Abstract
The study examined the effect of gestational (GA) and postnatal (PNA) age on speech sound perception in infants. Auditory ERPs were recorded in response to speech sounds (CV syllables) in 50 infant NICU patients (born at 24–40 weeks gestation) prior to discharge. Efficiency of speech perception was quantified as absolute difference in mean amplitudes of ERPs in response to vowel (/a/–/u/) and consonant (/b/–/g/, /d/–/g/) contrasts within 150–250, 250–400, 400–700 ms after stimulus onset. Results indicated that both GA and PNA affected speech sound processing. These effects were more pronounced for consonant than vowel contrasts. Increasing PNA was associated with greater sound discrimination in infants born at or after 30 weeks GA, while minimal PNA-related changes were observed for infants with GA less than 30 weeks. Our findings suggest that a certain level of brain maturity at birth is necessary to benefit from postnatal experience in the first 4 months of life, and both gestational and postnatal ages need to be considered when evaluating infant brain responses.
Keywords: ERP, preterm, speech perception, gestational age, postnatal age
Infants born prematurely are at high risk for poor neurodevelopmental outcomes (Allen, 2008; Hack, 2007; Stephens & Vohr, 2009); the younger their gestational age at birth, the greater the risk of severe developmental and cognitive delays. Birth during the late preterm or early term period may also be associated with cognitive and behavioral deficits (Kramer, 2009; van Baar et al., 2009; Yang et al., 2010). A combination of nervous system immaturity, susceptibility to brain injury (e.g., periventricular leukomalacia, intraventricular hemorrhage) and adverse events during the neonatal intensive care unit (NICU) stay (e.g., mechanical ventilation, sepsis, atypical sound environment) may contribute to the abnormal neurodevelopment of premature infants. However, it is difficult to accurately determine the degree of neurodevelopmental maturity and neural function of infants prior to their discharge from the NICU. Currently, the prognosis for poor motor, cognitive or communication outcomes is based solely on structural imaging findings (MRI, head ultrasound) and clinical events. These measures are helpful when extremes of injury are present but are limited when assessing the functional status of most infants at the time of discharge.
Observing infant brain activity associated with information processing offers a different way to assess neural function. Unlike a head ultrasound or an MRI, functional measures may provide details about the speed and efficiency of brain processing. Recording of event-related potentials (ERPs) has proved to be a valuable tool in gaining insights into early perceptual and cognitive processes of newborns and very young infants who are unable to comprehend standard test instructions or provide a reliable behavioral response (e.g., Guttorm et al., 2003; Key et al., 2007; Molfese & Molfese 1979a, 1985, 1997; Molfese et al., 2002). ERPs are a portion of the electroencephalogram (EEG) time-locked to the onset of a stimulus (e.g., tone, speech sound). The advantage of ERPs lies in their ability to track the brain’s response to stimulation with millisecond-level precision (Molfese et al., 2001, 2002; Key et al., 2005). ERPs are particularly useful for studying early brain development because testing does not require an overt behavioral response from the participants, can be conducted at patient bedside, and does not require sedation.
Measures of auditory processing have been widely used to assess brain functioning in infants because the ability to discriminate sounds develops early (Alho et al., 1990; Cheour et al., 2002a,b; Kushnerenko et al., 2001, 2002; Leppanen et al., 1997, 2004). Furthermore, auditory ERPs can be recorded even in a sleeping infant (e.g., Cheour et al., 2002c; Martynova et al., 2003). Studies using ERPs in full-term healthy infants consistently demonstrate that the brains of newborns and young infants are sensitive to differences in tones (Alho et al., 1990; Ceponiene et al. 2002; Fellman & Huotilainen, 2006) as well speech sounds, including vowels (e.g., Cheour et al., 1997; Cheour-Luhtanen et al., 1995; Molfese & Searock, 1986) and consonants varying in voice onset time (e.g., Molfese & Molfese 1979a; Pang et al., 1998) and place of articulation (Dehaene-Lambertz & Dehaene 1994; Dehaene-Lambertz 2000; Key et al., 2007). Because similar results have been obtained using a wide range of stimuli and experimental paradigms, individual differences in auditory ERPs may reflect an underlying perceptual ability. This ability supports a range of verbal and cognitive processes that emerge at later developmental stages and therefore can be predictive of later functioning during childhood (Molfese & Molfese, 1985, 1997; Molfese, 2000; Guttorm et al., 2003; Leppanen et al., 1997; Lyytinen, 1997; Lyytinen et al., 2003).
A few studies using ERP and magnetoencephalography (MEG) have focused on preterm infants. Auditory discrimination could be detected in premature infants as young as 27 gestational weeks, however, the amplitude of brain responses was reduced compared to that of full-term controls (Holst et al., 2005; see also Cheour-Luhtanen et al., 1996; deRegnier et al., 2000, 2002, 2008). In addition to increasing ERP amplitudes with longer gestational age, the distribution of ERPs differences across scalp locations also varied with gestational age at birth. Speech sound differences were observed at the frontal and temporal sites for preterm infants (born at 24–32 weeks gestation), and at the midline frontal–central sites for the full-term infants (GA 39–41 weeks), suggesting GA-related differences in neural processes supporting speech perception (Therien et al., 2004).
A separate line of research examined the effect of postnatal auditory experience on ERPs. In full-term newborns, brain processing of stimuli varies with auditory experience (larger amplitudes for more familiar stimuli; deRegnier et al., 2002) and targeted exposure to specific sounds can result in increased amplitude differences between such stimuli (Cheour et al., 2002c). For premature infants, prolonged postnatal auditory experience (compared to that of full-term newborns) could potentially provide a compensatory effect to offset the immaturity of the brain at birth (Ostfeld et al., 2000; deRegnier et al., 2002; Lickliter, 2000; Philbin, 2000). However, the auditory environment of a NICU may be developmentally inappropriate (e.g., overstimulating) and therefore insufficient for proper brain development (Huppi et al., 1996; Graven & Brown, 2008). Thus, in addition to the separate effects of GA and PNA on infant brain responses, these two variables may interact. In a recent study comparing ERPs of premature infants born before or after 30 weeks gestation and tested at the age equivalent of 35 weeks gestation (late preterm), Bisiacchi et al (2009) reported smaller amplitudes in the extremely preterm infants despite their longer postnatal auditory experience. When premature infants were tested at the age equivalent to 40 weeks gestation (average chronological age of 3 months), their ERPs were more similar to those of full-term newborns and not the full-term controls matched on the postnatal age (Fellman et al., 2004; see also Cheour-Luhtanen et al., 1996).
While these studies provided valuable information about the role of gestational and postnatal age in infant brain functioning, more research is needed to fully understand the individual and combined contributions of these variables. Prior studies utilized primarily oddball designs for stimulus presentation, where rare presentations of one sound are randomly interspersed among frequent presentations of a different sound. Although an effective measure of sound discrimination (Naatanen, 2008), the oddball design may confound basic perceptual processes with auditory memory. Also, analysis of oddball paradigms in infants typically focuses on the mismatch negativity (MMN) response as the index of stimulus discrimination. However, not all infants generate an MMN response (Cheour et al., 1998; Leppanen, Eklund, & Lyytinen, 1997), even when they are able to discriminate stimuli. Furthermore, most prior studies used non-speech stimuli (tones) and the development of auditory processes for tone versus speech may have a different time course (Jusczyk & Bertoncini, 1988) and different predictive value for determining risk of poor developmental outcomes. The effects of postnatal age were often inferred from comparisons of populations tested while still premature (e.g., 35 weeks) or during later childhood, when a wide range of postnatal influences could contribute to the observed developmental outcomes. Finally, gestational age and postnatal age were often treated as categorical rather than continuous variables, leading to loss of information and statistical power equivalent to reducing the sample by 38–60% (Cohen, 1983).
Therefore, in the present study, we attempt to expand prior findings regarding the impact of gestational and postnatal age on infant brain responses by (1) focusing on speech sound perception (using syllables to examine vowel and consonant discriminations), (2) using an equiprobable stimulus presentation that assesses perceptual processes independent of memory ability (e.g., Bisiacchi et al., 2009), (3) including infants with wider gestational (24–40 weeks) and postnatal ages (.03–4.5 months) and using these variables as continuums.
We hypothesize that gestational age and postnatal age will affect speech sound discrimination with greater absolute values for ERP mean amplitude differences between sounds observed for infants with greater GA (born closer to 40 weeks gestation) and for those tested at older postnatal age (greater PNA). We also anticipate that the GA and PNA will have a combined effect, reflected in the interaction of these two variables, such that brain responses of more premature infants tested at an older PNA will be different from those of infants with greater GA tested at the same or younger PNA. Furthermore, these differences may also vary by hemiscalp or electrode location.
Method
Participants
Fifty infants (29 males, 21 females) cared for at the Monroe Carell Junior Children’s Hospital neonatal intensive care unit at Vanderbilt Children’s Hospital (2006–2009) participated in the study. Infants were recruited according to the Vanderbilt IRB-approved protocols and parents provided written consent within one week of the infants’ birth. All infants received serial cranial ultrasounds to evaluate for the presence of white matter damage during their intensive care stay per standard of care. ERP testing was performed after 32 weeks post-conceptional age and when infant head circumference reached at least 31 cm. All infants passed auditory brainstem response (ABR) hearing screening. The mean gestational age (GA) at birth for the study population was 29.6 weeks (SD=4.94 weeks; range 24–40), and the mean postnatal age (PNA) was 2.09 months (SD=1.01 month, range 0.03–4.5). Gestational age at birth was derived from the best obstetric estimate of the gestation, based on the mother’s last menstrual period, obstetric measurements, and ultrasound measurements taken in the first trimester of pregnancy (Andersen et al., 1981). The average birth weight was 1494.54 g (SD=886.22g). Mean maternal education was 4.59 (SD=1.28) as assessed by the Hollingshead index of socioeconomic status education codes ranging from 1 (less than seventh grade) to 7 (graduate or professional training). Additional 7 infants were excluded from the study due to germinal matrix hemorrhage extending into the ventricles (intraventricular hemorrhage Grade II or above in the Papile classification; Papile et al., 1983), evidence of hydrocephalus, or diagnosis of auditory neuropathy.
Stimuli
The stimuli included six computer-synthesized consonant-vowel syllables (i.e. /ba/, /da/, /ga/, /bu/, /du/, /gu/), from the "transition-only" stimulus series employed by Stevens and Blumstein (1978). Thus, none of the stimuli contained initial noise bursts. The tokens selected for the present study were the ones most accurately identified by the adult subjects in the above study as members of their respective phonetic categories. These were stimulus tokens 1, 7, and 14 from the /ba, da, ga/ continuum, and tokens 1, 7, and 13 from the /bu, du, gu/ continuum, respectively. The five-formant CVs were synthesized on a Klatt (cascade) synthesizer, so that the amplitude of individual formants was modulated as a function of the respective formant frequencies, as in natural speech. To further improve the naturalness of the stimuli, the vowel /u/ was slightly diphthongized. The central frequencies of the steady-state portion of the formants were kept constant across the different consonants and only varied as a function of the vowel sounds. Duration of F1 transition ranged between 15 and 45 ms across tokens depending on the syllable-initial consonant as well as on the following vowel. Transition duration for all the other formants was always 40 ms. It was followed by a 250 ms steady state vowel. Rise and decay times were equivalent across sounds.
Electrodes
A high-density array net of 124 Ag/AgCl electrodes embedded in soft sponges (Geodesic Sensor Net, EGI, Inc., without the lower eye channels) was used to record the ERPs of the infants. Electrode impedance levels were below 40 kOhm before and after testing. During data collection, data were sampled at 250Hz with the filters set to .1–30 Hz. All electrodes were referred to Cz (vertex) and then re-referenced offline during data analysis to an average reference.
Procedure
Each infant was tested in his/her patient room while lying on their back in the bassinet/crib or being held in the supine position by a caregiver. The high-density array of soft sensors does not create any localized pressure points and minimizes any potential discomfort. No restraint was used beyond the typical infant swaddling routinely performed by nurses and parents. All tests were scheduled to maximize the chance of the participants being in a calm, relaxed state (e.g., shortly after feeding; not immediately after clinical procedures). The syllables were presented at 75 dB SPL(A) as measured at the infant’s ear through a speaker positioned approximately one meter above the mid-line of the infant’s head. The infant's head was centered below the speaker so that the speaker-to-ear distance was equal for both ears. All sounds were presented in random order, 25 times each, for a total of 150 trials. Interstimulus intervals varied randomly from 1.8–2.8 seconds to prevent habituation. Recording of the brainwaves was controlled by Net Station software (v. 4.2; EGI, Inc.). Stimulus presentation was controlled by E-Prime (v. 1.2, PST, Inc., Pittsburgh, PA). The entire test session lasted 7–10 minutes, while infant's electroencephalogram (EEG) and behavior were continuously monitored. In cases when EEG was contaminated by artifacts for more than two consecutive trials, stimulus presentation could be manually suspended and then resumed when the source of noise was addressed and EEG signal was optimal again.
Data Analysis
Individual ERPs were obtained by segmenting the ongoing EEG based on each stimulus onset to include a 100-ms prestimulus baseline and a 900 ms post-stimulus interval. ERPs were baseline corrected and referenced to an average reference. Trials contaminated by ocular and movement artifacts were rejected from further analysis using an automated screening algorithm in NetStation followed by a manual review. The same automated screening criteria were applied to all participants and set as follows: for the eye channels, voltage in excess of 140 µV was interpreted as an eye blink and voltage above 55 µV was considered to reflect eye movements. Any channel with voltage exceeding 200 µV was considered bad. Data from electrodes with poor signal quality were reconstructed using spherical spline interpolation procedures available in NetStation software (see also Srinivasan, Tucker, & Murias, 1998). If more than 15 electrodes (12% of the array) within a trial were deemed bad, the entire trial was discarded. For a data set to be included in the statistical analyses, each stimulus condition had to have a minimum of 10 clean trials. Trial retention rates were comparable across conditions (M /ba/ = 14.86, SD=3.97; M /da/ = 14.90, SD=4.11; M /ga/ = 15.10, SD=3.63; M /bu/ = 14.82, SD=3.70; M /du/ = 14.86, SD=3.90; M /gu/ = 15.14, SD=4.10).
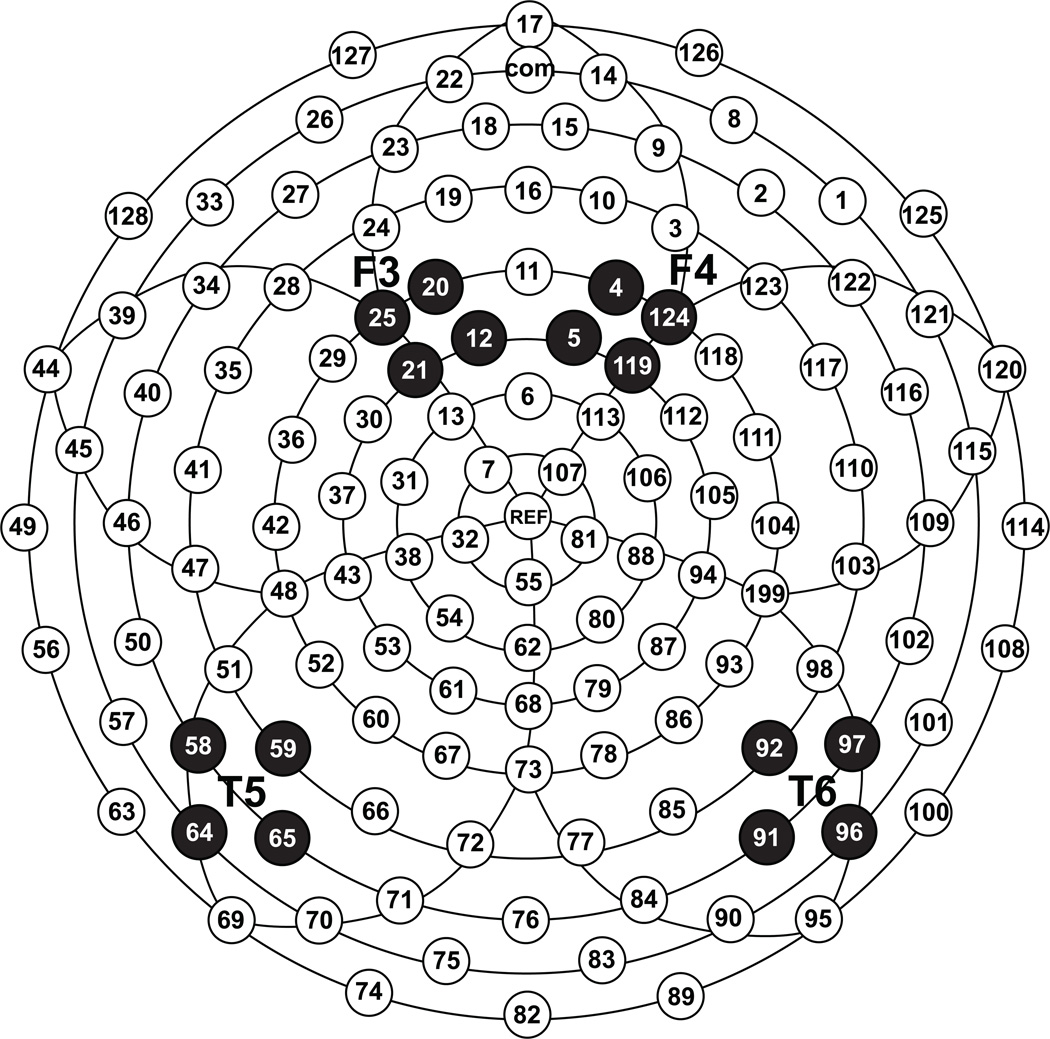
Following artifact screening, individual ERPs were averaged and baseline-corrected by subtracting the average microvolt value across the 100-ms prestimulus interval from the post-stimulus segment. To reduce the number of analyses, data for individual stimuli were averaged across vowels to create three consonant variables (/b/, /d/, /g/) and two vowel variables (/a/, /u/). Next, mean amplitudes were calculated for each of these variables within three consecutive temporal intervals: 150–250ms, 250–400ms, and 400–700ms. The intervals were determined based on analyses used in prior infant ERP studies and by examining the morphology of the grand-averaged waveform. Furthermore, only data from electrodes corresponding to the traditional posterior temporal (T5/T6) and frontal (F3/F4) scalp locations (Figure 1), previously identified as sensitive to sound differences in full-term infants (e.g., Key et al., 2007) and preterm infants (e.g., Therien et al., 2004), were used in the analyses.
Figure 1.
Electrode layout and selected clusters used in the study.
Because the equiprobable design does not provide a prediction for the direction of differences in ERP amplitudes between any of the stimulus sounds, sound differentiation was quantified as the difference in mean amplitudes between two stimuli (/a/–/u/, /b/–/g/, /d/–/g/). Furthermore, because there is no theoretical rationale for expecting any sound in a pair to elicit a waveform of greater amplitude and because the direction of such differences varied across infants, we chose to use absolute values as the dependent measure. Incidentally, prior studies utilizing an oddball paradigm have on several occasional reported a reversal in the expected direction of mismatch responses in preterm vs. term infants (Mikkola et al., 2007; Leppanen et al., 2004).
Main analytic model
A random coefficients model for repeated measurements (Rogosa & Saner, 1995), also known as random regression (Hedeker & Gibbons, 2006), hierarchical linear modeling (Raudenbush & Bryk, 2002), multilevel modeling (Snijders & Bosker, 1999), or linear mixed effects modeling (Pinheiro & Bates, 2000) was used to address the research questions. This analysis can be thought of as a modern analysis of variance (ANOVA) for repeated measures when there are correlated errors that violate ANOVA’s assumption of independent errors. The term “mixed” means that there are two levels, Level 1: 12 repeated measurements nested within infant, and Level 2: 50 infants with their between-infant characteristics, such as gestational age. In a mixed model the two levels can interact, such as a group by time interaction showing different trajectories over time. Just as in an ANOVA, the main effects and interactions are orthogonal contrasts representing independent findings where the presence of one effect does not inform about whether the other effects occur.
This two-level approach controls for the correlated errors that occur with repeated measurements of an individual better than more traditional analysis of variance models (Nich & Carroll, 1997). The multilevel model also provides an intraclass correlation coefficient (ICC) to estimate the size of individual differences among infants. The independent variables were sex, hemiscalp, electrode location, temporal window (150–250, 250–400, 400–700 ms), gestational age (weeks), and postnatal age (months). Gestational age, postnatal age, and their interaction, were the main variables of interest. The other variables served as controls to increase power and precision by explaining important sources of variance. The outcome variables in these analyses were absolute values of differences in mean amplitudes for /a/–/u/, /b/–/g/ and /d/–/g/ contrasts.
The main analysis focused on the effects of GA, PNA, and their interaction, or combined effect, on sound discrimination ability. Significance tests were done using continuous forms of these ages because dichotomization reduces power similar to a 50% reduction in sample size (Cohen, 1983). However, interpreting 2- and 3-way interactions with continuous variables can be difficult, so dichotomized forms of GA and PNA were used for illustrating the results in the interaction plots where interactions are reflected by non-parallel lines. Models were run using SAS PROC MIXED (Littell et al., 2006) assuming linearity for the GA and PNA effects.
To reduce over-fitting of the model given a relatively small sample, we report only the six terms involving the effects of interest (gestation, age, and their interaction), producing 6 × 3 = 18 tests. In the main analysis, postnatal age and gestational age are centered on the sample mean as continuous standardized variables. To present 2- and 3-way interactions in interpretable trellis plots (Cleveland, 1985), follow-up analyses were conducted with postnatal age dichotomized at the sample median and gestational age dichotomized at 30 weeks, a critical point in prenatal development due to both cerebral vascular stability and cortical network maturation (Levene at al., 2001). We recognize the problems with dichotomizing variables (Cohen, 1983) and use that approach only to make plots. Effect sizes were calculated using approximate effect sizes from Type III F-ratios based on meta-analytic formulas from the literature (Rosenthal & DiMatteo, 2001).
In addition to the mixed model, we describe important variables, such as length of gestation, postnatal age, and sound discrimination ability with tables, histograms, and nonparametric loess curves to examine linearity of the effects.
Results
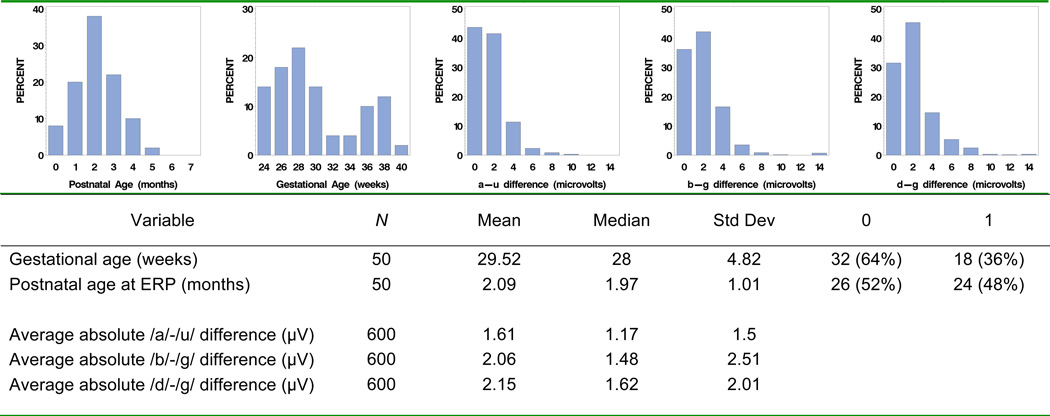
Descriptive statistics for postnatal age and gestational age are presented in Figures 2a and 2b. Postnatal age was nearly normally distributed (skew = 0.13, kurtosis = −0.17). The distribution of gestational ages was somewhat bimodal (under 30, over 30 weeks). The sample’s bimodality could be due to the nature of the Vanderbilt NICU as a tertiary care referral center: extremely preterm infants (<30 weeks GA) and full term infants with birth complications are referred to this NICU, while moderately preterm infants can be cared for in secondary care level NICUs throughout the state.
Figure 2.
Descriptive statistics: gestational age (weeks), postnatal age at ERP (months), and ERP differences (µV).
Descriptive statistics for differences in mean ERP amplitudes used as indices of differences in sound perception appear in Figures 2c–e. The number of observations is 600 (50 infants, each with 12 data points: 3 temporal windows × 2 electrode locations × 2 hemiscalp sides). While the raw difference scores were approximately normal, the absolute differences are positively skewed (skew = 1.82, 6.64, 2.22 for /a/–/u/, /b/–/g/ and /d/–/g/ contrasts respectively). The positive skew is an expected result associated with the use of the absolute values.
Results for the random coefficient model are presented in Table 1. This analytic approach controls for individual differences in sound discrimination ability among infants, a required step because the repeated observations per infant violate the independence requirement of analysis of variance. The ICCs suggest that a large proportion of the total variance (30% to 44%) is due to individual differences among infants ***(some infants had persistently higher difference scores while others had psome infants had persistentlyrsistently lower difference scores. These significant individual differences should not occur if difference scores were due to random noise*** (Raudenbush & Xiaofeng, 2000).
Table 1.
Type III significance tests
| Effect | Significance (Probability of alpha) |
Effect size (Cohen’s d) | ||||
|---|---|---|---|---|---|---|
| a – u | b – g | d – g | a – u | b – g | d – g | |
| 1. Sex | 0.25 | 0.15 | 0.70 | 0.10 | 0.12 | 0.03 |
| 2. Hemiscalp | 0.001 | 0.009 | 0.75 | 0.28 | 0.22 | 0.03 |
| 3. Electrode | 0.46 | 0.14 | 0.02 | 0.06 | 0.12 | 0.20 |
| 4. Temporal window | 0.51 | 0.0002 | <.0001 | 0.07 | 0.24 | 0.48 |
| 5. Postnatal Age (months) | 0.38 | 0.0003 | 0.008 | 0.07 | 0.30 | 0.22 |
| 6. Gestational Age (weeks) | 0.07 | 0.004 | 0.02 | 0.15 | 0.24 | 0.20 |
| 7. Postnatal Age*Gestational Age | 0.89 | 0.02 | 0.08 | 0.01 | 0.19 | 0.15 |
| 8. Postnatal Age*Hemiscalp | 0.65 | <.0001 | 0.86 | 0.04 | 0.58 | 0.01 |
| 9. Gestational Age*Hemiscalp | 0.93 | <.0001 | 0.57 | 0.01 | 0.41 | 0.05 |
| 10. Postnatal Age*Electrode | 0.27 | <.0001 | 0.0099 | 0.09 | 0.35 | 0.22 |
| 11. Gestational Age* Electrode | 0.13 | 0.011 | 0.09 | 0.12 | 0.21 | 0.14 |
| 12. Postnatal Age*Temporal window | 0.86 | 0.20 | 0.001 | 0.03 | 0.11 | 0.22 |
| 13. Gestational Age*Temporal window | 0.80 | 0.35 | 0.03 | 0.04 | 0.09 | 0.16 |
| 14. Postnatal Age*Gestational Age*Hemiscalp | 0.03 | 0.06 | 0.25 | 0.18 | 0.16 | 0.10 |
| 15. Postnatal Age*Gestational Age*Electrode | 0.11 | 0.02 | 0.33 | 0.13 | 0.20 | 0.08 |
| 16. Postnatal Age*Gestational Age*Temporal Window | 0.08 | 0.27 | 0.003 | 0.13 | 0.10 | 0.20 |
To reduce problems associated with multiple significance tests, only results for the 6 prestated hypotheses are reported. The 6 hypotheses appear boxed in bold font to mark statistical significance (p < .05). The other 10 effects are control variables to increase precision. According to Cohen’s (1992) guidelines, effect size d values of .2/.5/.8 may be considered small/medium/large.
Effects of Gestational Age
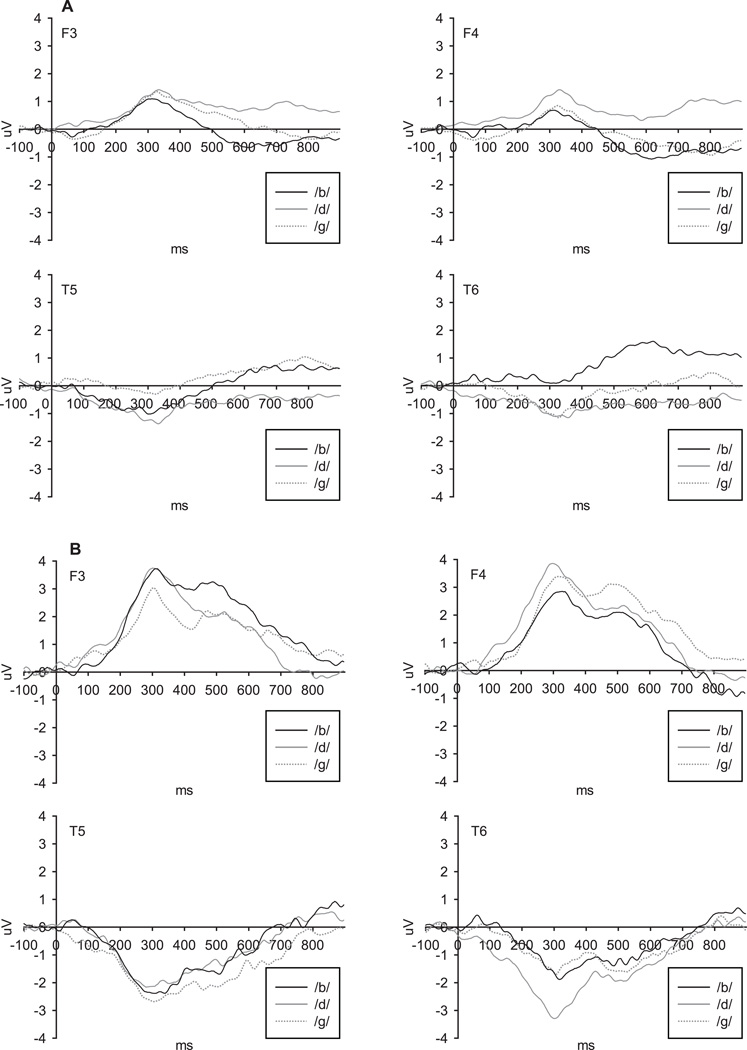
A main effect of gestational age was present for the /b/–/g/ and /d/–/g/ contrasts, F (1,579) = 8.46, p=.004 and F (1, 579) = 5.79, p=.02, respectively. Averaged ERP waveforms are presented in Figure 3. As would be expected, differences in ERP responses to consonants were greater in infants born after a longer gestation period (closer to full-term; Figure 4a). There was no main effect of gestational age on ERPs for the /a/–/u/ vowel contrast, F(1, 579) = 3.20, p = .07.
Figure 3.
Average ERP waveforms for consonant contrasts in infants with gestational age (A) less than 30 weeks; (B) over 30 weeks.
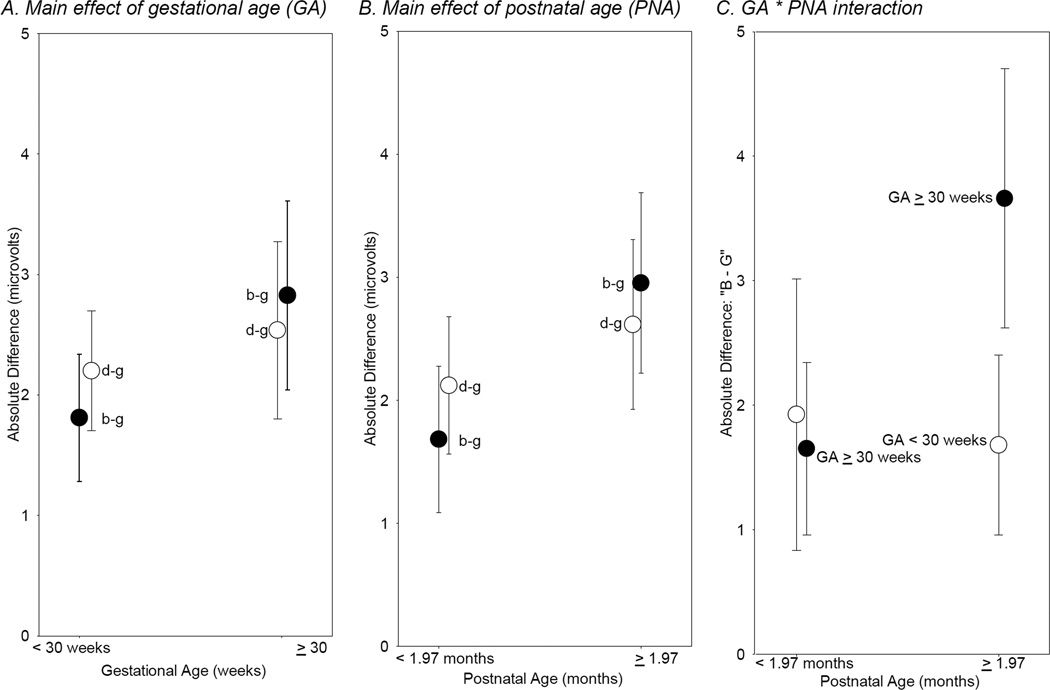
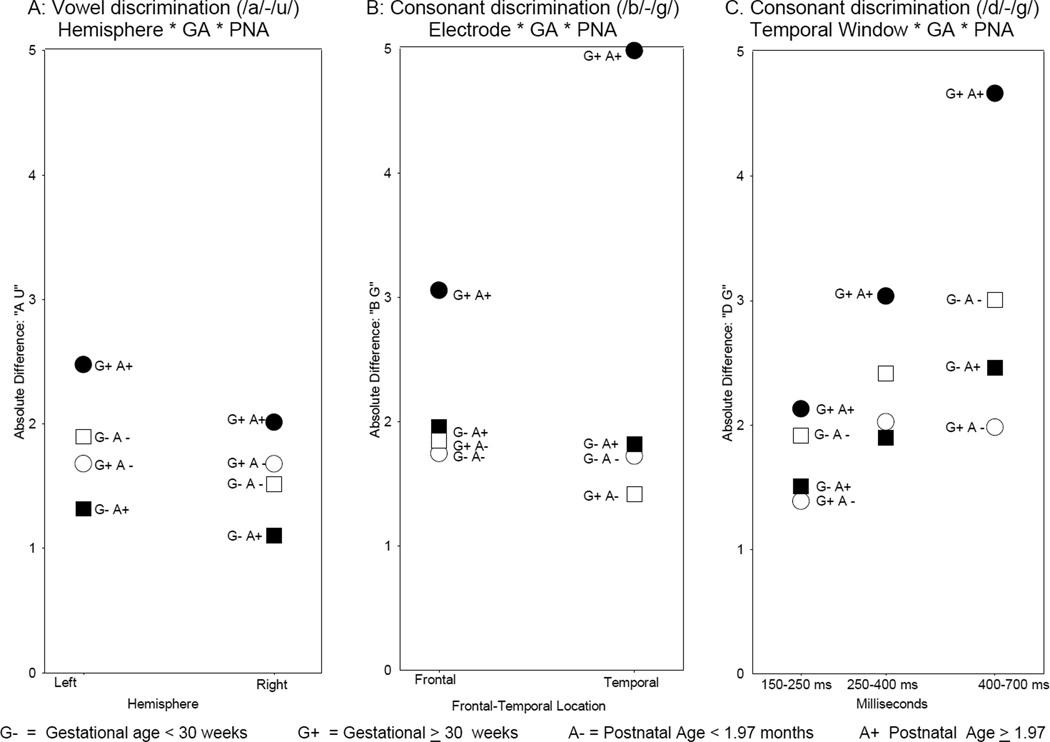
Figure 4.
Significant (p < .05) effects of gestational age and postnatal age.
Note: Figure shows mean absolute discrimination scores (µV) as a function of gestational age and postnatal age. Error bars show 95% confidence intervals. For illustration purposes only, postnatal age was dichotomized with a median split (1.97 months). Gestational age was dichotomized at 30 weeks. Significance tests were done with continuous data, as shown in Table 1.
Effects of Postnatal Age
A main effect of postnatal age was observed for both /b/–/g/ and /d/–/g/ consonant contrasts, F (1, 579) = 13.33, p=.0003 and F (1, 579) = 7.21, p=.008, respectively. As would be expected, ERPs of infants who were older at the time of testing showed greater amplitude differences between the sounds (Figure 4b). There was no main effect of postnatal age for the /a/–/u/ vowel contrast F (1, 579) = 0.78, p= .38.
Combined Effects of Gestational and Postnatal Age
A Gestational Age × Postnatal Age × Hemiscalp interaction was observed for the /a/–/u/ vowel comparison, F (1, 579) = 4.77, p=.03. The effect could be attributed to greater ERP differences between the vowels at the left than right hemiscalp sites in infants born closer to full term who were tested at an older age compared to all other infants (Figure 5a). However, this interaction has a small effect size (see Table 1) and may possibly be spurious.
Figure 5.
Significant (p < .05) three-way GA * PNA Interactions
Note: Figure shows GA*PNA interactions with hemisphere, location, and time window. Each point represents a mean absolute difference (µV). For illustration purposes only, postnatal age was dichotomized with a median split (1.97 months). Gestational age was dichotomized at 30 weeks. Significance tests were done with continuous data, as shown in Table 1. Confidence intervals were wide but are not marked to improve figure clarity.
The /b/–/g/ contrast was characterized by a significant Postnatal Age × Gestational Age interaction, F (1, 579) = 5.47, p=.02, indicating that for infants born at a younger gestational age, increasing postnatal age was not associated with increased differences in stimulus processing, which remained low (an average difference score of about 2 µV). However for infants born closer to full-term, increasing postnatal age was related to greater differences in mean amplitudes for the two sounds (Figure 4c) suggesting that the effects of PNA may differ among infants with GA of 30 weeks or more and those born earlier in gestation.
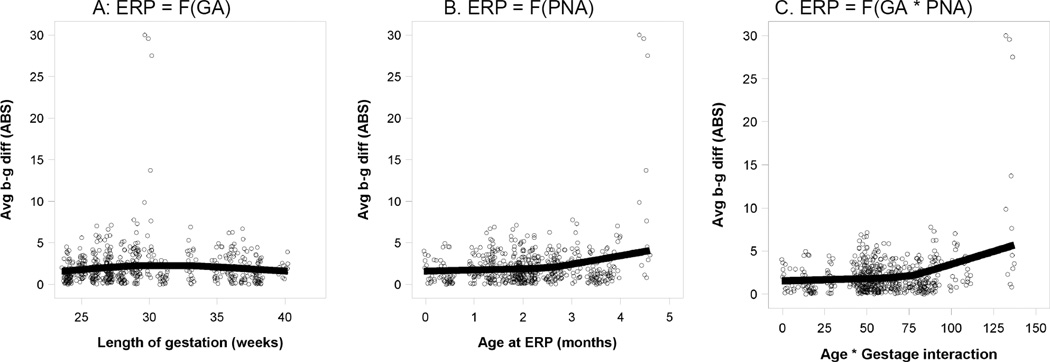
As described in the method section, the models assumed linear effects for GA and PNA, as the literature provides no proven alternative. As an exploratory test of linearity, we plotted three continuous-variable scattergrams (Figure 6) representing individual amplitude differences for the /b/–/g/ contrast for the main effects and interaction of GA and PNA that appeared as categorical data in Figure 4a–c. To relax the assumption of linearity, we used a non-parametric loess fit (Cleveland, 1993) rather than forcing the best fit lines to be straight.
Figure 6.
Significant GA and PNA Main Effects and Interaction
Panels A & B are main effects, the effect of a single variable on discrimination. Panel C shows the interaction, the combined effect of GA and PNA, calculated as the product of the two variables. As seen in Panel C, the interaction is an increase in discrimination ability when both GA and PNA are high. These three effects are orthogonal (statistically independent) in the sense of making distinct contributions to speech discrimination ability.
The main effect of GA appears to be nonlinear (Figure 6a). Discriminating ability for /b/–/g/ increases up to 30 weeks gestation, after which it may level off or decline slightly. For PNA (Figure 6b), there is a small increase in /b/–/g/ discrimination during the first weeks of postnatal life, which then becomes more pronounced between 2–3 months of age. This same accelerating effect also appears in the interaction (Figure 6c). These graphic results are exploratory, suggesting that there may be non-linear relationships in the interaction of GA and PNA.
Differential effects of PNA on ERPs of infants born at different GA are further reflected in a 3-way interaction of Gestational Age × Postnatal Age × Electrode, F (1, 579) = 5.49, p=.02. As shown in Figure 5b, infants born later in gestation and tested at an older age showed greater sound differentiation at frontal and especially at temporal scalp locations compared to all other infants.
A 3-way interaction of Gestational Age × Postnatal Age × Temporal Window, F (2, 579) = 5.74, p=.003, was significant for the /d/–/g/ consonant contrast. This effect was due primarily to greater amplitude difference between /d/–/g/ consonants in the later temporal window (400–700ms) in infants born closer to full-term and tested at the older age compared to all other infants (Figure 5c).
Discussion
The purpose of the study was to examine the effect of gestational age and postnatal age on speech sound processing in NICU patients. While prior studies have addressed each of these two variables independently, no study to date focused on their potential interaction or used both ages as continuous variables.
As would be expected, both vowel and consonant differentiation, quantified as an absolute difference in mean amplitudes of ERP waveforms in response to the contrasting stimuli, were impacted by both gestational age and postnatal age. An increase in either gestational or postnatal age was associated with greater ERP differences in response to various speech sounds. Additionally, gestational and postnatal ages interacted, although the specific effects varied across speech sound contrasts.
Brain responses to vowels were least affected by changes in either gestational or postnatal age. No main effects of either gestational or postnatal age were present for the /a/–/u/ comparison, probably because it is a simpler sound contrast. Compared to consonants, vowels are relatively long and temporally stable sounds that greatly differ from one another in frequency (Korczak & Stapells, 2010), and thus they could be successfully differentiated even by an immature auditory system. The combined effect of both gestational and postnatal ages on vowel perception was associated only with hemiscalp differences – more mature and chronologically older infants demonstrated greater ERP differences to vowels on the left side of the scalp. The effect size was small, most likely due to increased inter-subject variability in the present sample. Recent neuroimaging studies showed that hemisphere lateralization for sound discrimination develops over time during the 3rd trimester of pregnancy and initially, the right hemisphere matures faster (Mento et al., 2010; Perani et al., 2011). Left-hemisphere bias for speech may be more experience-dependent and slower to develop, although it may already be present in full-term newborns (Kotilahti et al., 2010; Molfese et al., 1976). Because over 50% of our sample was born prior to or early in the 3rd trimester, many of these infants might not have developed such lateralization patterns at the time of the ERP recording.
The two consonant contrasts reflected similar effects of gestational and postnatal ages when the latter were considered separately. Overall, infants born later in gestation or chronologically older at the time of ERP testing evidenced greater differences in ERP amplitude to various consonants. However, the combined effect of gestational and postnatal ages, reflected by interactions, was not identical for the two consonant contrasts. The /b/–/g/ contrast appeared more sensitive to the combined effects of gestational and postnatal age. In particular, infants born at an earlier gestational age did not show the same increase in ERP amplitude difference with increasing postnatal age as infants born later in gestation. Similar findings were reported by Fellman et al (2004) who tested the ability to detect differences in tone frequency in preterm infants at corrected gestational age of 40 weeks and noted that up to 3 months of postnatal auditory exposure did not accelerate the maturation of auditory processing. In that study, brain responses of premature infants were more similar to ERPs of full-term newborns than to those of full-term infants matched on postnatal age. Together these results suggest that during the first 4 months of life, a minimal level of brain maturity is required to benefit from postnatal auditory experience (see also Nelson, 2002; Mento et al., 2010). Recent investigation focused on the development of a more complex ability to distinguish native language patterns from foreign alternatives demonstrated that brain immaturity may continue to constrain the effects of postnatal experience even beyond 4 months of age (Pena, Pittaluga, & Mehler, 2010). However, the question of whether a delay in developing better speech and language processing ability in premature infants is related exclusively to the immaturity of the brain at birth or to the increased rate of medical complications and potentially neurotoxic intervention as well as the adverse sound environment experienced during the NICU stay would require further study.
The combined effect of gestational and postnatal ages on /b/–/g/ differentiation also varied by electrode location – a greater difference in ERP amplitudes was observed at temporal than frontal scalp locations for infants with greater gestational and postnatal age, while in all other infants discrimination effects were comparable across sites. Although direct correspondence between scalp-recorded ERPs and activity in the underlying cortex cannot be assumed, several prior mapping studies demonstrated that activity at temporal scalp locations may be associated with stimulus-specific processing performed by brain sources in the temporal cortex (e.g., Giard et al., 1994; Huotilainen et al., 1998; Papanicolaou et al., 1990). On the other hand, frontal scalp activity may reflect nonspecific stimulus detection and/or integration of subcortical and cortical information; it has a wider range of possible brain sources including temporal lobe as well as the motor cortex and/or the cingulate gyrus (Giard et al., 1994). The temporal cortex may be particularly affected by gestation length as suggested by recent fMRI studies. In a sample of school-age children who were born between 28–41 weeks gestation and had not experienced typical perinatal risk factors, greater gray matter density in the temporal areas supporting auditory perception and processing was associated with longer gestation (Davis et al, 2011). Reductions in cortical volume of mid-temporal regions were also reported in a sample of 8-year-olds who were born between 26–33 weeks gestation and had diverse perinatal risk history (Peterson et al., 2000).
The effects of the gestational and postnatal ages on the /d/–/g/ contrast varied across the three temporal windows included in the analysis. The greatest differences in brain responses to /d/–/g/ sound pair were observed in the 400–700ms temporal window between chronologically older infants born at a later gestational age and all other infants. In prior studies of auditory processes in infants, this time interval was interpreted to reflect engagement of attentional and/or memory processes (Ceponiene et al., 2002; Kushnerenko et al., 2002) rather than basic perceptual analysis that is typically associated with the earlier time intervals (Key et al., 2005).
The difference in the combined effects of gestational and postnatal ages on the two consonant contrasts used in the current study requires further consideration. Both consonant pairs have been extensively used to study speech perception in full-term infants and resulted in comparable findings. Differential sensitivity to the effects of gestational and postnatal age effects could suggest that processing of these sounds relies on different brain mechanisms. While all consonants in question vary in place of articulation, /b/–/g/ are farther apart on that continuum, potentially making this contrast easier to perceive. The /d/–/g/ consonants are closer together, and therefore could benefit from or require the engagement of attentional processes as basic sensory and perceptual analyses may be insufficient for accurate discrimination of these sounds by a less mature and/or less experienced auditory system. Delays or alterations in the development of efficient speech sound perception processes in infant brain may have consequence for a range of developmental outcomes. A number of existing ERP studies in typical newborns demonstrated connections between the brain’s ability to differentially respond to various spoken syllables at birth and risk status for learning disabilities as well as performance on standardized assessments of language and reading skills at 3–8 years of age (Guttorm et al., 2003; Molfese, Molfese, & Espy, 1999; Molfese, 2000). In premature infants, atypical brain responses to speech stimuli during the first year of life were also related to lower language ability at 2 years of age (Jansson-Verkasalo et al., 2010). Studies examining the association between NICU infants’ sensitivity to specific sound contrasts (e.g., /b/–/g/ vs. /d/–/g/) and neurocognitive developmental outcomes are currently underway in our laboratory.
While the study has a number of strengths, it also presents several limitations. First, although the study sample size was large for an ERP study, performing 18 significance tests (6 hypothesized effects × 3 temporal windows) on a sample of 50 participants increases the likelihood of spurious results. Thus, our results would need to be replicated in new samples. Second, this study is a cross-sectional study of 50 infants tested on a single occasion. To demonstrate that the maturation rate is reduced by prematurity more conclusively, a longitudinal study with repeated measurements over time is needed. We are currently gathering longitudinal data in these infants to examine the rate of auditory development in the months after birth. Also, while the absolute ERP differences showed significant stimulus discrimination, they were not normally distributed. The high skew of absolute values may distort significance tests that assume normality. However, according to (Tabachnick & Fidell, 2007) when there are more than 20 degrees of freedom, "… the F test is said to be robust to violations of normality…" The present study had 600 observations. In future research, "generalized" statistical models (Liang & Zeger, 1986) may employ non-normal distributions that fit ERP data better than the normal distribution. Another issue is the possibility that variability in absolute values of differences in ERP amplitudes could be due to differences in signal-to-noise ratio among infants. We attempted to offset this limitation by first computing mean amplitude values within the designated temporal windows for each sound stimulus separately and then computing the difference values. Mean amplitude measures are less affected by noise and more tolerant of individual variability in latencies (Luck, 2005). However, follow-up studies should take into consideration potential differences in noise levels when examining effects of gestational and postnatal age on ERPs.
In summary, the present study demonstrates that gestational age and postnatal age independently and in combination, affect sound discrimination in infants as measured by ERPs. The effect of postnatal age is more pronounced in infants born later in gestation. However, ERP responses are not affected equally: simpler vowel discrimination is less sensitive to the effects of gestational or postnatal age than more complex consonant contrasts. The /b/–/g/ discrimination is particularly affected by differences in both gestational and postnatal age. Prior evidence relating activity in the temporal regions to auditory perception and comprehension of verbal language (Binder et al., 2000, 2009; Schmithorst et al., 2006; Karunanayaka et al., 2007), suggests that the altered neural pattern observed in premature infant’s auditory ERPs may be indicative of an increased risk for cognitive deficits (e.g., deRegnier et al 2005; Fellman et al, 2004). This has already been demonstrated in full-term infants at risk for dyslexia (e.g., Molfese, 2000; Guttorm et al., 2003), but still needs to be investigated in the preterm population. Therefore, future studies need to examine sensitivity and specificity of the auditory ERP measures (e.g., /b/–/g/ discrimination) as predictors of language processing and cognitive development for individual infants.
Acknowledgments
This research was supported by National Institute of Child Health and Development Grant P30HD15052 to the Vanderbilt Kennedy Center for Research on Human Development, by a Hazinski-Turner Award to Dr. Nathalie L. Maitre, and by a grant from the Gerber Foundation to Dr. Judy L. Aschner. This study was supported in part by the Vanderbilt CTSA grant [UL1RR024975-01] from NCRR/NIH
We are grateful to Ms. Dorita Jones and Ms. Amber Vinson for assistance with acquisition and processing of the ERP data.
References
- Alho K, Sainio K, Sajaniemi N, Reinikainen K, Näätänen R. Event-related brain potential of human newborns to pitch change of an acoustic stimulus. Electroencephalography and Clinical Neurophysiology. 1990;77(2):151–155. doi: 10.1016/0168-5597(90)90031-8. http://dx.doi.org/10.1016/0168-5597(90)90031-8. [DOI] [PubMed] [Google Scholar]
- Allen MC. Neurodevelopmental outcomes of preterm infants. Current Opinions in Neurology. 2008;21(2):123–128. doi: 10.1097/WCO.0b013e3282f88bb4. http://dx.doi.org/10.1097/WCO.0b013e3282f88bb4. [DOI] [PubMed] [Google Scholar]
- Andersen HF, Johnson TR, Jr, Flora JD, Jr, Barclay ML. Gestational age assessment: II. Prediction from combined clinical observations. American Journal of Obstetrics and Gynecology. 1981;140:770–774. [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. http://dx.doi.org/10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cerebral Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. http://dx.doi.org/10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Bisiacchi P, Mento G, Suppiej A. Cortical auditory processing in preterm newborns: An ERP study. Biological Psychology. 2009;82:176–185. doi: 10.1016/j.biopsycho.2009.07.005. http://dx.doi.org/10.1016/j.biopsycho.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Kushnerenko E, Fellman V, Renlund M, Suominen K, Näätänen R. Event-related potential features indexing central auditory discrimination by newborns. Brain Research. Cognitive Brain Research. 2002;13(1):101–113. doi: 10.1016/s0926-6410(01)00093-3. http://dx.doi.org/10.1016/S0926-6410(01)00093-3. [DOI] [PubMed] [Google Scholar]
- Cheour M, Alho K, Sainio K, Reinikainen K, Renlund M, Aaltonen O, Näätänen R. The mismatch negativity to changes in speech sounds at the age of 3 months. Developmental Neuropsychology. 1997;13(2):167–174. http://dx.doi.org/10.1080/87565649709540676. [Google Scholar]
- Cheour M, Ceponiele R, Lehtokoski A, Luuk A, Alli J, Alho K, Näätänen R. Development of language-specific phoneme representations in the infant brain. Nature Neuroscience. 1998;1:351–353. doi: 10.1038/1561. http://dx.doi.org/10.1038/1561. [DOI] [PubMed] [Google Scholar]
- Cheour M, Ceponiene R, Leppanen P, Alho K, Kujala T, Renlund M, Näätänen R. The auditory sensory memory trace decays rapidly in newborns. Scandinavian Journal of Psychology. 2002a;43(1):33–39. doi: 10.1111/1467-9450.00266. http://dx.doi.org/10.1111/1467-9450.00266. [DOI] [PubMed] [Google Scholar]
- Cheour M, Kushnerenko E, Ceponiene R, Fellman V, Näätänen R. Electric brain responses obtained from newborn infants to changes in duration in complex harmonic tones. Developmental Neuropsychology. 2002b;22(2):471–479. doi: 10.1207/S15326942DN2202_3. http://dx.doi.org/10.1207/S15326942DN2202_3. [DOI] [PubMed] [Google Scholar]
- Cheour M, Martynova O, Näätänen R, Erkkola R, Sillanpaa M, Kero P, Hamalainen H. Speech sounds learned by sleeping newborns. Nature. 2002;415:599–600. doi: 10.1038/415599b. http://dx.doi.org/10.1038/415599b. [DOI] [PubMed] [Google Scholar]
- Cheour-Luhtanen M, Alho K, Kujala T, Sainio K, Reinikainen K, Renlund M, Näätänen R. Mismatch negativity indicates vowel discrimination in newborns. Hearing Research. 1995;82(1):53–58. doi: 10.1016/0378-5955(94)00164-l. http://dx.doi.org/10.1016/0378-5955(94)00164-L. [DOI] [PubMed] [Google Scholar]
- Cheour-Luhtanen M, Alho K, Sainio K, Rinne T, Reinikainen K, Pohjavuori M, Näätänen R. The ontogentically earliest discriminative respnse of the human brain. Psychophysiology. 1996;33:478–481. doi: 10.1111/j.1469-8986.1996.tb01074.x. http://dx.doi.org/10.1111/j.1469-8986.1996.tb01074.x. [DOI] [PubMed] [Google Scholar]
- Cleveland WS. The elements of graphing data. Monterey, Calif.: Wadsworth Advanced Books and Software; 1985. [Google Scholar]
- Cleveland WS. Visualizing data. Murray Hill, N.J: AT&T Bell Laboratories; 1993. [Google Scholar]
- Cohen J. The cost of dichotomization. Applied Psychological Measurement. 1983;7(3):249–253. http://dx.doi.org/10.1177/014662168300700301. [Google Scholar]
- Davis E, Buss C, Muftuler L, Head K, Hasso A, Wing D, Sandman C. Children’s brain development benefits from longer gestation. Frontiers in Psychology, 2, Article 1. 2011:1–7. doi: 10.3389/fpsyg.2011.00001. http://dx.doi.org/10.3389/fpsyg.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambert G, Dehaene S. Speed and cerebral correlates of syllable discrimination in infants. Nature. 1994;370:292–295. doi: 10.1038/370292a0. http://dx.doi.org/10.1038/370292a0. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G. Cerebral specialization for speech and non-speech stimuli in infants. Journal of Cognitive Neuroscience. 2000;12:449–460. doi: 10.1162/089892900562264. http://dx.doi.org/10.1162/089892900562264. [DOI] [PubMed] [Google Scholar]
- deRegnier R, Nelson CA, Thomas KM, Wewerka S, Georgieff MK. Neurophysiologic evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. Journal of Pediatrics. 2000;137:777–784. doi: 10.1067/mpd.2000.109149. http://dx.doi.org/10.1067/mpd.2000.109149. [DOI] [PubMed] [Google Scholar]
- deRegnier R-A, Wewerka S, Georgieff M, Mattia F, Nelson C. Influences of postconceptional age and postnatal experience on the development of auditory recognition memory on the newborn infant. Developmental Psychobiology. 2002;41:216–225. doi: 10.1002/dev.10070. http://dx.doi.org/10.1002/dev.10070. [DOI] [PubMed] [Google Scholar]
- deRegnier R-A. Neurophysiologic evaluation of brain function in extremely premature newborn infants. Seminars in Perinatology. 2008;32:2–10. doi: 10.1053/j.semperi.2007.12.003. http://dx.doi.org/10.1053/j.semperi.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Fellman V, Huotilainen M. Cortical auditory event-related potentials in newborn infants. Seminars on Fetal & Neonatal Medicine. 2006;11:452–458. doi: 10.1016/j.siny.2006.07.004. http://dx.doi.org/10.1016/j.siny.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Fellman V, Kushnerenko E, Mikkola K, Ceponiene R, Leipala J, Näätänen R. Atypical auditory event-related potentials in preterm infants during the first year of life: a possible sign of cognitive dysfunction? Pediatric Research. 2004;56(2):291–297. doi: 10.1203/01.PDR.0000132750.97066.B9. http://dx.doi.org/10.1203/01.PDR.0000132750.97066.B9. [DOI] [PubMed] [Google Scholar]
- Giard M, Perrin F, Echallier J, Thevenet M, Froment J, Pernier J. Dissociation of temporal and frontal components of the human auditory N1 wave: A scalp current density and dipole model analysis. Electroencephalography and Clinical Neurophysiology. 1994;92(3):238–252. doi: 10.1016/0168-5597(94)90067-1. http://dx.doi.org/10.1016/0168-5597(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Graven S, Brown J. Auditory development in the fetus and infant. Newborn and Infant Nursing Reviews. 2008;8(4):187–193. http://dx.doi.org/10.1053/j.nainr.2008.10.010. [Google Scholar]
- Guttorm TK, Leppänen PHT, Tolvanen A, Lyytinen H. Event-related potential in newborns with and without familial risk for dyslexia: Principal component analysis reveals differences between the groups. Journal of Neural Transmission. 2003;110:1059–1074. doi: 10.1007/s00702-003-0014-x. http://dx.doi.org/10.1007/s00702-003-0014-x. [DOI] [PubMed] [Google Scholar]
- Hack M. Survival and neurodevelopmental outcomes of preterm infants. J Pediatr Gastroenterol Nutr. 2007;45 Suppl 3:S141–S142. doi: 10.1097/01.mpg.0000302959.55428.05. http://dx.doi.org/10.1097/01.mpg.0000302959.55428.05. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. Longitudinal data analysis. Hoboken, NJ: Wiley-Interscience; 2006. [Google Scholar]
- Holst M, Eswarana H, Lowery C, Murphy P, Norton J, Preiss H. Development of auditory evoked fields in human fetuses and newborns: A longitudinal MEG study. Clinical Neurophysiology. 2005;116:1949–1955. doi: 10.1016/j.clinph.2005.04.008. http://dx.doi.org/10.1016/j.clinph.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Schuknecht B, Boesch C, Bossi E, Felblinger J, Fusch C, Herschkowitz N. Structural and neurobehavioral delay in postnatal brain development of preterm infants. Pediatrics Research. 1996;39(5):895–901. doi: 10.1203/00006450-199605000-00026. http://dx.doi.org/10.1203/00006450-199605000-00026. [DOI] [PubMed] [Google Scholar]
- Huotilainen M, Winkler I, Alho K, Escera C, Virtanen J, Ilmoniemi RJ, Näätänen R. Combined mapping of human auditory EEG and MEG responses. Electroencephalography & Clinical Neurophysiology. 1998;108:370–379. doi: 10.1016/s0168-5597(98)00017-3. http://dx.doi.org/10.1016/S0168-5597(98)00017-3. [DOI] [PubMed] [Google Scholar]
- Jansson-Verkasalo E, Ruusuvirta T, Huotilainen M, Alku P, Kushnerenko E, Suominen K, Hallman M. Atypical perceptual narrowing in prematurely born infants is associated with compromised language acquisition at 2 years of age. BMC Neuroscience. 2010;11:88. doi: 10.1186/1471-2202-11-88. http://dx.doi.org/10.1186/1471-2202-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusczyk P, Bertoncini J. Viewing the development of speech perception as an innately guided learning process. Language and Speech. 1988;31(3):217–238. doi: 10.1177/002383098803100301. http://dx.doi.org/ 10.1177/002383098803100301. [DOI] [PubMed] [Google Scholar]
- Karunanayaka PR, Holland SK, Schmithorst VJ, Solodkin A, Chen EE, Szaflarski JP, Plante E. Age-related connectivity changes in fMRI data from children listening to stories. Neuroimage. 2007;34:349–360. doi: 10.1016/j.neuroimage.2006.08.028. http://dx.doi.org/10.1016/j.neuroimage.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Key A, Dove G, Maguire M. Linking brainwaves to the brain: An ERP primer. Developmental Neuropsychology. 2005:183–215. doi: 10.1207/s15326942dn2702_1. http://dx.doi.org/10.1207/s15326942dn2702_1. [DOI] [PubMed] [Google Scholar]
- Key A, Ferguson M, Molfese D, Peach K, Lehman C, Molfese V. Smoking during pregnancy affects speech discrimination ability in newborn infants. Environmental Health Perspectives. 2007;115(4):623–629. doi: 10.1289/ehp.9521. http://dx.doi.org/10.1289/ehp.9521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korczak P, Stapells D. Effects of various articulatory features of speech on cortical event-related potentials and behavioral measures of speech-sound processing. Ear & Hearing. 2010;31:491–504. doi: 10.1097/AUD.0b013e3181d8683d. http://dx.doi.org/10.1097/AUD.0b013e3181d8683d. [DOI] [PubMed] [Google Scholar]
- Kotilahti K, Nissila I, Nasi T, Lipiainen L, Noponen T, Merilainen P, Fellman V. Hemodynamic responses to speech and music in newborn infants. Human Brain Mapping. 2010;31:595–603. doi: 10.1002/hbm.20890. http://dx.doi.org/10.1002/hbm.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS. Late preterm birth: appreciable risks, rising incidence. Journal of Pediatrics. 2009;154:159–160. doi: 10.1016/j.jpeds.2008.09.048. http://dx.doi.org/10.1016/j.jpeds.2008.09.048. [DOI] [PubMed] [Google Scholar]
- Kushnerenko E, Cheour M, Ceponiene R, Fellman V, Renlund M, Soininen K, Näätänen R. Central auditory processing of durational changes in complex speech patterns by newborns: an event-related brain potential study. Developmental Neuropsychology. 2001;19(1):83–97. doi: 10.1207/S15326942DN1901_6. http://dx.doi.org/10.1207/S15326942DN1901_6. [DOI] [PubMed] [Google Scholar]
- Kushnerenko E, Ceponiene R, Balan P, Fellman V, Huotilainen M, Näätänen R. Maturation of the auditory event-related potentials during the first year of life. NeuroReport. 2002;13:47–51. doi: 10.1097/00001756-200201210-00014. http://dx.doi.org/10.1097/00001756-200201210-00014. [DOI] [PubMed] [Google Scholar]
- Leppänen PHT, Eklund KM, Lyytinen H. Event-related brain potentials to change in rapidly presented acoustic stimuli in newborns. Developmental Neuropsychology. 1997;13:175–204. http://dx.doi.org/10.1080/87565649709540677. [Google Scholar]
- Leppanen PHT, Guttorm TK, Pihko E, Takkinen S, Eklund KM, Lyytinen H. Maturational effects on newborn AERPs measured in the mismatch negativity paradigm. Experimental Neurology. 2004;1:91–101. doi: 10.1016/j.expneurol.2004.06.002. http://dx.doi.org/10.1016/j.expneurol.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Levene MI, Chervenak FA, Whittle M. Fetal and neonatal neurology and neurosurgery. 3rd ed. Edinburgh, UK: Churchill Livinstone Press; 2001. [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. http://dx.doi.org/10.1093/biomet/73.1.13. [Google Scholar]
- Lickliter R. Atypical perinatal sensory stimulation and early perceptual development: insights from developmental psychobiology. Journal of Perinatology. 2000;20(8 Pt 2):S45–S54. doi: 10.1038/sj.jp.7200450. http://dx.doi.org/10.1038/sj.jp.7200450. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenger O. SAS System for Mixed Models, Second Edition. Cary, N.C.: SAS Institute; 2006. [Google Scholar]
- Luck S. An Introduction to Event-Related Pontential Technique. Cambridge, Massachussetts: The MIT Press; 2005. [Google Scholar]
- Lyytinen H. In search of precursors of dyslexia: A prospective study of children at risk for reading problems. In: Snowling M, editor. Dyslexia: Biology, cognition and intervention. London: Whurr Publishers; 1997. pp. 97–107. [Google Scholar]
- Lyytinen H, Leppänen PHT, Richardson U, Guttorm TK. Brain functions and speech perception in infants at risk for dyslexia. In: Csépe V, editor. Dyslexia: Different Brain, Different Behaviour. Neuropsychology and Cognition Series. Dorthrecht: Kluwer; 2003. pp. 113–152. [Google Scholar]
- Martynova O, Kirjavainen J, Cheour M. Mismatch negativity and late discriminative negativity in sleeping human newborns. Neuroscience Letters. 2003;340(2):75–78. doi: 10.1016/s0304-3940(02)01401-5. http://dx.doi.org/10.1016/S0304-3940(02)01401-5. [DOI] [PubMed] [Google Scholar]
- Mento G, Suppiej A, Altoe G, Bisiacchi P. Functional hemispheric asymmetries in humans: Electrophysiological evidence from preterm infants. European Journal of Neuroscience. 2010;31:565–574. doi: 10.1111/j.1460-9568.2010.07076.x. http://dx.doi.org/10.1111/j.1460-9568.2010.07076.x. [DOI] [PubMed] [Google Scholar]
- Mikkola K, Kushnerenko E, Partanen E, Serenius-Sirve S, Leipala J, Huotilainen M, Fellman V. Auditory event-related potentials and cognitive function of preterm children at five years of age. Clinical Neurophysiology. 2007;118(7):1494–1502. doi: 10.1016/j.clinph.2007.04.012. http://dx.doi.org/10.1016/j.clinph.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Molfese DL. Predicting dyslexia at 8 years using neonatal brain responses. Brain and Language. 2000;72:238–245. doi: 10.1006/brln.2000.2287. http://dx.doi.org/10.1006/brln.2000.2287. [DOI] [PubMed] [Google Scholar]
- Molfese DL, Molfese VJ. Hemisphere and stimulus differences as reflected in the cortical responses of newborn infants to speech stimuli. Developmental Psychology. 1979a;15:505–511. http://dx.doi.org/10.1037/0012-1649.15.5.505. [Google Scholar]
- Molfese DL, Molfese VJ. Infant speech perception: Learned or innate? In: Whitaker HA, Whitaker H, editors. Advances in Neurolinguistics: Vol. 4. New York: Academic Press; 1979b. [Google Scholar]
- Molfese DL, Molfese VJ. Electrophysiological indices of auditory discrimination in newborn infants: The bases for predicting later language development. Infant Behavior and Development. 1985;8:197–211. http://dx.doi.org/10.1016/S0163-6383(85)80006-0. [Google Scholar]
- Molfese DL, Molfese V. Discrimination of language skills at five years of age using event related potentials recorded at birth. Developmental Neuropsychology. 1997;13:135–156. http://dx.doi.org/10.1080/87565649709540674. [Google Scholar]
- Molfese DL, Molfese VJ, Espy KA. The predictive use of event-related potentials in language development and the treatment of language disorders. Developmental Neuropsychology. 1999;16:358–360. http://dx.doi.org/10.1207/S15326942DN1603_19. [Google Scholar]
- Molfese DL, Molfese VJ, Kelly S. The use of brain electrophysiology techniques to study language: A basic guide for the beginning consumer of electrophysiology information. Learning Disabilities Quarterly. 2001;24:177–188. http://dx.doi.org/10.2307/1511242. [Google Scholar]
- Molfese DL, Molfese V, Modglin A, Kelly S, Terrell S. Reading and cognitive abilities: Longitudinal studies of brain and behavior changes in young children. Annals of Dyslexia. 2002;52:99–119. http://dx.doi.org/10.1007/s11881-002-0008-7. [Google Scholar]
- Molfese D, Nunez V, Seibert S, Ramanaiah N. Cerebral asymmetry: Changes in factors affecting its development. Annals of the New York Academy of Sciences. 1976;280:821–833. doi: 10.1111/j.1749-6632.1976.tb25545.x. http://dx.doi.org/10.1111/j.1749-6632.1976.tb25545.x. [DOI] [PubMed] [Google Scholar]
- Molfese D, Searock K. The use of auditory evoked responses at one year of age to predict language skills at 3 years. Australian Journal of Communication Disorders. 1986;14:35–46. [Google Scholar]
- Näätänen R. Mismatch negativity (MMN) as an index of central auditory system plasticity. International Journal of Audiology. 2008;47 Suppl. 2:S16–S20. doi: 10.1080/14992020802340116. http://dx.doi.org/10.1080/14992020802340116. [DOI] [PubMed] [Google Scholar]
- Nelson CA. Neural development and life-long plasticity. In: Lerner RM, Jacobs F, Wetlieb D, editors. Promoting Positive Child, Adolescent, and Family Development: Handbook of Program and Policy Interventions. Thousand Oaks, CA: Sage Publications; 2002. pp. 31–60. [Google Scholar]
- Nich C, Carroll K. Now you see it, now you don't: A comparison of traditional versus random-effects regression models in the analysis of longitudinal follow-up data from a clinical trial. Journal of Consulting and Clinical Psychology. 1997;65(2):252–261. doi: 10.1037//0022-006x.65.2.252. http://dx.doi.org/10.1037/0022-006X.65.2.252. [DOI] [PubMed] [Google Scholar]
- Novak GP, Kurtzberg D, Kreuzer JA, Vaughan HG. Cortical responses to speech sounds and their formants in normal infants: maturational sequence and spatiotemporal analysis. Electroencephalography and Clinical Neurophysiology. 1989;73:295–305. doi: 10.1016/0013-4694(89)90108-9. http://dx.doi.org/10.1016/0013-4694(89)90108-9. [DOI] [PubMed] [Google Scholar]
- Ostfeld BM, Smith RH, Hiatt M, Hegyi T. Maternal behavior toward premature twins: implications for development. Twin Research. 2000;3(4):234–241. doi: 10.1375/136905200320565201. http://dx.doi.org/10.1375/136905200320565201. [DOI] [PubMed] [Google Scholar]
- Papanicolaou A, Baumann S, Rogers R, Saydjari C, Amparo E, Eisenberg H. Localization of auditory response sources using magnetoencephalography and magnetic resonance imaging. Archives of Neurology. 1990;47(1):33–37. doi: 10.1001/archneur.1990.00530010041016. http://dx.doi.org/10.1001/archneur.1990.00530010041016. [DOI] [PubMed] [Google Scholar]
- Papile LA, Munsick-Bruno G, Schaefer A. Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. Journal of Pediatrics. 1983;103(2):273–277. doi: 10.1016/s0022-3476(83)80366-7. http://dx.doi.org/10.1016/S0022-3476(83)80366-7. [DOI] [PubMed] [Google Scholar]
- Pena M, Pittaluga E, Mehler J. Language acquisition in premature and full-term infants. Proceedings of the National Academy of Sciences. 2010;107(8):3823–3828. doi: 10.1073/pnas.0914326107. http://dx.doi.org/10.1073/pnas.0914326107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Saccuman M, Scifo P, Awander A, Spada D, Baldoli C, Friederici A. Neural language networks at birth. Proceedings of the National Academy of Science. 2011;108(38):16056–16061. doi: 10.1073/pnas.1102991108. http://dx.doi.org/10.1073/pnas.1102991108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B, Vohr B, Staib L, Cannistraci C, Dolberg A, Schneider K, Ment L. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. Journal of the American Medical Association. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. http://dx.doi.org/10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Philbin MK. The influence of auditory experience on the behavior of preterm newborns. Journal of Perinatology. 2000;20(8 Pt 2):S77–S87. doi: 10.1038/sj.jp.7200453. http://dx.doi.org/10.1038/sj.jp.7200453. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York: Springer; 2000. http://dx.doi.org/10.1007/978-1-4419-0318-1. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd ed. Newbury Park, CA: Sage Publications, Inc.; 2002. [Google Scholar]
- Raudenbush SW, Xiaofeng L. Statistical power and optimal design for multisite randomized trials. Psychological Methods. 2000;5(2):199–213. doi: 10.1037/1082-989x.5.2.199. http://dx.doi.org/10.1037/1082-989X.5.2.199. [DOI] [PubMed] [Google Scholar]
- Rogosa D, Saner H. Longitudinal data analysis examples with random coefficient models. Journal of Educational and Behavioral Statistics. 1995;20(2):149–170. http://dx.doi.org/10.3102/10769986020002149. [Google Scholar]
- Rosenthal R, DiMatteo MR. Meta analysis: Recent developments in quantitative methods for literature reviews. Annual Review of Psychology. 2001;52:59–82. doi: 10.1146/annurev.psych.52.1.59. http://dx.doi.org/10.1146/annurev.psych.52.1.59. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Plante E. Cognitive modules utilized for narrative comprehension in children: a functional magnetic resonance imaging study. Neuroimage. 2006;29:254–266. doi: 10.1016/j.neuroimage.2005.07.020. http://dx.doi.org/10.1016/j.neuroimage.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders T, Bosker R. Multilevel Analysis: An introduction to basic and advanced multilevel modeling. Thousand Oaks CA: Sage Publications; 1999. [Google Scholar]
- Srinivasan R, Tucker DM, Murias M. Estimating the spatial Nyquist of the human EEG. Behavior Research Methods, Instruments, and Computers. 1998;30:8–19. http://dx.doi.org/10.3758/BF03209412. [Google Scholar]
- Stephens BE, Vohr BR. Neurodevelopmental outcome of the premature infant. Pediatr Clin North Am. 2009;56(3):631–646. doi: 10.1016/j.pcl.2009.03.005. http://dx.doi.org/10.1016/j.pcl.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Stevens KN, Blumstein SE. Invariant cues for place of articulation in stop consonants. Journal of the Acoustical Society of America. 1978;64:1358–1368. doi: 10.1121/1.382102. http://dx.doi.org/10.1121/1.382102. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5th ed. Boston: Allyn and Bacon; 2007. [Google Scholar]
- Therien JM, Worwa CT, Mattia FR, deRegnier R-A. Altered pathways for auditory discrimination and recognition memory in premature newborns. Developmental Medicine and Child Neurology. 2004;46:816–824. doi: 10.1017/s0012162204001434. http://dx.doi.org/10.1111/j.1469-8749.2004.tb00447.x. [DOI] [PubMed] [Google Scholar]
- van Baar AL, Vermaas J, Knots E, de Kleine MJ, Soons P. Functioning at school age of moderately preterm children born at 32 to 36 weeks’ gestational age. Pediatrics. 2009;124:251–257. doi: 10.1542/peds.2008-2315. http://dx.doi.org/10.1542/peds.2008-2315. [DOI] [PubMed] [Google Scholar]
- Yang S, Platt RW, Kramer MS. Variation in child cognitive ability by week of gestation among healthy term births. American Journal of Epidemiology. 2010;171:399–406. doi: 10.1093/aje/kwp413. http://dx.doi.org/10.1093/aje/kwp413. [DOI] [PMC free article] [PubMed] [Google Scholar]