Abstract
C5aR is a G protein-coupled receptor for the anaphylatoxin C5a and mediates many pro-inflammatory reactions. C5aR signaling has also been shown to regulate T cell immunity, but its sites and mechanism of action in this process remains uncertain. Here, we created a green fluorescence protein (GFP) knock-in mouse and used GFP as a surrogate marker to examine C5aR expression. GFP was knocked into the 3′-untranslated region (3′-UTR) of C5aR by gene targeting. We show that GFP is expressed highly on Gr-1+CD11b+ cells in the blood, spleen and bone marrow (BM), and moderately on CD11b+F4/80+ circulating leukocytes and elicited peritoneal macrophages. No GFP is detected on resting or activated T lymphocytes, nor on splenic myeloid or plasmacytoid dendritic cells. In contrast, 5–20% cultured BM-derived dendritic cells expressed GFP. Interestingly, GFP knock-in prevented cell surface but not intracellular C5aR expression. We conclude that C5aR is unlikely to play an intrinsic role on murine T cells and primary DCs. Instead, its effect on T cell immunity in vivo may involve CD11b+F4/80+ or other C5aR-expressing leukocytes. Further, our data reveal a surprising role of the 3′UTR of C5aR mRNA in regulating C5aR protein targeting to the plasma membrane.
Introduction
The complement system, comprising a potent and carefully regulated proteolytic cascade in blood, is an important component of the innate immune system and its activation in response to pathogens and other noxious stimuli is a keystone of innate host defense. Complement activation leads to the clearance of invading pathogens through opsonization, direct targeted lysis, and by generating the pro-inflammatory anaphylatoxins which serve as local mediators of inflammation (1, 2). One of the anaphylatoxins generated in response to complement activation is C5a, a 74-amino acid split product of the N-terminal region of C5, which has paradigmatic functions as a local inflammatory mediator including induction of leukocyte chemotaxis and increasing vascular permeability (3).
The functional responses attributed to C5a result from its interaction with its cognate 7-transmembrane G-protein coupled receptors (GPCR), C5aR and C5L2 of the rhodopsin family of proteins (4). C5L2 binds both C5a and its degradation product C5adesArg, the biological significance of which has been a matter of dispute (5, 6). Some studies have provided evidence that C5L2 is a decoy receptor incapable of signaling, whereas others have suggested a functional role for C5L2 in certain disease settings, possibly in cooperation with C5aR (7, 8). In contrast, the biology of C5aR has been well established. Binding of C5a to the extracellular, N-terminal region of C5aR allows conformational changes in the intracellular, C-terminal regions of the receptor resulting in G-protein coupling, intracellular signaling and a wide range of physiological responses (9, 10). C5a production and C5aR signaling have been implicated in a large number of inflammatory disorders and C5aR blockade has long been pursued as a method of clinical intervention in autoimmune and inflammatory disorders (4).
It has become increasingly clear that the role of complement in informing the immune system does not stop with innate immunity but rather presents a variety of signals to the adaptive immune system. It is well established that complement can serve as a natural adjuvant for the humoral immune response and other aspects of the B cell response (11). Furthermore, the relevance of complement to the development and maintenance of T cell immunity has emerged in recent years (2, 12, 13). The role of C5aR in T cell immunity in particular has garnered much attention and works from several laboratories have implicated C5aR in antigen presenting cell (APC) and T cell function, and in models of T cell-mediated diseases (14–21). The mechanism by which C5a impacts these T cell responses remains to be established. It has been postulated that local complement activation, via the alternative pathway (AP), produces C5a which can act on both APCs and T cells directly to regulate antigen uptake, co-stimulatory molecule expression, and T cell expansion and differentiation (15, 22–24). Other studies described a role of C5aR signaling more specifically on APCs, e.g. on the differentiation and function of primary and bone marrow (BM)-derived dendritic cells (DCs) (17, 25).
A main barrier to fully understanding the mechanism of action of the C5a:C5aR axis in T cell immunity has been the lack of clarity regarding C5aR expression on T cells and APCs. Elicited peritoneal macrophages as well as primary and BM-derived DCs have been used as APCs in the study of C5aR signaling in T cell immunity (15, 17, 20, 24–27). While it is well recognized that C5aR is expressed on peritoneal macrophages and blood monocytes, reported findings of C5aR expression on T cells and DCs have been varied in the literature (15, 17, 28, 29). Here, we created a C5aR/GFP knock-in mouse by gene targeting and used GFP as a sensitive surrogate marker to examine C5aR expression. Our studies shed new light on the expression and regulation of C5aR and represent a major step forward in understanding the sites and mechanism of action of C5aR signaling in T cell biology.
Materials and Methods
Mice
C5aR−/− mice on a C57BL/6 background were described previously (14, 30) Wild-type (WT) C57BL/6, OT-I and OT-II T cell receptor (TCR) transgenic mice were purchased from Jackson Laboratories (Bar Harbor, ME). OT-I and OT-II mice were crossed with C5aR GFP knock-in (GFP-KI) mice to derive OTI-GFP-KI and OT-II-GFP-KI mice, respectively. Gender- and age-matched WT and transgenic mice were used throughout this study. Mice were housed in a specific pathogen-free facility, and all experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania.
Generation of C5aR GFP knock-in mice
GFP-KI mice were generated by gene targeting. A schematic of the targeting strategy is shown in Figure 1A. We used the pND1 plasmid to construct the targeting vector (31). Our strategy was to insert GFP into the 3′-UTR and simultaneously flox the coding region of C5aR with two loxP sites. If successful, this strategy would have produced a C5aR GFP knock-in as well as a C5aR floxed mouse for conditional knockout studies. The short arm homologous sequence is a 5.1 kb fragment containing a segment of 3′ UTR and the immediate downstream flanking sequence of the C5aR gene. This fragment was amplified by PCR from 129/SvEv mouse genomic DNA using the following primers: 5′-GAT-ATC-GCA-GTG-GCT-AGG-CAG-ATA-CA-3′ (upstream, italicized for EcoRV site), 5′-GAG-AAC-ATG-CTT-GTC-ACT-CCA-3′ (downstream). It was cloned into pCR2 (Invitrogen) and then excised by EcoRI restriction digestion and subcloned into pND1 at EcoRI site. The long arm of homologous sequence contained two segments (5.3 kb and 1.5kb) separated by a loxP site. The 5.3 kb segment, corresponding to an intron sequence immediately upstream of exon 2, was amplified by PCR using the following primers: 5′-GCG-GCC-GCT-GCT-GGG-ATC-ACA-GTT-ATG-CA-3′ (upstream, italicized for NotI site), 5′-TCT-AGA-AAG-CCC-AGG-ATG-AAC-TGA-AAT-C-3′ (downstream, italicized for XbaI site). The 1.5 kb fragment corresponded to the coding sequence and a potion of the 3′-UTR of exon 2. It was amplified by PCR using the following two primers: 5′-TCT-AGA-ATA-ACT-TCG-TAT-AAT-GTA-TGC-TAT-ACG-AAG-TTA-TCA-GAG-TGA-GTT-TAA-GAC-CAG-TCT-3′ (upstream, italicized for XbaI site and underlined for loxP sequence), 5′-CTC-GAG-AGG-TGG-GCA-ACG-TAG-CCA-AGA-A-3′ (downstream, italicized for XhoI site). The 5.3 kb and 1.5 kb fragments were separately cloned into pCR2 and then excised with NotI/XbaI and XbaI/XhoI restriction digestion, respectively. They were then ligated into a pND1 vector, containing the short arm and prepared by NotI/XhoI digestion, in a three-piece ligation reaction. Finally, a GFP cassette containing an internal ribosomal entry site (IRES) upstream and an SV40 RNA polyadenylation signal downstream (IRES-GFP-SV40) was ligated at XhoI site into the pND1 plasmid containing the long and short homologous arms. To assemble the IRES-GFP-SV40 gene cassette, we used the retroviral vector Migr-1 which contained an IRES sequence upstream of the enhanced GFP (eGFP) cDNA (32). We first amplified by PCR the SV-40 sequence (0.2 kb) using the XE-63 plasmid (kindly provided by Dr. Xie Wen, University of Pittsburgh) as a template and the following two primers: 5′-GTC-GAC-CTG-TTC-ATG-ATC-ATA-ATC-AGC-C-3′ (upstream, italicized for SalI site), 5′-GTC-GAC-CTC-GAG-ACC-ACA-ACT-AGA-ATG-CAG-TGA-3′ (downstream, italicized for SalI site and underlined for XhoI site). The SV-40 was cloned into pCR2 and then excised by SalI restriction digestion and subcloned into Migr-1 vector at SalI site. The IRES-GFP-SV40 cassette was then excised from Migr-1 by XhoI digestion and cloned into pND1 as outlined above. The Expand Long Template PCR system (Roche) was used in all PCR reactions.
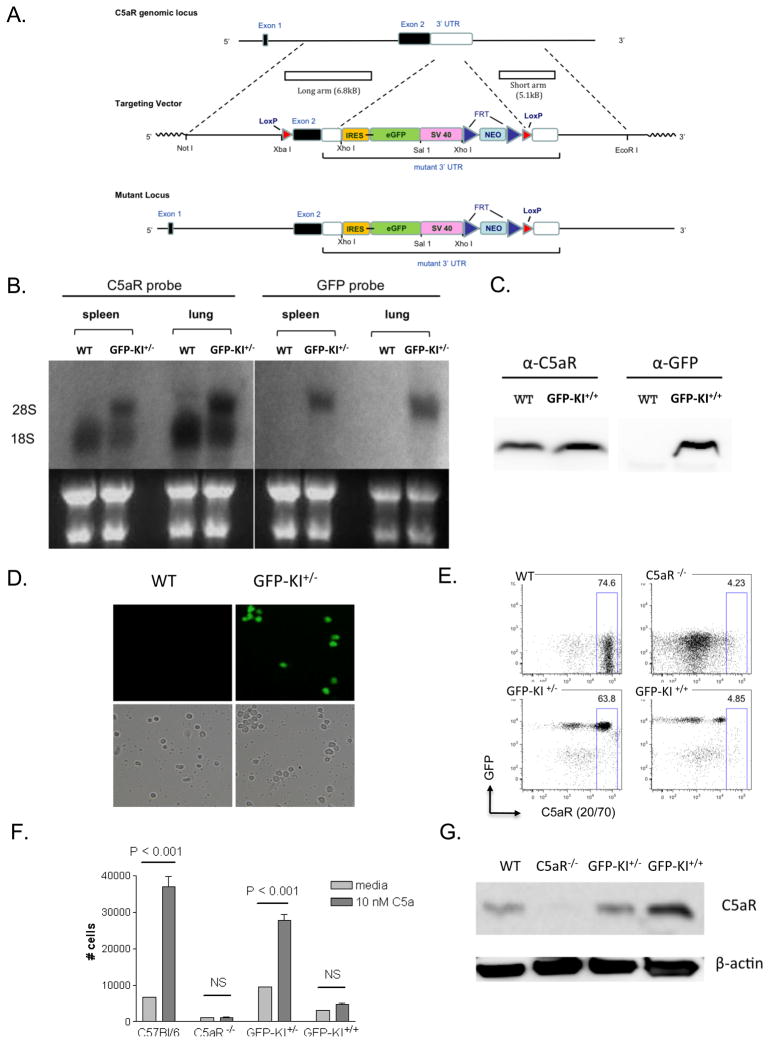
Figure 1. Generation of a C5aR GFP knock-in mouse.
(A) Schematic of the gene targeting strategy. An IRES-eGFP-SV40 gene cassette was inserted into the 3′ UTR of the C5aR gene by homologous recombination. (B) Northern blot demonstrating the presence of a larger chimeric C5aR-GFP mRNA species in the spleen and lung of GFP-KI+/− mice. Note that WT and mutant mRNA bands in the GFP-KI+/− mouse were of similar intensity, suggesting that transcription of the mutant C5aR gene allele was not affected. (C) Western blot analysis showing that C5aR protein was detected in the cell lysates of both WT and GFP-KI+/− mouse peritoneal macrophages, whereas GFP was detected only in GFP-KI+/− mouse cell lysate. (D) Peripheral blood smear showing fluorescent PBMCs in GFP-KI+/− but not WT mice. (E) FACS analysis of peritoneal neutrophils showing GFP+ cells in GFP-KI+/− and GFP-KI+/+ mice but not in WT controls. It also shows staining of C5aR with mAb 20/70 in WT and GFP-KI+/− mice. C5aR staining was absent on C5aR−/− cells and markedly reduced GFP-KI+/+ cells. (F) Neutrophil assays showing GFP-KI+/+ mouse neutrophils did not respond to C5a stimulation, confirming the essential absence of C5aR on their surface. (G) Western blot demonstrating higher intracellular C5aR protein levels in the cell lysate of elicited peritoneal macrophages of GFP-KI+/+ mice.
Gene targeting in embryonic stem (ES) cells was performed according to standard protocols (33). The targeting vector was linearized with NotI digestion and transfected into ES cells (TL-1, a cell line derived from 129/SvEv mouse strain) (34). The neomycin (NEO) and diphtheria toxin (DT) genes on the vector allowed for double selection of ES cells which have undergone homologous recombination and incorporated GFP and loxP into the C5aR gene locus on one strand of their genomic DNA. Positive clones were screened by Southern blot analysis of EcoRV-digested ES cell DNAs using a 420 bp probe 3′ to the short arm. The cDNA probe was amplified by PCR using the following two primers: 5′-CTC-TAT-GTA-GTC-CTG-GCT-GTC-T-3′ (upstream), 5′-GCA-GCC-TCA-CTA-CTA-TCA-GCT-T-3′ (downstream). Positive clones were expanded, confirmed in second-round screening and microinjected into C57BL/6 mouse blastocysts before being transferred to pseudopregnant females. Male chimeras were subsequently mated with female C57BL/6 mice (Jackson Laboratories) to achieve germ line transmission. Unless otherwise noted, GFP-KI+/− mice and wild-type (WT) littermate controls were utilized for all GFP expression studies.
Northern Blot Analysis
Total RNAs from mouse tissues were isolated using the Trizol Reagent (Life Technologies). RNA samples (10 μg each lane) were separated on a 1% formaldehyde-agarose gel and transferred onto a nylon membrane (Hybond-N; Amersham) via capillary action overnight in 20× SSC. The membrane was cross-linked under UV and hybridized first with a 32P-labeled C5aR cDNA probe. After probing, the membrane was stripped by boiling in 0.1× SSC-0.1% SDS and rehybridized with a 32P-labeled GFP cDNA probe. Both cDNA probes were labeled with 32P using random primers. Northern hybridizations were conducted in QuikHyb solution (Stratagene) at 68°C for 1 h. The membrane was washed, first in 2× SSC-0.1% SDS at 55°C for 15 min and then in 0.1× SSC-0.1% SDS at 55°C, and exposed to X-ray film.
Western Blot Analysis
Western blot was performed according to standard protocols (35). Peritoneal macrophages were lysed in RIPA buffer and protein concentrations were determined by Pierce BCA protein assay kit (Thermo Scientific). The following antibodies were used according to manufacturer’s instructions: CD88 (C5aR; clone H-100; Santa Cruz Biotechnology, Santa Cruz), GFP (clone B34, Convance), β-actin (clone AC-15, Invitrogen). Total proteins (40 μg per well) were separated by SDS-PAGE under reducing conditions and then transferred to a polyvinylidene difluoridemembrane (Millipore). Signals were visualized by the ECL system (Amersham Bioscience) and detected by the FUJI image reader.
Neutrophil Migration Assay
Neutrophils were isolated from mice via peritoneal lavage with PBS 6hr post injection of 1mL 4% w/v Brewer thioglycollate (Becton Dickinson Microbiology System). Transwell chemotaxis assay was performed as previously described (36). Briefly, peritoneal neutrophils were suspended in DMEM with 10% FBS in the top well of a Costar Transwell 24 well plate (Corning). Media or media containing 10 nm recombinant murine C5a (Hycult) was added to the bottom chamber and incubated for 6hr, after which time an accurate count of the number of neutrophils which had migrated to the bottom chamber was determined by running the contents of the bottom chamber through a flow cytometer.
Cell isolation
PBMCs were isolated from peripheral blood collected via either retroorbital bleeding or cardiac puncture using 0.2 M EDTA as anti-coagulant (1/10 volume). Red blood cells (RBCs) were lysed in 1x RBC lysis buffer (eBioscience). For cytometric analyses, single cell suspensions of splenocytes were obtained by first removing spleens and then carefully sectioning with a sharp scapel followed by mechanical disruption and finally passing through 70 μm nylon cell strainers (BD Falcon). Inguinal and axillary lymph nodes (LN) were removed and mechanically disrupted as per spleen, but did not require RBC lysis before analysis. Bone marrow (BM) was isolated by removing femur and flushing of the interior with either PBS (for flow cytometry) or sterile RPMI (for BMDC). RBCs were subsequently lysed as before.
Flow Cytometry
Following fresh isolation cells were first incubated with 0.5 μg Fc receptor blocking antibody (2.4G2) for 15 min at 4 C in 20 μl FACS buffer (PBS supplemented with 2% fetal calf serum, 0.1% sodium azide and 1 mM EDTA). Cells were washed twice in staining buffer, and were stained with a variable panel of antibodies covering the cell surface antigens utilized in analysis. Panels were made up of some combination of flurochromes including PE, PE-Texas Red, PerCP-Cy5.5, PE-Cy7, APC, Alexa Flour 700, APC-Alexa Flour 750, and Pacific Blue. All antibodies were obtained from eBioscience except anti-C5aR (clone 20/70, Cedarlane). Isotype controls for antibodies (rat IgG1/2a/2b and Armenian hamster IgG) were obtained in the appropriate color from eBioscience. Aqua Live/Dead (Invitrogen) was included with every sample to gate out dead cells, with only Aqua low cells being used in further analysis. Samples were analyzed on a FACSCanto flow cytometer (BD) and data were analyzed with the FlowJo software (TreeStar). Data are representative of 3–5 independent experiments.
Immunostaining and Fluorescence Microscopy
Peritoneal neutrophils were isolated according to the above protocol and were fixed in 2% paraformaldehyde (Electron Microscopy Services) for 15 minutes before permeabilization in 0.5% Triton X-100 (LabExpress). Cells were then stained with a combination of biotinylated anti-C5aR mAb (clone 20/70, Cedarlane) and streptavidin-Alexa Flour 594 (Invitrogen), to detect C5aR, and Hoechst 33528 (Invitrogen) to counterstain nuclei. Images were acquired on a LSM 710 (Zeiss) confocal microscope with Zen 2010 software (Zeiss) and analyzed with ImageJ.
FLT3-L secreting tumor implantation
GFP-KI mice and WT controls were implanted with 5 × 106 FLT3 Ligand-secreting B16 melanoma cells (kindly gifted by Dr. U.H. Von Andrian, Harvard Medical School) by subcutaneous route according to published protocols (37, 38). After 15 days, tumor-bearing mice were sacrificed and splenocytes isolated as described above and further subjected to cytometric analyses.
T cell activation assays
Non-specific T cell activation was performed using plate-bound anti-mouse CD3 mAb (BD Pharmingen, 5μg/ml for plate-coating) in the presence 2 μg/ml soluble anti-mouse CD28 mAb (BD Pharmingen) or Concavalin A (ConA, 1μg/mL, Sigma) in 200μl complete medium (CM, DMEM containing 10% FBS, 2 mM L-glutamine, 10 mM Hepes, 0.1 mM nonessential amino acids, 50 μM 2-mercaptoethanol, 1mM sodium pyruvate and 100 U/ml penicillin-streptomycin) for 6 hours. Cells were collected hourly in CM and analyzed using flow cytometry. All reagents and columns used for cell isolation were purchased from Miltenyi Biotec (Auburn, CA). Splenocytes from OT-I-GFP-KI+/− and OT-II-GFP-KI+/− mice were prepared by lysis of red blood cells with 1x RBC lysis buffer and passage through a cell strainer as before. In each well of a 96-well U-bottomed plate, 1 × 106 splenocytes were plated in 200μl CM. OVA peptides (OVA257-264 for OT-I and OVA323-339 for OT-II, ABBIOTEC) were added at 10 μM at the time of cell mixing. Triplicate wells were set up for each condition. 48 hours post-mixing, cells were collected for flow cytometric analysis and proliferation was measured with the Celltiter Cell proliferation kit (Promega).
In vivo TLR stimulation
Lipopolysaccharide (LPS, E coli 026:B6; Sigma) and CpG 1826 (5′-TCCATGACGTTCCTGACGTT-3′, synthesized by Oligos Etc.) were injected i.p. into GFP-KI mice or non-transgenic littermate controls at 20 mg/kg. Animals were sacrificed after 12h and relevant tissues harvested for analysis. Poly(I:C) (high MW, Invivogen) was injected i.p. at 15 mg/kg and animals again sacrificed after 12 hours for analysis.
Bone marrow-derived macrophage and DC cultures
Bone marrow-derived macrophages (BMDMs) were generated as previously described.(40) Briefly, BM cells were isolated as described above and plated in RMPI 1640 containing 10 mM HEPES, 10% FBS and 15% (v/v) of L-cell conditioned media (LCM), which was produced according to previous protocols (39). Cells were collected at day 7 and stained for flow cytometry. BMDCs were generated as previously described (40). BM cells were cultured in RMPI 1640 (10% FCS, 2 mM L-glutamine, 50 μM β-mercaptoethanol, 100 U pen/strep, 20 ng/ml GM-CSF, 10 ng/mL IL-4). Medium was changed every other day. On day 6, DCs were stimulated with LPS at 1μg/mL and cells were collected 24 hours later for flow cytometry analysis.
Results
Generation of C5aR GFP-KI mice
We used gene targeting to insert a GFP cDNA gene cassette into the 3′-UTR and to simultaneously flox the second exon of mouse C5aR (Fig 1A). We obtained several targeted ES cell clones that had the GFP cassette correctly inserted but without the upstream LoxP site incorporated as intended (Fig 1A and analysis data not shown). The latter outcome was a result of preferential homologous recombination between the C5aR gene and sequences downstream of the 5′ loxP in the targeting vector. Insertion of the GFP cDNA into 3′-UTR of the C5aR gene would allow both genes to be transcribed bicistronically under the control of the C5aR gene promoter, producing a single chimeric mRNA from which C5aR and GFP can be synthesized as separate proteins.
We successfully produced heterozygous (GFP-KI+/−) and homozygous (GFP-KI+/+) GFP knock-in mice from one of the positive ES clones. Northern blot analysis revealed two C5aR mRNA species in the spleen and lung of GFP-KI+/− mice, one of normal size and the other of a larger size (Fig 1B). As expected, only the larger C5aR mRNA species could be detected by a GFP cDNA probe (Fig 1B), confirming the presence of a bicistronic C5aR-GFP mRNA in GFP-KI+/− mice. Western blot analysis showed that C5aR protein was present in peritoneal macrophages of both WT and GFP-KI+/+ mice, whereas GFP protein could be detected only in GFP-KI+/+ mice (Fig 1C). Similarly, green fluorescent peripheral blood mononuclear cells (PBMC) were easily detected by immunofluorescence microscopy on a blood smear of GFP-KI+/−mice but not of WT mice (Fig 1D). Collectively, these data established that GFP mRNA was transcribed under the C5aR gene promoter in GFP-KI+/− and GFP-KI+/+ mice and GFP protein was successfully synthesized and easily detectible.
By FACS analysis, we also detected high levels of GFP in elicited peritoneal neutrophils of GFP-KI+/− and GFP-KI+/+ mice but not of WT controls (Fig 1E). Unexpectedly, while C5aR expression was detected by FACS on WT and GFP-KI+/− cells, it was markedly reduced on GFP-KI+/+ mouse cells (Fig 1E). Furthermore, in a chemotactic assay neutrophils from WT and GFP-KI+/− mice, but not GFP-KI+/+ mice, responded to C5a stimulation (Fig 1F). These results suggested that insertion of GFP into 3′ UTR of the C5aR gene interfered with C5aR expression on the cell surface. The inhibitory effect on C5aR expression appeared to be post-translational, as Western blot analysis detected a higher level C5aR protein in the cell lysate of GFP-KI+/+ mouse cells (Fig 1G). Immunostaining of permeablized peritoneal neutrophils from GFP-KI+/+ mice confirmed the presence of intracellular C5aR protein that was localized to the perinuclear region (supplemental Fig 1). In light of this result, we used GFP-KI+/− mice in subsequent GFP expression studies to avoid potential underestimation of C5aR expression in case the receptor is auto-regulated.
GFP expression on peripheral blood cells and in secondary lymphoid organs
Using GFP-KI+/− mice, we first set out to identify GFP expressing cells in the periphery and secondary lymphoid organs including the spleen and lymph nodes. We found that about 10–20% of PBMCs in GFP-KI+/− mice were positive for GFP (Fig. 2A). Staining with cell type-specific markers showed that a majority of Gr-1+ cells and approximately half of CD11b+ cells expressed GFP (Fig 2A). In contrast, very few CD8+, CD4+ and B220+ PBMCs expressed GFP (Fig 2A). These results demonstrated that GFP-expressing PBMCs were primarily neutrophils and monocytes, and that peripheral T and B lymphocytes were negative for GFP expression. In a separate analysis, we further examined GFP expression on three distinct populations of PBMCs defined by Gr-1 and CD11b double staining (Fig 2B). Gr-1+CD11b+ cells were negative for F4/80 and CD11c and had high GFP expression. These cells represented neutrophils and granulocytes. CD11b+ Gr-1low cells expressed low levels of F4/80, CD11c and GFP, and these cells most likely represented monocytes. Gr-1−/CD11b− cells had no expression of F4/80, CD11c or GFP and were presumably consisted of lymphocytes (Fig 2B).
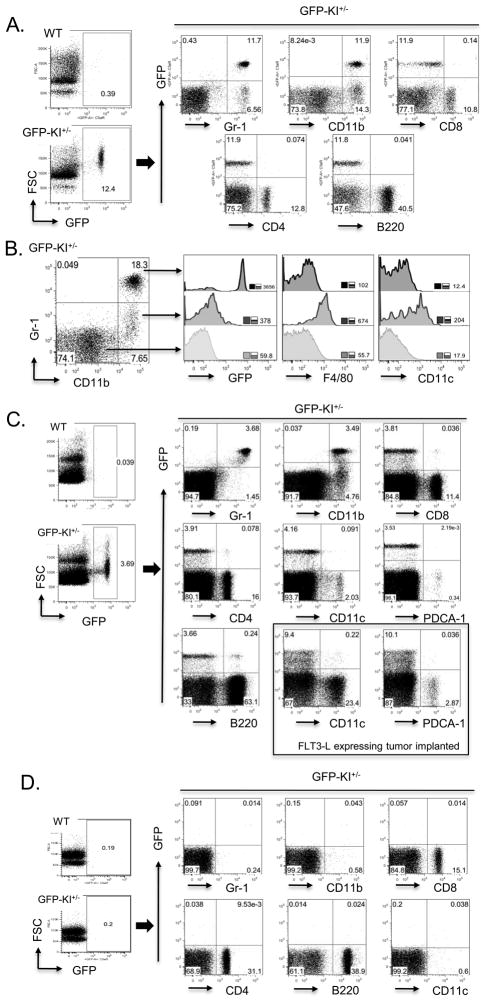
Figure 2. FACS analysis of GFP expression on naïve GFP-KI+/− mouse PBMCs, spleen and lymph node cells.
(A) No GFP+ cells were present in WT mouse PBMCs, whereas ~ 10–20% of PBMCs from GFP-KI+/− mice were GFP+. Most Gr-1+ PBMCs and approximately half of CD11b+ PBMCs were GFP+. Very few CD8+, CD4+ or B220+ PBMCs expressed GFP. (B) CD11b and Gr-1 double staining defined 3 distinct populations with different levels of GFP expression. Gr-1high/CD11bhigh cells expressed high levels of GFP, Gr-1low/CD11bhigh cells expressed low levels of GFP and Gr-1−/CD11b− cells expressed no GFP. Inserts numbers represent mean fluorescence intensity of stains. (C) No GFP+ cells were present in the WT mouse spleen while 2–5% GFP-KI+/− mouse splenocytes were positive for GFP with the majority coinciding with Gr-1 and CD11b expression. Very few cells bearing lymphocyte markers (CD4, CD8, B220) or dendritic cell markers (CD11c, PDCA-1) showed GFP expression. Dramatically expanded mDC (CD11c+) and pDC (PDCA-1+) populations in mice with implanted FLT3-L-expressing tumor cells also did not appreciably express GFP. (D) GFP is essentially absent from resting inguinal lymph node cells. Gate percentages are displayed and data are representative of 3 independent experiments.
Similar results were obtained from analysis of splenocytes of naïve mice. In general, approximately 3–5% splenocytes were positive for GFP (Fig 2C). As in PBMCs, GFP expression was mostly coincidental with Gr-1 staining, and to a lesser extent, with CD11b staining (Fig 2C), and very few splenic T (CD8+ or CD4+) and B (B220+) lymphocytes expressed GFP (Fig 2C). Notably, we also failed to detect GFP expression on splenic CD11c+ or PDCA-1+ cells, suggesting that C5aR is expressed neither on myeloid dendritic cells (mDCs) nor on plasmacytoid DCs (pDCs) of the mouse spleen (Fig 2C). To further examine GFP expression on splenic DCs, we implanted GFP-KI+/− mice with FLT-3L-expressiing tumor cells to expand the DC populations (37, 38). As in naïve mice, we found that splenic mDCs and pDCs from FLT-3L-stimulated GFP-KI+/− mice were also essentially negative for GFP expression (Fig 2C). Finally, no GFP was observed on any of the inguinal lymph node cells (lymphocytes) of naïve mice (Fig 2D). Importantly, staining of native C5aR with the mAb 20/70 detected good correlations between GFP and C5aR signals both in GFP+ and GFP− spleen cell populations (supplemental Fig 2).
GFP expression on bone marrow (BM) cells, peritoneal and BM-derived macrophages
In contrast to peripheral blood or secondary lymphoid organs, C5aR expression was more prevalent on BM cells. We found that approximately 50% of primary BM cells expressed GFP (Fig. 3A). As is the case in peripheral blood and the spleen, much of GFP expression tracked with Gr-1+ and CD11b+ cells which likely represented the pool of mature and maturing neutrophils that can be mobilized following immune challenges (41). Interestingly, we detected small populations of CD11clowGFP+ cells and B220+GFP+ cells (Fig 3A). We wondered if these might be the same cells, representing a type of C5aR-expressing pDCs or their precursors with a phenotype of CD11cintB220+ as previously described (42). Further analysis showed however that both cell populations were Gr-1+, and that there was little overlap between the CD11c and B220 markers (Fig 3A). Thus, they appeared to represent minor sub-populations of the Gr-1+C5aR+ cell lineage with unknown significance.
Figure 3. GFP expression on primary bone marrow cells and macrophages from different sources.
(A) Close to half of primary BM cells were positive for GFP and most GFP+ cells are also Gr-1+ and CD11b+. Small populations of CD11clowGFP+ and B220+GFP+ cells were present in the BM. Both populations were also Gr-1+ but they appeared to be separate populations. (B) The majority of resident peritoneal macrophages were negative for GFP but a small population had high levels of GFP expression. Thioglycollate-elicited peritoneal macrophages were relatively homogeneous in expressing moderate levels of GFP. BM-derived macrophages appeared to have two separate populations with regard to GFP expression, with GFP-expressing cells constituting the larger population. Data are representative of 3 independent experiments.
We found that resident peritoneal macrophages consisted of two distinct populations differing in GFP expression. The majority of the cells were negative for GFP, while a small population (~10% of F4/80+ cells) expressed high levels of GFP (Fig 3B). The pattern of thioglycollate-elicited peritoneal macrophages was quite different in that most cells expressed intermediate levels of GFP (Fig 3B). We also examined GFP expression on BM-derived macrophages (BMDM) and detected low to intermediate levels of GFP on a majority of the cells (Fig 3B).
GFP expression on activated T cells
The finding that GFP is essentially absent from T lymphocytes and splenic DCs contradicted recent reports describing C5aR signaling on these cell types (15, 22, 24, 25). It has been suggested that local complement activation in the immunological synapse produce C5a which triggers C5aR signaling both on T cells and on DCs, providing a co-stimulatory signal to enhance T cell activation (15, 43, 44). To determine if C5aR expression might be induced upon T cell activation, we examined GFP expression on polyclonal T cells activated by anti-CD3/anti-CD28 antibodies or concanavalin A (ConA). As shown in Fig 4A, either treatment strongly and time-dependently activated GFP-KI+/− mouse T cells as indicated by CD69 and CD25 up-regulation. However, no GFP expression was observed on the activated T cells (Fig 4B).
Figure 4. GFP is not expressed on activated T cells.
(A) Splenocytes from GFP-KI+/− mice were left untreated (NS) or stimulated with plate-bound anti-CD3 and soluble anti-CD28 or with ConA. Samples were collected at hourly intervals and CD3-gated T cells were analyzed by FACS for the expression of CD25 and CD69, two activation markers. (B) Analysis of GFP levels on CD3-gated T cells from GFP-KI+/−mouse splenocytes at various time points after anti-CD3/anti-CD28 or ConA stimulation. WT CD3+ cells (top trace) are displayed as a negative control and Gr-1+ (second from top trace) cells are plotted as a positive control for GFP expression. Insert numbers represent mean fluorescence intensity. Data are representative of 3 independent experiments. (C) Splenocytes from OT-I-GFP-KI+/− and OT-II- GFP-KI+/−mice were stimulated with OVA257-264 and OVA323-339, respectively, as a specific antigen or with anti-CD3/anti-CD28 as a non-specific activator (positive control). CD8-gated (for OT-I) or CD4-gated (for OT-II) T cells were then analyzed for activation markers CD25 and CD69 expression at 48 hrs. (D) Proliferation assays of splenocytes stimulated as described in panel A. (E) Despite robust activation by specific peptide antigens, GFP was not induced on OT-I CD8+ or OT-II CD4+ T cells. Insert numbers represent mean fluorescence intensity. Data are representative of two independent experiments.
In separate experiments, we assessed GFP expression on T cells activated by specific antigens. We crossed GFP-KI+/− mice with OT-I or OT-II TCR transgenic mice and generated OT-I-GFP-KI+/− and OT-II-GFP-KI+/− mice. The OT-I and OT-II mice bear transgenic T cell receptors that recognize specific ovalbumin peptides, in the context of MHC class I and II, respectively, to activate CD8+ or CD4+ T cells. Splenocytes from OT-I-GFP-KI+/− mice were stimulated with 10 μM OVA257-264 for 72h before being collected and analyzed via flow cytometry for activation markers and proliferation. Non-specific stimulation with anti-CD3/anti-CD28 was included as a control. Using CD69 and CD25 expression and cell proliferation assays, we showed that CD8+ T cells from both WT and OT-I-GFP-KI+/− mice were activated by anti-CD3/anti-CD28 stimulation but only cells from OT-I-GFP-KI+/− mice were activated by OVA257-264 (Fig 4, C and D). Importantly, we found no GFP expression on OVA peptide-activated TCR transgenic CD8+ T cells (Fig 4E). Likewise, we detected no GFP expression on TCR transgenic CD4+ T cells from OT-II-GFP-KI+/− mice activated by OVA323-339 peptide (Fig 4, C–E). Taken together, our results suggested that C5aR expression is not induced on activated T cells.
GFP expression in peripheral blood and the spleen following systemic inflammatory challenge
We next studied GFP expression on peripheral leukocytes and in the spleen in response to inflammatory challenge. We treated GFP-KI+/− mice with LPS, poly(I:C) or CpG, respective ligand for TLR4, TLR3 and TLR9, and examined GFP expression 12 hr later by FACS. We found that systemic inflammation triggered by activation of any of the three TLRs caused a profound increase in GFP+ cells in PBML and in the spleen (Fig 5). For example, the percentage of GFP+ cells in peripheral blood increased from a baseline of 10–20% to 50–80%, depending on the length of time of stimulation and specific ligand utilized (Fig 5A and data not shown). Likewise, about 3% of splenocyes in naïve mice were GFP+ but such cells constituted 10–20% of the splenocytes in TLR ligand-stimulated mice (Fig 5B). As observed in naïve mice, the vast majority of GFP+ cells in the blood and spleen of stimulated mice were found to express Gr-1 and CD11b (Fig 5), suggesting that systemic inflammation caused rapid and large-scale mobilization of neutrophils and monocytes, presumably by driving their migration from the BM to peripheral sites. None of the treatment conditions caused GFP expression on circulating or splenic T and B lymphocytes (data not shown). Notably, systemic inflammation also did not induce C5aR expression on splenic CD11c+ DCs, as very few of the CD11cint and CD11chigh cells expressed GFP regardless of the nature and length of time of TLR ligand stimulation (Fig 5B and data not shown). On the other hand, we observed a moderate expansion in the spleen of GFP+ cells with a CD11clowGr-1+ phenotype (Fig 5C). It is not known if these cells have DC function or are simply part of the Gr-1+ neutrophil/granulocyte lineage.
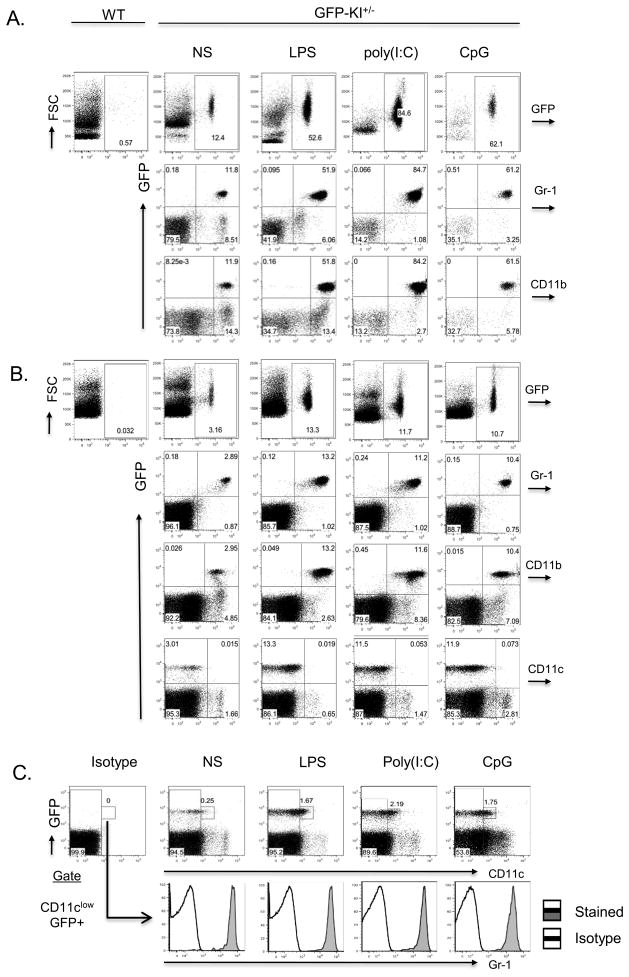
Figure 5. GFP expression on PBMCs and in the spleen following systemic TLR stimulation.
(A) GFP+ cells were markedly increased (5–8 fold) in PBMCs 12 hrs after GFP-KI+/− mice were treated with LPS, poly(I:C) or CpG. As in naïve mice, most GFP+ cells in TLR ligand-stimulated mice were Gr-1+ and CD11b+. (B). GFP+ cells were also significantly expanded (3–5 fold) in the spleens of TLR ligand-treated GFP-KI+/− mice when analyzed 12 hrs after stimulation. Again, the expanded GFP+ cell population was found to consist mainly of neutrophils (Gr-1+ and CD11b+). Note that TLR activation did not increase the number of GFP+CD11chigh cells in the spleen of GFP-KI+/− mice. (C) A population of GFP+CD11clow cells expanded moderately in the spleen of TLR ligand-treated GFP-KI+/− mice. These cells were all Gr-1+. NS, non-stimulated. Data are representative of more than 3 independent experiments.
GFP expression on BM-derived DCs
One type of DCs commonly used in T cell immune response studies in vitro are BM-derived DCs (BMDCs). Given our findings that a substantial fraction of BM cells are GFP+, we investigated if DCs differentiated in vitro from BM in the presence of GM-CSF and IL-4 might express C5aR. As shown in Fig 6A, we produced CD11c+ BMDCs in culture that expressed characteristic DC markers including MHC class II, CD80, CD11b and CD86. Interestingly, in multiple independent experiments, we found that 5–20% of CD11c+ BMDCs expressed GFP (Fig 6B). GFP expression was constitutive and independent of the maturation status of the BMDC, as stimulation with LPS, a prototypical inducer of DC maturation, did not significantly augment GFP expression (Fig 6B).
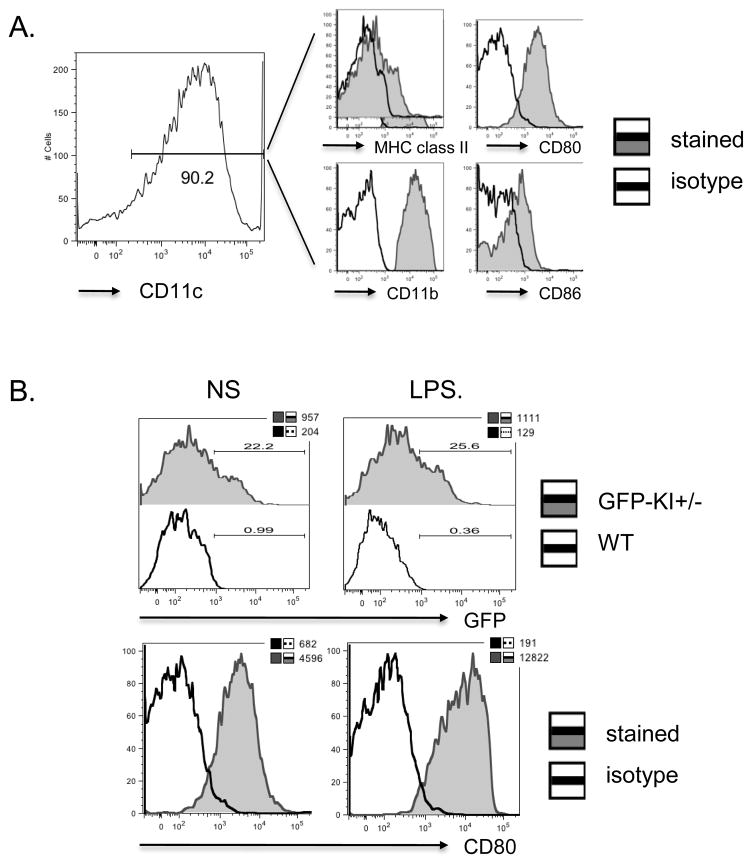
Figure 6. GFP expression on BMDCs under resting and stimulated conditions.
(A) BMDCs differentiated from GFP-KI+/− mouse BM cells in vitro showed characteristic DC markers including CD11c, CD11b, MHC class II, CD80 and CD86. Open histograms represent antibody isotype controls. (B) Representative histograms of GFP expression on BMDCs. In multiple independent experiments, about 15–25% CD11C+ BMDCs were found to express GFP. Stimulation with LPS increased CD86 but not GFP expression on BMDCs. In the GFP histograms, open and filled histograms represent WT and GFP-KI+/− BMDCs, respectively. Numbers above the gating bars indicate percentage of GFP+ cells and insert numbers indicate mean fluorescence intensity. In the CD80 histograms, open and filled histograms represent isotype control and CD80-specifici antibody staining, respectively. Insert numbers indicate mean fluorescence intensity. Data are representative of 3 independent experiments.
Discussion
C5aR mediates many of the pro-inflammatory activities of activated complement. It also plays a key role in the regulation of T cell immunity by complement and in the crosstalk between complement and TLRs, topics that have attracted much attention recently. Most evidence supporting a role of C5aR signaling in T cell immune responses came from disease modeling studies in mice, including T cell-mediated allogeneic organ transplantation, experimental autoimmune encephalomyelitis, allergic asthma and viral infection (14–16, 18, 20, 21, 43–46). How C5aR signaling impacted T cell reaction in these settings remains incompletely understood. Some studies have indicated an intrinsic role of C5aR signaling on T cells and antigen-presenting cells (APC) such as macrophages and DCs in providing co-stimulation during T cell activation (15, 17, 24, 25), whereas other models have considered the possibility of indirect regulation, e.g. through the generation of pro-inflammatory cytokines by cells other than T cells or DCs via C5aR-dependent mechanisms (13, 14, 19, 20, 30). Of relevance to these hypotheses is knowledge of C5aR expression and regulation on T cells and various APCs. Unfortunately, there is considerable uncertainty on this issue, particularly with regard to C5aR expression on T cells and DCs. Part of the reason may relate to limitations in prior studies in antibody specificity, choice of appropriate controls and activation status of the cells. Additionally, there may be species differences between human and experimental animals.
In the present study, we generated a GFP knock-in mouse by gene targeting and used GFP as a surrogate marker to track C5aR expression on cells of the immune system under various conditions. A similar approach has been used previously for the examination of other immunologically relevant promoters, including CD83, IL-23, and IL-13(47–49). The advantage of this approach is the non-invasive nature of the labeling which allows for direct visualization of cells without antibody staining of the target protein, thus eliminating potential nonspecific bindings of primary and secondary antibodies. Using this approach, we demonstrated that C5aR was most abundantly expressed on Gr-1+CD11b+ neutrophils in peripheral blood, the spleen and BM. It was also detected on blood monocytes in naive mice but at a much lower level than that on neutrophils. In the resting mouse spleen, a similar pattern was observed, i.e. the vast majority of Gr-1+ neutrophils expressed high levels of C5aR whereas CD11b+Gr-1− splenic macrophages were either negative or had low levels of C5aR expression. Of interest, we found a significant difference in C5aR expression between resident and thioglycollate-elicited peritoneal macrophages. Only a small fraction of resident peritoneal macrophages were positive for GFP while most elicited F4/80+ cells expressed GFP. We also found that TLR activation profoundly increased the abundance of C5aR-expressing cells in peripheral blood and the spleen. However, the increased cells were overwhelmingly Gr-1+CD11b+ neutrophils which expressed similar levels of C5aR to those in naïve mice. Thus, this outcome represented the well-known phenomenon of neutrophil mobilization and migration from the BM to peripheral sites in response to pathogen infection (41).
A clear finding of the present study is the lack of C5aR expression on T lymphocytes both in peripheral blood and in secondary lymphoid organs. We did not detect GFP expression on naïve T cells, nor on T cells activated by non-discriminative stimulators such as ConA and anti-CD3/CD28 mAbs or by specific antigens. Using similar bicistronic gene constructs, GFP has been detected as a surrogate marker for genes expressed in T cells (48, 49), which rules out potential technical explanations for the failure to observe GFP expression in T cells. Furthermore, we observed good correlations between GFP and native C5aR expression in both GFP+ and GFP− cell populations (supplemental Fig 2). Thus, our data do not support an intrinsic role of C5aR signaling on T cells during their activation. Likewise and somewhat unexpectedly, we did not detect significant C5aR expression on CD11chigh and CD11cint splenic mDCs or pDCs from naïve or TLR ligand-stimulated mice, nor on splenic DCs expanded by FLT-3L stimulation. We did however note an increase in CD11clowGFP+ cells in TLR ligand-stimulated mouse spleens. Further analysis showed these cells to express high levels of Gr-1. Interestingly, cells with similar characteristics (CD11clowGr-1+GFP+) were also observed in the BM, suggesting that they may have migrated from BM to peripheral sites as did other Gr-1+ cells under inflammatory conditions. It is not known if these CD11clowGFP+Gr-1+cells represented a subset of neutrophils or an uncharacterized type of DCs with APC function. In this context, it is relevant to mention a group of Gr-1+ cells that have previously been characterized as inflammatory monocytes or their downstream progeny, the so-called ‘inflammatory’ DCs that serve as important informers of the adaptive immune response to infection through antigen trapping, presentation and cytokine production (50).
If T lymphocytes and conventional DCs (as defined by CD11chigh and CD11cint) do not express C5aR, how do we explain the effects of C5aR on T cell immunity in various in vivo models? Firstly, C5aR signaling could play a role on macrophages which almost certainly can function as APCs in vivo. Indeed, many of the published in vitro studies demonstrating a role of C5aR signaling within the immunological synapse used elicited peritoneal macrophages as APCs (19, 20, 22, 26, 44). Secondly, although we did not find C5aR expression on CD11chigh and CD11cint DCs from naïve and TLR ligand-stimulated mouse spleens, we cannot yet exclude the possibility that DCs differentiated and matured in other end organs under certain disease settings (e.g. asthma, EAE, organ transplantation etc) may up-regulate C5aR expression in response to specific cytokine stimulation. In support of this possibility, we found that a sub-group of DCs differentiated in vitro from BM cells in the presence of GM-CSF and IL-4 expressed C5aR. Hopefully, the availability of the C5aR GFP-KI mouse will now allow this hypothesis to be tested experimentally. Thirdly, as mentioned above, it is possible that some CD11clow(or even CD11c−)C5aR+ cells, such as the CD11clowGr-1+GFP+ cells we have observed or other C5aR+ cells in the monocytic cell lineage, may function as unconventional APCs in vivo. Finally, C5aR signaling on cells other than T cells or APCs may extrinsically regulate T cell activation and differentiation by affecting the production of proinflammatory cytokines and chemokines (13). It is likely that one or several of these mechanisms in concert work to influence T cell immunity and link the complement and adaptive immune systems.
The finding that 3′-UTR of the mouse C5aR gene regulates C5aR protein targeting to the cell surface was rather unexpected. We found that C5aR was largely absent from the cell surface of GFP-KI+/+ mice. This outcome was not due to decreased C5aR mRNA stability which 3′-UTR is generally known to regulate. We detected normal levels of the bicistronic C5aR-GFP mRNA, and both GFP and cytoplasmic C5aR protein were made at normal, and in the case of C5aR, higher levels. To our knowledge, this is only the second example of a 3′-UTR sequence critically regulating cell surface expression of a GPCR protein. Previously, it has been reported that disruption of the 3′-UTR of the β2-adrenergic receptor (β2-AR) mRNA interfered with its recognition by the RNA-binding protein HuR, and this in turn led to impaired cell surface expression of β2-AR (51, 52). Nuclear binding of HuR to the 3′UTR of β2-AR mRNA is thought to prevent translation of the β2-AR mRNA while allowing its transit to the cell periphery where unknown mechanisms release the translational block and the locally-translated β2-AR protein can efficiently traffic to the cell surface. Disruption of the 3′ UTR of β2-AR mRNA or knockdown of HuR led to premature initiation of translation, resulting in overproduction of β2-AR protein in perinuclear polyribosomes but defective trafficking to the cell surface (51). This is reminiscent of the increase in C5aR protein in the GFP-KI+/+ cytosol seen by Western blot and its perinuclear localization on immunofluorescence microscopy in our study. It is likely that a similar mechanism accounted for impaired cell surface expression of C5aR in GFP-KI+/+ mice.
In summary, we have generated and used a novel GFP knock-in mouse to comprehensively characterize C5aR expression. Our results shed new light on the regulation of C5aR in vivo and have implications for understanding the mechanism of action of C5aR signaling in T cell immune responses. The GFP-KI mouse described here may serve as a valuable tool to further elucidate the biology of C5aR in health and disease.
Supplementary Material
Abbreviations used
- GFP
green fluorescent protein
- KI
knock-in
- 3′-UTR
3′-untranslated region
- PBMC
peripheral blood mononuclear cell
- BM
bone marrow
- DC
dendritic cells
- BMDC
bone marrow-derived dendritic cell
- mDC
myloid dendritic cells
- pDC
plasmacytoid dendritic cells
- IRES
internal ribosomal entry site
- GPCR
G-protein coupled receptor
- NEO
neomycin
- DT
diphtheria toxin
- APC
antigen-presenting cell
- TLR
toll-like receptor
- OVA
ovalbumin
- CM
complete medium
- β2-AR
β2-adrenergic receptor
- ConA
concanavalin A
- TCR
T cell receptor
- LPS
lipopolysaccharide
- GM-CSF
granulocyte-macrophage colony stimulating factor
- DMEM
Dulbecco’s modified Eagle’s medium
- WT
wild-type
- RBC
red blood cell
- ES cell
embryonic stem cell
Footnotes
Supported by National Institutes of Health grants AI44970, GM92108 and AI49344 (to W.-C.S.) and a pre-doctoral fellowship from the American Heart Association (09PRE2080124 to J. D.).
References
- 1.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 2.Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 3.Haas PJ, van Strijp J. Anaphylatoxins: their role in bacterial infection and inflammation. Immunol Res. 2007;37:161–175. doi: 10.1007/BF02697367. [DOI] [PubMed] [Google Scholar]
- 4.Monk PN, Scola AM, Madala P, Fairlie DP. Function, structure and therapeutic potential of complement C5a receptors. Br J Pharmacol. 2007;152:429–448. doi: 10.1038/sj.bjp.0707332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerard N, Lu B, Liu P, Craig S, Fujiwara Y, Okinaga S, Gerard C. An Anti-inflammatory Function for the Complement Anaphylatoxin C5a-binding Protein, C5L2. J Biol Chem. 2005;280:39677–39680. doi: 10.1074/jbc.C500287200. [DOI] [PubMed] [Google Scholar]
- 6.Chen N, Mirtsos C, Suh D, Lu Y, Lin W, McKerlie C, Lee T, Baribault H, Tian H, Yeh W. C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature. 2007;446:203–207. doi: 10.1038/nature05559. [DOI] [PubMed] [Google Scholar]
- 7.Bamberg CE, Mackay CR, Lee H, Zahra D, Jackson J, Lim YS, Whitfeld PL, Craig S, Corsini E, Lu B, Gerard C, Gerard NP. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem. 2010;285:7633–7644. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raby AC, Holst B, Davies J, Colmont C, Laumonnier Y, Coles B, Shah S, Hall J, Topley N, Kohl J, Morgan BP, Labeta MO. Toll-like receptor activation enhances C5a-induced pro-inflammatory responses by negatively modulating the second C5a receptor, C5L2. Eur J Immunol. 2011 doi: 10.1002/eji.201041350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buhl AM, Avdi N, Worthen GS, Johnson GL. Mapping of the C5a receptor signal transduction network in human neutrophils. Proc Natl Acad Sci U S A. 1994;91:9190–9194. doi: 10.1073/pnas.91.19.9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabiet M, Huet E, Boulay F. The N-formyl peptide receptors and the anaphylatoxin C5a receptors: An overview. Biochimie. 2007;89:1089–1106. doi: 10.1016/j.biochi.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll MC. Complement and humoral immunity. Vaccine. 2008;26(Suppl 8):I28–33. doi: 10.1016/j.vaccine.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 13.Dunkelberger JR, Song WC. Role and mechanism of action of complement in regulating T cell immunity. Mol Immunol. 2010;47:2176–2186. doi: 10.1016/j.molimm.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang C, Miwa T, Shen H, Song W. Complement-Dependent Enhancement of CD8+ T Cell Immunity to Lymphocytic Choriomeningitis Virus Infection in Decay-Accelerating Factor-Deficient Mice. J Immunol. 2007;179:3178–3186. doi: 10.4049/jimmunol.179.5.3178. [DOI] [PubMed] [Google Scholar]
- 15.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim AHJ, I, Dimitriou D, Holland MCH, Mastellos D, Mueller YM, Altman JD, Lambris JD, Katsikis PD. Complement C5a Receptor Is Essential for the Optimal Generation of Antiviral CD8+ T Cell Responses. J Immunol. 2004;173:2524–2529. doi: 10.4049/jimmunol.173.4.2524. [DOI] [PubMed] [Google Scholar]
- 17.Peng Q, Li K, Wang N, Li Q, Asgari E, Lu B, Woodruff TM, Sacks SH, Zhou W. Dendritic cell function in allostimulation is modulated by C5aR signaling. J Immunol. 2009;183:6058–6068. doi: 10.4049/jimmunol.0804186. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Miwa T, Hilliard B, Chen Y, Lambris JD, Wells AD, Song WC. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med. 2005;201:567–577. doi: 10.1084/jem.20040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang C, Miwa T, Song WC. Decay-accelerating factor regulates T-cell immunity in the context of inflammation by influencing costimulatory molecule expression on antigen-presenting cells. Blood. 2011;118:1008–1014. doi: 10.1182/blood-2011-04-348474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang C, Zhang X, Miwa T, Song WC. Complement promotes the development of inflammatory T-helper 17 cells through synergistic interaction with Toll-like receptor signaling and interleukin-6 production. Blood. 2009;114:1005–1015. doi: 10.1182/blood-2009-01-198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohl J, Baelder R, Lewkowich IP, Pandey MK, Hawlisch H, Wang L, Best J, Herman NS, Sproles AA, Zwirner J, Whitsett JA, Gerard C, Sfyroera G, Lambris JD, Wills-Karp M. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J Clin Invest. 2006;116:783–796. doi: 10.1172/JCI26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lalli PN, Strainic MG, Lin F, Medof ME, Heeger PS. Decay accelerating factor can control T cell differentiation into IFN-gamma-producing effector cells via regulating local C5a-induced IL-12 production. J Immunol. 2007;179:5793–5802. doi: 10.4049/jimmunol.179.9.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Lin F, Strainic MG, An F, Miller RH, Altuntas CZ, Heeger PS, Tuohy VK, Medof ME. IFN-gamma and IL-17 production in experimental autoimmune encephalomyelitis depends on local APC-T cell complement production. J Immunol. 2008;180:5882–5889. doi: 10.4049/jimmunol.180.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–1766. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weaver DJ, Jr, Reis ES, Pandey MK, Kohl G, Harris N, Gerard C, Kohl J. C5a receptor-deficient dendritic cells promote induction of Treg and Th17 cells. Eur J Immunol. 2010;40:710–721. doi: 10.1002/eji.200939333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou W, Patel H, Li K, Peng Q, Villiers MB, Sacks SH. Macrophages from C3-deficient mice have impaired potency to stimulate alloreactive T cells. Blood. 2006;107:2461–2469. doi: 10.1182/blood-2005-08-3144. [DOI] [PubMed] [Google Scholar]
- 27.Peng Q, Li K, Anderson K, Farrar CA, Lu B, Smith RAG, Sacks SH, Zhou W. Local production and activation of complement up-regulates the allostimulatory function of dendritic cells through C3a-C3aR interaction. Blood. 2008;111:2452–2461. doi: 10.1182/blood-2007-06-095018. [DOI] [PubMed] [Google Scholar]
- 28.Soruri A, Kim S, Kiafard Z, Zwirner J. Characterization of C5aR expression on murine myeloid and lymphoid cells by the use of a novel monoclonal antibody. Immunology Letters. 2003;88:47–52. doi: 10.1016/s0165-2478(03)00052-x. [DOI] [PubMed] [Google Scholar]
- 29.Wetsel RA. Structure, function and cellular expression of complement anaphylatoxin receptors. Curr Opin Immunol. 1995;7:48–53. doi: 10.1016/0952-7915(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song W. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura Y, Miwa T, Zhou L, Song WC. Activator-specific requirement of properdin in the initiation and amplification of the alternative pathway complement. Blood. 2008;111:732–740. doi: 10.1182/blood-2007-05-089821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pear WS, Miller JP, Xu L, Pui JC, Soffer B, Quackenbush RC, Pendergast AM, Bronson R, Aster JC, Scott ML, Baltimore D. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92:3780–3792. [PubMed] [Google Scholar]
- 33.Ramirez-Solis R, Davis AC, Bradley A. Gene targeting in embryonic stem cells. Methods Enzymol. 1993;225:855–878. doi: 10.1016/0076-6879(93)25054-6. [DOI] [PubMed] [Google Scholar]
- 34.Labosky PA, Barlow DP, Hogan BL. Mouse embryonic germ (EG) cell lines: transmission through the germline and differences in the methylation imprint of insulin-like growth factor 2 receptor (Igf2r) gene compared with embryonic stem (ES) cell lines. Development. 1994;120:3197–3204. doi: 10.1242/dev.120.11.3197. [DOI] [PubMed] [Google Scholar]
- 35.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. 1979. Biotechnology. 1992;24:145–149. [PubMed] [Google Scholar]
- 36.Wuyts A, Menten P, Van Osselaer N, Van Damme J. Assays for chemotaxis. Methods Mol Biol. 2004;249:153–166. doi: 10.1385/1-59259-667-3:153. [DOI] [PubMed] [Google Scholar]
- 37.Shi GP, Villadangos JA, Dranoff G, Small C, Gu L, Haley KJ, Riese R, Ploegh HL, Chapman HA. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity. 1999;10:197–206. doi: 10.1016/s1074-7613(00)80020-5. [DOI] [PubMed] [Google Scholar]
- 38.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 39.Davies JQ, Gordon S. Isolation and culture of murine macrophages. Methods Mol Biol. 2005;290:91–103. doi: 10.1385/1-59259-838-2:091. [DOI] [PubMed] [Google Scholar]
- 40.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol. 2008;181:5183–5188. doi: 10.4049/jimmunol.181.8.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nikolic T, Dingjan GM, Leenen PJ, Hendriks RW. A subfraction of B220(+) cells in murine bone marrow and spleen does not belong to the B cell lineage but has dendritic cell characteristics. Eur J Immunol. 2002;32:686–692. doi: 10.1002/1521-4141(200203)32:3<686::AID-IMMU686>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 43.Lalli PN, Zhou W, Sacks S, Medof ME, Heeger PS. Locally produced and activated complement as a mediator of alloreactive T cells. Front Biosci (Schol Ed) 2009;1:117–124. doi: 10.2741/S11. [DOI] [PubMed] [Google Scholar]
- 44.Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, Xu Y, Medof ME. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201:1523–1530. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baelder R, Fuchs B, Bautsch W, Zwirner J, Kohl J, Hoymann HG, Glaab T, Erpenbeck V, Krug N, Braun A. Pharmacological Targeting of Anaphylatoxin Receptors during the Effector Phase of Allergic Asthma Suppresses Airway Hyperresponsiveness and Airway Inflammation. J Immunol. 2005;174:783–789. doi: 10.4049/jimmunol.174.2.783. [DOI] [PubMed] [Google Scholar]
- 46.Li Q, Peng Q, Xing G, Li K, Wang N, Farrar CA, Meader L, Sacks SH, Zhou W. Deficiency of C5aR prolongs renal allograft survival. J Am Soc Nephrol. 2010;21:1344–1353. doi: 10.1681/ASN.2009090977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lechmann M, Shuman N, Wakeham A, Mak TW. The CD83 reporter mouse elucidates the activity of the CD83 promoter in B, T, and dendritic cell populations in vivo. Proc Natl Acad Sci U S A. 2008;105:11887–11892. doi: 10.1073/pnas.0806335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Awasthi A, Riol-Blanco L, Jager A, Korn T, Pot C, Galileos G, Bettelli E, Kuchroo VK, Oukka M. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 51.Tholanikunnel BG, Joseph K, Kandasamy K, Baldys A, Raymond JR, Luttrell LM, McDermott PJ, Fernandes DJ. Novel mechanisms in the regulation of G protein-coupled receptor trafficking to the plasma membrane. J Biol Chem. 2010;285:33816–33825. doi: 10.1074/jbc.M110.168229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Subramaniam K, Chen K, Joseph K, Raymond JR, Tholanikunnel BG. The 3′-untranslated region of the beta2-adrenergic receptor mRNA regulates receptor synthesis. J Biol Chem. 2004;279:27108–27115. doi: 10.1074/jbc.M401352200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.