Abstract
The soy compound genistein has been observed preclinically to inhibit bladder cancer growth with one potential mechanism being inhibition of epidermal growth factor receptor phosphorylation (p-EGFR). A phase 2 randomized, placebo-controlled trial investigated whether daily, oral genistein (300 or 600 mg/day as the purified soy extract G-2535) for 14–21 days before surgery alters molecular pathways in bladder epithelial tissue in 59 subjects diagnosed with urothelial bladder cancer (median age 71). G-2535 treatment was well tolerated; observed toxicities were primarily mild to moderate gastrointestinal or metabolic and usually not attributed to study drug. Genistein was detected in plasma and urine of subjects receiving G-2535 at concentrations greater than placebo subjects’ but were not dose-dependent. Reduction in bladder cancer tissue p-EGFR staining between the placebo arm and the combined genistein arms was significant at the protocol-specified significance level of 0.10 (p=0.07). This difference was most prominent when comparing the 300 mg group vs placebo (p=0.015), but there was no significant reduction in p-EGFR staining between the 600 mg group and placebo. No difference in normal bladder epithelium p-EGFR staining was observed between treatment groups. No significant differences in tumor tissue staining between treatment groups was observed for COX-2, Ki-67, activated caspase 3, Akt, p-Akt, MAPK, or p-MAPK. No significant differences in urinary Survivin or BLCA-4 levels between treatment groups were observed. Genistein displayed a possible bimodal effect (more effective at the lower dose) on bladder cancer tissue EGFR phosphorylation that should be evaluated further, possibly in combination with other agents.
INTRODUCTION
Urothelial bladder cancer is the fifth most commonly diagnosed noncutaneous malignancy in the United States (US) (1) and the second most prevalent in middle-aged and elderly males (2). The clinical course is characterized by frequent occurrences and recurrences throughout the urothelium following initial management of non-muscle-invasive bladder cancers (3) and by extremely aggressive treatment of cancers that are more advanced or invade into the muscularis propria of the bladder. Because of this, it is the most expensive cancer to treat over the lifetime of a patient (4). Urothelial cancer is dichotomized by tumor grade; low grade represents slightly more than 50% of all newly diagnosed malignancies and high grade slightly less than 50% (5) (2) . Many molecular changes characterize low- and high-grade disease, but in reality, patients often develop low- and high-grade lesions simultaneously and recurrences often differ in grade from prior tumors (6). Although more commonly associated with high-grade disease, altered expression of epidermal growth factor receptor (EGFR) is found in both low- and high-grade bladder cancer (7). Other molecular features also co-exist in both tumor grades (8).
Urothelial cancer is a particularly appropriate target for preventive strategies, due to its biologic and clinical behavior, as well as its histologic and molecular characteristics. Unfortunately, there is minimal evidence of any advantage in tertiary chemoprevention trials using a variety of agents that are well tolerated by elderly patients (9–11). Though study design may be a factor in this lack of efficacy (9), it is possible that specifically targeted agents fail because redundant molecular pathways in the tumor make it refractory to such approaches. It was hypothesized that a natural agent with a high probability of excellent tolerance affecting many molecular pathways would be more effective.
The isoflavone genistein, a component of soy, has receptor tyrosine kinase-inhibiting activities (12) as well as phytoestrogenic effects (13). As a result, it inhibits the growth of estrogen receptor (ER)-positive human breast cancer (MCF-7) cells in vitro and in vivo (14). Genistein has also been shown in a number of cancer cell line studies to inhibit downstream modulation of genes involved in cell cycle growth, apoptosis, angiogenesis, invasion, and motility, as well as adhesion, through a number of signaling pathways, including those involving epidermal growth factor (EGF) and protein kinase B (AKT) (15–20). There is also in vitro evidence for inhibition of EGF receptor (EGFR)-mediated activity through direct competition with ATP for tyrosine kinase (Ki, 14 µM) in A431 cells, but not serine or threonine kinases(21,22) .
Epidemiologic data indicate that populations with high dietary soy intake have lower incidences of bladder cancer than those following more Western diets (23, 24). Genistein has demonstrated anti-urothelial cancer activity in vitro (25,26) ; reported mechanisms include inhibition of cyclin B expression, induction of apoptosis, development of G2–M cell cycle arrest, inhibition of EGFR activity and EGF-mediated responses such as proliferation and cell motility (26) and modulation of cyclooxygenase (COX)-2 expression and activity (27–29) . Vascular endothelial growth factor (VEGF) induction of COX-2 activity is inhibited by genistein in endothelial cells, another critical target for tumor growth (30). Importantly, many markers of these molecular processes can be assessed with immunohistochemistry (IHC) in formalin-fixed, paraffin-embedded tissue.
The goal of the current study was to determine if short-term oral ingestion of 300 or 600 mg genistein/day from the soy extract G-2535 in patients diagnosed with a bladder tumor by office cystoscopy alters molecular pathways in specimens removed at transurethral resection of bladder tumor (TURBT) or cystectomy. Changes in EGFR phosphorylation at tyrosine 1068, and downstream signaling molecules as well as Ki-67 and activated caspase 3 were measured in normal-appearing and tumor urothelium tissue sections after surgery. Additionally, the influence of genistein on urine levels of survivin, an apoptosis inhibitor associated with bladder cancer and predictive of superficial bladder cancer recurrence (31, 32), and BLCA-4, a nuclear matrix protein and promising bladder cancer marker (33) were assessed in in voided urine during and at completion of therapy.
MATERIALS AND METHODS
Subject Eligibility and Recruitment
Men and women were recruited by research personnel from urology clinics at participating institutions (University of Wisconsin-Madison; University of Rochester Medical School, NY; University of Alabama-Birmingham; University of Iowa; South Orange County Medical Research Center, CA; Urology San Antonio Research, TX; AccuMed Research Associates LLC, NY) in the UW Chemoprevention Consortium. Prior to determining eligibility, potential participants underwent informed consent per federal, state, and local institutional standards. Eligibility criteria included: age ≥18 years old; evidence of an initial or recurrent primary bladder tumor by cystoscopy with no evidence of distant metastases; diagnostic cystoscopy within 60 days of study enrollment and at least 45 days after bladder treatment with intravesicular agents; planned subsequent TURBT or cystectomy (partial or complete); ECOG performance status 0 or 1; for women of childbearing potential, a negative pregnancy test; for men, agreement to use adequate contraception prior to study entry and during study duration; no concomitant thyroid or nonsteroidal antiinflammatory drugs (81 mg aspirin/day allowed); no concomitant use of soy- or genistein-containing supplements; no history of pelvic irradiation; and normal organ function. Organ function parameters included the following: in marrow, white blood cells (WBC) ≥3,000 mm3, platelets ≥100,000 mm3, and hemoglobin ≥10 g/dL; in liver, bilirubin ≤1.4 mg/dL and aspartate aminotransferase ≤3 times normal; in renal, creatinine ≤2.0 mg/dL; in metabolic functions, serum calcium ≤ 10.2 mg/dL and amylase ≤3 times normal; in electrolytes, sodium ≥125 and ≤155 mmol/L, potassium ≥3.2 and ≤6 mmol/L, chloride ≥85 and ≤114 mmol/L, and carbon dioxide ≥11 mEQ/dL; and in thyroid, thyroid-stimulating hormone (TSH) within 1.3 times the upper range of normal and normal thyroxine (T4).
Trial Design
Participants meeting the eligibility criteria were randomized (1:1:1) to one of three arms: G-2535, one capsule taken orally bid, for a dose of 300 mg genistein/day; G-2535, two capsules taken orally bid, for a dose of 600 mg genistein/day; or placebo, one or two capsules taken orally bid for 14–21 days until the day prior to planned TURBT or cystectomy, or up to 30 days if surgery was delayed. Randomization was based on permuted blocks of size six, stratified by clinical suspicion into non-muscle-invasive (low malignant potential or low-grade or high grade, stage Ta, Tis, T1) vs. muscle invasive (stage T2+). Participants had safety labs (eligibility lab work plus serum lipase, T4, and TSH levels) done at baseline, after one week of study therapy, and on the morning of surgery. Urine for biomarkers survivin and BLCA-4 was collected at baseline, after one week of study therapy, and on the morning of surgery. Blood and urine for genistein and daidzein concentrations were collected at baseline, after one week of study therapy, and on the morning of surgery.
The primary objective of the study was to compare tumor tissue EGFR phosphorylation between treatment groups. Secondary objectives included assessment of EGFR, Ki67, activated caspase 3, Akt, p-Akt, mitogen-activated protein kinase (MAPK), phosphorylated –MAPK (p-MAPK) and COX-2 by IHC in tumor tissues; assessment of tumor EGFR, urine survivin and BLCA-4 levels; plasma and urine genistein and daidzein concentrations; and safety and tolerance between treatment groups.
Study Agent
G-2535 capsules and matching placebo capsules were manufactured by The Solae Company [Solae (St. Louis, MO); formerly Protein Technologies International, Inc. (PTI)]. The active pharmaceutical ingredient, G-2535, is a purified soy extract containing ≥97% total unconjugated isoflavones and composed primarily of genistein and daidzein in a 2:1 ratio (genistein:daidzein). Each blue gelatin capsule contained enough drug substance to deliver 150 mg genistein and 75 mg daidzein. Bottles containing 60 G-2535 capsules or matching placebo capsules were distributed to the clinical research sites by McKesson Health Solutions (now Fisher BioServices, DCP Repository, Germantown, MD). Subjects randomized to the 600 mg/day dose received two bottles and those randomized to the 300 mg/day dose received one bottle. The placebo group of subjects also received either one or two bottles of placebo capsules to avoid potential bias.
Genistein doses of 300 and 600 mg/day were based on the observed pharmacokinetics and safety of the similar soy extract PTI G-2535 in prior NIH-supported and NCI, DCP-sponsored human studies (34,35), including one in prostate cancer patients (36) . Both doses were projected to result in urinary concentrations of genistein above the 18–37 µmol/L needed to slow EGF-induced proliferation of human bladder cancer cell lines in culture, or to inhibit EGF-induced bladder cancer cell motility in chemotaxis assays (26) . In addition, ingestion of 600 mg genistein in the form of PTI G-2535 resulted in plasma concentrations of 10–20 µM after 12 hours, which inhibited protein tyrosine phosphorylation in peripheral lymphocytes for >24 hrs (34).
Collection of Blood (Plasma) and Urine for Concentrations of Genistein and Daidzein
Prior to first study drug administration, at 12 hours postdose on day 8, and on the morning of surgery, 10 mL of whole blood was drawn into a heparinized tube, centrifuged for 10 minutes, with plasma aliquoted into two NUNC tubes, and frozen at −70°C prior to transport and eventual analysis at the UWCCC Analytical Laboratory. Urine was collected at the time of blood collection for plasma concentrations (prior to first study drug administration, 12 hours postdose on day 8, and on the morning of surgery). The time of the last void and the time urine was collected with total volume were recorded; four 2 mL aliquots of urine were placed into 3.6 mL NUNC tubes, stored immediately at 4°C, and frozen at −70°C within 1–4 hours.
Genistein and daidzein concentrations were evaluated in plasma and urine samples as previously described (37). Briefly, plasma and urine samples were extracted with methyl t-butyl ether. Analysis was by reverse-phase high-performance liquid chromatography (HPLC) with ultraviolet detection at 260 nm; the mobile phase consisted of 75% 0.05M ammonium formate/25% acetonitrile. All standard curves had an R2 greater than 0.995. The lower limit of quantitation was 3.9 ng/mL for genistein and 3.1 ng/mL for daidzein. Genistein interday variability over two months was 16.6% for high standard (n=4) and 1.6% for low standard (n=4) in both matrices. Recovery of genistein from plasma was >90% based on comparison with H2O standards. Daidzein plasma interday variability over two months was 9% for high standard (n=6) and 22% for low standard (n=4); urine interday variability over 11 days was 9% for high standard (n=5) and 7% for low standard (n=5). Recovery of daidzein from plasma was approximately 60% based on comparison with H2O standards.
Urine Collection for Survivin
Urine by clean catch was obtained prior to drug administration at baseline, at 12 hours postdose on day 8, and presurgery, at the end of the study; 4 mL was immediately stored at 4°C, then aliquoted into 1.7 mL eppendorf tubes within 1–4 hours, and frozen at −70° C. Survivin was assayed in urine by the laboratory of Dr. Robert Weiss (Yale University) using the R&D Duoset IC human total survivin ELISA (R&D DYC647), and assayed in triplicate after centrifugation and dilution of the supernatant (two parts buffer to one part urine). Each microplate assayed included a standard calibration curve prepared in duplicate. Samples and control urines were assayed in triplicate, and average absorbance values used. . Control samples included urine from a high stage, high grade bladder cancer patient (male) collected at the time of cystectomy, and urine from a 58 year old female with no history of bladder cancer.
Urine Collection for BLCA-4
Urine by clean catch was obtained prior to first drug administration at baseline, at 12 hours postdose on day 8, and the morning presurgery, at the end of the study; 15 mL was immediately stored at 4°C, aliquoted into 50 mL conical tubes within 1–4 hours, and frozen at −70°C. Assays were performed in the laboratory of Dr. Robert Getzenberg at the Johns Hopkins University School of Medicine. BLCA-4 in urine was evaluated utilizing an adaptation of a protocol that has previously been described (38). 100 µl of urine was plated into a 96-well Maxisorp (Nunc 442404) plate in duplicate. The plates were then sealed with adhesive film and incubated overnight shaking at 300 rpm on a IKA MTS 2/4 microtiter plate shaker. The following morning, the plates were washed three times with 200 µl of freshly prepared 0.05% Tween 20 in PBS utilizing a Beckman Coulter MW96 plate washer. The plates were then blocked with 200 ul of 1% BSA, 1% dry milk and 0.05% Tween 20 in PBS, per well, for 30 minutes at room temperature with shaking. After the blocking step, the washing step was then repeated. 100 µl of BLCA-4 monoclonal antibodiy (QCB/Biosource – Lot#0106193) diluted 1:50 in 1% BSA, 1% dry milk and 0.05% Tween 20 in PBS was then added and incubated at room temperature for two hours. After the inclubation, the plates were washed again and then 100 ul of goat anti-mouse secondary antibody 1 mg/ml, diluted 1:2500 in 1% BSA, 1% dry milk and 0.05% Tween 20 in PBS (IgG) that was human serum absorbed and perioxidase labeled ((Kirkegaard & Perry Laboratories, Inc., #074-1806, Lot #070775) was added. After a two hour incubation, the plates were washed again and developed with 100 µl of SureBlue TMB microwell peroxidase substrate (Kirkegaard & Perry Laboratories, Inc #52-00-03). The absorbance was read at 630 nm with the BMG LabTech PeraStar plate reader. Data were collected using the MARS Data Analysis software Ver1.20 R2 and then exported to Microsoft Excel where data points were corrected for background
Tissue
Paraffin blocks or 10 unstained slides of normal-appearing or tumor tissue from the TURBT or cystectomy were obtained from each subject. Tissue biomarkers (total and phosphorylated EGFR (p-EGFR), Ki67, activated caspase 3, Akt, , p-Akt, MAPK, p-MAPK, and COX-2) were evaluated from malignant urothelium and normal-appearing urothelium both remote and neighboring the tumor in the Immunocytochemistry Laboratory of the Department of Pathology and Laboratory Medicine at the University of Rochester Medical Center. Immunostaining was done via either DAKO cytomation EnVision Plus system-HRP (Dako Corporation, Glostrup, Denmark), a polymer based proprietary system, or standard Streptavidin-HRP procedure with 3-amino-9-ethylcarbamide (AEC) as the chromagen (HRP, Dako, Carpinteria, CA). IHC scoring (IHS) was based on the German immunoreactive score; this method has been shown to approximate data generated from image analysis-based scoring systems (39). The IHS score was calculated by combining an estimate of the percentage of immunoreactive cells (quantity score) with an estimate of the staining intensity (staining intensity score), as follows: 0, no staining; 1, 1–10% of cells stained; 2, 11–50% of cells stained; 3, 51–80% of cells stained; 4, 81–100% of cells stained. Staining intensity was rated on a scale of 0 to 3, with 0, negative; 1, weak; 2, moderate; and 3, strong. The IHS was determined by multiplying quantity and staining intensity scores to produce scores ranging from 0–4, 6, 8, 9, and 12. An IHS score of 9–12 was considered strongly immunoreactive; 5–8, moderate; 1–4, weak; and 0, negative. Sections in which the staining could not be well characterized were considered equivocal.
The rabbit polyclonal anti-p-EGFR (TYR1068) antibody (Cell Signaling Technology, Danvers, MA) was selected for the primary IHC endpoint based on recognition of the activated conformation of EGFR with the phosphorylated C-terminal tyrosine (40). The antibody was optimized for IHC using positive and negative controls, given the basal layer of the epidermis has been shown to be the best single positive control (41). In addition, a control tissue microarray containing 23 different normal tissues and neoplasms was used in the workup. The tyramide catalyzed signal amplification system, GenPoint (K0620, Dako, Carpinteria, CA) was used to generate an optimal immunostaining signal . The DAKO Autostainer Universal Staining System was used for all staining, under stringently controlled conditions. Positive control standards were used for each run. In addition, two primary antibodies to nonphosphorylated EGFR (mouse monoclonals H11 from DAKO and 31G7 from Zymed) were worked up in a similar manner, as well as the DAKO EGFR PharmDX clinical diagnostic kit with rigid and extensive quality control procedures for automated staining. The other IHC assays for Ki67, activated caspase-3, Akt, MAPK, p-MAPK, COX-2, and survivin were validated using standard antibodies.
STATISTICAL ANALYSES
For sample size estimation/justification, the primary study endpoint of EGFR phosphorylation measured by IHS was treated as a continuous variable. Power was computed using a two-sample t-test to test IHS differences in post treatment tissue specimens between placebo and G-2535 groups at a two-sided significance level of 0.1. A total sample size of 36–93 was required to ensure 0.85 power for a range 0.6–1.0 of the effect size, i.e., the difference in mean IHS between the placebo and G-2535-treated groups combined divided by the standard deviation of the IHS assumed to be common. Assuming 10% random dropouts, 60 subjects (20 each in placebo, 300, and 600 mg genistein/day groups) were planned for enrollment to ensure a total of 54 subjects for primary comparison, giving at least 0.85 power to detect the effect size of 0.8 or greater. If the rate of random dropouts was 20%, leaving a total of 48 subjects for primary comparison, the study would have at least 0.82 power to detect the effect size of 0.8 or greater. Because of the small number of promising chemopreventive agents, it was felt important to have high power albeit a higher false positive probability than usual. The primary comparison between placebo and G-2535 was done using two-sample Wilcoxon rank-sum test at a 0.1 level of significance as specified in the protocol for testing the differences in IHS in post-treatment tissue specimens between the placebo and G-2535 groups. Secondary endpoints were examined also using two-sample Wilcoxen rank-sum test. No adjustments were made for multiple endpoints. Safety data described using NCI CTCAE version 3.0 were summarized descriptively as proportions, and compared between the placebo and G-2535-treated groups using nonparametric tests such as Fisher’s exact test or Kruskal-Wallis test. A dose-response relation was assessed using one degree-of-freedom analysis of covariance or Jonckheere-Terpstra test depending on the outcome.
RESULTS
Subjects
Eighty-two subjects signed local IRB-approved informed consent were screened for the study from seven sites between June 2006 and June 2008; 72 were male, 10 were female. Twenty-two of these were screen failures. Of the 60 remaining subjects, median age was 71 years (mean 70; range 46–97), including eight women and 52 men; 51 completed the study; one randomized subject dropped out of the study before receiving drug. Subjects were predominantly white, non-Hispanic (98%), with an ECOG performance status of zero (88%) or one (12%). Forty-eight (80%) of the tumors were non-muscle-invasive; 12 subjects (20%) had muscle-invasive bladder cancers (four in each treatment group). All recorded subject characteristics appeared consistent with randomization among the three groups except for an imbalance in baseline weight (and BMI) in male subjects between arms (placebo and 300 mg groups were lighter/smaller BMI than the 600 mg group (p<0.05). Baseline subject characteristics are summarized in Table 1.
Table 1.
Baseline Patient and Disease Characteristics
| Treatment Group | Placebo | 300 mg | 600 mg | |
|---|---|---|---|---|
| Number of patients | 20 | 20 | 20 | |
| Age | Mean ± S. D. | 72.0 ± 12.7 | 68.6 ± 9.2 | 68.7 ± 9.2 |
| Median | 74 | 68.5 | 68.5 | |
| Range | 46–97 | 51–83 | 50–81 | |
| Sex | Male:Female | 15:5 | 19:1 | 18:2 |
| Race | White, non-Hispanic | 20 | 20 | 19 |
| Hispanic or Latino | 0 | 0 | 1 | |
| ECOG | 0 | 14 | 17 | 19 |
| 1 | 3 | 3 | 1 | |
| Weight (kg)* | Mean +S. D. | 78.3 ± 20.1 | 80.6 ± 14.1 | 90.5 ± 11.0 |
| Range | 47.7–134.9 | 52.6–111.4 | 70.7–112.3 | |
| Body Mass Index** |
Mean | 27.0 ± 7.2 | 26.3 ± 4.4 | 29.5 ± 3.3 |
| Range | 16.7–44.0 | 18.7–35.4 | 24.4–39.05 | |
P-Value for males only (Placebo vs 600 mg)=0.008; P-Value (300 mg vs 600 mg)=0.02
P-Value for males only (Placebo vs 600 mg)=0.009; P-Value (300 mg vs 600 mg)=0.031
Study drug compliance was generally good. Eight (14%) subjects met the criteria for noncompliance (lack of documentation of taking >95% of doses), including five on the placebo arm, and three on the treatment arms (300 mg – 1; 600 mg – 2). Of the eight subjects who dropped out of study after receiving drug, one was due to adverse events (AEs); these included grade 1 hypothyroidism considered unrelated to study drug, and grade 1 hypoaesthesia, grade 2 dyspepsia, and grade 2 esophageal pain considered possibly related to study drug.
Safety
G-2535 was well tolerated; the majority of subjects experienced no or mild side effects. Table 2 displays the worst grade of adverse event (CTCAE v3) by subject in toxicity categories which at least one grade 3 or worse adverse event occurred in a non-placebo subject, as well as all categories combined (Overall). Examining subjects by worst post-baseline AE, shows 12 subjects with no AE, 35 with grade 1 (mild), 8 with grade 2 (moderate), 4 with grade 3 (severe) and 1 subject with grade 4 (life-threatening). The grade 4 event was grade 4 paroxysmal supraventricular tachycardia occurring in a placebo subject. Metabolic abnormalities (elevations in serum amylase or lipase, slight increases in serum creatinine, or decreased serum calcium) observed in numerous subjects were not related to dose. Gastrointestinal adverse events (AEs) across all treatment groups included constipation; diarrhea, nausea and heartburn were also not dose-related. . Examination of all AEs revealed no relationship between severity or frequency and increasing dose (p=0.12, Jonckheere-Terpstra test).
Table 2.
Percent of Subjects with Worst Grade of Adverse Event for CTCAE v3 Category (Includes Categories Where at Least One Grade 2 or Worse Event Occurred in a Non-Placebo Arm)
| CTCAE Category | Grade | Placebo (%) | 300 mg (%) | 600 mg (%) | |
|---|---|---|---|---|---|
| Overall* | 0 | (None) | 7 (35%) | 2 (10%) | 3 (15%) |
| 1 | (Mild) | 10 (50%) | 14 (70%) | 11 (55%) | |
| 2 | (Moderate) | 1 (5%) | 2 (10%) | 5 (25%) | |
| 3 | (Severe) | 1 (5%) | 2 (10%) | 1 (5%) | |
| 4 | (Life-Threatening) | 1 (5%) | 0 (0%) | 0 (0%) | |
| Metabolic/Laboratory | 0 | (None) | 5 (25%) | 7 (35%) | 7 (35%) |
| 1 | (Mild) | 13 (65%) | 11 (55%) | 11 (55%) | |
| 2 | (Moderate) | 1 (5%) | 0 (0%) | 2 (10%) | |
| 3 | (Severe) | 1 (5%) | 2 (10%) | 0 (0%) | |
| 4 | (Life-Threatening) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Gastrointestinal | 0 | (None) | 16 (80%) | 17 (85%) | 16 (80%) |
| 1 | (Mild) | 4 (20%) | 2 (10%) | 2 (10%) | |
| 2 | (Moderate) | 0 (0%) | 1 (5%) | 1 (5%) | |
| 3 | (Severe) | 0 (0%) | 0 (0%) | 1 (5%) | |
| 4 | (Life-Threatening) | 0 (0%) | 0 (0%) | 0 (0%) | |
p=0.12 Jonckheere Terpstra
Comparisons of treatment groups and all gradable AEs revealed no significant differences between the placebo and treatment groups but some trends toward the treatment groups were evident. The proportion of subjects with any AE in the 300 mg genistein group appeared greater than placebo group (p=0.058, χ2 test) The proportion of subjects with an AE in the 600 mg genistein group as compared to placebo was less evident (p=0.144). Comparisons of investigator-determined relationships between AEs and study drug (definitely-, probably-, possibly-, unlikely-, or not-related) between treatment groups also revealed a possible difference between the 300 mg genistein group and placebo. The 300 mg genistein group had a stronger relationship between AEs and study treatment than placebo subjects (p=0.022, Wilcoxon rank-sum test).
G-2535 is a purified soy extract containing ≥ 97% total unconjugated isoflavones, composed primarily of genistein and daidzein in a 2:1 ratio (genistein:daidzein). Therefore, subjects in the lower genistein dose group received approximately 300 mg genistein/day and 150 mg daidzein/day, while those in the high-dose group received 600 mg genistein/day and 300 mg daidzein/day. Overall, as shown in Table 3, no significant differences were seen in genistein and daidzein plasma or urine concentrations at baseline between the placebo and treatment groups, although both were detected in some subjects. While consumption of soy supplements was prohibited, soy intake in the diet was allowed; plasma and urine concentrations at baseline are most likely related to dietary consumption. As a group, subjects receiving placebo had no significant differences in genistein concentration from baseline to day 8 to presurgery, suggesting that dietary consumption of soy remained relatively stable during the study. On day 8, mean ± S.D. plasma genistein trough levels were 1.8 ± 7.0 ng/mL in the placebo group, 23.0 ± 69.6 ng/mL in the 300 mg genistein/day group, and 11.2 ± 14.2 ng/mL in the 600 mg genistein/day group. Both the 300 and 600 mg genistein/day groups were significantly different from the placebo group, but there was no significant difference in genistein concentrations between the two genistein-treated groups. Median trough concentrations of genistein and diadzein reflect the finding that most subjects receiving active drug had detectable peaks of genistein and diadzein in plasma below the lower limits of quantification (3.7 ng/ml for genistein, 3.1 ng/ml for diadzein). At presurgery, plasma genistein levels were zero in the placebo group, 22.9 ± 82.4 ng/mL in the 300 mg genistein/day group, and 3.4 ± 5.9 ng/mL in the 600 mg genistein/day group. Again, there was a significant difference between the treatment and placebo groups and no difference between the two genistein-treated groups. The higher mean concentrations in the 300 mg genistein/day group are attributable to one individual with baseline genistein concentrations of approximately 200 ng/mL (10 times higher than any other subject’s baseline level) and slight increases in genistein levels on day 8 and presurgery. This result is likely secondary to high dietary soy intake, which frequently results in plasma levels exceeding those from supplements (42). Urinary concentrations of both genistein and daidzein were consistently higher than plasma concentrations, as has been previously reported (43).
Table 3.
Total Genistein and Daidzein Concentration in Plasma and Urinea
| Treatment Group |
Timepoint | N | Genistein (ng/mL) | Daidzein (ng/mL) | ||
|---|---|---|---|---|---|---|
| Plasma | Urine | Plasma | Urine | |||
| Baseline | Placebo | 17 | 0 ± 0 (0) |
59.3 ± 98.5 (21.1) |
2.2 ± 6.6 (0) |
305 ± 420 (61.7) |
| 300 mg | 18 | 22.6 ± 93.9 (0) |
212 ± 551 (47.4) |
0.6 ± 2.5 (0) |
650 ± 1610 (281.0) |
|
| 600 mg | 18 | 0 ± 0 (0) |
34.9 ± 39.0 (24.9) |
24.7 ± 79 (0) |
1545 ± 5815 (135.9) |
|
| Day 8 | Placebo | 15 | 1.8 ± 7.0 (0) |
57.2 ± 116 (23.6) |
3.9 ± 6.8 (0) |
236 ± 258 (120.0) |
| 300 mg | 19 | 23.0 ± 69.6 (0–3.7) |
1235 ± 2128 (115.5) |
240 ± 982 (0–3.1) |
796 ± 1054 (452.8) |
|
| 600 mg | 18 | 11.2 ± 14.2 (0–3.7) |
648 ± 762 (288.0) |
4.3 ± 6.4 (0–3.1) |
975 ± 1121 (486.7) |
|
| Pre-surgery | Placebo | 15 | 0 ± 0 (0) |
34.2 ± 33.8 (23.9) |
1.3 ± 3.3 (0) |
177 ± 230 (72.4) |
| 300 mg | 19 | 22.9 ± 82.4 (0–3.7) |
848 ± 977 (507.2) |
54.2 ± 219 (0–3.1) |
981 ± 741 (852.9) |
|
| 600 mg | 18 | 3.4 ± 5.9 (0–3.7) |
1055 ± 1129 (680.0) |
96.1 ± 315 (0–3.1) |
1693 ± 2731 (1026.5) |
|
Mean ± standard deviation
(median values)
Tissue Endpoints
Tissue endpoints are summarized in Table 4 and a representative EGFR phosphorylation IHC is shown in Figure 1. Strong bladder tumor tissue EGFR phosphorylation, determined by IHC (3+ staining), occurred in 14 of 15 subjects in the placebo group, with lower amounts in the two genistein-treated groups. For the primary endpoint, comparing all genistein-treated subjects (300 and 600 mg) to placebo subjects, for the reduction (3+ staining vs. 0, 1+, or 2+ staining) in p-EGFR a p-value of 0.07 was observed. According to the protocol study design, in which power was designed to test the difference between the placebo and combined genistein groups at a two-sided significance level 0.1, this would be considered significant. In addition, examination of the treatment groups separately found a significant reduction in tumor tissue p-EGFR in subjects randomized to genistein at 300 mg/day (p=0.015) but not 600 mg/day (p=0.44) compared with placebo. The reduction in p-EGFR tissue staining between the placebo and 300 mg genistein/day groups was greatest in subjects with muscle-invasive bladder cancer (p=0.047) compared with non-muscle-invasive cancer (p =0.062).
Table 4.
Phosphorylated EGFR in Normal-appearing and Tumor Tissue
| Tissue Type | Grade | Placebo n (%) | 300 mg n (%) | 600 mg n (%) |
|---|---|---|---|---|
| N=9 | N=14 | N=8 | ||
| Benign | Negative | 2 (22%) | 1 (7%) | 2 (25%) |
| Weak | 4 (44%) | 4 (29%) | 4 (50%) | |
| Moderate | 3 (33%) | 8 (57%) | 2 (25%) | |
| Strong | 0 (0%) | 1 (7%) | 0 (0%) | |
| N=15 | N=19 | N=18 | ||
| Tumor* | Negative | 0 (0%) | 0 (0%) | 0 (0%) |
| Weak | 1 (7%) | 5 (26%) | 0 (0%) | |
| Moderate | 0 (0%) | 4 (21%) | 3 (17%) | |
| Strong | 14 (93%) | 10 (53%) | 15 (83%) | |
P-Value (Placebo vs All Genistein)=0.07; P-Value (Placebo vs 300 mg)=0.015
Figure 1. Representative Staining for phosphorylated EGFR.
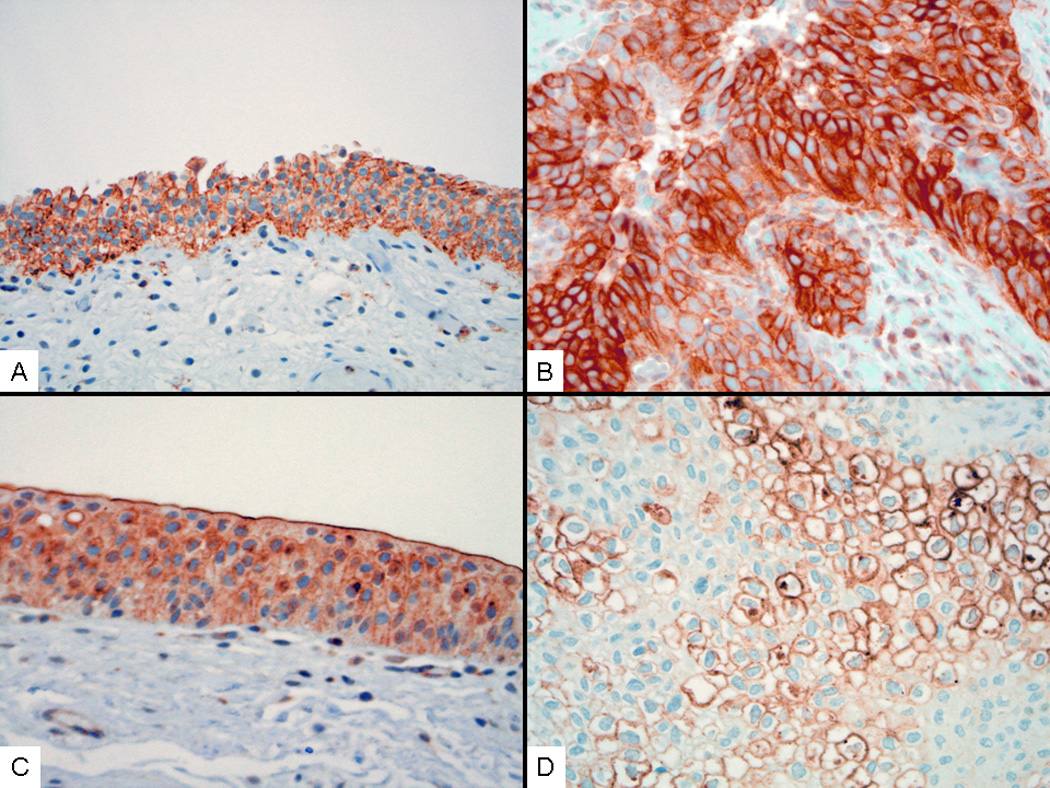
A) Benign urothelium from patient treated with placebo with membrane staining for phosphorylated-EGFR, IRS score moderate. B) Urothelial carcinoma from patient treated with placebo with membrane staining for phosphorylated-EGFR, IRS score strong. C) Benign urothelium from patient treated with 300 mg Genistein with membrane staining for phosphorylated-EGFR, IRS score Weak. D) Urothelial carcinoma from patient treated with 300mg Genistein with membrane staining for phosphorylated-EGFR, IRS score Weak. Immunohistochemical stain with hematoxylin counterstain, original magnification 400×
Normal bladder epithelium was also available from a majority of subjects for examination. In normal-appearing tissue, p-EGFR staining was in general less intense than in tumor tissue, especially for placebo subjects. An examination of the difference in p-EGFR between tumor and normal-appearing epithelium within each subject revealed a significantly greater proportion of placebo subjects with markedly less p-EGFR staining in normal-appearing tissue (e.g., 3+ to 1+ or 0; 2+ to 0) as compared to the 300 mg group (p=0.0152) and a trend toward a significant difference when comparing placebo to all G-2535 subjects (p=0.063). This was primarily due to stronger tumor p-EGFR staining in placebo subjects than in those in treatment groups. EGFR staining intensity in tumor vs. normal-appearing epithelium did not differ between the treatment (together or alone) and placebo groups. Significant differences in staining were also not found between treatment groups for tumor tissue expression of COX-2 (p=0.32), Ki67 (p=0.35), or activated caspase 3 (p =0.52). Assessment of tumor expression of Akt (p=0.76), p-Akt (p =0.38), MAPK (p =0.41), and p-MAPK (p =0.35) also failed to reveal any differences between placebo subjects and those treated with G-2535, but similar to p-EGFR, tumor tissue from subjects in the 300 mg genistein/day group had a trend toward reduced phosphorylation of MAPK (p =0.15).
Survivin excretion in urine (Table 5) exhibited moderate intra- and intersubject variability. Treatment groups (300 and 600 mg genistein/day) showed a nonsignificant trend toward reduced amounts on day 8, and for the 600 mg genistein/day group, at presurgery compared to baseline. BLCA-4 excretion was unchanged across all time points and groups (Table 6).
Table 5.
Urine Survivin Concentrationa
| Treatment Group | N | Baseline (pg/mL) |
Day 8 (pg/mL) |
Presurgery (pg/mL) |
|---|---|---|---|---|
| Placebo | 16 | 16 ± 37 | 18 ± 33 | 29 ± 43 |
| Genistein: 300 mg/day | 18 | 71 ± 172 | 60 ± 175 | 84 ± 238 |
| Genistein: 600 mg/day | 19 | 47 ± 109 | 24 ± 41 | 30 ± 50 |
Mean concentration ± standard deviation
Table 6.
Urine BLCA-4a
| Treatment Group | N | Baseline (O.D. | Day 8 (O.D.) | Presurgery (O.D.) |
|---|---|---|---|---|
| Placebo | 17 | 0.46 ± 0.24 | 0.53 ± 0.20 | 0.49 ± 0.34 |
| Genistein: 300 mg/day | 20 | 0.52 ± 0.37 | 0.54 ± 0.42 | 0.50 ± 0.39 |
| Genistein: 600 mg/day | 19 | 0.55 ± 0.34 | 0.50 ± 0.43 | 0.44 ± 0.31 |
mean ± standard deviation
DISCUSSION
The doses of G-2535 chosen for this study, 150 and 300 mg genistein bid, produced plasma concentrations that have been shown to rapidly silence protein tyrosine phosphorylation in peripheral lymphocytes within three to six hours and maintain that effect for over 24 hours in a previous study with a similar isoflavone mixture containing lower concentrations of genistein(34). These doses also achieved urinary concentrations of unconjugated genistein that can block EGFR phosphorylation and impede EGF-induced proliferation and bladder cell motility; 18.5 to 37 µM genistein has been shown to slow EGF-induced proliferation and cell motility of human bladder cancer cell lines in culture (26). These concentrations have also been shown to inhibit VEGF-induced COX-2 protein expression in human endothelial cells and in mouse macrophages in vitro (27). In previous clinical studies, doses of similar extracts delivering the same genistein doses were well tolerated by both men and women, including men with prostate cancer (34–36). Given the similar age range of bladder cancer patients in this study to prostate cancer patients treated in previous studies at 300 and 600 mg genistein/day, the same genistein doses from G-2535 were thought to be appropriate.
While this study found no significant differences in parameters other than EGFR phosphorylation, the lower dosage initiated a clear, although not statistically significant, trend of reduced expression of p-MAPK (p=0.145) in tumors, primarily in subjects in whom genistein reduced EGFR phosphorylation in tumor tissue, indicating that EGFR signaling was probably affected by genistein.
G-2535 was less effective at reducing EGFR phosphorylation in normal epithelium (not shown), again indicating that EGFR activation may occur at a later stage in bladder cancer development.
This first prospective study examining the effects of genistein and other isoflavones in presurgical bladder cancer patients was not designed to assess therapeutic or preventive effects on tumors histologically. No patients were tumor-free at surgery; such a brief exposure time was not expected to accomplish this. However, G-2535 was well tolerated and no significant AEs were thought to be due to study drug. Since much of this study was aimed at testing tolerance and molecular response in this age and population, an effect was considered to occur if there were more responders in the treatment groups than in the placebo group. A significant (p=0.07; of note for this initial exploratory study significance was defined as p<0.10 rather than the usual p<0.05) reduction of EGFR phosphorylation was seen in the genistein-treated subjects as compared to placebo with the greatest effect seen in the 300 mg genistein/day group, and maximum serum levels were seen at eight days. That a lesser effect was seen in the higher dose should not be interpreted as indicating that genistein had no effect. Evidence from other studies found that genistein can induce a bimodal response, rendering higher doses less effective than lower ones (13), as do other biologic response modifiers and natural substances (44). The possible bimodal effect (13) was hypothesized to be related to differing concentration effects upon estrogen-receptor modulated events, whether such an effect could be observed relative to EGFR phosphorylation independent or dependent of estrogen-receptor related effects is unknown. A formal dose-finding study was beyond the scope of this initial study but might be appropriate. Whether related to the lack of any observed correlation between EGFR phosphorylation inhibition and dose or genistein concentrations, blood levels of genistein were roughly as high at 300 mg/day as at 600 mg/day. The genistein and diadzein plasma concentrations assessed ≥ 24 hours after the last G-2535 (genistein) dose produced results consistent with the G-2535 pharmacokinetics reported by Takimoto et al. (45) who also observed marked interpatient variability, minimal to no dose relationship and a relatively short half- life of total and free genistein.
The most efficient study design to evaluate the effect of genistein upon normal and abnormal bladder epithelium would have had patients serve as their own controls. However, it was believed that a predrug/biopsy was so beyond the standard of care the accrual would have been impossible – particularly for patients with non-muscle invasive disease (80% of subjects). Thus an interpretation of any effect of Genistein must be interpreted in the context that stratification permitted comparison of similar groups.
While our study is the first prospective clinical trial to observe a significant biological effect of genistein in bladder epithelium, other studies have also observed evidence of genistein’s potential anti-neoplastic effects. An epidemiologic study in Shanghai found that individuals with higher soy product exposure by self-report had a significantly higher, rather than lower, risk of developing bladder cancer. While the authors did control for a variety of risk factors, only 61 bladder cancers (54 histologically confirmed) developed in 18,244 men over 10 years of follow-up (0.026% per patient-year). Even for the highest quartile, daily mean soy isoflavone ingestion was 1–2% of the 300 mg genistein dose in this study. The authors postulated that byproducts were formed during tofu production using soybeans soaked in possibly carcinogenic chlorinated municipal Shanghai water (46–47). Other epidemiologic studies have reported a protective effect for dietary soy on other cancers such as breast and prostate (48–52).
Occasionally, patients with bladder cancer in the US have diets high in soy. This was the case in four subjects, as determined by detectable or “therapeutic” levels of genistein in their serum at study entry. Dietary logs or questionnaires could not really determine whether this was a chronic issue or reflected large quantities ingested for brief periods immediately preceding study entry. However, if these four subjects are eliminated, the effects of genistein were even more dramatic on EGFR phosphorylation (not shown).
The phytoestrogenic properties of genistein were not investigated in this study (13,14, 53). While a minority of subjects were postmenopausal women, hormonal landmarks were not recorded. Bladder cancer is primarily a disease of men (54), but ERβ has been implicated as a promoting factor for disease progression (55). Indeed, selective ER modifiers (SERMs) have been tested in experimental systems with some protective effects against bladder cancer (56). As stated earlier whether variable estrogenic effects by genistein could explain a possible bimodal effect in bladder epithelium is unknown. Clinical trials are ongoing with SERMs, but it is unclear where substances with estrogenic properties fit into human bladder carcinogenesis and progression.
While genistein was, in part, selected because it had multiple effects on urothelial cancer cells in culture, in vivo, and in other tumor systems, the primary effect assessed in this study was inhibition of EGFR phosphorylation. The effects of 300 mg genistein/day on EGFR phosphorylation were more dramatic than the 600 mg/day dose; and those who had a response tended towards having reduced downstream signaling, e.g. MAPK phosphorylation. That these secondary effects did not reach statistical significance is perhaps more reflective of study design and numbers of subjects than lack of effect. Additionally, effects on other pertinent downstream events such as proliferation (determined by Ki-67 expression), apoptosis, (caspase 3 expression), and apoptotic-inhibiting markers such as survivin, were not seen, which may indicate more molecular redundancy in bladder cancer than lack of an effect of genistein per se. However, it is unlikely that this agent or oral soy products, when used alone, would have successful preventive or therapeutic properties. To be clinically active, such products would probably need to be combined with other treatments.
Acknowledgments
Grant Support: The work was supported by NIH contract N01 CN35153 and grants P30 CA014520 and 1UL1RR025011.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009 Jan 1;115(1):68–74. doi: 10.1002/cncr.23986. [DOI] [PubMed] [Google Scholar]
- 3.Messing EM. Campbell-Walsh Urology. 9th ed. Philadelphia: Saunders Elsevier; 2007. Urothelial Tumors of the Bladder. [Google Scholar]
- 4.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21(18):1315–1330. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 5.Messing EM, Young TB, Hunt VB, Gilchrist KW, Newton MA, Bram LL, et al. Comparison of bladder cancer outcome in men undergoing hematuria home screening versus those with standard clinical presentations. Urology. 1995 Mar;45(3):387–397. doi: 10.1016/s0090-4295(99)80006-5. [DOI] [PubMed] [Google Scholar]
- 6.Borhan A, Reeder JE, O'Connell MJ, Wright KO, Wheeless LL, di Sant'Agnese PA, et al. Grade progression and regression in recurrent urothelial cancer. J Urol. 2003;169:2106–2109. doi: 10.1097/01.ju.0000067160.09881.45. [DOI] [PubMed] [Google Scholar]
- 7.Messing EM. Clinical implications of the expression of epidermal growth factor receptors in human transitional cell carcinoma. Cancer Res. 1990 Apr 15;50(8):2530–2537. [PubMed] [Google Scholar]
- 8.Black PC, Dinney CP. Growth factors and receptors as prognostic markers in urothelial carcinoma. Curr Urol Rep. 2008 Jan;9(1):55–61. doi: 10.1007/s11934-008-0011-6. [DOI] [PubMed] [Google Scholar]
- 9.Messing E, Kim KM, Sharkey F, Schultz M, Parnes H, Kim D, et al. Randomized prospective phase III trial of difluoromethylornithine vs placebo in preventing recurrence of completely resected low risk superficial bladder cancer. J Urol. 2006 Aug;176(2):500–504. doi: 10.1016/j.juro.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 10.Golijanin DJ, Kakiashvili D, Madeb RR, Messing EM, Lerner SP. Chemoprevention of bladder cancer. World J Urol. 2006 Nov;24(5):445–472. doi: 10.1007/s00345-006-0123-x. [DOI] [PubMed] [Google Scholar]
- 11.Studer UE. Advanced prostatic carcinoma--which hormone therapy when? Urologe A. 1995 Sep;34(5):361–366. [PubMed] [Google Scholar]
- 12.Peterson G. Evaluation of the biochemical targets of genistein in tumor cells. J Nutr. 1995 Mar;125(3 Suppl):784S–789S. doi: 10.1093/jn/125.suppl_3.784S. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh CY, Santell RC, Haslam SZ, Helferich WG. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998 Sep 1;58(17):3833–3838. Erratum in: Cancer Res 1999 Mar 15;59(6):1388. [PubMed] [Google Scholar]
- 14.Rowell C, Carpenter DM, Lamartiniere CA. Chemoprevention of breast cancer, proteomic discovery of genistein action in the rat mammary gland. J Nutr. 2005 Dec;135(12 Suppl):2953S–2959S. doi: 10.1093/jn/135.12.2953S. [DOI] [PubMed] [Google Scholar]
- 15.Pavese JM, Farmer RL, Bergan RC. Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 2010 Sep;29(3):465–482. doi: 10.1007/s10555-010-9238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Sarkar FH. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res. 2002 Jul;8(7):2369–2377. [PubMed] [Google Scholar]
- 17.Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH. Inactivation of NF-kappaB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene. 2003 Jul 24;22(30):4702–4709. doi: 10.1038/sj.onc.1206583. [DOI] [PubMed] [Google Scholar]
- 18.Chinni SR, Alhasan SA, Multani AS, Pathak S, Sarkar FH. Pleotropic effects of genistein on MCF-7 breast cancer cells. Int J Mol Med. 2003 Jul;12(1):29–34. [PubMed] [Google Scholar]
- 19.Kim SH, Kim SH, Kim YB, Jeon YT, Lee SC, Song YS. Genistein inhibits cell growth by modulating various mitogen-activated protein kinases and AKT in cervical cancer cells. Ann N Y Acad Sci. 2009 Aug;1171:495–500. doi: 10.1111/j.1749-6632.2009.04899.x. [DOI] [PubMed] [Google Scholar]
- 20.Sliva D. Suppression of cancer invasiveness by dietary compounds. Mini Rev Med Chem. 2008 Jun;8(7):677–688. doi: 10.2174/138955708784567412. [DOI] [PubMed] [Google Scholar]
- 21.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- 22.Akiyama T, Ogawara H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-w. [DOI] [PubMed] [Google Scholar]
- 23.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21:113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 24.Silverman DT, Hartge P, Morrison AS, Devesa SS. Epidemiology of bladder cancer. Hematol Oncol Clin North Amer. 1992;6:1–30. [PubMed] [Google Scholar]
- 25.Su SJ, Yeh TM, Lei HY, Chow NH. The potential of soybean foods as a chemoprevention approach for human urinary tract cancer. Clin Cancer Res. 2000;6:230–236. [PubMed] [Google Scholar]
- 26.Theodorescu D, Laderoute KR, Calaoagan JM, Guilding KM. Inhibition of human bladder cancer cell motility by genistein is dependent on epidermal growth factor receptor but not p21ras gene expression. Int J Cancer. 1998;78:775–782. doi: 10.1002/(sici)1097-0215(19981209)78:6<775::aid-ijc16>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 27.Liang YC, Huang YT, Tsai SH, Lin-Shiau SY, Chen CF, Lin JK. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis. 1999;20:1945–1952. doi: 10.1093/carcin/20.10.1945. [DOI] [PubMed] [Google Scholar]
- 28.Swami S, Krishnan AV, Moreno J, Bhattacharyya RB, Peehl DM, Feldman D. Calcitriol and genistein actions to inhibit the prostaglandin pathway: potential combination therapy to treat prostate cancer. J Nutr. 2007 Jan;137(1 Suppl):205S–210S. doi: 10.1093/jn/137.1.205S. [DOI] [PubMed] [Google Scholar]
- 29.Swami S, Krishnan AV, Moreno J, Bhattacharyya RS, Gardner C, Brooks JD, et al. Inhibition of prostaglandin synthesis and actions by genistein in human prostate cancer cells and by soy isoflavones in prostate cancer patients. Int J Cancer. 2009 May 1;124(9):2050–2059. doi: 10.1002/ijc.24161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akarasereenont PC, Techatraisak K, Thaworn A, Chotewuttakorn S. The expression of COX-2 in VEGF-treated endothelial cells is mediated through protein tyrosine kinase. Mediators Inflamm. 2002;11:17–22. doi: 10.1080/09629350210311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith SD, Wheeler MA, Plescia J, Colberg JW, Weiss RM, Altieri DC. Urine detection of survivin and diagnosis of bladder cancer. JAMA. 2001 Jan 17;285(3):324–328. doi: 10.1001/jama.285.3.324. [DOI] [PubMed] [Google Scholar]
- 32.Konety BR, Nguyen TS, Dhir R, Day RS, Becich MJ, Stadler WM, et al. Detection of bladder cancer using a novel nuclear matrix protein, BLCA-4. Clin Cancer Res. 2000 Jul;6(7):2618–2625. [PubMed] [Google Scholar]
- 33.Hausladen DA, Wheeler MA, Altieri DC, Colberg JW, Weiss RM. Effect of intravesical treatment of transitional cell carcinoma with bacillus Calmette-Guerin and mitomycin C on urinary survivin levels and outcome. J Urol. 2003 Jul;170(1):230–234. doi: 10.1097/01.ju.0000063685.29339.24. [DOI] [PubMed] [Google Scholar]
- 34.Bloedon LT, Jeffcoat AR, Lopaczynski W, Schell MJ, Black TM, Dix KJ, et al. Safety and pharmacokinetics of purified soy isoflavones: single-dose administration to postmenopausal women. Am J Clin Nutr. 2002 Nov;76(5):1126–1137. doi: 10.1093/ajcn/76.5.1126. [DOI] [PubMed] [Google Scholar]
- 35.Busby MG, Jeffcoat AR, Bloedon LT, Koch MA, Black T, Dix KJ, et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr. 2002;75:126–136. doi: 10.1093/ajcn/75.1.126. [DOI] [PubMed] [Google Scholar]
- 36.Miltyk W, Craciunescu CN, Fischer L, Jeffcoat RA, Koch MA, Lopaczynski W, et al. Lack of significant genotoxicity of purified soy isoflavones (genistein, daidzein, and glycitein) in 20 patients with prostate cancer. Am J Clin Nutr. 2003;77:875–882. doi: 10.1093/ajcn/77.4.875. [DOI] [PubMed] [Google Scholar]
- 37.King RA, Bursill DB. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am J Clin Nutr. 1998;67:867–872. doi: 10.1093/ajcn/67.5.867. [DOI] [PubMed] [Google Scholar]
- 38.Van Le TS, Miller R, Barder T, Babjuk M, Potter DM, Getzenberg RH. Highly specific urine-based marker of bladder cancer. Urology. 2005 Dec;66(6):1256–1260. doi: 10.1016/j.urology.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Shim V, Gauthier ML, Sudilovsky D, Mantei K, Chew KL, Moore DH, et al. Cyclooxygenase-2 expression is related to nuclear grade in ductal carcinoma in situ and is increased in its normal adjacent epithelium. Cancer Res. 2003 May 15;63(10):2347–2350. [PubMed] [Google Scholar]
- 40.Rojas M, Yao S, Lin YZ. Controlling epidermal growth factor (EGF)-stimulated Ras activation in intact cells by a cell-permeable peptide mimicking phosphorylated EGF receptor. J Biol Chem. 1996 Nov 1;271(44):27456–27161. doi: 10.1074/jbc.271.44.27456. [DOI] [PubMed] [Google Scholar]
- 41.Calvo E, Malik SN, Siu LL, Baillargeon GM, Irish J, Chin SF, et al. Assessment of erlotinib pharmacodynamics in tumors and skin of patients with head and neck cancer. Ann Oncol. 2007 Apr;18(4):761–767. doi: 10.1093/annonc/mdl495. [DOI] [PubMed] [Google Scholar]
- 42.Gardner CD, Chatterjee LM, Franke AA. Effects of isoflavone supplements vs. soy foods on blood concentrations of genistein and daidzein in adults. J Nutr Biochem. 2009 Mar;20(3):227–234. doi: 10.1016/j.jnutbio.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vergne S, Titier K, Bernard V, Asselineau J, Durand M, Lamothe V, et al. Bioavailability and urinary excretion of isoflavones in humans: effects of soy-based supplements formulation and equol production. J Pharm Biomed Anal. 2007 Mar 12;43(4):1488–1494. doi: 10.1016/j.jpba.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Raju J, Swamy MV, Cooma I, Patlolla JM, Pittman B, Reddy BS, et al. Low doses of beta-carotene and lutein inhibit AOM-induced rat colonic ACF formation but high doses augment ACF incidence. Int J Cancer. 2005 Feb;20(113)(5):798–802. doi: 10.1002/ijc.20640. [DOI] [PubMed] [Google Scholar]
- 45.Takimoto CH, Glover K, Huang X, Hayes SA, Gallot L, Quinn M, Jovanovic BD, Shapiro A, Hernandez L, Goetz A, Llorens V, Lieberman R, Crowell JA, Poisson BA, Bergan RC. Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones administered to individuals with cancer. Cancer Epidemiol Biomarkers Prev. 2003 Nov;12(11 Pt 1):1213–1221. [PubMed] [Google Scholar]
- 46.Sun CL, Yuan JM, Arakawa K, Low SH, Lee HP, Yu MC. Dietary soy and increased risk of bladder cancer: the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev. 2002 Dec;11(12):1674–1677. [PubMed] [Google Scholar]
- 47.Sun CL, Yuan JM, Wang XL, Gao YT, Ross RK, Yu MC. Dietary soy and increased risk of bladder cancer: A prospective cohort study of men in Shanghai, China. Int J Cancer. 2004;112:319–323. doi: 10.1002/ijc.20384. [DOI] [PubMed] [Google Scholar]
- 48.Wu AH, Koh WP, Wang R, Lee HP, Yu MC. Soy intake and breast cancer risk in Singapore Chinese Health Study. Br J Cancer. 2008 Jul 8;99(1):196–200. doi: 10.1038/sj.bjc.6604448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Messina M, Barnes S. The role of soy products in reducing risk of cancer. J Natl Cancer Inst. 1991 Apr 17;83(8):541–546. doi: 10.1093/jnci/83.8.541. [DOI] [PubMed] [Google Scholar]
- 50.Jian L. Soy, isoflavones, and prostate cancer. Mol Nutr Food Res. 2009 Feb;53(2):217–226. doi: 10.1002/mnfr.200800167. [DOI] [PubMed] [Google Scholar]
- 51.Messina M, Watanabe S, Setchell KD. Report on the 8th International Symposium on the Role of Soy in Health Promotion and Chronic Disease Prevention and Treatment. J Nutr. 2009 Apr;139(4):796S–802S. doi: 10.3945/jn.108.104182. Epub 2009 Feb 18. [DOI] [PubMed] [Google Scholar]
- 52.Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008 Oct 8;269(2):226–242. doi: 10.1016/j.canlet.2008.03.052. Epub 2008 May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miodini P, Fioravanti L, DiFronzo G, Cappelletti V. The two phyto-oestrogens genistein and quercetin exert different effects on oestrogen receptor function. BJU. 1999;80:1150–1155. doi: 10.1038/sj.bjc.6690479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009 Jan 1;115(1):68–74. doi: 10.1002/cncr.23986. [DOI] [PubMed] [Google Scholar]
- 55.Shen SS, Smith CL, Hsieh JT, Yu J, Kim IY, Jian W, et al. Expression of estrogen receptors-alpha and -beta in bladder cancer cell lines and human bladder tumor tissue. Cancer. 2006 Jun 15;106(12):2610–2616. doi: 10.1002/cncr.21945. [DOI] [PubMed] [Google Scholar]
- 56.Sonpavde G, Okuno N, Weiss H, Yu J, Shen SS, Younes M, et al. Efficacy of selective estrogen receptor modulators in nude mice bearing human transitional cell carcinoma. Urology. 2007 Jun;69(6):1221–1226. doi: 10.1016/j.urology.2007.02.041. [DOI] [PubMed] [Google Scholar]


