Abstract
The reinforcement sensitivity theory (RST) relates individual differences in reward sensitivity to the activation of the behavioral approach system (BAS). Dopamine-related brain structures have been repeatedly associated with reward processing, but also with cognitive processes such as task switching. In the present study, we examined the association between reward sensitivity and the event-related fMRI BOLD response with set switching in 31 males. As expected, the right inferior frontal cortex (rIFG) and the striatum (i.e. the left putamen) were involved in set-switching activity for the overall sample. Interindividual differences in Gray's reward sensitivity were related to stronger activity in the rIFG and the ventral striatum. Thus, trait reward sensitivity contributed to the modulation of brain responsiveness in set-switching tasks. Having considered previous research, we propose that higher BAS activity is associated with a stronger reward to process a better implementation of goal-directed tasks and the diminished processing of secondary cues.
Keywords: sensitivity to reward, personality, behavioral approach system, ventral striatum, set switching
INTRODUCTION
Gray (1987; Gray and McNaughton, 2000) defined a behavioral activation system (BAS) that underlies individual differences in positive incentive motivation and impulsivity. Dopaminergic neurotransmission has been proposed to play a central role in BAS functioning because of the implication of the mesolimbic and mesocortical pathways in reward-directed behavior (Depue and Collins, 1999; Pickering and Gray, 1999). Neuroendocrine, neuroimaging and genetic research on individual differences in dopaminergic activity have confirmed the proposed role of this neurotransmitter in the regulation of BAS activity (Depue et al., 1994; Farde et al., 1997; Ebstein et al., 2000; Yasuno et al., 2001).
Several behavioral studies have been conducted to ascertain the cognitive mechanisms involved in BAS using personality questionnaires such as the BIS–BAS scales (Carver and White, 1994) or the Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ; Torrubia et al., 2001). These studies have served to confirm and extend the hypotheses derived from the reinforcement sensitivity theory (RST) model (for reviews, see Corr, 2004; Ávila et al., 2008). Basically, individuals with a more active BAS have better appetitive learning but an impaired processing of aversive cues when responding to reward (Gupta, 1990; Patterson and Newman, 1993; Pickering et al., 1995; Ávila, 2001). In other words, the processing of secondary aversive cues diminishes when these individuals expect a reward.
Dopamine has been traditionally related to reward processing (Wise, 2008). However, some proposals have extended its role in cognition to a more generalized processing of set switching, an executive function that requires online maintenance and the updating of the sensorimotor associations between the stated sets of sensory and motor representations that are to be intermittently updated (upheld, reversed or replaced altogether) in a context-sensitive way (Cools, 2006; Boulougouris and Tsaltas, 2008). This relationship arose from experimental studies in patients with Parkinson’s disease which show that low activity in dopaminergic pathways impairs the ability to disengage from old and irrelevant response sets (Hayes et al., 1998; Gaunlett-Gilbert et al., 1999).
Patterson and Newman (1993) have proposed that BAS activity was specifically related to the ability to switch from automatic to controlled processing when contingencies impel to do so. Situations in which previously rewarded responses were followed by punishment or nonreward are the most illustrative cases because unexpected feedback would entail the need for controlled processing. Some behavioral studies have shown that a BAS overactivity (measured with personality questionnaires) was associated with a better ability to disengage from aversive stimuli (Patterson et al., 1987; Ávila, 2001) and in general, with a better ability for disengagement from previous irrelevant stimuli (Ávila and Parcet, 1997, 2001; Pickering and Gray, 1999; Poy et al., 2004) and to set switching (Ávila et al., 2003). Thus, individuals with a more active BAS had a lower facility to switch from automatic to controlled processing when faced with unexpected feedback. It is noteworthy that the action of the BAS has been proposed to be mediated by dopamine-related structures (see Pickering and Gray, 1999; Ávila et al., 2008).
In the present study, we investigated the neural basis of set switching by adapting the paradigm used in our previous study to fMRI (Ávila et al., 2003). Previous studies have related performance in these tasks to the activation of both reward-related brain structures such as the striatum (Graham et al., 2009; Zastrow et al., 2009) and the right inferior frontal cortex (Aron and Poldrack, 2006; Robbins, 2007). We predicted that these structures would be implicated in the set-switching process and that BAS activity (measured with the SR scale) would be positively related with their activation.
METHODS
Subjects
Total of 31 male, right-handed students at the Universitat Jaume I of Castelló were selected for this study (mean age = 25.0, s.d. = 5.9). All the participants completed the Sensitivity to Reward scale of the SPSRQ (Torrubia et al., 2001). The mean score was 10.23 (s.d. = 4.21, ranges 3–21) and scores followed a normal distribution; thus this distribution was consistent with those obtained from other samples (Torrubia et al., 2001; Barrós-Loscertales et al., 2010). The research project was approved by the ethical committee of the Universitat Jaume I and was in accordance with the Declaration of Helsinki guidelines. All the subjects participating in this experiment were paid for their collaboration.
Task
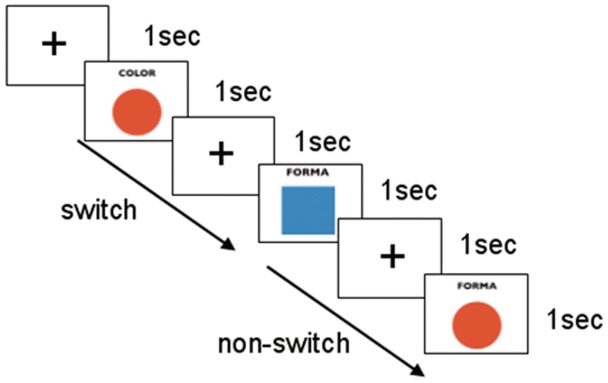
The stimulus consisted of a square or a circle presented on a white background. The participants were presented with MRI-adapted goggles (Resonance Technologies, Inc., Northridge, CA, USA). Each figure could be alternatively filled in with red or blue. A label was presented at the same time as the stimulus at the top of the figure, indicating whether the subject should pay attention to the shape (‘Forma’) or color (‘Color’) of the figure. Eight different combinations were available by mixing two possible shapes, two colors and two labels. The participants were instructed to push one of the two buttons of the ResponseGrip (Nordic NeuroLab AS, Bergen, Norway) with their right hand when they recognized an upcoming stimulus according to the following rules: one button was pushed if the figure was a circle (when Shape) or red (when Color), whereas the other button was pushed if the figure was a square (when Shape) or blue (when Color). The response buttons were counterbalanced across subjects. A fixation cross was presented 1 s before the stimulus in order to help the subject maintain the fixation point in the center of the screen. Each subject was trained with an 8-min session before entering the scanner. They also underwent a further 5-min training inside the scanner while running a short fMRI session so there was less possibility of arousal effects during the proper experiment sessions which followed.
Presentation software (Neurobehavioral Systems, Inc., Albany, CA, USA) which ran on a Microsoft XP platform was used to program the task. Each time bin, intended as the time needed to acquire a whole brain volume, was set to 2000 ms. Visual presentation was synchronized with the scanner via the SyncBox (Nordic NeuroLab AS, Bergen, Norway) equipment. Each stimulus lasted 1000 ms and was placed at the beginning of a time bin. The stimuli were pseudo-randomly interleaved by 0, 1, 2 or 3 blank time bins (null events with a fixation cross).
The experimental session consisted of two subsequent runs of 7 min and 5 s each, with a differently randomized presentation of sequences. Each run consisted in 215 volume acquisitions. Within these, a total of 100 stimuli were presented in order to obtain 30 ‘switch’ and 30 ‘nonswitch’ conditions per run. A ‘switch’ event was defined as the condition of changing the set to be used in order to answer the upcoming stimulus in relation to the previous one (shape–color or color–shape trials). Coherently, a ‘nonswitch’ event was assumed to happen when the subject answered the upcoming stimulus with the same set as the previous one (shape–shape or color–color). Figure 1 illustrates the experimental conditions. The possibility of a switch or a nonswitch condition was considered only between subsequent time bins (2 s); when one or more null events was/were set between two stimuli, the upcoming stimulus was considered a ‘first event’ and was modeled separately. To ensure good spectral density in variance for both ‘switch’ and ‘nonswitch’ conditions, a total of 115 null events were introduced into each run. Finally, it is important to notice that, compared with the procedure used by Ávila et al., (2003), the present procedure reduces the magnitude of switch costs because the interstimulus interval was increased to 2 s.
Fig. 1.
Task conditions.
MRI parameters
All the sessions were performed in a 1.5 T MRI Siemens Avanto scanner (Siemens, Erlangen, Germany). The acquisition plane was axial and oriented so that both the rostral and caudal extremities of the corpus callosum lay on the same plane. The fMRI sessions parameters were as follows: Single-Shot Echo-Planar, Tr/Te = 2000/45 ms, axial matrix = 64 × 64, slice thickness = 4 mm, FOV = 210 × 210 mm, resulting voxel size = 3.28 × 3.28 × 4 mm, number of slices = 29 interleaved, flip angle = 90°.
Data analysis
Data analysis was performed with SPM5 (The Wellcome Institute of Neurology, London, UK). Images were first realigned and unwrapped, then normalized to the MNI space by using the standard EPI template and by setting the mean realigned image as a reference. Smoothing was applied with a Gaussian kernel of 8 mm. In the first-level analysis, a full factorial design including both runs was set for each subject by modeling the switch events, the nonswitch events and the first event within each trial, separately. Translational and rotational realignment corrections were set as regressors. A high pass filter was introduced with a value of 128 s. The convolution was performed by using the canonical HRF. At this level of analysis, contrast images were defined for each subject as the difference between the switch and nonswitch events, averaged over the two runs. The resulting contrast images of parameter estimates were used in the second-level analysis to explore task related activations and association of this activation with Sensitivity to Reward scores. A voxel-by-voxel regression analysis of switch-repeat contrast images was performed by including the SR scale scores as a covariate of interest within the general linear model in framework SPM5. The statistics resulting from each voxel were transformed to Z-scores and displayed as SPMs within the standard space.
Task-related switch activation was studied by the definition of a priori regions of interest (ROIs) in the right inferior frontal gyrus and the striatum. The definition of the ROI in the right inferior was based on the areas activated by task switching in previous studies (Aron and Poldrack, 2006; Robbins, 2007; Graham et al., 2009; Zastrow et al., 2009), while the striatum was defined with the coordinate system from the Montreal Neurological Institute template (Tzourio-Mazoyer et al., 2002). These two ROIs were defined with the structural templates using the WFU Pickatlas (Maldjian et al., 2003). A statistical threshold of P < 0.005 uncorrected (k > 20) was used to study the activation in the right inferior frontal gyrus a priori ROIs, which is in line with previous functional imaging investigations of the neural correlates of the BAS (Beaver et al., 2006). Likewise for a discrete anatomical structure such as the striatum, we applied a small volume correction (SVC) for multiple comparisons at a statistical threshold of P < 0.05 (FWE corrected).
RESULTS
Behavioral data
The reaction times (RTs) and accuracy data appear in Table 1. As expected, RTs were faster for the control nonswitching trials than for the switching trials [F (1,29) = 7.66, P < 0.01]. Similarly, accuracy was better for the control trials than for the switching trials [F (1,29) = 38.54, P < 0.001]. The correlations between the SR scores and performance were all nonsignificant. However, a positive correlation between the SR scores and errors during the switching trials was obtained once the errors in the control condition had been controlled (P < 0.10; Table 1).
Table 1.
Means, standard deviations and correlations with the SR scores of the behavioral data
| Variable | Mean (s.d.) | Correlation with SR |
|---|---|---|
| RTs | ||
| Shifting | 799 (129) | 0.02 |
| Nonshifting | 770 (100) | −0.14 |
| Shifting minus nonshifting | 29 (57) | 0.21 |
| Errors | ||
| Shifting | 6.3 (5.89) | 0.11 |
| Nonshifting | 4.7 (5.73) | 0.25 |
| Shifting minus nonshifting | 1.6 (3.04) | 0.30 |
All the correlations were non significant (P > 0.05).
The fMRI data
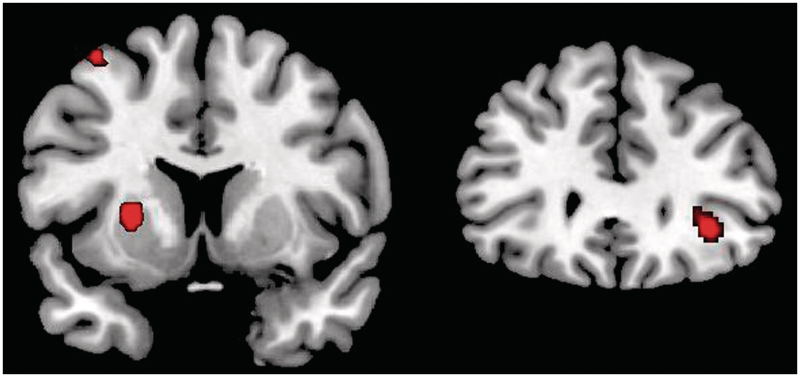
As expected, the average effect among all the subjects revealed activation in the right inferior frontal cortex (MNI 34, 32, − 6; k = 47; T = 4.17, P < 0.001, uncorrected) and the left striatum affecting the putamen (MNI −24, 6, 4; k = 82; T = 4.32; P < 0.02, FWE-SVC) during task completion (Figure 2). Other activations for the overall task appear in Supplementary Table S1 (P < 0.005, uncorrected).
Fig. 2.
The results obtained for the switch vs nonswitch contrast (P < 0.0005, uncorrected). Activation foci were located on the left putamen (left) and the right inferior frontal gyrus (right).
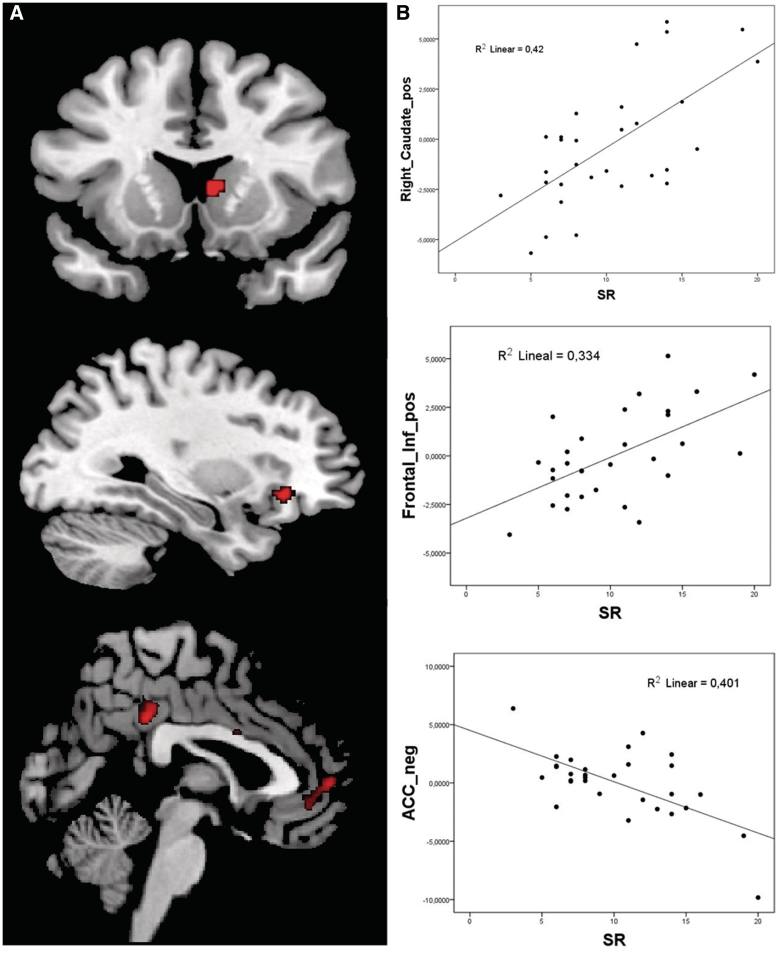
The regression analysis yielded a significant positive correlation between the SR scores and activation in the right ventral striatum (MNI 6, 12, 6; k = 26; T = 3.75; P < 0.05, FWE-SVC) and the right inferior frontal cortex (MNI 30, 28, −8; k = 32, T = 3.44, P < 0.001, uncorrected) (Figure 3A). The other activations appear in Supplementary Table S2 (P < 0.005, uncorrected). No negative correlation between the SR scores and the activation in the ROIs was found. It is noteworthy that the SR scores correlated negatively with the activation in the rostral anterior cingulate (MNI 8, 38, −2; k = 166; T = 4.14; P < 0.001, uncorrected, see Figure 3B). The other negative correlations appear in Supplementary Table S2.
Fig. 3.
Results of the regression analyses (P < 0.005, uncorrected). (A) Brain areas positively and negatively correlated with the SR scores, including the right striatum (upper), the right inferior frontal cortex (middle) and rostral ACC (lower); (B) The scatterplots depict the correlation between standardized SR scores and the BOLD response in the local maxima.
DISCUSSION
This study was designed to investigate the brain mechanisms involved in the higher ability of individuals with a more active BAS to automatically disengage from the semantic information derived from previous stimuli once they had disappeared and to reconfigure conditions of relevancy to a new set (Patterson et al., 1987; Ávila and Parcet, 1997, 2001; Ávila et al., 2003; Smillie and Jackson, 2005). For this purpose, we adapted a task-switching procedure employed in previous studies to fMRI (Hayes et al., 1998; Ávila et al., 2003; Gu et al., 2008). When compared with our previous behavioral study (Ávila et al., 2003), the present procedure replicated the switching effect, but not the negative correlation with the BAS scores. However, individuals with higher SR scale scores presented a more activated right striatum and right inferior frontal cortex than low scorers, i.e. the areas related to response reconfiguration and inhibition.
Adaptation of the task employed by Avila et al. (2003) to the fMRI yielded a significant, but reduced switch cost. Previous results using similar procedures to investigate task switching have obtained similar magnitudes of the switch costs and brain areas related to task switching (Gu et al., 2008). However, this difference is important between both procedures and should be borne in mind to interpret the lack of SR effects on behavioral measures derived from the task. The main change in the task was made given the need to regulate successive trials using a fixed 2-s interstimulus interval, whereas the second stimulus in the seminal study appeared immediately after the response to the first stimulus. This change considerably reduced the magnitude of the time required for switching and has probably precluded the replication of the BAS-associated effect. This lack of replication of the personality effect may not only affect the interpretation of the neural differences, but the presence of these correlates may also make the interpretation of the neural results difficult (see Price and Friston, 1999, for a discussion).
Performance on task-switching appears to be a product of an interaction between task-set inertia (the persistence of a set from the previous trial), exogenous task-set activation and endogenous control (Robbins, 2007). The brain activity associated with switching the cognitive set revealed larger oxygen consumption in the right inferior frontal cortex (rIFG) for switch trials when compared with nonswitch trials. This activation pattern is largely consistent with previous literature, indicating that this brain area is fundamental in inhibitory processes (Xue et al., 2008). The right inferior frontal gyrus is an area that is consistently related to inhibitory control, task switching and set switching across different paradigms. Its role seems to be related to reactive inhibition to an exogenous cue, which needs endogenous control to reconfigure the response to the new relevant conditions (Robbins, 2007).
The overall task also yielded a significant activation in the left putamen, similar to that reported in previous fMRI studies using either set-switching tasks such as the WCST (Monchi et al., 2001, 2006) or set-switching tasks similar to those employed in the present study (Rubia et al., 2006; Zastrow et al., 2009). The fact that a significant increase in activity was lateralized to the left putamen is consistent with the required contralateral motor response (Monchi et al., 2001). Moreover, the present study provides further evidence that the putamen is involved in the execution of nonroutine actions that require an internal reconfiguration of the relevant set (Monchi et al., 2006). Cunnington et al. (2002) reported a significant activity of the putamen during self-initiated movements, but not during externally triggered alternating movements. The fact that putamen activation appears when comparing responses that require a switch in the relevant set (shape–color or color–shape) with responses to the same kind of stimuli that do not require set-switching (shape–shape and color–color) shows that the left putamen specifically participates in this internal set reconfiguration.
The analysis of brain activation as a function of BAS activity has revealed the relevant role of the right inferior frontal cortex and the striatum in set-switching. Previous research has associated the right IFG with efficiency in stopping and switching in normal (Aron and Poldrack, 2006) and pathological samples (Rubia et al., 1999). In addition, its activity is modulated by drugs that improve the inhibitory control (Chamberlain et al., 2009). This research clearly relates the stronger activity in this area with a better ability to inhibit the representation of the previous set and to also reconfigure the response to the new relevant set.
As expected, the SR scores positively correlated with the activation of the right ventral striatum. Previous studies obtained similar results using reward processing paradigms. Beaver et al. (2006) showed a stronger activation in the right ventral striatum (and other reward brain areas) in participants with high BAS scale scores while they looked at pictures of appetizing food. Similarly, Hahn et al. (2009) used the task widely employed by Knutson et al. (2001) to investigate individual differences in processing cues for reward. Again, a very similar pattern of activation involving the right ventral striatum correlated positively with the SR scores. Importantly, a previous anatomical study in our laboratory using the VBM technique also revealed a reduction of gray matter volume in the right ventral striatum in individuals with a more active BAS (Barrós-Loscertales et al., 2006). Later, a number of studies from different laboratories using different paradigms all focused on the BAS activity-related brain activation being linked with the right ventral striatum.
One important aspect is the different role of striatum nuclei in this task. The left putamen participates uniquely in the switch process by implementing the correct motor program. Laterality is in the left part of the brain because responses are made with the right hand. This effect seems to be independent of personality. The right caudate, however, seems to act as a moderator of motivation-related switch activity. In individuals with higher reward sensitivity this nucleus is activated when a relevant stimulus indicates that a cognitive shift should be made. Then the putamen and the caudate seem to play different roles in this task.
The results of the present study also show a negative relationship between the SR scores and the activation of the right rostral anterior cingulate. This area is anatomically connected to the ventral striatum and appears to be involved in the selection of appropriate motor responses and also in planning sequential movements. Its role could be related to the employment of the attentional resources required to select the correct actions by detection of error and by monitoring action performance (Isomura and Takada, 2004). These authors suggested that the rostral ACC may play critical roles in performing appropriate actions with attention and in checking the performance to acquire rewards efficiently. Thus a negative correlation between BAS activity and the activation of the rostral anterior cingulate may reflect a tendency to reassure responses to changing conditions.
Previous behavioral studies investigating BAS-associated cognitive processes have revealed differences in the processing of reward cues, appetitive learning and aversive learning. In short, individuals with a more active BAS have shown better appetitive learning (Gupta 1990; Ávila and Parcet, 2000, 2001) and impaired aversive learning (Patterson et al., 1987 Ávila et al., 1995; Ávila, 2001). In all cases, the experimental procedures implied continuous responding for reward and sporadic appearances of aversive, secondary stimuli. In such circumstances, a more active BAS predisposes to persevering in dominant, appetitive responses and to ignoring secondary information, whereas a less active BAS predisposes to greater reflection after processing unexpected, infrequent information (Patterson and Newman, 1993). As dopamine is not merely related to reward processing, we may extend these results to switching situations by considering that dopamine may not only mediate processing of reward, but may also play a more general role in processing relevant stimuli and in preparing and programming goal-directed behavior (Robbins and Everitt, 1995; Pickering and Gray, 2001). Along these lines, previous ERP research has shown that increased dopamine-active agents such as caffeine decrease switch costs by enhancing anticipatory control processes (Tieges et al., 2006). This response of the dopaminergic cells would suppress the influence of past stimuli when coping with new stimuli, improving the ability to flexibly maintain and coordinate two task sets during task switching. Therefore, individuals with a more active BAS would experiment greater activity in the dopaminergic neurons that easily disengage attention from the influence of past stimuli, thus allowing a faster set reconfiguration. This process is advantageous for situations requiring continuous switching between two different tasks, but is disadvanteagous for situations requiring a modification of an overlearned response program. These processess would be mediated by the right ventral striatum and right inferior cortex and also by its connection to the rostral ACC.
There is growing interest in the literature in investigating how the presence of motivational components may modulate performance in task-switching tasks. In this sense, the results of the present study are consistent with recent fMRI data and show that the striatum (Aarts et al., 2010) and the inferior frontal cortex (Savine and Braver, 2010) are brain areas involved in task switching, but that their activity is modulated by the presence of reward cues. Aarts et al. (2010) found that those individuals with stronger dopamine activity in the striatum (the 9-repeat allele of the DAT1 polymorphism) have reduced switch costs and a stronger BOLD response in the striatum than 10-R carriers when faced with a reward cue. Similarly, Savine and Braver (2010) observed how the inferior frontal cortex may treat reward and switch cues as equivalent signals, which indicates that high salience or motivational priority is associated with available task-set information. As the individuals with higher SR scale scores showed more incentive motivation (see Avila et al., 2008), we may interpret consistently with the above studies that stronger activity in the striatum and the right inferior frontal cortex reflects the action of the reward system on the cognitive system related to task switching. Then, the increased dopamine activity associated with stronger BAS activity would serve to enhance the updating of information, which would facilitate task switching.
This study has several limitations. First, the sample was formed by males. Although this approach is acceptable, especially when investigating the personality dimensions related to reward sensitivity, future studies should determine if it is possible to generalize the results to females. Second, the design synchronized the stimulus presentation with the volume acquisition, thus opening up the possibility of measuring the BOLD response inadequately. However, the considerable number trials, the inclusion of an important number of null trials and the use of a small TR may counteract this problem.
In conclusion, our results not only indicate associations between individual differences in reward sensitivity and activity in the right frontal and striatum during set-switching tasks, but also show how brain correlates are consistent with the behavioral differences observed in these tasks. BAS overactivity is associated with faster switching and this pattern seems to depend on a stronger activation in these brain areas. Conversely, BAS underactivity was seen to be associated with more caution and responding, which is related to activity in the rostral ACC. The potential utility of these findings to understand pathologies related to reward processing requires further study.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This study was supported by two grants from the Spanish Ministry of Science and Technology (the BrainGlot Project, reference CSD2007-00012, of the Consolider-Ingenio 2010 program and grant SEJ2007-65929/PSIC) and one grant from the Universitat Jaume I (ref P1_1B2008-35).
REFERENCES
- Aarts E, Roelofs A, Franke B, Rijpkema M, Fernández G, Helmich RC, Cools R. Striatal dopamine mediates the interface between motivational and cognitive control in humans: evidence from genetic imaging. Neuropsychopharmacology. 2010;35:1943–51. doi: 10.1038/npp.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. Journal of Neuroscience. 2006;26(9):2424–33. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ávila C. Distinguishing BIS-mediated and BAS-mediated disinhibition mechanisms: a comparison of disinhibition models of Gray and Patterson and Newman. Journal of Personality and Social Psychology. 2001;80:311–24. doi: 10.1037/0022-3514.80.2.311. [DOI] [PubMed] [Google Scholar]
- Ávila C, Barrós A, Ortet G, Parcet MA, Ibáñez MI. Set-shifting and sensitivity to reward: A dopamine mechanism for explaining disinhibitory disorders. Cognition and Emotion. 2003;17:951–9. [Google Scholar]
- Ávila C, Moltó J, Segarra P, Torrubia R. Sensitivity to primary or secondary reinforcers, what is the mechanism underlying passive avoidance deficits in extraverts? Journal of Research in Personality. 1995;29:373–94. [Google Scholar]
- Ávila C, Parcet MA. Impulsivity and anxiety differences in cognitive inhibition. Personality and Individual Differences. 1997;23:1055–64. [Google Scholar]
- Ávila C, Parcet MA. The role of Gray's impulsivity in anxiety-mediated differences in resistance to extinction. European Journal of Personality. 2000;14:185–98. [Google Scholar]
- Ávila C, Parcet MA. Personality and inhibitory deficits in the Stop-signal Task: the mediating role of Gray’s anxiety and impulsivity. Personality and Individual Differences. 2001;29:975–86. [Google Scholar]
- Ávila C, Parcet MA, Barrós-Loscertales A. A cognitive neuroscience approach to individual differences in sensitivity to reward. Neurotoxicity Research. 2008;14:191–203. doi: 10.1007/BF03033810. [DOI] [PubMed] [Google Scholar]
- Barrós-Loscertales A, Meseguer V, Sanjuán A, et al. Striatum gray matter reduction in males with an overactive Behavior Activation System. European Journal of Neuroscience. 2006;24:2071–4. doi: 10.1111/j.1460-9568.2006.05084.x. [DOI] [PubMed] [Google Scholar]
- Barrós-Loscertales A, Ventura-Campos N, Sanjuán-Tomás A, Belloch V, Parcet MA, Avila C. Behavioral activation system modulation on brain activation during appetitive and aversive stimulus processing. Social Cognitive and Affective Neuroscience. 2010;5:18–28. doi: 10.1093/scan/nsq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. Journal of Neuroscience. 2006;26:5160–6. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Tsaltas E. Serotonergic and dopaminergic modulation of attentional processes. Progress in Brain Research. 2008;172:517–42. doi: 10.1016/S0079-6123(08)00925-4. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–33. [Google Scholar]
- Chamberlain SR, Hampshire A, Müller U, et al. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biological Psychiatry. 2009;65:550–5. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neuroscience and Biobehavioral Reviews. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Corr PJ. Reinforcement sensitivity theory and personality. Neuroscience and Biobehavioral Reviews. 2004;28:317–32. doi: 10.1016/j.neubiorev.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage. 2002;15:373–85. doi: 10.1006/nimg.2001.0976. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation and extraversion. Behavioral and Brain Sciences. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Depue RA, Luciana M, Arbisi P, Collins P, Leon A. Dopamine and the structure of personality: Relation of agonist-induced dopamine activity to Positive Emotionality. Journal of Personality and Social Psychology. 1994;67:485–98. doi: 10.1037//0022-3514.67.3.485. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Benjamin J, Belmaker RH. Personality and polymorphisms of genes involved in aminergic neurotransmission. European Journal of Pharmacology. 2000;410:205–14. doi: 10.1016/s0014-2999(00)00852-9. [DOI] [PubMed] [Google Scholar]
- Farde L, Gustavsson JP, Jonsson E. D2 dopamine receptors and personality traits. Nature. 1997;385:590. doi: 10.1038/385590a0. [DOI] [PubMed] [Google Scholar]
- Gaunlett-Gilbert J, Roberts RC, Brown VJ. Mechanisms underlying attentional set-shifting in Parkinson’s disease. Neuropsychologia. 1999;37:605–16. doi: 10.1016/s0028-3932(98)00049-9. [DOI] [PubMed] [Google Scholar]
- Graham S, Phua E, Soon CS, et al. Role of medial cortical, hippocampal and striatal interactions during cognitive set-shifting. Neuroimage. 2009;45:1359–67. doi: 10.1016/j.neuroimage.2008.12.040. [DOI] [PubMed] [Google Scholar]
- Gray JA. The neuropsychology of personality and emotion. In: Stahl SM, Iversen SD, Goodman EC, editors. Cognitive Neurochemistry. Oxford: Oxford University Press; 1987. [Google Scholar]
- Gray JA, McNaughton NJ. The Neuropsychology of Anxiety. Oxford: Oxford Medical Publications; 2000. [Google Scholar]
- Gu BM, Park JY, Kang DH, et al. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain. 2008;1311:55–64. doi: 10.1093/brain/awm277. [DOI] [PubMed] [Google Scholar]
- Gupta S. Personality and reinforcement in verbal operant conditioning: A test of Gray's theory. Psychology Studies. 1990;35:157–62. [Google Scholar]
- Hahn T, Dresler T, Ehlis AC, et al. Neural response to reward anticipation is modulated by Gray's impulsivity. Neuroimage. 2009;46:1148–53. doi: 10.1016/j.neuroimage.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Hayes AE, Davidson MC, Keele SW, Rafal RD. Toward a functional analysis of the basal ganglia. Journal of Cognitive Neuroscience. 1998;10:178–98. doi: 10.1162/089892998562645. [DOI] [PubMed] [Google Scholar]
- Isomura Y, Takada M. Neural mechanisms of versatile functions in primate anterior cingulate cortex. Reviews in the Neurosciences. 2004;15:279–91. doi: 10.1515/revneuro.2004.15.4.279. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Monchi O, Ko JH, Strafella AP. Striatal dopamine release during performance of executive functions: A [(11)C] raclopride PET study. Neuroimage. 2006;33:907–12. doi: 10.1016/j.neuroimage.2006.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, et al. Card-sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related fMRI. Journal of Neuroscience. 2001;21:7733–41. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CM, Kosson DS, Newman JP. Reaction to punishment, reflectivity, and passive avoidance learning in extraverts. Journal of Personality and Social Psychology. 1987;52:565–75. doi: 10.1037//0022-3514.52.3.565. [DOI] [PubMed] [Google Scholar]
- Patterson CM, Newman JP. Reflectivity and learning from aversive events: toward a psychological mechanism for Syndromes of Disinhibition. Psychological Review. 1993;100:716–36. doi: 10.1037/0033-295x.100.4.716. [DOI] [PubMed] [Google Scholar]
- Pickering AD, Díaz A, Gray JA. Personality and reinforcement: an exploration using a maze-learning task. Personality and Individual Differences. 1995;18:541–58. [Google Scholar]
- Pickering AD, Gray JA. The neuroscience of personality. In: Pervin L, John O, editors. Handbook of Personality. New York: Guilford Press; 1999. pp. 277–99. [Google Scholar]
- Pickering AD, Gray JA. Dopamine, appetitive reinforcement, and the neuropsychology of human learning: an individual differences approach. In: Eliasz A, Angleitner A, editors. Advances in Individual Differences Research. Lengerich, Germany: PABST Science Publishers; 2001. pp. 113–149. [Google Scholar]
- Poy R, Eixarch MC, Ávila A. On the relationship between attention and personality: covert visual orienting of attention in anxiety and impulsivity. Personality and Individual Differences. 2004;36:1471–81. [Google Scholar]
- Price CJ, Friston KJ. Scanning patients with tasks they can perform. Human Brain Mapping. 1999;8:102–8. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2007;362:917–32. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Arousal systems and attention. In: Gazzaniga MS, editor. The Cognitive Neurosciences. Cambridge: MIT Press; 1995. pp. 703–20. [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Bullmore ET. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. American Journal of Psychiatry. 1999;156:891–6. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Savine AC, Braver TS. Motivated cognitive control: reward incentives modulate preparatory neural activity during task-switching. Journal of Neuroscience. 2010;30:10294–305. doi: 10.1523/JNEUROSCI.2052-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Smillie LD, Jackson C. The appetitive motivation scale and other BAS measures in the prediction of Approach and Active Avoidance. Personality and Individual Differences. 2005;38:981–994. [Google Scholar]
- Rubia K, Smith AB, Woolley J, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27:973–93. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieges Z, Snel J, Kok A, Wijnen JG, Lorist MM, Ridderinkhof RK. Caffeine improves anticipatory processes in task switching. Biological Psychology. 2006;73:101–13. doi: 10.1016/j.biopsycho.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Torrubia R, Ávila C, Moltó J, Caseras X. The sensitivity to punishment and sensitivity to reward questionnaire (SPSRQ) as a measure of Gray's anxiety and impulsivity dimensions. Personality and Individual Differences. 2001;31:837–62. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotoxicity Research. 2008;14:169–83. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Aron AR, Poldrack RA. Common neural substrates for inhibition of spoken and manual responses. Cerebral Cortex. 2008;18:1923–32. doi: 10.1093/cercor/bhm220. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Suhara T, Sudo Y, et al. Relation among dopamine D2 receptor binding, obesity and personality in normal human subjects. Neuroscience Letters. 2001;300:59–61. doi: 10.1016/s0304-3940(01)01552-x. [DOI] [PubMed] [Google Scholar]
- Zastrow A, Kaiser S, Stippich C, et al. Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. American Journal of Psychiatry. 2009;166:608–16. doi: 10.1176/appi.ajp.2008.08050775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.