Abstract
In the spring of 2009, New York City (NYC) experienced the emergence and rapid spread of pandemic influenza A H1N1 virus (pH1N1), which had a high attack rate in children and caused many school closures. During the 2009 fall wave of pH1N1, a school-located vaccination campaign for elementary schoolchildren was conducted in order to reduce infection and transmission in the school setting, thereby reducing the impact of pH1N1 that was observed earlier in the year. In this paper, we describe the planning and outcomes of the NYC school-located vaccination campaign. We compared consent and vaccination data for three vaccination models (school nurse alone, school nurse plus contract nurse, team). Overall, >1,200 of almost 1,600 eligible schools participated, achieving 26.8% consent and 21.5% first-dose vaccination rates, which did not vary significantly by vaccination model. A total of 189,902 doses were administered during two vaccination rounds to 115,668 students at 998 schools included in the analysis; vaccination rates varied by borough, school type, and poverty level. The team model achieved vaccination of more children per day and required fewer vaccination days per school. NYC’s campaign is the largest described school-located influenza vaccination campaign to date. Despite substantial challenges, school-located vaccination is feasible in large, urban settings, and during a public health emergency.
Keywords: H1N1 influenza, Vaccination, Elementary schoolchildren
In the spring of 2009, New York City (NYC) experienced the emergence and rapid spread of pandemic influenza A H1N1 virus (pH1N1).1 Due to a high attack rate and initial concern about disease severity, over 50 NYC schools with high rates of influenza-like illness (ILI) were closed between April and June 2009. A population-based survey conducted in June 2009 estimated that 12% of New Yorkers (~1 million people) had ILI in the previous 30 days.2 The attack rate was highest among children, with approximately 21% of 0–17 year olds reporting ILI. Because of the high attack rates in children and to reduce infection and transmission in the school setting, as well as to reduce community spread of pH1N1, the NYC Department of Health and Mental Hygiene (DOHMH) launched a school-located vaccination campaign during the 2009 fall wave of pH1N1.
Schools are increasingly recognized as optimal locations for vaccinations since they provide the opportunity to reach almost all children and have the potential to reach children who do not have direct access to a healthcare provider.3 Vaccination of children in the school setting has been shown to reduce absenteeism of students and school staff due to influenza or ILI.4–6 In addition, vaccinating school-aged children may impact community influenza transmission.7 Vaccinating schoolchildren reduced adult influenza deaths in Japan8 and medically attended acute respiratory illness in adults 35 years old and over,9 and vaccinating children and adolescents with inactivated influenza vaccine protected unimmunized residents of rural communities against influenza.10 This paper describes the planning and implementation of the 2009 DOHMH school-located pH1N1 vaccination campaign, outcomes, and key lessons learned.
Methods
Program Description
NYC is composed of five boroughs: Bronx, Brooklyn, Manhattan, Queens, and Staten Island and has a population of over 8.3 million people.10 Almost three million residents are foreign-born, and nearly half speak a language other than English at home.10 The NYC school system is the largest in the country, with over 1,600 public schools and 900 nonpublic schools and approximately 1.4 million students.11,12 Over 1,300 DOHMH nurses provide school health services: approximately 800 in public elementary schools, 196 in special education schools, 190 in nonpublic schools, and 114 in either public intermediate schools or high schools.
The school-located pH1N1 vaccination campaign targeted elementary school students aged 4 years and older. Inactivated and live, attenuated (LAIV) pH1N1 monovalent vaccines were offered. Upon completion of the first dose (round 1), a second dose was offered to children <10 years of age based on the Centers for Disease Control and Prevention’s (CDC) recommendations (round 2).13 The vaccination campaign operated from October 2009 through March 2010. All public elementary schools and public schools with elementary schoolchildren were mandated to participate in the program by the Department of Education (DOE), which numbered 925 schools, including 149 special education schools serving cognitively delayed, emotionally delayed, or disabled children. The 652 eligible nonpublic schools with elementary schoolchildren were offered the opportunity but were not mandated to participate. Whether or not schools were mandated, parental consent was required to administer vaccine.
Planning Timeline and Vaccination Strategies
Campaign planning began in August 2009 and was led by the DOHMH. A multi-agency planning committee was formed, including DOE and NYC Office of Emergency Management. Vaccine and ancillary supplies were provided by the federal government; additional costs (contract nurses, supplies and equipment, and logistics) were funded by the CDC Public Health Emergency Response grant.
Three main strategies were employed to provide vaccine. In schools with an enrollment of <400 students, the on-site school nurse was responsible for vaccinating children with signed consent forms; this was in addition to regular duties and largely not done as dedicated clinics. The school nurse was given 18 days to complete round 1 of vaccination and 15 days to complete round 2. For schools with 400–600 students, a supplemental contract nurse was assigned for 3–4 days to assist the school nurse with vaccinations. In schools with >600 students, mobile vaccination teams or simply “teams” (described below) were assigned for 1–2 days per school. Participating nonpublic schools with nurses and/or physicians onsite had the option of receiving supplies and vaccinating students on their own; since only 19 schools did this and implementation was different for each one, this was not considered a primary model and associated data are not included in the ensuing analysis. Eighty three public school-based health centers were provided with vaccine but not managed by this campaign.
Implementation, Assumptions, and Oversight
DOHMH created 42 teams and nine “strike teams” that were each staffed with eight to nine people: one team leader, three to four support staff, and four nurse vaccinators. The strike teams were created to assist in completing vaccination at schools when a team was unable to do so. DOHMH staff and staff members from eight other city agencies1 were deployed for an 8–10 week period to serve as team support staff. The team nurses were staffed by contract agencies; teams were overseen by DOHMH field supervisors who traveled between schools to assist and troubleshoot.
Assuming a maximum 50% parental consent rate, supplies and vaccine were ordered and delivered to each school to support vaccination of half the enrolled students; DOHMH provided 50% LAIV and 50% inactivated vaccine for each school as supply allowed. Schools that did not have both a school nurse and at least 50 consents were not eligible to participate in the campaign. Based on data from NYC senior center vaccination clinics, DOHMH assumed that each of four nurses supported by a team could vaccinate up to 100 students per school day (300–400 students per team). A school nurse and contract nurse together were expected to vaccinate 40 students per day, while a school nurse alone was expected to vaccinate 10 students per day. Initially, it was assumed that children who submitted consents past the deadline would not be vaccinated and that only second doses would be given during round 2. These assumptions were modified during the campaign since a significant number of consents were received after the deadline and some children still needed a first dose during the second round.
To oversee day-to-day operations, DOHMH implemented a command and control structure staffed by 25 people, including five regional coordinators who were each assigned to oversee activities in one borough. These staff held daily meetings to review emerging issues and implement real-time improvements. The operation was managed from the DOHMH Department Emergency Operations Center. In total, 1,402 staff were involved with implementation: 25 command and control; 167 staff from DOHMH and 239 staff from other City agencies served as team leaders, support staff for the teams, and field monitors; 571 school health nurses; and 400 contract nurses.
Campaign Promotion
A mayoral press conference in early September 2009 announced the launch of the campaign. Throughout the campaign, additional press conferences and press releases provided the public with information regarding campaign status and reminded parents of the opportunity. Media coverage and advertisements in television, radio, and print encouraged individuals to get vaccinated.
An informational packet that was translated into nine languages2 included an introductory letter, Consent/Screening Form, the Vaccine Information Statement, and a Health Insurance Portability and Accountability Act Notice of Privacy. Informational packets were sent home with students and parents were asked to return the screening/consent forms to the school within 3 days of receipt. Each principal was asked to identify a school designee who would be responsible for collecting the consent forms and reviewing them for completeness.
Data Collection and Analysis
The school nurse or school designee at each school was responsible for entering the number of consents received daily into a customized, web-based application called ILI Tracker. To facilitate collection of information for billing and entry into the Citywide Immunization Registry3 (CIR), DOHMH developed a scannable consent form which was collected after each round of vaccination. After an outside vendor scanned and validated (through human review) any fields that were questionable, the data were transmitted electronically to be uploaded into the CIR.
Lessons learned were collected via debriefing sessions with the planning team, vaccination teams, and oversight staff during and after the campaign. Postcampaign online surveys were distributed to public school principals, school nurses, and all campaign staff.
Consent rates (number of consents/total school enrollment) and vaccination rates (number of first-dose vaccinations/total school enrollment) were calculated by school type (public vs. nonpublic) and by NYC borough. Data sources were ILI tracker for consents, DOE for school enrollments, and CIR for vaccinations. Comparisons of the three primary vaccination models were made with regard to: number of schools, number of consents, average consent rate, number of first doses of vaccine, and average vaccination rate per day. For analyses of consent and vaccination rates, only the subset of schools that had both consent data in ILI tracker and vaccination data in CIR were included.
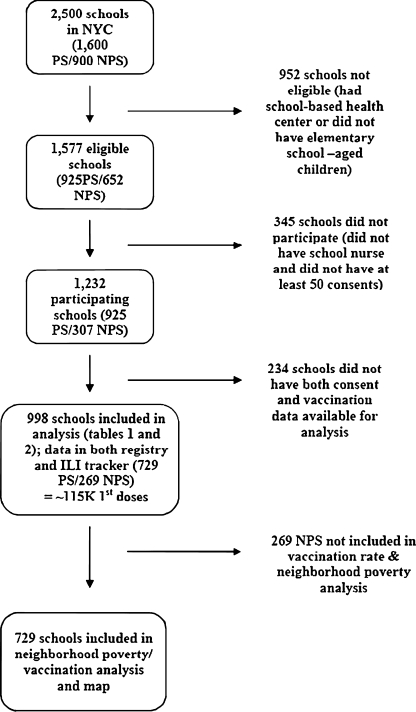
To examine whether area socioeconomic status impacted consent and vaccination rates, average consent rates among 729 participating public schools in each of 42 neighborhoods were plotted against neighborhood poverty levels, and average vaccination rates were computed, mapped, and compared to a map of neighborhood poverty levels. Neighborhood poverty indicates resource availability and can serve as a proxy for individual income, both of which can impact a person’s health status. Neighborhood poverty was defined as the percent of residents in an area living below the federal poverty level, according to the US Census 2000. In examining health disparities, the DOHMH has defined a high-poverty neighborhood as one that has >30% of its residents living below poverty, a medium-poverty neighborhood as one with 20%–29.9% of residents below poverty, and a low-poverty neighborhood as one that has <20% of its residents living below the poverty level.14 Nonpublic schools were not included for this analysis as the majority of their students attend schools outside of their neighborhoods. Data were analyzed using STATA 7.0 (STATA Corp., College Station, TX, USA). A summary of schools included in each stage of the campaign and analysis is provided in Figure 1.
FIGURE 1.
Flow chart of schools included in the analysis, school-located pandemic H1N1 influenza vaccination campaign, New York City 2009–2010. PS public schools, NPS nonpublic schools.
Results
Vaccination Outcomes
A total of 1,232 schools with an enrollment of 571,282 students participated in the campaign. All eligible elementary public schools (n = 925) and 47% (307/652) of eligible nonpublic elementary schools participated.
Of 1,232 participating schools, 998 (81%) had both vaccination records in the CIR and consent data in ILI tracker, and were included in this analysis. These 729 public and 269 nonpublic schools accounted for 189,902 administered vaccine doses; 115,668 children received a first dose and 74,234 of those (64.2%) received a second dose in school. Of all pH1N1 vaccine doses given, 45% were inactivated vaccine and 55% were LAIV. Of vaccinated students, 49.9% were male and the median age was 8 years.
The 998 matched schools combined had 537,353 students, an overall consent rate of 26.8% (144,060/537,353), and a first-dose vaccination rate of 21.5% (115,668/537,353). Consent levels between public and nonpublic school students were similar (26.6% vs. 28.0%, respectively), as were vaccination rates (21.8% vs. 20.3%, respectively). Vaccination rates were lower than consent rates because some children with signed consent forms had missing data, were absent on vaccination day, or had contraindications to vaccination. Consent and vaccination rates varied by borough (Table 1) with rates lowest in Staten Island (consent, 20.5%; vaccination, 16.9%) and highest in Manhattan (consent, 34.6%; vaccination, 27.5%). A comparison of vaccination models, which is also a comparison of school size, is shown in Table 2. Overall, the team model was used most often and predominantly in public schools. Similar consent rates and average vaccination rates were seen across all models; approximately 27% of students had consents, leading to an overall vaccination rate of ~21%. Teams achieved an average of 123 first dose vaccinations per vaccination day. The school nurse plus contract nurse and school nurse models achieved an average of 14 and nine first dose vaccinations per vaccination day, respectively.
Table 1.
Enrollment, and consent and vaccination rates by borough (998 schools), school-located pandemic H1N1 influenza vaccination campaign, New York City 2009–2010
| Manhattan | Bronx | Brooklyn | Staten Island | Queens | |
|---|---|---|---|---|---|
| Total enrollment | 71,015 | 106,187 | 157,828 | 40,687 | 161,636 |
| Overall consent ratea | 34.6% | 25.7% | 22.7% | 20.5% | 29.7% |
| Overall vaccination ratea (percent of enrollment) | 27.5% | 18.4% | 19.4% | 16.9% | 24.2% |
aExcludes one Queens, one Bronx, one Manhattan, and two Brooklyn schools without recorded consents
Table 2.
Comparison of vaccination models (998 schools), school-located pandemic H1N1 influenza vaccination campaign, New York City 2009–2010
| School nurse only (<400 students) | School nurse + contract nurse (400–600 students) | Team (>600 students) | |
|---|---|---|---|
| Number of schools (includes public and nonpublic) | 350 | 221 | 427 |
| Number of nonpublic schools | 160 | 45 | 64 |
| Total enrollment | 97,954 | 110,052 | 329,347 |
| Average enrollment/school | 280 | 498 | 771 |
| Total number of consents | 25,547a | 26,821 | 91,692b |
| Average consent rate | 26.1%a | 24.4% | 27.8%b |
| Total number of first dose vaccines | 20,769 | 21,143 | 73,756 |
| Average vaccination rate (percent of enrollment) | 21.2%a | 19.2% | 22.4%b |
| Average number vaccinated per vaccination day | 9 | 14 | 123 |
aExcludes four schools without recorded consents
bExcludes one school without recorded consents
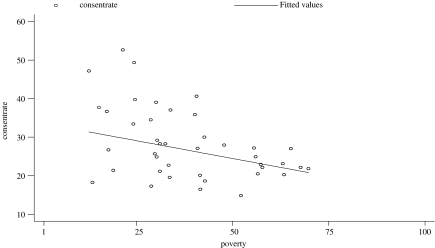
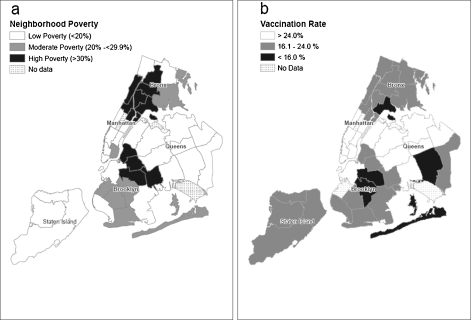
As neighborhood poverty levels increased, average consent levels of schools in those neighborhoods linearly decreased (Figure 2, r = −0.34, p < 0.0001). There was a strong correlation between neighborhood-level consent and vaccination rates (r = 0.89). Figure 3 displays poverty level and average vaccination rates by neighborhood. Average vaccination rates ranged from 13.1% in Rockaway (Queens) to 46% in Greenwich Village–Soho (Manhattan).
FIGURE 2.
Consent rates by neighborhood poverty (729 schools), school-located pandemic H1N1 influenza vaccination campaign, New York City 2009–2010. r = −0.34; 95% CI −0.28, −0.41.
FIGURE 3.
a Map of poverty level by neighborhood. b Map of vaccination rates by neighborhood (729 schools), school-located pandemic H1N1 influenza vaccination campaign, New York City 2009–2010. Source: US Census 2000.
Lessons Learned and Recommendations
A sufficient planning period is critical for a large and complex campaign. Scheduling schools and organizing teams, school nurses, and contract nurses was complicated by several factors, such as the large number and type of schools, schedule revisions to accommodate state testing days and parent–teacher conferences, and the scheduling of round 2. Late schedule changes caused confusion among principals and teams. When planning future campaigns, it is important that jurisdictions weigh implementation options against operational capacity. From NYC’s experience, complexity could be reduced by implementing just one vaccination model; multiple strategies made the implementation of protocols difficult to manage. For instance, using teams even for smaller schools could work well if they were scheduled for half days or staffed with fewer team members. Providing only one dose of seasonal influenza vaccine in the future, while informing the parent or guardian about the possible need for a second, would also greatly lessen the logistical burden.
Critical to the success of NYC’s campaign was the contract with the Strategic National Stockpile receiving, staging, and storing vendor to manage ordering, packaging, delivery, and resupply of vaccine and supplies. This capacity has been established since 2002. Working with an experienced vendor who had an existing relationship and understanding of DOHMH operations reduced the logistical challenges inherent in such a compressed and far-reaching campaign.
A robust information technology (IT) system that manages large amounts of data is critical. The planning team did not have adequate time to work with the agency’s IT department to develop the necessary data system. Therefore, disparate systems were used to track staff, payroll, consents, vaccinations, supplies, and the schedule. When components of the campaign changed, these systems were unable to meet evolving data management needs. IT should be involved early in the planning process to develop the necessary system, and staff with program evaluation and analytic expertise should be consulted to anticipate data needs for evaluation. A web-based registration and scheduling site for principals to choose their vaccination days, as used by the Hawaii Department of Health for their seasonal influenza vaccination program, would address many of the scheduling challenges we faced.15
We recommend clear protocols for identifying children to be vaccinated and for withdrawing consents, which is crucial for minimizing vaccination errors (vaccinating the wrong child or vaccinating a child without consent). Putting the onus on children to identify themselves correctly is problematic as some young children may not know their names and birthdays or be too shy to speak to vaccination staff. Thus, we recommend involvement of school staff during vaccination days, ideally those who can assist in the identification of children. One solution that was used in some schools was to put name tags on the children before they were sent for vaccination.
The level of outreach conducted by each school varied greatly and likely impacted consent rates. For example, some schools had parent volunteers that contacted other parents and reminded them about returning consent forms. Others had nurses and principals actively involved in outreach. In addition to materials that were sent home to parents with the students, some schools utilized other methods to promote the campaign and increase consent form return, including school websites, newsletters, emails, phone calls, and auto-dial reminders. Future campaigns should provide outreach activities and materials to each school and measure their impact. In order to increase consent rates, forms should be easy to understand, as postcampaign surveys of nurses found that the number of different languages spoken by parents and the length and complexity of the forms may have been barriers to completion. Consent forms could be sent home with children multiple times to increase the chances that they reach parents, since it has been found that a major reason for parents not returning consent forms is because they never received them.16
The structure implemented for oversight was critical throughout the campaign. Having staff with appropriate expertise in a central location facilitated effective communications and the rapid troubleshooting needed to manage a large and fast-moving campaign.
Discussion
Despite the condensed planning period and with substantial resources (>$15 million in staff time and resources),17 the DOHMH was able to offer pH1N1 vaccine to over 500,000 elementary children. Overall, the NYC school-located pH1N1 vaccination campaign was successful, reaching over 1,200 schools twice and over 115,000 students who may have otherwise been at risk for acquiring pH1N1 infection.
NYC’s school-located first-dose vaccination rate reached 21.5%, which is lower than published findings from other jurisdictions.4,15,18,19 There are several possible reasons for the lower rate. First, the condensed planning period did not allow for comprehensive outreach. Second, there was significant media coverage related to the 2009 pandemic and the pH1N1 vaccine, but it is unknown whether news stories positively or negatively impacted vaccination rates. Third, NYC experienced its first wave of the pandemic during the previous spring, with relatively low levels of disease and panic in the fall, and therefore, perhaps less concern about the need for vaccination. Fourth, the school-located campaign was one of three pH1N1 vaccine distribution strategies implemented by DOHMH. The other two were via healthcare providers and large scale community “Points of Dispensing” sites;20 over half the 252,837 vaccinated 4–10-year-old children in NYC were vaccinated outside of the school setting. Promotional materials received by parents could have motivated some to have their child vaccinated outside of the school setting. While reasons for nonconsent for school-located vaccination were not investigated during this campaign, 38% of parents in national opinion polls who chose not to get their children vaccinated indicated that they were concerned about side effects.21
The number of children vaccinated per day by the team model, in particular, was considerably lower than our planning assumption (123 vs. 300–400), which was overly optimistic based on experience with vaccination clinics held at senior centers. This may be because consent numbers were low and teams could have completed more vaccinations had there been more students to vaccinate, or because the students were younger and possibly less cooperative, requiring more time to vaccinate compared to seniors. Nevertheless, we consider the team model as the best approach to deliver influenza vaccine in the school setting. This approach should be considered in jurisdictions with large school enrollment as more children are vaccinated per day compared to the other models and it requires fewer vaccination days overall, resulting in less disruption to the medical room and school nurses’ daily responsibilities.
The inverse relationship observed between vaccination rate and poverty level has been described elsewhere.18 Our geographic analysis highlighted differences in pH1N1 vaccination rates by neighborhood, underscoring the continued importance of targeted outreach, not only in neighborhoods with high poverty and low vaccination rates, but also in low poverty areas with low vaccination rates. Interestingly, neighborhoods identified as having the lowest pH1N1 school vaccination rates coincided with neighborhoods previously noted to have lower coverage for influenza vaccination in persons 65 and older.22 Although we did not systematically collect data on promotional efforts in schools according to their area poverty levels, factors influencing the acceptance of vaccination in urban areas, as well as the direct relationship between poverty and consent and vaccination rates, should be further explored as they may affect the success of future school-located campaigns.
There were some limitations to our analysis. The accuracy and completeness of consent data, reported by school nurses or a school designee, varied greatly among schools and a reliable system for validating the information was not developed. Under-reporting may have occurred due to busy school medical rooms and/or unfamiliarity with the online reporting system, resulting in an under-estimation of the consent rate. At least 5,000 forms were not entered into the registry because of missing information; however, this omission resulted in a relatively small (<3%) underestimation of the number of doses administered and children vaccinated. Finally, our analysis was limited to 998 schools that had vaccination and consent data. However, this subset of schools was likely representative of all schools included in the campaign as they were located in every borough and their students had identical characteristics (e.g., age and sex) as all students vaccinated through the school-located program who appeared in the registry.
New York City’s school-located pH1N1 vaccination campaign is the largest described citywide school-located influenza vaccination campaign to date. There were substantial expenses, challenges, and major lessons learned. This paper illustrates the complexities of planning and implementing such a campaign, and contributes to the growing literature on school-located influenza vaccination programs by demonstrating its feasibility in a large, urban setting and during a public health emergency. Had pH1N1 infection been more severe, the opportunity to vaccinate school-age children would have been even more essential in reducing morbidity and mortality. As such, school-located vaccinations should be considered during future seasonal influenza seasons and pandemics.
Acknowledgments
This campaign would not have been possible without the contributions from the NYC Department of Education, the Office of School Health, and the NYC Office of Emergency Management. The authors also thank the following agencies for providing the additional personnel necessary to staff this campaign: NYC Department of Transportation, Department of Finance, Department of Housing Preservation and Development, Department of Citywide Administrative Services, Human Resources Administration, NYC Housing Authority, Parks Department, and the Department of Environmental Protection. The authors would like to thank the following individuals for providing data used in this paper: Angel Aponte, Tai Baker, Christine Edillon, Richard Fox, Ayaan Gedi, Christopher Goranson, Janet King, Vassiliki Papadouka, Nora Puffet, Susan Resnick, and Gina Romeo. The authors would also like to thank Stephanie Keating, Carole Marchese, Monica Marquez, Meghan Mcginty, Despina Zaharakis, and Thomas Farley for reviewing this manuscript.
Footnotes
NYC Department of Transportation, Department of Finance, Department of Housing Preservation and Development, Department of Citywide Administrative Services, Human Resources Administration, NYC Housing Authority, Parks Department, and the Department of Environmental Protection
Arabic, Bengali, Chinese, French, Haitian-Creole, Korean, Russian, Spanish, and Urdu
The Citywide Immunization Registry is a population-based database that contains immunizations of children 0–18 years of age. All providers in NYC are required to participate by law (New York City Department of Health and Mental Hygiene. New York City Health Code, section 11.04. Available at: http://www.nyc.gov/html/doh/downloads/pdf/cir/healthcode2005.pdf. Accessed January 2, 2009).
This publication was supported by the Public Health Emergency Preparedness (PHEP) Cooperative Agreement (grant number: 5U90TP221298-08) and the Public Health Emergency Response Grant (funding opportunity number: CDC-RFA-TP09-902-H1N109) from the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC.
References
- 1.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Dawood FS, Jain S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360(25):2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 2.Hadler JL, Konty K, McVeigh KH, et al. Case fatality rates based on population estimates of influenza-like illness due to novel H1N1 influenza: New York City, May–June 2009. PLoS One. 2010;5(7):e11677. doi: 10.1371/journal.pone.0011677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cawley J, Hull HF, Rousculp MD. Strategies for implementing school-located influenza vaccination of children: a systematic literature review. J Sch Health. 2010;80(4):167–175. doi: 10.1111/j.1746-1561.2009.00482.x. [DOI] [PubMed] [Google Scholar]
- 4.Davis MM, King JC, Jr, Moag L, Cummings G, Magder LS. Countywide school-based influenza immunization: direct and indirect impact on student absenteeism. Pediatrics. 2008;122(1):e260–5. doi: 10.1542/peds.2007-2963. [DOI] [PubMed] [Google Scholar]
- 5.King JC, Jr, Stoddard JJ, Gaglani MJ, et al. Effectiveness of school-based influenza vaccination. N Engl J Med. 2006;355(24):2523–2532. doi: 10.1056/NEJMoa055414. [DOI] [PubMed] [Google Scholar]
- 6.Ghendon YZ, Kaira AN, Elshina GA. The effect of mass influenza immunization in children on the morbidity of the unvaccinated elderly. Epidemiol Infect. 2006;134(1):71–78. doi: 10.1017/S0950268805005650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loeb M, Russell ML, Moss L, et al. Effect of influenza vaccination of children on infection rates in Hutterite communities: a randomized trial. JAMA. 2010;303(10):943–950. doi: 10.1001/jama.2010.250. [DOI] [PubMed] [Google Scholar]
- 8.Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med. 2001;344(12):889–896. doi: 10.1056/NEJM200103223441204. [DOI] [PubMed] [Google Scholar]
- 9.Piedra PA, Gaglani MJ, Kozinetz CA, et al. Herd immunity in adults against influenza-related illnesses with use of the trivalent-live attenuated influenza vaccine (CAIV-T) in children. Vaccine. 2005;23(13):1540–1548. doi: 10.1016/j.vaccine.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Census Bureau. American FactFinder, Fact Sheet for New York City, New York. http://factfinder.census.gov. Accessed May 4, 2010.
- 11.Statistical summaries—Data About Schools—New York City Department of Education. http://schools.nyc.gov/AboutUs/data/stats/default.htm. Accessed May 4, 2010.
- 12.NYSED:IRS: Directory of public and non-public schools and administrators in New York State. May 19, 2010. http://www.emsc.nysed.gov/irts/schoolDirectory/. Accessed June 8, 2010.
- 13.Centers for Disease Control and Prevention. Use of influenza A (H1N1) 2009 monovalent vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR 2009;58 (No. RR-10):1–8. [PubMed]
- 14.Myers C, Olson C, Kerker B, Thorpe L, Greene C, Farley T. Reducing health disparities in New York City: health disparities in life expectancy and death. New York, NY: New York City Department of Health and Mental Hygiene, 2010. http://www.nyc.gov/html/doh/downloads/pdf/episrv/disparitiesone.pdf. Accessed July 19, 2011.
- 15.Effler PV, Chu C, He H, et al. Statewide school-located influenza vaccination program for children 5–13 years of age, Hawaii, USA. Emerg Infect Dis. 2010;16(2):244–250. doi: 10.3201/eid1602.091375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodruff BA, Unti L, Coyle K, Boyer-Chuanroong L. Parents’ attitudes toward school-based hepatitis B vaccination of their children. Pediatrics. 1996;98(3 Pt 1):410–413. [PubMed] [Google Scholar]
- 17.Kansagra S, McGinty M, Maldin B. Cost comparison of two mass vaccination campaigns against H1N1 in New York City. December 15, 2011. http://ajph.aphapublications.org/doi/pdf/10.2105/AJPH.2011.300363. Accessed January 9, 2012. [DOI] [PMC free article] [PubMed]
- 18.Carpenter LR, Lott J, Lawson BM, et al. Mass distribution of free, intranasally administered influenza vaccine in a public school system. Pediatrics. 2007;120(1):e172–8. doi: 10.1542/peds.2006-2603. [DOI] [PubMed] [Google Scholar]
- 19.Talbot HK, Poehling KA, Williams JV, et al. Influenza in older adults: impact of vaccination of school children. Vaccine. 2009;27(13):1923–1927. doi: 10.1016/j.vaccine.2009.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rinchiuso-Hasselmann A, McKay RL, Williams CA, et al. Protecting the public from H1N1 through points of dispensing (PODs) Biosecur Bioterror. 2011;9(1):13–21. doi: 10.1089/bsp.2010.0049. [DOI] [PubMed] [Google Scholar]
- 21.Steelfisher GK, Blendon RJ, Bekheit MM, Lubell K. The public’s response to the 2009 H1N1 influenza pandemic. N Engl J Med. 2010: 362–e65 [DOI] [PubMed]
- 22.Community Health Survey, 2008: NYC DOHMH. EpiQuery: NYC Interactive Health Data. December 31, 2009. http://www.nyc.gov/html/doh/html/survey/chsdata.shtml. Accessed June 16, 2010.