Abstract
Our two closest living primate relatives, chimpanzees (Pan troglodytes) and bonobos (Pan paniscus), exhibit significant behavioral differences despite belonging to the same genus and sharing a very recent common ancestor. Differences have been reported in multiple aspects of social behavior, including aggression, sex, play and cooperation. However, the neurobiological basis of these differences has only been minimally investigated and remains uncertain. Here, we present the first ever comparison of chimpanzee and bonobo brains using diffusion tensor imaging, supplemented with a voxel-wise analysis of T1-weighted images to specifically compare neural circuitry implicated in social cognition. We find that bonobos have more gray matter in brain regions involved in perceiving distress in both oneself and others, including the right dorsal amygdala and right anterior insula. Bonobos also have a larger pathway linking the amygdala with the ventral anterior cingulate cortex, a pathway implicated in both top–down control of aggressive impulses as well as bottom–up biases against harming others. We suggest that this neural system not only supports increased empathic sensitivity in bonobos, but also behaviors like sex and play that serve to dissipate tension, thereby limiting distress and anxiety to levels conducive with prosocial behavior.
Keywords: chimpanzee, bonobo, brain, social cognition
INTRODUCTION
Chimpanzees (Pan troglodytes) and bonobos (Pan paniscus) are our closest living primate relatives, having diverged from a common ancestor with humans ∼6 million years ago (Goodman et al., 1990), and from each other just 1–2 million years ago (Becquet et al., 2007; Hey, 2010). Despite their close phylogenetic relationship, chimpanzees and bonobos exhibit noteworthy differences in temperament and behavior, with bonobos exhibiting less severe aggression (Goodall, 1986; Kano, 1992; Wrangham and Peterson, 1996; Wrangham, 1999; Parish and de Waal, 2000), more adult play (Palagi, 2006), greater variety and frequency of sexual behaviors (de Waal, 1987, 1995; Parish, 1996), greater stress reactivity (Wobber et al., 2010b), greater social tolerance (Hare et al., 2007; Hare and Kwetuenda, 2010) and behaviors that may be indicative of greater empathy (de Waal, 1997) compared with chimpanzees.
The neurobiological bases of these species differences in behavior have been only minimally investigated, largely due to the very limited availability of bonobo brains. A comparative in vivo MRI study found no significant differences in brain size between the two species (n = 4 bonobos and n = 6 chimpanzees) after controlling for body size (Rilling and Insel, 1999). A later study based on a sub-set of these MRI scans showed that the dorsal sector of the frontal lobe, as contrasted with the orbital and mesial sectors, occupies a larger proportion of the frontal lobe in chimpanzees than in bonobos (Schenker et al., 2005). Another study used the same MRI scans from living bonobos, supplemented with additional post-mortem scans, to compare the volume of seven brain regions in chimpanzees and bonobos. Chimpanzees were found to have a larger cerebellum than bonobos after adjusting for brain size, and bonobos had greater leftward asymmetries in the striatum and motor hand area compared with chimpanzees (Hopkins et al., 2009). Finally, a histological study compared the volume of the amygdaloid complex, including the basolateral division specifically, in a sample of great apes that included three chimpanzees and two bonobos. Although the sample size was too small to conclusively demonstrate significant species differences, there was a suggestion that bonobos had a larger lateral nucleus after correcting for brain size (Barger et al., 2007).
Here, for the first time, we examine interspecies differences using two approaches that have the potential to reveal detailed brain-wide differences both in gray matter regions, and in the white matter circuitry that connects these regions. First, we use voxel-based morphometry (VBM) to show differences between local gray matter architecture in the two species. Second, we use MR diffusion imaging and tractography to show differences in white matter pathways. By focusing on brain regions that show inter-species differences both in local gray matter architecture and in associated white matter pathways, we are able to highlight evolutionary changes in neural circuitry at the network level.
In light of the above-specified differences in social behavior, we hypothesized that bonobos would have increased gray matter as well as increased white matter connectivity in brain regions implicated in empathy, emotion regulation, sexual behavior and anxiety. These regions include the anterior insula, amygdala, hypothalamus, and orbitofrontal cortex.
METHODS
Subjects
This study included neuroimaging data from a total of six bonobos and seven chimpanzees. In vivo, T1-weighted scans were obtained from three bonobos and four chimpanzees. In addition, diffusion-weighted scans were acquired from an independent sample of three bonobo and three chimpanzee cadaver brains. No animals were sacrificed for the purposes of this study, and all procedures were approved by the Emory University Animal Care and Use Committee. All cadaver brains were immersion fixed in formalin. Post-mortem intervals are listed for those specimens that they are known for in Supplementary Table S1. Demographic characteristics for both samples are provided in Table 1. All animals were at least 8 years of age, and adult brain weight is achieved by 7 years in chimpanzees (Herndon et al., 1999).
Table 1.
Sample demographics
| Species | Specimen type | Name | Sex | Age (years) | Brain volume (mm3) | Body weight (g) |
|---|---|---|---|---|---|---|
| Bonobo | In vivo | Jill | F | 11.5 | 374 338 | 36 700.00 |
| Bonobo | In vivo | Brian | M | 8 | 342 148 | 35 000.00 |
| Bonobo | In vivo | Lorel | M | 28 | 356 332 | 41 500.00 |
| Chimpanzee | In vivo | Laz | M | 20 | 415 924 | 59 500.00 |
| Chimpanzee | In vivo | Kengee | F | 8 | 390 058 | 43 000.00 |
| Chimpanzee | In vivo | Merv | M | 21 | 428 801 | 62 500.00 |
| Chimpanzee | In vivo | Mary | F | 36 | 357 827 | 44 000.00 |
| Bonobo | Cadaver | Mambo | M | 15 | 407 862 | |
| Bonobo | Cadaver | Psuke | M | 27 | 352 108 | |
| Bonobo | Cadaver | Tamuli | F | 13 | 318 844 | |
| Chimpanzee | Cadaver | Polyanna | F | 14 | 397 504 | |
| Chimpanzee | Cadaver | Jenda | F | 48 | 354 375 | |
| Chimpanzee | Cadaver | Artifee | F | 28 | 346 373 |
Image acquisition
In vivo scans.
As described previously (Rilling and Insel, 1999; Rilling and Seligman, 2002), prior to scanning, animals were anesthetized with Ketamine (10 mg/kg) and then weighed. For ketamine injections, animals were placed in a squeeze cage. Animals were encouraged to voluntarily present a hip for i.m. injection, but in some cases it was necessary to use the squeeze mechanism to restrain the animals for injection. Subjects were scanned in a supine position using the human head coil. Throughout the scan, animals received a continuous IV infusion of propofol (10–20 mg/kg/h) for anesthesia. T1-weighted images of the entire brain were acquired with a 1.5 T Phillips NT scanner (Philips Medical Systems, The Netherlands) using a gradient-echo protocol with the following parameters: slice thickness = 1.2 mm, slice interval = 0.6 mm, repetition time (TR) = 19.0 ms, echo time (TE) = 8.5 ms, number of signals averaged = 8, matrix = 256 × 256, field of view (FOV) = 180 mm. In one chimpanzee, the field of view was increased to 200 mm to accommodate the entire brain, and slice thickness and interval were adjusted to 1.4 and 0.7 mm, respectively.
Post-mortem scans.
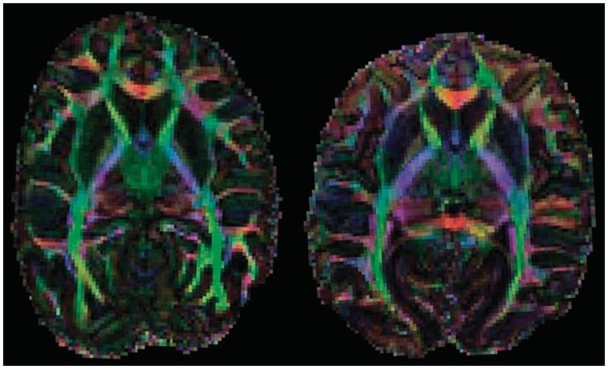
Brains were removed from the formalin solution that they were stored in, rinsed with phosphate buffer, patted dry with paper towels and then sealed in a thin-walled plastic bag for scanning. Diffusion-weighted scans were acquired on a 4.7 T Bruker scanner with a spin-echo sequence at 1.5 mm isotropic resolution with a b value of 4500 s mm−2 and 60 diffusion directions (Figure 1). We acquired two sets of diffusion-weighted data for subsequent averaging as well as five volumes with no diffusion weighting. Scan duration was 24 h.
Fig. 1.
Horizontal section through FA color map in one chimpanzee (left) and one bonobo (right).
Image analysis
In vivo scans.
Brain volumes were estimated from the brain masks generated with the brain extraction tool in FSL (Smith, 2002). A VBM style analysis (Ashburner and Friston, 2000; Good et al., 2001) was used to compare local gray matter volume estimates between chimpanzees and bonobos. This technique enables comparisons to be made across the entire brain, at every voxel (i.e. smallest volume element) of an image. Importantly, the technique controls for differences in brain size between the two comparison groups, thereby identifying differences in local gray matter volume estimates relative to whole brain size. T1 images were analyzed with FSL-VBM (Smith et al., 2004). The first step was to create a combined chimpanzee and bonobo gray matter template. So as not to bias the template toward one species, an equal number of chimpanzees and bonobos were included. Therefore, we began by brain extracting images from three of the four chimpanzees and from all three bonobos using BET (Smith, 2002). Next, tissue-type segmentation was carried out using FAST4 (Zhang et al., 2001). The resulting gray matter partial volume images were then aligned to geometric mid-space using the affine registration tool FLIRT (Jenkinson and Smith, 2001; Jenkinson et al., 2002). The weighted average of all aligned images then served as the target to which all original images were non-linearly aligned using FNIRT (Andersson et al., 2007a,b), which uses a b-spline representation of the registration warp field (Rueckert et al., 1999). The resulting weighted average constituted the template for the following analysis.
All of the native gray matter images (n = 3 bonobos and n = 4 chimpanzees) were then non-linearly registered to this template. The registered partial volume images were then modulated (to allow the measurement of local expansion or contraction) by dividing by the Jacobian of the warp field. The modulated, segmented images were then smoothed with an isotropic Gaussian kernel with a sigma of 2 mm. Finally, local gray matter volume estimates were compared between chimpanzees and bonobos using a voxel-wise two sample t-test. The resulting t-statistic image was thresholded at t = 4.0 which is equivalent to a two-tailed P-value of 0.01 with five degrees of freedom. Multiple comparison correction was not implemented due to limited statistical power associated with the small sample size and the accompanying risk of type 2 errors. However, as discussed above, by focusing on regions that show differences both in gray matter volume and, independently, in related white matter anatomy, we are able to further limit the chances of type I errors.
Post-mortem scans
Diffusion-weighted images were analyzed using the diffusion toolbox in the FSL software package (Smith et al., 2004). Each diffusion-weighted volume was affine-registered to a B0 volume to correct for eddy-current effects before averaging the two acquisitions, and DTIFIT was used to calculate the diffusion tensor at each voxel, from which a fractional anisotropy (FA) image was calculated. A bias-free FA template was created using affine followed by nonlinear registration as described above. Individual FA images (n = 3 bonobos and n = 3 chimpanzees) were then non-linearly registered to the template (Andersson et al., 2007a,b). Two methods were then used to compare FA between species. To compare the peak anisotropy along white matter tracts, we used tract-based spatial statistics [TBSS, (Smith et al., 2006)]. To compare the volume of local white matter pathways, we compared the Jacobians of the non-linear warp fields derived from the FA images. These Jacobians reflect the extent to which each template voxel must be either expanded or compressed to match the individual image. Both Jacobian values and FA values were compared between chimpanzees and bonobos using a two sample t-test. In parallel with the VBM analysis, the resulting t-statistic image was thresholded at t = 4.0. Like the VBM analysis described above, comparisons are made across the entire brain, at each brain voxel.
Tractography.
Tractography methods can reconstruct white matter fiber tracts based on magnitude and direction of anisotropic water diffusion in each voxel of a diffusion-weighted image. The probabilistic tractography algorithm in FSL, with adaptation to a multi-fibre model, was used for pathway reconstruction (Behrens et al., 2007). The Jacobian comparison of FA images described above yielded a difference in the bilateral ventral posterior frontal lobe. This region of interest (ROI) was warped from template space into individual subject space and then used as a seed mask for tractography. The ROI extended into gray matter. Therefore, an exclusion mask was placed in the gray matter ventral to the ROI extending back to the amygdala in order to restrict the pathway to white matter. For each subject, the total number of streamlines sent from their mask (i.e. 5000 times the number of voxels in the mask) was calculated. The average of these six values was multiplied by 0.001 and this threshold was applied to the pathway identified in all six subjects. The resulting pathway volumes were compared between chimpanzees and bonobos using a two sample t-test. To visualize group results, pathways were also thresholded more liberally at 0.0005 times the total sample number and warped back into template space. Individual pathways were then binarized and summed to create an overlap image that was then thresholded to show only those voxels where at least three of the six subjects showed evidence of a potential connection.
Finally, affine registration was used to warp tractography results into T1 space so that pathway terminations could be compared with regions where local gray matter volume estimates differed between species.
RESULTS
Overall brain size
Brain volumes obtained from the T1 scans indicated that the average chimpanzee brain volume (398 cc, n = 4) was slightly but significantly larger than the average bonobo brain volume (358 cc, n = 3; P < 0.05) (Table 1). These volume estimates are somewhat larger than other published values (Stephan et al., 1981; Herndon et al., 1999; Rilling and Insel, 1999), in part because they include some meninges and space between sulci at the brain periphery.
VBM results
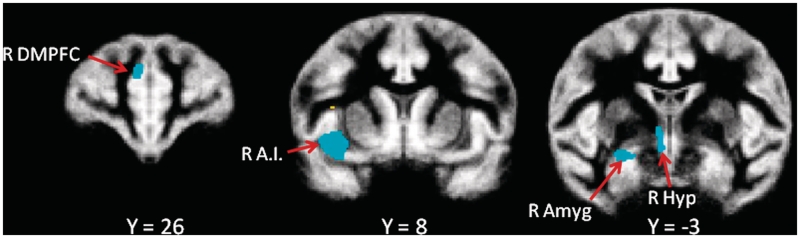
VBM results show that after controlling for differences in overall brain size, bonobos had significantly higher local gray matter volume estimates than chimpanzees in limbic and paralimbic areas, and in portions of frontal and parietal cortex. On the other hand, chimpanzees had higher local gray matter volume estimates than bonobos in portions of parietal and occipital cortex (Table 2). More specifically, bonobos had higher local gray matter volume estimates in several areas of particular relevance to social cognition, and these were among the largest differences overall. These areas included the right anterior insula, right dorsal amygdala, right hypothalamus and right dorsomedial prefrontal cortex (Figure 2 and Supplementary Figure S1).
Table 2.
Brain regions showing species differences in local gray matter volume estimates at a threshold of t >4.0 (P < 0.01 uncorrected for multiple comparisons)
| Brain region | No. of voxels |
|---|---|
| Bonobo > chimpanzee | |
| L dlpfc (area 46) | 86 |
| R dmpfc | 64 |
| R dorsal premotor cortex | 49 |
| R ventral premotor cortex (45) | 45 |
| R anterior insula | 943 |
| Hypothalamus and adjacent basal forebrain | 215 |
| R dorsal amygdala | 174 |
| R insula | 20 |
| L temporal pole | 28 |
| L mid insula/claustrum | 19 |
| R amygdalo-hippocampal region | 49 |
| L post-central sulcus/anterior intraparietal sulcus? | 129 |
| L anterior STG | 181 |
| L entorhinal cortex | 25 |
| R intraparietal sulcus | 346 |
| R pontine nuclei | 117 |
| R inferior posterior parietal cortex | 25 |
| L inferotemporal cortex (IT) | 30 |
| Bilateral cerebellum | 59 |
| L posterior cingulate sulcus | 88 |
| Anterior parieto-occiptal sulcus | 90 |
| R calcarine sulcus | 33 |
| Left visual cortex | 148 |
| Left dorsal visual cortex | 117 |
| Chimpanzee > bonobo | |
| R frontoorbital sulcus (44/45) | 10 |
| R Dorsal insula | 10 |
| L frontal operculum | 47 |
| R anterior STG | 57 |
| R dorsal STS | 45 |
| R intraparietal sulcus/post-central sulcus | 23 |
| L tectum | 18 |
| Retrosplenial cortex | 23 |
| L temporo-parietal junction (TPJ) | 54 |
| R calcarine sulcus | 92 |
| L posterior intraparietal sulcus (IPS) | 159 |
| R extrastriate visual cortex | 581 |
| R fusiform gyrus | 99 |
| R calcarine sulcus (V1) | 77 |
| L calcarine sulcus (V1) | 1410 |
| R dorsomedial extrastriate | 130 |
| L lunate sulcus | 137 |
| R occipital pole | 161 |
| L occipital pole | 91 |
Spatial extent of the regions is given in number of 0.8 mm isotropic voxels.
Fig. 2.
Differences in local gray matter volume estimates between chimpanzees and bonobos based on analysis of T1-weighted images. The t-statistic image is thresholded at t > 4.0 and overlaid on the common species gray matter template. The figure shows three coronal sections through the gray matter template at different anterior–posterior levels. Regions in blue have higher local gray matter volume estimates in bonobos; regions in yellow have higher local gray matter volume estimates in chimpanzees. The Y-coordinate is relative to Y = 0 at the anterior commissure.
FA analysis
FA images were analyzed using two complementary techniques—TBSS analysis of FA, and FA-based warp field comparisons. It is important to note that these two techniques measure different aspects of white matter tracts. TBSS was developed specifically to measure changes in local diffusion anisotropy in a way that was robust to changes in local geometries such as tract thicknesses. By comparison, Jacobian comparisons explicitly measure the local volumetric change in tract geometry.
FA TBSS analysis
TBSS identified several regions where chimpanzees had higher FA values than bonobos (Table 3, Supplementary Figure S2). These were concentrated along visual system pathways, including the right optic tract, splenium of the corpus callosum and left parieto-occipital white matter. Chimpanzees also had higher FA in the left cerebellar white matter. There were no regions where bonobos had significantly higher FA than chimpanzees.
Table 3.
Brain regions showing species differences in white matter fractional anisotropy (FA) as determined by tract-based spatial statistics (TBSS) at a threshold of t > 4.0 with at least 10 contiguous voxels
| Brain region | No. of voxels |
|---|---|
| Bonobo >chimpanzee | |
| None | |
| Chimpanzee > bonobo | |
| R genu | 10 |
| R inferior frontal white matter | 17 |
| R genu | 10 |
| R optic tract | 14 |
| R cerebral peduncle | 10 |
| Splenium of corpus callosum | 13 |
| L lateral splenium | 10 |
| L inferior parietal white matter | 14 |
| L parietal/occipital white matter | 37 |
| R parietal/occipital white matter | 13 |
| L cerebellar white matter | 50 |
Spatial extent of the regions is given in number of 1.0 mm isotropic voxels.
FA Jacobian comparison
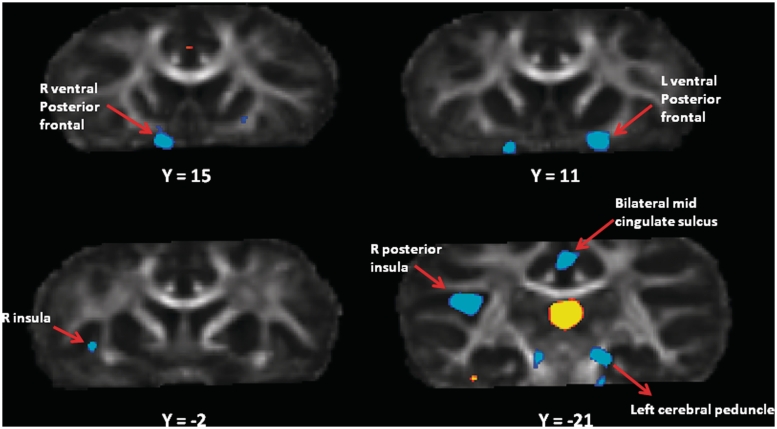
Comparison of the warpfields that registered each subject's FA image to the FA template revealed several regions of relative expansion in bonobos compared with chimpanzees (Table 4, Supplementary Figure S3). It is important to note that the contrast that drives the Jacobian estimation comes from FA, which highlights white matter. Changes in the local Jacobian therefore reflect changes in local white matter volumes, even if they appear at the boundary between gray and white matter on the average template. Of particular relevance to social cognitive differences between the two species is expansion in bilateral ventral frontal lobe in bonobos (Figure 3), as well as in the right anterior insula. On the other hand, compared with bonobos, chimpanzees showed pronounced relative enlargement of the white matter in the vicinity of the medial thalamus (Figure 3). Chimpanzees also showed expansion relative to bonobos in primary motor cortex, the ventral temporal lobe, portions of the parietal and occipital lobes and in cerebellar white matter (Supplementary Figure S3).
Table 4.
Brain regions in which the Jacobian warp field of the FA images differed between species at a threshold of t > 4.0 with at least 10 contiguous voxels
| Brain region | No. of voxels |
|---|---|
| Bonobo > chimp | |
| L inferior frontal gyrus | 118 |
| Genu of corpus callosum | 10 |
| L head of caudate | 32 |
| R lateral inferior frontal cortex | 81 |
| R ventral posterior frontal white matter | 828 |
| L medial orbital gyrus and white matter | 505 |
| R mid-insula | 236 |
| R anterior STS | 186 |
| R superior frontal gyrus | 21 |
| L ventral anterior thalamus | 231 |
| Bilateral mid cingulate sulcus | 975 |
| R dorsal posterior insula | 1441 |
| R cerebral peduncle | 532 |
| L cerebral peduncle | 1037 |
| L putamen | 35 |
| R posterior intraparietal sulcus | 94 |
| R inferior lateral extrastriate cortex | 2935 |
| L anterior bank of lunate (V2) | 158 |
| R visual cortex | 55 |
| Chimpanzee > bonobo | |
| R ventral rim of cingulate sulcus | 30 |
| R motor cortex (precentral gyrus) | 797 |
| MD and adjacent midline thalamic nuclei | 2657 |
| R medial temporal white matter | 29 |
| R ventrolateral thalamus | 69 |
| R perirhinal cortex | 455 |
| R hippocampus | 51 |
| L fusiform gyrus | 555 |
| R intraparietal sulcus | 22 |
| R posterior STS | 237 |
| R optic radiation | 301 |
| L optic radiation | 84 |
| L optic radiation | 124 |
| L optic radiation | 239 |
| L posterior bank of parieto-occipital sulcus (extrastriate visual cortex) | 162 |
| R superior cerebellar peduncle | 34 |
| R lateral cerebellum | 1165 |
| R lateral cerebellum | 50 |
| L lateral cerebellum | 843 |
| L lateral cerebellum | 84 |
| L dorsal extrastriate cortex | 31 |
| L dorsal extrastriate cortex | 162 |
| R dorsal extrastriate cortex | 18 |
| R ventral occipital cortex | 128 |
| L ventral occipital cortex | 52 |
Spatial extent of the regions is given in number of 0.8 mm isotropic voxels.
Fig. 3.
Differences in Jacobian warp fields between chimpanzees and bonobos based on analysis of diffusion-weighted images. The t-statistic image is thresholded at t > 4.0 and overlaid on the common species FA template. The figure shows four coronal sections through the FA template at different anterior–posterior levels. Regions in blue are larger in bonobos (larger Jacobians from template to subject space) and regions in yellow are larger in chimpanzees. The Y-coordinate is relative to Y = 0 at the anterior commissure.
Tractography results
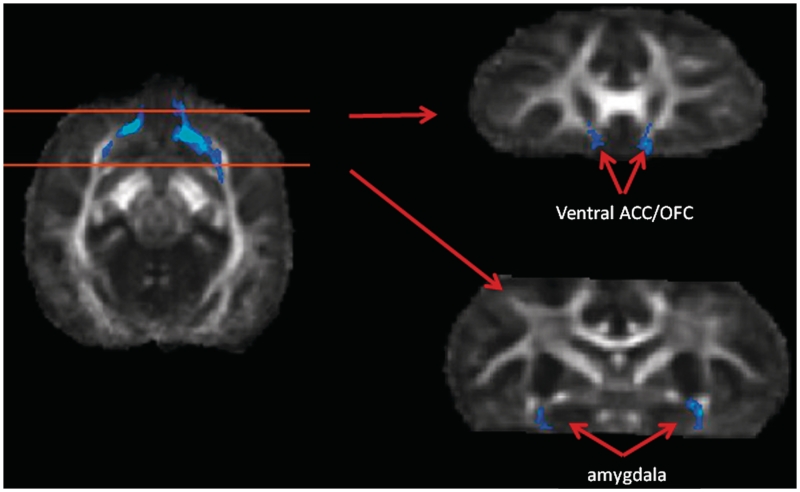
To determine whether the species difference in ventral posterior frontal lobe volume identified in the FA comparison was related to the VBM results, we used tractography to delineate the pathway passing through this ROI. The pathway was tracked in native diffusion space for each of the three individual chimpanzee brains and each of the three individual bonobo brains and then warped back into FA template space to visualize the group average trajectory. The resulting tract connected the ventral anterior cingulate cortex with the amygdala bilaterally (Figure 4 and Supplementary Figure S4). Pathway volumes calculated in native space revealed that all three bonobos had a larger pathway than all three chimpanzees in both the left [t(4) = 5.06, P < 0.01) and right [t(4) = 2.82, P < 0.05] hemisphere (Table 5). Warping the average pathway into T1 space revealed that it made contact with the region of expanded amygdala volume in bonobos (Supplementary Figure S5).
Fig. 4.
Group tractography result from posterior ventral frontal ROI derived from the jacobian comparison shown in Figure 3. Tracts from all three bonobos and all three chimpanzees were registered to template space, binarized and summed. The resulting map shows voxels where at least three of six subjects had a possible connection.
Table 5.
Amygdala to ventral ACC pathway volumes in bonobos and chimpanzees
| Species | Left volume (mm3) | Right volume (mm3) |
|---|---|---|
| Bonobo (Mambo) | 1676.31 | 1135.27 |
| Bonobo (Psuke) | 2010.43 | 1577.59 |
| Bonobo (Tamuli) | 2025.62 | 1980.17 |
| Chimpanzee (Artifee) | 1104.92 | 1080.23 |
| Chimpanzee (Jenda) | 749.87 | 364.50 |
| Chimpanzee (Polyanna) | 429.08 | 358.82 |
DISCUSSION
VBM analyses of T1 scans revealed that bonobos have more gray matter than chimpanzees in the hypothalamus, the right dorsal amygdala and the right anterior insula. DTI analyses additionally showed that the pathway connecting the amygdala with the ventral anterior cingulate cortex is larger in bonobos than chimpanzees. These species differences in brain anatomy can be plausibly linked with known species differences in temperament and behavior.
Anxiety and stress reactivity
Bonobos exhibit a stronger stress hormone response to feeding competition (Wobber et al., 2010b), and have been described as more nervous than chimpanzees (de Waal, 1997). The larger right amygdala, hypothalamus and right anterior insula of bonobos are a potential neural substrate for this difference in stress and anxiety. The amygdala difference is concentrated in its dorsal aspect, in the vicinity of the central nucleus (Amunts et al., 2005; Barger et al., 2007), a region strongly implicated in fear and anxiety that is known to activate the hypothalamic–pituitary–adrenal (HPA) axis and autonomic nervous system by way of connections with the hypothalamus (Davis, 1997; Ledoux, 1998). Interestingly, this nucleus is larger in human patients with generalized anxiety disorder compared with healthy controls (Etkin et al., 2009). Human subjects who are prone to anxiety have increased activity in the anterior insula during emotion processing (Paulus and Stein, 2006). The anterior insula also tracks risk (Bossaerts, 2010) and predicts risk-averse choices (Kuhnen and Knutson, 2005), and bonobos are more risk-averse than chimpanzees (Heilbronner et al., 2008).
Sexual behavior
Compared with chimpanzees, bonobos have higher rates of sexual interactions in captivity (de Waal, 1995) but not in the wild (Stanford, 1998; Hashimoto, 2002). In both contexts, bonobos exhibit a greater diversity of sexual behaviors, including all possible age–sex combinations (de Waal, 1987; Parish and de Waal, 2000). The hypothalamus and amygdala are key nodes of a network that controls sexual behavior in non-human animals (Newman, 1999; Breedlove et al., 2007). Functional neuroimaging experiments have similarly implicated the hypothalamus and amygdala in the human response to visual sexual stimuli (Karama et al., 2002; Hamann et al., 2004). Given that bonobos use sex to reduce tension (de Waal, 1987; Parish, 1996; Hare et al., 2007; Hohmann et al., 2008), the enlarged bonobo amygdala could be eliciting fear and anxiety, while simultaneously motivating sexual behavior that helps dissipate it.
Play
Bonobos are thought to have retained certain juvenile characteristics into adulthood (Shea, 1983; Wrangham and Pilbeam, 2001; Lieberman et al., 2007) (i.e. paedomorphism), including extraordinary playfulness (de Waal, 1997). In addition to sex, bonobos use play to diffuse social tension (Enomoto, 1990) and rates of play are higher among adult bonobos than adult chimpanzees (Palagi, 2006). After controlling for overall brain size and body size, both amygdala and hypothalamus size are correlated with frequency of social play across primate species (Lewis and Barton, 2006), so the larger amygdala and hypothalamus of bonobos could also relate to this difference in play frequency.
Empathy
Anecdotal observations have led to the suggestion that bonobos may be more empathic than chimpanzees (de Waal, 1997). Psychopathy, a disorder marked by lack of empathy, is associated with reduced size and function of the amygdala (Gordon et al., 2004; Yang et al., 2009), as well as reduced functional connectivity between the amygdala and ventromedial prefrontal cortex (VMPFC) (Marsh et al., 2008). These results and others have led to the hypothesis that the pathway from the amygdala to the VMPFC is involved in perceiving distress in others and in learning to avoid behaviors that provoke such distress (Blair, 2008). Our results show that bonobos have more gray matter in the dorsal amygdala and a larger pathway linking the amygdala with VMPFC compared with chimpanzees. There is evidence that testosterone impairs the functioning of this pathway in humans (van Wingen et al., 2010), and chimpanzee males have both higher baseline testosterone metabolite levels (Sannen et al., 2004), as well as a more pronounced increase in testosterone in response to feeding competition (Wobber et al., 2010b) compared with bonobo males. Thus, species differences in pathway size may be augmented by differences in testosterone-mediated functional suppression. There is also suggestive evidence, in the form of the ratio of the second to fourth finger length (2D : 4D), that chimpanzees may have higher prenatal androgen levels than bonobos (or humans) (McIntyre et al., 2009). Prenatal testosterone levels are inversely correlated with measures of empathy in human children (Chapman et al., 2006). Thus, organizational effects of prenatal androgens could contribute to species differences in the size of the pathway linking the amygdala and VMPFC, as well as other empathy-related structures.
The anterior insula is also strongly implicated in empathy, presumably through a mechanism that involves simulation of visceral somatic states (Singer et al., 2009), and disorders characterized by empathic deficits are associated with reduced size and function of the anterior insula (Bird et al., 2010) (Veit et al., 2002; Birbaumer et al., 2005; Sterzer et al., 2007; Seeley, 2010). Bonobos have more gray matter in the anterior insula than chimpanzees, specifically within the ventral region that is consistently associated with social–emotional processing (Allman et al., 2010; Kurth et al., 2010) and that reliably coactivates with the amygdala (Mutschler et al., 2009). Thus, the neurobiological characteristics that differentiate chimpanzees from bonobos bear a striking similarity to those that differentiate patients with empathy deficits from normal controls.
Social tolerance
Bonobos exhibit highly tolerant food sharing in the wild (Kuroda, 1984; White, 1994; Fruth and Hohmann, 2002) [but see (Jaeggi et al., 2010)], and bonobos showed greater tolerance of co-feeding than chimpanzees in direct experimental comparisons (Hare et al., 2007; Hare and Kwetuenda, 2010; Wobber et al., 2010a). Bonobos may therefore be better at inhibiting selfish behaviors than chimpanzees are (Hare et al., 2007). This may be supported by their larger pathway linking ventral ACC with the amygdala, a pathway that has been implicated in emotion regulation in humans (Davidson et al., 2000; Pezawas et al., 2005). This same pathway might also be involved in controlling aggressive impulses, another behavior that differs between these two species.
Aggression
Chimpanzees exhibit more severe aggression than bonobos. Within social groups, chimpanzee aggression involves male aggression against females, male infanticide and intense male competition for dominance status. In contrast, male aggression against females is rare in bonobos (perhaps due to strong bonds among females), male infanticide has not yet been observed and reconciliation following conflict is more common and more often initiated by the aggressor than among chimpanzees (Goodall, 1986; de Waal, 1987; Parish, 1996; Wrangham and Peterson, 1996; Parish and de Waal, 2000). Chimpanzees are strongly xenophobic. Between group interactions are typically hostile and can involve lethal aggression (Wrangham, 1999). In bonobos, intergroup encounters can be hostile, but in contrast to chimpanzees, also affiliative (Idani, 1990; Kano, 1992). Wrangham and Pilbeam (2001) suggests that bonobos evolved due to selection against aggression and that they share a number of traits with domesticated versions of wild animals that also show reduced aggression. For example, both bonobos and dogs are less aggressive, as well as more juvenilized, than chimpanzees and wolves, respectively.
Evidence suggests that the pathway linking the ventral ACC and the amygdala normally functions to restrain aggression via both top–down suppression of aggressive impulses from the amygdala (Davidson et al., 2000; Meyer-Lindenberg et al., 2006), and through a bottom–up relay of perceived distress in others to VMPFC that inhibits anti-social behavior (Blair, 2008). Thus, underdevelopment of this pathway, as found in chimpanzees, might be expected to augment both impulsive aggression due to lack of top–down control, as well as instrumental aggression due to lack of bottom–up biases against harming others. As mentioned above, this effect may be compounded by higher testosterone levels in male chimpanzees that inhibit functionality in this pathway, especially since testosterone is positively correlated with aggression in male chimpanzees (Muller and Wrangham, 2004).
Synthesis
Our results can be usefully interpreted within a recently proposed model of the neurobiology of human empathy and callousness (Blair, 2008; Shirtcliff et al., 2009). Callous individuals are characterized by hypoarousal of the stress system in response to their own distress. Because we normally empathize with others by simulating their distress (Singer and Lamm, 2009), callous individuals are also deficient in representing the distress of others. For example, criminal psychopaths are autonomically insensitive to the suffering and distress of others (Blair et al., 1997). These psychological deficits are paralleled by deficits in the structure and function of empathy-related areas including the amygdala and anterior insula, as well as the connections linking the amygdala to VMPFC. Further, deficits in empathy may contribute to increased aggression insofar as harming others will be less aversive to those with a reduced capacity to experience distress. In parallel, we suggest that the enlarged dorsal amygdala, anterior insula and amygdala to vACC pathway in bonobos allows them to more strongly represent distress of both self and others compared with chimpanzees. This translates into greater empathy, as well as reduced aggression. However, excessive distress and anxiety can interfere with helping behavior (Batson, 1998). In bonobos, this may be prevented in part by a stronger regulatory pathway from ventral ACC to amygdala and in part by increased rates of play, sexual behavior and reconciliation that serve to limit anxiety and distress to a level that supports prosocial behavior.
Other species differences
The above discussion is focused on species differences in the neural system involved in understanding others’ emotions. However, humans have an additional neural system for understanding others that involves making cognitive inferences about others’ thoughts. Both the medial prefrontal cortex and the temporo-parietal junction have been consistently implicated in this function (Gallagher and Frith, 2003; Saxe and Kanwisher, 2003). Thus, it is interesting that bonobos also have higher local gray matter volume estimates in the dorsomedial prefrontal cortex compared with chimpanzees. Although the question of whether chimpanzees possess a theory of mind is still debated (Byrne and Whiten, 1992; Tomasello et al., 2003; Penn and Povinelli, 2007), our results suggest that further investigation of bonobo mentalizing capacities may be of interest. Indeed, a recent experimental study concluded that bonobos were more skilled than chimpanzees at tasks related to theory of mind (Herrmann et al., 2010).
Our FA analysis revealed a large area of the medial thalamus that is larger in chimpanzees than bonobos. This difference is concentrated in the region of the medial dorsal thalamic nucleus and could reflect differences in the white matters axons that penetrate the medial dorsal nucleus, or alternatively differences in the adjacent white matter pathway—the fornix. Chimpanzees also showed expansion relative to bonobos in several visual cortical regions, including portions of both the ventral and dorsal visual streams, and chimpanzees showed higher FA values along visual system white matter pathways, which might imply greater myelination or fiber density in these pathways (Pierpaoli and Basser, 1996; Beaulieu, 2002; Le Bihan, 2003). Although the functional significance of these differences is uncertain, we note that chimpanzees outperform bonobos on tasks requiring the use of tools (Herrmann et al., 2010) and also more often use tools in the wild (Schaik et al., 1999; Hohmann and Fruth, 2003). Neural specializations may have evolved to support this ability, and human tool use relies heavily on the dorsal and ventral visual streams (Frey, 2007). Finally, in both the FA and T1 analyses, chimpanzees showed evidence of expansion in the right superior temporal sulcus relative to bonobos. This region is involved in the perception of biological motion and also in understanding intentions behind actions (Perret et al., 1985; Allison et al., 2000), raising the possibility that chimpanzees may excel at this aspect of social cognition relative to bonobos.
Limitations
Access to bonobo brains is limited due to their endangered status, and our sample size is accordingly limited. We therefore cannot exclude the possibility that some of our group differences are type 1 errors due to chance sampling. This concern is mitigated to some extent by the overlapping results from the independent FA and T1 data sets, particularly with respect to consistent differences in the right anterior insula and amygdala in favor of bonobos, and consistent differences in visual cortical areas in favor of chimpanzees. Moreover, some of our results converge with earlier results, such as the finding that chimpanzees have a larger cerebellum than bonobos (Hopkins et al., 2009) and bonobos have a larger lateral amygdala nucleus after controlling for brain size (Barger et al., 2007). Our group differences could also be explained by a confounding variable. For example, rearing history can impact brain development and subsequent anatomy (Sanchez et al., 1998). Although we do not have information on the rearing history of all of the animals included in this study, we do know that two of our bonobo subjects were mother-reared and two of our chimpanzee subjects were nursery-reared by humans (Schenker et al., 2005). Although we cannot exclude this as a potential explanation for our results, the nursery-reared chimpanzees were not raised in an impoverished social environment, but instead had frequent human contact along with the opportunity for daily play with other chimpanzees. Thus, we favor heritable species differences as the most likely factor driving these neurobiological differences. Finally, given evidence for developmental delay and paedomorphosis in bonobos, it would be informative to compare the two species at earlier developmental stages to determine how these differences in adult brain anatomy arise in ontogeny.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The authors thank Brain Hare, Scott Lilienfeld, Jennifer Mascaro, Frans de Waal and Patricia Whitten, as well as our two anonymous reviewers, for their many helpful comments on this article. The authors also thank William Hopkins for supplying two post-mortem bonobo brains for scanning.
This work was supported by the Emory University Research Committee, the Center for Behavioral Neuroscience, the Yerkes Base Grant (NIH RR-00165), and by a visiting research fellowship to JKR from Jesus College at Oxford University.
REFERENCES
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Sciences. 2000;4:267–78. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Allman JM, Tetreault NA, Hakeem AY, et al. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Structure and Function. 2010;214:495–517. doi: 10.1007/s00429-010-0254-0. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomical Embryology. 2005;210:343–52. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. 2007a. http://www.fmrib.ox.ac.uk/analysis/techrep/
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation, aka Spatial normalisation. 2007b. http://www.fmrib.ox.ac.uk/analysis/techrep/
- Ashburner J, Friston KJ. Voxel-based morphometry - the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Barger N, Stefanacci L, Semendeferi K. A comparative volumetric analysis of the amygdaloid complex and basolateral division in the human and ape brain. American Journal of Physical Anthropology. 2007;134:392–403. doi: 10.1002/ajpa.20684. [DOI] [PubMed] [Google Scholar]
- Batson CD. Altruism and prosocial behavior. In: Gilbert DT, Fiske ST, Lindzey G, editors. The Handbook of Social Psychology. Boston: McGraw-Hill; 1998. pp. 282–316. [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomedical. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Becquet C, Patterson N, Stone AC, Przeworski M, Reich D. Genetic structure of chimpanzee populations. PLoS Genetics. 2007;3:e66. doi: 10.1371/journal.pgen.0030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34:144–55. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, et al. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Archives in General Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, Singer T. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain. 2010;133:1515–25. doi: 10.1093/brain/awq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2008;363:2557–65. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Jones L, Clark F, Smith M. The psychopathic individual: a lack of responsiveness to distress cues? Psychophysiology. 1997;34:192–8. doi: 10.1111/j.1469-8986.1997.tb02131.x. [DOI] [PubMed] [Google Scholar]
- Bossaerts P. Risk and risk prediction error signals in anterior insula. Brain Structure and Function. 2010;214:645–53. doi: 10.1007/s00429-010-0253-1. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Rosenzweig MR, Watson NV. In: Biological Psychology: An Introduction to Behavioral, Cognitive and Clinical Neuroscience. 5th edn. Sunderland, MA: Sinaur Associates; 2007. Sex: evolutionary, hormonal, and neural bases; pp. 354–84. [Google Scholar]
- Byrne RW, Whiten A. Cognitive evolution in primates: evidence from tactical deception. Man. 1992;27:609–27. [Google Scholar]
- Chapman E, Baron-Cohen S, Auyeung B, Knickmeyer R, Taylor K, Hackett G. Fetal testosterone and empathy: evidence from the empathy quotient (EQ) and the "reading the mind in the eyes" test. Social Neurosciences. 2006;1:135–48. doi: 10.1080/17470910600992239. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation - A possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Davis M. Neurobiology of fear responses: the role of the amygdala. Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- de Waal FB. Tension regulation and nonreproductive functions of sex in captive bonobos Pan paniscus. National Geogrpahic Research. 1987;3:318–35. [Google Scholar]
- de Waal FB. Sex as an alternative to aggression in the bonobo. In: Abramson P, Pinkerton S, editors. Sexual Nature, Sexual Culture. Chicago: University of Chicago Press; 1995. pp. 37–56. [Google Scholar]
- de Waal FB. Bonobo: the Forgotten Ape. Berkeley: University of California Press; 1997. [Google Scholar]
- Enomoto T. Social play and sexual behavior of the bonobo (Pan paniscus) with special reference to flexibility. Primates. 1990;31:469–80. [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives in Genenal Psychiatry. 2009;66:1361–72. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- Frey SH. What puts the how in where? Tool use and the divided visual streams hypothesis. Cortex. 2007;43:368–75. doi: 10.1016/s0010-9452(08)70462-3. [DOI] [PubMed] [Google Scholar]
- Fruth B, Hohmann G. How bonobos handle hunts and harvests: why share food? In: Boesch C, Hohmann G, Marchant LF, editors. Behavioural Diversity in Chimpanzees and Bonobos. New York: Cambridge University Press; 2002. pp. 231–243. [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of “theory of mind”. Trends in Cognitive Sciences. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Cambridge, MA: Harvard University Press; 1986. [Google Scholar]
- Goodman M, Tagle DA, Fitch DH, et al. Primate evolution at the DNA level and a classification of hominoids. Journal of Molecular Evolution. 1990;30:260–6. doi: 10.1007/BF02099995. [DOI] [PubMed] [Google Scholar]
- Gordon HL, Baird AA, End A. Functional differences among those high and low on a trait measure of psychopathy. Biological Psychiatry. 2004;56:516–21. doi: 10.1016/j.biopsych.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nature Neuroscience. 2004;7:411–6. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- Hare B, Kwetuenda S. Bonobos voluntarily share their own food with others. Current Biology. 2010;20:R230–1. doi: 10.1016/j.cub.2009.12.038. [DOI] [PubMed] [Google Scholar]
- Hare B, Melis AP, Woods V, Hastings S, Wrangham R. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Current Biology. 2007;17:619–23. doi: 10.1016/j.cub.2007.02.040. [DOI] [PubMed] [Google Scholar]
- Hashimoto TFTaC. Why female bonobos have a lower copulation rate during estrus than chimpanzees. In: Boesch GH, Marchant L, editors. Behavioral Diversity of Chimpanzees and Bonobos. Cambridge: Cambridge University Press; 2002. pp. 156–67. [Google Scholar]
- Heilbronner SR, Rosati AG, Stevens JR, Hare B, Hauser MD. A fruit in the hand or two in the bush? Divergent risk preferences in chimpanzees and bonobos. Biological Letter. 2008;4:246–9. doi: 10.1098/rsbl.2008.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Tigges J, Anderson DC, Klumpp SA, McClure HM. Brain weight throughout the life span of the chimpanzee. Journal of Computer Neurology. 1999;409:567–72. [PubMed] [Google Scholar]
- Herrmann E, Hare B, Call J, Tomasello M. Differences in the cognitive skills of bonobos and chimpanzees. PLoS ONE. 2010;5:e12438. doi: 10.1371/journal.pone.0012438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hey J. The divergence of chimpanzee species and subspecies as revealed in multipopulation isolation-with-migration analyses. Molecular Biology Evolution. 2010;27:921–33. doi: 10.1093/molbev/msp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann G, Fruth B. Culture in bonobos? Between-species and within-secies variation in behavior. Current Anthropology. 2003;44:563–71. [Google Scholar]
- Hohmann G, Mundry R, Deschner T. The relationship between socio-sexual behavior and salivary cortisol in bonobos: Tests of the tension regulation hypothesis. American Journal of Primatology. 2008;71:223–32. doi: 10.1002/ajp.20640. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Lyn H, Cantalupo C. Volumetric and lateralized differences in selected brain regions of chimpanzees (Pan troglodytes) and bonobos (Pan paniscus) Americal Journal of Primatology. 2009;71:988–97. doi: 10.1002/ajp.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idani G. Relations between unit-groups of bonobos at Wamba: encounters and temporary fusions. African Study Monographs. 1990;11:153–86. [Google Scholar]
- Jaeggi AV, Stevens JM, Van Schaik CP. Tolerant food sharing and reciprocity is precluded by despotism among bonobos but not chimpanzees. American Journal of Physical Anthropology. 2010;143:41–51. doi: 10.1002/ajpa.21288. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kano T. The Last Ape: Pygmy Chimpanzee Behavior and Ecology. Stanford, CA: Stanford University Press; 1992. [Google Scholar]
- Karama S, Lecours AR, Leroux JM, et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Human Brain Mapping. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–70. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Kuroda S. Interaction over food among pygmy chimpanzees. In: Susman RL, editor. The Pygmy Chimpanzee: Evolutionary Biology and Behavior. New York: Plenum Press; 1984. pp. 301–24. [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function. 2010;214:519–34. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nature Review of Neuroscience. 2003;4:469–80. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Ledoux J. Fear and the brain: where have we been, and where are we going? Biological Psychiatry. 1998;44:1229–38. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Lewis KP, Barton RA. Amygdala size and hypothalamus size predict social play frequency in nonhuman primates: a comparative analysis using independent contrasts. Journal of Computer Psychology. 2006;120:31–7. doi: 10.1037/0735-7036.120.1.31. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Carlo J, de Leon MP, Zollikofer CPE. A geometric morphometric analysis of heterochrony in the cranium of chimpanzees and bonobos. Journal of Human Evolution. 2007;52:647–62. doi: 10.1016/j.jhevol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165:712–20. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- McIntyre MH, Herrmann E, Wobber V, et al. Bonobos have a more human-like second-to-fourth finger length ratio (2D:4D) than chimpanzees: a hypothesized indication of lower prenatal androgens. Journal of Human Evolution. 2009;56:361–5. doi: 10.1016/j.jhevol.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6269–74. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Wrangham RW. Dominance, aggression and testosterone in wild chimpanzees: a test of the “challenge hypothesis”. Animal Behavior. 2004;67:113–23. [Google Scholar]
- Mutschler I, Wieckhorst B, Kowalevski S, et al. Functional organization of the human anterior insular cortex. Neuroscience Letter. 2009;457:66–70. doi: 10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Annual New York Academy of Sciences. 1999;877:242–57. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Palagi E. Social play in bonobos (Pan paniscus) and chimpanzees (Pan troglodytes): implications for natural social systems and interindividual relationships. American Journal of Physical Anthropology. 2006;129:418–26. doi: 10.1002/ajpa.20289. [DOI] [PubMed] [Google Scholar]
- Parish AR. Female relationships in bonobos (Pan paniscus) Human Nature. 1996;7:61–96. doi: 10.1007/BF02733490. [DOI] [PubMed] [Google Scholar]
- Parish AR, de Waal FB. The other “closest living relative”. How bonobos (Pan paniscus) challenge traditional assumptions about females, dominance, intra- and intersexual interactions, and hominid evolution. Annual New York Academy of Sciences. 2000;907:97–113. [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biology of Psychiatry. 2006;60:383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Penn DC, Povinelli DJ. On the lack of evidence that non-human animals possess anything remotely resembling a ‘theory of mind'. Philosophical Transactions of Royal Society of London, Series B Biological Sciences. 2007;362:731–44. doi: 10.1098/rstb.2006.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret DI, Smith PA, Potter DD, et al. Visual cells in the temporal cortex sensitive to face view and gaze direction. Proceedings of Royal Soceity of London, Series B Biological Science. 1985;223:293–317. doi: 10.1098/rspb.1985.0003. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature of Neuroscience. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. Journal of Human Evolution. 1999;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Seligman RA. A quantitative morphometric comparative analysis of the primate temporal lobe. Journal of Human Evolution. 2002;42:505–33. doi: 10.1006/jhev.2001.0537. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Transactions on Medical Imaging. 1999;18:712–21. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Research. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Sannen A, Van Elsacker L, Heistermann M, Eens M. Urinary testosterone-metabolite levels and dominance rank in male and female bonobos (Pan paniscus) Primates. 2004;45:89–96. doi: 10.1007/s10329-003-0066-4. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19:1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schaik CPv, Deaner RO, Merrill MY. The conditions for tool use in primates: implications for the evolution of material culture. Journal of Human Evolution. 1999;36:719–41. doi: 10.1006/jhev.1999.0304. [DOI] [PubMed] [Google Scholar]
- Schenker NM, Desgouttes AM, Semendeferi K. Neural connectivity and cortical substrates of cognition in hominoids. Journal of Human Evolution. 2005;49:547–69. doi: 10.1016/j.jhevol.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Seeley WW. Anterior insula degeneration in frontotemporal dementia. Brain Structure and Function. 2010;214:465–75. doi: 10.1007/s00429-010-0263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea BT. Paedomorphosis and neoteny in the pygmy chimpanzee. Science. 1983;222:521–2. doi: 10.1126/science.6623093. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Vitacco MJ, Graf AR, Gostisha AJ, Merz JL, Zahn-Waxler C. Neurobiology of empathy and callousness: implications for the development of antisocial behavior. Behavior Science Law. 2009;27:137–71. doi: 10.1002/bsl.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences. 2009 doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Singer T, Lamm C. The social neuroscience of empathy. Annual New York Academy Sciences. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stanford CB. The social behavior of chimpanzees and bonobos. Current Anthropology. 1998;39:399–420. [Google Scholar]
- Stephan H, Frahm H, Baron G. New and revised data on volumes of brain structures in insectivores and primates. Folia Primatologica. 1981;35:1–29. doi: 10.1159/000155963. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37:335–42. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Call J, Hare B. Chimpanzees understand psychological states – the question is which ones and to what extent. Trends in Cognitive Sciences. 2003;7:153–6. doi: 10.1016/s1364-6613(03)00035-4. [DOI] [PubMed] [Google Scholar]
- van Wingen G, Mattern C, Verkes RJ, Buitelaar J, Fernandez G. Testosterone reduces amygdala-orbitofrontal cortex coupling. Psychoneuroendocrinology. 2010;35:105–13. doi: 10.1016/j.psyneuen.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Veit R, Flor H, Erb M, et al. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neuroscience Letters. 2002;328:233–6. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- White F. Food sharing in wild pygmy chimpanzees (Pan paniscus) In: Roeder JJ, Thierry B, Anderson JR, Herrenschmidt N, editors. Current Primatology: Social Development, Learning and Behavior. Vol. II. Strasbourg: Universite Louis Pasteur; 1994. pp. 1–10. [Google Scholar]
- Wobber V, Hare B, Maboto J, Lipson S, Wrangham R, Ellison PT. Differential changes in steroid hormones before competition in bonobos and chimpanzees. Proceedings of the National Academy of Sciences of the United States of America. 2010b;107:12457–62. doi: 10.1073/pnas.1007411107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobber V, Wrangham R, Hare B. Bonobos exhibit delayed development of social behavior and cognition relative to chimpanzees. Current Biology. 2010a;20:226–30. doi: 10.1016/j.cub.2009.11.070. [DOI] [PubMed] [Google Scholar]
- Wrangham R, Pilbeam D. African apes as time machines. In: Galdikas BMF, Briggs NE, Sheeran LK, Shapiro GL, Goodall J, editors. All Apes Great and Small. New York: Plenum; 2001. pp. 5–17. [Google Scholar]
- Wrangham RW. The evolution of coalitionary killing. Yearbook of Physical Anthropology. 1999;42:1–30. doi: 10.1002/(sici)1096-8644(1999)110:29+<1::aid-ajpa2>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- Wrangham RW, Peterson D. Demonic Males: Apes and the Origins of Human Violence. Boston: Houghton Mifflin; 1996. [Google Scholar]
- Yang Y, Raine A, Narr KL, Colletti P, Toga AW. Localization of deformations within the amygdala in individuals with psychopathy. Archives in General Psychiatry. 2009;66:986–94. doi: 10.1001/archgenpsychiatry.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions of Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.