Abstract
TIGIT is a newly identified receptor expressed on T cells that binds to CD155 on the dendritic cell surface driving them to a more tolerogenic phenotype. Given that TIGIT contains an ITIM motif in its intracellular domain and considering the potential importance of the TIGIT/CD226 pathway in human autoimmune disease, we investigated the specific role of TIGIT in human CD4+ T cells. Using an agonistic anti-TIGIT mAb, we demonstrate a direct inhibitory effect on T cell proliferation with a decrease in expression of T-bet, GATA3, IRF4 and RORc with inhibition of cytokine production, predominately IFNγ. Knockdown of TIGIT expression by shRNA resulted in an increase of both T-bet and IFNγ mRNA and protein expression with concomitant decrease in IL-10 expression. Increases in IFNγ with TIGIT knockdown could be overcome by blocking CD226 signaling indicating that TIGIT exerts immunosuppressive effects by competing with CD226 for the same CD155 ligand. These data demonstrate that TIGIT can inhibit T cell functions by competing with CD226 and can also directly inhibit T cells in a T cell intrinsic manner. Our results provide evidence for a novel role of this alternative co-stimulatory pathway in regulating human T cell responses associated with autoimmune disease.
Introduction
T cells are driven into cell cycle after T cell receptor recognition of antigen presented by major histocompatibility complex (MHC) and a “second signal” first elucidated with the CD80/CD86 — CD28 co-stimulatory pathway (1). While this has become a major tenant in immunology, it has become clear that there are a multitude of signals integrated by the T cell ultimately leading to either entry into cell cycle, expansion and differentiation or to non-responsiveness or apoptosis. Curiously, many of these costimulatory pathways have multiple receptor-ligand pairs one of which results in positive signaling events while the other transmits negative signals, such as CD28/CTLA-4 (2). A first step in deciphering how T cells integrate these second signals requires an understanding of the individual components of the costimulatory pathways.
Genome wide association scans in patients with autoimmune diseases have identified allelic variants in a number of T cell co-stimulatory molecular pathways as genetic risk factors for disease pathogenesis. This includes allelic variants in the CTLA-4 — CD86 pathway (3–5) and CD226 that has been associated with risk to develop both type 1 diabetes and multiple sclerosis (6, 7). Costimulatory pathways regulate the functional outcome of T cell activation and allelic variations altering the balance between positive and negative costimulatory signals may increase the risk of autoimmune diseases (2). These genetic investigations may be particularly useful in focusing on key nodal points that modulate immune response.
CD226 was first identified by Shibuya and coworkers as having a role in enhancing cytotoxic function of NK cells demonstrating that CD226 is associated with LFA-1 to induce IFNγ production in naïve CD4+ T cells (8, 9). CD226 binds to two different cell surface ligands, CD155 (poliovirus receptor, PVR) and CD112 (10, 11). T cell Ig and ITIM domain (TIGIT) is a transmembrane glycoprotein belonging to a PVR family of type 1 proteins that also binds to CD155 and CD112 ligands (12). TIGIT is expressed on peripheral memory and regulatory CD4+ T cells and NK cells and is up-regulated following activation on naïve CD4+ T cells. CD155 is a high-affinity receptor for TIGIT expressed on monocytes and CD11c+ human dendritic cells (DCs) (12). TIGIT contains an Ig-like V-type (immunoglobulin-like) domain and an ITIM in its cytoplasmic domain suggesting that receptor occupancy may trigger a negative signaling event. Similar to the costimulatory CD28/CTLA-4 — CD80/CD86 pathway where CD28 engagement induces positive and CTLA-4 negative signaling while the CTLA-4/CD28 pathway has been intensively investigated, little is known regarding the TIGIT/CD226 pathway, particularly in human T cells.
Grogan and co-workers described that engagement of TIGIT by CD155 on human DCs enhanced the production of interleukin 10 while diminishing the production of interleukin 12p40 (12). In addition to effects on APCs, we demonstrated a T-cell intrinsic, inhibitory effect of TIGIT dependent T cell-signaling pathway in murine models of experimental autoimmune encephalomyelitis (EAE) (13). Specifically, we demonstrated that loss of TIGIT expression in mice results in hyperproliferative T cell responses and increased susceptibility to EAE. By generating an agonistic anti-TIGIT mAb, we demonstrated that TIGIT directly inhibited murine T cell responses even in the absence of APCs, demonstrating a T cell intrinsic inhibitory effect of TIGIT.
Considering the potential importance of the TIGIT/CD226 pathway in human autoimmune disease and based on our murine studies (13), we investigated the specific role of TIGIT in human CD4+ T cell activation in the absence of APCs where CD155 expression on T cells provided a co-stimulatory signal. Using an agonistic anti-TIGIT mAb, we demonstrate a direct inhibitory effect on T cell proliferation with a decrease in expression of T-bet, GATA3, IRF4 and RORc with inhibition of cytokine production, predominately IFNγ. Knockdown of TIGIT expression by short hairpin RNA (shRNA) expression constructs introduced into human CD4+ T cells resulted in an increase of both T-bet and IFNγ mRNA and protein expression. These effects could be overcome by blocking CD226 signaling indicating that TIGIT exerts immunosuppressive effects by competing with CD226 for the same ligand CD155. Furthermore, we also show that TIGIT expression and function are not altered in untreated relapsing remitting (RR) multiple sclerosis (MS) patients, suggesting that this pathway could potentially be targeted therapeutically to inhibit the hyperactivated status of memory CD4+ T cells in patients. These data provide evidence for a novel role of this alternative co-stimulatory pathway in regulating human T cell responses associated with autoimmune disease.
Materials and Methods
Cell-culture reagents and antibodies
Peripheral venous blood was obtained from healthy control volunteers in compliance with Institutional Review Board protocols at Yale University School of Medicine. Total CD4+ T cells were isolated by negative selection (CD4+ T cell isolation kit II, Miltenyi Biotec, Auburn, CA) and sorted on a FACS Aria (BD Biosciences, San Jose, CA).
Cells were cultured in 96-well plates in RPMI 1640 medium with 5% human AB serum (Gemini Bio-Products, Woodland, CA). The antibodies used for stimulation were anti-CD3 (clone UCHT1) and anti-CD28 (clone 28.2) (BD Biosciences) at 1 µg/ml. Recombinant human IL-2 was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) and was used at 10 U/ml.
Antibodies to human TIGIT (VSTM3) were kindly provided by ZymoGenetics, Inc. (a wholly-owned subsidiary of Bristol-Myers Squibb) and were generated by immunizing BALB/C mice with soluble human TIGIT protein (14). None of the five mAbs against human TIGIT were capable of blocking the binding of soluble human TIGIT to cells expressing human CD155. However, two of these mAbs showed an agonistic activity measured in T cell proliferation assays (14). For our functional assays, we use the clone 318.28.2.1 (isotype IgG1) plate-bound at 50 µg/ml. The endotoxin level in the antibody solution was <1EU/ml. All antibodies were dialyzed before performing functional assays. Mouse Anti-TIGIT Functional Grade Monoclonal Antibody (clone MBSA43) was obtained from eBioscience (San Diego, CA). Blocking anti-human CD155 (clone SKII.4) and LEAF™ purified mouse IgG1 isotype control were purchased from Biolegend (San Diego, CA) and blocking anti-human CD226 (clone DX11) from Abcam (Cambridge, MA).
Proliferation Assay and Apoptosis
Carboxyfluoresceinsuccinimidyl ester (CFSE)–labeled memory T cells (104/well) were in the presence of plate-bound agonistic anti-TIGIT at 50 µg/ml or IgG isotype control. Cells were stimulated anti-CD3, anti-CD28 and IL-2 (10 U/ml). Fluorescence was assessed on day 5 of stimulation on a LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar, Ashland, OR). Apoptosis was measured with FITC Annexin V Apoptosis detection kit I (BD Biosciences) according to the manufacturer’s instructions.
Cell Activation and Intracellular Staining
Cell populations were stimulated with anti-CD3, anti-CD28 and IL-2 in the presence of agonistic anti-TIGIT. At day 4, cells were restimulated with PMA (50 ng/mL) and ionomycin (250 ng/mL) for 4 hours in the presence of GolgiStop (BD Biosciences). Cells were fixed and made permeable (Foxp3 Staining Buffer Set; eBioscience) and incubated with anti-IFNγ (clone B27), anti-IL13 (clone JES10-5A2), anti-IL4 (clone 8D4-8), anti-IL9 (clone MH9A4) and anti-IL17 (clone BL168) from Biolegend. Staining with LIVE/DEAD Fixable Dead Cell Stain Kit (Molecular Probes) was performed before fixation to allow gating on viable cells.
Enzyme-Linked Immunosorbent Assay
ELISA measurement of IFNγ and IL-10 were performed with purified coating and biotinylated detection antibodies: human IFNγ mAb (clone 2G1) and human IFNγ mAb biotin-labeled (Thermo Scientific, Rockford, IL) and rat anti-human and viral IL-10 and biotin anti-human and viral IL-10 (BD Biosciences).
Quantification of mRNA expression levels by real time PCR
RNA was isolated using QIAGEN RNeasy Micro Kit (QIAGEN, Valencia, CA) and converted to cDNA by RT with random hexamers and Multiscribe RT (TQMN, Reverse Transcription Reagents; Applied Biosystems, Foster City, CA). For mRNA gene expression assays, probes were purchased from Applied Biosystems and the reactions run on a 7500 Fast Real-Time PCR System (Applied Biosystems). To evaluate IL-2 gene expression, memory T cells were incubated in the presence of anti-TIGIT mAb or an isotype control for 2 days (probe Hs_00914135_m1 from Applied Biosystems). Values are represented as the difference in Ct values normalized to β2-microglobulin for each sample as per the following formula: Relative RNA expression = (2−dCt) × 1000.
CD226 silencing by siRNA
Predesigned siRNA for human CD226 and a negative control siRNA were obtained from Applied Biosystems. Purified CD4+ T cells (1 × 106) were transfected with either negative control siRNA or CD226 siRNA (s20972) by electroporation, using the Nucleofactor Device (Amaxa Inc.). Transfected cells were incubated at 37°C for 30 min, followed by addition of 20 U/ml of human IL-2 and transfer of the cells to a 96-well plate coated with anti-CD3/CD28 mAbs. On day 1 and 2 after transfection, cells were collected for RNA extraction to assess gene expression. On day 3 CD226 surface expression and IFNγ production were determined.
Lentiviral shRNA Knock Down of CD226 and TIGIT
Lentiviruses expressing shRNAs were obtained from the library of The RNAi Consortium (TRC) (15). We initially tested five different hairpins directed against CD226 and TIGIT and compared their efficiency by real-time PCR against a control hairpin. Tested target sequences are shown in Supplemental Table 1, indicated as shCD226, shTIGIT-1 and shTIGIT-2 were the most efficient (Suppl. Table 1).
Human CD4+ T cells were plated on a 96-well plate at a concentration of 105 cells per well and activated with anti-CD3 and anti-CD28 at 1 µg/ml. After 24 h, the medium was carefully removed, we added 22 µl of virus and polybrene at 6 µg/ml and we centrifuged the plate at 2250 rpm for 30 min at RT. After the centrifugation, all medium was removed and fresh supplemented RPMI medium were added. After 48 hr cells were selected with 2 µg/ml of puromycin and collected for analysis 72 hr after selection.
Statistical analysis
A standard 2-tailed Student’s t test was used for statistical analysis; P values less than 0.05 were considered statistically significant.
Results
TIGIT, CD155 and CD226 are upregulated on human CD4+ T cells after activation
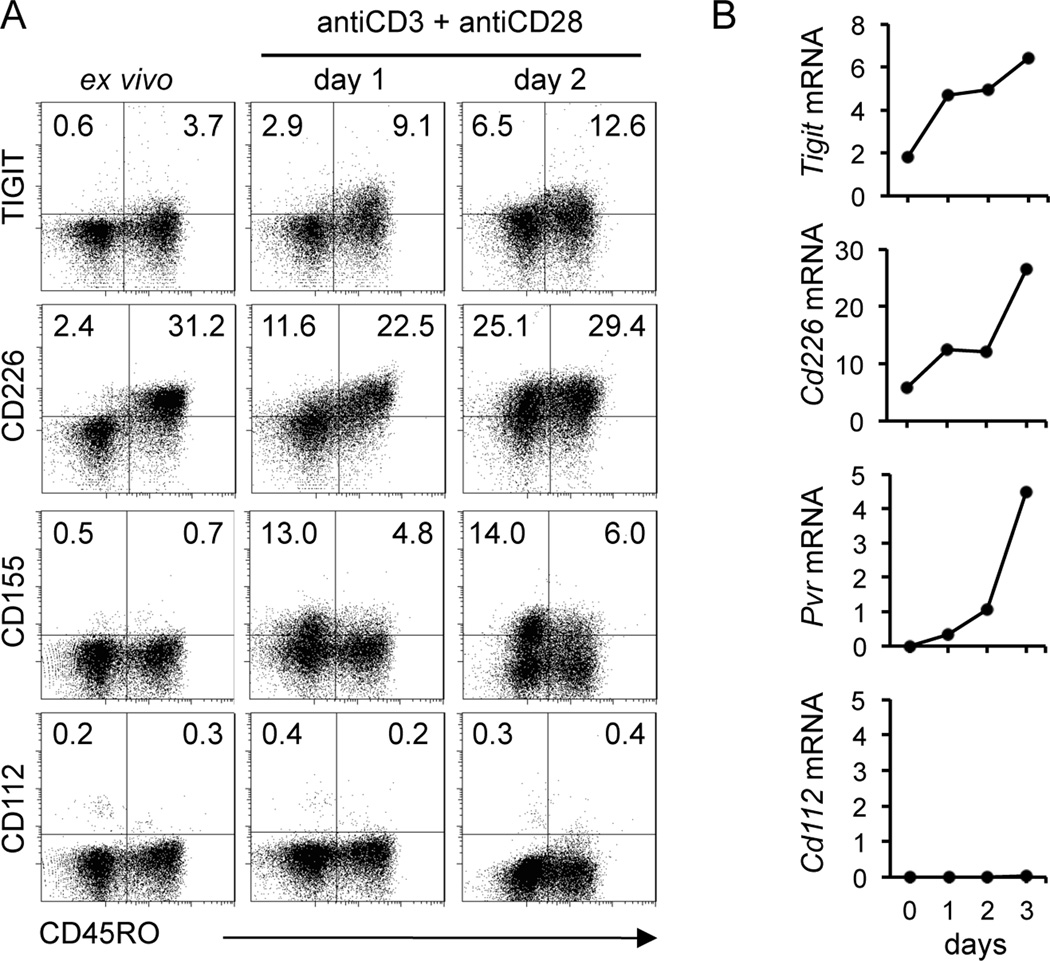
To investigate the role of TIGIT on T cell function, we began by examining TIGIT expression in human CD4+ T cells ex vivo and after activation with anti-CD3 and anti-CD28 mAbs. As shown in Figure 1, TIGIT was not expressed on ex vivo naïve T cells (CD4+ CD45RO−) but its expression was induced upon stimulation in the CD45RO+ population. Similarly, we confirmed the upregulation of CD226 in both naïve and memory populations. Interestingly, CD155, the ligand for both CD226 and TIGIT, was also induced after activation, both on naïve and memory T cell, although there was a higher frequency of CD155+ T cells in CD45RO− population (Fig. 1A). We confirmed upregulation of TIGIT, CD226 and CD155 gene expression by real-time RT-PCR on FACS-sorted memory T cells (Fig. 1B). The second ligand for CD226 and TIGIT, CD112 was not expressed on CD4+ T cells ex vivo and was not upregulated upon activation.
Figure 1.
Upregulation of TIGIT, CD226 and CD155 in human CD4+ T cells after activation. (A) Dot plots displaying the cell surface expression of TIGIT, CD226, CD155 and CD112 on naïve CD4+ T cells (CD45RO−) and memory T cells (CD45RO+) ex vivo and after activation with anti-CD3 and anti-CD28 for 2 days. (B) Kinetics of gene expression of TIGIT, CD226 and their ligands CD155 and CD112 on sorted memory T cells (CD4+ CD62L+ CD25lowCD45RO+). Data are normalized relative to beta 2 microglobulin. Data are representative of four donors.
TIGIT signaling inhibits T cell proliferation
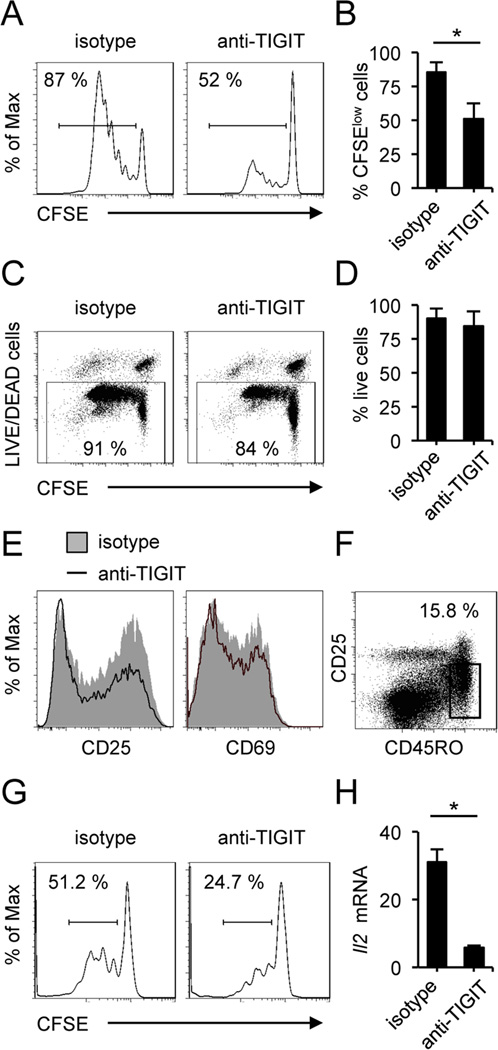
As TIGIT contains an ITIM that could trigger a negative regulation of T cell functions, we investigated whether TIGIT can also directly inhibit T cell proliferation. T cells were stimulated in the presence of an agonistic anti-TIGIT mAb (clone 318.28.2.1) for 5 days and T cell proliferation was assessed by CFSE dilution assay after gating on viable cells. As depicted in Figure 2, this agonistic anti-TIGIT mAb efficiently inhibited human CD4+ T cell proliferation (Fig. 2A and 2B). No significant differences were observed in frequency of viable cells (Fig. 2C and 2D). Recently a new functional antibody against human TIGIT (clone MBSA43) has been generated; when we tested this antibody in the same conditions, we did not find an effect on T cell proliferation, although in certain conditions, we observed a slight increase in IFNγ production. (Supplemental Fig. 1). Furthermore, TIGIT ligation did not cause a significant increase in the frequency of early or late apoptotic cells (Supplemental Fig. 2). To address the mechanism by which TIGIT engagement leads to inhibition of proliferation, we evaluated the expression of cell-surface markers of activation by flow cytometry. When primary CD4+ T cells were cultured in the presence of the agonistic anti-TIGIT (clone 318.28.2.1), they showed decreased expression of CD25 and CD69 as compared to isotype control (Fig. 2E). To exclude the possibility that anti-TIGIT could act on the small percentage of regulatory T cells increasing suppression, we isolated CD4+CD62L+CD45RO+CD25low memory T cells by FACS sorting (Fig. 2F) and repeated proliferation assays excluding any other cell type. We confirmed that TIGIT has intrinsic inhibitory effect on human memory T cell proliferation (Fig. 2G). To better characterize the mechanism by which TIGIT decreases T cell proliferation, we also quantified the production of IL-2 by real-time PCR. Importantly, IL-2 gene expression was significantly downregulated in the presence of anti-TIGIT (clone 318.28.2.1) (Fig. 2H), consistent with the decrease in TCR signaling after TIGIT ligation. Taken together, these data indicate the engagement of TIGIT by its ligands can deliver an inhibitory signal resulting in decreased T cell activation, IL-2 production and T cell receptor-mediated proliferation.
Figure 2.
TIGIT cell-intrinsic signaling inhibits T cell proliferation. (A) Proliferation of human CD4+ T cells labeled with CFSE and activated with anti-CD3 and anti-CD28 with plate-bound agonistic anti-TIGIT or isotype-matched control antibody. On day 5, CFSE dilution was analyzed by flow cytometry after gating on viable cells. (B) Percentages of dividing cells after activation represent the CFSElow cells (n=8). Mean values are shown ± SD. (C) Viability was assessed in all the experiments. (D) No significant differences were observed in the frequency of viable cells. (E) Flow cytometry of the surface expression of CD25 and CD69 as markers of activation of human CD4+ T cells activated in presence of agonistic anti-TIGIT or isotype control, assessed on day 5 after activation. (F) Sorting strategy for memory T cells (CD4+ CD62L+ CD25low CD45RO+) purity. (G) CFSE proliferation assay of memory T cells in the presence of agonistic anti-TIGIT. Proliferation was assessed on day 5 after gating on viable cells. Percentages of proliferating cells are depicted above the histograms. (H) Quantitative real-time RT-PCR analysis of relative IL-2 gene expression in FACS-sorted memory T cells. Data shown are representative of four different donors.
Furthermore, no significant differences were found in TIGIT expression on ex vivo and activated FACS-sorted memory T cells from untreated relapsing remitting (RR) MS patients compared to age-matched healthy donors (Supplemental Fig. 3). In agreement with the results obtained with healthy donor T cells, the inhibitory effects of TIGIT signaling are functional in T cells from MS patients, suggesting that activation of TIGIT signaling may represent a new approach for the treatment of human autoimmune disease.
TIGIT signaling inhibits cytokine production
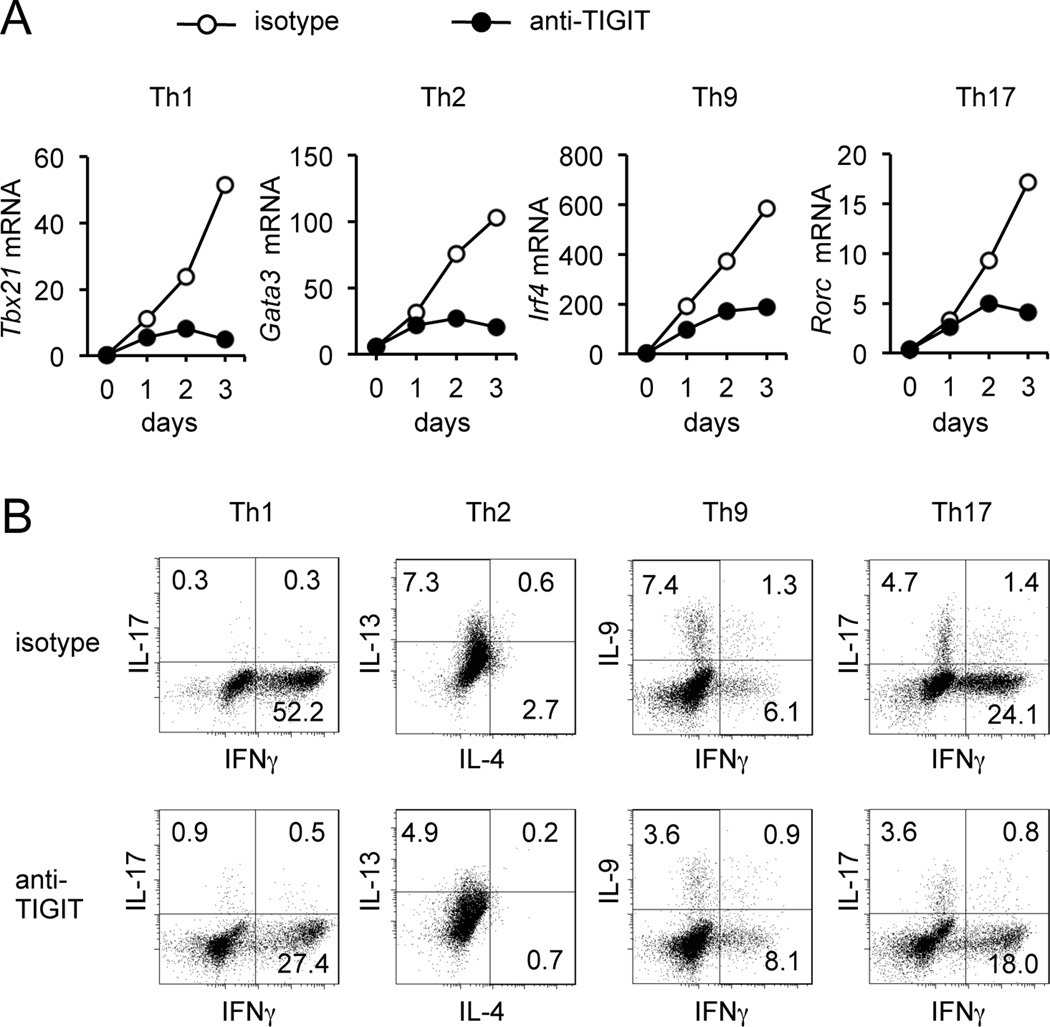
To further study the direct mechanism of the inhibitory effect of TIGIT on T cell function, we then investigated the role of TIGIT signaling on cytokine production by CD4+CD62L+CD45RO+CD25low memory T cells. To specifically investigate whether the effect of TIGIT signaling is more prominent for a specific functional T cell subset, we incubated memory T cells with plate-bound anti-TIGIT (clone 318.28.2.1) or isotype control mAb and stimulated them in Th1, Th2, Th9 and Th17 conditions for 3 days. TIGIT ligation efficiently inhibited expression of transcription factors T-bet, GATA3, IRF4 and RORc specific for regulating Th1, Th2, Th9 and Th17 cells, respectively (Fig. 3A). We next assessed cytokine production by intracellular staining in anti-TIGIT treated cells. Consistent with the decrease in T-bet expression, the frequency of IFNγ-producing T cells was significantly reduced with signaling by anti-TIGIT mAb (Fig. 3B). To a lesser extent, production of IL-13, IL-9 and IL-17 were also reduced in T cells stimulated with anti-TIGIT mAb for 4 days. These data clearly demonstrate that TIGIT suppresses T cell responses directly in a T cell-intrinsic manner.
Figure 3.
TIGIT cell-intrinsic signaling inhibits T cell cytokine production. (A) Time course of transcription factors expression in memory T cells stimulated with IL-12 (Th1) IL-4 (Th2), TGFβ+IL-4 (Th9) and TGFβ+IL-1β/IL-6/IL-21 (Th17) and cultured in presence agonistic anti-TIGIT or isotype control for 3 days. (B) Intracellular cytokine staining for IFNγ, IL-13, IL-4, IL-9 and IL-17 in memory T cells cultured in the same conditions than A for 4 days. Data shown are representative of at least four independent experiments.
CD226 knockdown decreases T-bet expression and IFNγ production in human CD4+ T cells
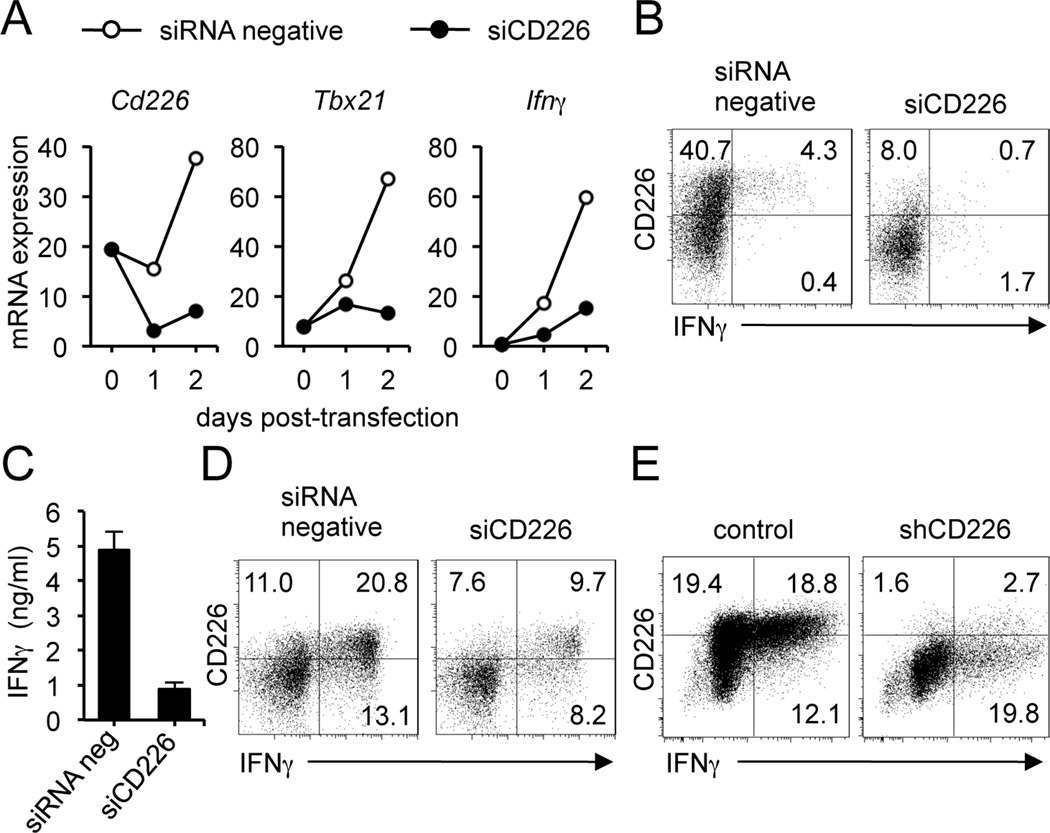
In addition to the inhibitory cell-intrinsic role for TIGIT on T cells, we wished to examine whether TIGIT has a second mechanism of negative immunoregulation by competing with CD226 for the same CD155 ligand. To specifically explore the contribution of the CD226 and TIGIT receptors, we performed a small interfering RNA (siRNA) knockdown of CD226 on ex vivo CD4+ T cells. A decrease in CD226 expression resulted in a decrease in T-bet and IFNγ mRNA expression (Fig. 4A). On day 3, we stimulated cells with PMA and ionomycin for 4 hours and analyzed CD226 surface expression versus IFNγ production, gating on viable cells. As shown in Fig 4B, siCD226 effectively reduced CD226 expression with a concomitant decrease in IFNγ production. We observed similar results by measuring IFNγ levels in the supernatants of cells transfected with siCD226 or siRNA negative (Fig. 4C). After confirming CD226 depletion, cells from replicate wells were stimulated with IL-12 for 3 more days and we observed that decrease in CD226 expression correlates with a lower percentage of IFNγ producing cells (Fig. 4D). We validated these results by lentiviral transduction of short hairpin RNA (shRNA) into human CD4+ T cells to specifically downregulate CD226 expression. We used the vector pLKO-1, containing a gene encoding puromycin resistance, which allowed us to select T cells with stable integration of shRNA constructs. We tested five different shRNA constructs to knockdown CD226, using the target sequence indicated as shCD226 the most efficient in depleting CD226 expression (Table S1). The decrease in CD226 cell surface expression resulted in approximately 27% reduction in IFNγ-producing cells (Fig 4E). Taken together, our data strongly suggest that CD226, possibly through its interaction with CD155, can trigger a positive signal in T cells inducing T-bet expression and IFNγ production.
Figure 4.
Knockdown of CD226 expression in human CD4+ T cells. (A) CD226 gene expression in human CD4+ T cells transfected with specific siCD226 or negative siRNA, assessed by real-time RT-PCR on day 1 and 2 after electroporation. (B) Flow cytometry of the surface expression of CD226 and IFNγ intracellular staining on human CD4+ T cells transfected with siRNA or control, and stimulated with PMA and ionomycin for 4 hr in the presence of GolgiStop. Cells were stained with LIVE/DEAD Fixable Dead Cell Stain Kit before fixation to allow gating on viable cells. (C) Measurement of IFNγ in the supernatants of cells transfected with siCD226 or negative siRNA. (D) Flow cytometry of the surface expression of CD226 and IFNγ intracellular staining on CD4+ T cells transfected as in B, and then stimulated with IL-12 for 3 days. (E) Flow cytometry of the surface expression of CD226 and IFNγ intracellular staining on human CD4+ T cells transduced with shCD226 or control by lentiviral transduction. Data are representative of three independent experiments. Mean values are shown ± SEM.
TIGIT knockdown increases T-bet expression and IFNγ production in human CD4+ T cells
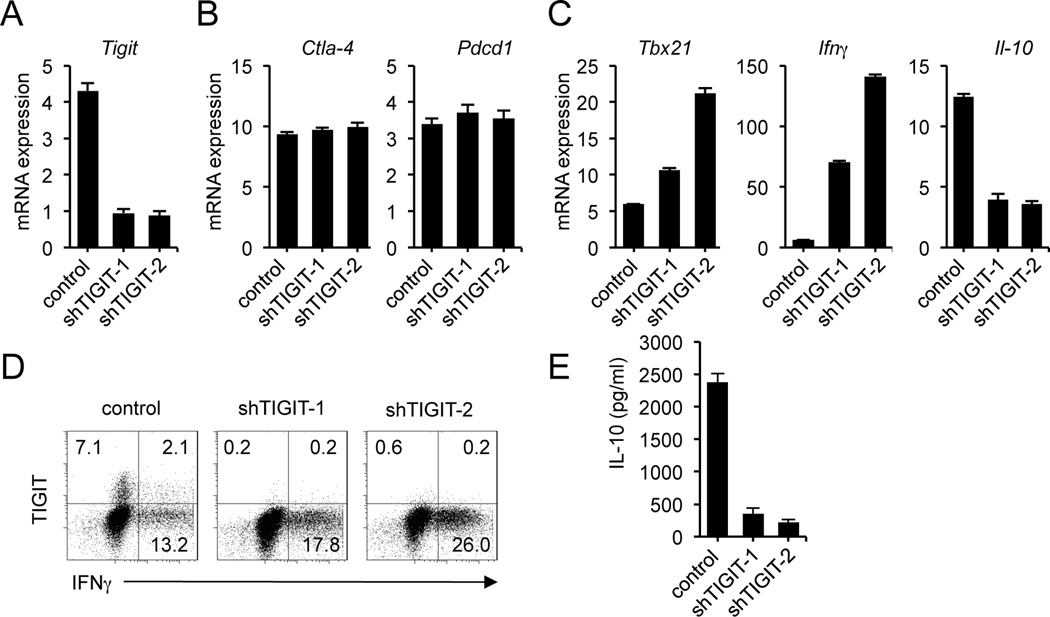
To better understand the role of TIGIT in this regulatory pathway, we used a specific shRNA to stably downregulate TIGIT expression in human CD4+ T cells. We tested five different shRNA constructs to knockdown TIGIT; one directed against the 3′ untranslated region of TIGIT mRNA (shTIGIT-1) and the other against the coding sequence (shTIGIT-2) that were efficient in down-regulating TIGIT expression (Table S1). On day 3 after puromycin selection, shTIGIT-1 and shTIGIT-2 decreased the expression of TIGIT mRNA over 80% (Fig. 5A) but not of other negative T cell regulators such as CTLA-4 and PD-1 (Fig. 5B).
Figure 5.
Knockdown of TIGIT expression in human CD4+ T cells by lentiviral transduction with two specific shRNAs. (A) Quantitative real-time RT-PCR analysis of relative TIGIT gene expression in human CD4+ T cells infected with lentivirus containing two TIGIT-specific shRNA sequences or an empty vector, assessed on day 3 after puromycin selection. (B) Gene expression of other negative regulators, CTLA-4 and PD-1 was measured to exclude non-target effects. (C) Relative quantification of T-bet (Tbx21), IFNγ and IL-10 mRNA in T cells transduced with shTIGIT-1, shTIGIT-2 or an empty vector. (D) Flow cytometry of the surface expression of TIGIT and IFNγ intracellular staining on human CD4+ T cells infected with TIGIT-specific shRNA or control, selected with puromycin and stimulated with PMA and ionomycin for 4 hr in the presence of GolgiStop. Cells were stained with LIVE/DEAD Fixable Dead Cell Stain Kit before fixation to allow gating on viable cells. Data are representative of four independent experiments with three individual donors. (E) Levels of IL-10 in the supernatants derived from the same cultures as in C quantified by ELISA. Mean values are shown ± SEM.
To determine the effect of knocking down TIGIT expression on cytokine production, we examined the expression of transcription factor T-bet (Tbx21), the master transcription factor that induces IFNγ production. Knockdown of TIGIT mRNA expression was accompanied by increases in both T-bet and IFNγ mRNA expression (Fig. 5C). In contrast, deletion of TIGIT resulted in a significant reduction of IL-10 gene expression in cells transduced with both of the shTIGIT-1 and shTIGIT-2 (Fig. 5C) suggesting a central role of TIGIT in reciprocally regulating pro-inflammatory (IFNγ) and anti-inflammatory (IL-10) cytokines.
To confirm these results at the protein level, we stimulated the puromycin-resistant cells with PMA and ionomycin for 4 hrs and then assayed TIGIT expression and cytokine production by intracellular staining. Consistent with a role of TIGIT in reducing T cell responses, knockdown of TIGIT increased IFNγ production in cells transduced with shTIGIT-1 and shTIGIT-2 (Fig. 5D) confirming that TIGIT may act directly by inhibiting IFNγ production in human T cells. Measurement of IL-10 secretion in transduced CD4+ T cells revealed that depletion of TIGIT was accompanied by a decrease in IL-10 production (Fig. 5E), suggesting a cell intrinsic role for TIGIT in IL-10 production in the absence of APCs.
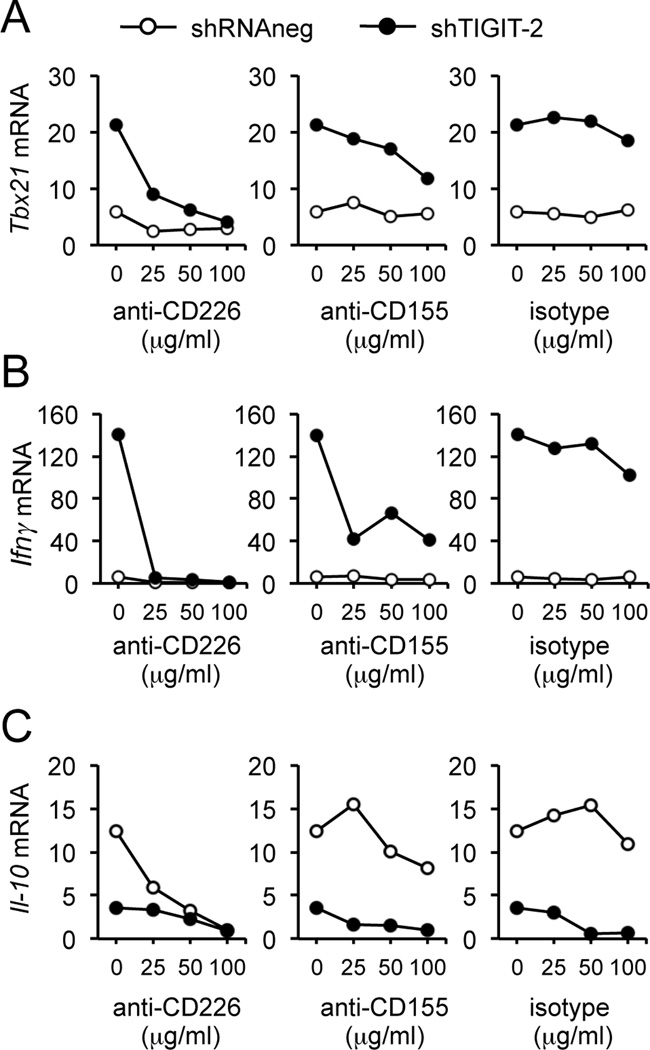
IFNγ secretion after TIGIT knockdown is mediated by CD155 engagement of CD226
To examine the mechanism for the increased production of IFNγ with knockdown of TIGIT, we cultured with mAbs directed against either CD155 or CD226 that were expressed on T cells. As shown in Figure 6, adding either anti-CD155 or anti-CD226 mAbs blocked the increased T-bet and IFNγ gene expression induced by TIGIT knockdown. Interestingly, blockade of CD226 or CD155 also decreased IL-10 gene expression (Fig. 6C). These data indicate that in the absence of TIGIT, CD226-CD155 interaction induces T-bet and IFNγ production whereas IL-10 production requires TIGIT for its induction. These data further support our hypothesis that CD226-CD155 provides positive costimulation to induce IFNγ production whereas TIGIT is required for IL-10 production.
Figure 6.
Blockade of CD226 signaling with anti-CD226 or anti-CD155 decreased the effects of TIGIT knockdown. (A) Quantitative real-time PCR analysis of relative T-bet (Tbx21) expression in TIGIT-depleted CD4+ T cells incubated for 4 days in the presence of increasing concentrations of plate-bound blocking anti-CD226, blocking anti-CD155 or isotype control IgG. (B) IFNγ gene expression. (C) IL-10 gene expression in the same conditions than in A. Data shown are representative of four different donors.
Discussion
Allelic variants in the TIGIT/CD226 pathway have recently been identified as risk factors for developing human autoimmune diseases (7). However, the mechanisms associated with human CD4+ T cell expression of these receptors are not known. Here, we demonstrate that engagement of TIGIT with an agonistic mAb triggers a direct inhibitory effect on T cell entry into cell cycle with a decrease in expression of the T-bet, GATA3, IRF4 and RORc transcription factors associated with inhibition of cytokine production, predominately IFNγ. TIGIT expression represses T-bet as knockdown of TIGIT induces T-bet mediated IFNγ secretion while decreasing IL-10. The increase in IFNγ was overcome by blocking CD226 signaling indicating that TIGIT exerts immunosuppressive effects by competing with CD226 for the CD155 ligand similar to the CD28/CTLA-4 pathway. These data provide evidence for a novel role of this alternative co-stimulatory pathway in differentially regulating pro-inflammatory (IFNγ) vs. anti-inflammatory (IL-10) cytokine production in a T cell intrinsic manner.
Our recent investigations in the EAE model of multiple sclerosis have demonstrated a critical role of TIGIT as a negative regulator of autoimmune responses (13). The phenotype of TIGIT-deficient mice includes augmented T cell proliferation upon immunization and higher production of proinflammatory cytokines leading to greater EAE susceptibility (13). These data in the EAE model were confirmed in a collagen-induced arthritis (CIA) model of arthritis where administration of soluble TIGIT Fc-fusion protein significantly inhibited the severity of disease whereas a blocking anti-TIGIT accelerates disease development (14). Taken together, these studies show an intrinsic T cell function of TIGIT in regulating autoimmune responses and indicate a key role for the TIGIT-CD155 pathway in autoimmune diseases.
In humans, it has been reported that TIGIT expressed on T cells only exerts its immunosuppressive effects indirectly by modulating cytokine production by dendritic cells (12). In that study, Yu et al. concluded that TIGIT does not have a T-cell intrinsic function (12). The likely explanation for the discrepancy between their results and the present investigation is that the anti-TIGIT mAb (10A7) was not agonistic and perhaps recognized a different TIGIT determinant. In another study performed by Levin et al., two of five anti-human TIGIT showed an agonistic effect efficiently inhibiting T cell proliferation (14). In our investigations, one of these clones was selected for functional assays (clone 318.28.2.1) while the others could bind TIGIT but didn’t show any agonistic effect. Consistent with data reported by Levin et al. (14), we demonstrated that engagement of TIGIT by its ligands can deliver an inhibitory signal resulting in decreased T cell activation, IL-2 production and T cell receptor-mediated proliferation. Thus, the agonistic function of the TIGIT mAbs in vitro and in vivo in the EAE model suggests its utility therapeutically, particularly with our recent finding of Th1 Tregs in patients with MS where IFNγ expression inhibits the function of regulatory T cells (16).
TIGIT intrinsic mechanism of negative regulation of T cell functions may also have implications in tumor immunology. Similar to PD-1 ligands, TIGIT ligands CD155 and CD112 are over-expressed in certain human tumors, including colorectal carcinomas (17), gastric cancer (18) and neuroblastomas (19). CD226-CD155 interaction has been implicated as a major trigger for NK cell antitumor activity (20) while TIGIT-CD155 interaction inhibits NK cell cytotoxicity (21). However the role of TIGIT in tumor settings remains to be elucidated. Our data demonstrate that TIGIT is critical for the regulation of lymphocyte function and can efficiently limit T-cell-dependent immune responses. This mechanism may enable tumors overexpressing CD155 to escape from immune attack. The clinical relevance of modulation of a negative regulator to augment immune responses directed at tumors has been recently proved with the generation of an antibody against CTLA-4 (Ipilimumab) that enhances immune activation against cancer cells, resulting in significant antitumor effects (22).
Here, we demonstrated that TIGIT signaling could also directly inhibit T cell activation by a second mechanism where TIGIT acts as negative regulator by competing with CD226 for binding to its ligand CD155 to inhibit T cell responses. This new costimulatory pathway is similar to the CD28/CTLA-4 pathway that binds to B7-1 (CD80) and B7-2 (CD86) ligands. Interestingly CTLA-4 interacts with at least 20-fold higher avidity that CD28 does (23). In a similar manner, TIGIT is a negative regulator that binds CD155 with higher affinity (119 nM) compared to the CD226-CD155 interaction (1–3 nM) (12). Therefore, once expressed, TIGIT can effectively compete for binding to the same CD155 ligand similar to CD28/CTLA4 interaction with B7-1/B7-2. In this regard, therapies based on interfering with the costimulatory CTLA-4 pathway have recently been approved for clinical use. Specifically, a CTLA-4 fusion protein (Abatacept) has been approved to treat rheumatoid arthritis (24). Thus, the TIGIT/CD226 pathway is an attractive target for therapeutic manipulation.
In addition to its immunosuppressive effects on DCs, here we demonstrate that TIGIT exerts multiple mechanisms of peripheral tolerance: (i) the direct inhibition of T cell proliferation and production of pro-inflammatory cytokines; ii) induction of anti-inflammatory cytokines such as IL-10 and (iii) the blockade of CD226 positive costimulatory signaling. To better understand the role of each receptor in this complex network, we performed these experiments in the absence of APCs. Given that T cells can express CD155 but not CD112, we can exclude that the observed effects are due to CD112 signaling. To dissect the underlying molecular mechanisms of this regulatory pathway, we first downregulated CD226 expression on human T cells and observed a decrease in T-bet expression and IFNγ production. This result is consistent with previous reports showing that introduction of mutant (Y-F322) CD226 into naive CD4+ T cells suppresses their growth initiated by CD3 and LFA-1 ligations in the absence of IL-2, suggesting that CD226 functions as a signal transducer of LFA-1 upon triggering T cell activation (9). When we specifically knocked-down TIGIT expression, we obtained the opposite results; an increase in T-bet and IFNγ expression, consistent with the inhibitory effects triggered by the agonistic anti-TIGIT mAb. These data demonstrate that TIGIT ligation initiates a negative signaling cascade and by blocking CD226 or CD155 in TIGIT-depleted cells, the increases in IFNγ production are due to an enhanced CD226-CD155 interaction.
Interestingly, lack of TIGIT resulted in a decrease in IL-10 production. Accordingly, studies in TIGIT−/− mice showed that IL-10 was not induced by Ag-specific stimulation in TIGIT−/− T cell cultures, suggesting that production of IL-10 by T cells was impaired (13). One possible explanation for this defect in IL-10 production could be that TIGIT signaling directly activates IL-10 production but agonistic anti-TIGIT mAb failed to increase IL-10 expression (data not shown). Alternatively, in the absence of TIGIT, CD226 can interact with CD155, increasing IFNγ while repressing IL-10 gene expression. However, blocking CD226-CD155 interaction did not increase IL-10 expression. It has been reported that TIGIT, through its interaction with CD155, can modulate Erk activity and increase IL-10 production by DCs (12). It is intriguing to speculate that T cells expressing CD155 might be regulated in a similar manner and thus TIGIT depletion impaired IL-10 production by CD155-expressing T cells.
TIGIT/CD226 pathway is a novel regulatory pathway of T cell function associated to human autoimmune disease. Manipulation of the TIGIT/CD226 axis in vitro regulated T cell function by cell-intrinsic and cell-extrinsic mechanisms. In order to examine the potential therapeutic intervention of this pathway in patients with autoimmune diseases, we demonstrate that TIGIT expression is not altered in untreated RRMS patients and an agonistic antibody to TIGIT has an inhibitory effect on CD4+ T cells from MS patients. Although further studies are needed to test the therapeutic benefits of targeting inhibitory pathways, modulation of the TIGIT/CD226 axis may allow more targeted manipulation of human CD4+ T cells in autoimmune diseases.
Supplementary Material
Acknowledgements
We thank the blood donors and MS patients for their participation. Antibodies to human TIGIT were provided by ZymoGenetics, Inc, (a wholly-owned subsidiary of Bristol-Myers Squibb) under Material Transfer Agreement.
This work was supported by the National Institutes of Health grants U19AI070352, R01NS024247, P01AI03971, and P01NS038037 (D.A.H.). D.A.H. is a Jacob Javits Scholar of the National Institute of Neurological Disorders and Stroke. E.L. was supported by “Ministerio de Educación y Ciencia-Becas MEC/Fulbright y Cátedras Príncipe de Asturias-Plan nacional de I-D+I 2008–2011” and is recipient of a Postdoctoral Scholarship in the Beatriu de Pinós Programme (2011–2013) (AGAUR-Government of Catalonia).
Footnotes
Disclosures
The authors have no conflicting financial of interests.
References
- 1.Sharpe AH. Mechanisms of costimulation. Immunol Rev. 2009;229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vijayakrishnan L, Slavik JM, Illes Z, Greenwald RJ, Rainbow D, Greve B, Peterson LB, Hafler DA, Freeman GJ, Sharpe AH, Wicker LS, Kuchroo VK. An autoimmune disease-associated CTLA-4 splice variant lacking the B7 binding domain signals negatively in T cells. Immunity. 2004;20:563–575. doi: 10.1016/s1074-7613(04)00110-4. [DOI] [PubMed] [Google Scholar]
- 3.International Multiple Sclerosis Genetics Consortium; Wellcome Trust Case Control Consortium 2. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maier LM, Hafler DA. Autoimmunity risk alleles in costimulation pathways. Immunol Rev. 2009;229:322–336. doi: 10.1111/j.1600-065X.2009.00777.x. [DOI] [PubMed] [Google Scholar]
- 5.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, Ronningen KS, Guja C, Ionescu-Tirgoviste C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SC. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 6.Hafler JP, Maier LM, Cooper JD, Plagnol V, Hinks A, Simmonds MJ, Stevens HE, Walker NM, Healy B, Howson JM, Maisuria M, Duley S, Coleman G, Gough SC, Worthington J, Kuchroo VK, Wicker LS, Todd JA. CD226 Gly307Ser association with multiple autoimmune diseases. Genes Immun. 2009;10:5–10. doi: 10.1038/gene.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.IMSGC. The expanding genetic overlap between multiple sclerosis and type I diabetes. Genes Immun. 2009;10:11–14. doi: 10.1038/gene.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, Kitamura T, Nicholl J, Sutherland GR, Lanier LL, Phillips JH. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4:573–581. doi: 10.1016/s1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- 9.Shibuya K, Shirakawa J, Kameyama T, Honda S, Tahara-Hanaoka S, Miyamoto A, Onodera M, Sumida T, Nakauchi H, Miyoshi H, Shibuya A. CD226 (DNAM-1) is involved in lymphocyte function-associated antigen 1 costimulatory signal for naive T cell differentiation and proliferation. J Exp Med. 2003;198:1829–1839. doi: 10.1084/jem.20030958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, Vitale M, Moretta L, Lopez M, Moretta A. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198:557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tahara-Hanaoka S, Shibuya K, Onoda Y, Zhang H, Yamazaki S, Miyamoto A, Honda S, Lanier LL, Shibuya A. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112) Int Immunol. 2004;16:533–538. doi: 10.1093/intimm/dxh059. [DOI] [PubMed] [Google Scholar]
- 12.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 13.Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186:1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levin SD, Taft DW, Brandt CS, Bucher C, Howard ED, Chadwick EM, Johnston J, Hammond A, Bontadelli K, Ardourel D, Hebb L, Wolf A, Bukowski TR, Rixon MW, Kuijper JL, Ostrander CD, West JW, Bilsborough J, Fox B, Gao Z, Xu W, Ramsdell F, Blazar BR, Lewis KE. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur J Immunol. 2011;41:902–915. doi: 10.1002/eji.201041136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G, Piqani B, Eisenhaure TM, Luo B, Grenier JK, Carpenter AE, Foo SY, Stewart SA, Stockwell BR, Hacohen N, Hahn WC, Lander ES, Sabatini DM, Root DE. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 16.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17:673–675. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masson D, Jarry A, Baury B, Blanchardie P, Laboisse C, Lustenberger P, Denis MG. Overexpression of the CD155 gene in human colorectal carcinoma. Gut. 2001;49:236–240. doi: 10.1136/gut.49.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tahara-Hanaoka S, Shibuya K, Kai H, Miyamoto A, Morikawa Y, Ohkochi N, Honda S, Shibuya A. Tumor rejection by the poliovirus receptor family ligands of the DNAM-1 (CD226) receptor. Blood. 2006;107:1491–1496. doi: 10.1182/blood-2005-04-1684. [DOI] [PubMed] [Google Scholar]
- 19.Castriconi R, Dondero A, Corrias MV, Lanino E, Pende D, Moretta L, Bottino C, Moretta A. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 2004;64:9180–9184. doi: 10.1158/0008-5472.CAN-04-2682. [DOI] [PubMed] [Google Scholar]
- 20.Chan CJ, Andrews DM, McLaughlin NM, Yagita H, Gilfillan S, Colonna M, Smyth MJ. DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J Immunol. 2010;184:902–911. doi: 10.4049/jimmunol.0903225. [DOI] [PubMed] [Google Scholar]
- 21.Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins AV, Brodie DW, Gilbert RJ, Iaboni A, Manso-Sancho R, Walse B, Stuart DI, van der Merwe PA, Davis SJ. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 24.Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, Russell A, Dougados M, Emery P, Nuamah IF, Williams GR, Becker JC, Hagerty DT, Moreland LW. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.