Abstract
Failure to efficiently clear apoptotic cells is linked to defects in development and the onset of autoimmunity. Complement component C1q is required for efficient engulfment of apoptotic cells in mice and humans, however, the molecular mechanisms leading to C1q-dependent engulfment are not fully understood. Here we used primary mouse macrophages to identify and characterize a novel molecular mechanism for macrophage-mediated C1q-dependent engulfment of apoptotic cells. We found that macrophage activation with C1q resulted in cycloheximide-sensitive enhanced engulfment, indicating a requirement for de novo protein synthesis. To investigate the cycloheximide-sensitive pathway, C1q-elicited macrophage transcripts were identified by microarray. C1q triggered the expression of Mer tyrosine kinase and the Mer ligand, Gas6: a receptor-ligand pair that mediates clearance of apoptotic cells. Full-length native C1q, and not the collagen-like tail or heat-denatured protein, stimulated Mer expression. This novel pathway is specific to C1q since MBL, a related collectin, failed to up regulate Mer expression and function. Soluble Mer-Fc fusion protein inhibited C1q-dependent engulfment of apoptotic cells indicating a requirement for Mer. Moreover, Mer deficient macrophages failed to respond to C1q with enhanced engulfment. Our results suggest that C1q elicits a macrophage phenotype specifically tailored for apoptotic cell clearance, and these data are consistent with the established requirement for C1q in prevention of autoimmunity.
Introduction
C1q is the recognition component of the classical complement pathway, and C1q is also required for efficient clearance of apoptotic cells (1). C1q deficiency is the strongest known susceptibility factor for lupus: C1q deficient humans develop lupus with >90% penetrance (2). A leading theory for the development of lupus is that failure to efficiently clear apoptotic cells provides a reservoir of self-antigen resulting in autoimmunity and immune complex deposition (3). “Bridging molecules” normally mediate clearance of apoptotic cells by binding to both the apoptotic cell and the phagocyte (4). For example, Growth arrest-specific 6 (Gas6) is a well described bridging molecule required for the efficient clearance of apoptotic cells. Gas6 binds to phosphatidylserine, exposed on the apoptotic cell surface, and also binds to members of the TAM (Tyro3, Axl, Mer) family of tyrosine kinases (TK) expressed by phagocytes (5). C1q is often depicted as a bridging molecule, however, the mechanisms leading to C1q-dependent engulfment of apoptotic cells and prevention of autoimmunity are not fully understood.
The ability of C1q to enhance phagocytosis was first described for engulfment of antibody and complement coated particles (6, 7). Subsequently, it became evident that the larger family of C1q-related proteins called “defense collagens” or “collectins”, including mannose binding lectin (MBL) and surfactant protein-A (SP-A), shared the ability to enhance phagocytosis of multiple targets (8, 9). Korb et al. made the important discovery that C1q bound specifically to apoptotic cells (10). This discovery led to numerous studies that demonstrated that C1q and the collectins enhanced engulfment of apoptotic cells [reviewed in (11)].
It is generally accepted that C1q enhances engulfment by bridging apoptotic cells to phagocytes, and therefore, C1q deficiency leads to a failure in apoptotic cell clearance causing subsequent accumulation of apoptotic cell bodies. Importantly, MBL and other C1q-related collectins also enhance engulfment, but MBL deficiency does not lead to autoimmunity (12). In addition, the C1q/collectin-dependent phagocytosis pathway described to date is not specific for apoptotic cells since C1q/collectins enhance phagocytosis of multiple target particles including antibody and complement opsonized particles as well as apoptotic cells [reviewed in (13)]. These data suggest the existence of an alternative C1q-dependent engulfment mechanism with specificity for C1q and apoptotic cells. Since C1q alters monocyte/macrophage gene expression (14, 15), we hypothesized that C1q alters gene expression required for clearance of apoptotic cells. In support of our hypothesis, herein we identify and characterize a novel C1q-dependent apoptotic cell engulfment mechanism that requires Mer, a TAM family member which regulates engulfment of apoptotic cells and autoimmunity (16, 17).
Materials and Methods
Reagents
All reagents were purchased from Fisher (Pittsburgh, PA) unless otherwise indicated. Dulbecco’s modified Eagle’s medium (DMEM) and RPMI 1640 were purchased from Gibco/Molecular Probes/Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Hyclone Laboratories (Logan, UT) and heat inactivated for 30 minutes at 56°C. C1q was isolated from plasma-derived normal human serum by ion-exchange chromatography, followed by size-exclusion chromatography according to the method of Tenner et al. (18) and modified as described (19). Commercially available purified C1q was also purchased from Complement Technology Inc. (Tyler, Texas). Purified C1q collagen-like tails were kindly provided by Dr. Andrea Tenner (University of California Irvine). Mannose binding lectin (MBL), goat anti-mouse Mer antibody, Mer-Fc and goat anti-mouse Gas 6 were purchased from R&D Systems (Minneapolis, MN). Rabbit anti-mouse Mer antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Whole goat IgG was purchased from Jackson Immuno Research Laboratory, Inc. (West Grove, PA). PE conjugated Rat anti-mouse F4/80 was purchased from eBiosciences (San Diego, CA). Protein G beads were purchased from GE Healthcare Life Sciences (Piscataway, NJ).
Mice
MerKD kinase domain deletion mutant mice were developed as described (20), and obtained from Jackson Laboratories (Bar Harbor, ME) on a C57Bl/6 background and referred to as mertk−/−. C3-deficient mice on a mixed background were kindly provided by Dr. Mary Ann McDowell (University of Notre Dame) and were described previously (21). CD93 knockout mice were kindly provided by Dr. Marina Botto & Dr. Mark Walport (Imperial College, London, U.K.) and were back crossed onto C57Bl/6 for 11 generations. Macrophage specific LRP deficient mice were kindly provided by Dr. Dudley Strickland (University of Maryland School of Medicine) and were described previously (22). All techniques were approved by the University of Notre Dame Institutional Animal Care and Use Committee.
Cell culture
Bone marrow derived macrophages (BMDM) were generated as previously described (23). Briefly, femurs and tibias were isolated from mice and bone marrow was flushed from bones with DMEM supplemented with 2% FBS and 100 units/ml penicillin G sodium/100 µg/ml streptomycin sulfate (Pen/Strep). Marrow was washed with PBS 10 mM EDTA, red cells were lysed and cells were cultured in DMEM supplemented with 10% FBS, 10 mM HEPES, and Pen/Strep at 5% CO2 for 2–4 hours in a pre-adhesion step to remove resident fibroblasts and macrophages. Non adherent cells were cultured in BMDM media (DMEM, 10% FBS, 15% L929 conditioned media, Pen/Strep, and 10 mM HEPES pH 7.4) at 5% CO2. Following maturation for 4 days, 6 ml fresh media was added and cells were considered fully mature at 7 days. Media was replenished every two to three days to ensure cell viability. To isolate peritoneal macrophages, peritoneal exudate was harvested from mice 72 hours post injection of 1 ml 4% sterile Brewer’s thioglycollate (Sigma, St. Louis, MO). The cell suspension was subject to hypotonic solution to lyse red blood cells and then washed in phagocytosis buffer (RPMI 1640, Pen/Strep, and 0.5% MgCl2) before experimentation. The percentage of F4/80 macrophages in exudate preparation was greater than 80% as assessed by flow cytometric analysis. The human Jurkat T cell line was obtained from ATTC (Manassas, VA) and maintained in DMEM supplemented with 10% FBS and Pen/Strep. Murine thymocytes were harvested from C57BL/6 mice at 3–4 weeks of age. To generate a single cell suspension of thymocytes, thymi were placed in a sterile cell strainer (100 µM nylon mesh) and disaggregated with a 1 ml syringe plunger. Thymocytes were washed in PBS and red blood cells were lysed. Thymocytes were cultured in RPMI media supplemented with 10 mM sodium hypoxanthine and 1.6 mM thymidine (Invitrogen).
Phagocytosis assays
Sheep erythrocytes suboptimally opsonized with IgG (EAIgG) were prepared as previously described (24) and the EAIgG phagocytosis assays were performed described (22). Percent phagocytosis was defined as the number of cells ingesting at least one target divided by the total number of cells counted multiplied by 100. Phagocytic index was defined as the number of ingested targets per 100 cells counted. Slides were scored by individuals blinded to treatment. Flow cytometric apoptotic cell phagocytosis assays were performed as described (22), and percent phagocytosis was defined as (CD11b- and CFSE-positive cells) divided by the total CD11b-positive cells multiplied by 100. For microscopy based apoptotic cell phagocytosis assays, murine thymocytes were treated with 3 µM dexamethasone (Sigma) for 5 hours at 37°C in complete RPMI media supplemented with 10 mM sodium hypoxanthine and 1.6 mM thymidine (Invitrogen), washed with complete media, and labeled with 5 µM CFSE (Invitrogen) for 30 minutes at 37°C followed by an additional wash in complete media. Untreated control cells were incubated for the same period without dexamethasone. Treatment with dexamethasone for this time period routinely yielded greater than 60% apoptotic cells as measured by Annexin V/Propidium Iodide labeling (Bio Vision Inc., Mountain View CA). Eight well Lab Tek Chamber Slides (Nalge Nunc International, Rochester, NY) were coated with 250 µl C1q or human serum albumin (HSA) (Baxter, Deerfield, IL) at a concentration of 4 µg/ml and washed with PBS prior to addition of cells. Phagocytes were resuspended at 1.0 × 105 cells/ml in phagocytosis buffer, and 250 µl of the cell suspension was added to the wells. Cells were allowed to adhere for 5 hours at 37°C, and 5% CO2 prior to the addition of targets. In some conditions MerFc (40 µg/ml) was added to the macrophages for 1 hour prior to the addition of targets. Apoptotic or live thymocytes were added at a 1:10 ratio of macrophage to target and slides were centrifuged for 3 minutes at 700 rpm (98 × g) and then incubated for 1 hour at 37°C, 5% CO2. At the indicated time point, slides were washed extensively with PBS, fixed with 3.7% paraformaldehyde and stained with PE labeled anti-mouse CD11b (eBioscience, San Diego CA). A minimum of 200 cells per experimental condition were quantified. Percent phagocytosis was calculated as described above.
Western Blot
BMDM were seeded at 1.0 × 106 cells/ml in one well Lab Tek Chamber Slides that had been pre-coated with 2ml of 4 µg/ml HSA, C1q, heat inactivated C1q or MBL. At the indicated time point, BMDM lysates were generated with RIPA buffer supplemented with a cocktail of protease inhibitors and phosphatase inhibitors. Total protein concentration was determined by BCA according to manufacturer’s instructions (Thermo Scientific, Rockford, IL) and 10–20 µg of protein was loaded on 7.5% gels under reducing conditions. Proteins were transferred to PVDF membrane and blocked for 2 hours or overnight. Membranes were probed with rabbit anti-mouse Mer antibody for 2 hours, washed and incubated with anti-rabbit HRP conjugated antibody (Jackson ImmunoResearch, West Grove, PA). For immunoprecipitation experiments, lysates were pre-cleared with whole goat IgG and protein G beads then incubated with fresh protein G beads and goat anti-mouse Mer antibody. Gas 6 was precipitated with Mer-Fc (200nM) from concentrated cell supernatants of macrophages stimulated with C1q using protein G beads. Gas 6 was detected with goat anti-mouse Gas 6 antibody.
Microarray analysis
Microarray and Bioinformatics analyses were performed in collaboration with the University of Notre Dame Genomics and Bioinformatics Core Facility. Briefly, total RNA was extracted using the TRIzol (Sigma, St. Louis, MO) method from bone marrow derived macrophages adhered to HSA or C1q for 4 or 18 hours. The resulting RNA was precipitated using sodium acetate and washed with ethanol to remove contaminants. Randomly selected RNA samples were analyzed with an Agilent 2100 Bioanalyzer to verify sample integrity prior to cDNA synthesis. Double stranded cDNA was generated using the Trans Plex Complete Whole Transcriptome Amplification Kit (Sigma, St. Louis, MO) according to the manufacturer’s instructions and the resulting product was precipitated with sodium acetate, washed with ethanol to remove residual primers and nucleotides, and stored at −20°C until labeled. Randomly selected cDNA were analyzed with an Agilent 2100 Bioanaly to verify sample integrity prior to labeling. cDNA was labeled with Cy3 using NimbleGen One-color DNA labeling kits according to manufacturer’s instructions (Roche NimbleGen, Inc., Madison, WI), lyophilized, and stored at −20°C overnight. Labeled product was resuspended in hybridization buffer containing alignment oligos and unique sample tracking controls, loaded onto a custom murine gene expression microarray designed by NimbleGen, and hybridized overnight. After extensive washing, the array microchips were dried and then scanned using a NimbleGen MS 200 Microarray Scanner. Array data was extracted and analyzed using NimbleScan software v2.5. The mean fluorescence intensity was derived from a log2 transformation of the data and normalized using quantile normalization method. Heat maps were generated using Genesis Software. The data was compared for statistical significance using Student’s t test and q value for control of false discovery rate (FDR).
The data discussed in this publication have been deposited in the National Center for Biotechnology Information’s (NCBI) Gene Expression Omnibus (GEO) and are accessible through GEO Series accession number GSE35280 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE35280).
Quantitative Real Time Polymerase Chain Reaction
Total RNA was isolated as described above. qRT-PCR reactions were assembled with SABiosciences (Frederick, MD) SYBR Green qPCR Master Mix according to the manufacturer’s instructions and amplified using Applied Biosystems (Carlsbad, CA) Real Time PCR system. Gene expression was quantified from standard curves prepared from a series of dilutions of control template cDNA with either GAPDH primers or gene specific primers. All reactions were carried out in triplicate. Negative controls included reactions in which template cDNA or enzyme were omitted. The amplification specificity of the reactions was confirmed by melting-curve analysis. Results were normalized to GAPDH and reported as relative gene expression. The primers used were: Protein S (forward: 5’-GCT TTC CAC TTG AGC CAA CAC CTT-3’; reverse: 5’-TGT GCT CTC AGC AGC TTA GGT TGA-3’), C3 (forward: 5’-GGG CTG TTA ATT GGT TGA TTC TG C3-3’; reverse: 5’-GAT GAG GAC GAA GGC TGT G-3’), C1qb (forward: 5’-AGA AGC ATC ACA GAA CAC CAG-3’; reverse: 5’-ACA TGG AGA AAA CCT AGA AGC AG-3’), GAPDH (forward: 5’-CCA ATG TGT CCG TCG TGG ATC-3’; reverse: 5’-GTT GAA GTC GCA GGA GAC ACC-3'), MFG-E8 (forward: 5’-TAG CCC ACA GTG ATG ATG GT-3’; reverse: 5’-TCT TGT GGG AGT TGT TGT CC-3’), GAS-6 (forward: 5’-TCA AGA AGC AGT TGG TGG TC-3’; reverse: 5’-GTG CCA TCC ACT TCT AGG GT-3’), Mer (forward: 5’-CAC AAT GAC AAA GGA CTG ACG-3’; reverse: 5”-AGT AGC CAT CAA AAC CAG GG-3’).
Detection of Gas6
Gas6 protein levels in culture supernatants and lysates were assayed by ELISA according to manufacturer’s instructions (AdipoBiotech, Beijing, China). BMDM were seeded at 1 × 106 cells/ml in 1 well Lab Tek Chamber slides that had been pre-coated with 4 µg/ml HSA, C1q or MBL. At the indicated time point, BMDM lysates were generated with RIPA buffer supplemented with a cocktail of protease and phosphatase inhibitors. Total protein concentration was determined by BCA according to manufacturer’s instructions (Thermo Scientific, Rockford, IL) and equal protein concentrations were assayed within groups and between experiments.
Statistics
Statistics were performed using Prism; GraphPad (Version 5.02). Statistical comparisons between groups were performed as indicated in the figure legends.
Results
C1q-dependent engulfment of apoptotic cells requires de novo protein synthesis
Fraser et al. reported that C1q regulated the expression of proinflammatory cytokines from human monocytes, macrophages and dendritic cells (14,15). Based on these data, we hypothesized that C1q-dependent gene expression may also regulate clearance of apoptotic cells. To investigate this possibility, we compared the effects of short term and prolonged stimulation with C1q on the ability of mouse bone marrow derived macrophages to phagocytose antibody coated targets and apoptotic cells. In agreement with previously published observations, macrophages stimulated with C1q for 30 minutes showed enhanced phagocytosis of erythrocytes suboptimally opsonized with IgG (EAIgG) (6). C1q enhanced both the percent of macrophages engulfing EAIgG (Fig. 1A), as well as the number of EAIgG ingested per macrophage (data not shown). C1q adhered macrophages showed a 2.6 fold increase in phagocytosis compared to control macrophages (Fig. 1A, 44 ± 3.6 vs 17 ± 2.3, respectively). While C1q is known to enhance engulfment of numerous target particles, including antibody and complement coated particles, pathogens and apoptotic cells [reviewed in (13)], under these experimental conditions, C1q failed to enhance engulfment of non-opsonized apoptotic cells (Fig. 1B). These experiments were conducted in the absence of serum to eliminate the effect of factors (i.e. bridging molecules) which have been previously shown to influence engulfment of apoptotic cells.
Figure 1. C1q enhances engulfment of apoptotic cells via a cycloheximide-sensitive mechanism.
(A and B) Macrophages were cultured in chamber slides pre-coated with 4 µg/ml C1q (black bars) or the control protein human serum albumin (HSA) (white bars) for 30 minutes and then fed sheep erythrocytes suboptimally opsonized with IgG (EAIgG) for an additional 30 minutes (A, n=4) or apoptotic Jurkat cell sat a 1:3 macrophage to apoptotic cell ratio for 1 hour (B, n=3). Bars represent the average ± SEM. ***p< 0.001, Student’s t test.(C and D) Phagocytosis assays were performed as in A and B except macrophages were adhered to protein coated wells for five hours instead of 30 minutes prior to feeding EAIgG (C) or apoptotic cells (D). Bars represent the average ± SEM from three to six experiments. *p < 0.05; ***p< 0.001, Student’s t test. (E and F) BMDM were cultured as in C and D in the presence and absence of 100 µM cycloheximide. Bars represent the average ± SEM from three independent experiments. **p< 0.01; ***p< 0.001, two-way ANOVA, Bonferroni multiple comparison tests.
Next we investigated whether prolonged macrophage stimulation with C1q influenced macrophage phagocytosis. Macrophages were stimulated with C1q or control protein for five hours before the addition of targets. As expected, similar to short term stimulation with C1q, prolonged stimulation with C1q resulted in enhanced engulfment of EAIgG; there was a 1.6-fold C1q-dependent enhancement of phagocytosis of EAIgG over control (Fig. 1C, 58 ± 1.2 vs 37 ± 1.9, respectively). Interestingly, and in contrast to short term stimulation with C1q, macrophages stimulated with C1q for five hours ingested twice as many non-opsonized apoptotic cells compared to control macrophages (Fig. 1D, 22 ± 3.1 vs 12 ± 2.4, respectively). Together, these data demonstrate that prolonged stimulation with C1q enhances the ability of macrophages to engulf apoptotic cells.
Since prolonged macrophage activation with C1q resulted in enhanced engulfment of apoptotic cells (Fig. 1D), we hypothesized that de novo macrophage protein synthesis was required for the C1q-dependent effect. To assess the requirement for protein synthesis, macrophages were treated with cycloheximide, and C1q-dependent engulfment of apoptotic cells was measured. Cycloheximide treatment completely abolished the C1q-mediated engulfment of apoptotic cells (Fig. 1F). Importantly, cycloheximide treatment did not alter C1q-dependent engulfment of EAIgG. Macrophages stimulated with C1q for five hours ingested three fold more EAIgG compared to control macrophages in the presence and absence of cycloheximide (Fig. 1E). Together, these data demonstrate the existence of a novel C1q-dependent phagocytosis pathway for apoptotic cells that requires de novo protein synthesis. The data also demonstrate that this novel pathway is distinct from the previously described function of C1q in triggering a direct enhancement of phagocytic function [reviewed in (11)], and suggest a role for C1q-dependent gene transcription in engulfment of apoptotic cells.
C1q stimulates expression of proteins required for engulfment of apoptotic cells
Since C1q-dependent enhanced engulfment of apoptotic cells required protein synthesis, we performed a microarray of C1q elicited macrophage transcripts to identify genes involved in apoptotic cell engulfment. Cluster analysis of macrophage gene transcripts following 4 (Fig. 2A) or 18 (Fig. 2B) hour adhesion to C1q demonstrated that C1q upregulated a number of genes encoding well characterized proteins required for engulfment of apoptotic cells. The proteins included C1q, C3, milk fat globule- epidermal growth factor (MFG-E8), Mer tyrosine kinase (Mer), as well as the Mer ligands, Growth arrest-specific 6 (Gas6) and protein S (Fig. 2A, 2B).
Figure 2. C1q elicits macrophage expression of apoptotic cell engulfment proteins including Mer tyrosine kinase and the Mer ligand Gas6.
Macrophages were cultured in chamber slides pre-coated with 4 µg/ml C1q (black bars) or HSA (white bars) for 4 (A) and 18 (B) hours prior to microarray analysis (as described in Materials and Methods). (A and B) Cluster analysis of gene transcripts following 4 (A) or 18 hour (B) adhesion to protein. The data are from five biological replicates for each condition. (C and D) Quantitative real-time PCR was performed with mRNA from macrophages treated as described in A and Bafter 4 (C) and 18 (D) hour adhesion to C1q or control protein (n=3, *p< 0.05, Student’s t test). (E) Macrophages were treated as in (D) and Mer expression quantified by qRT-PCR (n=3 *p< 0.05, Student’s t test). (F) Mer protein expression was detected from lysates of C1q-adherent macrophages by immunoprecipitation and western blot. (G) Mer expression was detected from macrophage cell lysates following stimulation with C1q in the presence and absence of 100 µM cycloheximide. (H) Gas6 protein levels in conditioned media were measured by ELISA following 5 hour adhesion to C1q (n=3, *p< 0.05, Student’s t test). Bars represent the mean ± SEM.
Quantitative real-time PCR (qRT-PCR) confirmed the microarray macrophage gene signature observed following adhesion to C1q. Consistent with the microarray data, we observed enhanced expression of mRNA encoding C1q, C3, MFG-E8, Mer and the Mer ligands, Gas6 and protein S (Fig. 2C–E). Mer is a member of the TAM family of receptor tyrosine kinases which also includes Tyro 3 and Axl. Mer is expressed on phagocytic cells including macrophages and dendritic cells, and has a non-redundant role in clearance of apoptotic cells (17,25). Importantly, Mer deficiency results in failure to clear apoptotic cells in vivo and onset of autoimmunity (16,17,26–29), similar to C1q deficiency (1). Because of the importance of Mer in apoptotic cell clearance, we confirmed C1q-dependent enhanced Mer mRNA and protein expression by qRT-PCR (Fig. 2E) and immunoprecipitation/western blot, respectively (Fig. 2F). As expected, pretreatment of macrophages with cycloheximide prior to stimulation with C1q inhibited Mer expression (Fig. 2G). Additionally, five hour macrophage stimulation with C1q resulted in twice as much Gas6 protein compared to control macrophages as measured by ELISA (Fig. 2H, 2900 ± 350 vs 1400 ± 130, respectively). Surprisingly, stimulation with C1q failed to enhance MFG-E8 protein levels above control as measured by ELISA (data not shown).
Full length native C1q and not the C1q collagen-like tail, elicits phagocytosis and Mer expression independent of LRP and CD93
To investigate whether soluble C1q enhanced Mer expression, macrophages were incubated with increasing concentration of soluble C1q for five hours. There was a dose-dependent increase in Mer expression with increasing concentrations of C1q up to physiologic concentrations (Fig. 3A). Moreover, physiologic C1q concentration (75 µg/ml) stimulated Mer expression to similar levels as 4 µg/ml immobilized C1q: the C1q concentration used for macrophage stimulation for the majority of the experiments reported herein (data not shown). To demonstrate that the effects reported herein were specific to C1q, C1q was heat inactivated at 56°C for 1 hour. Heat inactivated C1q failed to enhance Mer expression (Fig. 3B) and engulfment of antibody coated targets and apoptotic cells (Fig. 3C, 3D). The collagen-like tails of C1q are sufficient for triggering C1q-dependent engulfment of EAIgG (7), therefore, the ability of C1q-tails to upregulate Mer expression was tested. C1q-tails failed to stimulate Mer expression when compared to full-length C1q (Fig. 3E), but enhanced engulfment of EAIgG (data not shown), suggesting that the whole C1q molecule is necessary for Mer expression. A number of putative receptors have been proposed to mediate C1q-dependent signaling on macrophages including CR1 (CD35), CD93 and LRP (CD91) [reviewed in (11)]. Since CR1 is not expressed by murine macrophages [(30) and our unpublished results], we focused our attention on CD93 and LRP and tested whether these receptors were required for C1q-dependent Mer expression using CD93 or LRP-deficient macrophages. C1q upregulated Mer expression in both CD93 and LRP-deficient macrophages at levels comparable to wild type demonstrating that these receptors are not required for C1q dependent upregulation of Mer (Fig. 3F).
Figure 3. Full length native C1q and not the C1q collagen-like tail, elicits phagocytosis and Mer expression independent of LRP and CD93.
(A) Cell lysates from macrophages treated with increasing concentrations of C1q in solution were analyzed for Mer expression by western blot. (B) Cell lysates from macrophages adhered to wells pre-coated with 4 µg/ml HSA, C1q or heat inactivated (Δ) HSA and C1q were analyzed for Mer expression by western blot. (C and D) Phagocytosis of EAIgG (C) or apoptotic Jurkatcells (D) was performed as described in the legend to figure 1, except ΔC1q was used as an additional control (black bars). Data is expressed as fold change in phagocytosis relative to control from three independent experiments. Bars represent the mean ± SEM from three experiments. *p < 0.05, **p< 0.01, one-way ANOVA, Bonferroni multiple comparison tests. (E) Cell lysates from macrophages adhered to wells pre-coated with 4 or 8 µg/ml HSA, C1q or C1q-tails (C1qT) were analyzed for Mer expression by western blot. (F) Cell lysates from CD93+/+, CD93−/−, LRP+/+ and LRP−/− macrophages adhered to wells pre-coated with 4 µg/ml HSA or C1q were analyzed for Mer expression by western blot.
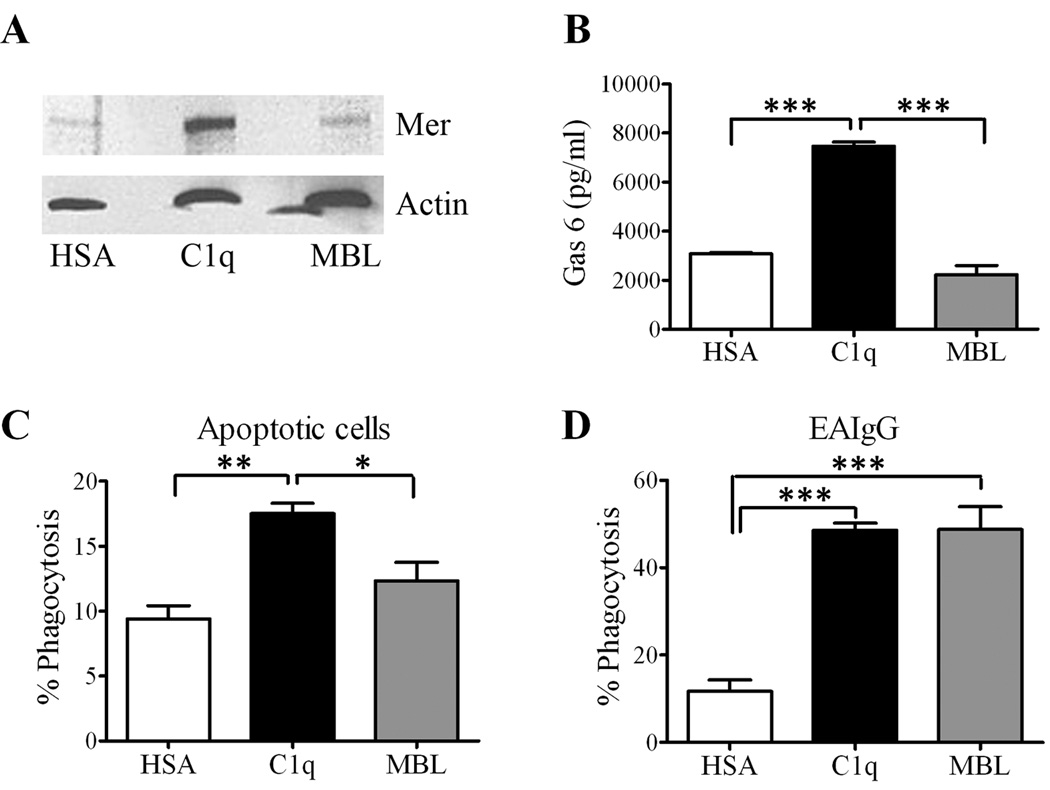
MBL fails to enhance engulfment of apoptotic cells and expression of Mer and Gas6
In addition to C1q, other members of the defense collagen family have been reported to stimulate macrophage phagocytosis. Using recombinant protein, Arora et al. identified a six-amino acid motif in the collagen-like tail of MBL that is conserved in C1q, and required for enhanced FcR-mediated phagocytosis (31). Similar to C1q, MBL has been implicated in facilitating ingestion of apoptotic cells by acting as a bridging molecule [reviewed in (11)]. However, despite a function for MBL in enhanced phagocytosis of apoptotic cells, MBL deficiency is not associated with autoimmunity (12). Therefore, we hypothesized that C1q, and not MBL, would activate the novel engulfment pathway resulting in Mer-dependent engulfment of apoptotic cells. As predicted, C1q, but not MBL, increased macrophage Mer expression and Gas6 protein levels as measured by western blot (Fig. 4A, Supplemental Fig. 1) and ELISA (Fig. 4B), respectively. Macrophages stimulated with C1q expressed significantly more Gas6 compared to control and MBL activated macrophages (Fig. 4B). To investigate the effects of MBL on phagocytosis, macrophages were adhered to C1q or MBL for five hours and then fed apoptotic cells. As shown previously, C1q enhanced engulfment of apoptotic cells compared to the control (Fig. 4C, 18 ± 0.8 vs 9.4 ± 1.0, respectively), however, MBL failed to significantly enhance engulfment above control levels (Fig. 4C, 12 ± 1.4 vs 9.4 ± 1.0, respectively). Consistent with previously published observations, MBL enhanced FcR-mediated phagocytosis at levels comparable to C1q when compared to the control (Fig. 4D) indicating that MBL was biologically active. Together, these data suggest that C1q, and not MBL, enhances engulfment of apoptotic cells via upregulation of Mer and the Mer ligand, Gas6.
Figure 4. C1q, but not MBL, enhances Mer expression and engulfment of apoptotic cells.
(A and B) Cell lysates from macrophages adhered to wells pre-coated with 4 µg/ml HSA, C1q or MBL were analyzed for Mer expression by western blot (A) or Gas6 expression by ELISA (B). (B) Bars represent average of triplicate samples from three experiments ± SD. ***p< 0.001, one-way ANOVA, Bonferroni multiple comparison tests.(C and D) Macrophages cultured on slides pre-coated with 4 µg/ml C1q, HSA or MBL for 5 hours were fed apoptotic Jurkat cells at a 1:3 macrophage to apoptotic cell ratio for an additional hour (C) or EAIgG for an additional 30 minutes (D). Data is expressed as fold change in phagocytosis relative to control. Bars represent the average of three independent experiments ± SEM. *p < 0.05, **p< 0.01, ***p< 0.001, one-way ANOVA, Bonferroni multiple comparison tests.
Mer tyrosine kinase is required for C1q-dependent engulfment of apoptotic cells
To determine whether Mer expression was required for the observed C1q-dependent engulfment of apoptotic cells, experiments were performed with macrophages from Mer-deficient mice (mertk−/−). Mertk−/− mice lack the tyrosine kinase signaling domain in the cytoplasmic tail (20) and fail to express Mer protein (32). Both wild type and mertk−/− bone marrow derived macrophages responded to C1q with enhanced FcR-mediated phagocytosis, there was a three- to five- fold enhanced FcR-mediated phagocytosis above control levels (Fig. 5A). However, while wild type macrophages responded normally to C1q, mertk−/− macrophages failed to respond to C1q with enhanced engulfment of apoptotic cells (Fig. 5B, n=4). To confirm that the C1q-elicited response was conserved in other phagocytes, experiments were also carried out with thioglycollate-elicited peritoneal macrophages (PMφ). Similarly, prolonged activation of wild type PMφ with C1q resulted in enhanced Mer expression (Supplemental Fig. 2) and engulfment of apoptotic cells, and C1q-dependent engulfment was significantly reduced in mertk−/−PMφ (Supplemental Fig. 2). As expected, mertk−/− bone marrow macrophages failed to show increased Mer expression upon stimulation with C1q compared to wild type macrophages (Fig. 5E).
Figure 5. C1q mediated engulfment of apoptotic cells is dependent on Mer.
Engulfment assays were performed as described in the legend to Figure 1 using wild type (WT) and mertk−/− macrophages. (A and B) C1q-adherent macrophages were fed EAIgG (A) or apoptotic Jurkatcells (B). Bars represent the mean fold change from four to six individual experiments ± SEM. **p< 0.01, two-way ANOVA, Bonferroni multiple comparison tests. (C and D) C1q-dependent engulfment was assessed in WT and C3−/− macrophages. Data is expressed as fold change in phagocytosis relative to control from three independent experiments. Bars represent the mean ± SEM. *p < 0.05, Student’s t test. (E) Cell lysates from WT and mertk−/− macrophages adhered to wells pre-coated with 4 µg/ml HSA, C1q or MBL were analyzed for Mer expression by western blot
Since C1q upregulated macrophage expression of complement components including C3 (Fig. 2A–D), the role of C3 in the C1q-dependent enhanced phagocytosis was investigated using C3−/− macrophages. As expected, C1q-elicited FcR-mediated phagocytosis was similar between wild type and C3−/− macrophages (Fig. 5C). As in previous experiments, wild type and C3−/− macrophages were allowed to adhere to C1q or control protein for five hours and then fed apoptotic cells. In contrast to mertk−/−macrophages, C3−/− macrophages responded to C1q with enhanced engulfment of apoptotic cells similar to wild type controls (Fig. 5D). There was no significant difference in C1q-dependent engulfment of apoptotic cells between wild type and C3−/− macrophages (Fig. 5D).
C1q-dependent engulfment of apoptotic cells is inhibited by soluble Mer
The Mer-Fc fusion protein has been shown to inhibit Mer-dependent engulfment by competing for Mer ligands in solution (32). To determine if Mer ligands were required for C1q-dependent engulfment of apoptotic cells, macrophages were stimulated with C1q for five hours and then cultured in the presence or absence of recombinant Mer-Fc fusion protein prior to co-culture with apoptotic thymocytes. Apoptotic primary mouse thymocytes were used as targets to confirm that the observed C1q-dependent engulfment pathway was observed in a cell autologous system since previous experiments had utilized mouse phagocytes and a human cell line (Jurkat) as targets. C1q enhanced the engulfment of apoptotic murine thymocytes, similar to apoptotic Jurkats, as measured by flow cytometry (data not shown) and microscopy (Fig. 6A, 6B). As predicted, C1q enhanced engulfment of apoptotic thymocytes over control (Fig. 6B, 53 ± 8.6 vs 21 ± 4.5 vs, respectively) and Mer-Fc inhibited the C1q-dependent engulfment of apoptotic thymocytes (Fig. 6A, 6B). To ascertain whether Mer-Fc was binding Gas6 in our culture system, culture media from macrophages stimulated with C1q was incubated with Mer-Fc followed by precipitation with protein G agarose. Western blotting and immunodetection with an antibody against Gas 6 showed a single band corresponding to Gas 6 of similar molecular weight to that found in murine serum (Fig. 6C).
Figure 6. C1q mediated engulfment of apoptotic cells is inhibited by Mer-Fc.
Macrophages were cultured in chamber slides pre-coated with 4 µg/ml C1q (black bars) or HSA (white bars) for 4 hours at 37°C, 5% CO2. Mer-Fc was added to culture wells for 1 hour after which macrophages were fed apoptotic mouse thymocytes at a 1:10 macrophage to apoptotic cell ratio for an additional hour. (A) Representative photomicrographs of PE -conjugated anti CD11b labeled macrophages (red) fed CFSE labeled apoptotic thymocytes (green) and adhered to HSA or C1q in the presence or absence of Mer-Fc. Inset in the upper right panel shows higher magnification (63×) of cells. Arrows indicate apoptotic thymocytes that are associated with macrophages, and arrow heads indicate apoptotic thymocytes that are not associated with macrophages. Photomicrographs were captured with a 40× object. (Scale bars: 50 µm; Inset, 10 µm). (B) Bars represent the mean ± SEM from three experiments. *p< 0.05, two-way ANOVA, Bonferroni multiple comparison tests. A minimum of 200 cells per experimental condition were quantified. (C) Mer-Fc precipitated Gas 6 from macrophage conditioned media following 18 hour adhesion to C1q.
Discussion
The ability of C1q and MBL to enhance phagocytosis has been well documented in the literature [reviewed in (13)]. The results presented here are in accordance with previous published observations and show that brief macrophage stimulation with either C1q or MBL enhances phagocytosis of antibody coated targets (Fig. 1A, 4D). However, unlike the previously described effects of C1q on macrophages phagocytosis, here we show that prolonged macrophage stimulation with C1q elicited expression of Mer tyrosine kinase as well as Mer ligands (Fig. 2, 4A, 4B). Mer was essential for the C1q-dependent engulfment of apoptotic cells since treatment with soluble Mer-Fc fusion protein (Fig. 6) inhibited C1q-dependent engulfment of apoptotic cells, and Mer-deficient macrophages failed to respond to C1q with enhanced engulfment of apoptotic cells (Fig. 5B). This novel pathway is specific for C1q since MBL failed to elicit Mer and Gas 6 expression (Fig. 4A, 4B). Together these data demonstrate a novel C1q-specific mechanism for clearance of apoptotic cells.
The importance of C1q-dependent engulfment of apoptotic cells in prevention of autoimmunity has been appreciated for more than a decade (1,10). However, previous studies to identify C1q-dependent molecular mechanisms were guided by the assumption that C1q provided a direct and immediate signal for enhanced phagocytosis (33,34). The direct pathway was confirmed in this study by measuring C1q/MBL-dependent phagocytosis of EAIgG (Fig. 4D). However, this pathway is not specific for apoptotic cells since C1q also triggers engulfment of antibody and complement coated particles (6,7). Moreover, this immediate signaling pathway is not specific for C1q since the collectins, including MBL and SP-A, trigger a similar enhancement (8,9,34). Together, these data fail to provide a satisfactory explanation for the observation that C1q deficiency (and not MBL or SP-A deficiency) results in a specific failure to clear apoptotic cells and resultant autoimmunity.
The data presented herein provides a new hypothesis: that C1q specifically elicits a macrophage phenotype optimized for efficient clearance of apoptotic cells. While the data demonstrate that Mer is required for C1q-dependent engulfment of apoptotic cells in murine BMDM (Fig. 5B, 6), it should be noted that additional C1q-triggered BMDM transcripts were identified by microarray that are required for efficient engulfment of apoptotic cells. The significance of these transcripts was not explored in this study however, C1q-dependent expression of other engulfment proteins may be important in clearance in specific microenvironments and/or in other phagocyte populations. In addition, the mechanism leading to C1q-dependent upregulation of Mer remains to be determined. While LRP and CD93 have been implicated as receptors or co-receptors required for C1q-dependent signaling (33,34), we found that neither protein was required for C1q-dependent expression of Mer (Fig. 3F). Two recent reports have demonstrated a role for nuclear receptor signaling in upregulation of Mer (35,36). Nuclear receptors LXR and PPARδ have been proposed to act as sensors for apoptotic cells. Binding of apoptotic cell derived lipids to nuclear receptors stimulates nuclear receptor-dependent expression of engulfment machinery required for efficient clearance of apoptotic cells and prevention of autoimmunity. The mechanism(s) leading to C1q-dependent macrophage activation remain to be elucidated, however based on these studies, nuclear receptors may be a viable avenue for investigation of C1q-dependent macrophage activation.
Data presented here demonstrate that sub-physiologic concentrations of soluble C1q stimulate Mer expression (Fig. 3A). This is a significant finding because C1q is a soluble protein that circulates in blood, and therefore this study suggests that under normal physiology, circulating C1q may program macrophages to respond efficiently to apoptotic cells. In addition, C1q is upregulated in response to injury (37), and therefore, increased C1q concentration following injury may further upregulate the Mer clearance system and promote the resolution of inflammation.
C1q-deficiency is the strongest known genetic link to autoimmunity associated with lupus, and it is widely accepted that autoimmunity occurs, at least in part, due to a failure to clear apoptotic cells (1,2,10,38). Therefore, the identification of a novel molecular mechanism leading to C1q-dependent engulfment of apoptotic cells has important implications in the development of therapeutics for autoimmune diseases. Our data demonstrate that C1q-dependent expression of Mer is required for C1q-dependent engulfment of apoptotic cells (Fig. 5B, 6). However, while a specific deficiency in Mer has been associated with lupus-like autoimmunity in mice, the association has not yet been reported in humans. Mer is a member of the TAM family of receptor tyrosine kinases that include Axl and Tyro3. TAM receptor signaling is thought to occur through hetero- and/or homodimerization. In addition scavenger receptor A (SR-A) signals via Mer during SR-A mediated apoptotic cell engulfment (39). Lu et al. demonstrated that deletion of all three TAM receptors resulted in profound autoimmunity in mice (40,41). More recent studies further indicate a critical role for Mertk, and not Axl or Tyro3, in prevention of autoimmunity in mice (28,42). It is possible that C1q regulates the expression of other TAM receptors and/or other engulfment proteins, such as SR-A, that may contribute to clearance of apoptotic cells and prevention of autoimmunity. Mer plays a non-redundant role in clearance of apoptotic cells in the retina, and Mer deficiency results in retinitis pigmentosa (RP) due to a failure in retinal pigment epithelial cell phagocytosis of photoreceptor outer segments (43,44). It is interesting to note that it was recently reported that C1q levels are elevated in the retina of a mouse model of RP, and lack of C1q resulted in heightened susceptibility to RP, which is consistent with a role for C1q in apoptotic cells clearance (45). Therefore, it will be important to measure levels of Mer and other engulfment proteins in vivo under conditions where deficiency in C1q results in pathology due to inefficient clearance of apoptotic cells.
The data presented here establish a previously unknown link between two well characterized and important clearance systems, C1q and Mer tyrosine kinase. Identification of the signaling mechanisms downstream of C1q that result in upregulation of Mer, and characterization of C1q-dependent expression of Mer and other engulfment proteins in vivo during normal physiology and autoimmune disease will provide additional insight into the contribution of this molecular pathway to pathogenesis.
Supplementary Material
Acknowledgements
The authors are grateful to Mallary Greenlee-Wacker, Nicholas Burley, Stephanie Jones, Thomas Heitker, and the cell biology special studies laboratory at the University of Notre Dame for technical assistance. The authors are also grateful to Carlos Brisenofor careful review of the manuscript.
Footnotes
This work was supported by a research enhancement grant and research support funds grant from Indiana University School of Medicine, NIH NIAID grant 1R56AI080877 and a genomics pilot grant from the University of Notre Dame Eck Institute for Global Health to SSB.
The authors have no conflicting financial interests.
Reference List
- 1.Botto M, Dell'Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 2.Pickering MC, Botto M, Taylor PR, Lachmann PJ, Walport MJ. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv. Immunol. 2000;76:227–324. doi: 10.1016/s0065-2776(01)76021-x. [DOI] [PubMed] [Google Scholar]
- 3.Walport MJ. Lupus, DNase and defective disposal of cellular debris. Nat. Genet. 2000;25:135–136. doi: 10.1038/75963. [DOI] [PubMed] [Google Scholar]
- 4.Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J. Cell Biol. 2010;189:1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothlin CV, Lemke G. TAM receptor signaling and autoimmune disease. Curr. Opin. Immunol. 2010;22:740–746. doi: 10.1016/j.coi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobak DA, Gaither TA, Frank MM, Tenner AJ. Modulation of FcR function by complement: subcomponent C1q enhances the phagocytosis of IgG-opsonized targets by human monocytes and culture-derived macrophages. J. Immunol. 1987;138:1150–1156. [PubMed] [Google Scholar]
- 7.Bobak DA, Frank MM, Tenner AJ. C1q acts synergistically with phorbol dibutyrate to activate CR1-mediated phagocytosis by human mononuclear phagocytes. Eur. J. Immunol. 1988;18:2001–2007. doi: 10.1002/eji.1830181220. [DOI] [PubMed] [Google Scholar]
- 8.Tenner AJ, Robinson SL, Borchelt J, Wright JR. Human pulmonary surfactant protein (SP-A), a protein structurally homologous to C1q, can enhance FcR-and CR1-mediated phagocytosis. J. Biol. Chem. 1989;264:13923–13928. [PubMed] [Google Scholar]
- 9.Tenner AJ, Robinson SL, Ezekowitz RA. Mannose binding protein (MBP) enhances mononuclear phagocyte function via a receptor that contains the 126,000 M(r) component of the C1q receptor. Immunity. 1995;3:485–493. doi: 10.1016/1074-7613(95)90177-9. [DOI] [PubMed] [Google Scholar]
- 10.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J. Immunol. 1997;158:4525–4528. [PubMed] [Google Scholar]
- 11.Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol. Immunol. 2007;44:33–43. doi: 10.1016/j.molimm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J. Immunol. 2005;174:3220–3226. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 13.Fraser DA, Tenner AJ. Directing an appropriate immune response: the role of defense collagens and other soluble pattern recognition molecules. Curr. Drug Targets. 2008;9:113–122. doi: 10.2174/138945008783502476. [DOI] [PubMed] [Google Scholar]
- 14.Fraser DA, Bohlson SS, Jasinskiene N, Rawal N, Palmarini G, Ruiz S, Rochford R, Tenner AJ. C1q and MBL, components of the innate immune system, influence monocyte cytokine expression. J. Leukoc. Biol. 2006;80:107–116. doi: 10.1189/jlb.1105683. [DOI] [PubMed] [Google Scholar]
- 15.Fraser DA, Laust AK, Nelson EL, Tenner AJ. C1q differentially modulates phagocytosis and cytokine responses during ingestion of apoptotic cells by human monocytes, macrophages, and dendritic cells. J. Immunol. 2009;183:6175–6185. doi: 10.4049/jimmunol.0902232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, Matsushima G, Reap EA. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J. Exp. Med. 2002;196:135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 18.Tenner AJ, Lesavre PH, Cooper NR. Purification and radiolabeling of human C1q. J. Immunol. 1981;127:648–653. [PubMed] [Google Scholar]
- 19.Young KR, Jr, Ambrus JL, Jr, Malbran A, Fauci AS, Tenner AJ. Complement subcomponent C1q stimulates Ig production by human B lymphocytes. J. Immunol. 1991;146:3356–3364. [PubMed] [Google Scholar]
- 20.Camenisch TD, Koller BH, Earp HS, Matsushima GK. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J. Immunol. 1999;162:3498–3503. [PubMed] [Google Scholar]
- 21.Bohlson SS, Strasser JA, Bower JJ, Schorey JS. Role of complement in Mycobacterium avium pathogenesis: in vivo and in vitro analyses of the host response to infection in the absence of complement component C3. Infect. Immun. 2001;69:7729–7735. doi: 10.1128/IAI.69.12.7729-7735.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lillis AP, Greenlee MC, Mikhailenko I, Pizzo SV, Tenner AJ, Strickland DK, Bohlson SS. Murine Low-Density Lipoprotein Receptor-Related Protein 1 (LRP) Is Required for Phagocytosis of Targets Bearing LRP Ligands but Is Not Required for C1q-Triggered Enhancement of Phagocytosis. J. Immunol. 2008;181:364–373. doi: 10.4049/jimmunol.181.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roach T, Slater S, Koval M, White L, Cahir McFarland ED, Okumura M, Thomas M, Brown E. CD45 regulates Src family member kinase activity associated with macrophage integrin-mediated adhesion. Curr. Biol. 1997;7:408–417. doi: 10.1016/s0960-9822(06)00188-6. [DOI] [PubMed] [Google Scholar]
- 24.Bohnsack JF, Kleinman HK, Takahashi T, O'Shea JJ, Brown EJ. Connective tissue proteins and phagocytic cell function. Laminin enhances complement and Fc-mediated phagocytosis by cultured human macrophages. J. Exp. Med. 1985;161:912–923. doi: 10.1084/jem.161.5.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behrens EM, Gadue P, Gong SY, Garrett S, Stein PL, Cohen PL. The mer receptor tyrosine kinase: expression and function suggest a role in innate immunity. Eur. J. Immunol. 2003;33:2160–2167. doi: 10.1002/eji.200324076. [DOI] [PubMed] [Google Scholar]
- 26.Shao WH, Eisenberg RA, Cohen PL. The Mer receptor tyrosine kinase is required for the loss of B cell tolerance in the chronic graft-versus-host disease model of systemic lupus erythematosus. J. Immunol. 2008;180:7728–7735. doi: 10.4049/jimmunol.180.11.7728. [DOI] [PubMed] [Google Scholar]
- 27.Shao WH, Zhen Y, Eisenberg RA, Cohen PL. The Mer receptor tyrosine kinase is expressed on discrete macrophage subpopulations and mainly uses Gas6 as its ligand for uptake of apoptotic cells. Clin. Immunol. 2009;133:138–144. doi: 10.1016/j.clim.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao WH, Kuan AP, Wang C, Abraham V, Waldman MA, Vogelgesang A, Wittenburg G, Choudhury A, Tsao PY, Miwa T, Eisenberg RA, Cohen PL. Disrupted Mer receptor tyrosine kinase expression leads to enhanced MZ B-cell responses. J. Autoimmun. 2010;35:368–374. doi: 10.1016/j.jaut.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao WH, Zhen Y, Rosenbaum J, Eisenberg RA, McGaha TL, Birkenbach M, Cohen PL. A protective role of Mer receptor tyrosine kinase in nephrotoxic serum-induced nephritis. Clin. Immunol. 2010;136:236–244. doi: 10.1016/j.clim.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin BK, Weis JH. Murine macrophages lack expression of the Cr2–145 (CR2) and Cr2–190 (CR1) gene products. Eur. J. Immunol. 1993;23:3037–3042. doi: 10.1002/eji.1830231146. [DOI] [PubMed] [Google Scholar]
- 31.Arora M, Munoz E, Tenner AJ. Identification of a site on mannan-binding lectin critical for enhancement of phagocytosis. J. Biol. Chem. 2001;276:43087–43094. doi: 10.1074/jbc.M105455200. [DOI] [PubMed] [Google Scholar]
- 32.Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, Graham DK. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109:1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nepomuceno RR, Henschen-Edman AH, Burgess WH, Tenner AJ. cDNA cloning and primary structure analysis of C1qR(P), the human C1q/MBL/SPA receptor that mediates enhanced phagocytosis in vitro. Immunity. 1997;6:119–129. doi: 10.1016/s1074-7613(00)80419-7. [DOI] [PubMed] [Google Scholar]
- 34.Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Diaz M, Gallardo G, de Galarreta CR, Salazar J, Lopez F, Edwards P, Parks J, Andujar M, Tontonoz P, Castrillo A. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, Ricardo-Gonzalez RR, Goh YP, Eagle AR, Dunn SE, Awakuni JU, Nguyen KD, Steinman L, Michie SA, Chawla A. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat. Med. 2009;15:1266–1272. doi: 10.1038/nm.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan R, Tenner AJ. Differential regulation of Abeta42-induced neuronal C1q synthesis and microglial activation. J. Neuroinflammation. 2005;2:1. doi: 10.1186/1742-2094-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MJ. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J. Exp. Med. 2000;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Todt JC, Hu B, Curtis JL. The scavenger receptor SR-A I/II (CD204) signals via the receptor tyrosine kinase Mertk during apoptotic cell uptake by murine macrophages. J. Leukoc. Biol. 2008;84:510–518. doi: 10.1189/jlb.0307135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothlin CV, Lemke G. TAM receptor signaling and autoimmune disease. Curr. Opin. Immunol. 2010;22:740–746. doi: 10.1016/j.coi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 42.Williams JC, Wagner NJ, Earp HS, Vilen BJ, Matsushima GK. Increased hematopoietic cells in the mertk−/− mouse peritoneal cavity: a result of augmented migration. J. Immunol. 2010;184:6637–6648. doi: 10.4049/jimmunol.0902784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat. Genet. 2000;26:270–271. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- 44.Nandrot E, Dufour EM, Provost AC, Pequignot MO, Bonnel S, Gogat K, Marchant D, Rouillac C, Sepulchre de CB, Bihoreau MT, Shaver C, Dufier JL, Marsac C, Lathrop M, Menasche M, Abitbol MM. Homozygous deletion in the coding sequence of the c-mer gene in RCS rats unravels general mechanisms of physiological cell adhesion and apoptosis. Neurobiol. Dis. 2000;7:586–599. doi: 10.1006/nbdi.2000.0328. [DOI] [PubMed] [Google Scholar]
- 45.Humphries MM, Kenna PF, Campbell M, Tam LC, Nguyen AT, Farrar GJ, Botto M, Kiang AS, Humphries P. C1q enhances cone photoreceptor survival in a mouse model of autosomal recessive retinitis pigmentosa. Eur. J. Hum. Genet. 2011 doi: 10.1038/ejhg.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.