SUMMARY
Variation in mechanical loading is known to influence chondrogenesis during joint formation. However, the interaction among chondrocyte behavior and variation in activity patterns is incompletely understood, hindering our knowledge of limb ontogeny and function. Here, the role of endurance exercise in the development of articular and physeal cartilage in the humeral head was examined in 14 miniature swine (Sus scrofa domesticus). One group was subjected to graded treadmill running over a period of 17 weeks. A matched sedentary group was confined to individual pens. Hematoxylin and eosin staining was performed for histomorphometry of cartilage zone thickness, chondrocyte count and cell area, with these parameters compared multivariately between exercised and sedentary groups. Comparisons were also made with femora from the same sample, focusing on humerus–femur differences between exercised and sedentary groups, within-cohort comparisons of humerus–femur responses and correlated changes within and across joints. This study shows conflicting support for the chondral modeling theory. The humeral articular cartilage of exercised pigs was thinner than that of sedentary pigs, but their physeal cartilage was thicker. While articular and physeal cartilage demonstrated between-cohort differences, humeral physeal cartilage exhibited load-induced responses of greater magnitude than humeral articular cartilage. Controlling for cohort, the humerus showed increased chondrocyte mitosis and cell area, presumably due to relatively greater loading than the femur. This represents the first known effort to evaluate chondral modeling across multiple joints from the same individuals. Our findings suggest the chondral response to elevated loading is complex, varying within and among joints. This has important implications for understanding joint biomechanics and development.
KEY WORDS: cartilage, mechanobiology, limbs, chondrogenesis, joints, mammals, plasticity
INTRODUCTION
Chondral modeling is defined as the adaptive ontogenetic response of cartilage to regional variation in hydrostatic pressure, resulting in increased chondrocyte mitosis and synthesis of the extracellular matrix (ECM), influencing overall joint topography, congruence and size (Frost, 1979; Frost, 1999; Hamrick, 1999; Murray et al., 2001; Carter and Wong, 2003; Plochocki et al., 2009). Such chondrogenic changes are believed to facilitate normal joint movement and minimize dangerously high regional contact stresses (Frost, 1979). Chondral modeling theory predicts regional or widespread thickening of cartilage, changes in cellular and extracellular cartilage composition, and differential mineralization and ossification of hyaline tissue, thereby directly influencing bony morphology through increased bone deposition. There is evidence that chondral modeling occurs at articular and physeal cartilage surfaces (Hammond et al., 2010) and it is hypothesized to occur at sites of fascial, tendinous and ligamentous insertion (Frost, 1979).
Postnatal variation in mechanical loading has been shown to yield changes in chondrocyte proliferation and metabolism, as well as changes in the synthesis and proteoglycan content of the ECM, ultimately influencing adult form (Eggli et al., 1988; Kiviranta et al., 1988; Wu and Chen, 2000; Liu et al., 2001; Carter and Wong, 2003; Ravosa et al., 2007; Ravosa et al., 2008a; Ravosa et al., 2008b; Hammond et al., 2010). Chondrocytes, in various stages of development, are the only cells located in cartilage. They are responsible for the production and maintenance of the ECM, and regulate the numerous changes thought to occur during chondral modeling (Kiviranta et al., 1988). It has been suggested that a range of physiological loading results in maximal stimulation of regional cartilage growth (Frost, 1979). Frost observed that cartilage synthesis is reduced under routinely high compressive loads, but enhanced under moderate loads, and hypothesized that unequal mechanical loads are responsible for the observed patterns of morphological variation in adult cartilage (Frost, 1979). Accordingly, cartilage regions subjected to greater loads are posited to display a decrease in chondrocyte density and an increase in cell area compared with less heavily loaded regions (Eggli et al., 1988).
In a re-evaluation of chondral modeling theory, Hamrick discussed the effect of hydrostatic pressure on both the range of cartilage loading magnitude and loading frequency (Hamrick, 1999). Under moderately increased levels of mechanical stimuli, chondrocytes are predicted to exhibit increased division and ECM synthesis in order to produce uniform hydrostatic pressure throughout the tissue. In this regard, in vitro cyclic loading resulted in differentially greater chondrocyte proliferation in immature vs more mature hypertrophic chondrocytes (Wu and Chen, 2000). This suggested that certain effects of mechanical loading may vary with age. Another in vitro study examined the effect of vibration on proteoglycan synthesis in articular cartilage of rabbits, and found that moderate frequencies of vibration, applied intermittently, resulted in an increase in proteoglycan content, whereas higher frequencies inhibited proteoglycan synthesis (Liu et al., 2001). Similar findings have been observed in the jaw joints of growing rabbits subjected to long-term elevation of masticatory stresses (Ravosa et al., 2007; Ravosa et al., 2008a; Ravosa et al., 2008b). An in silico analysis suggests that intermittent hydrostatic pressures would result in cartilage maintenance, but repetitive tensile strains would result in elevated cartilage modeling (Carter and Wong, 2003). Lastly, while cartilage plasticity involves a number of changes at the protein and molecular levels (e.g. Carvalho et al., 1995; Mizoguchi et al., 1996; Grodzinsky et al., 2000; Wong and Carter, 2003), chondral modeling theory has largely focused on tissue and cellular changes (e.g. Frost, 1979; Eggli et al., 1988; Hamrick, 1999; Wu and Chen, 2000).
Comparisons of articular and physeal cartilage have demonstrated tissue-level differences in the ontogenetic responses to altered loading conditions, specifically the pathological conditions of chondrocytes, the relative expression of collagen type I, II, X and XI, and the molecular underpinnings of angiogenesis (Wardale and Duance, 1993; Wardale and Duance, 1994; Stempel et al., 2011). There is evidence that articular chondrocytes derive from a unique cellular subpopulation prior to early embryological differentiation (Hyde et al., 2007). While both cartilage types express many of the same transcription factors during development, such transcription factors play different roles in articular vs physeal cartilage (Dy et al., 2010). Further variation in the distribution of collagen type XII underscores embryological differences between these two cartilage types, suggesting the possibility of differing responses to similar stimuli at very early ages (Gregory et al., 2001). This could contribute to different load-induced plasticity, which deserves further investigation, particularly in an experimental setting where one can examine both cartilage types within the same joint.
In this regard, a recent study found dissimilar results regarding the plasticity responses of articular and physeal cartilage to altered limb loading (Hammond et al., 2010). In particular, support for the predictions of chondral modeling theory, including increased cell proliferation and cartilage height, was observed only for the physeal cartilage. While neither cartilage height nor cellularity discriminated well between the articular cartilage in the proximal femur of exercise vs sedentary pigs, both parameters in the physeal cartilage were highly successful in discriminating between loading cohorts. Such dissimilarities in the response of different cartilage types highlight the possibility of site-specific variability in chondrogenesis and, ultimately, variation in cartilage proportions within the same joint of members of a given loading cohort (Hammond et al., 2010). Additional in vivo data on load-induced responses of the ECM as well as chondrocyte proliferation and hypertrophy would be invaluable for further illuminating issues regarding intralimb variation in chondrogenesis as it relates to altered loading regimes.
With this in mind, a further examination of the humeri from the same subjects examined previously (Hammond et al., 2010) represents a unique opportunity to evaluate the chondral response in different joints in the same experimental animals. By comparing differences in loaded vs unloaded cohorts in another joint from the same organism, one can better evaluate the general relevance of the predictions of chondral modeling theory. In addition, as prior work has demonstrated that domestic pigs are ‘forelimb dominant’, experiencing greater vertical reaction forces in the forelimb vs the hindlimb (Thorup et al., 2007; Von Wachenfelt et al., 2009a; Von Wachenfelt et al., 2009b; Von Wachenfelt et al., 2010), comparisons of multiple joints in the same individuals may be informative about the relative magnitude of cartilaginous responses in different joints subjected to the same locomotor regimes. By detailing how multiple joints respond to loading within a given organism, we can enhance our understanding of joint formation and norms of reaction in mammalian limbs. Such information would be valuable not only to biomedical research exploring the applicability of exercise as a therapeutic method but also to paleontological fields where locomotor reconstructions of fossil material could benefit greatly from a better understanding of the adaptive response of joints.
To determine the adaptive responses of cartilage to altered loads in the growing limb, the proximal humerus of exercised (Ex) and sedentary (Sed) juvenile miniature pigs (Sus scrofa domesticus) was evaluated. Following the expectations of chondral modeling theory, it was hypothesized that an increase in mechanical loading of the proximal humerus would result in chondrocyte hypertrophy and proliferation, with corresponding increases in cartilage height (Frost, 1979; Frost, 1999; Hamrick, 1999; Murray et al., 2001; Carter and Wong, 2003; Plochocki et al., 2009). As pig forelimbs are known to be loaded more heavily than their hindlimbs (Thorup et al., 2007; Von Wachenfelt et al., 2009a; Von Wachenfelt et al., 2009b; Von Wachenfelt et al., 2010), chondral modeling was further examined via cohort-controlled analyses of the proximal humerus and proximal femur. For such comparisons, we predicted that the humerus would exhibit elevated chondrocyte hypertrophy and proliferation as well as greater cartilage height than the femur. To the best of our knowledge, this represents the first study to evaluate adaptive chondrogenesis in two limb joints from the same experimental sample.
MATERIALS AND METHODS
Sample
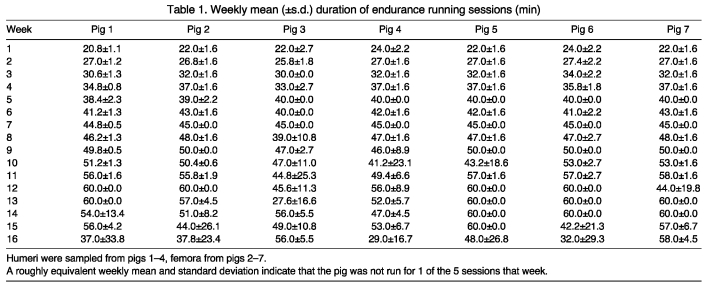
Fifteen castrated male juvenile miniature Yucatan swine (S. s. domesticus, Erxleben 1777) (Sinclair Bio Resources, Auxvasse, MO, USA) began the protocol at 8 months of age and were killed in the 17th week of participation in the experiment. Pigs are skeletally immature until approximately 6 years of age (Dyce et al., 2002); therefore, these individuals can be considered juveniles for the extent of the study period. The pigs were divided into two groups composed of seven exercised and eight sedentary control animals. Exercised pigs completed treadmill running 5 days a week, while members of the sedentary cohort were raised without exercise for the same time period. All other aspects of the experimental protocol were identical between groups. Treadmill running consisted of four stages: a 5 min warm-up (2.0–2.5 m.p.h., where 1 m.p.h.=1.609 km h–1), 15 min sprint (4.0–7.0 m.p.h.), a period of endurance running (3.0–5.0 m.p.h.), and a 5 min cool-down (2.0–2.5 m.p.h.). The endurance running period varied among both individuals and exercise sessions, and was determined behaviorally by the subject’s willingness to run (Table 1); all subjects showed age-related increases in the weekly mean amount of time they were able to perform endurance running. Ultimately, seven right humeri from each group were preserved, with four specimens per group of sufficient quality for histomorphometry [additional details on the housing and care of experimental subjects, as well as sample acquisition and preparation can be found elsewhere (Hammond et al., 2010)]. All in vivo procedures were approved by the University of Missouri Animal Care and Use Committee under protocol 472-2.
Table 1.
Weekly mean (±s.d.) duration of endurance running sessions (min)
One additional caveat to consider is the effect of castration on chondrogenesis. Earlier research has shown that castration, and thereby the absence of testosterone, can increase apoptosis and decrease chondrocyte proliferation in rabbits (Irie et al., 2005). While in this case all the animals were castrated and therefore the absence of testosterone is likely not underlying differences between groups, it is possible that all individuals exhibited less chondrocyte proliferation than they would have if left intact. Given the relatively young age of the sample, this is likely to have been less an issue than if the period of experimental modification were continued into subadult and adult stages.
Measurements
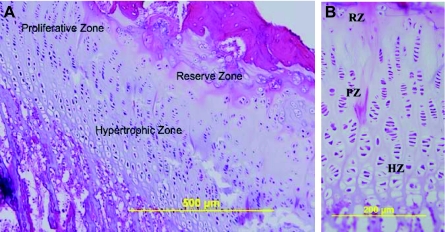
After dissection, coronal sections of proximal humerus bone and cartilage were prepared using standard histological methods. Hematoxylin and eosin stain (H&E) was used to identify articular and physeal cartilage for histomorphometry. Cartilage for both sites was imaged at four regions with an Olympus BX41 microscope (Fig. 1). Physeal cartilage zones (reserve, proliferative, hypertrophic; Fig. 2A) were delineated based on cell morphology (Niehoff et al., 2004) (Fig. 2B) and measured as a linear mean in each standard section. Articular cartilage was measured as total mean height because zones could not be sufficiently defined based on cell morphology. In each cartilage zone, ratios of ‘scaled cell counts’ in a 300 μm wide standard column height were calculated by taking a raw count of cell number and dividing that value by the height of a cartilage region. A ‘cell area’ A was calculated for both articular and physeal chondrocytes from the formula A=(0.5h×0.5l×π), where h and l are the height and length measurement, respectively, of the cell. Corresponding histomorphometric data were available from a similar study of the proximal femur (Hammond et al., 2010). When comparing Ex humeri to Sed humeri, a mean for each of the four regions sampled was calculated for the articular cartilage variables, with one mean per region per zone calculated for the physeal cartilage variables.
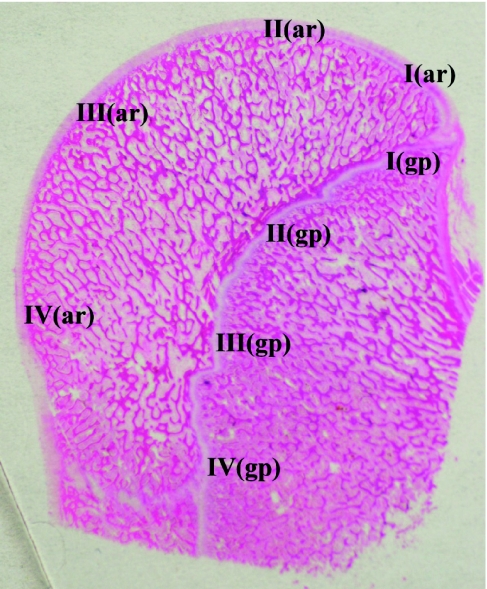
Fig. 1.
Illustration of 8 sampled regions from the humeral head (Ar, articular; gp, physeal). Regions are referred to as articular regions I–IV and physeal regions I–IV. This is a longitudinal section of humeral head, with the epiphyses oriented towards the left.
Fig. 2.
Morphology of growth plate cartilage. (A) The reserve, proliferative and hypertrophic zones. Oriented with the epiphyseal plate towards the top right corner of the image. (B) Characteristic morphology of reserve cells (RZ), proliferative cells (PZ) and hypertrophic cells (HZ).
It is important to note that these are two-dimensional measurements from two-dimensional images of what are in fact three-dimensional structures. In all cases, considerable caution was employed to ensure that specimen orientation was consistent for histological sections, and thus histomorphometric parameters, at mid-joint. Such a protocol is crucial for the within- and between-joint comparisons performed herein. While outside of the scope and goals of this study, another goal is to perform more exhaustive analyses of cartilage histomorphometry so as to more thoroughly characterize within-joint three-dimensional variation in cartilage form and function.
Analyses
Between-group scaled cell counts, cell area and cartilage height were compared via a series of discriminant function analyses (DFA). DFA is a multivariate statistical test that uses a priori knowledge of group composition to examine the extent a set of parameters correctly sorts the members of a cohort. To ensure that a given variable would not have an undue influence on such multivariate analyses, only variables of similar scale were included in a given DFA. Thus, ratio data, linear measurements and cell counts were analyzed separately, controlling for articular and physeal cartilage. For each DFA performed, each individual was represented by one mean value for each variable included. As an example, for the DFA of articular variables comparing Sed humeri with Ex humeri, each individual was represented by one mean scaled cell count, one mean cartilage height and one mean cell area. To accommodate constraints on the number of variables that could be included in a given DFA related to the sample sizes, it was necessary to separately analyze some data of similar scale from similar cartilage types. Thus, in certain cases physeal values were sorted into central regions (identified as 2 and 3) and peripheral regions (identified as 1 and 4). We chose the variables based on joint site, with central regions closer to the middle of the physis and peripheral regions near the edges of the physis. Because of the smaller sample sizes, only DFAs with a mean correct classification percentage above 75% were considered noteworthy, indicating that three out of four individuals were correctly classified.
Non-parametric ANOVA (Mann–Whitney U-test) was used solely to identify a variable(s) that may be underlying the between-group variation detected via DFA. Thus, in addition to following the convention where statistical significance between groups is evaluated at P≤0.05, mean trends were indicated for those variables occurring at P≤0.10 and P≤0.20. In the absence of a univariate trend, notable variation detected by DFA was considered to be driven by the totality of the multivariate pattern for a given cohort.
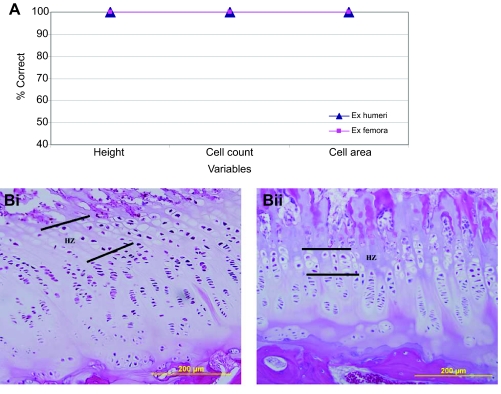
DFA and ANOVA were also used to evaluate differences between humerus and femur parameters within each pig, controlling for locomotor behavior. When comparing humeri with femora, elements were represented by one mean value, pooling the four measured regions for each variable, ultimately yielding one value for each articular cartilage variable and one value per zone for each physeal cartilage variable. DFAs for this sample examined height (including all articular and physeal values), cell count (including all articular and physeal values) and cell area (including only physeal values, as articular cell areas could not be accurately measured for the femora because of insufficient clarity of cell borders).
Similar articular and physeal cartilage variables were tested for strength of within-cohort associations via Pearson correlation coefficients (r); specifically, total height, cell count and cell area in the humerus and total height and cell count in the femur, as were homologous variables within articular and physeal cartilage across joints of the same cohort. Both r-values and P-values from bivariate regressions are reported. Correlations were tested for all Sed and Ex variables in both humeral and femoral samples. Statistical significance was evaluated at P≤0.05, with trends indicated for comparisons occurring at P≤0.20.
RESULTS
Between-cohort comparisons in the humerus
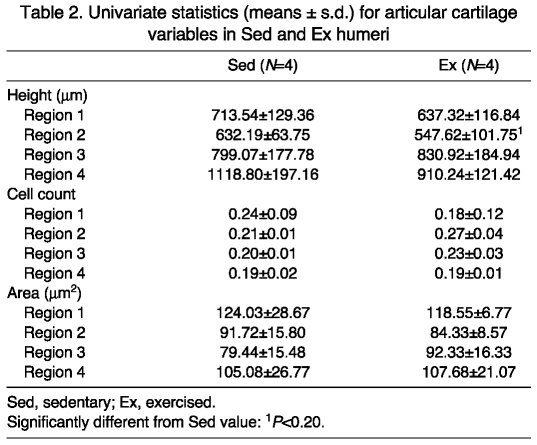
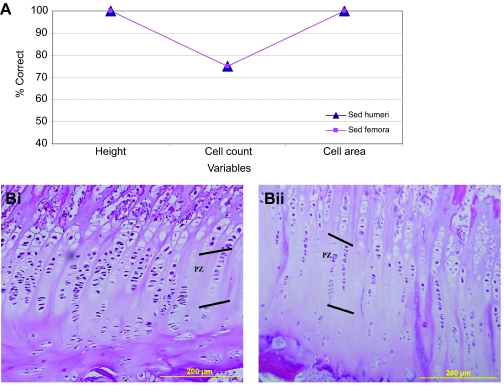
For DFA of articular cartilage height, 75% of Sed individuals were classified correctly, while 50% of Ex individuals were classified correctly (Fig. 3A). Using ANOVA (Table 2), region 2 tended to have a higher mean height in the Sed vs Ex group, with all other regions tending to be similar between the two groups.
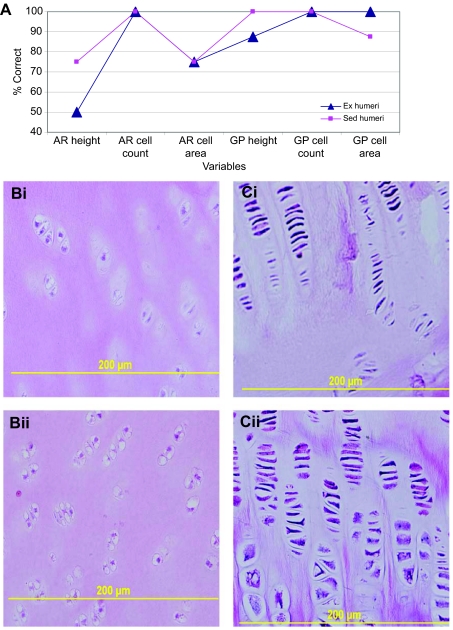
Fig. 3.
Ex and Sed humeri DFA results. (A) Line graph of the percentage correct for articular (AR) and physeal (GP) height, scaled cell count and cell area. Physeal values for peripheral and central regions have been averaged. (B) Examples of the articular cell counts for humeri from sedentary (Sed, i) and exercised (Ex, ii) pigs. (C) Examples of the physeal cell counts for Sed (i) and Ex (ii) humeri. The growth plate is oriented towards the top of the image.
Table 2.
Univariate statistics (means ± s.d.) for articular cartilage variables in Sed and Ex humeri
DFA for articular scaled cell counts had 100% of the members of both loading cohorts classified correctly (Fig. 3A,B). Based on ANOVA, all regions tended to be similar among cohorts (Table 2).
A DFA of articular cartilage cell area correctly classified 75% of the members of each loading cohort (Fig. 3A). ANOVA indicated that means for all regions tended to be similar between the two groups (Table 2).
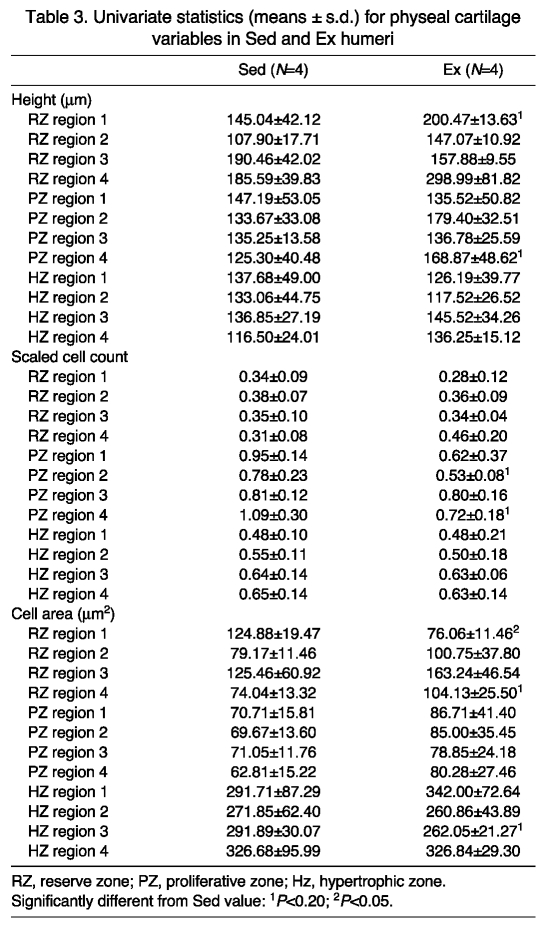
For physeal cartilage heights, in the peripheral regions, 100% of Sed individuals were classified correctly, whereas only 75% of Ex individuals were classified correctly. In the central regions, members of both groups sorted equally well at 100% accuracy (Fig. 3A). The reserve zone region 1 tended to have a higher mean in the Ex group, while regions 3 and 4 tended to be similar between Sed and Ex groups (Table 3). For the proliferative zone, the mean for region 4 tended to be greater in the Ex group, with regions 1, 2 and 3 having means similar between Sed and Ex cohorts. For the hypertrophic zone, all means tended to be similar in the two cohorts (Table 3).
Table 3.
Univariate statistics (means ± s.d.) for physeal cartilage variables in Sed and Ex humeri
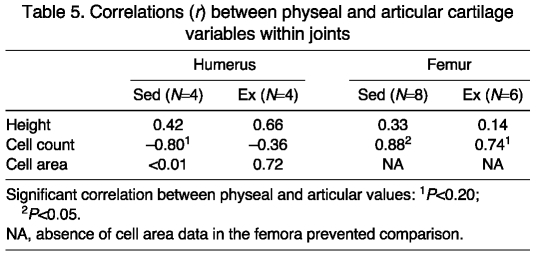
DFA for physeal scaled cell counts had 100% of all individuals in each group classified correctly for peripheral physeal regions and central physeal regions (Fig. 3A,C). ANOVA showed that all reserve regions tended to be similar for the two groups (Table 3). Proliferative zone regions 2 and 4 means tended to be greater in the Sed group, while means for regions 1 and 3 were similar for the two cohorts. Hypertrophic zone regions all tended to be similar between treatment cohorts.
Physeal cell area DFA had 100% of the members in both loading cohorts classified correctly for peripheral physeal regions (Fig. 3A). In the central physeal regions, 75% of Sed individuals were classified correctly, whereas all Ex individuals sorted properly. In the reserve zone, ANOVA demonstrated that the mean for region 4 tended to be greater for the Ex cohort, while the mean for region 1 was significantly higher in the Sed group (Table 3). Regions 2 and 3 were similar between groups. In the proliferative zone all regions were similar between cohorts. In the hypertrophic zone, the mean for region 3 tended to be higher in the Sed cohort, and the means for all other regions were similar in the two groups.
Within-cohort comparisons in the humerus and femur
Sedentary cohort
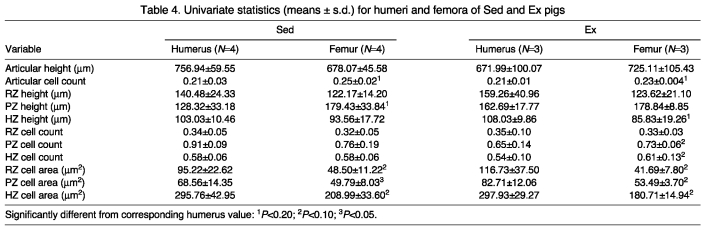
All cartilage heights were classified correctly via DFA for both limb elements (Fig. 4A). Mean articular cartilage height was similar in all regions. ANOVA indicated that in the physeal cartilage, the proliferative zone mean tended to be higher in the femur, while the reserve and hypertrophic zones were roughly equal (Table 4).
Fig. 4.
Sed humeri and femora DFA results. (A) Line graph of the percentage correct for cartilage height, cell count and cell area (both articular and physeal). Physeal values for peripheral and central regions have been averaged. (B) Examples of the proliferative zone cell areas for humeri (i) and femora (ii). The growth plate is oriented towards the bottom of the image. Diagonal lines delineate a region representative of the proliferative zone (PZ).
Table 4.
Univariate statistics (means ± s.d.) for humeri and femora of Sed and Ex pigs
Combined articular and physeal scaled cell count DFA classified 75% of the limb elements correctly (Fig. 4A). Mean articular scaled cell counts tended to be higher in the femur (Table 4). Physeal regions exhibited similar means in the reserve and hypertrophic zones, while the mean for the proliferative zone was similar between the two limbs (Table 4).
All cell areas were classified correctly for both limb elements (Fig. 4A,B). In all three physeal zones, the mean for the humerus tended to be higher than the mean for the femur.
Exercised cohort
Combined articular and physeal cartilage heights were classified correctly via DFA in 100% of both limb elements (Fig. 5A). ANOVA demonstrated that mean articular cartilage height tended to be similar in the humerus and femur (Table 4). In the physeal cartilage, the mean for the hypertrophic zone tended to be higher in the humerus, with the reserve and proliferative zone means being similar between the two groups.
Fig. 5.
Ex humeri and femora DFA results. (A) Line graph of the percentage correct for cartilage height, cell count and cell area (both articular and physeal). (B) Examples of the hypertrophic zone heights for humeri (i) and femora (ii). The growth plate is oriented towards the bottom of the image. Lines delineate a region representative of the hypertrophic zone (HZ).
Scaled cell counts were classified correctly in 100% of the limb elements (Fig. 5A). Mean articular cell counts tended to be greater in the femur than in the humerus (Table 4). While the reserve zone means were similar, means for the proliferative and hypertrophic zones tended to be higher in the femur than in the humerus.
Cell areas were classified correctly in 100% of both limb elements (Fig. 5A). In all three zones, means for the humerus tended to be higher than for the femur (Table 4).
Comparisons of articular and physeal variables in the humerus and femur
In Sed humeri, the correlation between articular and physeal cell counts was not significant, exhibiting only a negative trend. Sed correlations for humerus cartilage heights and cell areas were not significant (Table 5). None of the Ex correlations for humerus cell counts, cartilage heights or cell areas were statistically significant (Table 5).
Table 5.
Correlations (r) between physeal and articular cartilage variables within joints
In Sed femora, articular and physeal cell counts were significantly positively correlated, whereas the correlation between cartilage heights was not significant (Table 5). In Ex femora, the correlation between articular and physeal cell counts was not significant, showing only a positive trend (Table 5). The Ex correlation for femur cartilage heights was not significant.
In the Sed cohort, the correlation between humeral and femoral total articular height was significantly negatively correlated. There were no significant correlations for total physeal height, articular or physeal cell count or physeal cell area between the humerus and femur (Table 6).
Table 6.
Correlations (r) of within-cohort humerus and femur articular and physeal cartilage
In the Ex cohort, the correlation between humeral and femoral total articular height was significantly negatively correlated. There were no significant correlations for total physeal height, articular or physeal cell count, or physeal cell area between the humerus and femur (Table 6).
DISCUSSION
Chondral modeling theory predicts that growing joints subjected to elevated mechanical stresses exhibit an adaptive response, including elevated chondrocyte mitosis and hypertrophy, which results in increased cartilage formation and larger joints (Frost, 1979; Frost, 1999; Hamrick, 1999; Murray et al., 2001; Carter and Wong, 2003; Plochocki et al., 2009). This hypothesis was investigated in two ways in a sample of growing pigs subjected to different loading regimes: (1) the effects of exercise vs sedentary locomotion on cartilage plasticity in the proximal humerus; and (2) behavior-controlled comparisons of cartilage histomorphometry between the proximal humerus and proximal femur. The former set of comparisons was performed between cohorts that differed postnatally in both loading magnitude and loading frequency, while the latter suite of analyses contrasted groups that differed in loading magnitude.
The humerus
When comparing humerus parameters across cohorts, differences were observed between the responses of articular vs physeal cartilage to greater loading. The articular cartilage in the Ex group was characterized by greater cell proliferation, which is consistent with the predictions of chondral modeling theory. However, a lack of correspondingly greater cartilage heights in the Ex group indicates that there is not a corresponding increase in ECM production. In fact, such a result may even suggest a relative decrease in the amount of ECM generated by articular chondrocytes under increased loading. This finding is opposite to predictions that greater loads will result in an increase in cartilage height. There was also no associated increase in articular chondrocyte size in the Ex group, which is also contrary to predictions of chondral modeling theory.
The physeal cartilage demonstrated the most differences in regional cartilage heights and scaled cell counts between cohorts, but the results were somewhat contradictory. While a greater cartilage height in the Ex group suggests elevated ECM production in accordance with predictions, the lack of an increase in scaled cell count is contrary to predictions that greater mechanical loading also results in increased chondrocyte mitosis. Moreover, mean cell area demonstrated notable regional variation. The significantly greater mean cell area in reserve zone region 1 of the Sed group contradicts expectations that an increase in mechanical loading will result in greater cell area, and the majority of physeal cartilage regions across all three zones did not show a tendency for a larger cell area in the Ex cohort. As bone calcification and growth progresses, it is the hypertrophy of physeal chondrocytes that contributes most to this process (Farnum et al., 2002). We would therefore expect an increase in the cell area of hypertrophic zone chondrocytes in the Ex cohort, as this is the primary mechanism by which new (load-induced) bone occurs. However, this effect was not observed in the hypertrophic chondrocytes of Ex humeri.
When considering the traditional predictions of chondral modeling theory, the articular and physeal cartilage showed differing responses to mechanical loading. The articular cartilage was most responsive via greater cellularity without a corresponding increase in cartilage height, suggesting that articular cartilage responds to loading by greater chondrocyte mitosis without increasing ECM production. This runs counter to predictions that mechanical loading induces higher cartilage viscoelasticity via elevated ECM production. In contrast, physeal cartilage responded to loading with an increase in height and cell area in the relative absence of an increase in cellularity, essentially producing an opposite signal to that observed in articular cartilage.
Other studies examining specific responses of cartilage to mechanical loading have yielded possible explanations for such variable responses, which in some cases also contradict the predictions of chondral modeling. While the differences in articular and physeal responses found here could be the result of a depth-dependent chondrogenic response (Wu and Herzog, 2006) or a change in load magnitude as it is transmitted distally across the joint, it is also possible that this variation is simply a reflection of inherent biological differences among articular and physeal cartilage. Specifically, articular and growth plate cartilage in pigs differ in both the types and relative amounts of collagen present, and growth plate cartilage is known to contain less total collagen than articular cartilage (Wardale and Duance, 1993). In the absence of variation in other ECM components or cartilage proportions that also influence tissue mechanical properties, this would suggest that the growth plate is perhaps less viscoelastic than articular cartilage. Earlier work has demonstrated that different growth plate zones have different elastic moduli, with the stiffness gradually increasing from the reserve zone to the calcification zone (Rhadakrishnan et al., 2004). This implies not only that chondrocytes in different zones are experiencing the induced load differentially but also that the unknown compressive stresses combined with the variable elastic modulus make it difficult to infer how individual zones experience a given load.
Prior research has also shown that p21, a cyclin-dependent kinase inhibitor that plays an important role in the terminal differentiation of chondrocytes, is upregulated in chondrocytes undergoing hypertrophic differentiation (Stewart et al., 1997). It is possible that this cell-specific upregulation could be playing a role in the cell count differences observed here, particularly in the hypertrophic zone of the growth plate, in which all regions were found to be equivalent among Sed and Ex humeri. In conjunction with those findings, certain growth factors are found to be more heavily expressed in the reserve zone of growth plate cartilage than the comparable surface zone of articular cartilage (Yamane et al., 2007), likely resulting in the widely acknowledged lack of proliferation of articular chondrocytes and potentially contributing to the higher cell counts observed in the reserve and proliferative zones of the growth plate cartilage from this study.
It has previously been suggested that cyclic, highly compressive loads can lead to fluid exudation in the superficial zone of articular cartilage (Carter and Wong, 2003). If this is occurring in the articular cartilage of Ex humeri, then it is possible that articular chondrocytes did increase ECM production in response to loading, as predicted by the chondral modeling theory, but this increased production simply resulted in maintained articular cartilage size and shape so as to compensate for fluid lost in exudation. When considering activity levels insufficient to induce gross changes in joint size or shape, this kind of maintenance would be useful in maintaining joint integrity in the face of increased loads. This could further complicate paleontological reconstructions of locomotor behavior, as it must be accepted that the soft tissue of joints may have the ability to mitigate certain variations in activity level, preventing them from influencing the ultimate skeletal form. Alternatively, disparity in cell counts in Ex vs Sed humeri also suggests a differential ECM response as opposed to cellularity, perhaps a load-induced change that may augment tissue viscoelasticity via an increase in ECM volume surrounding a chondrocyte (Eggli et al., 1988). This would result in greater cartilage viscoelasticity without a necessary increase in height or cellularity, which could contribute positively to the ability of cartilage to respond to elevated loading.
The larger relative cell area in some humerus articular regions and physeal zones observed in response to mechanical loading may be related to an increase in the number of cytoskeletal proteins (i.e. intermediate filaments) and glycogen within the chondrocytes, which contribute to cell structural integrity (Eggli et al., 1988; Benjamin and Ralphs, 1998). This interpretation is further supported by an in vitro study that found remodeling of actin myofilaments in articular chondrocytes can be induced by both static and cyclic mechanical loads, and that this remodeling may reverse after time (Knight et al., 2006). Alternatively, it has been suggested that such a load-induced increase in area is a temporary swelling due to elevated matrix production within the cell, which would subside when loading ceases (Paukkonen et al., 1985). It is therefore worth noting that all Ex pigs were exercised the day before they were killed, while Paukkonen et al. (Paukkonen et al., 1985) make no predictions regarding the duration of this temporary swelling.
Regional variation in cartilage responses may be explained by Frost’s predictions that areas of very high loading will show a decrease in variables relevant to chondral modeling, with an increase visible in nearby areas of moderate loading (Frost, 1999). Without in vivo data or in silico estimates of forces distributed across the articular surface of the pig humeral head, it is difficult to gauge the functional significance of regional variation in cartilage parameters. Interestingly, a recent study demonstrated the presence of regional differences in articular chondrocyte responses to mechanical loading, suggesting the possibility that even under uniform loads different cartilage regions may not respond uniformly (Bevill et al., 2009). As chondrocytes are particularly sensitive to the three-dimensional microenvironment and differentiate in response to local signals (Lemare et al., 1998; Goldring, 2004a; Goldring, 2004b), the expression of cartilage ECM elements likely exhibit regional variation due to subtle differences in loading patterns in distinct joint regions (Bayliss et al., 1983; Nakano and Scott, 1989; Mow et al., 1990; Mizoguchi et al., 1996; Tanaka et al., 2000). It is unknown how velocity-related changes in limb posture affect load orientation. However, as it is unlikely that loads are distributed evenly across the joint surface during walking or running, disparity in regional loads could also account for regional differences observed in this study.
Comparison with femur
Within-joint analyses
Overall, the articular cartilage appears to have a more uniform response to increased loading across joints than the physeal cartilage. When comparing the results of the within-humerus comparisons made here with those obtained previously for pig femora (Hammond et al., 2010), there is not a consistent signal for both articular and physeal cartilage changes. The femur and humerus exhibited similar patterns of increased chondrocyte mitosis in response to mechanical loading for articular chondrocytes. The Ex femora, but not the Ex humeri, exhibited an increase in physeal cell proliferation, decreased physeal zone heights and decreased articular zone heights
A number of factors could contribute to the observed differences between the humerus and femur. The growth plate may be more responsive to mechanical loading than the articular cartilage regardless of the location because it is the primary site of limb elongation. Thus, as a site of active chondrogenesis, the growth plate has a greater potential for load-induced tissue plasticity than the articular cartilage (Ravosa et al., 2008b). As the proximal humerus and femur fuse at different ages (Dyce, 2002), the differences in physeal cartilage response could be attributable to the two joints being at different stages of ossification. Previous work in diverse vertebrates has also demonstrated that musculoskeletal plasticity is not uniform across ontogeny, with reaction norms being greater in younger organisms (Goldspink, 1970; Hinton and McNamara, 1984; Meyer, 1987; Bouvier, 1988; Rubin et al., 1992; Ravosa et al., 2008b). For this reason, it is possible that individuals of either younger or older ages subjected to the same experimental regimen could yield somewhat different results (Bertram and Swartz, 1991; Pearson and Lieberman, 2004; Hoverman and Relyea, 2007; Ravosa et al., 2008a; Ravosa et al., 2008b).
However, differential loading between the humerus and the femur could be responsible for a number of the differences observed between these two elements. Previous studies have examined differences in ground reaction forces (GRFs) between the forelimb and hindlimb in pigs, noting that substrate surface can significantly impact the magnitude of the vertical GRFs (Von Wachenfelt et al., 2009a; Von Wachenfelt et al., 2009b; Von Wachenfelt et al., 2010). As research cited above only examined the effects of walking, it is unknown how the GRF differential is affected by elevated loads during running, as is the case with this study. Alternatively, the effects of mechanically loading limbs may be more individually variable than predicted by chondral modeling theory.
Controlling for cohort
As previous work has demonstrated increased vertical GRFs in the domestic pig forelimb compared with the hindlimb (Thorup et al., 2007; Von Wachenfelt et al., 2009a; Von Wachenfelt et al., 2009b; Von Wachenfelt et al., 2010), we should expect that humeral cartilages will demonstrate increased ECM production, chondrocyte mitosis and hypertrophy compared with femoral cartilages. This study yielded conflicting results in this regard.
When considering articular chondrocyte mitosis and cartilage height together, certain observations are noteworthy. In the articular cartilage, the similar heights of Sed humeri vs Sed femora and Ex humeri vs Ex femora combined with the lower cell counts suggest that there may be an overall increase in ECM volume, much as predicted by Eggli et al. (Eggli et al., 1988). Alternatively, this may relate more to the presence of routinely high loads and corresponding decreases in chondrogenesis (cf. Frost, 1999). As loading frequency is likely to be the same across limbs when exercise level is increased, it is unlikely these findings are the result of a frequency-induced variation in loads between limbs.
The physeal cartilage demonstrated trends that varied among cartilage zones. None of the zones exhibited a mean cell count that was greater in the humerus, and only the hypertrophic zone of Ex humeri exhibited a greater mean height. Contrary to predictions, the proliferative zone of Sed femora exhibited a greater mean cartilage height than that of the Sed humeri. However, for both Sed and Ex humeri, all three zones exhibited a larger mean cell area than their femoral counterparts. The similar cell counts in conjunction with the shorter zone height in the Sed humeri suggest additional support for the role of ECM exudation during increased loading (cf. Carter and Wong, 2003).
Overall, this further indicates that different physeal zones may respond differently to similar loading regimens. In this regard, Ueki et al. (Ueki et al., 2008) showed that mechanical loading of the growth plate of Wistar rats resulted in increased chondrocyte proliferation and matrix formation. For the current study, this suggests that elevated mechanical loading could result in lower scaled cell counts for the reserve and hypertrophic zones and higher counts for the proliferative zone, as the loading regimen increases the rate at which reserve zone cells enter the proliferative phase and hypertrophic cells become incorporated into the bony matrix of the limb element. Work by Amini et al. found zonal differences in chondrocyte bulk strains, with greater strains recorded in the proliferative and hypertrophic chondrocytes, as well as a decrease of the cell to matrix volume ratio following compression in the reserve and hypertrophic zones, coupled with an increase in the proliferative zone (Amini et al., 2010). This demonstrates further support for possible alternative explanations for findings that contradict traditional chondral modeling theory.
The differences observed here in the within-joint comparisons and within-cohort comparisons could also result from variability in loading parameters. In the case of the within-joint comparison, where Ex humeri were compared with Sed humeri, an increase in both the magnitude and frequency of loading could be contributing to our results, whereas within-cohort comparisons across joints are likely affected solely by loading magnitude. Previous work has attempted to address the differential role of loading magnitude vs frequency in cartilage growth and remodeling. Specifically, it has been suggested that cyclic loads of moderate magnitude applied to an in vitro mesenchymal sample result in the maintenance of chondrocyte viability and chondrogenesis (Pelaez et al., 2009). An in vitro study of chick mesenchymal cells yielded results that implied both magnitude and frequency were integral to connective tissue development (Elder et al., 2001). Further in vitro research comparing the effect of static vs dynamic loads demonstrated that, while static loading leads to suppression of chondrocyte matrix metabolism, dynamic loads lead to matrix synthesis (Davisson et al., 2002). An in vitro study of articular cartilage showed that high loads applied with moderate frequency can in fact lead to cartilage degradation and this may be a contributing factor in the onset of osteoarthritis (Thibault et al., 2002). These findings may account for the greater uniformity observed among behaviorally similar cohorts (i.e. Sed humerus vs femur, Ex humerus vs femur). For example, all but one (Sed cell count) articular and physeal variables discriminate, respectively, Ex and Sed humeri from Ex and Sed femora with 100% accuracy, and all physeal zone cell areas follow the predicted trend of being larger in the more heavily loaded groups.
It should be noted that previous work (Stokes et al., 2002; Stokes et al., 2006; Stokes et al., 2007) has shown that prolonged compression leads to a decrease in cartilage growth, while prolonged distraction results in increased growth. However, the cyclic loading experienced by these study animals is distinctly different from static loading in that the former includes periods of unloading. Cyclic loading of immature rat knees has been shown to result in the growth of physeal cartilage (Zhang et al., 2010); specifically, increased height of proliferative and hypertrophic zones. While this finding follows Frost’s predictions that moderate, cyclic loads result in chondrogenesis (Frost, 1999), in our study physeal cartilage height increases were largely restricted to the reserve zone. This could suggest variability in the response of cartilage from different joints. Indeed, a greater understanding of chondral modeling requires additional data on the singular roles of loading magnitude and loading frequency as well as how such factors interact to influence chondrogenesis.
By testing for an association between similar articular and physeal variables, we were able to assess whether there are loading-specific patterns of covariation in cartilage parameters and whether low loading levels resulted in a decoupling of cartilage parameters. Interestingly, increased loading appears to influence the relationship between articular and physeal values within joints. However, this response does not appear to be uniform either between variables or across joints. In the humerus, increased loading appears to increase the likelihood of covariation between both articular and physeal cartilage height and chondrocyte hypertrophy, but decreases the likelihood of covaration between articular and physeal chondrocyte proliferation. Contrastingly, increased loading appears to decouple both height and cell proliferation in the femora. As cartilage height is at least in part a byproduct of chondrocyte size and proliferation, it must be considered that the correlation between articular and physeal height in both humeri and femora is being influenced by the changes in the other two variables and ECM synthesis. While we can assume that these two cartilage types are experiencing similar loading magnitudes by being located in the same region of the same limb, this decoupling of chondrocyte proliferation in both the humerus and femur could be an indication that there is a greater disparity in how the increased stress is experienced in the two types of cartilage than when considering the lower loading levels and frequency experienced by sedentary individuals. The general lack of a similar pattern for cartilage in the humerus and femur further reinforces the possibility that the effects of loading on joints must be considered individually for each skeletal element considered.
When considering the relationship between cartilage of the same type across the two joints examined here, different patterns emerge. While we cannot assume that the loads being experienced by homologous types of cartilage are uniform across joints, we can do so for loading frequency. Increased load frequency appears to have very little effect on the relationship between articular cartilage in the humerus and the femur. In the physeal cartilage, only chondrocyte proliferation appears to be more closely linked between the humerus and the femur by an increase in loading frequency. This could indicate that load magnitude is more influential than load frequency for both cartilage types. It could also suggest that both cartilage sites are responding with relative uniformity to increased loading frequency. Ultimately these comparisons indicate that the differential experience of load magnitude within joints is more disruptive to the relationship between cartilage types than a change of load frequency is to cartilage of the same type located in different joints.
CONCLUSION
Chondral modeling theory posits a series of predictions regarding cartilage responses to elevated mechanical loads. These predictions include an increase in chondrocyte mitosis, greater chondrocyte area and an increase in ECM production. Ultimately, this should result in thicker cartilage that better maintains joint integrity during routine loading. The aim of this study was to test these predictions both within and across joints subjected to one of two loading regimens.
Our results have several implications for evaluating chondral modeling theory. In particular, it is apparent that articular and physeal cartilage respond differently to mechanical loading during development. This suggests that chondral modeling theory should account for cartilage type and function as well as the distinctly growth-related nature of physeal cartilage. Moreover, humeral articular cartilage responded disparately to mechanical loading via greater chondrocyte mitosis, while physeal cartilage exhibited an increase in cartilage height and cell area. Additionally, increased loading and load frequency may impact covariation patterns for different cartilage types within joints as well as similar cartilage types across joints. These findings correspond favorably to prior work on the proximal femur in pigs, where the physeal cartilage was differentially responsive to elevated loading (Hammond et al., 2010).
There are several explanations for why these two cartilage types exhibited different responses to similar loading regimes. It is possible that chondral modeling may be subject to a threshold effect, with articular and physeal cartilage reaching a response threshold at different force magnitudes. When examining more heavily loaded groups, articular cartilage expressed increased cellularity within humeri, but decreased cellularity between Sed and Ex humeri and femora, while the physeal cartilage exhibited greater heights within humeri and greater cell area when comparing Sed humeri with femora and Ex humeri with femora. Alternatively, articular cartilage may dissipate loads sufficiently after a period of altered loading such that it no longer responds with increased chondral modeling, with forces transmitted to the physeal cartilage where the remaining adaptive changes are observed in the joint due in part to the growth-related nature of physeal cartilage. This latter explanation suggests that tissue location, both within and between cartilage types, is likely to play a role in the adaptive nature of chondrogenesis.
There are also broader implications that should be considered. Previous work has shown that an exercise regimen implemented during development can have a lifelong impact, increasing bone strength and fatigue life (Warden et al., 2007). As is well known, elevated loading can also lead to increased modeling and remodeling of bone (e.g. Bouvier and Hylander, 1981; Lanyon and Rubin, 1985; Biewener et al., 1986). In the light of such evidence, studies that examine the chondral response to exercise should in turn consider the long-term effects on bone. Such knowledge would benefit those disciplines that rely on an understanding of the plasticity response of bone to activity and loading.
Additional explanations for responses observed herein of cartilage to mechanical loading appear to contradict those of chondral modeling theory. These findings suggest that there are multiple solutions to the biological process whereby cartilage responds to mechanical loading so as to optimize joint congruence, facilitate joint movement and minimize traumatic joint stresses. This implies that the specific predictions of chondral modeling theory as to how cartilage achieves that goal may not hold under all circumstances. The fact that the intensity of the load, as well as the type of cartilage and developmental stage of the chondrocytes could play a role in the response of cartilage suggests that, at the very least, the predicted responses of chondrocytes to loading should be regarded separately for articular vs physeal cartilage, different cartilage sites and zones, and disparate anatomical joints. The complexity of this process might be more fully understood via an integrative analysis of cartilage responses at the tissue, cellular, protein and molecular level. Investigations that characterize the cascade of chondrogenic events during ontogeny as well as the local and global effects of stresses on joint formation will undoubtedly increase our knowledge of the mechanobiology of connective tissues. Future research should also pursue integrative analysis of multiple joints and multiple joint regions in the same individuals, and record data not only on chondral changes but also on the specific loads experienced at each site. Clearly, there are extrinsic and intrinsic influences on regional and local variation in chondrogenic stimuli. Coupled with the fact that there are strain-mediated, site-specific osteogenic thresholds throughout the skeleton (Goodship et al., 1979; Rawlinson et al., 1995; Hylander and Johnson, 1997; Ravosa et al., 2010), this suggests it is highly likely that a similar scenario applies to both hard and soft connective tissues.
ACKNOWLEDGEMENTS
Many thanks to Harold Laughlin s lab, particularly David Harah for access to specimens, Bobby Colley for access to laboratory machinery, and the University of Missouri Veterinary Medical Diagnostic Laboratory for histological preparations. We also thank Carol Ward, Erin Franks, Casey Holliday, Mike Plavcan, Greg Blomquist, Andy Biewener and two anonymous reviewers for valuable feedback.
FOOTNOTES
FUNDING
K.A.C. and A.S.H. are supported by Life Sciences Fellowships from the University of Missouri. This study was partly funded by the National Institutes of Health [grant no. PO1-HL52490 to H. Laughlin]. Deposited in PMC for release after 12 months.
REFERENCES
- Amini S., Veilleux D., Villemure I. (2010). Tissue and cellular morphological changes in growth plate explants under compression. J. Biomech. 43, 2582–2588 [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Venn M., Maroudas A., Ali S. Y. (1983). Structure of proteoglycans from different layers of human articular cartilage. Biochem. J. 209, 387–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M., Ralphs R. J. (1998). Fibrocartilage in tendons and ligaments – an adaptation to compressive load. J. Anat. 193, 481–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram J. E., Swartz S. M. (1991). The law of bone transformation: a case of crying Wolff? Biol. Rev. Camb. Philos. Soc. 66, 245–273 [DOI] [PubMed] [Google Scholar]
- Bevill S. L., Briant P. L., Levenston M. E., Andriacchi T. P. (2009). Central and peripheral region tibial plateau chondrocytes respond differently to in vitro dynamic compression. Osteoarth. Cartil. 17, 980–987 [DOI] [PubMed] [Google Scholar]
- Biewener A. A., Swartz S. M., Bertram J. E. A. (1986). Bone modeling during growth: dynamic strain equilibrium in the chick tibiotarsus. Calcif. Tissue Int. 39, 390–395 [DOI] [PubMed] [Google Scholar]
- Bouvier M. (1988). Effects of age on the ability of the rat temporomandibular joint to respond to changing functional demands. J. Dent. Res. 67, 1206–1212 [DOI] [PubMed] [Google Scholar]
- Bouvier M., Hylander W. L. (1981). Effect of bone strain on cortical bone structure in macaques (Macaca mulatta). J. Morphol. 167, 1–12 [DOI] [PubMed] [Google Scholar]
- Carter D. R., Wong M. (2003). Modelling cartilage mechanobiology. Philos. Trans. Biol. Sci. 358, 1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho R. S., Yen E. H., Suga D. M. (1995). Glycosaminoglycan synthesis in the rat articular disc in response to mechanical stress. Am. J. Orthod. Dentofacial Orthop. 107, 401–410 [DOI] [PubMed] [Google Scholar]
- Davisson T., Kunig S., Chen A., Sah R., Ratcliffe A. (2002). Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. J. Orthop. Res. 20, 842–848 [DOI] [PubMed] [Google Scholar]
- Dy P., Smits P., Silvester A., Penzo-Mendez A., Dumitriu B., Han Y., de la Motte C. A., Kingsley D. M., Lefebvre V. (2010). Synovial joint morphogenesis requires the chondrogenic action of Sox5 and Sox6 in growth plate and articular cartilage. Dev. Biol. 340, 346–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyce K. M., Sack W. O., Wensing C. J. G. (2002). Textbook of Veterinary Anatomy. Philadelphia: Saunders/Elsevier; [Google Scholar]
- Eggli P. S., Hunziker E. B., Schenk R. K. (1988). Quantitation of structural features characterizing weight-and less-weight-bearing regions in articular cartilage: a stereological analysis of medial femoral condyles in young adult rabbits. Anat. Rec. 222, 217–227 [DOI] [PubMed] [Google Scholar]
- Elder S. H., Goldstein S. A., Kimura J. H., Soslowsky L. J., Spengler D. M. (2001). Chondrocyte differentiation is modulated by frequency and duration of cyclic compressive loading. Ann. Biomed. Eng. 29, 476–482 [DOI] [PubMed] [Google Scholar]
- Farnum C. E., Lee R., O Hara K., Urban J. P. G. (2002). Volume increase in growth plate chondrocytes during hypertrophy: the contribution of organic osmolytes. Bone 30, 574–581 [DOI] [PubMed] [Google Scholar]
- Frost H. M. (1979). A chondral modeling theory. Calcif. Tissue Int. 28, 181–200 [DOI] [PubMed] [Google Scholar]
- Frost H. M. (1999). Joint anatomy, design and arthroses: insights of the Utah paradigm. Anat. Rec. 255, 162–174 [DOI] [PubMed] [Google Scholar]
- Goldring M. B. (2004a). Human chondrocyte cultures as models of cartilage-specific gene regulation. In Methods in Molecular Medicine: Human Cell Culture Protocols, 2nd edn (ed. Picot J.), pp. 69–96 Totowa, NJ: Humana Press; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring M. B. (2004b). Immortalization of human articular chondrocytes for generation of stable, differentiated cell lines. In Methods in Molecular Medicine: Cartilage and Osteoarthritis, Vol. 1, Cellular and Molecular Tools (ed. Sabatini M., Pastoureau P., de Ceuninck F.), pp. 23–36 Totowa, NJ: Humana Press; [DOI] [PubMed] [Google Scholar]
- Goldspink G. (1970). Morphological adaptation due to growth and activity. In Physiology and Biochemistry of Muscle as a Food (ed. Briskey E. J., Cassens R. G., Marsh B. B.), pp. 521–536 Madison, WI: University of Wisconsin Press; [Google Scholar]
- Goodship A. E., Lanyon L. E., McFie H. (1979). Functional adaptation of bone to increased stress. An experimental study. J. Bone Joint Surg. 61, 539–546 [PubMed] [Google Scholar]
- Gregory K. E., Keene D. R., Tufa S. F., Lunstrom G. P., Morris N. P. (2001). Developmental distribution of collagen type XII in cartilage: association with articular cartilage and the growth plate. J. Bone Miner. Res. 16, 2005–2016 [DOI] [PubMed] [Google Scholar]
- Grodzinsky A. J., Levenston M. E., Jin M., Frank E. H. (2000). Cartilage tissue remodeling in response to mechanical forces. Ann. Rev. Biomed. Eng. 2, 691–713 [DOI] [PubMed] [Google Scholar]
- Hammond A. S., Ning J., Ward C. V., Ravosa M. J. (2010). Mammalian limb loading and chondral modeling during ontogeny. Anat. Rec. 293, 658–670 [DOI] [PubMed] [Google Scholar]
- Hamrick M. W. (1999). A chondral modeling theory revisited. J. Theor. Biol. 201, 201–208 [DOI] [PubMed] [Google Scholar]
- Hinton R. J., McNamara J. A. (1984). Effect of age on the adaptive response of the adult temporomandibular joint. A study of induced protrusion in Macaca mulatta. Angle Orthod. 54, 154–162 [DOI] [PubMed] [Google Scholar]
- Hoverman J. T., Relyea R. A. (2007). How flexible is phenotypic plasticity? Developmental windows for trait induction and reversal. Ecology 88, 693–705 [DOI] [PubMed] [Google Scholar]
- Hyde G., Dover S., Aszodi A., Wallis G. A., Boot-Handford R. P. (2007). Lineage tracing using matrilin-1 gene expression reveals that articular chondrocytes exist as the joint interzone forms. Dev. Biol. 304, 825–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylander W. L., Johnson K. R. (1997). In vivo bone strain patterns in the zygomatic arch of macaques and the significance of these patterns for functional interpretations of craniofacial form. Am. J. Phys. Anthropol. 102, 203–232 [DOI] [PubMed] [Google Scholar]
- Irie T., Aizawa T., Kokubun S. (2005). The role of sex hormones in the kinetics of chondrocytes in the growth plate. J. Bone Joint Surg. Br. 87, 1278–1284 [DOI] [PubMed] [Google Scholar]
- Kiviranta I., Tammi M., Jurvelin J., Saamanen A. M., Helminen H. J. (1988). Moderate running exercise augments glycosaminoglycans and thickness of articular cartilage in the knee joint of young beagle dogs. J. Orthop. Res. 6, 188–195 [DOI] [PubMed] [Google Scholar]
- Knight M. M., Toyoda T., Lee D. A., Bader D. L. (2006). Mechanical compression and hydrostatic pressure induce reversible changes in actin cytoskeletal organisation in chondrocytes in agarose. J. Biomech. 39, 1547–1551 [DOI] [PubMed] [Google Scholar]
- Lanyon L. E., Rubin C. T. (1985). Functional adaptation in skeletal structures. In Functional Vertebrate Morphology (ed. Hildebrand M., Bramble D. M., Liem K. F., Wake D. B.), pp. 1–25 Cambridge: Harvard; [Google Scholar]
- Lemare F., Steimberg N., Le Griel C., Demignot S., Adolphe M. (1998). Dedifferentiated chondrocytes cultured in alginate beads: restoration of the differentiated phenotype and of the metabolic responses to interleukin-1β. J. Cell. Physiol. 176, 303–313 [DOI] [PubMed] [Google Scholar]
- Liu J., Sekiya I., Asai K., Tada T., Kato T., Matsui N. (2001). Biosynthetic response of cultured articular chondrocytes to mechanical vibration. Res. Exp. Med. 200, 183–193 [PubMed] [Google Scholar]
- Meyer A. (1987). Phenotypic plasticity and heterochrony in Cichlasoma managuense (Pisces, Cichlidae) and their implications for speciation in cichlid fishes. Evolution 41, 1357–1369 [DOI] [PubMed] [Google Scholar]
- Mizoguchi I., Takahashi I., Nakamura M., Sasano Y., Sato S., Kagayama M., Mitani H. (1996). An immunohistochemical study of regional differences in the distribution of type I and type II collagens in rat mandibular condylar cartilage. Arch. Oral Biol. 41, 863–869 [DOI] [PubMed] [Google Scholar]
- Mow V. C., Fithian D. C., Keely M. A. (1990). Fundamentals of articular cartilage and meniscus biomechanics. In Articular Cartilage and Knee Joint Function (ed. Ewing J. W.), pp. 1–18 New York: Raven Press; [Google Scholar]
- Murray R. C., Vedi S., Birch H. L., Lakhani K. H., Goodship A. E. (2001). Subchondral bone thickness, hardness and remodeling are influenced by short-term exercise in a site specific manner. J. Orthop. Res. 19, 1035–1042 [DOI] [PubMed] [Google Scholar]
- Nakano T., Scott P. G. (1989). A quantitative chemical study of glycosaminoglycans in the articular disc of bovine temporomandibular joint. Arch. Oral Biol. 34, 749–757 [DOI] [PubMed] [Google Scholar]
- Niehoff A., Kersting U. G., Zaucke F., Morlock M. M., Bruggemann G. (2004). Adaptation of mechanical, morphological and biochemical properties of the rat growth plate to dose-dependent voluntary exercise. Bone 35, 899–908 [DOI] [PubMed] [Google Scholar]
- Paukkonen K., Selkäinaho K., Jurvelin J., Kiviranta I., Helminen H. J. (1985). Cells and nuclei of articular cartilage chondrocytes in young rabbits enlarged after non-strenuous physical exercise. J. Anat. 142, 13–20 [PMC free article] [PubMed] [Google Scholar]
- Pearson O. M., Lieberman D. E. (2004). The aging of Wolff s Law: ontogeny and responses to mechanical loading in cortical bone. Am. J. Phys. Anthropol. 125, 63–99 [DOI] [PubMed] [Google Scholar]
- Pelaez D., Huang C. H., Cheung H. S. (2009). Cyclic compression maintains viability and induces chondrogenesis of human mesenchymal stem cells in fibrin gel scaffolds. Stem Cells Dev. 18, 93–102 [DOI] [PubMed] [Google Scholar]
- Plochocki J. H., Ward C. V., Smith D. E. (2009). Evaluation of the chondral modeling theory using fe-simulation and numeric shape optimization. J. Anat. 214, 768–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan P., Lewis N. T., Mao J. J. (2004). Zone-specific micromechanical properties of the extracellular matrices of growth plate cartilage. Ann. Biomed. Eng. 32, 284–291 [DOI] [PubMed] [Google Scholar]
- Ravosa M. J., Kunwar R., Stock S. R., Stack M. S. (2007). Pushing the limit: masticatory stress and adaptive plasticity in mammalian craniomandibular joints. J. Exp. Biol. 210, 628–641 [DOI] [PubMed] [Google Scholar]
- Ravosa M. J., López E. K., Menegaz R. A., Stock S. R., Stack M. S., Hamrick M. W. (2008a). Using Mighty Mouse to understand masticatory plasticity: myostatin-deficient mice and musculoskeletal function. Integr. Comp. Biol. 48, 345–359 [DOI] [PubMed] [Google Scholar]
- Ravosa M. J., López E. K., Menegaz R. A., Stock S. R., Stack M. S., Hamrick M. W. (2008b). Adaptive plasticity in the mammalian masticatory complex: you are what, and how, you eat. In Primate Craniofacial Biology and Function (ed. Vinyard C. J., Ravosa M. J., Wall C. E.), pp. 293–328 New York: Springer Academic Publishers; [Google Scholar]
- Ravosa M. J., Ross C. F., Williams S. H., Costley D. B. (2010). Allometry of masticatory loading parameters in mammals. Anat. Rec. 293A, 557–571 [DOI] [PubMed] [Google Scholar]
- Rawlinson S. C. F., Mosley J. R., Suswillo R. F. L., Pitsillides A. A., Lanyon L. E. (1995). Calvarial and limb bone cells in organ and monolayer culture do not show the same early responses to dynamic mechanical strain. J. Bone Miner. Res. 10, 1225–1232 [DOI] [PubMed] [Google Scholar]
- Rubin C. T., Bain S. D., McLeod K. J. (1992). Suppression of the osteogenic response in the aging skeleton. Calcif. Tissue Int. 50, 306–313 [DOI] [PubMed] [Google Scholar]
- Stempel J., Fritsch H., Pfaller K., Blumer M. J. F. (2011). Development of articular cartilage and the metaphyseal growth plate: the localization of TRAP cells, VEGF, and endostatin. J. Anat. 218, 608–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. C., Farnum C. E., MacLeod J. N. (1997). Expression of p21CIP1/WAF1 in chondrocytes. Calcif. Tissue Int. 61, 199–204 [DOI] [PubMed] [Google Scholar]
- Stokes I. A., Mente P. L., Iatridis J. C., Farnum C. E., Aronsson D. D. (2002). Enlargement of growth plate chondrocytes modulated by sustained mechanical loading. J. Bone Joint Surg. Am. 84, 1842–1848 [DOI] [PubMed] [Google Scholar]
- Stokes I. A. F., Aronsson D. D., Dimock A. N., Cortright V., Beck S. (2006). Endochondral growth in growth plates of three species at two anatomical locations modulated by mechanical compression and tension. J. Orthop. Res. 24, 1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes I. A. F., Clark K. C., Farnum C. E., Aronsson D. D. (2007). Alterations in the growth plate associated with growth modulation by sustained compression or distraction. Bone 41, 197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Kawashiri S., Kumagai S., Takatsuka S., Narinobou M., Nakagawa K., Tanaka S. (2000). Expression of matrix metalloproteinase-2 in osteoarthritic fibrocartilage from human mandibular condyle. J. Oral Pathol. Med. 29, 314–320 [DOI] [PubMed] [Google Scholar]
- Thibault M., Poole A. R., Buschmann D. (2002). Cyclic compression of cartilage/bone explants in vitro leads to physical weakening, mechanical breakdown of collagen and release of matrix fragments. J. Orthop. Res. 20, 1265–1273 [DOI] [PubMed] [Google Scholar]
- Thorup V. M., Togersen F. A., Jorgensen B., Jensen B. R. (2007). Biomechanical gait analysis of pigs walking on solid concrete floor. Animal 1, 708–715 [DOI] [PubMed] [Google Scholar]
- Ueki M., Tanaka N., Tanimoto K., Nishio C., Honda K., Lin Y.-Y., Tanne Y., Ohkuma S., Kamiya T., Tanaka E., et al. (2008). The effect of mechanical loading on the metabolism of growth plate chondrocytes. Ann. Biomed. Eng. 36, 793–800 [DOI] [PubMed] [Google Scholar]
- Von Wachenfelt H., Pinzke S., Nilsson C. J. (2009a). Gait and force analysis of provoked pig gait on clean and fouled concrete surfaces. Biosys. Eng. 104, 534–544 [Google Scholar]
- Von Wachenfelt H., Pinzke S., Nilsson C. J., Olsson O., Ehlorsson C. J. (2009b). Force analysis of unprovoked pig gait on clean and fouled concrete surfaces. Biosys. Eng. 104, 250–257 [Google Scholar]
- Von Wachenfelt H., Nilsson C. J., Pinzke S. (2010). Gait and force analysis of provoked pig gait on clean and fouled rubber mat surfaces. Biosys. Eng. 106, 86–96 [Google Scholar]
- Wardale J. R., Duance V. C. (1993). Quantification and immunolocalisation of porcine articular and growth plate cartilage collagens. J. Cell Sci. 105, 975–984 [DOI] [PubMed] [Google Scholar]
- Wardale J. R., Duance V. C. (1994). Characterisation of articular growth plate cartilage collagens in porcine osteochondrosis. J. Cell Sci. 107, 47–59 [DOI] [PubMed] [Google Scholar]
- Warden S. J., Fuchs R. K., Castillo A. B., Nelson I. R., Turner C. H. (2007). Exercise when young provides lifelong benefits to bone structure and strength. J. Bone Miner. Res. 22, 251–249 [DOI] [PubMed] [Google Scholar]
- Wong M., Carter D. R. (2003). Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone 33, 1–13 [DOI] [PubMed] [Google Scholar]
- Wu J. Z., Herzog W. (2006). Analysis of the mechanical behavior of chondrocytes in unconfined compression tests for cyclic loading. J. Biomech. 39, 603–616 [DOI] [PubMed] [Google Scholar]
- Wu Q., Chen Q. (2000). Mechanoregulation of chondrocyte proliferation, maturation, and hypertrophy: ion-channel dependent transduction of matrix deformation signals. Exp. Cell Res. 256, 383–391 [DOI] [PubMed] [Google Scholar]
- Yamane S., Cheng E., You Z. (2007). Gene expression profiling of mouse articular and growth plate cartilage. Tissue Eng. 13, 2163–2173 [DOI] [PubMed] [Google Scholar]
- Zhang P., Hamamura K., Turner C. H., Yokota H. (2010). Lengthening of mouse hindlimbs with joint loading. J. Bone Miner. Metab. 28, 268–275 [DOI] [PubMed] [Google Scholar]