Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) mutation ΔF508CFTR still causes regulatory defects when rescued to the apical membrane, suggesting that the intracellular milieu might affect its ability to respond to cAMP regulation. We recently reported that overexpression of the Na+/H+ exchanger regulatory factor NHERF1 in the cystic fibrosis (CF) airway cell line CFBE41o-rescues the functional expression of ΔF508CFTR by promoting F-actin organization and formation of the NHERF1–ezrin–actin complex. Here, using real-time FRET reporters of both PKA activity and cAMP levels, we find that lack of an organized subcortical cytoskeleton in CFBE41o-cells causes both defective accumulation of cAMP in the subcortical compartment and excessive cytosolic accumulation of cAMP. This results in reduced subcortical levels and increased cytosolic levels of PKA activity. NHERF1 overexpression in CFBE41o-cells restores chloride secretion, subcortical cAMP compartmentalization and local PKA activity, indicating that regulation of ΔF508CFTR function requires not only stable expression of the mutant CFTR at the cell surface but also depends on both generation of local cAMP signals of adequate amplitude and activation of PKA in proximity of its target. Moreover, we found that the knockdown of wild-type CFTR in the non-CF 16HBE14o-cells results in both altered cytoskeletal organization and loss of cAMP compartmentalization, whereas stable overexpression of wt CFTR in CF cells restores cytoskeleton organization and re-establishes the compartmentalization of cAMP at the plasma membrane. This suggests that the presence of CFTR on the plasma membrane influences the cytoskeletal organizational state and, consequently, cAMP distribution. Our data show that a sufficiently high concentration of cAMP in the subcortical compartment is required to achieve PKA-mediated regulation of CFTR activity.
Key words: Cystic fibrosis, cAMP, PKA, NHERF1, airways cells, FRET
Introduction
The cystic fibrosis transmembrane conductance regulator (CFTR) protein is responsible for regulated chloride conductance in airway and intestinal epithelia and in exocrine glands (Bear et al., 1992; Quinton, 1983; Riordan, 1993). In addition to transepithelial chloride transport, CFTR plays a crucial role in fluid homeostasis and influences a large number of cell functions, including the transport of other electrolytes (reviewed by Li and Naren, 2010). The key pathway regulating CFTR activity involves elevation of cAMP and activation of protein kinase A (PKA) (Gadsby and Nairn, 1999), where PKA-mediated phosphorylation of CFTR opens the anion permeation pathway in the channel and allows efflux of Cl– ions (Cheng et al., 1991).
Cystic fibrosis (CF) is caused by mutations in the CFTR gene. Deletion of phenylalanine in position 508 of the CFTR (ΔF508) is the most common disease-associated mutation and results in the synthesis of an improperly folded CFTR. Although being partially functional and responsive to cAMP and PKA regulation, ΔF508CFTR is unable to reach the cell membrane and undergoes endoplasmic reticulum (ER)-associated degradation (Gelman et al., 2002; Ward and Kopito, 1994; Ward et al., 1995). This results in severely reduced or absent CFTR at the cell surface and defective cAMP-dependent Cl– conductance in affected tissues.
Expression of ΔF508CFTR at the cell surface can be partially rescued either by lowering the temperature (Denning et al., 1992; Bear et al., 1992) or by using small ‘corrector’ molecules that act as chemical chaperones by releasing the mutated protein from ER-mediated degradation (Brown et al., 1996; Zeitlin, 2000). Large-scale screenings have been undertaken to identify rescue agents that could restore correct processing of the mutant CFTR and could thus be used as therapeutics (Pedemonte et al., 2005; Sloane and Rowe, 2010). However, the rescued ΔF508CFTR, despite localization to the plasma membrane, exhibits persistent regulatory defects, including reduced channel gating (Al-Nakkash and Hwang, 1999; Hwang et al., 1997). By contrast, studies using the isolated protein in cell-free systems have indicated that ΔF508CFTR retains normal PKA-dependent regulation and activity relative to wild-type (wt) CFTR (Li et al., 1993). Altogether, these data suggest that, rather than the mutation itself, the intracellular milieu plays an important role in determining the ability of ΔF508CFTR to respond to cAMP regulation. Understanding the mechanisms involved in this regulation is critical for overcoming defective channel gating of misfolded ΔF508CFTR, and has important implications for selection of appropriate pre-clinical models for drug discovery.
It is now well documented that receptors, ion channels or transporters, signaling intermediates and their effectors are compartmentalized into regulatory complexes in epithelial cells, and that such compartmentalization increases the specificity and the efficiency of signaling (Huang et al., 2001). A growing body of evidence supports the view that CFTR membrane expression and activity depend on a high level of organization of cytoskeletal F-actin (Cantiello, 2001; Ganeshan et al., 2007; Haggie et al., 2004; Okiyoneda and Lukacs, 2007). In airway epithelial cells, it has been demonstrated that CFTR regulation depends on the organization of a multiprotein complex involving F-actin and the scaffolding proteins NHERF1 and ezrin (Sun et al., 2000b). This multiprotein complex is important for maintaining CFTR in highly restricted domains at the plasma membrane (Jin et al., 2007). In addition, this complex plays a key role in the control of CFTR function because ezrin, an A-kinase-anchoring protein, tethers PKA in the vicinity of CFTR, allowing cAMP-dependent control of Cl– efflux (Moyer et al., 2000; Short et al., 1998; Sun et al., 2000a).
We have shown that overexpression of NHERF1 can rescue CFTR functional expression in CFBE41o-cells, a human CF airway epithelial cell line homozygous for the ΔF508CFTR mutation, by promoting F-actin organization through the formation of the NHERF1–ezrin–actin complex (Guerra et al., 2005). The complex tethers ΔF508CFTR to the actin cytoskeleton, stabilizing the mutant CFTR on the apical membrane and delaying its internalization (Favia et al., 2010). As a consequence, cAMP-mediated control of Cl– efflux is restored (Guerra et al., 2005). In those studies, however, the contribution of PKA anchored to the sub-plasma-membrane compartment in rescuing functional control of the mutant CFTR had not been assessed.
Here, using real-time FRET reporters of PKA activity and of cAMP levels, we investigate the role of compartmentalized PKA and cAMP in the regulation of CFTR Cl– efflux. We find that the lack of an organized subcortical cytoskeleton in CFBE41o-cells results in defective accumulation of cAMP in the sub-plasma-membrane compartment and excessive accumulation of the second messenger in the cytosol and, consequently, increased PKA activity. Overexpression of wt CFTR in CFBE41o-cells restores both cytoskeletal organization and compartmentalization of cAMP and PKA. These findings uncover a novel mechanism whereby cells expressing ΔF508CFTR might be unable to effectively transduce signals in vivo as a consequence of inadequate subcortical cAMP accumulation and local PKA activation.
Results
Anchoring of PKA to AKAPs is required for NHERF1-mediated rescue of ΔF508CFTR activity
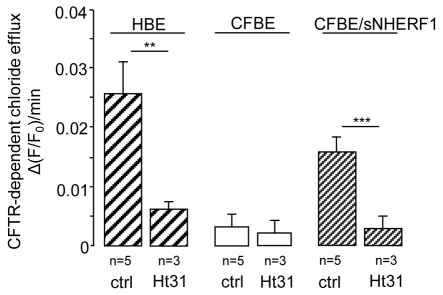
CFBE41o-cells (CFBE, expressing ΔF508CFTR), CFBE cells overexpressing NHERF1 (CFBE/sNHERF1) and 16HBE14o-cells (HBE, expressing wild-type CFTR), were treated with 10 μM forskolin (Frsk), a membrane-permeable adenylyl cyclase activator, and 100 μM IBMX, a non-selective phosphodiesterase inhibitor, to increase intracellular cAMP levels. In agreement with previous findings (Guerra et al., 2005), CFBE cells were unable to respond to cAMP with an increase in Cl– efflux, a defect that was rescued by overexpression of NHERF1 (Fig. 1, control conditions). To assess whether anchorage of PKA to ezrin is involved in the observed NHERF1-dependent recovery of Cl– efflux we measured CFTR activity in CFBE/sNHERF1 cells pre-treated with 100 μM s-Ht31, a membrane-permeable peptide that disrupts the interaction between PKA regulatory subunits and A-kinase anchor proteins (AKAPs) (Klussmann et al., 1999). As shown in Fig. 1, and in agreement with previous findings (Guerra et al., 2005), disruption of the PKA–AKAP interaction dramatically reduced the ability of wt CFTR to respond to cAMP modulation in HBE cells. In addition, treatment with the anchoring inhibitor s-Ht31 was sufficient to completely ablate the Cl– efflux recovery mediated by NHERF1 overexpression in CFBE/sNHERF1 cells, indicating that anchoring of PKA to AKAPs is critical for cAMP modulation of CFTR activity and that PKA anchoring is required for the rescuing effect exerted by overexpression of NHERF1.
Fig. 1.
s-Ht31 abolishes CFTR-dependent chloride efflux in HBE and CFBE/sNHERF1 cell monolayers. Measurements of CFTR-dependent Cl– efflux (expressed as the ΔF/F0 ratio) in HBE, CFBE and CFBE/sNHERF1 polarized monolayers, pre-incubated with s-Ht31 (Ht31) peptide (100 μM) for 45 minutes. Data are means ± s.e. ***P<0.001; **P<0.01.
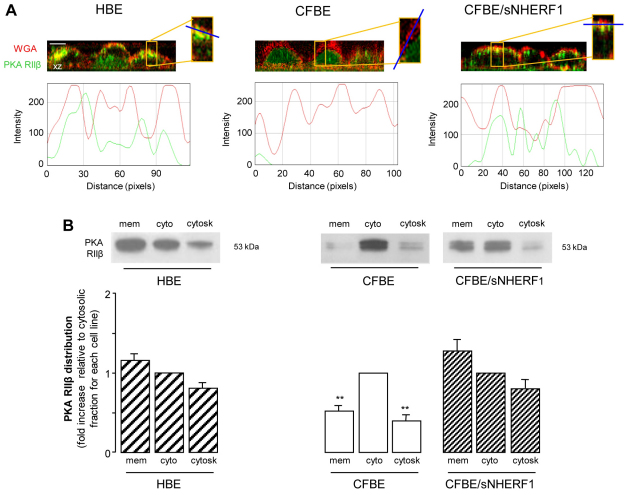
NHERF1 overexpression re-establishes subcortical PKA localization in CFBE cells
NHERF1 interacts with the AKAP ezrin and, by doing so, promotes subcortical cytoskeletal organization (Favia et al., 2010; Mohler et al., 1999). We therefore hypothesized that the rescuing effect of NHERF1 overexpression on CFTR-dependent Cl– efflux might involve recruitment of PKA at the sub-plasma-membrane region by the interaction of PKA regulatory subunits with ezrin. Confocal analysis of the distribution of the PKA RIIβ subunit, performed in polarized cell monolayers co-stained with TRITC-conjugated wheatgerm agglutinin (WGA–TRITC) to visualize the plasma membrane, showed that overexpression of NHERF1 in CFBE cells results in a relocalization of the PKA RIIβ subunit to the subapical membrane region, as shown by an increase in colocalization with WGA (Fig. 2A), a pattern that was very similar to that observed in HBE cell monolayers (Fig. 2A). It is interesting to note that when we analyzed both the level of PKA RIIβ expression in total lysates (see supplementary material Fig. S1) and PKA RIIβ distribution in the membrane, cytosolic and cytoskeletal fractions of the three cell lines (Fig. 2B), we found that NHERF1 overexpression in CFBE cells, although it did not alter total PKA expression, increased the amount of RIIβ recovered in the membrane fraction at the expense of the cytosolic fraction. Moreover, in line with confocal results, the analysis of PKA RIIβ distribution showed that, contrary to the localization in HBE and CFBE/sNHERF1 cells, in CFBE cells PKA RIIβ was significantly more concentrated in the cytosolic fraction than in the other fractions (Fig. 2B). These findings altogether indicate that the previously observed NHERF1-dependent cytoskeletal organization, through interaction with ezrin and F-actin, results not only in stabilization of ΔF508CFTR at the plasma membrane (Favia et al., 2010), but also in a large fraction of PKA to be restored in the subcortical compartment of CFBE/sNHERF1 cells.
Fig. 2.
PKA distribution in HBE, CFBE and CFBE/sNHERF1 cells. (A) Confocal immunofluorescence analysis in HBE, CFBE and CFBE/sNHERF1 polarized cell monolayers grown on permeable filters stained with antibody against PKA RIIβ (green) and counterstained with WGA–TRITC (red) to visualize the plasma membrane. The vertical section (x-z) was randomly acquired. Lower panels indicate fluorescence intensity profiles measured along the line segment indicated in blue. (B) Representative blots showing PKA RIIβ distribution in the membrane (mem), cytosolic (cyto) and cytoskeletal (cytosk) fractions and graphic representation of PKA RIIβ subcellular distribution, normalized to the level of PKA RIIβ in the cytosolic fraction, designated as 1, for each cell line. Data represent means ± s.e.; n=5. **P<0.01.
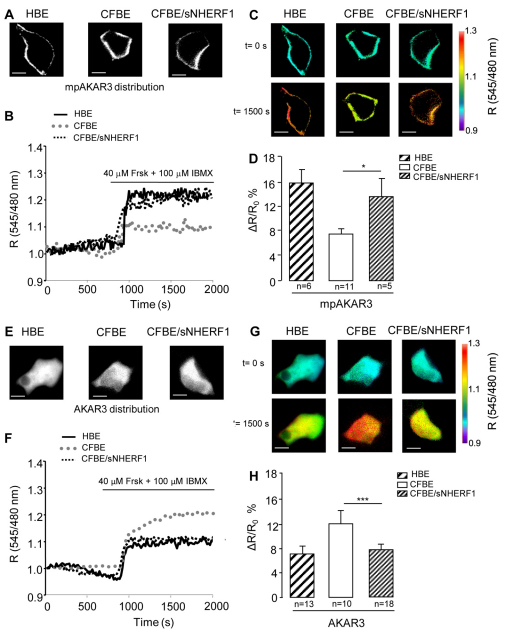
CFBE cells show altered localization of PKA activity
To assess whether this subcortical PKA activity is involved in the ability of overexpressed NHERF1 to rescue cAMP-dependent control of Cl– efflux in CFBE/sNHERF1 cells, we used real-time imaging and the PKA-activity reporter (Allen and Zhang, 2006), a FRET-based indicator that is targeted to the plasma membrane and measures PKA-mediated phosphorylation. When expressed in HBE, CFBE and CFBE/sNHERF1 cells, mpAKAR3 showed the expected localization at the plasma membrane (Fig. 3A). Cells expressing mpAKAR3 were treated with 40 μM Frsk and 100 μM IBMX, and PKA activity was measured over time as FRET change (an increase of the FRET emission ratio 545 nm/480 nm) induced by PKA-mediated phosphorylation of mpAKAR3. As shown in Fig. 3B–D, significantly higher PKA-mediated phosphorylation activity was detected at the plasma membrane of CFBE/sNHERF1 cells compared with CFBE cells, suggesting that overexpression of NHERF1 in CFBE cells re-establishes a level of sub-plasma-membrane PKA activity similar to that observed in HBE cells. To assess whether loss of subcortical PKA activity in CFBE cells associates with an increase in cytosolic PKA activity we used AKAR3, an untargeted, cytosolic version of the PKA activity FRET reporter (Fig. 3E). Indeed, when PKA activity was measured in the bulk cytosol of HBE, CFBE and CFBE/sNHERF1 cells expressing AKAR3 and treated with 40 μM Frsk and 100 μM IBMX, we found that PKA-mediated phosphorylation was significantly higher in the cytosol of CFBE cells than in the cytosol of CFBE/sNHERF1 and HBE cells (Fig. 3F–H). Overall, the above data indicate that cAMP-dependent regulation of CFTR activity relies on a subset of PKA localized in the subcortical compartment. The altered subcortical cytoskeleton previously observed in CFBE cells expressing the mutant ΔF508CFTR (Favia et al., 2010), results in depletion of the subcortical PKA pool and, consequently, in blunted local PKA-dependent regulation of CFTR. NHERF1 overexpression, by restoring subcortical localization of PKA activity, rescues Cl– efflux regulation.
Fig. 3.
FRET analysis of PKA activity in the sub-membrane region and bulk cytosol of epithelial cells. (A–D) Analysis of the membrane-targeted version of AKAR3, mpAKAR3. (E–H) Analysis of cytosolic AKAR3. Distribution of mpAKAR3 (A) and AKAR3 (E) reporters respectively, expressed in HBE, CFBE and CFBE/sNHERF1 cells. (B,F) Representative kinetics of PKA-mediated phosphorylation recorded in the cells shown in A and E, respectively, upon stimulation with 40 μM Frsk and 100 μM IBMX. R is the normalized 545 nm/480 nm value calculated at each acquisition time point. Black line, dotted gray line and dotted black line represent kinetics recorded respectively in HBE, CFBE and CFBE/sNHERF1 both in the bulk cytosol and at the plasma membrane. (C,G) Intensity-modulated pseudocolor images of the cells shown in A and E, respectively. For each cell the 545/480 nm emission ratio values before (time=0 seconds) and 1500 seconds after the addition of 40 μM Frsk and 100 μM IBMX are shown. (D,H) Summary of all experiments performed in B and F, respectively, showing FRET changes recorded in HBE, CFBE and CFBE/sNHERF1 cells expressing the mpAKAR3 or AKAR3 sensors upon stimulation with 40 μM Frsk and 100 μM IBMX. Data represent means ± s.e. Scale bars: 10 μm. ***P<0.001; *P<0.05.
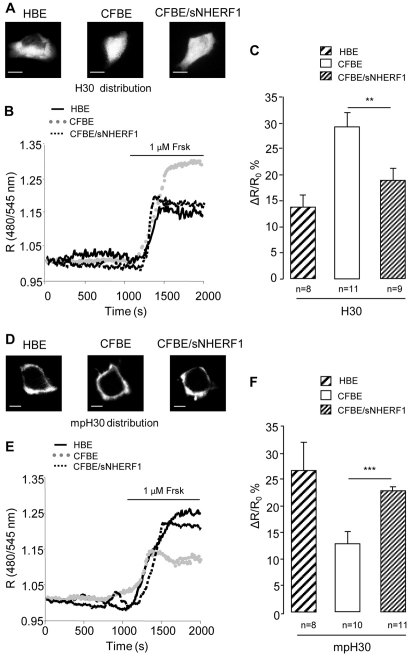
CFBE cells show altered cAMP compartmentalization that is rescued by overexpression of NHERF1
A possible explanation for the larger bulk cytosolic PKA activity detected in CFBE cells compared with CFBE/sNHERF1 and HBE cells, in spite of an equal or lower total PKA content (supplementary material Fig. S1), is that in CFBE cells cytosolic PKA is exposed to a higher concentration of cAMP than cytosolic PKA in CFBE/sNHERF1 and HBE cells. To assess this, we measured cAMP changes in cells expressing H30 (Ponsioen et al., 2004), a cytosolic FRET-based reporter for cAMP levels (Fig. 4A). As shown in Fig. 4, upon treatment with 1 μM Frsk, a significantly larger increase in cAMP was detected in the bulk cytosol of CFBE cells than in CFBE/sNHERF1 and HBE cells (Fig. 4B,C and supplementary material Fig. S2 for pseudocolor images). Interestingly, when we measured the cAMP response generated by 1 μM Frsk in the sub-plasma-membrane compartment by using a membrane-targeted version of H30 (mpH30) (Terrin et al., 2006) (Fig. 4D), we found that the subcortical cAMP response was significantly lower in CFBE cells than in CFBE/sNHERF1 and HBE cells (Fig. 4E,F and supplementary material Fig. S2). Thus, when compared with CFBE/sNHERF1 and HBE cells, CFBE cells appeared to accumulate a larger amount of cAMP in the bulk cytosol at the expense of the cAMP content in the sub-plasma-membrane compartment, in keeping with the observed difference in PKA activity (Fig. 3). It is important to note that again overexpression of NHERF1 in CFBE cells re-established the cAMP gradient observed in HBE cells, with a larger cAMP increase detected in the subcortical compartment compared with the bulk cytosol (Fig. 4F).
Fig. 4.
FRET analysis of cAMP distribution in HBE, CFBE and CFBE/sNHERF1 cells. (A–C) Analysis of the cAMP sensor H30. (D–F) Analysis of membrane-targeted mpH30. Localization of H30 (A) and mpH30 (D) in the three cell lines. (B,E) Representative kinetics of cAMP changes recorded in the cells shown in A and D, respectively. R is the normalized 480 nm/545 nm value calculated at each acquisition time point. Black line, dotted gray line and dotted black line represent kinetics recorded, respectively, in HBE, CFBE and CFBE/sNHERF1 cells, both in the bulk cytosol and at the plasma membrane. (C,F) Summary of FRET changes measured in HBE, CFBE and CFBE/sNHERF1 cells expressing H30 and mpH30 probes, respectively, upon treatment with 1 μM Frsk. Data are means ± s.e. Scale bars: 10 μm. ***P<0.001; **P<0.01.
The altered cAMP compartmentalization in CFBE cells does not require PDE activity
It has been previously reported that spatial control of cAMP signals relies on the activity of phosphodiesterases (PDEs). PDEs are the only enzymes that degrade cAMP and, doing so, they contribute to intracellular compartmentalization of cAMP (Barnes et al., 2005; Houslay et al., 2005). To assess whether the different levels of cAMP in the subcortical and cytosolic compartments observed in the three cell lines depend on differences in local PDE activity, HBE, CFBE and CFBE/sNHERF1 cells expressing either H30 or mpH30 were treated with 1 μM Frsk and 100 μM of the PDE inhibitor IBMX. Unfortunately, the amount of cAMP generated in these conditions resulted in probe saturation in both the subcortical and cytosolic compartment (supplementary material Fig. S3), thus preventing any conclusion on the contribution of PDEs in the observed differential compartmentalization of cAMP.
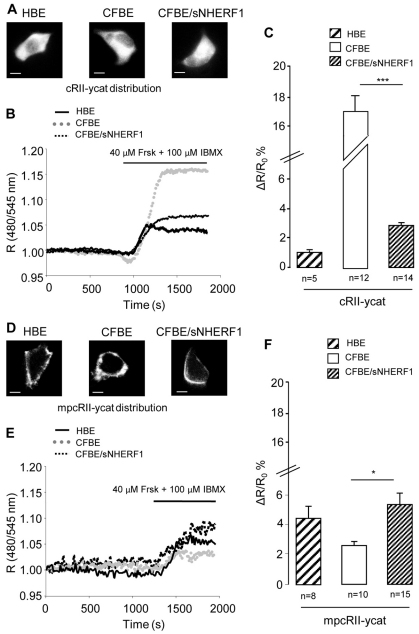
To overcome this limitation, we used a different cAMP FRET reporter (Zaccolo et al., 2000) expressed in both a cytosolic (cRII-ycat) and a plasma membrane-targeted (mpcRII-ycat) format (Fig. 5A,D). Using cRII-ycat and mpcRII-ycat we were able to measure non-saturated cAMP responses in the bulk cytosol and in the subcortical compartment respectively, upon treatment with 40 μM Frsk and 100 μM IBMX (Fig. 5B,C,E,F and supplementary material Fig. S4). The results clearly show that inhibition of PDEs does not affect the observed compartmentalization of cAMP and, when compared with HBE cells and CFBE/sNHERF1 cells, a much larger amount of cAMP still accumulates in the bulk cytosol of CFBE cells at the expense of the cAMP content in the sub-plasma-membrane compartment.
Fig. 5.
FRET analysis of cAMP response to Frsk and IBMX in HBE, CFBE and CFBE/sNHERF1. (A–C) Analysis of the cAMP sensor cRII-ycat. (D–F) Analysis of membrane-targeted mpcRII-ycat. Localization of cRII-ycat (A) and mpcRII-ycat (D) in the three cell lines (B,E) Representative kinetics of cAMP changes recorded in the cells shown in A and D, respectively. R is the normalized 480 nm/545 nm value calculated at each acquisition time point. Black line, dotted gray line and dotted black line represent kinetics recorded in HBE, CFBE and CFBE/sNHERF1 cells, respectively, both in the bulk cytosol and at the plasma membrane. (C,F) Summary of FRET changes measured in HBE, CFBE and CFBE/sNHERF1 cells expressing cRII-ycat and mpcRII-ycat probes, respectively, upon treatment with 40 μM Frsk and 100 μM IBMX. Data are means ± s.e. Scale bars: 10 μm. ***P<0.001; *P<0.05.
Spatial control of cAMP requires an intact subcortical cytoskeleton
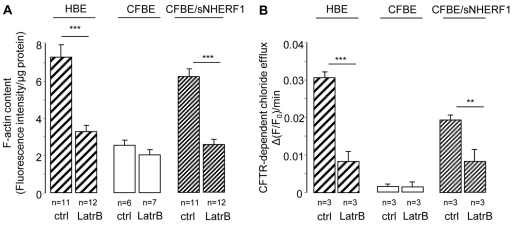
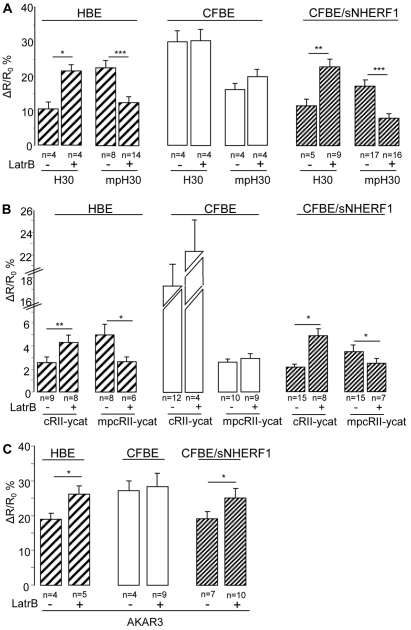
A possible explanation for the observed differences in the compartmentalization of cAMP in HBE and CFBE cells is that the well-organized subcortical cytoskeleton present in HBE cells might act as a physical barrier to cAMP diffusion and contribute to the accumulation of cAMP in the sub-plasma-membrane compartment, whereas the observed disorganized cytoskeletal structure in CFBE cells (Favia et al., 2010) allows cAMP to diffuse away from the site of synthesis at the plasma membrane and to accumulate in the cytosol. Based on this model, overexpression of NHERF1 in CFBE cells, by re-establishing cortical actin cytoskeletal organization, would restore the diffusional barrier and therefore the correct cAMP gradient. In keeping with this hypothesis, the content of polymerized actin (F-actin) measured in HBE and CFBE/sNHERF1 cells was significantly higher than that found in CFBE cells, and treatment with the F-actin-depolymerizing agent Latrunculin B (10 μM) reduced both F-actin assembly (Fig. 6A) and cAMP- and PKA-dependent chloride efflux (Fig. 6B) in HBE and CFBE/sNHERF1 cells, while having no effect in CFBE cells. This confirms that F-actin assembly plays a positive role in regulating the functional expression of CFTR-dependent chloride secretion. To further test whether a subcortical cytoskeletal barrier is responsible for the observed compartmentalization of cAMP, we measured the cAMP response in the bulk cytosol and at the plasma membrane of HBE, CFBE and CFBE/sNHERF1 cells in the absence and in the presence of Latrunculin B. As shown in Fig. 7A, F-actin depolymerization promoted cAMP accumulation in the cytosol at the expenses of subcortical cAMP both in HBE and CFBE/sNHERF1 cells in response to 1 μM Frsk, but had no effect on cAMP levels in either compartment in CFBE cells. Similar results were obtained when the cAMP response to 40 μM Frsk was measured in the presence of 100 μM IBMX (Fig. 7B), confirming that PDE activity is not critical in establishing the boundaries between the subcortical and cytosolic compartments in these cells. In further support of this hypothesis, Latrunculin B significantly increased PKA-mediated phosphorylation, as shown by detection of AKAR3 in the cytosol of both CFBE/sNHERF1 and HBE cells, whereas Latrunculin B was ineffective in CFBE cells (Fig. 7C).
Fig. 6.
Effect of Latrunculin B on F-actin content and on CFTR dependent chloride efflux in HBE, CFBE and CFBE/sNHERF1 cells. (A) Summary of F-actin content measurements performed in HBE, CFBE and CFBE/sNHERF1 cells, before and after treatment with the depolymerizing agent Latrunculin B (10 μM). (B) Summary of CFTR-dependent chloride effluxes performed in the three cell lines, before and after treatment with 10 μM Latrunculin B. CFTR-dependent chloride transport was calculated from the difference in alterations of Frsk-stimulated fluorescence measurements in the absence and presence of 5 μM CFTR inhibitor, CFTRinh-172. Each bar represents the mean ± s.e. ***P<0.001; **P<0.01.
Fig. 7.
Effect of Latrunculin B on cAMP distribution and PKA activity in HBE, CFBE and CFBE/sNHERF1 cells. (A) FRET changes in the presence of Latrunculin B in HBE, CFBE and CFBE/sNHERF1 cells, expressing either the H30 or mpH30 biosensors, as indicated upon treatment with 1 μM Frsk. (B) FRET changes in the presence of Latrunculin B in HBE, CFBE and CFBE/sNHERF1 cells, expressing either the cRII-ycat or mpcRII-ycat biosensor, as indicated, upon treatment with 40 μM Frsk and 100 μM IBMX. (C) Summary of FRET changes recorded in HBE, CFBE and CFBE/sNHERF1 cells expressing AKAR3 cytosolic sensor, upon stimulation with 40 μM Frsk and 100 μM IBMX. Data are means ± s.e. ***P<0.001; **P<0.01; *P<0.05.
CFTR expression at the plasma membrane is required for cytoskeletal organization and cAMP compartmentalization
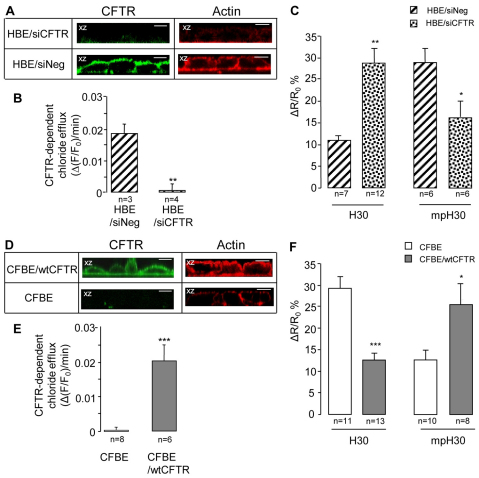
To assess whether the altered compartmentalization of cAMP and PKA activity observed in CFBE cells is a peculiarity of this cell line or rather might have a more general relevance in the physiopathology of cystic fibrosis, we genetically ablated CFTR expression in HBE cells using a small interfering RNA (siRNA) approach. After pre-incubation of HBE cells with CFTR specific siRNA (HBE/siCFTR) for 72 hours, the expression of apical CFTR as well as the CFTR-dependent chloride efflux was abolished (Fig. 8A,B). In addition, analysis of F-actin organization by confocal microscopy (Fig. 8A) showed a loss of cortical actin filaments in HBE cells treated with CFTR siRNA compared with levels in HBE cells transfected with a non-silencing control siRNA sequence. (supplementary material Fig. S5). When the cAMP response to Frsk (1 μM) was measured in either the cytoplasmic or in the sub-plasma-membrane compartments of HBE cells treated with CFTR siRNA (Fig. 8C), we found that CFTR knockdown reversed the cAMP distribution previously observed in HBE cells (compare Fig. 8C with Fig. 4C). However, stable overexpression of wt CFTR in CFBE cells rescued (1) the cortical actin cytoskeletal organization (Fig 8D), (2) the apical CFTR expression (Fig 8D), and (3) the CFTR-dependent chloride secretion (Fig. 8E). Importantly, it also re-established the compartmentalization of cAMP at the plasma membrane (Fig. 8F).
Fig. 8.
Effect of CFTR expression on cytoskeletal organization and cAMP compartmentalization. (A–C) HBE polarized monolayers grown on permeable filters preincubated for 72 hours with CFTR-specific siRNA (HBE/siCFTR) and with the scramble siRNA (siNeg). (D–F) CFBE cells stably transfected with wtCFTR (CFBE/wtCFTR) or untransfected as a control (CFBE). (A,D) Confocal immunofluorescence microscopy. In both cases, cells were immunoblotted with a primary mouse monoclonal antibody (CF3) raised against the extracellular first loop of CFTR (green) or were incubated with phalloidin to visualize F-actin (red). All images are in the vertical (x-z) plane. (B,E) Summary of CFTR-dependent chloride effluxes in HBE/siCFTR and CFBE/wtCFTR polarized cell monolayers performed as indicated in Fig. 6. (C,F) FRET analysis of cAMP distribution in HBE/siCFTR and in CFBE/wtCFTR cells, respectively, transfected with both H30 sensors. The experiments were performed as described in Fig. 4. Scale bars: 10 μm. ***P<0.001; **P<0.01; *P<0.05.
Altogether, these data demonstrate that both the cytoskeleton organization and the intracellular distribution of cAMP and PKA are dependent on the formation of the multi-protein complex CFTR–NHERF1–ezrin–actin, which is driven by the expression of wt CFTR on the apical membrane.
Discussion
It is well established that the ΔF508 mutation does not simply result in altered trafficking and stability of CFTR but also associates with an activity deficit in intact cells. A number of biochemical and electrophysiological studies have clearly shown that ΔF508CFTR rescued at the plasma membrane by a number of different methods, including growth at low temperature (Denning et al., 1992) and treatment with chemical chaperons or inhibitors of protein degradation, still exhibits additional defects that limit ion transport, including reduced channel gating (Al-Nakkash and Hwang, 1999; Hwang et al., 1997; Wang et al., 1998). However, studies in cell-free systems and on isolated protein have shown that ΔF508CFTR retains normal PKA-dependent regulation and activity relative to wt CFTR (Li et al., 1993). Such a discrepancy suggests that factors related to the intracellular environment might influence the regulation of ΔF508CFTR.
Interaction of NHERF1 with the C-terminus PDZ target domain of CFTR has been proposed to have a central role both in the trafficking of CFTR and its stabilization at the apical membrane of airway epithelial cells (Moyer et al., 2000; Short et al., 1998). In addition, the interaction between NHERF1, ezrin and PKA has been hypothesized to be essential not only for anchoring CFTR to the cytoskeleton (Short et al., 1998) and for stabilizing it at the cell surface (Swiatecka-Urban et al., 2002), but also for regulating CFTR activity (Liedtke et al., 2002; Sun et al., 2000a). In previous studies, we (Guerra et al., 2005) and others (Bossard et al., 2007) have shown that overexpression of NHERF1 induces a significant redistribution of the ΔF508 mutant from the cytoplasm to the cell surface and rescues its PKA-dependent activity in a number of cell types. A mechanism involving binding of NHERF1 to ezrin and Rho-kinase-mediated tethering of CFTR to the actin cytoskeleton appears to be involved in this process (Favia et al., 2010). Here we extend these findings and show that overexpression of NHERF1 in CFBE cells restores the localization of PKA to the sub-plasma-membrane compartment and that such localization is required for PKA-mediated regulation of Cl– efflux. In addition, we show that, by promoting cytoskeleton organization, NHERF1 overexpression ensures that a barrier to cAMP diffusion is reconstituted in the subcortical compartment of CFBE cells, which allows appropriate local accumulation of cAMP. These findings are compatible with a model in which effective regulation of ΔF508CFTR activity not only requires stable expression of the mutant CFTR at the cell surface, but also depends on the generation of a cAMP signal of adequate amplitude, as well as on a sufficient amount of the effector PKA being localized close to its target. In agreement with this hypothesis, we also observed that disaggregation of the cytoskeleton using the F-actin-depolymerizing agent Latrunculin B, a treatment that inhibits Cl– efflux in both HBE cells and CFBE/sNHERF1 cells, resulted in a significant reduction of the subcortical cAMP signal and in the associated local PKA activity. It is interesting to note that, although HBE cells have comparable amounts of PKA in the cytosol and at the plasma membrane, the activity of PKA is higher at the plasma membrane, presumably as a consequence of the higher cAMP concentration present in the sub-plasma-membrane compartment than in the cytosol. Therefore, it appears that the local cAMP concentration, rather than the absolute PKA content, determines the level of PKA activity in the two compartments.
The extent to which the alterations in the compartmentalization of cAMP and PKA observed in CFBE cells represent a generalized defect of human CF epithelial cells remains to be established. However, we found that genetic knockdown of CFTR in HBE cells results in an altered compartmentalization of cAMP and that altered compartmentalization of cAMP is rescued in CFBE cells by overexpression of wt CFTR. Altogether, these data indicate that altered local control of cAMP and PKA activity is not a peculiar feature of CFBE cells and that our findings might have a more general relevance in the physiopathology of cystic fibrosis. In addition, the present findings are relevant because CFBE cells are currently used by a number of laboratories to study ΔF508CFTR biogenesis and activity, as well as to test the effectiveness of small molecules selected as ‘correctors’ of CFTR misprocessing in high-throughput screenings (Galietta et al., 2001). The effectiveness of pharmacological correctors appears to be highly dependent on the cell background on which they are assayed (Pedemonte et al., 2010; Rowe et al., 2010) and a number of these molecules do not restore cAMP-mediated activation in CFBE cells despite their ability to stabilize ΔF508CFTR at the plasma membrane (Pedemonte et al., 2005; Rowe et al., 2010). The results presented here provide a possible rationale for such findings and indicate that in selecting pre-clinical models for screening compounds with corrector properties the integrity of the subcortical cAMP–PKA signal transduction apparatus should be taken into account as this might impact the ability of the small molecules to affect CFTR function. In this respect, it is interesting to note that a number of correctors identified in primary screenings using cell lines overexpressing ΔF508CFTR have been found to perform poorly when tested in primary airway epithelial cells (Pedemonte et al., 2010) and limited rescue of CFTR activity has been reported in clinical trials (McCarty et al., 2002; Rubenstein and Zeitlin, 1998).
PDEs have been shown to play a key role in the spatial control of cAMP–PKA signalling in a number of cell systems, including airway epithelial cells, where both PDE3 (Penmatsa et al., 2010) and PDE4 have been suggested to contribute to the local modulation of cAMP levels that control CFTR activity (Barnes et al., 2005). Although PDE activity has been shown to be sufficient to restrict cAMP diffusion to defined subcellular compartments in certain cell types (Oliveira et al., 2010; Terrin et al., 2006), it has been argued that the densely packed subcortical cytoskeleton might constitute an efficient barrier to cAMP diffusion and promote compartmentalized cAMP and PKA signals by allowing cAMP to accumulate locally to a level sufficient to activate PKA, while preventing activation of PKA in the bulk cytosol (Rich et al., 2000). Here we show that the actin cytoskeleton can indeed act as a diffusional barrier for cAMP and that PDEs, although involved in the control of local cAMP levels, do not seem to play a major role in the generation of the membrane-to-cytosol cAMP gradient in the cells used in this study. It will be important in the future to verify these results in primary human epithelial cells.
With respect to the CF phenotype, our studies show that the altered cytoskeletal organization in CFBE cells results in reduced levels of the second messenger in the subcortical compartment and excessive accumulation of cAMP in the cytosol, and it is interesting to consider that the consequence of such disrupted compartmentalization may be twofold. On the one hand, low subcortical cAMP might contribute to the deficient regulation of Cl– efflux; on the other hand, excessive cytosolic cAMP might also have deleterious effects, including increased activation of the pro-inflammatory transcription factor NF-κB (Zhong et al., 1998). In this respect, many studies have found that CF airway epithelial cells have constitutively active NF-κB (reviewed by Machen, 2006), a feature that has been linked to the hyperinflammatory phenotype observed in CF (Konstan and Davis, 2002).
Materials and Methods
Cell culture
The experiments were performed with human bronchiolar epithelial cell lines: 16HBE14o-, expressing wild-type CFTR; CFBE41o-derived from a cystic fibrosis patient, homozygous for the ΔF508 allele (ΔF508/ΔF508); CFBE/wtCFTR, CFBE41o-cells stably transfected with wt CFTR protein (generous gift from Dieter Gruenert, University of California at San Francisco, CA); and CFBE/sNHERF1, CFBE41o-cells stably transfected with full-length NHERF1 protein (Favia et al., 2010). The cells were grown in Eagle’s minimal essential medium (MEM) (EuroClone) supplemented with 10% fetal bovine serum (Gibco), L-glutamine, penicillin and streptomycin at 37°C under 5% CO2 and routinely grown in plastic flasks coated with an extracellular matrix containing fibronectin, collagen and bovine serum albumin.
F-actin content in adherent cells
Actin polymerization assay was performed as previously described (Clements et al., 2005; Favia et al., 2010). Briefly, confluent cells, grown on coated 35 mm dishes, were fixed with 3.7% formaldehyde and permeabilized in 0.1% Triton X-100 in PBS. The cells were then incubated with 0.25 μM Phalloidin–TRITC in buffer containing 20 mM KH2PO4, 2 mM MgCl2, 5 mM EGTA and 10 mM PIPES (pH 6.8 with KOH) for 1 hour. Cells were incubated in methanol at 4°C overnight to extract phalloidin linked to F-actin. After extraction, the cells were washed with PBS and a Bradford Coomassie Plus Protein Assay (Pierce) was performed to determine total cell protein content. Fluorescence emission at 565 nm on excitation at 540 nm was measured with a Cary Eclipse plate reader (Varian). Fluorescence emission values were normalized to protein levels for each sample.
Immunofluorescence and confocal analysis
Cell monolayers, grown on 0.4 μM pore size PET filter inserts (Falcon Becton-Dickinson Labware), were fixed in 3.7% paraformaldehyde and permeabilized in 0.1% Triton X-100 in PBS. The permeabilization was omitted for monolayers of cells stained for a plant lectin, wheatgerm agglutinin (WGA) TRITC conjugate (100 mg/ml) (Sigma), or stained with anti-CFTR (CF3, Abcam) mouse monoclonal antibody (dilution 1:500). To analyze the distribution of PKA, monolayers were incubated with anti-PKA RIIβ (dilution 1:100) antibody (BD Transduction Laboratories) according to the manufacturer’s protocol. Goat anti-mouse IgG conjugated to Alexa Fluor 488 (dilution 1:1000) was used as secondary antibody.
Monolayers were stained with 20 nM phalloidin–TRITC to visualize F-actin and then mounted onto slides with Prolong Gold antifade reagent with DAPI (Invitrogen) and examined with Leica TCS SP5 II microscope equipped with a laser-scanning confocal unit containing a He-Ne argon laser (Leica). Specimens were viewed through a Planapo 63×/1.25 oil immersion objective and images were acquired in the horizontal (x-y) and in the vertical (x-z) planes by the LAS-AF Version 2.2.1 software.
Transfection
Cells were plated on 24 mm glass coverslips and at 70–80% confluence were transiently transfected with plasmid constructs encoding for the FRET sensor, using Myrus-LT1 trans-it transfection reagent according to the manufacturer’s protocol. FRET imaging was conducted 24 hours later.
Transfection of siRNA targeting CFTR
At 70–80% confluence, 16HBE14o-cells were transiently transfected with siRNA targeting CFTR (ON-TARGET plus SMART pool CFTR; Dharmacon) or with scrambled siRNA (Dharmacon) as a control. Transfection was performed using DharmaFECT 4 siRNA Transfection Reagent (Dharmacon) according to the manufacturer’s protocol and the experiments were conducted 72 hours later.
Measurements of cAMP levels and PKA activity by FRET
FRET measurements were performed as described (Di Benedetto et al., 2008). Changes in cAMP concentration using the cRII-ycat, mpcRII-ycat, H30 and mpH30 sensors were monitored by measuring CFP (480 nm)/YFP (545 nm) fluorescence emission values upon excitation of the transfected cells at 430 nm. PKA-mediated phosphorylation using AKAR3 and mpAKAR3 was monitored by measuring YFP (545 nm)/CFP (480 nm) fluorescence emission values upon excitation of the transfected cells at 430 nm. The ratio images are presented in intensity modulated display mode (IMD) and ratio intensity is displayed stretched between the low and high renormalization value, according to a temperature-based look-up table with red (hot) and blue (cold), indicating high and low values, respectively, of cAMP level or PKA activity (Fig. 3 and supplementary material Figs S2, S4).
Cells were imaged with a Nikon ECLIPSE TE 2000-S microscope equipped with a cooled CCD camera controlled by the Metafluor 4.6 software (Meta Imaging 4.6; Universal Imaging, Downingtown, PA). The set-up of the microscope and the filters used for dual emission imaging studies were previously described (Cardone et al., 2005). FRET changes are expressed as ΔR/R0 where R is the fluorescence emission intensity ratio at time t, R0 is the value of this ratio at time =0 seconds and ΔR=R–R0. Optimised concentrations of forskolin where used with different reporters to match the different sensitivity of individual sensors to cAMP changes.
Cell fractionation
Fractionation was performed essentially as previously described (Favia et al., 2010). An aliquot of 10 μg of protein from each membrane, cytosolic and cytoskeleton fraction was separated by 9% Tris-HCl SDS-PAGE. The primary antibody used was anti-PKA RIIβ monoclonal antibody (BD Tranduction Laboratories, dilution 1:1000). The secondary antibody was anti-mouse IgG (Sigma). Immunocomplexes were detected with ECL plus reagent (Amersham Biosciences) and densitometric quantification and image processing were carried out using Adobe Photoshop and the Image software package (version 1.61, National Institutes of Health, Bethesda, MD).
Fluorescence measurements of apical chloride efflux
Chloride efflux was measured using the Cl–-sensitive dye MQAE. Confluent cell monolayers, grown on permeable filters, were loaded overnight in culture medium containing 5 mM MQAE at 37°C in a CO2 incubator and then inserted into a perfusion chamber that allowed independent perfusion of apical and basolateral cell surfaces. Fluorescence was recorded with a Cary Eclipse Varian spectrofluorimeter. To measure chloride efflux rate across the apical membrane, the apical perfusion medium was changed to a medium in which chloride was substituted with iso-osmotic nitrate. All experiments were performed at 37°C in HEPES-buffered bicarbonate-free media [Cl– medium 135 mM NaCl, 3 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 20 mM HEPES, 1 mM KH2PO4, 11 mM glucose, and Cl– free-medium: 135 mM NaNO3, 3 mM KNO3, 0.8 mM MgSO4, 1 mM KH2PO4, 20 mM HEPES, 5 mM Ca(NO3)2, 11 mM glucose]. The apical CFTR-dependent chloride secretion was measured as previously described (Guerra et al., 2005). CFTR-dependent chloride secretion was calculated as the difference in the rate of change of Frsk plus IBMX-stimulated fluorescence in the absence or presence of apical treatment with the specific CFTR inhibitor, CFTRinh-172 (Ma et al., 2002; Taddei et al., 2004).
Data analysis
Data are presented as mean ± s.e. for the number of samples indicated (n). Statistical comparisons were made using unpaired data Student’s t-test. Differences were considered significant when P<0.05 (*P<0.05; **P<0.01; ***P<0.001).
Acknowledgements
The authors would like to thank Jing Zhang (Johns Hopkins, Baltimore, MD) for the AKAR3 sensors.
Footnotes
Funding
This work was supported by the Foundation Leducq [grant number O6 CVD 02], the British Heart Foundation [grant number PG/07/091/23698] and the NSF National Institutes of Health CRCNS program [grant number IH R01 AA18060] to M.Z. and by the Italian Cystic Fibrosis Research Foundation [grant number FFC1/2009] with the contribution of the ‘Delegazione FFC della Valdadige’, ‘Nicola Petruzzi e Delegazione FFC di Molfetta’, ‘Gli amici di Thomas con la Delegazione FFC di Vibo Valentia’, ‘Delegazione FFC’, ‘La Bottega delle Donne’ di Montebelluna Treviso and the Italian Cystic Fibrosis Research Foundation [grant number FFC 4/2011] with the contribution of the ParkinGO Oasi srl and the Delegazione FFC di Avellino. We also wish to thank ‘Centro di Eccellenza di Genomica in Campo Biomedico e Agrario’, CEGBA. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.089086/-/DC1
References
- Al-Nakkash L., Hwang T. C. (1999). Activation of wild-type and deltaF508-CFTR by phosphodiesterase inhibitors through cAMP-dependent and -independent mechanisms. Pflugers Arch. 437, 553–561 [DOI] [PubMed] [Google Scholar]
- Allen M. D., Zhang J. (2006). Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem. Biophys. Res. Commun. 348, 716–721 [DOI] [PubMed] [Google Scholar]
- Barnes A. P., Livera G., Huang P., Sun C., O’Neal W. K., Conti M., Stutts M. J., Milgram S. L. (2005). Phosphodiesterase 4D forms a cAMP diffusion barrier at the apical membrane of the airway epithelium. J. Biol. Chem. 280, 7997–8003 [DOI] [PubMed] [Google Scholar]
- Bear C. E., Li C., Kartner N., Bridges R. D., Jensen T. J., Ramjeesingh M., Riordan J. R. (1992). Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell 68, 809–818 [DOI] [PubMed] [Google Scholar]
- Bossard F., Robay A., Toumaniantz G., Dahimene S., Becq F., Merot J., Gauthier C. (2007). NHE-RF1 protein rescues DeltaF508-CFTR function. Am. J. Physiol. Lung Cell Mol. Physiol. 292, L1085–L1094 [DOI] [PubMed] [Google Scholar]
- Brown C. R., Hong-Brown L. Q., Biwersi J., Verkman A. S., Welch W. J. (1996). Chemical chaperones correct the mutant phenotype of the delta F508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperones 1, 117–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantiello H. F. (2001). Role of actin filament organization in CFTR activation. Pflugers Arch. 443 Suppl. 1, S75–S80 [DOI] [PubMed] [Google Scholar]
- Cardone R. A., Bagorda A., Bellizzi A., Busco G., Guerra L., Paradiso A., Casavola V., Zaccolo M., Reshkin S. J. (2005). Protein kinase A gating of a pseudopodial-located RhoA/ROCK/p38/NHE1 signal module regulates invasion in breast cancer cell lines. Mol. Biol. Cell 16, 3117–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Rich D. P., Marshall J., Gregory R. J., Welsh M. J., Smith A. E. (1991). Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell 66, 1027–1036 [DOI] [PubMed] [Google Scholar]
- Clements R. T., Minnear F. L., Singer H. A., Keller R. S., Vincent P. A. (2005). RhoA and Rho-kinase dependent and independent signals mediate TGF-beta-induced pulmonary endothelial cytoskeletal reorganization and permeability. Am. J. Physiol. Lung Cell Mol. Physiol. 288, L294–L306 [DOI] [PubMed] [Google Scholar]
- Denning G. M., Anderson M. P., Amara J. F., Marshall J., Smith A. E., Welsh M. J. (1992). Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature 358, 761–764 [DOI] [PubMed] [Google Scholar]
- Di Benedetto G., Zoccarato A., Lissandron V., Terrin A., Li X., Houslay M. D., Baillie G. S., Zaccolo M. (2008). Protein kinase A type I and type II define distinct intracellular signaling compartments. Circ. Res. 103, 836–844 [DOI] [PubMed] [Google Scholar]
- Favia M., Guerra L., Fanelli T., Cardone R. A., Monterisi S., Di Sole F., Castellani S., Chen M., Seidler U., Reshkin S. J., et al. (2010). Na+/H+ exchanger regulatory factor 1 overexpression-dependent increase of cytoskeleton organization is fundamental in the rescue of F508del cystic fibrosis transmembrane conductance regulator in human airway CFBE41o-cells. Mol. Biol. Cell 21, 73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Nairn A. C. (1999). Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol. Rev. 79, S77–S107 [DOI] [PubMed] [Google Scholar]
- Galietta L. J., Springsteel M. F., Eda M., Niedzinski E. J., By K., Haddadin M. J., Kurth M. J., Nantz M. H., Verkman A. S. (2001). Novel CFTR chloride channel activators identified by screening of combinatorial libraries based on flavone and benzoquinolizinium lead compounds. J. Biol. Chem. 276, 19723–19728 [DOI] [PubMed] [Google Scholar]
- Ganeshan R., Nowotarski K., Di A., Nelson D. J., Kirk K. L. (2007). CFTR surface expression and chloride currents are decreased by inhibitors of N-WASP and actin polymerization. Biochim. Biophys. Acta 1773, 192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman M. S., Kannegaard E. S., Kopito R. R. (2002). A principal role for the proteasome in endoplasmic reticulum-associated degradation of misfolded intracellular cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 277, 11709–11714 [DOI] [PubMed] [Google Scholar]
- Guerra L., Fanelli T., Favia M., Riccardi S. M., Busco G., Cardone R. A., Carrabino S., Weinman E. J., Reshkin S. J., Conese M., et al. (2005). Na+/H+ exchanger regulatory factor isoform 1 overexpression modulates cystic fibrosis transmembrane conductance regulator (CFTR) expression and activity in human airway 16HBE14o-cells and rescues DeltaF508 CFTR functional expression in cystic fibrosis cells. J. Biol. Chem. 280, 40925–40933 [DOI] [PubMed] [Google Scholar]
- Haggie P. M., Stanton B. A., Verkman A. S. (2004). Increased diffusional mobility of CFTR at the plasma membrane after deletion of its C-terminal PDZ binding motif. J. Biol. Chem. 279, 5494–5500 [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Schafer P., Zhang K. Y. (2005). Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov. Today 10, 1503–1519 [DOI] [PubMed] [Google Scholar]
- Huang P., Lazarowski E. R., Tarran R., Milgram S. L., Boucher R. C., Stutts M. J. (2001). Compartmentalized autocrine signaling to cystic fibrosis transmem-brane conductance regulator at the apical membrane of airway epithelial cells. Proc. Natl. Acad. Sci. USA 98, 14120–14125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T. C., Wang F., Yang I. C., Reenstra W. W. (1997). Genistein potentiates wild-type and delta F508-CFTR channel activity. Am. J. Physiol. 273, C988–C998 [DOI] [PubMed] [Google Scholar]
- Jin S., Haggie P. M., Verkman A. S. (2007). Single-particle tracking of membrane protein diffusion in a potential: simulation, detection, and application to confined diffusion of CFTR Cl-channels. Biophys. J. 93, 1079–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klussmann E., Maric K., Wiesner B., Beyermann M., Rosenthal W. (1999). Protein kinase A anchoring proteins are required for vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J. Biol. Chem. 274, 4934–4938 [DOI] [PubMed] [Google Scholar]
- Konstan M. W., Davis P. B. (2002). Pharmacological approaches for the discovery and development of new anti-inflammatory agents for the treatment of cystic fibrosis. Adv. Drug Deliv. Rev. 54, 1409–1423 [DOI] [PubMed] [Google Scholar]
- Li C., Naren A. P. (2010). CFTR chloride channel in the apical compartments: spatiotemporal coupling to its interacting partners. Integr. Biol. 2, 161–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Ramjeesingh M., Reyes E., Jensen T., Chang X., Rommens J. M., Bear C. E. (1993). The cystic fibrosis mutation (delta F508) does not influence the chloride channel activity of CFTR. Nat. Genet. 3, 311–316 [DOI] [PubMed] [Google Scholar]
- Liedtke C. M., Yun C. H., Kyle N., Wang D. (2002). Protein kinase C epsilon-dependent regulation of cystic fibrosis transmembrane regulator involves binding to a receptor for activated C kinase (RACK1) and RACK1 binding to Na+/H+ exchange regulatory factor. J. Biol. Chem. 277, 22925–22933 [DOI] [PubMed] [Google Scholar]
- Ma T., Thiagarajah J. R., Yang H., Sonawane N. D., Folli C., Galietta L. J., Verkman A. S. (2002). Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J. Clin. Invest. 110, 1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machen T. E. (2006). Innate immune response in CF airway epithelia: hyperinflammatory? Am. J. Physiol. Cell Physiol. 291, C218–C230 [DOI] [PubMed] [Google Scholar]
- McCarty N. A., Standaert T. A., Teresi M., Tuthill C., Launspach J., Kelley T. J., Milgram L. J., Hilliard K. A., Regelmann W. E., Weatherly M. R., et al. (2002). A phase I randomized, multicenter trial of CPX in adult subjects with mild cystic fibrosis. Pediatr. Pulmonol. 33, 90–98 [DOI] [PubMed] [Google Scholar]
- Mohler P. J., Kreda S. M., Boucher R. C., Sudol M., Stutts M. J., Milgram S. L. (1999). Yes-associated protein 65 localizes p62(c-Yes) to the apical compartment of airway epithelia by association with EBP50. J. Cell Biol. 147, 879–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer B. D., Duhaime M., Shaw C., Denton J., Reynolds D., Karlson K. H., Pfeiffer J., Wang S., Mickle J. E., Milewski M., et al. (2000). The PDZ-interacting domain of cystic fibrosis transmembrane conductance regulator is required for functional expression in the apical plasma membrane. J. Biol. Chem. 275, 27069–27074 [DOI] [PubMed] [Google Scholar]
- Okiyoneda T., Lukacs G. L. (2007). Cell surface dynamics of CFTR: the ins and outs. Biochim. Biophys. Acta 1773, 476–479 [DOI] [PubMed] [Google Scholar]
- Oliveira R. F., Terrin A., Di Benedetto G., Cannon R. C., Koh W., Kim M., Zaccolo M., Blackwell K. T. (2010). The role of type 4 phosphodiesterases in generating microdomains of cAMP: large scale stochastic simulations. PLoS ONE 5, e11725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedemonte N., Sonawane N. D., Taddei A., Hu J., Zegarra-Moran O., Suen Y. F., Robins L. I., Dicus C. W., Willenbring D., Nantz M. H., et al. (2005). Phenylglycine and sulfonamide correctors of defective delta F508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Mol. Pharmacol. 67, 1797–1807 [DOI] [PubMed] [Google Scholar]
- Pedemonte N., Tomati V., Sondo E., Galietta L. J. (2010). Influence of cell background on pharmacological rescue of mutant CFTR. Am. J. Physiol. Cell Physiol. 298, C866–C874 [DOI] [PubMed] [Google Scholar]
- Penmatsa H., Zhang W., Yarlagadda S., Li C., Conoley V. G., Yue J., Bahouth S. W., Buddington R. K., Zhang G., Nelson D. J., et al. (2010). Compartmentalized cyclic adenosine 3′,5′-monophosphate at the plasma membrane clusters PDE3A and cystic fibrosis transmembrane conductance regulator into microdomains. Mol. Biol. Cell 21, 1097–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsioen B., Zhao J., Riedl J., Zwartkruis F., van der Krogt G., Zaccolo M., Moolenaar W. H., Bos J. L., Jalink K. (2004). Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 5, 1176–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton P. M. (1983). Chloride impermeability in cystic fibrosis. Nature 301, 421–422 [DOI] [PubMed] [Google Scholar]
- Rich T. C., Fagan K. A., Nakata H., Schaack J., Cooper D. M., Karpen J. W. (2000). Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J. Gen. Physiol. 116, 147–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. R. (1993). The cystic fibrosis transmembrane conductance regulator. Annu. Rev. Physiol. 55, 609–630 [DOI] [PubMed] [Google Scholar]
- Rowe S. M., Pyle L. C., Jurkevante A., Varga K., Collawn J., Sloane P. A., Woodworth B., Mazur M., Fulton J., Fan L., et al. (2010). DeltaF508 CFTR processing correction and activity in polarized airway and non-airway cell monolayers. Pulm. Pharmacol. Ther. 23, 268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein R. C., Zeitlin P. L. (1998). A pilot clinical trial of oral sodium 4-phenylbutyrate (Buphenyl) in deltaF508-homozygous cystic fibrosis patients: partial restoration of nasal epithelial CFTR function. Am. J. Respir. Crit. Care Med. 157, 484–490 [DOI] [PubMed] [Google Scholar]
- Short D. B., Trotter K. W., Reczek D., Kreda S. M., Bretscher A., Boucher R. C., Stutts M. J., Milgram S. L. (1998). An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J. Biol. Chem. 273, 19797–19801 [DOI] [PubMed] [Google Scholar]
- Sloane P. A., Rowe S. M. (2010). Cystic fibrosis transmembrane conductance regulator protein repair as a therapeutic strategy in cystic fibrosis. Curr. Opin. Pulm. Med. 16, 591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F., Hug M. J., Bradbury N. A., Frizzell R. A. (2000a). Protein kinase A associates with cystic fibrosis transmembrane conductance regulator via an interaction with ezrin. J. Biol. Chem. 275, 14360–14366 [DOI] [PubMed] [Google Scholar]
- Sun F., Hug M. J., Lewarchik C. M., Yun C. H., Bradbury N. A., Frizzell R. A. (2000b). E3KARP mediates the association of ezrin and protein kinase A with the cystic fibrosis transmembrane conductance regulator in airway cells. J. Biol. Chem. 275, 29539–29546 [DOI] [PubMed] [Google Scholar]
- Swiatecka-Urban A., Duhaime M., Coutermarsh B., Karlson K. H., Collawn J., Milewski M., Cutting G. R., Guggino W. B., Langford G., Stanton B. A. (2002). PDZ domain interaction controls the endocytic recycling of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 277, 40099–40105 [DOI] [PubMed] [Google Scholar]
- Taddei A., Folli C., Zegarra-Moran O., Fanen P., Verkman A. S., Galietta L. J. (2004). Altered channel gating mechanism for CFTR inhibition by a high-affinity thiazolidinone blocker. FEBS Lett. 558, 52–56 [DOI] [PubMed] [Google Scholar]
- Terrin A., Di Benedetto G., Pertegato V., Cheung Y. F., Baillie G., Lynch M. J., Elvassore N., Prinz A., Herberg F. W., Houslay M. D., et al. (2006). PGE(1) stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: role of compartmentalized phosphodiesterases. J. Cell Biol. 175, 441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Zeltwanger S., Yang I. C., Nairn A. C., Hwang T. C. (1998). Actions of genistein on cystic fibrosis transmembrane conductance regulator channel gating. Evidence for two binding sites with opposite effects. J. Gen. Physiol. 111, 477–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C. L., Kopito R. R. (1994). Intracellular turnover of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 41, 25710–25718 [PubMed] [Google Scholar]
- Ward C. L., Omura S., Kopito R. R. (1995). Degradation of CFTR by the ubiquitin-proteasome pathway. Cell 83, 121–127 [DOI] [PubMed] [Google Scholar]
- Zaccolo M., De Giorgi F., Cho C. Y., Feng L., Knapp T., Negulescu P. A., Taylor S. S., Tsien R. Y., Pozzan T. (2000). A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nat. Cell Biol. 2, 25–29 [DOI] [PubMed] [Google Scholar]
- Zeitlin P. L. (2000). Pharmacologic restoration of delta F508 CFTR-mediated chloride current. Kidney Int. 57, 832–837 [DOI] [PubMed] [Google Scholar]
- Zhong H., Voll R. E., Ghosh S. (1998). Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1, 661–671 [DOI] [PubMed] [Google Scholar]