Abstract
Interleukin 30 (IL30), the p28 subunit of IL27, interacts with Epstein-Barr virus induced gene 3 to form IL27, which modulates both pro- and anti-inflammatory responses during autoimmune or infectious disease. It also acts as a natural antagonist of glycoprotein 130 (gp130), thereby attenuating the signals of other gp130-associated cytokines. IL30 regulation via LPS has been reported by others, but the intercellular communication that induces IL30 expression is unknown. Here, we show that treatment with anti-CD3/CD28 antibodies plus CpG oligodeoxynucleotides induces robust expression of IL30, whereas either treatment alone induces only low expression of IL30. This observation in vitro mirrors murine model in which administration of CpG under inflammatory conditions in vivo induces IL30 expression. This robust induction of IL30 occurs through the coordination of helper CD4+ T cells and innate immune cells (such as macrophages) and, to a lesser degree, B cells via the CD40/CD154 signaling pathway. These findings reveal a previously unrecognized mechanism that integrates signaling pathways from T cells and macrophages at the cellular level to induce IL30 expression.
Introduction
The p28 subunit of interleukin 27 (IL27) can either be bound to Epstein-Barr virus induced gene 3 (EBI3) to form IL27 or can act independently (1–3). When acting alone, the p28 subunit is known as IL30 and acts as a natural antagonist of glycoprotein 130 (gp130) signaling (1–3). Consequently, the functions of IL30 are complex. On one hand, it has both pro- and anti-inflammatory properties as a subunit of IL27, and on the other hand, IL30 binds to gp130 and inhibits the signaling of many cytokines such as IL6, IL11, IL27, oncostatin M, leukemia inhibitory factor, cardiotrophin, cardiotrophin like cytokine, and ciliary neurotrophic factor (2, 4). Despite the importance of IL27 in regulating the host response to foreign and endogenous substances and its presence at the crossroads of potent signaling pathways such as IL6, gp130, and IL27, little is known about IL30 regulation.
Two key studies have laid the foundations for understanding the regulation of IL30 expression. Liu et al. showed that lipopolysaccharide (LPS) and interferon (IFN) γ treatment can induce expression of IL30 via cRel binding to a distal NF-κB site and interferon regulatory factor (IRF) 1 binding to a proximal interferon-stimulated response element site on IL30’s promoter in macrophages (5). This process was dependent on the MyD88 pathway. Molle et al. showed that IRF3 plays a critical role in inducing IL30 gene expression by the toll-like receptor (TLR) 4-TRIF-mediated pathway in dendritic cells (DC) (6).
Despite the in-depth understanding of IL30 regulation at a molecular level, little information is known about IL30 induction at the cellular level (5, 6). It is important to understand how this cytokine is upregulated in the context a real biological system in which various immune cells and cytokines affect such induction. To better mimic an in vivo scenario and take into consideration the interplay between various types of immune cells and the cytokine milieu present in organisms, splenocytes and mixtures of different types of immune cells (rather than macrophages alone) were used in this study. Likewise, various activation signals from different cell types were used either alone or in concert to examine the range of factors associated with IL30 induction. We reveal that simultaneous activation of two types of cells, CD4+ T cells and macrophages (and to a lesser degree B cells), in the same microenvironment is crucial in inducing the robust expression of IL30. This IL30 induction occurs via the CD40/CD154 signal pathway.
Materials and Methods
Reagents
Vendors for all reagents were as follows: thiol-modified CpG oligodeoxynucleotide (ODN) 1668 or control ODN (Sigma), anti-mouse CD3 (eBioscience), anti-mouse CD28 (Biolegend), anti-OX86 (Andrew Weinberg, Robert W. Franz Cancer Research Center, Earle A. Chiles Research Institute, Providence Cancer Center, Providence Portland Medical Center), anti-mouse 4-1BB (Shu-Hsia Chen, Mount Sinai Medical Center), activating anti-CD40 (Novus, NBP1-06657), anti-mouse TRL9-Pe (Imgenex) recombinant mouse IL12 and IFNγ (R&D systems), LPS (Sigma), lipoteichoic acid (Invivogen), poly I:C (Invivogen), concanamycin A (Sigma), and rat IgG (eBioscience).
Cell separation and coincubations
Splenocytes were prepared as previously described (7). Purification of DC, B cells, natural killer (NK) cells, and CD4+ T cells from splenocytes was performed using magnetic beads according to the manufacturer’s instructions (StemSep). Peritoneal exudate macrophages were obtained three days after intraperitoneal injection of 3% sodium thioglycolate medium (2mL per mouse, Sigma). Cells were seeded into 24 well plates, and after 3 hours, the cells were washed and fresh RPMI medium was added. 5×105 splenocytes were seeded in 0.75 ml of heat-inactivated RPMI media, activated with CD3 (2.0 µg/mL) and CD28 (0.5 µg/mL) (CD3/CD28) in the presence or absence of CpG ODN 1668 (1 µg/mL) (CPG) for 72 hours, and the IL30 levels in the supernatant were measured via IL27p28 and EBI3 ELISA) (R&D Systems and USCN Life Science Inc., respectively). When appropriate, splenocytes were treated with anti-CD40 (10 µg/mL), LPS (1 µg/mL on day 0 and 2), Poly I:C (50 µg/mL), lipoteichoic acid (LTA, 5 µg/mL), rIL12 (50 ng/mL), IFNγ (50 ng/mL), OX86 (2.0 µg/mL), or 4-1BB (2.0 µg/mL). Splenocytes depleted of various cell subsets were seeded as mentioned above. For the coincubation assay of CD4 T cells, B cells, DC, whole T cells (CD3+), NK cells, and macrophages, 2×105 cells of each type were seeded in 300 µL of heat-inactivated RPMI for 72 hours.
Mice
MyD88−/− splenocytes were harvested from mice generously provided by Dr. Samithamby Jeyaseelan (Louisiana State University). CD40−/−, CD154−/− and IFNγR1−/− mice were obtained from Jackson Laboratories. C57Bl/6, nude, and SCID mice where purchased from Harlan Laboratories. All experiments were performed using 6–8 week old mice. C7Bl/6 mice were immunized via injections into each foot pad with 50 ug of OVA (duck egg, MP Biomedicals) in 12.5 µL saline emulsified 1:1 in CFA containing 1.0 mg/mL of Mycobacterium tuberculosis (H37RA, heat killed and dried, Sigma). 24 hours after immunization, 10 µg of CpG or control nucleotides (GpC) ODNs were administered via IP injections into mice. Serum was collected 72 hours after immunization and was analyzed for IL30 production via ELISA. All animals procedures were approved by IACUC.
Flow Cytometry analysis
Splenocytes were treated as indicated in the figure, and at 48 or 72 hours post-incubation, TLR9 expression was analyzed via flow cytometry. Briefly, cells washed in PBS and permeabilized for 45’ at 4°C, were blocked for FcRs via incubation with anti-CD14 (5 µg/mL) for 20’ at 4°C. After blocking, cells were stained with anti-TLR9-Pe antibody for 30’ at 4°C. Cells were washed and analyzed by flow cytometry using Attune (Invitrogen).
Results
Two studies laid the ground work to understand and characterize the induction of IL30 at the molecular level in macrophages (5, 6). However, those studies were based on a single type of immune cell, and the role of cell-to-cell communication or interaction in the induction of IL30 was not reported, though intracellular interactions occur in every tissue and biological system. We used splenocytes as a first step toward understanding IL30 induction in biological systems similar to those found in vivo.
To test the coordination of different types of immune cells in inducing IL30, two signals that stimulate different cell types were independently or simultaneously applied to the cell mixtures. One set of stimulation signals was CD3/CD28, which mimics the first and second signals that activate T cells. The other stimulation signal was CpG, which activates cells via TLR9 receptor, which is present on many cells but primarily on antigen presenting cells (APCs) such as macrophages, DC, and B cells. To distinguish between IL27 and free IL30, we used the IL27p28 ELISA kit from R&D Systems, which specifically binds IL30 while showing only 7% cross-reactivity to recombinant IL27 (according to the manufacturer’s data sheet).
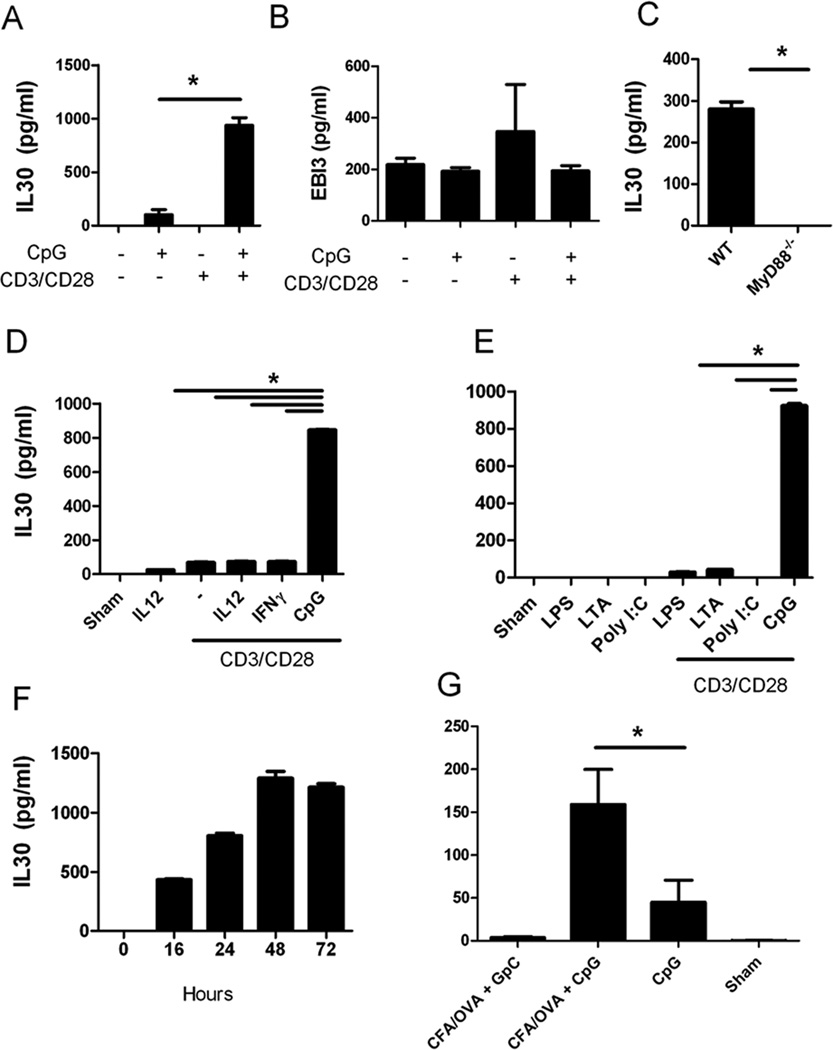
Treatment of cells with the combination of two types of cell signals (CD3/CD28/CpG) induced synergistic expression of IL30, whereas treatment of cells with either CD3/CD28 or CpG alone induced a relatively small increase in IL30 production (Fig. 1A). The effects of CD3/CD28/CpG were at least 10-fold higher compared to either individual stimulation signal, whereas treatment with CD3/CD28 and control isogenic CpG (in which CpG was switched to GpC) was defective in inducing IL30 expression (Sup. Fig.1). Others have also confirmed that a CpG signal alone is not effective in inducing IL30 expression in monocyte-derived DC (8). To detect if the combination treatment affects either both subunits of IL27 (IL30 and EBI3) or IL30 alone, the authors measured the levels of EBI3 protein via ELISA. Interestingly, the combination treatment induced IL30 specifically, as EBI3 levels by CD3/CD28/CpG treatment were not altered when compared to either treatment alone (Fig. 1B).
Figure 1. Coadministration of CD3/CD28 and CpG induce robust amounts of IL30 expression in splenocytes when compared to either signal alone.
A, B, Splenocytes were treated with CpG, CD3/CD28, CD3/CD28/CpG, or left untreated for 72 hours and analyzed for IL30 and EBI3 production by ELISA. C, Supernatants from wild type or MyD88−/− splenocytes treated with CD3/CD28/CpG for 72 hours were analyzed for IL30 production by ELISA. D–E, Supernatants from splenocytes treated as indicated for 72 hours were analyzed for IL30 production by ELISA. F, Supernatants from wild type splenocytes treated with CD3/CD28/CpG were collected at various time points and analyzed for IL30 production by ELISA. G, Non-immunized mice or mice were immunized with OVA/CFA were treated with CpG or GpC via IP. Serum was collected 48 hours post CpG treatment, and IL30 was quantified in the serum. N=5. *, P <0.05. N=3; data are representative of at least two independent experiments.
It is well established that the TLR9 ligand CpG can activate downstream genes through MyD88-dependent pathways. Thus, to examine the role of MyD88 in CD3/CD28- and CpG-induced IL30 expression, splenocytes from wild type and MyD88−/− mice were treated with CD3/CD28/CpG for 72 hours, and IL30 expression was measured using ELISA. IL30 protein expression induced by CD3/CD28/CpG was completely abolished in the absence of MyD88 (Fig. 1C).
Others have shown that both IFNγ and LPS can induce IL30 in macrophages (5). We were interested in comparing the effects of CD3/CD28/CpG with those of other known tertiary signals (such as IFNγ and the IFNγ-inducing cytokine IL12). The combination of two signals (CpG plus CD3/CD28) is much more effective than any of the other signal combinations, including the known IL30 inducer LPS (Fig. 1D). Therefore, these data suggest that activation of both T cells and TLR9-positive cells are needed to synergistically induce IL30 expression.
To determine whether this effect is TLR9-specific or other TLRs can induce similar effects, various TLR ligands were used in the presence of CD3/CD28 antibodies. Fig 1E shows that the enhanced expression of IL30 in coordination with T cell activation is specific only to TLR9 as stimulation with other TLR ligands did not induce expression of IL30. Kinetic studies further elucidated the mechanism of this TLR9-dependent activation and showed that maximal induction of IL30 via CD3/CD28/CpG occurs 48 to 72 hours post-treatment (Fig. 1F). On the other hand, LPS induction of IL30 occurs during the initial 24 hours (in agreement with Liu et al.’s observation), and this induction is reduced over time (Supplemental Fig. 2). Importantly, the IL30 induction by the TLR9 signal pathways is more robust (350 to about 1000 pg/mL) while LPS induces only up to 100 pg/mL.
A relevant question to address is whether this TLR9 and T cell stimulation-coordinated induction of IL30 occurs in vivo. Thus, mice were immunized with OVA/CFA in the hind foot pad, and these mice were treated with either CpG or control nucleotides (GpC). Indeed, mice immunized with OVA/CFA plus CpG produced high IL30 expression in the serum (Fig. 1G). Meanwhile, IL30 was not expressed at detectable levels in mice immunized with OVA/CFA plus control nucleotides (Fig. 1G). Although administration of CpG in the absence of immunization induced small amounts of IL30 in vivo, the levels induced were 3 folds lower than those from the administration of CpG plus T cell stimulation. Indeed, the in vivo data mirrors the in vitro data, supporting the observation that TLR9 engagement via CpG in the presence of activated T cells induces high levels of IL30.
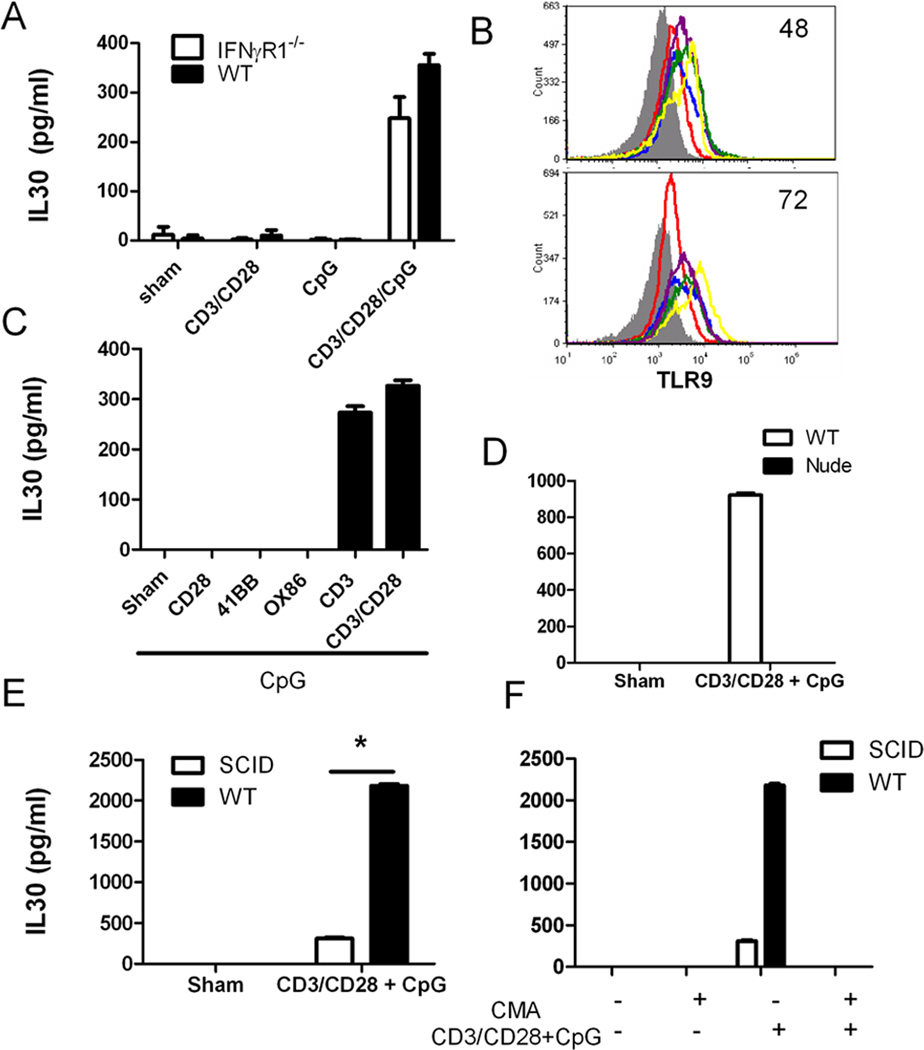
Finding the mechanism responsible for IL30 induction by CD3/CD28/CpG is important. In concert with others, we have previously discovered that IFNγ is a strong inducer of IL30 (5, 9), which led to the hypothesis that IFNγ is the mediator that effectively induces IL30 from this combination treatment. However, the absence of IFNγ signaling reduces this upregulation only by 30%, suggesting that other mechanisms also contribute significantly to the induction of Il30 (Fig. 2A). Since the peak stimulation of IL30 did not occur until 48–72 hours after administration, another possibility was that the co-stimulation treatment increases the levels of TLR9 which promotes the IL30 induction. However, we found that CpG alone is a better inducer of the TLR9 receptor than CD3/CD28/CpG treatment at 72 hours. Therefore, it is unlikely that the combination treatment induces IL30 via upregulation of TLR9 expression (Fig. 2B).
Figure 2. T cells play a crucial role in IL30 induction independently of IFNγ.
A, Supernatants from wild type or IFNγR1−/− splenocytes treated with CpG in absence or presence of CD3/CD28 for 72 hours were analyzed for IL30 production by ELISA. B, Splenocytes were left untreated (red), or were treated with CD3/CD28 (blue), CpG (yellow), CD3/CD28/CpG (purple), or CD3/CD28/CpG/CD40 for 48 or 72 hours, and analyzed for TLR9 expression via flow cytometry (shaded, isotype control). C, Splenocytes were treated with CpG in the presence or absence of various T cell-activating antibodies (CD28, 41BB, OX86, CD3, or CD3/CD28) for 72 hours, and IL30 expression in the supernatants was measured via ELISA. Supernatants from wild type and nude (D, Balb/c background) or wild type and SCID (E, C3H background) splenocytes were treated with CD3/CD28/CpG for 72 hours and then analyzed for IL30 production by ELISA. F, Supernatants from wild type and SCID splenocytes treated with concanamycin A alone (CMA), CD3/CD28/CpG, or a combination of concanamycin A and CD3/CD28/CpG for 72 hours were analyzed for IL30 production by ELISA. *, P <0.05. N=3.
The data above showed that either CD3/CD28 or CpG alone induces a very small amount of IL30 expression, yet the combination of these two treatments synergistically induces much higher IL30 expression. This result suggests that simultaneous activation of both T cells and TLR9+ cell signaling is crucial for inducing robust IL30 expression. To determine whether the T cell co-stimulation signal (CD28 stimulation) or the primary stimulation signal alone (CD3 stimulation) plus CpG is sufficient to induce robust IL30 expression, splenocytes were activated in the presence of CpG with various T cell activators, such as 4-1BB, OX86, CD28, CD3, or mixed CD3/CD28 agonist antibodies. Only in the presence of a primary T cell activation signal, anti-CD3 antibodies, can CpG induce a high level of IL30 production (Fig. 2A).
Immunocompromised nude mice were used to further confirm the role of T cells in high levels of IL30 induction. As predicted, the absence of functional T cells eliminated the CD3/CD28/CpG-mediated induction of IL30 (Fig. 2B). Similar results were also seen in immunodeficient SCID mice (Fig. 2C). These data suggest that T cell activation via CD3 is essential for maximal induction of IL30. To further confirm the crucial role of T cells in inducing IL30, the presence of concanamycin A, a known inhibitor of T cells (10), completely inhibited the CD3/CD28/CpG-induced IL30 induction (Fig. 2D).
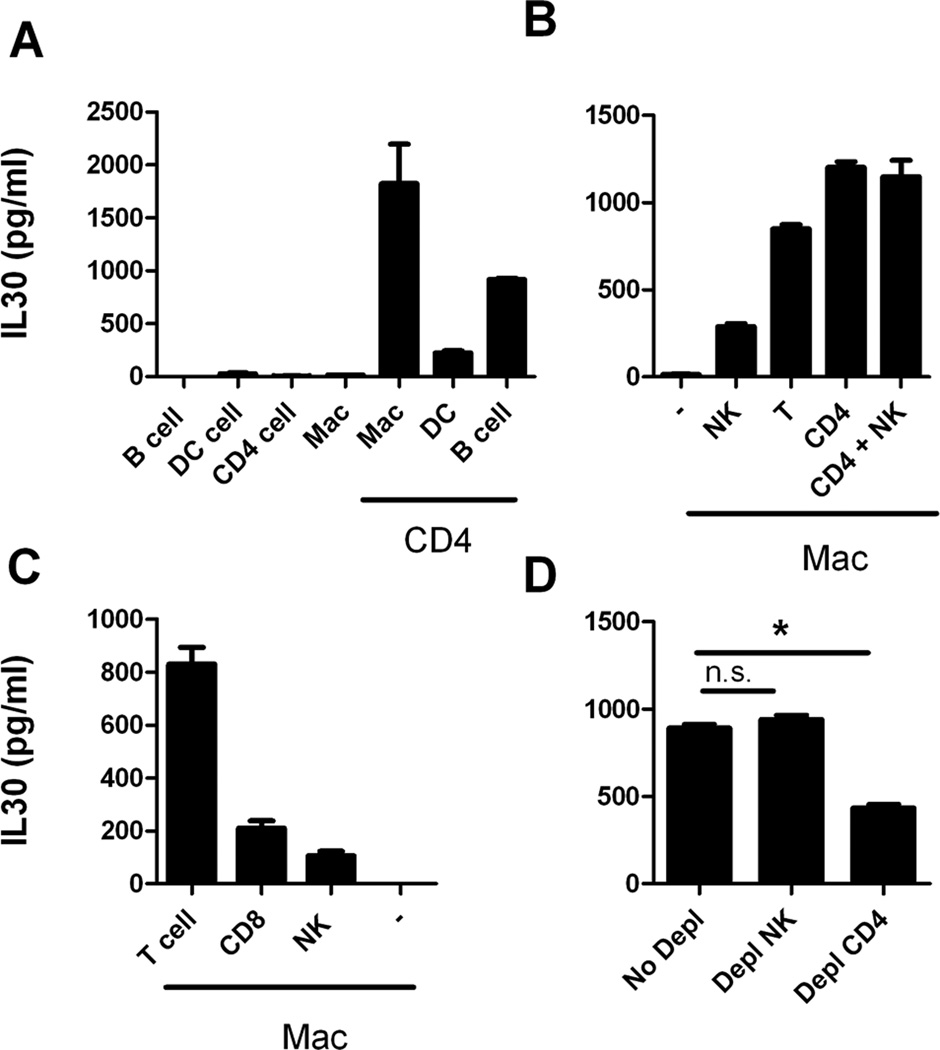
T cells are not an IL30-expressing cell type and have very low levels of TLR9 expression, which suggests that another cell type with effective TLR9-mediated activation is needed to coordinate with T cells to produce high levels of IL30. To understand the primary cell type in which TLR9 signaling was activated, we performed a series of cell mixture studies. Since APCs are normally enriched for TLR9 expression, the purified CD4+ T cells were co-incubated with various types of APCs, such as B cells, macrophages, and DC and the resulting supernatants were tested for IL30 expression.
We found that a mixture of macrophages and CD4+ T cells yields a high level of IL30 expression upon exposure to CD3/CD28/CpG (Fig. 3A). We also found that B cells and, to a lesser extent, DC play a role in IL30 induction. These results clearly indicate that interaction between CD4+ T cells and APCs are needed for inducing a high level of IL30 expression in the presence of the combination signals. Since macrophages induce the highest expression of IL30 (1700 pg/mL in macrophages compared to 1000 pg/mL in B cells), these cells were used in further studies. To confirm that CD4+ T cells and macrophages interact to induce high expression levels of IL30, macrophages were reconstituted with CD4+ T cells, CD4+ T and NK cells, or whole T cells (CD3+ cells). As expected, reconstitution of macrophages with CD4+ T cells induces the highest level of IL30 induction following CD3/CD28/CpG treatment (Fig. 3B). To determine whether CD8+ T cells also play an important role, purified CD8+ T cells, CD3+ T cells, or NK cells were coincubated with macrophages, the same combination treatment (CD3/CD28/CpG) was applied, and the levels of IL30 induction were compared (Fig. 3C). While both NK and CD8+ T cells boosted the level of CD3/CD28/CpG-induced IL30 expression from macrophages, the overall expression levels were much lower when compared to those of CD4+ T cells, suggesting that CD4+ T cells play a crucial role in IL30 biology.
Figure 3. Coordination between T cells and macrophages induces the highest expression of IL30.
A, Purified splenic B cells, DC, CD4+ T cells, or peritoneal macrophages were coincubated in the presence or absence of purified CD4+ T cells, treated with CD3/CD28/CpG for 72 hours and analyzed for IL30 expression in the supernatants by ELISA. B, Peritoneal macrophages were coincubated in the presence or absence of purified NK, whole T (CD3+ T), CD4+ T, or a combination of CD4+ T and NK cells; treated with CD3/CD28/CpG for 72 hours; and analyzed for IL30 expression in the supernatants via ELISA. C, Peritoneal macrophages were coincubated in the presence or absence of purified NK, T (CD3+ T), or CD8+ T cells; treated as in B; and analyzed for IL30 expression in the supernatant via ELISA. D, Splenocytes depleted of NK or CD4+ T cells were treated with CD3/CD28/CpG for 72 hours, and the supernatants were analyzed for IL30 expression via ELISA. *, P <0.05. N=3; data representative of at least two independent experiments.
The role of CD4+ T cells in boosting IL30 expression was further confirmed by cell depletion studies. Depletion of CD4+ T cells from splenocytes reduced IL30 expression the most, suggesting that this cell type plays an important role in upregulating IL30 expression (Fig. 3D). Depletion of NK cells did not have any effect. This suggests that CD4+ T cells are not the only cell type responsible for inducing IL30, and in absence of CD4+ T cells, other types or subtypes of cells (such as CD8+ T cells) may aid in the regulation of IL30.
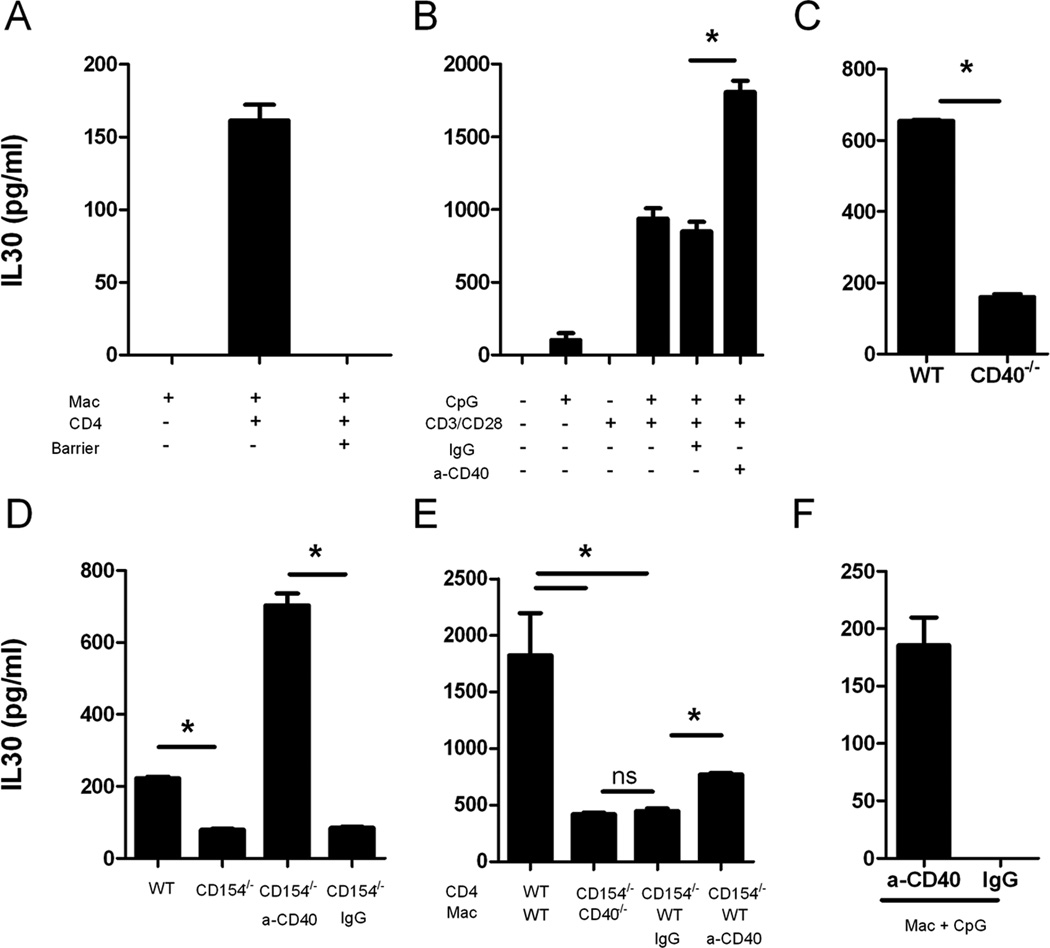
T cells can activate macrophages via cell contact (11). To determine whether macrophages and T cells need physical contact to induce IL30, macrophages and CD4+ T cells were coincubated in the presence or absence of a transwell barrier, treated with CD3/CD28/CpG, and analyzed for IL30 protein expression. In the presence of a barrier, CD3/CD28/CpG failed to induce any IL30 expression, suggesting that the expression of IL30 in macrophages requires direct cell-to-cell contact with CD4+ T cells (Fig. 4A).
Figure 4. The interaction between CD4+ T cell and macrophages induces IL30 through the CD40/CD154 signaling pathway.
A, Peritoneal macrophages were coincubated with purified CD4+ cells in the presence or absence of a transwell barrier, treated with CD3/CD28/CpG for 72 hours, and analyzed for IL30 expression in the supernatant via ELISA. B, Supernatants from splenocytes treated with CpG, CD3/CD28, CD3/CD28/CpG (combination treatment), or CD3/CD28/CpG in the presence of activating anti-CD40 for 72 hours were analyzed for IL30 production by ELISA. C, Supernatants from wild type or CD40−/− splenocytes treated with CD3/CD28/CpG for 72 hours were analyzed for IL30 production by ELISA. D, Splenocytes from wild type or CD154−/− mice were treated with CD3/CD28/CpG or CD154−/− splenocytes were treated with CD3/CD28/CpG in the presence of control or anti-CD40 antibodies for 72 hours, and IL30 expression was measured in the supernatants using ELISA. E, Peritoneal macrophages from wild type or CD40−/− mice were coincubated with purified CD4+ T cells from either wild type or CD154−/− mice, treated with CD3/CD28/CpG in the presence of control or anti-CD40 antibodies, and the supernatants were measured for IL30 expression by ELISA. F, Peritoneal macrophages were treated with CpG in the presence of control or anti-CD40 antibodies for 72 hours and analyzed for IL30 expression. *, P <0.05. N=3.
CD154 is one of the ligands on the T cell membrane that can bind to and activate the CD40 receptor on macrophages (12, 13). To demonstrate whether CD40/CD154 interaction between APCs and T cells accounts for the induction of IL30, we compared IL30 levels induced by CD3/CD28/CpG in wild type, CD40−/−, and CD154−/− splenocytes in different combinations (Fig. 4C and 4D). Absence of either CD40 or CD154 reduced IL30 expression by 60–70% compared to wild type, indicating that the CD40/CD154 interaction between the two cell types plays a major role in IL30 induction and, to a lesser extent, other pathways can compensate for the absence of CD40. To further confirm the role of CD40 in IL30 induction, we reconstituted the CD40 signaling in CD154−/− splenocytes using an anti-CD40 agonist antibody (Fig. 4D). Though the lack of CD154 inhibits IL30 expression by 60%, agonist anti-CD40 rescues such inhibition and induces a 9-fold increase in IL30 expression.
Our data have shown that maximal induction of IL30 requires the activation of both T cells and macrophages and physical interaction between these two types of cells (Figs. 3 and 4). The major mechanism accounting for a high level of IL30 induction is most likely the CD40/CD154 pathway. To provide additional evidence to support this conclusion, purified CD4+ T cells and peritoneal macrophages lacking either CD154 or CD40 were treated with CD3/CD28/CpG, and IL30 expression was analyzed 72 hours later. As expected, the lack of CD154 in T cells or CD40 in macrophages greatly reduced IL30 expression, which could be partially rescued by reconstitution with agonist anti-CD40 treatment (Fig. 4E). Anti-CD40 treatment rescued IL30 expression more efficiently in CD154−/− splenocytes than in CD154−/− T cells (Figs. 4D and 4E). One explanation for this phenomenon is that CD40 engages both splenic macrophages and B cells, resulting in higher expression of IL30 when compared to macrophages alone because the CD40/CD154 pathway is relevant not only in macrophages but also in B cells.
To determine whether CD40 stimulation alone can replace CD4+ T cells in inducing IL30, macrophages were stimulated with CpG and an anti-CD40 agonist antibody in the absence of CD4+ T cells. Though others have shown that CpG or CD40 ligands are not inducers of IL30 expression (8), stimulation with CpG and anti-CD40 induces higher IL30 expression than CpG alone (Fig. 4F). However, this expression is less efficient than that in the presence of CD4+ cells, most likely because CD4+ T cells provide continuous stimulation of CD40 over time and may provide other tertiary signals.
Discussion
Understanding the induction of IL30 has multiple implications in biology. Others have found that IL30 has anti-inflammatory properties because it inhibits pro-inflammatory cytokines such as IL6 in gp130 signaling (2). Supporting evidence for the anti-inflammatory role of this cytokine has been shown in other studies in which IL30 expression in myoblasts inhibited allogeneic T cell responses and prolonged graft survival (14–17). Likewise, single nucleotide polymorphisms of IL30 are associated with greater susceptibility to asthma and inflammatory bowel disease (14–17). Our group has also shown that IL30 inhibits IL12-, IFNγ- and ConA-mediated liver toxicity (9).
This study has made a step forward in understanding how IL30 is induced. While our group and others have reported that IFNγ and LPS are the two major inducers of IL30, here we show that IFNγ signaling is not required for inducing IL30 via the combination treatment. Additionally, the timing of induction is different between CpG/CD3/CD28 and LPS treatments. Liu et al. showed that LPS induces maximal IL30 levels between 12–24 hours in macrophages. Conversely, IL30 production in splenocytes is nearly absent 12–24 hours after the combination treatment, but it is robustly increased after 48 to 72 hours (Sup. Fig 2).
CpG has a strong capacity for inducing a Th1 response, but many studies have shown that CpG is prophylactic in inflammatory conditions. For example, administration of CpGs prior to onset of dextran sodium sulphate induced colitis ameliorates the colitis, inhibits release of proinflammatory cytokines, and induces regulatory properties in CD4+ T cells (18, 19). CpG has protective properties in Th2 mediated pathologies (20, 21). Certain CpGs, such as CpG 1668, have already been shown to have protective properties in an arthritis model (22). Additionally, CpG was able to inhibit autoimmune diabetes in NOD mice (23). These protective measures have been attributed to IL10 expression. Results from this study show that CpG administration during an on-going inflammatory condition (OVA/CFA) induces 3 fold higher levels of IL30 than either CpG or OVA/CFA alone. In coordination with the previous findings that IL30 has a protective role in liver injury models and CpG is prophylactic in a number of inflammatory conditions, this discovery suggests that the prophylactic properties of CpG are due not only to induction of IL10 but also to IL30 (9). Therefore, understanding the in vivo significance of this IL30 induction via CpG and whether such induction has anti-inflammatory properties must be addressed. Such a discovery is imperative as CpG could serve as a therapeutic modality in many chronic inflammatory disease models.
Previous work showed that MyD88, cRel, IRF1, and IRF3 are necessary to induce IL30 expression (5, 6). Though we have shown that MyD88 is critical in the induction of IL30, further work is necessary to understand whether the above-mentioned transcription factors are necessary to induce IL30 expression via CD3/CD28/CpG treatment. Interestingly, only activation of TLR9, and no other TLRs, induces robust IL30 expression at delayed time points. Epidemiological studies have suggested that low exposure to bacterial products in developing countries contributes to increased risk of developing inflammation of the gut (24, 25). It is tempting to speculate that since CpG is prophylactic in these conditions, simultaneous activation of TLR9 and T cells induces anti-inflammatory cytokines, such as IL30.
In summary, we have described a novel cellular mechanism that controls IL30 production. Our data reveal that coordination between an innate immune cell-derived signal and a helper T cell-derived signal is necessary to induce a high level of IL30 expression. These findings lay the groundwork for future studies to investigate how to manipulate IL30 production during inflammation, cancer, or autoimmune diseases.
Supplementary Material
Acknowledgements
The authors are grateful to Dr. Samithamby Jeyaseelan for providing us with MyD88−/− mice, Dr. Shu-Hsia Chen for 4-1BB antibodies, and Dr. Andrew Weinberg for OX86 antibodies.
Footnotes
This work was supported by NIH/NCI RO1 CA098928 and MD Anderson’s Cancer Center Support Grant CA016672.
Conflict of interest: The authors have no conflict of interest.
References
- 1.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 2.Stumhofer JS, Tait ED, Quinn WJ, 3rd, Hosken N, Spudy B, Goenka R, Fielding CA, O'Hara AC, Chen Y, Jones ML, Saris CJ, Rose-John S, Cua DJ, Jones SA, Elloso MM, Grotzinger J, Cancro MP, Levin SD, Hunter CA. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol. 11:1119–1126. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 4.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett. 2008;117:123–130. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Guan X, Ma X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J Exp Med. 2007;204:141–152. doi: 10.1084/jem.20061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molle C, Nguyen M, Flamand V, Renneson J, Trottein F, De Wit D, Willems F, Goldman M, Goriely S. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. J Immunol. 2007;178:7607–7615. doi: 10.4049/jimmunol.178.12.7607. [DOI] [PubMed] [Google Scholar]
- 7.Dibra D, Cutrera JJ, Xia X, Birkenbach MP, Li S. Expression of WSX1 in tumors sensitizes IL-27 signaling-independent natural killer cell surveillance. Cancer Res. 2009;69:5505–5513. doi: 10.1158/0008-5472.CAN-08-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnurr M, Toy T, Shin A, Wagner M, Cebon J, Maraskovsky E. Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway. Blood. 2005;105:1582–1589. doi: 10.1182/blood-2004-05-1718. [DOI] [PubMed] [Google Scholar]
- 9.Dibra D, Cutrera J, Xia X, Kallakury B, Mishra L, Li S. IL30-A novel anti-inflammatory cytokine candidate for prevention and treatment of inflammatory cytokine-induced liver injury. Hepatology. doi: 10.1002/hep.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanek Z, Mateju J, Curdova E. Immunomodulators isolated from microorganisms. Folia Microbiol (Praha) 1991;36:99–111. doi: 10.1007/BF02814487. [DOI] [PubMed] [Google Scholar]
- 11.Sypek JP, Wyler DJ. Cell contact-mediated macrophage activation for antileishmanial defence: mapping of the genetic restriction to the I region of the MHC. Clin Exp Immunol. 1985;62:449–457. [PMC free article] [PubMed] [Google Scholar]
- 12.Alderson MR, Armitage RJ, Tough TW, Strockbine L, Fanslow WC, Spriggs MK. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993;178:669–674. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner DH, Jr, Stout RD, Suttles J. Role of the CD40-CD40 ligand interaction in CD4+ T cell contact-dependent activation of monocyte interleukin-1 synthesis. Eur J Immunol. 1994;24:3148–3154. doi: 10.1002/eji.1830241235. [DOI] [PubMed] [Google Scholar]
- 14.Shimozato O, Sato A, Kawamura K, Chiyo M, Ma G, Li Q, Tagawa M. The secreted form of p28 subunit of interleukin (IL)-27 inhibits biological functions of IL-27 and suppresses anti-allogeneic immune responses. Immunology. 2009;128:e816–e825. doi: 10.1111/j.1365-2567.2009.03088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chae SC, Li CS, Kim KM, Yang JY, Zhang Q, Lee YC, Yang YS, Chung HT. Identification of polymorphisms in human interleukin-27 and their association with asthma in a Korean population. J Hum Genet. 2007;52:355–361. doi: 10.1007/s10038-007-0123-8. [DOI] [PubMed] [Google Scholar]
- 16.Imielinski M, Baldassano RN, Griffiths A, Russell RK, Annese V, Dubinsky M, Kugathasan S, Bradfield JP, Walters TD, Sleiman P, Kim CE, Muise A, Wang K, Glessner JT, Saeed S, Zhang H, Frackelton EC, Hou C, Flory JH, Otieno G, Chiavacci RM, Grundmeier R, Castro M, Latiano A, Dallapiccola B, Stempak J, Abrams DJ, Taylor K, McGovern D, Silber G, Wrobel I, Quiros A, Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmuda MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ, Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van Gossum A, Zelenika D, Franchimont D, Hugot JP, de Vos M, Vermeire S, Louis E, Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwillam R, Tremelling M, Delukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, Daly MJ, Heyman MB, Ferry GD, Kirschner B, Lee J, Essers J, Grand R, Stephens M, Levine A, Piccoli D, Van Limbergen J, Cucchiara S, Monos DS, Guthery SL, Denson L, Wilson DC, Grant SF, Daly M, Hakonarson H. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat Genet. 2009;41:1335–1340. doi: 10.1038/ng.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li CS, Zhang Q, Lee KJ, Cho SW, Lee KM, Hahm KB, Choi SC, Yun KJ, Chung SC, Chae HT. Interleukin-27 polymorphisms are associated with inflammatory bowel diseases in a Korean population. J Gastroenterol Hepatol. 2009;24:1692–1696. doi: 10.1111/j.1440-1746.2009.05901.x. [DOI] [PubMed] [Google Scholar]
- 18.Obermeier F, Dunger N, Strauch UG, Grunwald N, Herfarth H, Scholmerich J, Falk W. Contrasting activity of cytosin-guanosin dinucleotide oligonucleotides in mice with experimental colitis. Clin Exp Immunol. 2003;134:217–224. doi: 10.1046/j.1365-2249.2003.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obermeier F, Strauch UG, Dunger N, Grunwald N, Rath HC, Herfarth H, Scholmerich W, Falk J. In vivo CpG DNA/toll-like receptor 9 interaction induces regulatory properties in CD4+CD62L+ T cells which prevent intestinal inflammation in the SCID transfer model of colitis. Gut. 2005;54:1428–1436. doi: 10.1136/gut.2004.046946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broide D, Schwarze J, Tighe H, Gifford T, Nguyen MD, Malek S, Van Uden J, Martin-Orozco E, Gelfand EW, Raz E. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J Immunol. 1998;161:7054–7062. [PubMed] [Google Scholar]
- 21.Chiaramonte MG, Hesse M, Cheever AW, Wynn TA. CpG oligonucleotides can prophylactically immunize against Th2-mediated schistosome egg-induced pathology by an IL-12-independent mechanism. J Immunol. 2000;164:973–985. doi: 10.4049/jimmunol.164.2.973. [DOI] [PubMed] [Google Scholar]
- 22.Wu HJ, Sawaya H, Binstadt B, Brickelmaier M, Blasius A, Gorelik L, Mahmood U, Weissleder R, Carulli J, Benoist C, Mathis D. Inflammatory arthritis can be reined in by CpG-induced DC-NK cell cross talk. J Exp Med. 2007;204:1911–1922. doi: 10.1084/jem.20070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quintana FJ, Rotem A, Carmi P, Cohen IR. Vaccination with empty plasmid DNA or CpG oligonucleotide inhibits diabetes in nonobese diabetic mice: modulation of spontaneous 60-kDa heat shock protein autoimmunity. J Immunol. 2000;165:6148–6155. doi: 10.4049/jimmunol.165.11.6148. [DOI] [PubMed] [Google Scholar]
- 24.Duggan AE, Usmani I, Neal KR, Logan RF. Appendicectomy, childhood hygiene, Helicobacter pylori status, and risk of inflammatory bowel disease: a case control study. Gut. 1998;43:494–498. doi: 10.1136/gut.43.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gent AE, Hellier MD, Grace RH, Swarbrick ET, Coggon D. Inflammatory bowel disease and domestic hygiene in infancy. Lancet. 1994;343:766–767. doi: 10.1016/s0140-6736(94)91841-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.