Abstract
Objective
To determine the most cost-effective treatment option for patients with newly-diagnosed mild open-angle glaucoma (OAG): observation only, treatment with generic topical prostaglandin analogs (PGAs) or treatment with laser trabeculoplasty (LTP)
Methods
Using a Markov model with a 25 year time horizon, we compared the incremental cost effectiveness of treating newly-diagnosed mild OAG with PGAs, LTP, or observation only.
Results
The incremental cost effectiveness of LTP over no treatment is $16824/ quality-adjusted life year (QALY). By comparison, the incremental cost effectiveness of PGAs over no treatment is $14179/QALY and it provides greater health-related quality of life relative to LTP. If PGAs are 25% less effective due to poor patient adherence, LTP can confer greater value.
Conclusion
PGAs and LTP are both cost-effective options for the management of newly-diagnosed mild OAG. Assuming optimal medication adherence, PGAs confer greater value compared with LTP. However, when assuming more realistic levels of medication adherence (making them 25% less effective than the documented effectiveness reported in clinical trials), at today's prices for PGAs, LTP may be a more cost effective alternative.
Open angle glaucoma (OAG) is one of the leading causes of irreversible vision loss in the United States, affecting over 3 million individuals.1 With the aging of the U.S. population, the number of persons with OAG are expected to rise considerably in the coming decades.2 Randomized clinical trials have demonstrated that lowering intraocular pressure (IOP) can reduce the risk of developing OAG in persons with ocular hypertension3 and can reduce the risk of disease progression in patients with mild4, moderate5, and severe glaucoma.6 Effective interventions for lowering IOP include the use of topical glaucoma medications, laser trabeculoplasty (LTP), and incisional glaucoma surgery.
Several large randomized clinical trials have been conducted to compare the effectiveness of these different interventions for lowering IOP and preventing OAG progression. The Glaucoma Laser Trial (GLT) randomized a group of patients to treatment with a topical beta blocker versus treatment with argon laser trabeculoplasty (ALT).7 This study found that over 9 years of follow-up, 34% of individuals in the group treated with medications experienced disease progression enough to require laser or incisional surgery and 11% of patients in the group treated with ALT progressed to require additional laser or incisional surgery. While this trial demonstrated that laser surgery is as effective, if not more effective, than treatment with topical glaucoma medications, according to a recent Cochrane review, little is known about whether use of glaucoma medications or LTP is a more cost effective treatment option for patients with newly diagnosed OAG.8
Glaucoma medications have long been the mainstay of treatment for OAG. Over the past two decades an array of different classes of glaucoma medications have been commercially available including prostaglandin analogues (PGAs), beta blockers, alpha agonists, carbonic anhydrase inhibitors, and miotics.9 Advantages of treatment with glaucoma medications as compared with other treatment modalities are that most medications, especially the PGAs, are well tolerated and have few serious side effects. Should a patient experience a side effect from medication use, the side effects are often self-limiting upon medication discontinuation. Patients often prefer glaucoma medications because they are less invasive than other treatment options. However, since OAG is a chronic condition, regular use of glaucoma medications can become quite costly over time.10 In addition, several studies have demonstrated that medication adherence is a major challenge for many OAG patients.11 Moreover, a subset of patients do not respond to certain classes of pressure-lowering medications.
Laser trabeculoplasty is a relatively quick, minimally invasive outpatient procedure performed in the clinic setting. It is effective in most OAG patients and studies have shown that it can lower IOP 20-35%.12 If effective, LTP may reduce the long term need for glaucoma medications and the costs and side effects associated with chronic medication use. Furthermore, LTP may be a more effective treatment strategy in patients who struggle with medication adherence. Disadvantages of LTP include higher up-front costs and the risk of complications including inflammation and an acute elevation of IOP that, if left untreated, can lead to vision loss. Furthermore, there is a subset of patients who do not respond to LTP.13
Understanding whether one of these treatment options has greater incremental cost effectiveness, or confers a greater value, relative to the other is important for many different stakeholders including health policy makers, third party payers, eye care providers and most importantly, patients. With the dramatically rising costs of health care in the United States14, it is essential to find ways to curtail costs, ideally without sacrificing the quality of care provided.
The purpose of this study is to compare the incremental cost effectiveness of PGAs versus LTP in the treatment of individuals with newly diagnosed mild OAG. In addition, both of these treatment options will be compared with a control group of patients who are untreated to determine whether treatment of OAG (either using PGAs or LTP) is more cost effective than following OAG patients without treatment.
Methods
Study design
In this cost effectiveness analysis, we compared treatment with PGAs, LTP, and observation only for patients with newly diagnosed OAG over a 25 year time horizon. The target population was a hypothetical cohort of patients, 60 years of age with mild OAG. Age 60 was chosen as the starting age for the analysis because OAG is more common among older individuals and studies have found this to be the average age for developing incident OAG.15 In sensitivity analysis, we explore the impact of changing these model parameters (see below).
Markov Model
A Markov model is a mathematical method for quantifying the costs and health consequences of disease as patients transition through various disease stages over time.16-18 Such models can be useful in capturing the costs and benefits of treating chronic medical conditions such as glaucoma over time. In our Markov model, we assumed that each cycle through the model was 1 year long. Each cycle through the model, the costs and utilities were tabulated for each cohort. TreeAge Pro 2011 Health Care (TreeAge Software, Williamstown, Massachusetts) was used to capture the total costs and utilities for each of the three cohorts over 25 years (approximate life expectancy for individuals age 6019). We modeled mortality using data obtained from US life tables.20 We compared the incremental cost effectiveness of each of the three interventions with one another in the base model to determine the cost effectiveness of each of the three interventions. All costs were in 2010 USD and we assumed a 3% discounting rate per year.
Health States
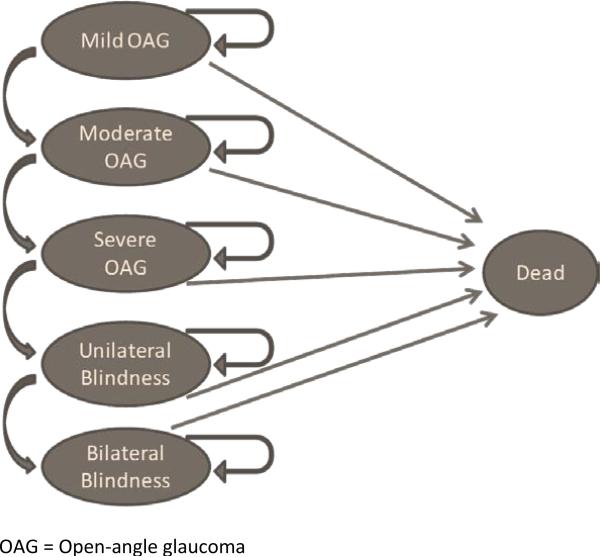
We considered five stages of disease: mild, moderate, and severe glaucoma followed by unilateral and bilateral blindness. For this analysis, we defined mild glaucoma as glaucomatous damage with a mean deviation of -6 decibels or less of visual field loss on standard automated perimetry. Moderate glaucoma was defined as glaucomatous damage with mean deviation of visual field loss of -6 to -12 decibels of mean deviation on standard automated perimetry. Severe glaucoma was defined as a mean deviation of -12 or worse on visual field testing. Unilateral blindness was defined as best corrected visual acuity of < 20/200 due to glaucoma in one eye and bilateral blindness is defined as best corrected visual acuity of < 20/200 in both eyes, caused by glaucoma.21
Progression From One Health State to Another
In the analysis, all patients started with mild glaucoma.(Figure 1) Patients were assumed to progress from less severe disease states to more severe disease states. Once a patient was in a more severe state they could either remain in that state or continue to progress to the next more severe disease state.
Figure 1.
Markov Model
For the untreated cohort, we determined disease progression from mild to moderate OAG based data from the untreated group in the Early Manifest Glaucoma Trial (EMGT).4 We used the progression rates from the GLT to capture progression from mild to moderate OAG for the PGA and LTP cohorts. To determine the proportion of individuals in the medication and LTP treatment groups who progressed from less severe to more severe disease states, we reviewed the data from the GLT study which captured, on a year by year basis for both groups, the proportion of individuals who required adjunctive glaucoma medical or surgical therapy because they were noted to have disease progression. Of note, at the time the GLT was conducted, topical beta blockers were prescribed as initial therapy and adjunctive medications included pilocarpine and dipiveferin, both of which now have been nearly universally replaced with newer agents.7 Since phase III clinical trials report that PGAs are approximately 30% more effective at lowering IOP as compared with topical beta blockers 22 to enable us to make our study findings more applicable to medications that are commonly prescribed today, we assumed that the medically treated group had a 30% reduced rate of disease progression relative to the rates of progression captured in the GLT for the medication group. In sensitivity analyses (see below), we tested the impact of varying the effectiveness of PGAs on the results of the analysis.
Once a patient had moderate OAG, we assumed 10% rate of progression to more severe disease states per year for each of the three groups. Because few studies have tracked long-term disease progression of glaucoma, we varied the rate of progression to more severe disease states from 5% to 15% per year in sensitivity analyses.
Model Validation
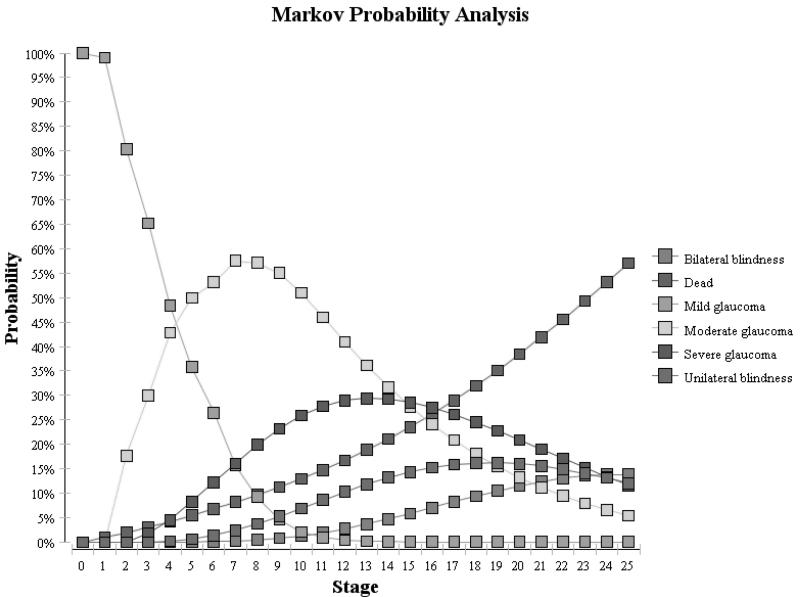
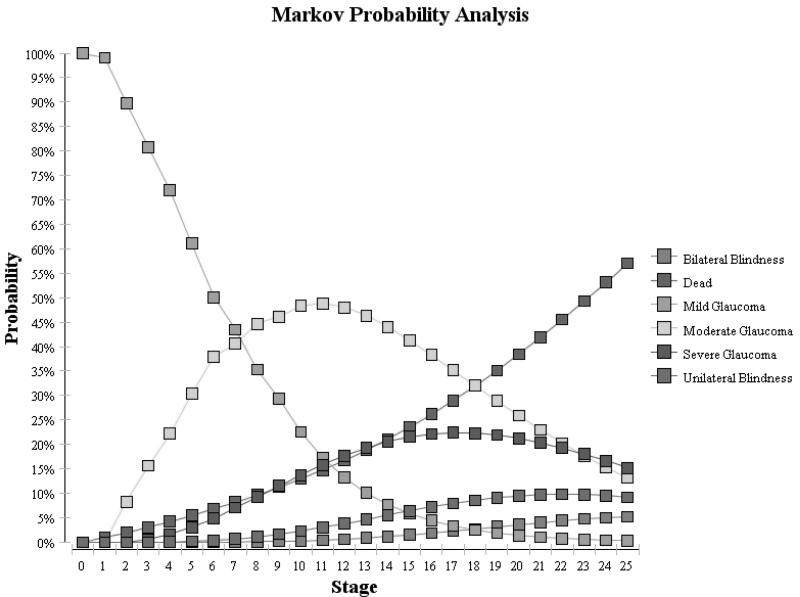
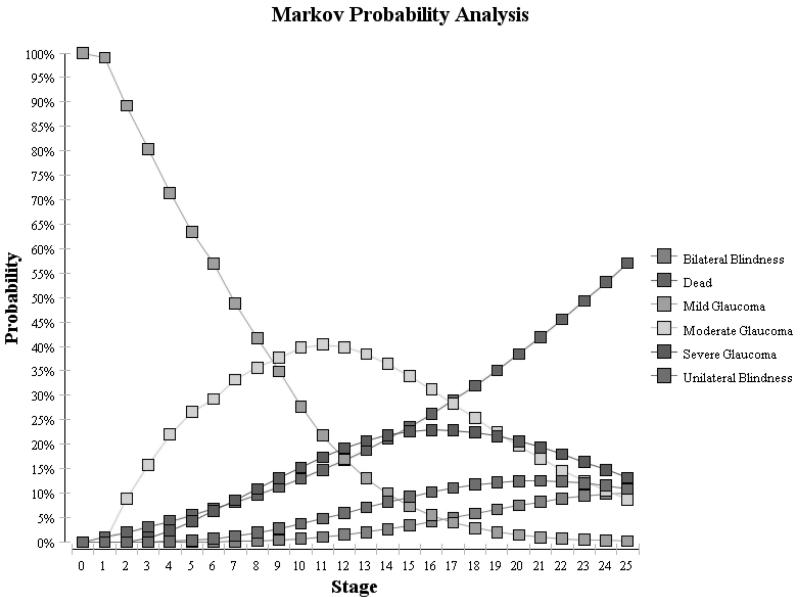
Using these assumptions, we generated Markov tracings for each of the 3 cohorts over the 25 years. (Figure 2) For the untreated cohort, these proportions were compared with findings from the St. Lucia study which assessed the natural history of untreated glaucoma and reported 16% of persons developed unilateral or bilateral blindness over 10 years.19 For both of the treated groups, we compared our cohorts to findings from studies by Chen and colleagues who reported that 14% of treated OAG patients experienced unilateral blindness and 4% of patient developed bilateral blindness from OAG over 15 years of follow-up.24 As Figure 2 demonstrates, we were able to achieve relatively similar rates of blindness in our Markov cohorts to those reported in these epidemiological studies.
Figure 2.
Cost-Effectiveness Plane Comparing the Three Interventions
Costs
Direct medical costs of glaucoma care were determined by using the 2010 Average Medicare Fee Schedule for services.25 Direct medical costs included costs of visits to eye care providers, ancillary glaucoma tests to monitor OAG patients and check for disease progression, costs of medical, laser, and surgical interventions, costs of treating side effects caused by the laser, and costs of requiring low vision aids for individuals who have progressed to unilateral or bilateral blindness. (Table 1)
Table 1.
Costs and Utilities Used in the Model
| Model Input | Item | Value | Range for Sensitivity analysis | |
|---|---|---|---|---|
| Costs | Medications (annual supply) | Prostaglandin Analogs | $330 | 0-1000 |
| Alpha Agonists | $1242 | 0-1500 | ||
| Beta Blockers | $435 | 0-500 | ||
| Laser surgery | Laser trabeculoplasty | $677 | 100-2000 | |
| Incisional surgery | Trabeculectomy | $2824 | 1000-4000 | |
| Initial evaluation | Visit | $190 | 100-200 | |
| Diagnostic Testing | $207 | 100-300 | ||
| Follow-up evaluation | Visit | $111 | 50-150 | |
| Diagnostic testing | $123 | 100-150 | ||
| Low Vision services | Unilateral low vision | $1000 | 500-4000 | |
| Bilateral low vision | $2000 | 1000-8000 | ||
| Side Effects | Laser trabeculoplasty | $1000 | 0-2000 | |
| Utilities | Glaucoma severity | Mild glaucoma | 0.92 | 0.8 – 0.99 |
| Moderate glaucoma | 0.89 | 0.7 – 0.95 | ||
| Severe glaucoma | 0.86 | 0.6 – 0.9 | ||
| Unilateral blindness | 0.47 | 0.4 – 0.8 | ||
| Bilateral Blindness | 0.26 | 0.2 – 0.5 | ||
| Side effects | Laser trabeculoplasty | 0.75 | 0.5-0.99 | |
| Other Parameters | Effectiveness | Prostaglandin analogs | 1.0 | 0.5 – 1.5 |
| Laser trabeculoplasty | 1.0 | 0.5 – 1.5 | ||
| Time Horizon (years) | 25 | 9-30 | ||
| Age at OAG diagnosis | 60 | 40-80 | ||
| Rate of OAG Progression (per year) | 10% | 5 – 15% |
All costs are in 2010 US dollars. All medication costs are for a 1 year supply of the medication. AWP = average wholesale price.
Costs of visits and diagnostic testing
All patients receiving treatment incurred the cost of an initial office consultation (Current Procedural Terminology (CPT) code, 99244), gonioscopy (CPT 92020), automated visual field testing (CPT 92083), scanning computerized imaging of the optic disc (CPT 92135), fundus photography (CPT 92250), and corneal pachymetry (CPT 76514). In the first year, individuals in all of the cohorts were assumed to incur costs for two follow-up examinations (CPT 92014), automated visual field testing, and scanning computerized imaging to determine whether they were stable or progressing. In each cycle through the model, patients who were classified as “stable” continued to incur costs of biannual examinations, annual automated visual field testing, and annual scanning computerized imaging. In each cycle through the model, patients in each cohort who were classified as “progressing” incurred costs of ocular examinations four times in the following year, visual field testing twice in the following year, and annual scanning computerized imaging to capture more close monitoring of these patients.
Costs of medical and surgical interventions
Costs of glaucoma medications were obtained using average wholesale prices from the Red Book.26 Since the average wholesale price for generic latanoprost has not yet been published, we determined the average annual charge for purchasing this product from two online pharmacies.27,28 In sensitivity analysis, we reran the model using the average wholesale price of a year supply of Travaprost (a commonly prescribed brand name PGA) of $750. The proportion of individuals in the LTP and PGA groups requiring adjunctive medications each year was obtained from the GLT. For those patients who required adjunctive medications, the cost of a year supply of timolol 0.5% was $435 and a year supply of brimonidine 0.1% was $1314. (Table 1). The cost of LTP and trabeculectomy were obtained from the 2010 average Medicare Fee Schedule for services using CPT codes 65855 and 66170, respectively. All individuals in the LTP cohort incurred the cost of LTP during the initial year in the model. Using information from the GLT, depending on disease severity, 2-25% of the patients in the glaucoma medication cohort per year incurred the cost of LTP, 2-10% of patients in the glaucoma medication group incurred the cost of trabeculectomy and 2-6% of persons in the LTP cohort incurred the cost of trabeculectomy each year.7
The proportion of patients who experience visually significant side effects following LTP is unknown as is the average costs associated with managing side effects following LTP. In the model we assumed that 5% of patients who underwent LTP experienced side effects from the laser. While the majority of these side effects are transient and self-limiting, rarely one can experience sight-threatening complications from a marked elevation of intraocular pressure following the procedure. Since it is challenging to fully capture in the model the costs of managing these different side effects, we assumed the cost of managing side effects to be $1000. This is likely an overestimation, but covers the costs of medications used to treat side effects and when necessary, incisional glaucoma surgery, in the rare event this was necessary. In sensitivity analysis, we explored the impact of altering the proportion of patients developing side effects from LTP from 0% to 5% and the costs of managing side effects from LTP from $0 to $1000.
Cost of Low Vision from Glaucoma
To capture the costs associated with need for low vision aids for unilateral blindness, we relied upon findings of a study by Lee and colleagues who captured the costs associated with different stages of glaucoma.29 Based on this study we assumed that the annual cost of low vision aides for unilateral blindness was $1000 and bilateral blindness was $2000. These costs were varied in sensitivity analyses. Since indirect costs such as lost productivity associated with vision loss from OAG are difficult to measure and may be included in utility estimates, we did not incorporate indirect costs into the Markov model. Patients in all interventions (including the untreated group) incurred this cost if their disease progressed to blindness.
Utilities
Since glaucoma treatment is not known to improve length-of-life, the value of therapy is conferred by the quality-of-life improvement gained from reducing the chances of peripheral vision loss and ultimately central vision loss and blindness caused by OAG. Utility analysis quantifies health-related quality-of-life, with anchors of 1.00 (perfect vision permanently) to 0.00 (death). Lee and colleagues quantified the utility values associated with different severities of glaucoma. These investigators determined the utilities of OAG ranged from 0.92 for mild disease to 0.86 for severe damage.30 Brown and colleagues reported the utility score from unilateral blindness is 0.47 and bilateral blindness is 0.26.31(Table 1) Finally, for the subset of beneficiaries who experienced side effects from the laser procedure, we assigned them a utility score of 0.75. While this utility score likely overestimates the loss of utility for many transient self-limiting side effects of the laser, we feel it captures utility loss associated with some of the more severe side effects associated with the laser procedure. This parameter was also varied in sensitivity analyses.
Sensitivity Analyses
Several sensitivity analyses were performed to capture the known variability that exists in estimates of costs, utilities, and transiting from one disease state to another. Table 1 shows the range of costs and utilities used when performing sensitivity analyses. An important factor that we varied in the sensitivity analysis was the effectiveness of the glaucoma medications. Since adherence with medical therapy is a known problem among individuals with OAG, by varying the effectiveness of the medications, we were able to determine how that factor influenced the results of the model. In addition, we conducted a two-way sensitivity analysis, simultaneously varying the effectiveness of glaucoma medications and LTP. Other one-way sensitivity analyses included restricting the time horizon to 9 years (the time frame of the actual GLT) rather than extrapolating the results out to 25 years; and increasing the utilities of mild, moderate, and severe OAG to be between 0.99 for mild disease and 0.9 for severe OAG since there is some evidence that even patients with advanced OAG experience little loss of health-related quality of life.25 In sensitivity analysis, we also explored the impact of changing the age of onset of OAG, the proportion of patients in the LTP group who experienced side effects, as well as the costs and utilities of side effects from LTP.
Results
Base Model
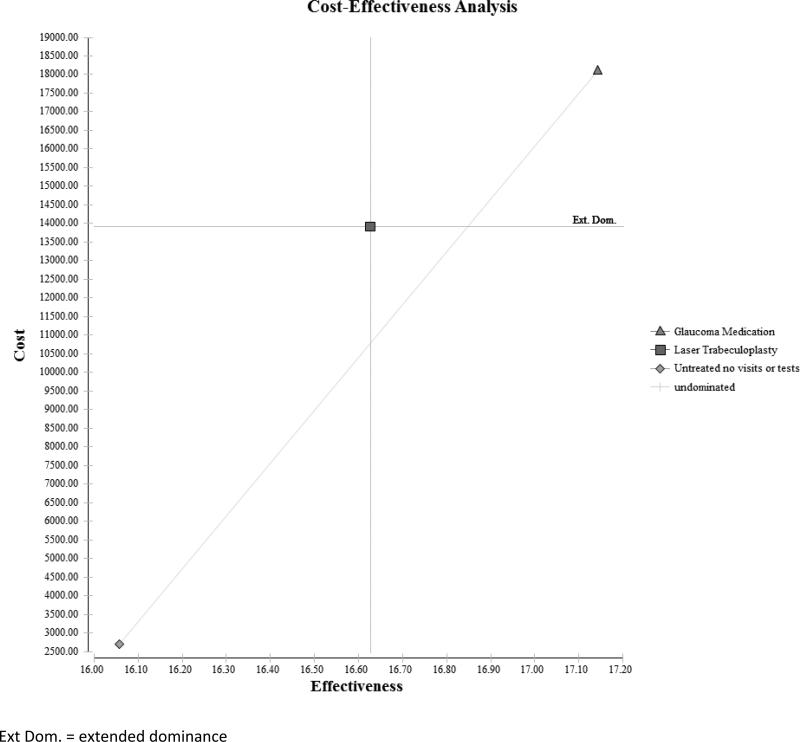
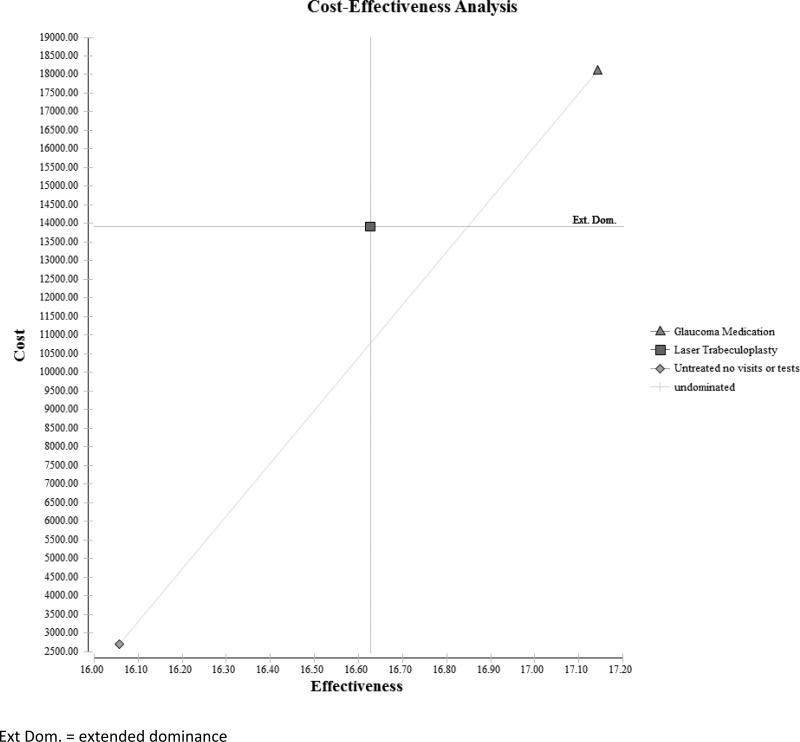
Over 25 years, the expected cost of untreated glaucoma for a single patient is $2700, the long-term cost of undergoing LTP is $13788, and the cost of receiving PGAs is $18101. Compared to a glaucoma patient who goes untreated, one who undergoes LTP accrues an additional $11088 in costs, and a patient treated with PGAs incurs an additional $15401 in costs. Over this same time period, the effectiveness for a glaucoma patient in the untreated group is 16.06 quality-adjusted life years (QALY), for a patient who underwent LTP is 16.71 QALYs, and for a patient receiving PGAs is 17.14 QALYs. Compared to an untreated patient, a patient who received LTP had 0.65 more QALYs, and one who received PGAs had 1.09 more QALYs over the 25 years. The incremental cost effectiveness of LTP over no treatment was $16824/QALY. But, the incremental cost effectiveness of PGAs over no treatment was $14179/QALY and it provides greater health-related quality of life relative to LTP.(Table 2 and Figure 3)
Table 2.
Cost Effectiveness of Treating Newly Diagnosed Mild Open-Angle Glaucoma Using Prostaglandin Analogs, Laser Trabeculoplasty, or No Treatment
| Intervention | Cost ($) | Incremental Costs | Effectiveness (QALYs) | Incremental Effectiveness | Incremental cost effectiveness |
|---|---|---|---|---|---|
| No Treatment | 2700 | 16.06 | |||
| LTP | 13788 | 11088 | 16.71 | 0.65 | $16824/ QALY |
| PGAs | 18101 | 15401 | 17.14 | 1.09 | $14179/ QALY |
LTP = laser trabeculoplasty; PGAs = prostaglandin analogues; QALY = quality-adjusted life year
Figure 3.
Cost-Effectiveness Plane Comparing the Three Interventions
Sensitivity Analysis
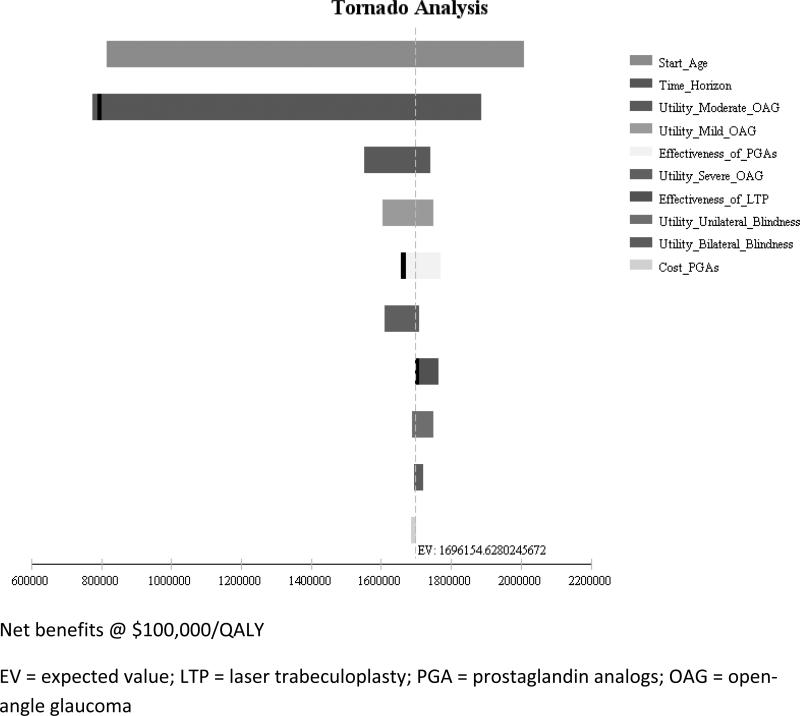
Sensitivity analyses were performed to determine whether the results of the Markov model would change by varying different model assumptions. A tornado diagram (eFigure 1) helped identify specific inputs that could be altered to make treatment with PGAs more or less cost effective relative to LTP. These inputs included the effectiveness of LTP and the effectiveness of PGAs.
eFigure 1.
Tornado Diagram Showing Impact of Varying Different Model Parameters
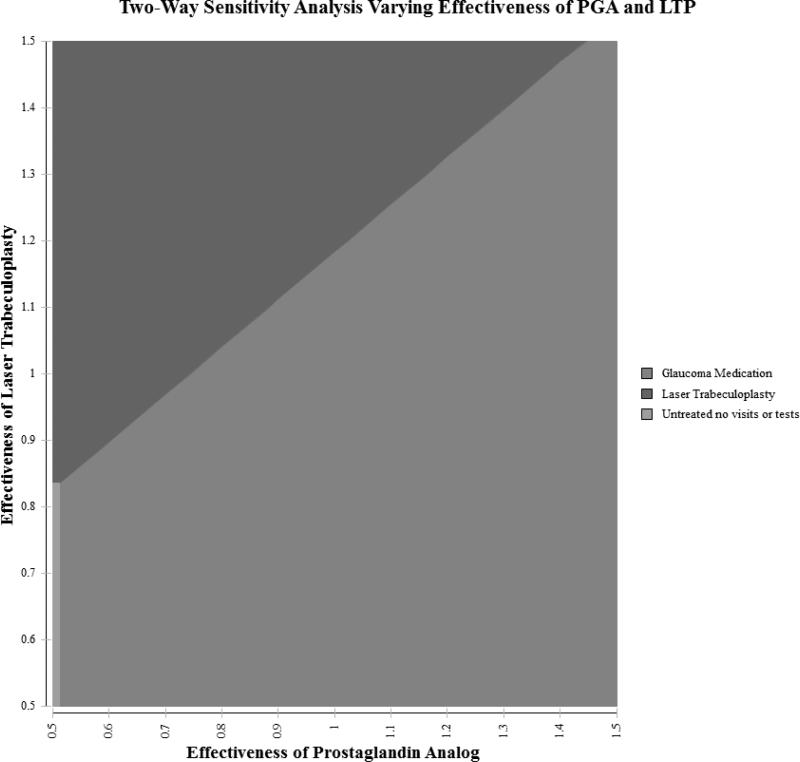
Figure 4 shows a two-way sensitivity analysis showing which treatment option would be preferred when simultaneously varying the effectiveness of both the LTP and PGAs relative to each other. Since prior studies have shown that many patients with OAG struggle with adherence to medication regimens and poorly adherent patients are more likely to experience disease progression, we examined lower effectiveness of PGAs. If PGAs are actually 25% less effective than the effectiveness reflected in the clinical trials due to poor adherence, than LTP could be the preferred option. Likewise, if PGAs were as effective as in clinical trials, but LTP were 20% or more effective than shown in the GLT trial, then LTP could be the preferred option. If PGAs were 50% less effective due to poor adherence and LTP is 17% less effective than estimates from the GLT, observation only could actually confer greater value.
Figure 4. Two-way Sensitivity Analysis Simultaneously Varying Effectiveness of Medication and Laser in Base Model.
Figure shows the impact of simultaneously varying the effectiveness of PGAs and LTP on the results of the model. Each axis captures the relative effectiveness of each intervention at delaying glaucoma progression. A value of 1.0 means the base case effectiveness as outlined in the paper and higher values represent higher effectiveness and lower values represent lower effectiveness. For example, a value of 0.75 means a 25% reduction in effectiveness. Each colored region represents the preferred intervention assuming a willingness to pay of $100,000 per quality-adjusted life year.
Other model inputs were varied in sensitivity analysis to determine whether they may affect the incremental cost effectiveness of LTP versus treatment with PGAs (Table 3). Since some studies have reported that there is little impact of advanced OAG on health-related quality of life,32 we reran the models assuming utility scores of 0.99 to 0.90 for mild to advanced OAG and this had little impact on the findings generated in the base model. Varying the time horizon had an impact on cost-effectiveness of these therapies. When we reran our Markov model over a time horizon of 9 years (the actual length of follow-up of the GLT) instead of the 25 years used in the base model we found that LTP over no treatment is $71893/QALY, but compared to LTP, the incremental cost effectiveness of PGAs is $158725/QALY. This is likely because the treatments incur high up-front costs, but the potential benefits of treating glaucoma may not be realized within 9 years given that glaucoma tends to be a slowly-progressing disease often taking more than 9 years to advance to a stage where it significantly reduces health-related quality of life. In addition, we were uncertain about the rates at which glaucoma progressed in individuals with and without treatment. In our base case, we had 10% of untreated and treated glaucoma patients worsen each year from moderate OAG to more advanced disease states. However, in sensitivity analysis, we looked to see whether the results would change if only 5% worsened each year (slow progression) and if 15% worsened each year (fast progression). In both cases, the incremental cost-effectiveness ratios of PGAs and LTP were both less than $50000 per QALY, and treatment with PGAs had a more favorable incremental cost-effectiveness ratio and provided better health-related quality of life when compared to LTP. Finally, varying the proportion of patients who develop side effects from LTP to 0% and the cost of managing side effects from LTP to $0 did not significantly impact the findings from the model.
Table 3.
Sensitivity Analyses
| Range | ICER Laser Trabeculoplasty | ICER Prostaglandin Analogues | |
|---|---|---|---|
| Effectiveness of PGAs | 0.5 | 16824/ QALY | (Dominated by LTP)^ |
| 0.75 | 16824/QALY | (Dominated by LTP)^ | |
| 1.0 | 16824* / QALY | 14179/ QALY | |
| 1.25 | 16824* / QALY | 9621/ QALY | |
| 1.5 | 16824* / QALY | 7266/ QALY | |
| Effectiveness of LTP | 0.5 | (Dominated by no treatment)^ | 14179 / QALY |
| 0.75 | 583432*/ QALY | 14179/ QALY | |
| 1.0 | 16824*/ QALY | 14179 / QALY | |
| 1.25 | 7750/ QALY | (Dominated by LTP)^ | |
| 1.5 | 4750/ QALY | (Dominated by LTP)^ | |
| Annual Cost of PGAs | $0 | 15928*/ QALY | 9665/ QALY |
| $250 | 16607*/ QALY | 13085/ QALY | |
| $500 | 17285*/ QALY | 16505/ QALY | |
| $750 | 17964*/ QALY | 22950/ QALY | |
| $1000 | 18643/ QALY | 30600/ QALY | |
| Time Horizon (years) | 9 | 71893/ QALY | 158725/ QALY |
| 20 | 20603*/ QALY | 19846/ QALY | |
| 25 | 16824*/ QALY | 14179/ QALY | |
| 30 | 15291*/ QALY | 11873/ QALY | |
| Age onset OAG | 40 | 14872*/ QALY | 12037/ QALY |
| 50 | 10513*/ QALY | 12587/ QALY | |
| 60 | 9853*/ QALY | 14179/ QALY | |
| 70 | 21547*/ QALY | 19536/ QALY | |
| 80 | 38383/ QALY | 40257/ QALY | |
| Utility for Different Severities of OAG | 0.9 / 0.95 / 0.99† | 14376*/ QALY | 12334/ QALY |
| Annual Rate of OAG Progression | 5% | 36839*/ QALY | 33720/ QALY |
| 10% | 16824*/ QALY | 14179 / QALY | |
| 15% | 10710*/ QALY | 8969 / QALY | |
| Cost of side effects from LTP | $0 | 16748*/ QALY | 14179/ QALY |
| $800 | 16809*/ QALY | 14179/ QALY | |
| $1600 | 16870*/ QALY | 14179/ QALY | |
| $2000 | 16900*/ QALY | 14179/ QALY | |
| Utility of side effects from LTP | 0.50 | 17149*/ QALY | 14179/ QALY |
| 0.79 | 16768*/ QALY | 14179/ QALY | |
| 0.89 | 16645*/ QALY | 14179/ QALY | |
| 0.99 | 16523*/ QALY | 14179/ QALY | |
| Proportion of LTP patients | 0% | 16535/QALY | 14179/QALY |
| who develop side effects | 2.5% | 16824/QALY | 14179 /QALY |
LTP strategy is dominated by PGA by extended dominance (lower QALYs at a higher $/QALY)
Utilities for different severities of glaucoma: 0.9 for severe OAG, 0.95 for moderate OAG, 0.99 for mild OAG
A strategy is considered “dominated” if it is more costly and less effective than the alternative Gray shading indicates the more cost-effective treatment option
OAG = open-angle glaucoma; LTP = laser trabeculoplasty; QALY = quality-adjusted life year; PGA = prostaglandin analogues; ICER = incremental cost effectiveness ratio
Discussion
In the 1980s, David Eddy published a troubling report questioning the value of treating patients with glaucoma. In his report he summarized the existing literature which he found demonstrated little evidence that lowering IOP had any impact on glaucoma progression.33 Eddy's report served as an impetus to the ophthalmological community to perform well-designed studies to assess the effectiveness of interventions for glaucoma. Over the past 25 years, there have been several large randomized clinical trials including OHTS, EMGT, CIGTS, and AGIS that demonstrated that patients with all different severities of glaucoma from patients with ocular hypertension to those with advanced glaucoma benefit from lowering eye pressure.3-6 While these trials have provided strong evidence supporting the effectiveness of lowering eye pressure to prevent glaucoma progression, the next question for policy makers and payers is whether these interventions are cost effective. A study by Rein and colleagues assessed the incremental cost effectiveness of screening for, identifying, and, treating patients with primary open-angle glaucoma.34 They found that glaucoma treatment was highly cost-effective when assuming optimistic treatment efficacy ($28,000/QALY) and in line with other health interventions when incorporating the costs of diagnostic testing and assuming conservative treatment efficacy ($46,000/QALY).
The focus of the present analysis was to perform a head-to-head comparison of the cost effectiveness of the most commonly prescribed topical medication class, PGAs versus LTP in the treatment of patients with newly-diagnosed OAG. When using data generated from the GLT, under the assumption that PGAs are 30% more effective than the medications that were available when the GLT was conducted and that patients have excellent adherence to these agents, we find treatment with PGAs to be more cost-effective and to provide better health-related quality of life than LTP. However, assuming more realistic patterns of medication adherence, LTP could be a preferred treatment. If PGAs were 25% less effective or LTP were 20% more effective, then LTP could provide better value for health benefits than PGAs.
Problems with glaucoma medication adherence have been well-documented in the literature.35,36 For PGAs to be effective, not only must the patient acquire the medications, but also remember to take them as prescribed, and properly administer the drops into their eyes.37,38 Each of those steps is critical for these medications to be effective at reducing intraocular pressure which can, in turn, help prevent glaucomatous progression. Studies haves shown that poor medication adherence is associated with worsening glaucoma.35,37,39,40
There are several study limitations that need to be acknowledged. These limitations can broadly be grouped into concerns about parameter values used in the model and limitations in how we accounted for LTP use. While we were able to obtain reasonable estimates of costs, utilities, and transition state probabilities for many of the variables included in the model from the literature, information on several parameters needed to be estimated since high-quality evidence is not available for all inputs. For all these assumptions, we were able to use sensitivity analyses to examine the impact of changing the assumptions on the findings.(Table 3) The GLT for which we relied upon to determine the likelihood of experiencing disease progression for the PGA and LTP groups only provided data for the first 9 years following treatment with one of these interventions. Since, on average, newly diagnosed 60 year old open-angle glaucoma patients live approximately 25 years, in the models we needed to extrapolate the findings from the GLT for the remaining years. In the models we assumed constant and equal progression rates from years 9 to 25 for those treated with medications or LTP. It is certainly possible that progression rates differ from years 9 to 25 among the groups. In sensitivity analysis, when we restrict our analyses to determining the incremental cost effectiveness of PGAs versus LTP for only the first 9 years we find that LTP may confer greater value. It is also unknown whether patients linearly progress from one severity level to another or whether rates of progression from mild to moderate disease occur more rapidly than from severe glaucoma to blindness. In our models we used findings from GLT and EMGT to determine progression from mild to moderate glaucoma for the 3 groups. Then we assumed a 10% yearly rate of progression to more severe disease states. These assumptions appeared to match blindness outcomes observed in longer-term studies and we have found that small deviations from these progression rates do not affect the conclusions of the study. However, if these assumptions were further off from actual disease progression, this may affect the study results. More research is needed to understand longer-term glaucoma progression in untreated and treated patients.
Our study had limitations in how we accounted for LTP use (type of laser, frequency of treatment). The information on the effectiveness of medications and LTP were obtained from the GLT, a study that was conducted 23 years ago. At that time, the only LTP unit commercially available was ALT. Since then selective laser trabeculoplasty (SLT) became available. Several studies comparing ALT with SLT have reported these procedures to be equally as effective at lowering IOP, however SLT may have fewer risks of side effects.12 It would be helpful to repeat such a cost-effectiveness analysis using the findings from the ongoing SLT/Med clinical trial which have yet to be published. Additionally, in this analysis we did not consider the role of repeat LTP before proceeding with other interventions in the model.
Finally, it is important to exercise care when generalizing these study findings to patients with other forms of glaucoma, those residing outside of the United States, those who are uninsured, and those living in communities who have no access to LTP as the variables may differ considerably from those used in the model for these groups.
Implications and Future Research
Our findings highlight the importance of medication adherence in determining which intervention is most cost effective. Identifying strategies to improve medication adherence will not only improve patient outcomes but also improve the cost-effectiveness of treating glaucoma with PGAs or other medications. Furthermore, if researchers can develop novel means of administering glaucoma medications (ex: intraocular injections) that reduce the need for adherence, assuming these medications are not overly costly, this too can improve the cost effectiveness of caring for patients with OAG.
In conclusion, this study shows that generic PGAs and LTP can both be cost-effective options for managing patients with newly diagnosed mild OAG. Assuming optimal medication adherence, generic PGAs confer greater value compared to LTP. However, when assuming more realistic levels of medication adherence, at today's prices for PGAs, LTP may be a more cost effective alternative.
eFigure 1a.
Glaucoma Severity Over Time for Untreated Cohort
eFigure 1b.
Glaucoma Severityeverity Over Time for Cohort Treated with Prostaglandin Analogs
eFigure 1c.
Glaucoma Severity Over Time for Cohort Treated with Laser Trabeculoplasty
Acknowledgements
The authors wish to thank Steven Kymes, Ph.D. for reviewing an earlier draft of this manuscript and for providing thoughtful feedback and suggestions.
Grant support: National Eye Institute K23 Mentored Clinician Scientist Award (1K23EY019511-01); American Glaucoma Society Clinician Scientist Grant, Blue Cross Blue Shield of Michigan Foundation, and an unrestricted grant from Research to Prevent Blindness
Footnotes
The authors have no proprietary or commercial interest in any material discussed in this manuscript
References
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–3. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 4.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: The Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 5.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in The Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–53. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 6.The AGIS Investigators The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–40. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 7.Glaucoma Laser Trial Research Group The Glaucoma Laser Trial (GLT) and glaucoma laser trial follow-up study: 7. Results. Am J Ophthalmol. 1995;120:718–31. doi: 10.1016/s0002-9394(14)72725-4. [DOI] [PubMed] [Google Scholar]
- 8.Burr J, Azuara-Blanco A, Avenell A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database Syst Rev. 2005:CD004399. doi: 10.1002/14651858.CD004399.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Stein JD, Ayyagari P, Sloan FA, et al. Rates of glaucoma medication utilization among persons with primary open-angle glaucoma, 1992 to 2002. Ophthalmology. 2008;115:1315–9. doi: 10.1016/j.ophtha.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Azuara-Blanco A, Burr J. The rising cost of glaucoma drugs. Br J Ophthalmol. 2006;90:130–1. doi: 10.1136/bjo.2005.079996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai JC. Medication adherence in glaucoma: approaches for optimizing patient compliance. CurrOpinOphthalmol. 2006;17:190–5. doi: 10.1097/01.icu.0000193078.47616.aa. [DOI] [PubMed] [Google Scholar]
- 12.Stein JD, Challa P. Mechanisms of action and efficacy of argon laser trabeculoplasty and selective laser trabeculoplasty. Curr Opin Ophthalmol. 2007;18:140–5. doi: 10.1097/ICU.0b013e328086aebf. [DOI] [PubMed] [Google Scholar]
- 13.Melamed S, Ben Simon GJ, Levkovitch-Verbin H. Selective laser trabeculoplasty as primary treatment for open-angle glaucoma: a prospective, nonrandomized pilot study. Arch Ophthalmol. 2003;121:957–60. doi: 10.1001/archopht.121.7.957. [DOI] [PubMed] [Google Scholar]
- 14.Truffer CJ, Keehan S, Smith S, et al. Health spending projections through 2019: the recession's impact continues. Health Aff (Millwood) 2010;29:522–9. doi: 10.1377/hlthaff.2009.1074. [DOI] [PubMed] [Google Scholar]
- 15.Deva NC, Insull E, Gamble G, et al. Risk factors for first presentation of glaucoma with significant visual field loss. Clin Experiment Ophthalmol. 2008;36:217–21. doi: 10.1111/j.1442-9071.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 16.Briggs AH, Claxton K, Sculpher MJ. Decision modelling for health economic evaluation. University Press; Oxford: 2006. [Google Scholar]
- 17.Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. Oxford University Press; New York: 2005. [Google Scholar]
- 18.Muennig P. Cost-Effectiveness Analysis in Health: a practical approach. 2nd ed. Jossey-Bass; San Francisco: 2008. [Google Scholar]
- 19.Arias E. United States life tables, 2004. Natl Vital Stat Rep. 2007;56:1–39. [PubMed] [Google Scholar]
- 20.Arias E. United States life tables, 2006. Natl Vital Stat Rep. 2010;58:1–40. [PubMed] [Google Scholar]
- 21.Hodapp E, Parrish RK, II, Anderson DR. Clinical decisions in glaucoma. C.V. Mosby; St. Louis: 1993. pp. 84–125. [Google Scholar]
- 22.Alm A, Camras CB, Watson PG. Phase III latanoprost studies in Scandinavia, the United Kingdom and the United States. Surv Ophthalmology. 1997;41:S105–10. doi: 10.1016/s0039-6257(97)80016-1. [DOI] [PubMed] [Google Scholar]
- 23.Wilson MR, Kosoko O, Cowan CL, Jr, et al. Progression of visual field loss in untreated glaucoma patients and glaucoma suspects in St. Lucia, West Indies. Am J Ophthalmol. 2002;134:399–405. doi: 10.1016/s0002-9394(02)01585-4. [DOI] [PubMed] [Google Scholar]
- 24.Chen PP. Blindness in patients with treated open-angle glaucoma. Ophthalmology. 2003;110:726–33. doi: 10.1016/S0161-6420(02)01974-7. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Department of Health and Human Services [November 14, 2010];Average Medicare Fee Schedule. 2010 Available at https://www.cms.gov/PhysicianFeeSched/.
- 26.Engel K, editor. Red Book. Thomson Healthcare; Montvale, NJ: 2008. [Google Scholar]
- 27.Drugstore.com [April 16, 2011];Latanoprost. Available at: http://www.drugstore.com/latanoprost/xalatan/2-5ml-bottle-0-005-solution/qxn61314054701.
- 28.SuperSaverMeds.com [April 16, 2011];Xalatan. Available at: http://www.supersavermeds.com/xalatan/
- 29.Lee PP, Walt JG, Doyle JJ, et al. A multicenter, retrospective pilot study of resource use and costs associated with severity of disease in glaucoma. Arch Ophthalmol. 2006;124:12–9. doi: 10.1001/archopht.124.1.12. [DOI] [PubMed] [Google Scholar]
- 30.Lee BS, Kymes SM, Nease RF, Jr, et al. The impact of anchor point on utilities for5 common ophthalmic diseases. Ophthalmology. 2008;115:898–903. doi: 10.1016/j.ophtha.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Brown MM, Brown GC, Sharma S, et al. Utility values associated with blindness in an adult population. Br J Ophthalmol. 2001;85:327–31. doi: 10.1136/bjo.85.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jampel HD, Schwartz A, Pollack I, et al. Glaucoma patients’ assessment of their visual function and quality of life. J Glaucoma. 2002;11:154–63. doi: 10.1097/00061198-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Eddy DM, Billings J. The quality of medical evidence: implications for quality of care. Health Aff (Millwood) 1988;7:19–32. doi: 10.1377/hlthaff.7.1.19. [DOI] [PubMed] [Google Scholar]
- 34.Rein DB, Wittenborn JS, Lee PP, et al. The cost-effectiveness of routine office-based identification and subsequent medical treatment of primary open-angle glaucoma in the United States. Ophthalmology. 2009;116:823–32. doi: 10.1016/j.ophtha.2008.12.056. [DOI] [PubMed] [Google Scholar]
- 35.Friedman DS, Okeke CO, Jampel HD, et al. Risk factors for poor adherence to eyedrops in electronically monitored patients with glaucoma. Ophthalmology. 2009;116:1097–105. doi: 10.1016/j.ophtha.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS). Invest Ophthalmol Vis Sci. 2007;48:5052–7. doi: 10.1167/iovs.07-0290. [DOI] [PubMed] [Google Scholar]
- 37.Sleath B, Blalock S, Covert D, et al. The Relationship between Glaucoma Medication Adherence, Eye Drop Technique, and Visual Field Defect Severity. Opthalmology. 2011 doi: 10.1016/j.ophtha.2011.05.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sleath B, Blalock SJ, Robin A, et al. Development of an instrument to measure glaucoma medication self-efficacy and outcome expectations. Eye (Lond) 2010;24:624–31. doi: 10.1038/eye.2009.174. [DOI] [PubMed] [Google Scholar]
- 39.Konstas AG, Maskaleris G, Gratsonidis S, Sardelli C. Compliance and viewpoint of glaucoma patients in Greece. Eye (Lond) 2000;14:752–6. doi: 10.1038/eye.2000.197. [DOI] [PubMed] [Google Scholar]
- 40.Rossi GC, Pasinetti GM, Scudeller L, et al. Do adherence rates and glaucomatous visual field progression correlate? Eur J Ophthalmol. 2010 Dec 3; doi: 10.5301/EJO.2010.6112. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]