Abstract
Cancer stem cells (CSCs) are implicated in resistance to ionizing radiation (IR) and chemotherapy. Honokiol, a biphenolic compound has been used in traditional Chinese Medicine for treating various ailments. In this study, we determined the ability of honokiol to enhance the sensitivity of colon CSCs to IR. The combination of honokiol and IR suppressed proliferation and colony formation while inducing apoptosis of colon cancer cells in culture. There were also reduced numbers and size of spheroids, which was coupled with reduced expression of CSC marker protein DCLK1. Flow cytometry studies confirmed that the honokiol-IR combination reduced the number of DCLK1+ cells. In addition, there were reduced levels of activated Notch-1, its ligand Jagged-1 and the downstream target gene Hes-1. Furthermore, expression of components of the Notch-1 activating γ-secretase complex, Presenilin 1, Nicastrin, Pen2 and APH-1 was also suppressed. On the other hand, the honokiol effects were mitigated when the Notch intracellular domain was expressed. To determine the effect of honokiol-IR combination on tumor growth in vivo, nude mice tumor xenografts were administered honokiol intraperitoneally and exposed to IR. The honokiol-IR combination significantly inhibited tumor xenograft growth. In addition, there were reduced levels of DCLK1 and the Notch signaling-related proteins in the xenograft tissues. Together, these data suggest that honokiol is a potent inhibitor of colon cancer growth that targets the stem cells by inhibiting the γ-secretase complex and the Notch signaling pathway. These studies warrant further clinical evaluation for the combination of honokiol and IR for treating colon cancers.
Keywords: DCLK1, tumor xenograft, γ-secretase complex, colonosphere, Notch-1
Introduction
Colon cancer remains incurable warranting discover of new strategies for therapy. Eradicating drug resistant colon cancer stem cells is one provocative area of investigation. This paper focuses on defining nontoxic approaches towards eradicating colon tumor stem cells. Honokiol (Fig. 1A) is a biphenolic compound from Magnolia officianalis that is used in traditional Chinese and Japanese medicine for the treatment of various ailments including ulcer, allergy, and bacterial infections. It is also used as a muscle relaxant and possesses antithrombotic activity (1, 2). Recent studies have demonstrated that it has anti-tumor activity with low toxicity (3, 4).
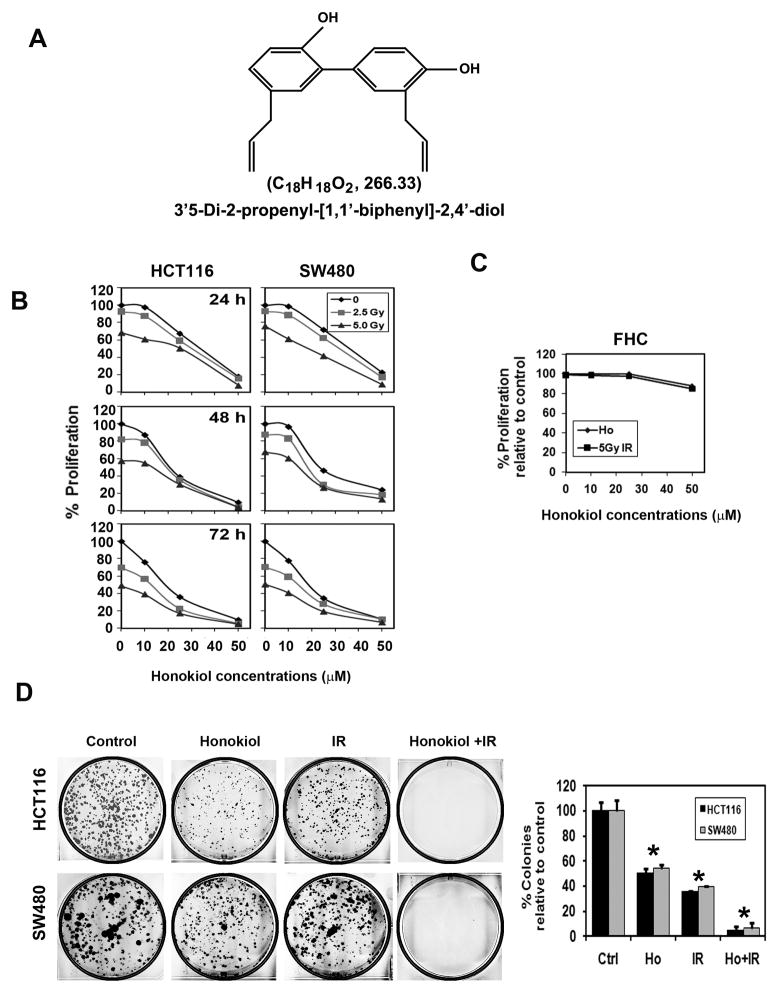
Figure 1. Combination of honokiol and IR inhibits colorectal cancer growth.
A, chemical structure of honokiol. B, HCT116 and SW480 cells were incubated with increasing doses of honokiol (0–50 μM), subsequently irradiated with increasing doses of IR (0–5 Gy), and proliferation was determined. The honokiol-IR combination resulted in a significant dose and time dependent decrease in cell proliferation in both cell lines when compared with untreated controls. Moreover, the combination showed an additive inhibition of proliferation compared to honokiol of IR alone (p<0.017). C, up to 50 μM honokiol and 5 Gy IR does not inhibit proliferation of FHC normal colonic epithelial cells. D, combination of honokiol and IR inhibits colony formation. HCT116 and SW480 cells were incubated with 25 μM honokiol for 24 h and subjected to 5.0 Gy IR. Following this, the cells were allowed to grow and form colonies. The honokiol and IR combination inhibited colony formation and showed that additive effect. Results are representative of three independent experiments.
Notch signaling is an evolutionarily conserved mechanism that affects proliferation, differentiation, and apoptotic programs thereby maintaining tissue homeostasis. Notch signaling also plays a fundamental role in the differentiation and maintenance of stem cells. Aberrant activation of the Notch signaling has been associated with the development of many cancers, including colon cancers (5, 6). More importantly, altered Notch activity has been shown to partially explain the apparent radioresistance present in the stem cell fraction in cancers (7). This suggests that targeting the Notch signaling pathway might affect growth of cancer stem cells (CSCs). In colon cancers, the levels of Notch-1 expression is associated with the pathologic grade, progression, and metastasis (5). Notch signaling is initiated when a ligand such as Jagged interacts with the notch transmembrane receptor (8), leading to two sequential proteolytic events, including one that occurs just inside the membrane by the γ-secretase complex (9). The enzyme complex is made up of four proteins presenilin, nicastrin, APH-1 (anterior pharynx-defective 1), and PEN-2 (presenilin enhancer 2), all of which are essential for activity. Cleavage by the γ-secretase complex releases the Notch intracellular domain (NICD), which in turn translocates into the nucleus of the cells, interacts with the C promoter-binding factor-1 (CBF1) transcriptional cofactor and transactivates target genes, such as those in the hairy and enhancer of split (Hes) and Hes related with YRPW motif (Hey) family proteins (10).
Tumorigenesis in the gut is thought to arise specifically in the stem cell population located at or near the base of the colonic crypts (11–13). Various proteins have been identified as potential markers for stem cells (14–18). We have demonstrated that doublecortin and CaM kinase-like-1 (DCLK-1), a microtubule-associated kinase expressed in postmitotic neurons (19) is an intestinal stem cell marker that is expressed in colon adenocarcinoma (20). In this article, we have determined the effect of combining honokiol and IR on the Notch signaling pathway and on colon cancer stem cells.
Materials and Methods
Cells and reagents
HCT116 and SW480 cells (ATCC, Manassas, VA) were grown in DMEM containing 10% fetal bovine serum (Sigma-Aldrich) and 1% antibiotic solution (Mediatech Inc) at 37°C in a humidified atmosphere containing 5% CO2. Normal colon epithelial cells (FHC, CRL-1831) were grown in Ham’s F12 medium 45%, DMEM 45%, 25 mM HEPES, 10 ng/ml cholera toxin, 0.005 mg/ml insulin, 0.005 mg/ml transferin, 100 ng/ml hydrocortisone, 10% fetal bovine serum and 1% antibiotic solution at 37°C in a humidified atmosphere of 5% CO2. All cells used in this study were within 20 passages after receipt or resuscitation (~3 months of noncontinuous culturing). The cell lines were not authenticated as they came from national repositories. Honokiol was purchased from LKT Laboratories, St Paul, MN. N-[N-(3, 5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT), a γ-secretase inhibitor (GSI) was purchased (Sigma-Aldrich) (Supplementary Fig. S1).
Proliferation and apoptosis assays
Cells in 96 well plates were treated with honokiol (0–50 μM) followed by IR (0–5 Gy). Proliferation was analyzed by hexoseaminidase assay (21, 22). For apoptosis, caspase 3/7 activity was measured using the Apo-one Homogeneous Caspase-3/7 Assay kit (Promega, Madison, WI).
Clonogenicity assay
Cells were treated with honokiol (25 μM) for 4 h, and then exposed to IR (0–5 Gy). 48 h following IR, the honokiol-containing medium was removed, and the cells were incubated for an additional 10 days. Treatments were performed in triplicate. The colonies obtained were formalin fixed and stained with hematoxylin. The colonies were counted and compared with untreated cells.
Western blot analysis
Cell lysates were subjected to polyacrylamide gel electrophoresis and blotted onto Immobilion polyvinylidene difluoride membranes (Millipore). Antibodies were purchased from Cell Signaling Technology., Abcam Inc., GenScript USA Inc., and Santa Cruz Biotechnology Inc., and specific proteins were detected by the enhanced chemiluminescence system (GE Health Care).
Flow cytometric analyses
24 h following honokiol (25 μM) and IR (5 Gy) exposure, cells were subjected to direct immunofluorescence staining (phycoerythrin conjugated DCLK1 or phycoerythrin conjugated CD133 antibody-followed by flow cytometric analyses. The samples were analyzed using a FACS Calibur analyzer (Becton Dickinson), capturing 10,000 events for each sample. Results were analyzed with ModFit LT™ software (Verity Software House).
Colonosphere assay
Cells were treated with honokiol (25 μM) for 4 h and then irradiated (5 Gy). After 7 days, the number and size of colonospheres were determined using Celigo (Cyntellect Inc). For second and third passages, cells were grown in the absence of honokiol and IR.
Plasmids and transfections
Cells were transfected with plasmid EF.hICN1.CMV.GFP encoding the Notch-1 intracellular domain (NICD) or the empty vector EF.v-CMV.GFP (Addgene Inc) and subsequently treated with honokiol (25μM) and IR (5 Gy). Cell proliferation and apoptosis were detected using hexoaminidase assay and Apo-one Homogeneous Caspase-3/7 Assay kit, respectively.
HCT116 xenograft tumors in mice
Five-week-old male athymic nude mice (Charles River) were utilized for in vivo experiments. They were maintained with water and standard mouse chow diet ad libidum and used in studies as per protocols approved by the University’s Animal Studies Committee. Animals were injected with 1×106 HCT116 cells into the flank and allowed to develop. Upon identification of a palpable tumor, honokiol (200 μg/kg body weight) was administered intraperitoneally daily for 21 d. Where applicable, animals were also irradiated at 5 Gy once a week for 3 weeks. At the end of treatment the animals were euthanized, and the tumors used for histology (hematoxylin & eosin), immunohistochemistry, and gene expression studies.
Immunohistochemistry
4 μm sections from paraffin embedded tissues were incubated overnight with primary antibodies followed by one hour with a broad spectrum secondary antibody and HRP- conjugate. The slides were developed with DAB, and counterstained by hematoxylin (Invitrogen). Slides were examined in Nikon Eclipse Ti microscope under a 40X objective.
Statistical analysis
All values are expressed as the mean ± SEM. Data was analyzed using an unpaired 2-tailed t test. A P value of <0.05 was considered statistically significant. For multiple comparisons, one way analysis of variance (ANOVA) was performed using Bonferroni corrections for multiple comparisons. To be considered significant, the P value must be less than 0.017. The SPSS V17 statistical software was used for these analyses.
Results
Honokiol and IR inhibit cell proliferation
Radiation therapy is commonly used in colorectal cancers either before or after surgery. However, it can have significant side effects suggesting that reducing the dose would be beneficial. Given that honokiol can reduce growth of colorectal cancer (CRC) cells, we determined its effect when combined with IR. The honokiol-IR combination significantly suppressed proliferation of CRC cells within 24 h at a dose of 25 μM, which continued significant suppression over the next 72 h (Fig. 1B). In contrast, the combination did not affect proliferation of normal colon cells (Fig. 1C). To determine the long-term effect of honokiol-IR treatment, cells were treated with honokiol for 4 h before exposure to 5 Gy IR. The cells were exposed to honokiol for an additional 24 h before allowing growth in normal medium. There were fewer colonies in both the two CRC cells with the combination treatment when compared to cells treated with either treatment alone (Fig. 1D), suggesting that the combination honokiol-IR treatment is effective.
Honokiol-IR combination induces apoptosis
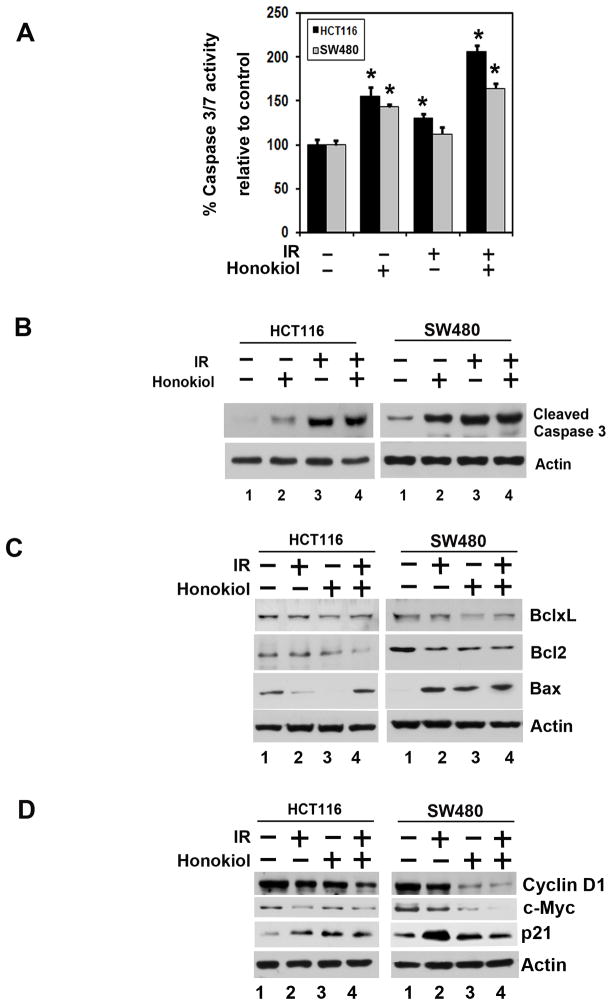
Caspase-3/7 are key effector molecules in the apoptosis pathway that initiate events that lead to the hallmarks of apoptosis, including DNA laddering and cellular morphological changes (23, 24). To determine whether honokiol-IR induced apoptosis, we determined caspase-3/7 activity. There was an increase in the caspase-3/7 activity following treatment with the combination of honokiol and IR (Fig. 2A). This was further confirmed by western blot analyses, demonstrating higher levels of activated caspase-3 in both CRC cells when compared either treatment alone (Fig. 2B). Further confirmation was obtained by western blot analyses for the anti-apoptotic Bcl2 and BclxL, and pro-apoptotic Bax proteins. While either honokiol or IR alone, inhibited Bcl2 and BclxL, there was a significant inhibition of the two proteins with the combination treatment (Fig. 2C). On the other hand, there was a significant increase in Bax protein with the individual treatment, with a further increase in response to the combination. These data suggest that honokiol is a potent inducer and an enhancer of radiation-induced apoptosis.
Figure 2. Honokiol and IR induce cancer cell apoptosis.
A, HCT116 and SW480 cells were treated with either 25 μM honokiol or 5 Gy IR or both for 48 h and tested for caspase 3/7 activity. The honokiol and IR combination induces apoptosis in both the cells, when compared to untreated controls (*p<0.05). B, honokiol and IR combination induces caspase 3, an apoptosis mediator. Cells were treated with 25 μM honokiol and 5 Gy IR. After 48 h, the lysates were analyzed by western blotting for caspase 3 protein. The combination of honokiol with IR resulted in increased levels of cleaved caspase 3 when compared to either treatment alone. C, lysates from cells treated with the combination of honokiol and IR were analyzed by western blotting for Bax and Bcl-2 family proteins. The honokiol-IR combination induces proapoptotic protein Bax and reduces expression of anti-apoptotic protein Bcl-2 family proteins when compared with untreated cells or cells treated with honokiol or IR alone. D, lysates from cells incubated with 25 μM of honokiol and exposed to 5 Gy IR were analyzed by western blotting for cyclin D1, c-myc and p21 expression. The honokiol-IR combination inhibits cyclin D1 and c-myc expression compared to either honokiol or IR alone while increasing p21 expression.
Cyclin D1 is a key cell cycle regulatory protein that functions as a cofactor for several transcription factors (25). Cyclin D1 overexpression has been linked to the development and progression of cancer (26). Similarly, c-myc is upregulated in cancers. In both CRC cells, honokiol and IR treatment resulted in reduced cyclin D1 and c-Myc, with the combination having an additive effect (Fig. 2D). On the other hand, there was a significant increase in p21WAF1 protein especially in response to the combination treatment (Fig. 2D).
Honokiol-IR targets cancer stem cells
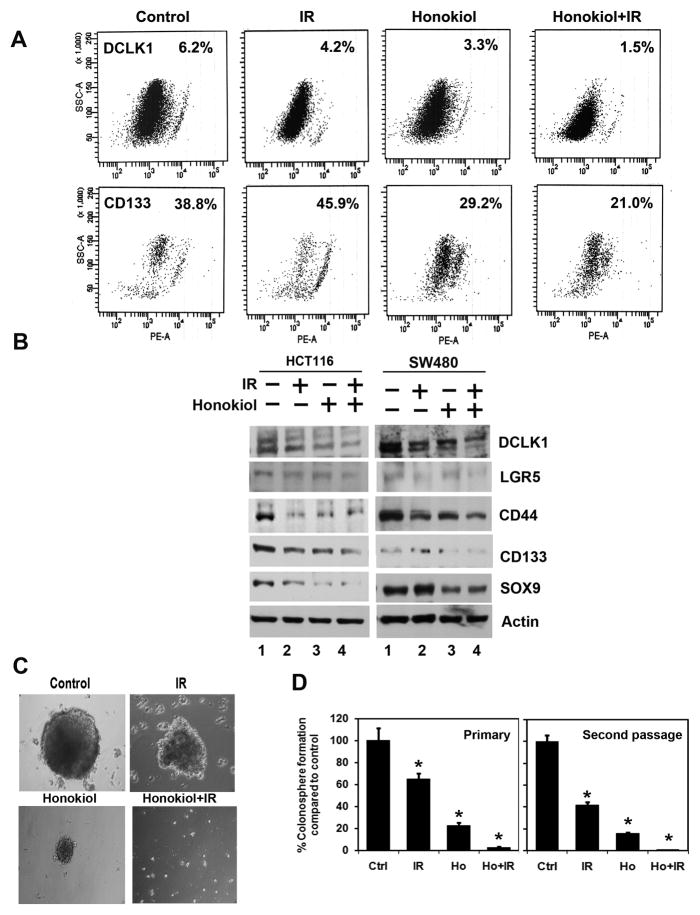
Colorectal tumors are thought to arise specifically in the stem cell population located at or near the base of the intestinal and colonic crypts. Markers used for identification of colon cancer stem cells include CD44, CD133, CD24, CD29, Lgr5 and DCLK-1 (20, 27). This cell population is capable of self-renewal and generating tumors resembling the primary tumor (16). We first determined the effects of honokiol-IR combination on DCLK1 and CD133 expression. Flow cytometric analyses showed a significant decrease in DCLK1+ and CD133+ SW480 cells with honokiol-IR combination (Fig. 3A). Western blot analyses confirmed these findings, as well reduction in other CSC markers (Fig. 3B). We confirmed these results by performing colonosphere formation assays, a hallmark for growth from CSCs. The honokiol-IR combination significantly inhibited primary and secondary SW480 colonosphere formation (Fig. 3C and D). Similar effects were obtained with tertiary colonosphere formation and also in HCT116 cells (data not shown).
Figure 3. Honokiol and IR affect cancer stem cells.
A, sorting of anti-DCLK1 and anti-CD133 antibodies-tagged SW480 cells by flow cytometry. 24 h after treatment, the honokiol-IR combination caused significant reduction in the number of DCLK1 and CD133 expressing cells. B, western blot analyses of lysates from the honokiol-IR combination showed significant reduction in cancer stem cell markers proteins DCLK1, LGR5, CD44, CD133 and SOX9 protein levels in both HCT116 and SW480 cells. C, D, SW480 cells were grown in specific spheroid growth media in low adherent plates and treated with 25 μM of Honokiol and 5.0 Gy IR. After one week, the colonospheres were photographed and counted. The primary spheroids were collected and separated into single cells and replated. The honokiol-IR combination significantly inhibited colonosphere formation (*P < 0.05).
Honokiol-IR inhibits Notch signaling by downregulating the γ-secretase complex
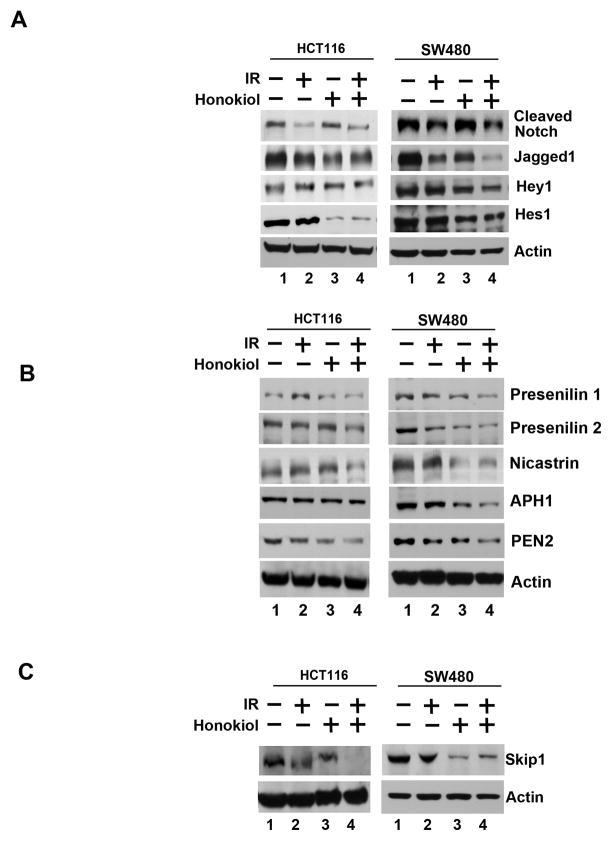
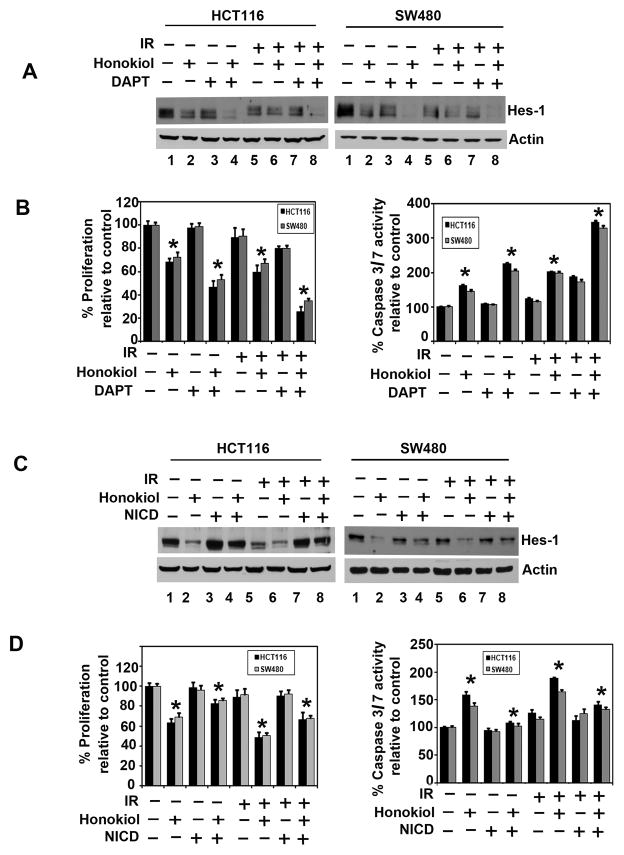
We next determined the effect of honokiol-IR combination on Notch signaling-related proteins in the two CRC cells. Both Notch-1 and its ligand, Jagged-1 were downregulated by the honokiol-IR combination (Fig. 4A). Further confirmation was obtained when reduced expression of Hey-1 and Hes-1 expression was observed (Fig. 4A). We next determined whether the γ-secretase complex comprising of Presenilin, Nicastrin, APH1 and PEN2 is affected. Treatment with the honokiol-IR combination resulted in downregulation in the expression of all four proteins (Fig. 4B). In addition, the combination inhibited expression of Skip1, a nuclear cofactor in NICD-mediated transcriptional activation (Fig. 4C). These data suggest that honokiol-IR mediated downregulation of the Notch signaling occurs in part through the inhibition of the γ-secretase complex. In addition, cotreatment of the honokiol-IR combination with DAPT further reduced Hes-1 expression (Fig. 5A), and proliferation (left panel) while inducing apoptosis (right panel) (Fig. 5B).
Figure 4. The honokiol-IR combination affects Notch signaling by inhibiting the γ-secretase complex.
A, lysates from cells treated with honokiol-IR combination caused significant reduction in the expression of cleaved Notch-1, its ligand Jagged-1 and its target genes Hes-1 and Hey-1 in both HCT116 and SW480 cells. B, the honokiol-IR combination also significantly reduced expression of γ-secretase complex proteins Presenilin-1 and 2, Nicastrin, APH1 and PEN2 in both HCT116 and SW480 cells. C, lysates from HCT116 and SW480 cells treated with the honokiol-IR combination demonstrated significant reduction in Skip1, a protein that plays an important role in the conversion from CBF1-mediated transcriptional repression to activation thereby allowing Notch intracellular domain to be a transcriptional trans-activator.
Figure 5. Honokiol-IR combination inhibits cell growth through inactivation of the γ-secretase complex.
A, combination of DAPT, a GSI with Honokiol-IR further inhibits proliferation and induce apoptosis. Cells were treated with DAPT (50 μM) and either honokiol (25 μM) alone or IR (5 Gy) and in combination for 24 h. Lysates were analyzed by western blotting. Hes-1 protein expression was further reduced with the combination of the two compounds and also combinations with IR. B, cells proliferation was significantly inhibited following treatment with the combination of DAPT and honokiol or IR when compared to each alone and all three combinations (*P <0.05) (left panel). DAPT also enhanced honokiol and IR induced apoptosis (right panel) (*P <0.05). C, ectopic expression of NICD overcomes honokiol and IR-mediated suppression of Hes-1 expression. Cells transiently expressing NICD were treated with honokiol and IR for 24 h. Lysate were analyzed by western blotting. Hes-1 was increased in the NICD expressing cells when compared to vector transfected controls. D, cells expressing NICD were treated with honokiol and IR and subsequently measured for proliferation (left panel) and apoptosis (right panel). Ectopic expression of NICD rescued honokiol and IR mediated inhibition of cell proliferation and apoptosis (*P <0.05).
We next determined whether lack of Notch-1 activation is the reason for reduced growth of colon cancer cells. For this, we expressed the intracellular domain NICD in HCT116 and SW480 cells. Western blot analyses demonstrated increased expression of Hes-1 following ectopic expression of NICD in both cell lines (Fig. 5C). Furthermore, while honokiol or IR alone inhibited the basal levels of Hes-1 expression, NICD rescued this inhibition resulting in increased Hes-1 expression. Moreover, ectopic expression of NICD reversed honokiol and IR mediated inhibition of cell proliferation (left panel) and induction of apoptosis (right panel) (Fig. 5D). Together, these data suggest that honokiol and IR inhibits the γ-secretase complex thereby affecting Notch signaling.
Honokiol radiosensitizes colon tumor xenografts
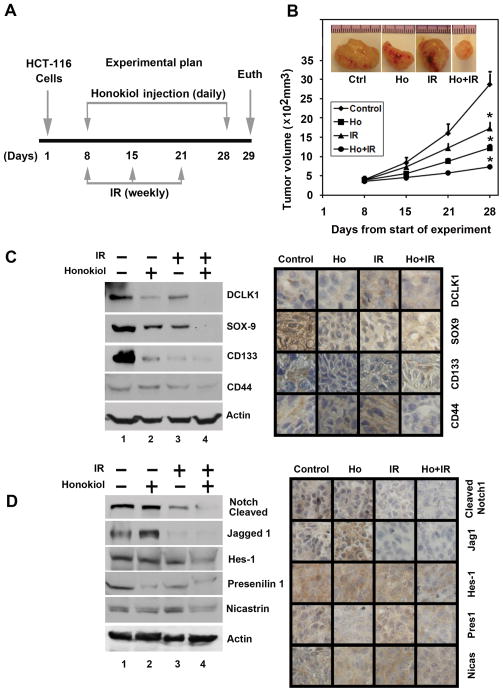
To evaluate the role of honokiol-IR combination on tumor growth in vivo, we next examined its effects on growth of HCT116 xenografts. Honokiol was administered intraperitoneally to mice bearing xenografts and irradiated as shown in Fig. 6A. While treatment with either honokiol or IR inhibited the growth of the tumor xenografts, there was reduction with the honokiol-IR combination (Fig. 6B). The excised tumors from control animals weighed ~3300 mg, those treated with honokiol and IR alone weighed ~1500 mg and~2300 mg, respectively. Moreover, the tumors from animals treated with the honokiol-IR combination weighed < 800 mg (Supplementary Fig. S2A). There was no apparent change in liver, spleen, or body weight in the animals (data not shown). These data imply that the honokiol-IR is a potential therapeutic combination for treating colon cancers but is relatively non-toxic to the animals. We also determined the effect of the combination on tumor vascularization by staining for endothelial-specific antigen CD31. As shown in supplementary Fig. S2B, treatment with the honokiol-IR combination leads to a significant reduction in CD31 staining and to the obliteration of the normal vasculature compared with either honokiol or IR alone (Supplementary Fig. S2B).
Figure 6. Honokiol radiosensitizes colon cancer xenografts, and inhibits stem cell related protein expression.
A, experimental plan, HCT116 cells were injected in to the flanks of nude mice and palpable tumors were allowed to develop for 7 days. Subsequently, honokiol (200 μg/kg bw) was injected daily intraperitoneally every day for 21 days. The mice were irradiated (5 Gy) weekly once for 3 weeks. On day 22, tumors were excised and subject to further analyses. B, Tumor volumes in honokiol-IR combination treated mice were smaller than either honokiol or IR alone (*P<0.05). C, western blot analysis showed that tissue lysates from the combination of honokiol and IR treated animals have significantly lower levels of cancer stem cell markers. (left panel). Immunohistochemistry shows that treatment with the honokiol-IR combination significantly lower reduced the expression of cancer stem cell markers (right panel). D, western blot analysis showed that tissue lysates from the combination of honokiol and IR treated animals have significantly lower levels of Notch-1, Jagged-1, Hes-1, and γ-secretase complex proteins. (left panel). Immunohistochemistry shows that the honokiol-IR combination treated animals have significantly lower levels of Notch-1, Jagged-1, Hes-1, and γ-secretase complex proteins in the tumor xenograft tissues (right panel).
To further investigate whether the honokiol-IR combination affects CSCs, we determined specific marker expression in the tumor tissues. Western blot analyses demonstrated that the honokiol-IR combination significantly reduced the expression of CSC proteins DCLK1, SOX-9, CD133 and CD44 (left panel), which was confirmed by immunohistochemistry (right panel) (Fig. 6C). These data suggest that the combination of honokiol and IR targets colon CSCs with high potency. Furthermore, treatment with the honokiol-IR combination resulted in significantly lower levels of activated Notch-1, its ligand Jagged 1 and the downstream target gene Hes-1 (left panel) (Fig. 6D). There was also a significant reduction in the expression of γ-secretase complex proteins, Presenilin 1 and Nicastrin (left panel) (Fig. 6D). Again, further confirmation of the downregulation was obtained by immunohistochemistry for the proteins in the xenograft tissue (right panel) (Fig. 6D). These data suggest that the honokiol-IR combination significantly affects the expression of Notch signaling-related proteins, which might contribute to the inhibitory effects of this treatment.
Discussion
Our results indicate that the honokiol-IR combination has a significant potential as an anti-colorectal cancer therapeutic strategy. Unlike other polyphenolic agents, which have been hindered by poor absorption and rapid excretion, honokiol exhibits a desirable spectrum of bioavailability (4). Significant systemic levels of honokiol can be obtained in preclinical models and it can also cross the blood brain barrier (4). Currently, it is not known whether honokiol has a single major target or several targets. However, it has several activities that make it desirable both as a therapeutic and as a chemopreventive agent. First, it is orally bioavailable and crosses the blood brain barrier. Second, it inhibits NF-κB activity in a manner that is different from other known inhibitors. Honokiol can also cause mitochondrial dysfunction in cancer cells (4). These data together suggest that honokiol could be used as an effective agent either alone or in combination with IR and/or chemotherapeutic drugs. While the compound alone can be administered as a chemopreventive agent, our data shows that it can be used in combination with other modalities such as chemotherapeutic drugs or radiation for therapeutic activity. Of course, there is a need to consider how the honokiol could be administered. For colorectal cancers, one mode could be oral administration, especially since it is water-soluble and has been shown to be bioavailable. This would also be an attractive chemopreventive strategy. Another delivery method could be an intravenous route of administration, which could have efficacy in therapeutic paradigms. In this regard, a recent study demonstrated that a biodegradable self-assembled PEG-PCL-PEG micelle encapsulating honokiol can be administered intravenously for effectively targeting colorectal cancers (28). More importantly, this method was found to be effective, stable and safe.
Honokiol disrupts many of the characteristic cancer-promoting events. Previous studies have shown that honokiol radiosensitizes lung cancer cells (29). Our current studies show a similar effect on colorectal cancer cells. The honokiol-IR combination inhibited proliferation of colorectal cancer cells and promoted apoptosis at a much higher rate than either one alone. Previous studies with LL/2 Lewis lung carcinoma cells showed that honokiol combined with radiotherapy can induce the cells to arrest in the G0/G1 phase and a corresponding decrease in the S-phase (29). Recently, we demonstrated that while honokiol induced a G0/G1 phase arrest and that IR alone induces G2/M arrest, the combination induced higher rates of G2/M arrest (30). Given that radiation is known to induce mitotic catastrophe (apoptosis during mitosis), we hypothesize that the compound probably drives the cells through the cell cycle but never makes it out of mitosis due to IR-induced catastrophe. Thorough analyses of the transition of the cell through the various phases of the cell cycle are needed along with the checkpoint related markers to confirm this phenomenon.
Recent studies have suggested that CSCs have the capacity to drive tumor resistance and recurrence to chemotherapeutic agents and radiation (31). Natural compounds such as curcumin and sulforaphane have been suggested to target CSCs (32–35). Our results suggest that the honokiol-IR combination is a potent inhibitor CSC based on two approaches. First, we determined that it inhibits the expression of DCLK1, a bonafide stem cell marker of the intestinal epithelial cells. The extracellular domain in DCLK makes it convenient to isolate and grow CSCs in vitro. DCLK1+ intestinal stem cells are quiescent and also label retaining cells. DCLK1+ cells are also numerically rare in human colorectal cancers and these cells are also quiescent. Others have suggested that the stem cell expresses proteins such as LGR5 and SOX9 (36, 37). Unfortunately these are not cell surface expressing proteins, and hence the need to resort to western blotting techniques. Moreover, these cells are not quiescent and are actively dividing. The possibility exist that these proteins are expressed by immediate progenitors of the quiescent stem cells and hence are rapidly dividing daughter cells. Nevertheless, our western blot analyses have demonstrated that the honokiol-IR combination also inhibits the expression of these proteins along with other such proteins including CD133 and CD44. This was also confirmed in vivo where the combination significantly reduced the expression of these markers and also the growth of the xenografts. Another method that is commonly used to demonstrate stemness is the growth of spheroids or colonospheres. The honokiol-IR combination inhibited colonosphere formation further suggesting that they target the CSCs.
The Notch pathway plays a critical role in colon cancer (38). Notch also has been shown to be important in stem cell renewal and vascular development (39). Notch signaling is also 10–30-fold higher in the stem cells when compare to other cell types (40). In our studies, we have determined that the honokiol-IR combination resulted in downregulation of the Notch ligand Jagged1 as well as all four essential members of the γ-secretase complex, the critical enzyme that cleaves and releases the NICD from the membrane. Therefore, honokiol-IR mediated inhibition of CRC cell growth is partly mediated via inactivation of Notch-1 activity. This was further confirmed by the combination of a GSI with honokiol-IR, which further inhibited proliferation and induced apoptosis. However, ectopic expression of NICD reversed the effects of honokiol and IR, and partially restored cell growth. Similarly, while the combination of honokiol and IR with a GSI further inhibited Hes-1 expression, the ectopic NICD partially rescued Hes-1 expression. Indeed studies have shown that targeting the γ-secretase complex using small molecule GSI inhibitors affect tumor initiating cells in mouse model of ERBB2 breast cancer (41). It would also be interesting to determine whether there are other clients for the γ-secretase complex and the role of these client proteins in CSC biogenesis. Our data suggest that ectopic expression of the Notch intracellular domain only partially rescues the honokiol effect. This suggests that there might be other pathways that could also mediate the honokiol-IR effect. In this regard, honokiol has been shown to block TNF-α induced NF-κB and Akt activation resulting in enhanced TNF-α–mediated cell death (42). A more recent study also demonstrated that honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine by affecting NF-κB (43,44). These data suggest that NF-κB could also play a role in radiosenzitising colon cancer stem cells.
Recent reports from experimental and clinical studies have also proposed that combinations of radio- or chemotherapy agents with natural preventive agents have greater than additive effects resulting in increased efficacy while reducing potential side effects (45). The current study provides evidence that treatment with the honokiol-IR combination results in a growth inhibition in vitro and in vivo. Furthermore, the combination treatment was more potent against colon CSCs. In addition, the honokiol-IR combination significantly suppressed Notch-1 activation. Taken together, these data suggest that the combination of honokiol and radiation to target colon CSCs is an attractive novel potential agent for the treatment and prevention colon cancer. Further studies are warranted to demonstrate the efficacy of the honokiol-IR combination in the clinical setting.
Supplementary Material
Acknowledgments
Grant/Funding Information: The work was supported by grants from the National Institutes of Health (SA), Cancer Center Pilot Project Program (DS) and from the Thomas O’Sullivan Foundation (DS). S. Anant is an Eminent Scientist of the Kansas Biosciences Authority.
We thank Ms. Lauren Larsen for her help during the writing of this manuscript. We also thank members of the Anant laboratory for their discussion during the course of this study.
Footnotes
Financial Disclosure: The authors have no conflict of interests to disclose.
Disclosure of Potential Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Fujita M, Itokawa H, Sashida Y. Studies on the components of Magnolia obovata Thunb. 3. Occurrence of magnolol and honokiol in M. obovata and other allied plants. Yakugaku Zasshi. 1973;93:429–34. doi: 10.1248/yakushi1947.93.4_429. [DOI] [PubMed] [Google Scholar]
- 2.Lee YJ, Lee YM, Lee CK, Jung JK, Han SB, Hong JT. Therapeutic applications of compounds in the Magnolia family. Pharmacol Ther. 2011;130:157–76. doi: 10.1016/j.pharmthera.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Teng CM, Chen CC, Ko FN, Lee LG, Huang TF, Chen YP, et al. Two antiplatelet agents from Magnolia officinalis. Thromb Res. 1988;50:757–65. doi: 10.1016/0049-3848(88)90336-2. [DOI] [PubMed] [Google Scholar]
- 4.Fried LE, Arbiser JL. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid Redox Signal. 2009;11:1139–48. doi: 10.1089/ars.2009.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Li B, Ji ZZ, Zheng PS. Notch1 regulates the growth of human colon cancers. Cancer. 2010;116:5207–18. doi: 10.1002/cncr.25449. [DOI] [PubMed] [Google Scholar]
- 6.Reedijk M, Odorcic S, Zhang H, Chetty R, Tennert C, Dickson BC, et al. Activation of Notch signaling in human colon adenocarcinoma. Int J Oncol. 2008;33:1223–9. doi: 10.3892/ijo_00000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26:2839–45. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miele L, Golde T, Osborne B. Notch signaling in cancer. Curr Mol Med. 2006;6:905–18. doi: 10.2174/156652406779010830. [DOI] [PubMed] [Google Scholar]
- 9.Miele L. Notch signaling. Clin Cancer Res. 2006;12:1074–9. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- 10.Miele L, Miao H, Nickoloff BJ. NOTCH signaling as a novel cancer therapeutic target. Curr Cancer Drug Targets. 2006;6:313–23. doi: 10.2174/156800906777441771. [DOI] [PubMed] [Google Scholar]
- 11.Sansom OJ, Reed KR, van de Wetering M, Muncan V, Winton DJ, Clevers H, et al. Cyclin D1 is not an immediate target of beta-catenin following Apc loss in the intestine. J Biol Chem. 2005;280:28463–7. doi: 10.1074/jbc.M500191200. [DOI] [PubMed] [Google Scholar]
- 12.de Lau W, Barker N, Clevers H. WNT signaling in the normal intestine and colorectal cancer. Front Biosci. 2007;12:471–91. doi: 10.2741/2076. [DOI] [PubMed] [Google Scholar]
- 13.Potten CS, Booth C, Tudor GL, Booth D, Brady G, Hurley P, et al. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation. 2003;71:28–41. doi: 10.1046/j.1432-0436.2003.700603.x. [DOI] [PubMed] [Google Scholar]
- 14.Du L, Wang H, He L, Zhang J, Ni B, Wang X, et al. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14:6751–60. doi: 10.1158/1078-0432.CCR-08-1034. [DOI] [PubMed] [Google Scholar]
- 15.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 16.Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells--old concepts, new insights. Cell Death Differ. 2008;15:947–58. doi: 10.1038/cdd.2008.20. [DOI] [PubMed] [Google Scholar]
- 17.Vermeulen L, Todaro M, de Sousa Mello F, Sprick MR, Kemper K, Perez Alea M, et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci U S A. 2008;105:13427–32. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 19.Lin PT, Gleeson JG, Corbo JC, Flanagan L, Walsh CA. DCAMKL1 encodes a protein kinase with homology to doublecortin that regulates microtubule polymerization. J Neurosci. 2000;20:9152–61. doi: 10.1523/JNEUROSCI.20-24-09152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sureban SM, May R, Ramalingam S, Subramaniam D, Natarajan G, Anant S, et al. Selective blockade of DCAMKL-1 results in tumor growth arrest by a Let-7a MicroRNA-dependent mechanism. Gastroenterology. 2009;137:649–59. 59 e1–2. doi: 10.1053/j.gastro.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J Immunol Methods. 1984;67:379–88. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- 22.Subramaniam D, May R, Sureban SM, Lee KB, George R, Kuppusamy P, et al. Diphenyl difluoroketone: a curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008;68:1962–9. doi: 10.1158/0008-5472.CAN-07-6011. [DOI] [PubMed] [Google Scholar]
- 23.Boatright KM, Salvesen GS. Caspase activation. Biochem Soc Symp. 2003;70:233–42. doi: 10.1042/bss0700233. [DOI] [PubMed] [Google Scholar]
- 24.Cho SG, Choi EJ. Apoptotic signaling pathways: caspases and stress-activated protein kinases. J Biochem Mol Biol. 2002;35:24–7. doi: 10.5483/bmbrep.2002.35.1.024. [DOI] [PubMed] [Google Scholar]
- 25.Krecicki T, Smigiel R, Fraczek M, Kowalczyk M, Sasiadek MM. Studies of the cell cycle regulatory proteins P16, cyclin D1 and retinoblastoma protein in laryngeal carcinoma tissue. J Laryngol Otol. 2004;118:676–80. doi: 10.1258/0022215042244769. [DOI] [PubMed] [Google Scholar]
- 26.Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26:630–7. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- 28.Gong C, Wei X, Wang X, Wang Y, Guo G, Mao Y, et al. Biodegradable self-assembled PEG-PCL-PEG micelles for hydrophobic honokiol delivery: I. Preparation and characterization. Nanotechnology. 2010;21:215103. doi: 10.1088/0957-4484/21/21/215103. [DOI] [PubMed] [Google Scholar]
- 29.Hu J, Chen LJ, Liu L, Chen X, Chen PL, Yang G, et al. Liposomal honokiol, a potent anti-angiogenesis agent, in combination with radiotherapy produces a synergistic antitumor efficacy without increasing toxicity. Exp Mol Med. 2008;40:617–28. doi: 10.3858/emm.2008.40.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He Z, Subramaniam D, Ramalingam S, Dhar A, Postier RG, Umar S, et al. Honokiol radiosensitizes colorectal cancer cells: enhanced activity in cells with mismatch repair defects. Am J Physiol Gastrointest Liver Physiol [Research Support, NIH, Extramural Research Support, Non-US Gov’t] 2011;301:G929–37. doi: 10.1152/ajpgi.00159.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milas L, Raju U, Liao Z, Ajani J. Targeting molecular determinants of tumor chemo-radioresistance. Semin Oncol. 2005;32:S78–81. doi: 10.1053/j.seminoncol.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 32.Yu Y, Kanwar SS, Patel BB, Nautiyal J, Sarkar FH, Majumdar AP. Elimination of Colon Cancer Stem-Like Cells by the Combination of Curcumin and FOLFOX. Transl Oncol. 2009;2:321–8. doi: 10.1593/tlo.09193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramaniam D, Ramalingam S, Houchen CW, Anant S. Cancer stem cells: a novel paradigm for cancer prevention and treatment. Mini Rev Med Chem. 2010;10:359–71. doi: 10.2174/138955710791330954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nautiyal J, Kanwar SS, Yu Y, Majumdar AP. Combination of dasatinib and curcumin eliminates chemo-resistant colon cancer cells. J Mol Signal. 2011;6:7. doi: 10.1186/1750-2187-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16:2580–90. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138:1681–96. doi: 10.1053/j.gastro.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178:635–48. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fre S, Pallavi SK, Huyghe M, Lae M, Janssen KP, Robine S, et al. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci U S A. 2009;106:6309–14. doi: 10.1073/pnas.0900427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer M, Yen WC, Kapoun AM, Wang M, O’Young G, Lewicki J, et al. Anti-DLL4 inhibits growth and reduces tumor-initiating cell frequency in colorectal tumors with oncogenic KRAS mutations. Cancer Res. 2011;71:1520–5. doi: 10.1158/0008-5472.CAN-10-2817. [DOI] [PubMed] [Google Scholar]
- 40.Sikandar SS, Pate KT, Anderson S, Dizon D, Edwards RA, Waterman ML, et al. NOTCH signaling is required for formation and self-renewal of tumor-initiating cells and for repression of secretory cell differentiation in colon cancer. Cancer Res. 2010;70:1469–78. doi: 10.1158/0008-5472.CAN-09-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kondratyev M, Kreso A, Hallett RM, Girgis-Gabardo A, Barcelon ME, Ilieva D, et al. Gamma-secretase inhibitors target tumor-initiating cells in a mouse model of ERBB2 breast cancer. Oncogene. 2012;5(31):93–103. doi: 10.1038/onc.2011.212. [DOI] [PubMed] [Google Scholar]
- 42.Ahn KS, Sethi G, Shishodia S, Sung B, Arbiser JL, Aggarwal BB. Honokiol potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through modulation of nuclear factor-kappaB activation pathway. Mol Cancer Res. 2006;4:621–33. doi: 10.1158/1541-7786.MCR-06-0076. [DOI] [PubMed] [Google Scholar]
- 43.Lee SY, Yuk DY, Song HS, Yoon do Y, Jung JK, Moon DC, Lee BS, Hong JT. Growth inhibitory effects of obovatol through induction of apoptotic cell death in prostate and colon cancer by blocking of NF-kappaB. Eur J Pharmacol. 2008;582:17–25. doi: 10.1016/j.ejphar.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 44.Arora S, Bhardwaj A, Srivastava SK, Singh S, McClellan S, Wang B, Singh AP. Honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells. PLoS One. 2011;6:e21573. doi: 10.1371/journal.pone.0021573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bello L, Carrabba G, Giussani C, Lucini V, Cerutti F, Scaglione F, et al. Low-dose chemotherapy combined with an antiangiogenic drug reduces human glioma growth in vivo. Cancer Res. 2001;61:7501–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.