: Chinese Map Publisher; 1982. [Google Scholar]
- 38.Baumann F, He J-S, Schmidt K, Kühn P, Scholten T. Pedogenesis, permafrost, soil temperature and soil moisture as controlling factors for soil nitrogen and carbon contents across the Qinghai-Tibetan Plateau. Global Change Biol. 2009;15:3001–3017. [Google Scholar]
- 39.Yang YH, Fang JY, Tang YH, Ji CJ, Zheng CY, et al. Storage, patterns and controls of soil organic carbon in the Tibetan grasslands. Global Change Biol. 2008;14:1592–1599. [Google Scholar]
- 40.Zimov SA, Schuur EAG, Chapin FS., III Permafrost and the global carbon budget. Science. 2006;312:1612–1613. doi: 10.1126/science.1128908. [DOI] [PubMed] [Google Scholar]
- 41.Cheng GD. Permafrost studies in the Qinghai-Tibet Plateau for road construction. J Cold Reg Eng. 2005;19:19–29. [Google Scholar]
- 42.Nan ZT, Li SX, Cheng GD. Prediction of permafrost distribution on the Qinghai-Tibet Plateau in the next 50 and 100 years. Sci China Ser D. 2005;48:797–804. [Google Scholar]
- 43.Wu S, Yin Y, Zheng D, Yang Q. Climate change in the Tibetan Plateau during the last three decades. Acta Geogr Sin. 2005;60:3–11. [Google Scholar]
- 44.Zhao L, Ping CL, Yang DQ, Cheng GD, Ding YJ, et al. Changes of climate and seasonally frozen ground over the past 30 years in Qinghai-Xizang (Tibetan) Plateau, China. Global Planet Change. 2004;43:19–31. [Google Scholar]
- 45.Böhner J, Lehmkuhl F. Environmental change modeling for Central and High Asia:Pleistocene, present and future scenarios. Boreas. 2005;34:220–231. [Google Scholar]
- 46.Cheng GD, Wu TH. Responses of permafrost to climate change and their environmental significance, Qinghai-Tibet Plateau. J Geophys Res Earth Surface. 2007;112 [Google Scholar]
- 47.Yang YH, Fang JY, Ji CJ, Han WX. Above- and belowground biomass allocation in Tibetan grasslands. J Veg Sci. 2009;20:177–184. [Google Scholar]
- 48.He JS, Wang ZH, Wang XP, Schmid B, Zuo WY, et al. A test of the generality of leaf trait relationships on the Tibetan Plateau. New Phytol. 2006;170:835–848. doi: 10.1111/j.1469-8137.2006.01704.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Wang JT, Chen W, Li B, Zhao K. Vegetation of Xizang (Tibet) Beijing: Science Press; 1988. 589 [Google Scholar]
- 50.Wang JT. The steppes and deserts of the Xizang Plateau (Tibet). Plant Ecol. 1988;75:135–142. [Google Scholar]
- 51.Fang JY, Piao SL, Tang ZY, Peng CH, Ji W. Interannual variability in net primary production and precipitation. Science. 2001;293:1723. doi: 10.1126/science.293.5536.1723a. [DOI] [PubMed] [Google Scholar]
- 52.He JS, Wang X, Flynn DFB, Wang L, Schmid B, et al. Taxonomic, phylogenetic, and environmental trade-offs between leaf productivity and persistence. Ecology. 2009;90:2779–2791. doi: 10.1890/08-1126.1. [DOI] [PubMed] [Google Scholar]
- 53.Breiman L, Friedman J, Olshen R, Stone C. Classification and regression trees. Belmont: Wadsworth International Group; 1984. 358 [Google Scholar]
- 54.SASInstitute. SAS/STAT User's guide, Version 8.01 (On-line Docs) Cary, NC: SAS Institute; 1999. [Google Scholar]
- 55.De'ath G, Fabricius KE. Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology. 2000;81:3178–3192. [Google Scholar]
- 56.Grace JB. Structural equation modeling and natural systems. Cambridge: Cambridge University Press; 2006. 365 [Google Scholar]
- 57.Grace JB, Pugesek BH. A structural equation model of plant species richness and its application to a coastal wetland. Am Nat. 1977;149:436–460. [Google Scholar]
- 58.Shipley B. Cause and correlation in biology: A user's guide to path analysis, structural equations and causal inference. Cambridge: Cambridge University Press; 2002. 317 [Google Scholar]
- 59.Grace JB, Keeley JE. A structural equation model analysis of post-fire plant diversity in California shrublands. Ecol Appl. 2006;16:503–514. doi: 10.1890/1051-0761(2006)016[0503:asemao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 60.Lamb EG. Direct and indirect control of grassland community structure by litter, resources, and biomass. Ecology. 2008;89:216–225. doi: 10.1890/07-0393.1. [DOI] [PubMed] [Google Scholar]
- 61.Shipley B, Lechowicz MJ, Wright IJ, Reich PB. Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology. 2006;87:535–541. doi: 10.1890/05-1051. [DOI] [PubMed] [Google Scholar]
- 62.Bentler PM. EQS 6 Structural equations program manual. Encino, CA: Multivariate Software, Inc; 2006. [Google Scholar]
- 63.Ripley EA. Grassland climate. In: Coupland RT, editor. Natural grasslands: Introduction and western hemisphere. Amsterdam: Elsevier; 1992. pp. 151–182. [Google Scholar]
- 64.Wu ZY. Vegetation of China. Beijing: Science Press; 1980. 1382 [Google Scholar]
- 65.Dong YS, Qi YC, Liu JY, Geng YB, Domroes M, et al. Variation characteristics of soil respiration fluxes in four types of grassland communities under different precipitation intensity. Chin Sci Bull. 2005;50:583–591. [Google Scholar]
- 66.Li LH, Wang QB, Bai YF, Zhou GS, Xing XR. Soil respiration of a Leymus chinensis grassland stand in the Xilin River Basin as affected by over-grazing and climate. Acta Phytoecol Sin. 2000;24:680–686. [Google Scholar]
- 67.Qi Y, Dong Y, Domroes M, Geng Y, Liu L, et al. Comparison of CO2 effluxes and their driving factors between two temperate steppes in Inner Mongolia, China. Adv Atmos Sci. 2006;23:726–736. [Google Scholar]
- 68.Wang G, Du R, Kong Q, Lu D. Experimental study on soil respiration of temperate grassland in China. Chin Sci Bull. 2004;49:642–646. [Google Scholar]
- 69.Yan L, Chen S, Huang J, Lin G. Differential responses of auto- and heterotrophic soil respiration to water and nitrogen addition in a semiarid temperate steppe. Global Change Biol. 2010;16:2345–2357. [Google Scholar]
- 70.Dennis JG. Distribution patterns of belowground standing crop in arctic tundra at Barrow, Alaska. Arct Alp Res. 1977;9:113–127. [Google Scholar]
- 71.Billings WD, Luken JO, Mortensen DA, Peterson KM. Arctic tundra: A source or sink for atmospheric carbon dioxide in a changing climate? Oecologia. 1982;53:7–11. doi: 10.1007/BF00377129. [DOI] [PubMed] [Google Scholar]
- 72.Nobrega S, Grogan P. Landscape and ecosystem-level controls on net carbon dioxide exchange along a natural moisture gradient in Canadian low arctic tundra. Ecosystems. 2008;11:377–396. [Google Scholar]
- 73.Mahecha MD, Reichstein M, Carvalhais N, Lasslop G, Lange H, et al. Global convergence in the temperature sensitivity of respiration at ecosystem level. Science. 2010;329:838–840. doi: 10.1126/science.1189587. [DOI] [PubMed] [Google Scholar]
- 74.Dornbush ME, Raich JW. Soil temperature, not aboveground plant productivity, best predicts intra-annual variations of soil respiration in central Iowa grasslands. Ecosystems. 2006;9:909–920. [Google Scholar]
- 75.Raich JW, Potter CS, Bhagawati D. Interannual variability in global soil respiration, 1980–94. Global Change Biol. 2002;8:800–812. [Google Scholar]
- 76.Burke IC, Lauenroth WK, Parton WJ. Regional and temporal variation in net primary production and nitrogen mineralization in grasslands. Ecology. 1997;78:1330–1340. [Google Scholar]
- 77.Davidson EA, Savage K, Verchot LV, Navarro R. Minimizing artifacts and biases in chamber-based measurements of soil respiration. Agric For Meteor. 2002;113:21–37. [Google Scholar]
- 78.Norman JM, Kucharik CJ, Gower ST, Baldocchi DD, Crill PM, et al. A comparison of six methods for measuring soil-surface carbon dioxide fluxes. J Geophys Res. 1997;102:28771–28778. [Google Scholar]
- 79.Zhou X, Wan S, Luo Y. Source components and interannual variability of soil CO2 efflux under experimental warming and clipping in a grassland ecosystem. Global Change Biol. 2007;13:761–775. [Google Scholar]
- 80.Editorial Board of Vegetation Map of China. Vegetation Atlas of China (1∶1,000,000) Beijing: Science Press; 2001. 260 [Google Scholar]