Abstract
API-1 is a novel small molecule inhibitor of Akt, which acts by binding to Akt and preventing its membrane translocation, and has promising preclinical antitumor activity. In this study, we reveal a novel function of API-1 in regulation of c-FLIP levels and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis, independent of Akt inhibition. API-1 effectively induced apoptosis in tested cancer cell lines including activation of caspase-8 and caspase-9. It reduced the levels of c-FLIP without increasing the expression of DR4 or DR5. Accordingly, it synergized with TRAIL to induce apoptosis. Enforced expression of ectopic c-FLIP did not attenuate API-1-induced apoptosis, but inhibited its ability to enhance TRAIL-induced apoptosis. These data indicate that downregulation of c-FLIP mediates enhancement of TRAIL-induced apoptosis by API-1, but is not sufficient for API-1-induced apoptosis. API-1-induced reduction of c-FLIP could be blocked by the proteasome inhibitor MG132. Moreover, API-1 increased c-FLIP ubiquitination and decreased c-FLIP stability. These data together suggest that API-1 downregulates c-FLIP by facilitating its ubiquitination and proteasome-mediated degradation. Since other Akt inhibitors including API-2 and MK2206 had minimal effects on reducing c-FLIP and enhancement of TRAIL-induced apoptosis, it is likely that API-1 reduces c-FLIP and enhances TRAIL-induced apoptosis independent of its Akt-inhibitory activity.
Keywords: API-1, Akt, TRAIL, c-FLIP, apoptosis, cancer
Introduction
API-1 (pyrido[2,3-d]pyrimidines) is a recently identified small molecule inhibitor of Akt, which acts through binding to Akt and blocking its membrane translocation (1). A previous study has shown that API-1 possesses promising anticancer activity, evidenced by its ability to suppress cell growth, induce apoptosis and inhibit the growth of cancer xenografts, particularly those with activated Akt, in nude mice (1).
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL; also called APO-2L) is a member of the tumor necrosis factor (TNF) family and is currently being tested in phase I oncology trials due to its unique ability to trigger apoptosis in various types of cancer cells with limited toxicity toward normal cells (2, 3). However, many primary tumors are inherently resistant to TRAIL-mediated apoptosis and require additional sensitization (4, 5).
TRAIL initiates apoptosis by binding to cell surface death receptor 4 (DR4) or 5 (DR5); this induces oligomerization of the death receptors and formation of the death inducing signaling complex (DISC), involving recruitment of the adaptor molecule FADD and subsequent caspase-8. DISC assembly promotes the autocleavage and activation of caspase-8, leading to further activation of the effector caspases (e.g., caspase-3) that eventually drive apoptotic death (6). Cellular FLICE-inhibitory protein (c-FLIP) is a truncated form of caspase-8 that lacks enzymatic activity. It can also be recruited to DISC, but suppresses apoptosis by blocking the activation of caspase-8 through competing with caspase-8 for binding to FADD (7). It has been well documented that elevated c-FLIP expression protects cells from death receptor–mediated apoptosis, whereas downregulation of c-FLIP by chemicals or small interfering RNA (siRNA) sensitizes cells to death receptor–mediated apoptosis (7, 8). Therefore, c-FLIP acts as a key inhibitor of TRAIL/death receptor-induced apoptosis. c-FLIP has multiple splice variants, however, only two of them have been well characterized at the protein levels: the 26 kDa short form (c-FLIPS) containing two death effector domains and the 55 kDa long form (c-FLIPL) containing an inactive caspase-like domain in addition to the two death effector domains (7, 9). The levels of c-FLIP, including both FLIPL and FLIPS are regulated by ubiquitin/proteasome-mediated degradation (10–12).
Although cancer cells possess intrinsic resistance to TRAIL, many anticancer agents can sensitize cancer cells to TRAIL-induced apoptosis through various mechanisms such as induction of DR5 and/or DR4 expression and/or downregulation of c-FLIP levels (13, 14). Akt has been suggested to positively regulate c-FLIP expression because activation or suppression of Akt accordingly increased or decreased the levels of c-FLIP (15). Recently, Akt1 was shown to directly interact with FLIPL and to phosphorylate it at S273, leading to stabilization of FLIPL (16). Thus, the current study primarily focused on determining whether API-1 negatively regulates c-FLIP levels and sensitizes cancer cells to TRAIL-induced apoptosis. Moreover, we have revealed the mechanisms by which API-1 reduces c-FLIP levels and enhances TRAIL-induced apoptosis.
Materials and Methods
Reagents
API-1 (NSC177233) was obtained from the National Cancer Institute (Bethesda, MD). API-2 (17) was provided by Dr. J. Q. Cheng (H Lee Moffitt Cancer Center and Research Institute, Tampa, FL). MK2206 was purchased from Active Biochem (Maplewood, NJ). The soluble recombinant human TRAIL was purchased from PeproTech, Inc. (Rocky Hill, NJ). The proteasome inhibitor MG132 and the protein synthesis inhibitor cycloheximide (CHX) were purchased from Sigma Chemical Co. (St. Louis, MO). Monoclonal anti-FLIP antibody (NF6) was obtained from Alexis Biochemicals (San Diego, CA). Mouse monoclonal anti-caspase-8 and rabbit polyclonal anti-caspase-9, anti-PARP, and anti-Akt antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Mouse monoclonal anti-caspase-3 antibody was purchased from Imgenex (San Diego, CA). Rabbit polyclonal anti-DR5 antibody was obtained from ProSci Inc. (Poway, CA). Mouse monoclonal anti-DR4 antibody (B-N28) was purchased from Diaclone (Stamford, CT). Rabbit monoclonal anti-p-Akt (S473) antibody was purchased from Epitomics, Inc (Burlingame, CA). Both polyclonal and monoclonal anti-actin antibodies were purchased from Sigma Chemical Co.
Cell lines and cell culture
Human non-small cell lung cancer (NSCLC) cell lines (H157, Calu-1 and H1299) and head and neck squamous cell carcinoma (HNSCC) cell lines (22A, Tr146 and SqCC/Y1) were described in our previous work (18). H157 cells were recently authenticated by Genetica DNA Laboratories, Inc. (Cincinnati, OH) by analyzing short tandem repeat DNA profile. The other cell lines have not been authenticated. The H157-Lac Z-5, H157-FLIPL-21, and H157-FLIPS-1 stable transfectants were established as described previously (19, 20). The 22A cells (pool) stably expressing Lac Z, FLIPL and FLIPS were described previously (21). These cell lines were cultured in PMRI-1640 or DMEM/F12 medium containing 5% fetal bovine serum at 37 C in a humidified atmosphere of 5% CO2 and 95% air.
Cell survival and apoptosis assays
Cells were seeded in 96-well cell culture plates and treated the next day with the given agents. The viable cell number was determined using sulforhodamine B (SRB) assay as described previously (22). Combination index (CI) for drug interaction (e.g., synergy) was calculated using the CompuSyn software (ComboSyn, Inc.; Paramus, NJ). Apoptosis was evaluated with annexin V-PE apoptosis detection kit purchased from BD Biosciences (San Jose, CA). We also detected caspase and PARP cleavage by Western blot analysis as described below, as additional indicators of apoptosis.
Western blot analysis
The procedures for preparation of whole-cell protein lysates and for Western blotting were the same as described before (23, 24). The quantification of Western blotting results was done with NIH Image J software (Bethesda, MD).
Immunoprecipitation (IP) for detection of ubiquitinated c-FLIP
H157-FLIPL-21 cells were transfected with HA-ubiquitin plasmid using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA) based on the manufacturer’s instructions. After 24 h, the cells were treated with API-1 or API-1 plus MG132 for 3 h. Cells were collected and lysed for IP of Flag-FLIPL using Flag M2 monoclonal antibody (Sigma) as previously described (19, 25) followed by detection of ubiquitinated FLIPL with Western blot analysis using anti-HA antibody (Abgent, San Diego, CA).
Results
API-1 effectively inhibits the growth and induces apoptosis of human NSCLC and HNSCC cells
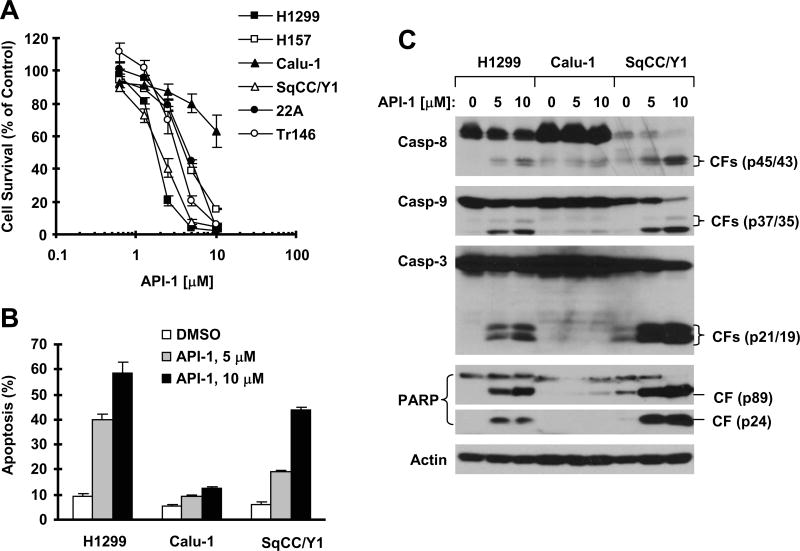
We first evaluated the single agent activity of API-1 on the growth of a panel of NSCLC and HNSCC cell lines. A 3-day exposure to API-1 effectively inhibited the growth of 5 (H1299, H157, SqCC/Y1, 22A and Tr146) of 6 tested cancer cell lines (Fig. 1A). The effective concentrations that decreased cell numbers by 50% (IC50s) ranged between 2 and 5 μM for these sensitive cell lines (Fig. 1A). Calu-1 was relatively insensitive to API-1 with an IC50 greater than 10 μM.
Fig. 1. API-1 inhibits the growth (A) and induces apoptosis (B) including caspase activation (C) in human cancer cells.
A, The indicated cancer cell lines were seeded in 96-well cell culture plates and treated the next day with API-1 at 0.625, 1.25, 2.5, 5 and 10μM. After 3 days, cell numbers were estimated using the SRB assay. Cell survival was expressed as the percentage of control (DMSO-treated) cells. Data are the means of four replicate determinations. Bars, ± SDs. B and C, The indicated cell lines were treated with the given concentrations of API-1 for 24 h and then harvested for measurement of apoptosis using annexin V staining (A) and for detection of caspase activation with Western blotting (C). Data are the means of duplicate experiments. Bars, ± SDs. CF, cleaved fragment.
We then determined whether API-1 induces apoptosis in these cell lines. Treatment of the representative H1299, Calu-1 and SqCC/Y1 cell lines with different concentrations of API-1 for 24 h dose-dependently increased annexin V-positive (or apoptotic) cells in H1299 and SqCC/Y1 cells (> 40% at 10 μM), but did so only minimally in Calu-1 cells (< 15% at 10 μM) (Fig. 1B). In agreement, we detected dose-dependent increase in cleavage of caspase-8, caspase-9, caspase-3 and PARP in H1299 and SqCC/Y1 cells, but this was not apparent in Calu-1 cells (Fig. 1C). These results clearly indicate that API-1 effectively induces apoptosis in API-1-sensitive NSCLC and HNSCC cell lines.
API-1 reduces c-FLIP levels without induction of DR4 and DR5 expression
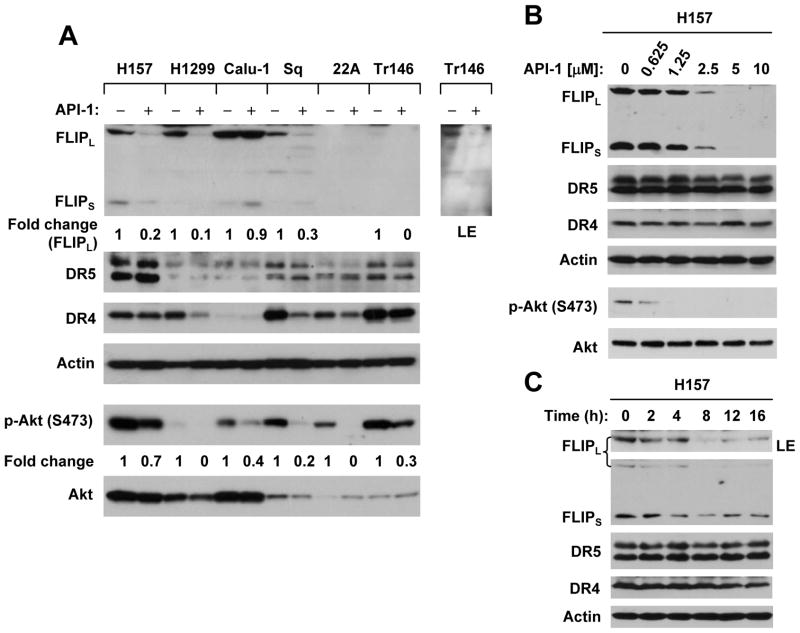
Since API-1 effectively activates caspase-8, we then asked whether API-1 modulates the levels of key proteins (e.g., c-FLIP, DR4 and DR5) involved in the death receptor-mediated apoptotic pathway. As presented in Fig. 2A, API-1 at 5 μM reduced the levels of c-FLIP in H157, H1299, SqCC/Y1 and Tr146 cells, but not in Calu-1 cells. c-FLIP levels in 22A cells were too low to be detected. API-1 did not increase the expression of either DR5 or DR4 in any of the cell lines. Rather, API-1 reduced the levels of DR4 in some cell lines (H1299, SqCC/Y1 and Tr146). Moreover, we conducted detailed dose-course and time-course studies of the effects of API-1 on the levels of c-FLIP, DR4 and DR5 in H157 cells. We found that API-1 could reduce c-FLIP levels even at 1.25 μM (Fig. 2B). The apparent reduction of c-FLIP in cells occurred after 4 h exposure to API-1 (Fig. 2C). Under these conditions, we did not see that API-1 increased the expression of either DR4 or DR5 (Figs. 2B and 2C). Thus, API-1 downregulates c-FLIP levels without induction of DR4 and DR5 expression in NSCLC and HNSCC cells.
Fig. 2. API-1 downregulates c-FLIP levels without increasing DR4 and DR5 expression.
A, The given cell lines were treated with 5μM API-1 for 24 h. B, H157 cells were treated with different concentrations of API-1 as indicated for 12 h. C, H157 cells were treated with 5μM API-1 for the indicated times. After the aforementioned treatments, the cells were subjected to preparation of whole-cell protein lysates and subsequent Western blot analysis for the given proteins. LE, longer exposure.
Additionally, we compared the effects of API-1 on Akt phosphorylation among the 6 NSCLC and HNSCC cell lines. As presented in Fig. 2A, API-1 inhibited the phosphorylation of Akt in all of the cell lines, albeit with varied potencies. Thus, it is clear that API-1 inhibits Akt phosphorylation.
API-1 synergizes with TRAIL to induce apoptosis
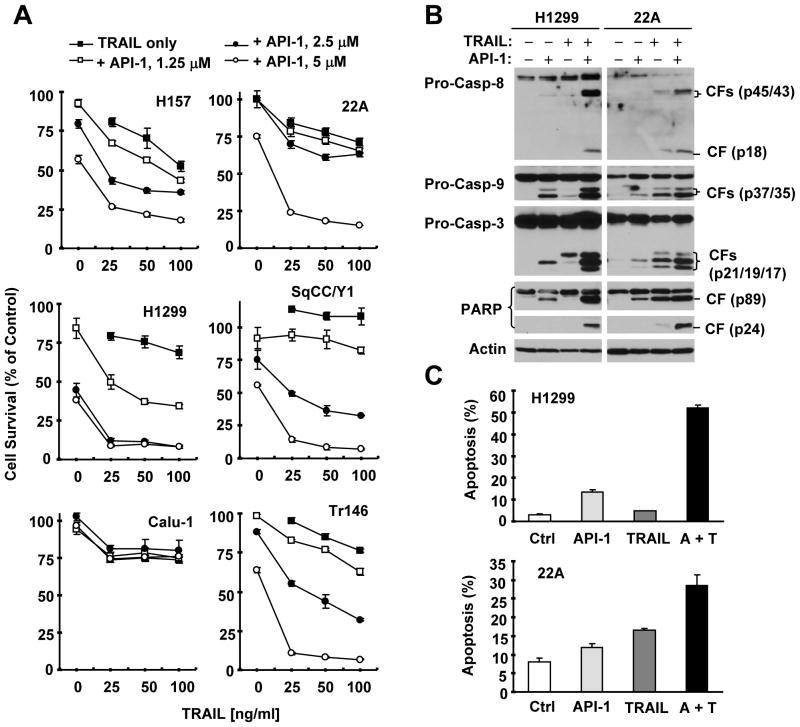
Given that API-1 reduces c-FLIP levels, we determined whether API-1 enhances TRAIL-induced apoptosis. As presented in Fig. 3A, the combination of API-1 at 5 μM or 2.5 μM and TRAIL (25 to 100 ng/ml) was much more effective than either agent alone in decreasing the survival of the tested NSCLC and HNSCC cell lines, except for Calu-1 cells. The CIs for these combinations were < 1 (supplemental Fig. S1), indicating that the combination of API-1 and TRAIL synergistically decreased the survival of these cancer cells. In agreement, the combination of API-1 (e.g., 5 μM) and TRAIL (e.g., 20 ng/ml) was also much more potent than either agent alone in increasing cleavage of caspase-8, caspase-9, caspase-3 and PARP in Western blot analysis (Fig. 3B) and in increasing the proportion of annexin V-positive cells as detected by the annexin V assay (Fig. 3C) in two representative cell lines, H1229 and 22A. For example, we detected approximately 5% and 12% of apoptotic cells in cells treated with TRAIL and API-1, respectively, but > 50% of apoptotic cells in H1299 cells exposed to the combination of API-1 and TRAIL (Fig. 3C), which is clearly greater than the sum of apoptosis induced by both single agents, further indicating that the combination of API-1 and TRAIL exerts more than additive (i.e., synergistic) apoptosis-inducing activity. Taken together, it is clear that the API-1 synergizes with TRAIL to induce apoptosis in NSCLC and HNSCC cells.
Fig. 3. API-1 enhances TRAIL-induced apoptosis as evaluated by cell survival (A), caspase activation (B), and annexin V staining (C).
A, The indicated cell lines were seeded in 96-well cell culture plates and treated the next day with the given concentrations of API-1 alone, TRAIL alone, or their respective combinations. After 24 h, cell numbers were estimated using the SRB assay. Data are the means of four replicate determinations. Bars, ± SDs. B and C, The indicated cell lines were treated with 20 ng/ml TRAIL alone, 5 μM API-1 alone, or their combination (A + T). After 24 h, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis (B) or for measurement of apoptosis using annexin V staining (C). CF, cleaved fragment. Columns, means of duplicate determinations; bars, ± SDs.
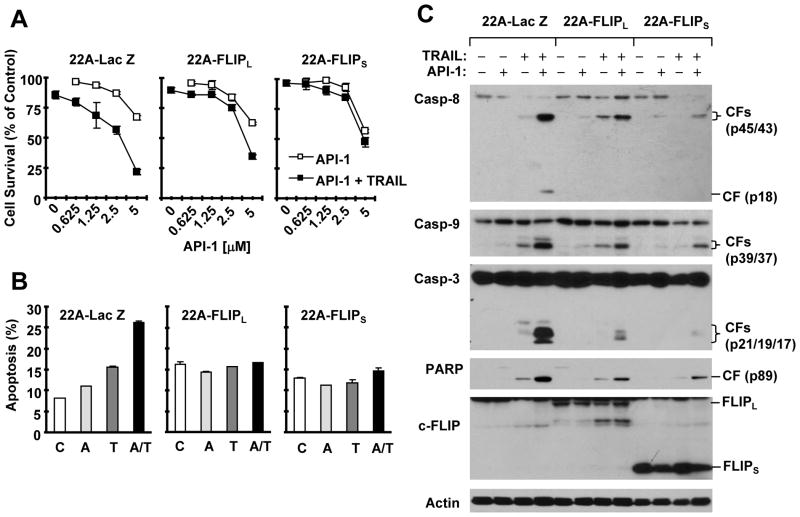
Enforced expression of ectopic c-FLIP attenuates the ability of API-1 to augment TRAIL-induced apoptosis
To demonstrate whether c-FLIP downregulation contributes to enhancement of TRAIL-induced apoptosis by API-1, we compared the effect of API-1 plus TRAIL on cell survival and apoptosis induction among 22A stable transfectants that express Lac Z (control), FLIPL and FLIPS. As demonstrated above, the combination of API-1 and TRAIL was more effective than either agent alone in decreasing the survival of 22A-Lac Z cells, but not of 22A-FLIPL or 22A-FLIPS cells (Fig. 4A). In agreement, the combination of API-1 and TRAIL was much more effective in increasing annexin V positive (apoptotic) cells and inducing the cleavage of caspase-8, caspase-9, caspase-3 and PARP in 22A-Lac Z cells than in 22A-FLIPS and 22A-FLIPL cells (Figs. 4B and 4C). Similar results were also generated from H157 cells that stably express Lac Z or FLIPL (supplemental Fig. S2). Enforced expression of FLIPL attenuated the ability of API-1 to augment TRAIL’s effects on decreasing cell survival (Fig. S2B), on increasing apoptotic populations (Fig. S2C), and on inducing cleavage of caspase-8, caspase-9, caspase-3 and PARP (Fig. S2D). These data taken together indicate that overexpression of c-FLIP protects cells from apoptosis induced by the API-1 and TRAIL combination, implying that c-FLIP downregulation contributes to enhancement of TRAIL-induced apoptosis by API-1.
Fig. 4. Enforced expression of ectopic c-FLIP attenuates API-1’s ability to decrease cell survival (A) and induce apoptosis (B) including activation of caspases (C).
A, The indicated cell lines were seeded in 96-well cell culture plates and treated the next day with DMSO, different concentrations of API-1 alone, 20 ng/ml TRAIL alone or API-1 plus TRAIL. After 24 h, cell numbers were estimated using the SRB assay. Cell survival was expressed as the percent of control (DMSO-treated) cells. Data are the means of four replicate determinations. Bars, ± SDs. B and C, The indicated cell lines were treated with DMSO, 5 μM API-1 alone, 20 ng/ml TRAIL alone or API-1 plus TRAIL. After 24 h, the cells were harvested for detection of apoptosis with annexin V staining (B) and for preparation of whole-cell protein lysates and subsequent Western blot analysis to detect the given proteins. Columns, means of duplicate experiments; bars, ± SDs. CF, cleaved fragment.
We also determined whether overexpression of c-FLIP confers resistance to API-1 alone and found that enforced expression of FLIPL or FLIPS did not affect the ability of API-1 to decrease cell survival in both H157 and 22A cells (data not shown). This suggests that c-FLIP downregulation may not be sufficient for API-1 to trigger apoptosis.
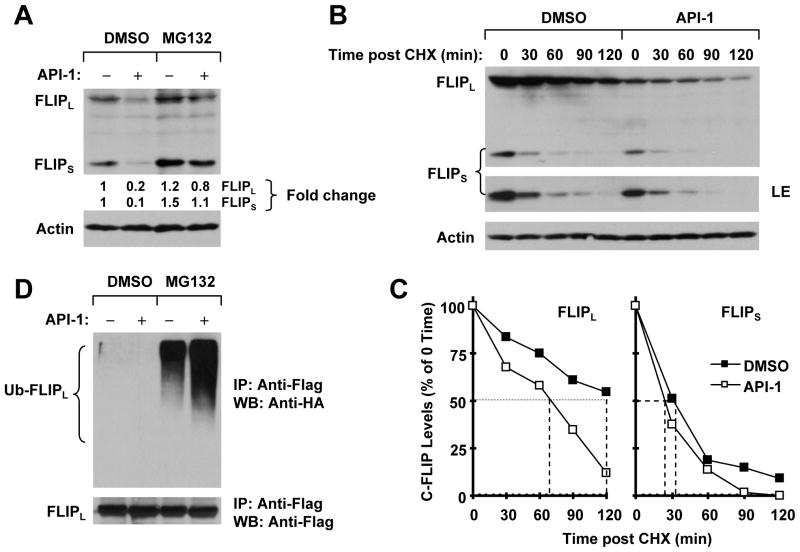
API-1 reduces c-FLIP levels through facilitating ubiquitin/proteasome-mediated degradation
To elucidate the mechanism by which API-1 reduces c-FLIP levels, we first tested whether proteasomal degradation is involved in this process, since c-FLIP is known to be regulated by an ubiquitin/proteasome-dependent mechanism. Thus, we treated H157 cells with API-1 in the absence and presence of the proteasome inhibitor MG132 and then detected c-FLIP with Western blot analysis. In the absence of MG132, API-1 decreased c-FLIP levels; however, the presence of MG132 increased basal levels of c-FLIP, particularly FLIPS, and prevented c-FLIP from reduction by API-1 (Fig. 5A). These data suggest that API-1 induces c-FLIP reduction through a proteasome-dependent mechanism. We next determined whether API-1 increases c-FLIP degradation by measuring its stability. To this end, CHX was added to H157 cells 5 h after DMSO or API-1 treatment. The cells were then harvested at the indicated times post-CHX for analysis of the c-FLIP degradation rate. The data shown in Figs. 5B and 5C revealed that the half-lives of FLIPS and FLIPL in DMSO-treated samples were about 34 and 120 min, respectively; on the contrary, in API-1 treated samples, their half-lives were reduced to approximately 24 and 69 min, respectively. Therefore, it is apparent that API-1 reduces c-FLIP protein stability. Furthermore, we determined whether API-1 increases c-FLIP ubiquitination. As presented in Fig. 5D, the highest level of ubiquitinated FLIPL was detected in H157-FLIPL-21 cells (which stably express ectopic flag-FLIPL) treated with API-1 plus MG132 compared with API-1 alone or MG132 alone, indicating that API-1 increases c-FLIP ubiquitination. Collectively, we conclude that API-1 facilitates ubiquitin/proteasome-mediated c-FLIP degradation, leading to downregulation of c-FLIP.
Figure 5. API-1 promotes ubiquitin/proteasome–mediated c-FLIP degradation (A and D) and decreases c-FLIP stability (B and C).
A, H157 cells were pretreated with 20 μM MG132 for 30 min and then co-treated with 5 μM API-1 for another 4 h. The cells were then harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis. B and C, H157 cells were treated with DMSO or 5 μM API-1 for 5 h. The cells were then washed with PBS three times and refed with fresh medium containing 10 μg/ml CHX. At the indicated times, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis (B). Protein levels were quantified with the NIH Image J software and were normalized to actin. The results were plotted as the relative c-FLIP levels compared with those at time 0 of CHX treatment (C). D, H157-FLIPL-21 cells, which stably express ectopic flag-FLIPL, were transfected with HA-ubiquitin plasmid using FuGENE 6 transfection reagent for 24 h. The cells were then pretreated with 20 μM MG132 for 30 min and then co-treated with 5 μM API-1 for 4 h. Whole-cell protein lysates were then prepared for immunoprecipitation (IP) using anti-Flag antibody followed by Western blot (WB) analysis using anti-HA antibody for detection of ubiquitinated FLIPL (Ub-FLIPL) and anti-Flag antibody for detection of ectopic FLIPL.
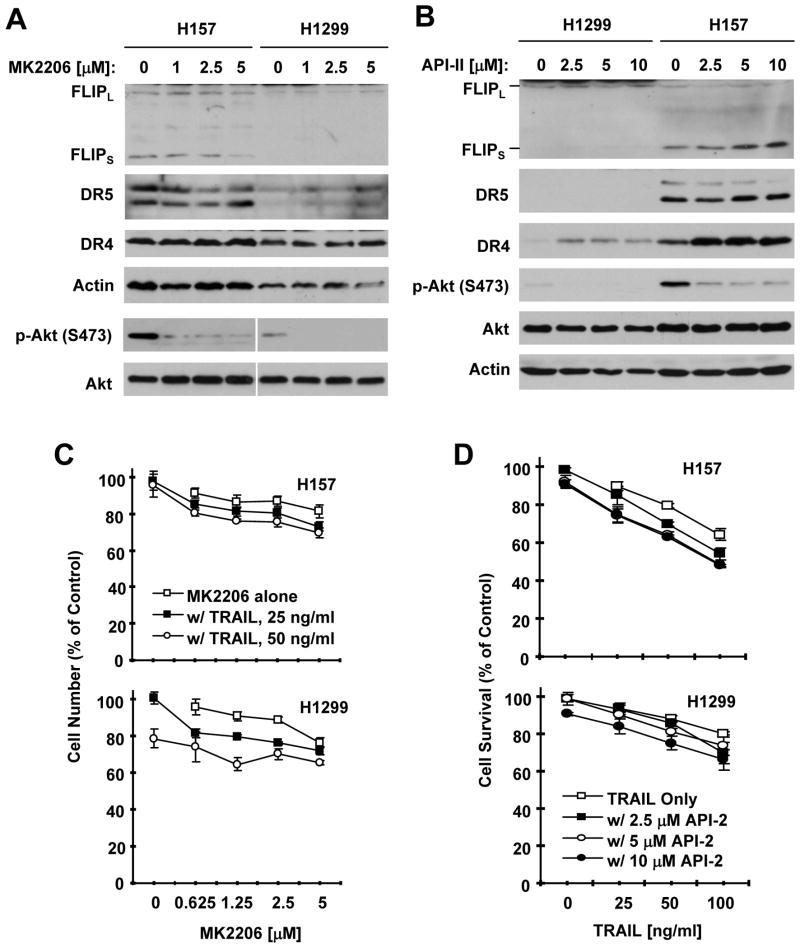
Other Akt inhibitors do not downregulate c-FLIP and enhance TRAIL-induced apoptosis
Since API-1 is an Akt inhibitor, we wanted to know whether the effects of API-1 on reducing c-FLIP levels and enhancing TRAIL-induced apoptosis are consequences of Akt inhibition. To this end, we tested whether two other allosteric Akt inhibitors, MK2206 (26) and API-2 (17), can also reduce c-FLIP levels and enhance TRAIL-mediated cell-killing. Both MK2206 and API-2 at concentrations ranging from 1 to 5 μM (MK2206) or 2.5 to 10 μM (API-2) effectively inhibited Akt phosphorylation in H1299 and H157 cells, but did not reduce c-FLIP levels in these cell lines (Figs. 6A and 6B). MK2006 did not increase the expression of DR5 or DR4 either (Fig. 6A). API-2 did not increase DR5 expression, but elevated DR4 levels (Fig. 6B). When combined with TRAIL, both MK2206 and API-2 exhibited only minimal increase in cell-killing (i.e., decreasing cell survival) in comparison with cell-killing effects by either agent alone (Figs. 6C and 6D). Thus, it is clear that other Akt inhibitors do not function in the same manner as API-1 in downregulating c-FLIP and in enhancing TRAIL-inducing cell-killing.
Figure 6. MK2206 and API-2 do not reduce c-FLIP levels (A and B) and only minimally enhance TRAIL-mediated cell-killing (C and D).
A and B, The indicated cell lines were treated with different concentrations of MK2206 or API-2 as indicated for 24 h and then subjected to preparation of whole-cell protein lysates and subsequent Western blot analysis for the given proteins. C and D, The indicated cell lines were seeded in 96-well cell culture plates and treated the next day with the given concentrations of MK2206 or API-2 alone, TRAIL alone, or TRAIL plus MK2206 or API-2. After 24 h, cell numbers were estimated using the SRB assay. Data are the means of four replicate determinations. Bars, ± SDs.
Discussion
In this study, we have demonstrated that API-1 effectively inhibits the growth of most NSCLC and HNSCC cell lines tested, with IC50s ranging from 1 μM to 5 μM. Moreover, API-1 effectively induces apoptosis in some NSCLC and HNSCC cell lines (Fig. 1). Thus, API-1 possesses promising single agent activity against NSCLC and HNSCC cells. When combined with TRAIL, synergistic induction of apoptosis, including decreased cell survival, induction of caspase cleavage and increased annexin V positive cells, occurred in most of the tested cell lines (Fig. 3). To the best of knowledge, this is the first report of the synergistic induction of apoptosis by the combination of API-1 and TRAIL in cancer cells. Given that TRAIL is being tested as a cancer therapeutic agent in clinical trials (6, 27), the further study of the potential application of the API-1 and TRAIL combination in cancer therapy (e.g., NSCLC and HNSCC) is warranted. Recently, targeting the Akt protein kinase or the TRAIL-mediated apoptotic pathway has been emerged as attractive strategies for cancer chemoprevention (13, 28–30). Indeed, a phase 0 chemoprevention trial on an orally active Akt inhibitor has been successfully conducted recently (31). Thus, the potential of the API-1 alone or in combination with TRAIL in cancer chemoprevention needs investigation as well.
We noted that, among the tested cancer cell lines, Calu-1 was the only cell line that exhibited resistance to API-1 alone or the combination of API-1 and TRAIL (Figs. 1 and 3). Thus understanding of the mechanisms by which API-1 induces apoptosis, including modulation of TRAIL-induced apoptosis, will be very helpful for guiding effective application of API-1 in future treatment of cancer in the clinic.
It is well known that cells can die of apoptosis primarily through the extrinsic death receptor-induced pathway and/or the intrinsic mitochondria-mediated pathway. Cross-talk between these two pathways is mediated by the truncated proapoptotic protein Bid (32). The activation of caspase-8 is the key step in the death receptor-mediated apoptosis, whereas caspase-9 activation is the key even in the mitochondria-mediated apoptotic pathway. Activated caspase-8 can also induce caspase-9 activation through Bid-mediated activation of the mitochondria-mediated apoptotic pathway (32). In this study, we found that API-1 activated both caspase-8 and caspase-9 (Fig. 1C), suggesting that API-1 either activates the death receptor-mediated apoptotic pathway or both the death receptor- and mitochondria-mediated apoptotic pathways, leading to induction of apoptosis.
DR4, DR5 and c-FLIP are key components in the regulation of TRAIL-induced apoptosis (6, 33). Modulation of the levels of these proteins in general (e.g., upregulation of DR4 and/or DR5 and/or downregulation of c-FLIP) results in sensitization of cancer cells to TRAIL-induced apoptosis (13, 14). We found that API-1 reduced c-FLIP levels without increasing DR4 or DR5 expression in the sensitive cancer cell lines (Fig. 2). Interestingly, Calu-1 cells, which are relatively resistant to API-1 or API-1 plus TRAIL, expressed the highest basal levels of c-FLIP, which was not reduced by API-1 (Fig. 2A). These results suggest that c-FLIP downregulation may play a critical role in mediating apoptosis induced by API-1 or by the combination of API-1 and TRAIL. Enforced expression of ectopic FLIPL or FLIPS did not confer resistance to API-1 alone, but indeed attenuated or abolished the effect of API-1 on enhancing TRAIL-induced apoptosis in both 22A and H157 cells (Figs. 4 and S2). Therefore, c-FLIP downregulation may not be sufficient for API-1 to initiate apoptosis, suggesting that other mechanisms are needed for API-1-induced apoptosis. However, it is clear that c-FLIP downregulation apparently plays a critical role in mediating synergistic induction of apoptosis by API-1and TRAIL.
It is known that enhancement of TRAIL induced apoptosis can be achieved through other mechanisms (e.g., inhibition of Bcl-2 family members) beyond downregulation of c-FLIP (34). Here we claim a critical role of c-FLIP downregulation in mediating enhancement of TRAIL-induced apoptosis by API-1, but does not exclude other potential mechanisms. We noted that c-FLIP protein was not detected in 22A cells (Fig. 2) and API-1 clearly enhanced TRAIL-induced apoptosis in this cell line (Fig. 3). It is possible that the downregulation of c-FLIP by API-1 in this cell line was not detected due to the sensitivity limitation of the assay. Of course, whether other mechanisms play a more important role than downregulation of c-FLIP in mediating enhancement of TRAIL-induced apoptosis by API-1 in this cell line cannot be ruled out and needs further investigation.
It is known that c-FLIP, including FLIPL and FLIPS, are rapidly turnover proteins subjected to regulation through ubiquitin/proteasome-mediated protein degradation (10–12). Some small molecules negatively regulate c-FLIP levels through this mechanism (19, 35, 36). In this study, we found that API-1 failed to decrease c-FLIP levels in the presence of a proteasome inhibitor, increased c-FLIP ubiquitination and reduced the stability of c-FLIP protein (Fig. 5). Thus, we conclude that API-1 reduces c-FLIP levels by facilitating its degradation through the ubiquitin/proteasome-dependent pathway. In the current study, we cannot rule out additional mechanisms accounting for c-FLIP downregulation induced by API-1 such as transcriptional regulation even though they are unlikely to be the primary mechanisms.
It has been suggested that Akt positively regulates c-FLIP expression (15). Recently, Akt1 was shown to directly interact with FLIPL and to phosphorylate it at S273, leading to stabilization of FLIPL (16). Given that API-1 is an Akt inhibitor, it is reasonable to speculate that API-1 may downregulate c-FLIP due to its Akt-inhibitory activity. To explore this, we tested the effects of two additional Akt inhibitors, MK2206 and API-2, on modulation of c-FLIP levels and TRAIL-induced apoptosis. Unfortunately, both MK2206 and API-2 failed to reduce c-FLIP levels or to detectably enhance TRAIL-induced cell killing although they effectively reduced p-Akt levels (Fig. 6), suggesting that inhibition of Akt does not necessarily result in c-FLIP downregulation and enhancement of TRAIL-induced apoptosis. Accordingly we suggest that the effects of API-1 on downregulation of c-FLIP and enhancement of TRAIL-induced apoptosis are unlikely secondary to Akt inhibition. Furthermore, we noted that API-1 downregulation of c-FLIP is not associated with its activity against Akt. In Calu-1 cells, API-1 did not reduce c-FLIP levels, but inhibited Akt phosphorylation (Fig. 2A). This piece of data further supports the notion that API-1 downregulates c-FLIP independent of Akt inhibition.
In summary, the current study has revealed a novel function of API-1 that induces c-FLIP degradation and synergizes with TRAIL to induce apoptosis of cancer cells. Moreover, our results warrant further evaluation of the potential of API-1 and TRAIL combination against cancer in the clinic.
Supplementary Material
Acknowledgments
Grant Support: Georgia Cancer Coalition Distinguished Cancer Scholar award (to S-Y. S.) and National Cancer Institute NIH SPORE P50 grant CA128613 (project 2 to S-Y. S and F. R. K).
We are grateful to Dr. J. Q. Cheng for generously providing some reagents and Dr. A. Hammond for editing the manuscript.
References
- 1.Kim D, Sun M, He L, Zhou QH, Chen J, Sun XM, et al. A small molecule inhibits Akt through direct binding to Akt and preventing Akt membrane translocation. J Biol Chem. 2010;285:8383–94. doi: 10.1074/jbc.M109.094060. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Kelley SK, Ashkenazi A. Targeting death receptors in cancer with Apo2L/TRAIL. Curr Opin Pharmacol. 2004;4:333–9. doi: 10.1016/j.coph.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Wajant H, Gerspach J, Pfizenmaier K. Tumor therapeutics by design: targeting and activation of death receptors. Cytokine Growth Factor Rev. 2005;16:55–76. doi: 10.1016/j.cytogfr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Newsom-Davis T, Prieske S, Walczak H. Is TRAIL the holy grail of cancer therapy? Apoptosis. 2009;14:607–23. doi: 10.1007/s10495-009-0321-2. [DOI] [PubMed] [Google Scholar]
- 5.Van Geelen CM, de Vries EG, de Jong S. Lessons from TRAIL-resistance mechanisms in colorectal cancer cells: paving the road to patient-tailored therapy. Drug Resist Updat. 2004;7:345–58. doi: 10.1016/j.drup.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J Clin Oncol. 2008;26:3621–30. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- 7.Wajant H. Targeting the FLICE Inhibitory Protein (FLIP) in cancer therapy. Mol Interv. 2003;3:124–7. doi: 10.1124/mi.3.3.124. [DOI] [PubMed] [Google Scholar]
- 8.Bagnoli M, Canevari S, Mezzanzanica D. Cellular FLICE-inhibitory protein (c-FLIP) signalling: a key regulator of receptor-mediated apoptosis in physiologic context and in cancer. Int J Biochem Cell Biol. 2010;42:210–3. doi: 10.1016/j.biocel.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Kataoka T. The caspase-8 modulator c-FLIP. Crit Rev Immunol. 2005;25:31–58. doi: 10.1615/critrevimmunol.v25.i1.30. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y, Suh N, Sporn M, Reed JC. An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. J Biol Chem. 2002;277:22320–9. doi: 10.1074/jbc.M202458200. [DOI] [PubMed] [Google Scholar]
- 11.Poukkula M, Kaunisto A, Hietakangas V, Denessiouk K, Katajamaki T, Johnson MS, et al. Rapid turnover of c-FLIPshort is determined by its unique C-terminal tail. J Biol Chem. 2005;280:27345–55. doi: 10.1074/jbc.M504019200. [DOI] [PubMed] [Google Scholar]
- 12.Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, et al. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–13. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Sun SY. Chemopreventive agent-induced modulation of death receptors. Apoptosis. 2005;10:1203–10. doi: 10.1007/s10495-005-2274-4. [DOI] [PubMed] [Google Scholar]
- 14.Elrod HA, Sun SY. Modulation of death receptors by cancer therapeutic agents. Cancer Biol Ther. 2007;7:163–73. doi: 10.4161/cbt.7.2.5335. [DOI] [PubMed] [Google Scholar]
- 15.Panka DJ, Mano T, Suhara T, Walsh K, Mier JW. Phosphatidylinositol 3-kinase/Akt activity regulates c-FLIP expression in tumor cells. J Biol Chem. 2001;276:6893–6. doi: 10.1074/jbc.C000569200. [DOI] [PubMed] [Google Scholar]
- 16.Shi B, Tran T, Sobkoviak R, Pope RM. Activation-induced degradation of FLIP(L) is mediated via the phosphatidylinositol 3-kinase/Akt signaling pathway in macrophages. J Biol Chem. 2009;284:14513–23. doi: 10.1074/jbc.M807918200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–9. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- 18.Sun SY, Yue P, Mao L, Dawson MI, Shroot B, Lamph WW, et al. Identification of receptor-selective retinoids that are potent inhibitors of the growth of human head and neck squamous cell carcinoma cells. Clin Cancer Res. 2000;6:1563–73. [PubMed] [Google Scholar]
- 19.Chen S, Liu X, Yue P, Schonthal AH, Khuri FR, Sun SY. CHOP-dependent DR5 induction and ubiquitin/proteasome-mediated c-FLIP downregulation contribute to enhancement of TRAIL-induced apoptosis by dimethyl-celecoxib in human non-small cell lung cancer cells. Mol Pharmacol. 2007;72:1269–79. doi: 10.1124/mol.107.037465. [DOI] [PubMed] [Google Scholar]
- 20.Raja SM, Chen S, Yue P, Acker TM, Lefkove B, Arbiser JL, et al. The natural product honokiol preferentially inhibits cellular FLICE-inhibitory protein and augments death receptor-induced apoptosis. Mol Cancer Ther. 2008;7:2212–23. doi: 10.1158/1535-7163.MCT-07-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao L, Yue P, Lonial S, Khuri FR, Sun SY. The NEDD8-activating enzyme inhibitor, MLN4924, cooperates with TRAIL to augment apoptosis through facilitating c-FLIP degradation in head and neck cancer cells. Mol Cancer Ther. 2011 doi: 10.1158/1535-7163.MCT-11-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun SY, Yue P, Dawson MI, Shroot B, Michel S, Lamph WW, et al. Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells. Cancer Res. 1997;57:4931–9. [PubMed] [Google Scholar]
- 23.Sun SY, Yue P, Wu GS, El-Deiry WS, Shroot B, Hong WK, et al. Mechanisms of apoptosis induced by the synthetic retinoid CD437 in human non-small cell lung carcinoma cells. Oncogene. 1999;18:2357–65. doi: 10.1038/sj.onc.1202543. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Yue P, Zhou Z, Khuri FR, Sun SY. Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Natl Cancer Inst. 2004;96:1769–80. doi: 10.1093/jnci/djh322. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Yue P, Schonthal AH, Khuri FR, Sun SY. Cellular FLICE-inhibitory protein down-regulation contributes to celecoxib-induced apoptosis in human lung cancer cells. Cancer Res. 2006;66:11115–9. doi: 10.1158/0008-5472.CAN-06-2471. [DOI] [PubMed] [Google Scholar]
- 26.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–67. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 27.Bellail AC, Qi L, Mulligan P, Chhabra V, Hao C. TRAIL agonists on clinical trials for cancer therapy. the promises and the challenges. Rev Recent Clin Trials. 2009;4:34–41. doi: 10.2174/157488709787047530. [DOI] [PubMed] [Google Scholar]
- 28.Crowell JA, Steele VE, Fay JR. Targeting the AKT protein kinase for cancer chemoprevention. Mol Cancer Ther. 2007;6:2139–48. doi: 10.1158/1535-7163.MCT-07-0120. [DOI] [PubMed] [Google Scholar]
- 29.Huang S, Ren X, Wang L, Zhang L, Wu X. Lung-Cancer Chemoprevention by Induction of Synthetic Lethality in Mutant KRAS Premalignant Cells In Vitro and In Vivo. Cancer Prev Res (Phila) 2011;4:666–73. doi: 10.1158/1940-6207.CAPR-10-0235. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Ren X, Alt E, Bai X, Huang S, Xu Z, et al. Chemoprevention of colorectal cancer by targeting APC-deficient cells for apoptosis. Nature. 2010;464:1058–61. doi: 10.1038/nature08871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid JM, Walden CA, Qin R, Ziegler KL, Haslam JL, Rajewski RA, et al. Phase 0 clinical chemoprevention trial of the Akt inhibitor SR13668. Cancer Prev Res (Phila) 2011;4:347–53. doi: 10.1158/1940-6207.CAPR-10-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 33.Falschlehner C, Ganten TM, Koschny R, Schaefer U, Walczak H. TRAIL and other TRAIL receptor agonists as novel cancer therapeutics. Adv Exp Med Biol. 2009;647:195–206. doi: 10.1007/978-0-387-89520-8_14. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–37. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 35.Fan S, Li Y, Yue P, Khuri FR, Sun SY. The eIF4E/eIF4G interaction inhibitor 4EGI-1 augments TRAIL-mediated apoptosis through c-FLIP Down-regulation and DR5 induction independent of inhibition of cap-dependent protein translation. Neoplasia. 2010;12:346–56. doi: 10.1593/neo.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Y, Liu X, Yue P, Benbrook DM, Berlin KD, Khuri FR, et al. Involvement of c-FLIP and survivin down-regulation in flexible heteroarotinoid-induced apoptosis and enhancement of TRAIL-initiated apoptosis in lung cancer cells. Mol Cancer Ther. 2008;7:3556–65. doi: 10.1158/1535-7163.MCT-08-0648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.