Abstract
The advent of three-dimensional echocardiography (3DE) has significantly improved the impact of non-invasive imaging on our understanding and management of cardiac diseases in clinical practice. Transthoracic 3DE enables an easier, more accurate and reproducible interpretation of the complex cardiac anatomy, overcoming the intrinsic limitations of conventional echocardiography. The availability of unprecedented views of cardiac structures from any perspective in the beating heart provides valuable clinical information and new levels of confidence in diagnosing heart disease. One major advantage of the third dimension is the improvement in the accuracy and reproducibility of chamber volume measurement by eliminating geometric assumptions and errors caused by foreshortened views. Another benefit of 3DE is the realistic en face views of heart valves, enabling a better appreciation of the severity and mechanisms of valve diseases in a unique, noninvasive manner. The purpose of this review is to provide readers with an update on the current clinical applications of transthoracic 3DE, emphasizing the incremental benefits of 3DE over conventional two-dimensional echocardiography.
Keywords: Three-dimensional echocardiography, Left ventricle, Right ventricle, Mitral valve, Aortic valve, Tricuspid valve, Left atrium
Introduction
The advent of three-dimensional echocardiography (3DE) represents a major innovation in cardiovascular ultrasound. Advancements in computer and transducers technology permit the acquisition of 3D data sets with adequate spatial and temporal resolution to assess most of the cardiac pathologies. In addition, 3D echocardiography enables the visualization of cardiac structures from virtually any perspective, providing a more anatomically sound and intuitive display, as well as an accurate quantitative evaluation of cardiac anatomy and function, thus offering solid elements for patient evaluation and management. Furthermore, 3DE sheds new lights in understanding pathophysiological aspects of underlying cardiac diseases.
Data regarding clinical applications of 3DE are burgeoning and gradually capturing an established place in the noninvasive clinical assessment of cardiac anatomy and function. Recently, EAE/ASE recommendations have been published, aiming to provide clinicians with a systematic approach to 3D image acquisition and analysis.1)
This review details the state-of-the-art 3DE applications in clinical practice, emphasizing the advantages of 3DE over conventional two-dimensional echocardiography (2DE) and its current limitations.
Three-Dimensional Echocardiography Technique: Image Acquisition and Display
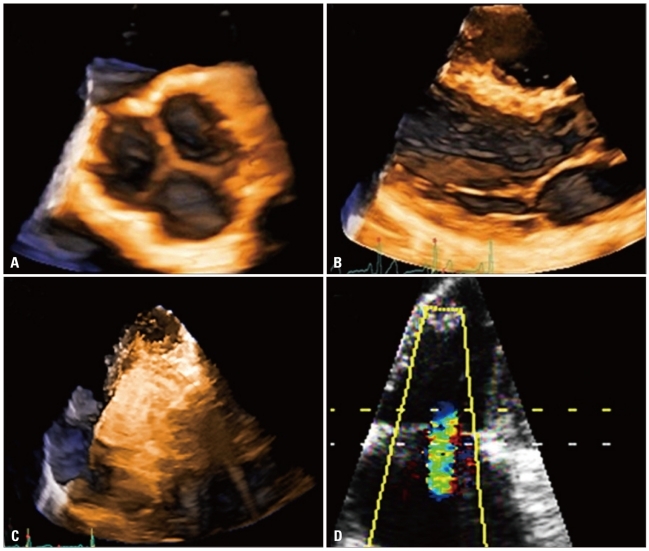
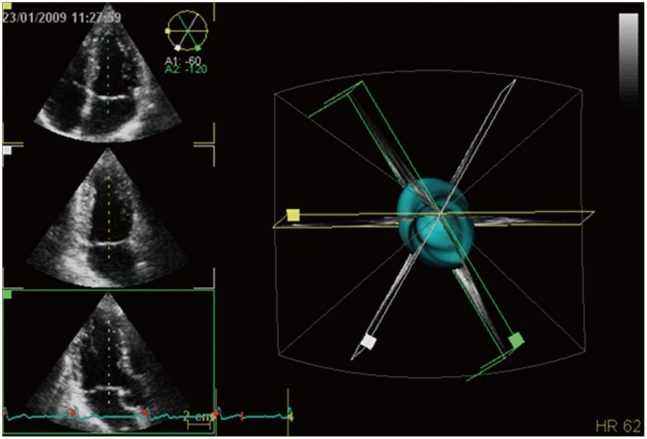
Currently, 3D data set acquisition can be easily implemented into standard echocardiographic examination by either switching among 2D and 3D probes or, with newest all-in-one-probes, by switching between 2D and 3D modalities available in the same probe. The latter probes are also capable to provide single-beat full-volume acquisition, as well as real-time 3D color Doppler imaging (Fig. 1).
Fig. 1.
Different acquisition modalities available with three-dimensional echocardiography: A: Zoom mode; it can acquired either single-beat (to encompass a specific region or structures like en-face valve display without the need of cropping) or multi-beat to improve spatial and temporal resolution while maintaining the small volume size (e.g. to assess rapidly moving structures like vegetations). B: Real-time acquisition with a limited depth (usually around 30° in elevation) generally used to monitor interventional procedures. C: Multi-beat full-volume (reaching up to 100° in elevation) to encompass a large part of the heart in order to assess spatial relationships between cardiac structures or quantitate size and function of cardiac chambers. D: Three-dimensional color mode to assess shape and extension of regurgitant jets and determine the location and size of cardiac defects.
3DE is the only imaging technique based on volumetric scanning able to show moving structures in the beating heart, in contrast to cardiac magnetic resonance (CMR) and computed tomography, which are based on post-acquisition 3D reconstruction from multiple tomographic images and displaying only 3D rendered snapshots. At present two different methods for 3D data acquisition are available: "real-time" (or "live" 3D mode) and multi-beat 3D mode (Fig. 2). In the real-time mode, a thin sector of a pyramidal 3D volume data set is obtained and visualized live, beat after beat as during 2D scanning. Imaging is usually available in several fashions, as narrow volume, zoom, wide-angle (full-volume) and color-Doppler modalities. Heart dynamics is shown in a realistic way, with instantaneous on-line volume rendered reconstruction. It allows fast acquisition from a single acoustic view of dynamic pyramidal data structures that can encompass the entire heart without the need of reference system, electrocardiogram (ECG) and respiratory gating. Real-time imaging is time-saving both for data acquisition and analysis. Although this acquisition mode overcomes rhythm disturbances or respiratory motion limitations, it still suffers of relatively poor temporal and spatial resolution.
Fig. 2.
Schematic representation of two-dimensional (i.e. tomographic; A) and single-beat three-dimensional (i.e. volumetric; B) of the left ventricular short-axis at mitral valve level. Volumetric rendering displays many more details and allow better appreciation of spatial relationship among cardiac structures. C shows the multi-beat acquisition modality: two to six consecutive single-beat sub-volumes are stitched together to obtain a full-volume with higher spatial and temporal resolution.
Conversely, multi-beat acquisition is realized through sequential acquisitions of narrow smaller volumes obtained from several ECG-gated consecutive heart cycles (from 2 to 6) that are subsequently stitched together to create a single volumetric data set. It provides large data sets with high temporal and spatial resolution, but more prone to artifacts due to patient or respiratory motion or irregular cardiac rhythms. The most appropriate acquisition mode for the specific clinical setting will be chosen in each individual case (Fig. 2).
3D data sets can be sectioned in several planes and rotated in order to visualize the cardiac structure of interest from any desired perspective, irrespective of its orientation and position within the heart. This allows the operator to easily obtain unique visualizations, that may be difficult or impossible to achieve using conventional 2DE (e.g. en-face views of the tricuspid valve or cardiac defects).
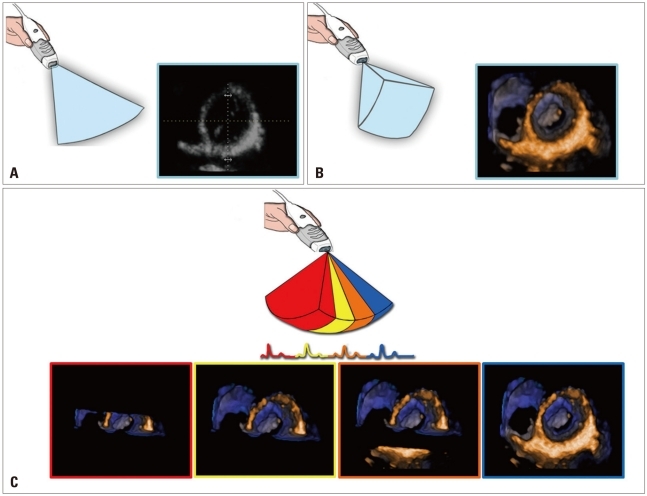
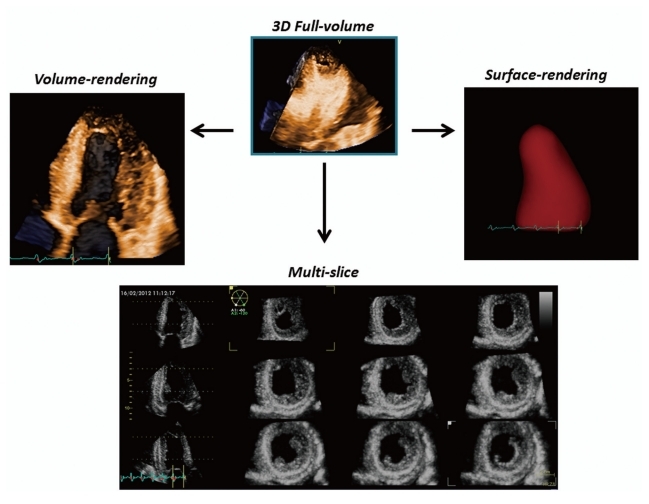
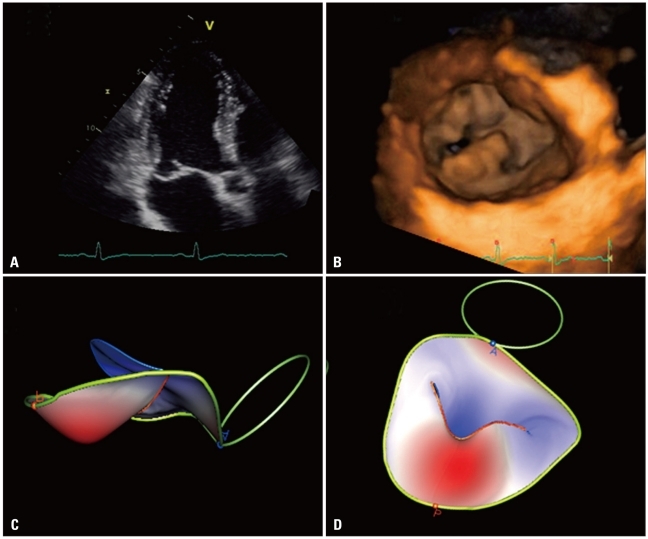
Acquisition of volumetric images generates the technical problem of rendering the depth perception on a flat, 2D monitor. 3D images can be visualized using three display modalities: volume rendering, surface rendering and tomographic slices (Fig. 3). In volume rendering modality, various color maps are applied to convey the depth perception to the observer. Generally, lighter shades (e.g. bronze, Fig. 4) are used for structures closer to the observer, while darker shades (e.g. blue, Fig. 4) are used for deeper structures. Surface rendering modality displays the 3D surface of cardiac structures, identified either by manual tracing or by using automated border detection algorithms on multiple 2D cross-sectional images of the structure/cavity of interest (Fig. 3 and 5B). This stereoscopic approach is useful for the assessment of shape and for a better appreciation of geometry and dynamic function during the cardiac cycle. Finally, the pyramidal data set can be automatically sliced in several tomographic views simultaneously displayed (Fig. 3). Cut planes can be orthogonal, parallel or free (any given plane orientation), selected as desired by the echocardiographer for obtaining optimized cross-sections of the heart in order to answer specific clinical questions and to perform accurate and reproducible measurements (Fig. 6).
Fig. 3.
From the same pyramidal three-dimensional data set, the left ventricle can be analyzed using different display modalities: volume rendering, to visualize morphology and spatial relationships among adjacent structures; surface-rendering, for quantitative purposes; and multi-slice (multiple two-dimensional tomographic views extracted automatically from a single 3D data set) for morphological and functional analysis at different regional levels.
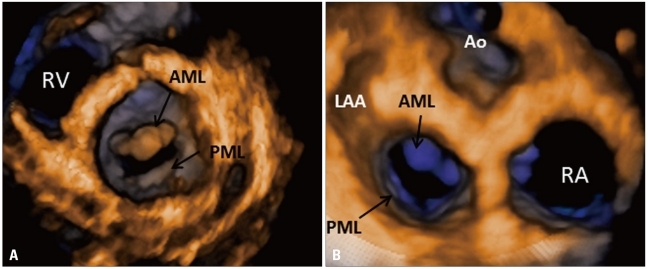
Fig. 4.
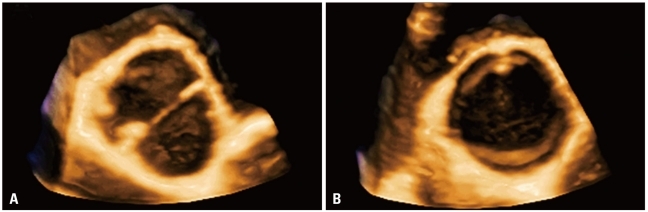
Normal mitral valve visualized en-face by transthoracic three-dimensional echocardiography: A: Left ventricular perspective. B: Left atrial perspective or "surgical view". RV: right ventricle, AML: acute myleogenous leukemia, PML: promyelocytic leukemia, LAA: left atrial appendage, Ao: aorta, RA: right atrium.
Fig. 5.
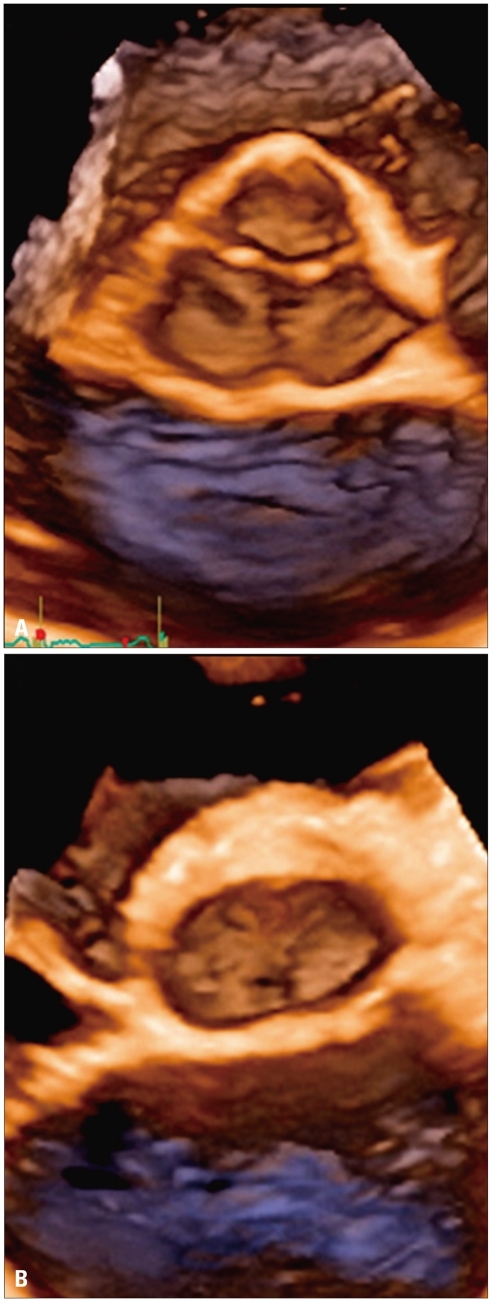
Degenerative mitral valve disease: A: Apical long-axis view showing a flail of posterior mitral leaflet. B: Volume rendering of the showing the location and extent of the prolapsing segment. C and D: Surface rendering of the valve leaflets, annulus and aortic annulus to provide an automated quantitative analysis of valve morphology and geometry useful to plan the surgical approach.
Fig. 6.
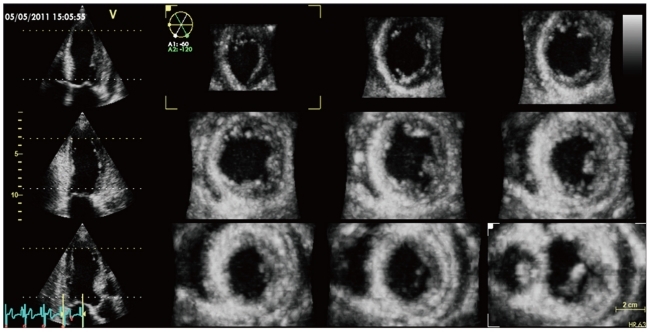
Multi-slice display of the left ventricle in a patient with antero-septal myocardial infarction. The three panels on the left show three apical views obtained by rotational slicing of the pyramidal data set. The nine panels on the right show nine short-axis views (from mitral valve level, indicated by the dashed white line on left panels - lower panel on the right, to the apical level, indicated by the dashed yellow line on left panels, upper panel on the left) obtained by parallel slicing of the pyramidal data set. All these views can be changed in both the angle of rotation (longitudinal views) and in the position within the ventricle (short axis views) according to the clinical need during postprocessing.
Clinical Applications
Left ventricular quantification
Noninvasive assessment of left ventricular (LV) geometry and function is critically important for clinical decision making and represents the most frequent indication for an echocardiographic study. Suitability for device implantation, indications to cardiac surgery or to treatment initiation in asymptomatic patients with LV systolic dysfunction are among the most critical decisions that rely on an accurate LV quantitation.2)
LV volume measurement by 2DE is highly experience-dependent, uses only partial information contained in few predefined cross-sections to assess global myocardial function, and relies on geometrical assumptions that may not be necessarily valid in all patients. Two-dimensional echocardiography has also shown a limited test-retest reproducibility for LV volumes and ejection fraction quantification.3) Geometric assumptions render the measurements of LV volume and ejection fraction particularly inaccurate in those patients in whom these parameters are most needed (i.e. patients with previous myocardial infarction or cardiomyopathies, whose LVs are asymmetric or distorted).
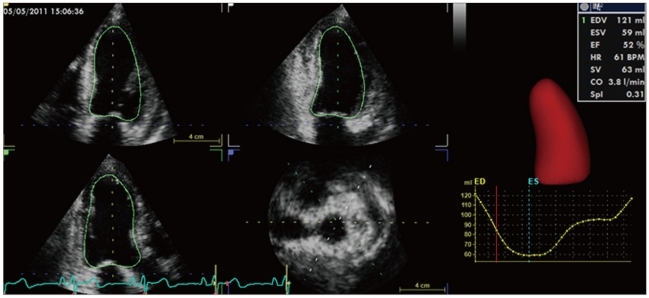
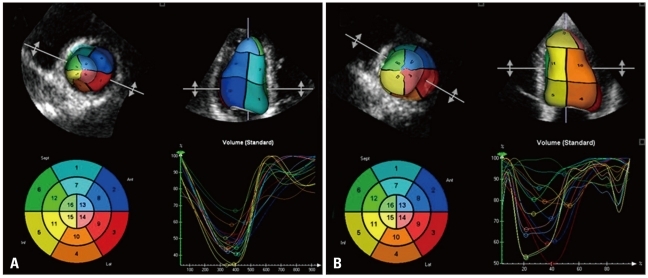
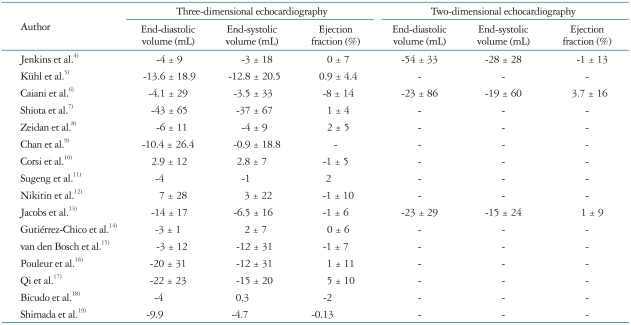
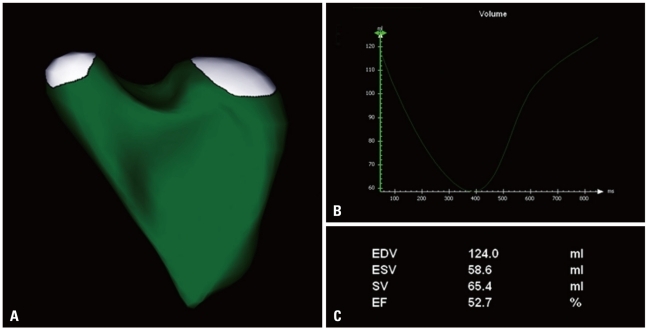
Three-dimensional LV data set analysis can now be performed using computerized automated or semi-automated endocardial surface detection softwares, which do not rely on geometric assumptions and require only minimal human intervention, therefore improving measurement reproducibility (Fig. 7). After identification of few anatomical landmarks (i.e. apex and mitral annulus reference points), the 3D LV cast can be automatically segmented into the standard 16 or 17 segments. The volume of the entire LV cavity, as well as the separate subvolumes corresponding to each of 16 or 17 segments can be measured frame-by-frame and plotted against time (Fig. 8).
Fig. 7.
Left ventricular volume and ejection fraction measurement using three-dimensional full-volume data set. The three longitudinal views (4-, 2-chamber, and long-axis vie and the adjustable short axis view are used to visualize the accuracy of the semiautomated endocardial tracking. On the right a time-volume curve showing the change in left ventricular volume during the cardiac cycle.
Fig. 8.
The endocardial surface can be subdivided in 16 or 17 color-coded areas corresponding to the left ventricular segmentation. Each segment can be assimilated to a pyramid with the base on the endocardium and the apex at the gravity center of the ventricle. The time to minimal volume of each segment can be measured on the corresponding time-volume curves and the standard deviation of the time to minimal volume of the 16/17 segments has been reported as an index of intraventricular dyssynchrony. A shows synchronous left ventricular contraction (all segments reach the minimal volume approximately at the same time). B shows a dyssynchronous left ventricular contraction showed by the large temporal dispersion of the time to minimal segmental volume.
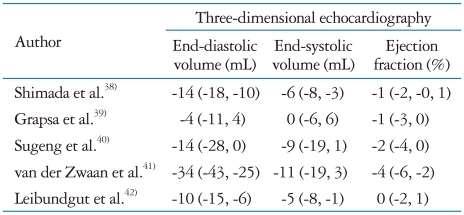
Three-dimensional echocardiography has been extensively validated against CMR (Table 1)4-19) and was demonstrated to be more time-saving, reproducible and accurate than conventional 2DE for LV volumes and ejection fraction measurement. The possibility of re-aligning planes and optimally adjusting the LV chamber size to its maximum longitudinal axis length is an important advantage offered by 3DE over conventional 2DE. Foreshortening of LV longitudinal axis is a major cause of volume underestimation by 2DE, which accounts for the larger bias observed in comparison with 3DE. However, despite eliminating LV apical foreshortening and geometric assumptions, 3DE still yields a systematic underestimation of LV volumes as shown in a meta-analysis of 95 studies having CMR as reference.19) A significant underestimation has been reported for LV end-systolic (-4.7 mL) and end-diastolic (-9.9 mL) volumes, whereas ejection fraction measurement revealed an excellent accuracy (-0.13%), probably due to a constant underestimation of both volumes. Female gender and presence of cardiac disease were associated with a larger extent of underestimation.
Table 1.
Differences between left ventricular volumes and function assessed by three-dimensional echocardiography and conventional two-dimensional echocardiography in comparison with cardiac magnetic resonance
Mean difference ± SD from cardiac magnetic resonance
As a rule, good image quality is a prerequisite for an accurate quantitation of global LV function using semi- or automated border detection algorithms. A manual editing of the automatically-identified endocardial surface may be required in order to ensure an accurate quantitative analysis, particularly in patients with suboptimal image quality.2),20) Some authors reported that LV volume measurements by 3DE are less when less than 60% of the endocardial border is visualized;21) in this setting, the use of contrast may improve the accuracy and reproducibility of measurements.22),23)
Simultaneous LV shape analysis (i.e. 3D sphericity index) is provided from the endocardial 3D surface reconstruction: as the LV becomes more globular, the sphericity index approaches unity. In patients with acute myocardial infarction, 3DE derived sphericity index has been demonstrated to be an earlier and more accurate predictor of LV remodeling than other clinical, electrocardiographic, and echocardiographic variables.24)
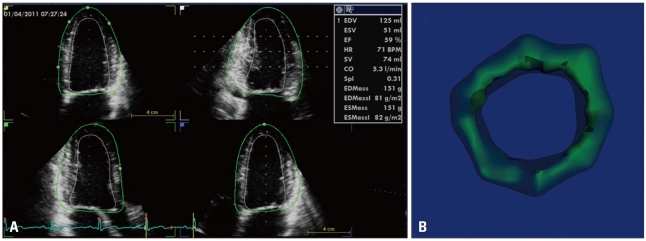
By adding an automated detection of the LV epicardial surface and applying 3D speckle-tracking analysis within the LV myocardial wall delimited between the endocardial and epicardial surfaces, additional parameters can be obtained from the same 3D data set: LV mass (Fig. 9), as well as myocardial deformation components (longitudinal, circumferential, radial and area strain) (Fig. 10).
Fig. 9.
Left ventricular mass measurement using three-dimensional echocardiography. Using automated or semi-automated endocardial and epicardial boundary detection endocardial and epicardial volumes are measured (A). By subtracting the left ventricular cavity volume from the epicardial volume, the volume of myocardium is obtained (B). Left ventricular mass is calculated by multiplying myocardial volume by its specific gravity (1.05 g/cm3).
Fig. 10.
Three-dimensional speckle-tracking analysis of left ventricular longitudinal myocardial deformation using two different platforms. Results can be displayed as bull's eye plots (A) and/or time-strain curves (B).
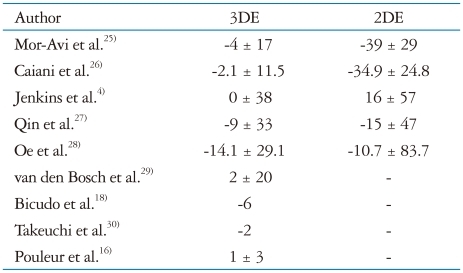
Left ventricular mass calculations by M-mode and 2DE are subject to the same limitations in reproducibility and accuracy affecting LV size and function. Several comparative studies (Table 2)4),16),18),25-30) have proven that 3DE is more accurate than M-mode or 2DE methods to calculate LV mass when CMR was used as reference standard. Inter-observer and test/re-test reproducibility were also improved by the 3DE approach.4) More accurate measurements of LV mass may facilitate its use as a surrogate outcome marker in trials involving antihypertensive medications.31)
Table 2.
Differences between left ventricular mass calculation by three-dimensional echocardiography and conventional two-dimensional echocardiography in comparison with cardiac magnetic resonance
Mean difference ± SD from cardiac magnetic resonance. 3DE: three-dimensional echocardiography, 2DE: two-dimensional echocardiography
3DE provides new opportunities of assessing regional LV wall motion. Conventional 2DE, being confined to several predefined views, actually shows only a very limited part of the whole LV myocardium (Fig. 11). Conversely, 3DE can display the entire myocardial volume in a multi-slice panel, allowing a comprehensive assessment of the whole LV circumference (Fig. 6). This display modality can improve the accuracy of visual regional wall motion assessment, because even limited wall motion abnormalities, localized in regions that are not apparent in conventional 2D views, can be visualized. Furthermore, with 3DE, a quantitative analysis of LV regional functional is possible by comparing regional volume variation of each pyramidal-shaped LV segment during the cardiac cycle (Fig. 8). A good correlation of quantitative 3DE analysis with 2DE regional wall motion score10) and CMR32) has been reported.
Fig. 11.
Schematic representation of the position of 3 apical views of left ventricle obtainable with conventional 2D echocardiography. As shown, the 3 longitudinal slices cover only a very limited part of left ventricular circumference and cannot be changed anymore once acquired, if proven unsatisfactory or foreshortened.
Very recently 3D speckle-tracking technology has been validated for regional wall motion analysis,33) providing an additional quantitative tool to objectively assess segmental myocardial deformation.
A more accurate evaluation of regional wall motion improves the diagnostic accuracy of pharmacological stress echocardiography in ischemic heart disease.34),35) In addition, since the recovery of wall motion abnormalities has been shown to occur fast in patients with limited coronary disease, the rapid acquisition of peak stress images is of paramount importance to detect single-vessel disease. Latest generation 3DE scanners allow a rapid image acquisition during peak stress, which may decrease the potential risk of missing transitory ischemia.35) Using 3DE, the operator is able to acquire all views from a single transducer location, significantly shortening the acquisition time. The views obtained by automatically slicing the 3D data sets acquired at different steps of the stress echocardiographic protocol can be re-aligned at any moment after acquisition and automatically displayed side-by-side to allow an easier detection of new wall motion abnormalities or of changes in wall motion abnormalities detected at baseline. Aggeli et al.34) and Badano et al.35) have reported an increased sensitivity of 3D stress echo to detect inducible ischemia in the territory of left anterior descendent coronary artery.
3DE is emerging as a promising tool to assess intraventricular mechanical dyssynchrony, to improve the selection of patients with heart failure who will benefit from biventricular pacing and to monitor post-implant LV remodeling.36),37) A systolic dyssynchrony index is calculated as the standard deviation of the time to minimum systolic volume of each LV segment (Fig. 8). A higher systolic dyssynchrony index denotes increasing intraventricular dyssynchrony. Optimal cut-off value of systolic dyssynchrony index remains to be defined (Table 3).38-43) 3DE can also be used to choose the optimal pacing lead position and to assess the response to cardiac resynchronization therapy, as the LV end-systolic volume decrease is considered a marker for reverse remodeling.44)
Table 3.
Differences between right ventricular volumes assessed by three-dimensional echocardiography and cardiac magnetic resonance
Mean difference (95% confidence interval) from cardiac magnetic resonance
Left ventricle
Advantages of 3DE:
In contrast with 2DE imaging, re-aligning planes on 3D data sets to identify LV maximum longitudinal axis eliminates apical foreshortening and optimizes volumetric quantification
3DE measurements of left ventricular volumes and function do not rely on geometric assumptions about its shape
A comprehensive and time-saving analysis of LV geometry and function can be obtained from a single 3D full-volume data set (volumes, sphericity, ejection fraction, regional wall motion, dyssynchrony, deformation, mass)
3DE allows both qualitative and quantitative assessment of regional wall motion in a faster, more accurate and comprehensive manner in comparison with 2DE
Limitations of 3DE:
Good image quality is a prerequisite for an accurate semi-automated or fully-automated LV quantitation
Regular cardiac rhythm and patient cooperation for breath holding are essential
Limited evidence exists regarding the reference values for LV parameters and the intervendor consistency of 3D quantitative parameters
The relatively low temporal resolution of 3DE limits the assessment of regional wall motion during stress
Right ventricular morphology and function
2DE quantification of right ventricular (RV) size and function is challenging, due to the anterior position of the RV in the chest, its complex asymmetric geometry, irregularity of the highly trabeculated endocardial border, impossibility to visualize in the same view both inflow and outflow tracts and lack of realistic geometrical models.45) 3DE was demonstrated to have a good accuracy in measuring RV volumes compared to CMR (Fig. 12).38)
Fig. 12.
A: Surface rendering of the right ventricle obtained by transthoracic three-dimensional echocardiography. B: Time-volume curve showing right volume changes during cardiac cycle. C: Results of the quantitative analysis are shown.
A good correlation between CMR and 3DE parameters reflecting RV geometry and function has been demonstrated, although, as for the LV, an underestimation of 3DE RV volumes is often reported (Table 3). Shimada et al.38) reported that the underestimation of RV volumes with 3DE was higher for larger end-diastolic volumes, whereas an overestimation was more likely for smaller end-systolic volumes. Age accounted for a part of the error, RV volumes being overestimated and RV ejection fraction underestimated in the elderly. Possible reasons for this systematic bias may be the poorly-defined endocardial border in a dilated RV, the non-inclusion of RV outflow tract in the analysis and the limited temporal resolution. In a recent study, Tamborini et al.46) proposed reference values for RV volumes and ejection fraction obtained from 245 normal subjects. RV volumes were significantly correlated with age, sex and body surface area.
Amaki et al.47) demonstrated that RV quantification by 3DE can be accurately performed also in patients with pulmonary hypertension. They reported that the abnormal end-diastolic interventricular septal convexity visualized by 3DE was a predictor of mortality. In addition, 3DE has been used to quantify the extent of the tricuspid tenting in the functional tricuspid regurgitation secondary to the increased RV afterload.48)
Using unique cut planes, 3DE can detect particular causes of pulmonary hypertension, such as interventricular or interatrial septal defects or complex congenital diseases. In congenital heart diseases, RV volumes are important predictors of patient outcome, may help in choosing the timing of interventions and in monitoring the post-surgical result. An added value of 3DE is the ability of quantitating RV function after surgery, when Tricuspid Annular Plane Systolic Excursion and RV myocardial velocities by tissue-Doppler are no longer reliable indicators of global systolic performance.49),50)
Right ventricle
Advantages of 3DE:
3DE provides a unique and reliable quantification of RV volumes by echocardiography, both in healthy subjects and in patients
3DE has a documented prognostic value in the assessment of patients with congenital and acquired heart diseases
Limitations of 3DE:
RV has an unfavourable position within the chest for transthoracic 3DE transducer
Severely dilated RV is often difficult to encompass in a pyramidal 3D data set
RV prominent trabeculations are the main source of error when tracing endocardial borders
RV outflow tract or anterior wall are sometimes difficult to be adequately imaged by 3DE
Congenital heart diseases
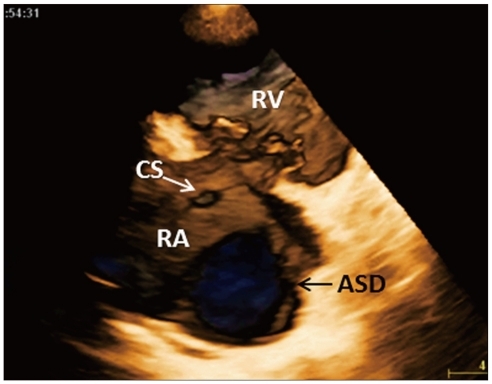
Children and young adults usually have excellent acoustic window and transthoracic 3DE represents the optimal technique to visualize and understand the complex anatomy of congenital heart disease. This technique allows a better appreciation of the position of cardiac structures in relation to each other and it is applicable in various congenital heart diseases, including valvular disease, shunts and aortic pathology.51-53) Monte et al.54) have reported the accuracy of 3D color Doppler in detecting patent foramen ovale in comparison with transthoracic and transesophageal contrast 2DE. 3DE enables novel views of congenital septal defects (Fig. 13) and improves the quantification of defect size as demonstrated by a closer correlation with surgical findings (r = 0.92 vs. r = 0.69 of the 2DE).55) Kasliwal et al.56) compared conventional echocardiographic methods with 3DE to assess the adequacy of rims for device closure and its relation to surroundings structures. 3DE derived maximal diameter and area of atrial septal defect showed the best correlations with invasively determined balloon occlusive diameter (r = 0.93, p = 0.0001).
Fig. 13.
En-face view of a large atrial septal defect from the right atrial perspective obtained by cropping the free wall of the right atrium from a full-volume three-dimensional data-set encompassing the base of the heart. The morphology and size of the defect can be appreciated and quantitated, as well as the extent of peripheral rims and relationships with surrounding cardiac structures. ASD: atrial septal defect, CS: coronary sinus, RA: right atrium, RV: right ventricle.
In patients who have undergone surgical (suture or patch) or percutaneous (occluder device) closure for ventricular or atrial septal defects, the entire shape, dimensions, and site of the patches or occluders, and their spatial relationships with surrounding structures could be clearly assessed on 3DE. Sinha et al.57) reported 4 clinical cases of atrial septal defects and patent foramen ovale, where 3DE and 3D color Doppler were used to assess the efficacy of Amplatzer transcatheter occluder device and postprocedure complications, such as presence and magnitude of residual shunt and device malposition. Kasliwal et al.56) also demonstrated the feasibility and the added diagnostic yield of 3DE in several patients with various congenital heart diseases: ventricular septal defects, patent ductus arteriosus, Valsalva sinus aneurysm, Ebstein anomaly and supramitral rings. 3DE gave additional information over standard 2DE by providing the spatial orientation of the anatomical structures.
Chamber volume quantification has been validated also in congenital diseases, in which 3D provides reliable and reproducible data to predict morbidity and mortality, to plan surgery and to monitor variations of chamber volumes and function, crucial for the management of congenital heart disease patient.41),58)
3DE has also been shown to reliably define the morphology and the anatomical details of bicuspid aortic valves (Fig. 14).59)
Fig. 14.
Bicuspid aortic valve displayed with closed (A) and open (B) leaflets.
Congenital heart disease
Advantages of 3DE:
3DE provides anatomic images in the beating heart, that are easily recognizable and interpreted by the surgeon, interventional cardiologist, pediatrician, congenital heart disease specialist, anatomist etc.
The additional dimension (depth) that is inherent to 3D images is particularly important when examining structures, such as the ventricular or atrial septum, or the atrioventricular junctions, which can now be visualized en face
3DE allows an easy appreciation of spatial orientation and a proper understanding of the (abnormal) relationship between adjacent cardiac structures
Limitations of 3DE:
3DE application in congenital diseases requires a specific training
Small children are difficult to image with the available 3D transducers
Patients with tachycardia or unable/unwilling to cooperate for respiratory maneuvers are particularly challenging with current 3DE technology
Heart valve morphology and function
Assessing the morphological and functional changes occurring in stenotic or regurgitant heart valves is key to the comprehensive diagnosis of heart valve diseases and to properly address their treatment. Since with 3DE we can obtain stereoscopic views of heart valve apparatus, 3DE findings have become pivotal to evaluate suitability for valve repair,60) provide surgical guidance, monitor interventional procedures61) and to assess effectiveness of treatment.62) Overcoming the necessity of mental reconstruction of valve anatomy from tomographic views, the surgeon and the echocardiographer share a common and reproducible view, allowing a better planning of patient's management.
Mitral valve
Mitral valve is a complex apparatus requiring anatomic integrity of its components and their correct functional relationship during the cardiac cycle for properly functioning. Due to the complex mitral annulus shape, a tomographic imaging modality like 2DE has several limitations in displaying the mitral valve morphology and geometry, which can be overcome by the volumetric display by 3DE.
The unique ability of 3DE to display en-face the atrio-ventricular valves both from the atrium or ventricle (Fig. 4 and 15) allows a better anatomical definition of mitral apparatus and its function in relation to the surrounding cardiac structures.63)
Fig. 15.
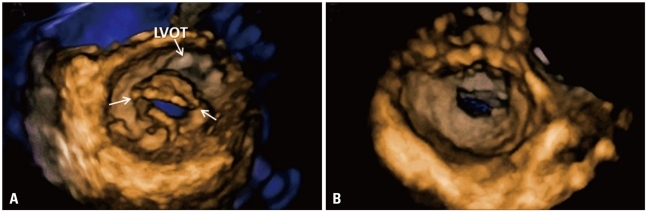
Rheumatic mitral stenosis. Volume rendering display from the left ventricular (A) and atrial (B) perspectives. The cut plane shown in A has been optimized to be perpendicular to the direction of the orifice to obtain an accurate orifice area planimetry. The thickened leaflets and fused commissures are well visualized (white arrows). LVOT: left ventricular outflow tract.
Mitral stenosis
To identify the best therapeutic strategy in patients with rheumatic mitral valve stenosis, clinical data and accurate measurements of mitral valve area are needed. Doppler based methods are heavily influenced by cardiac rhythm, haemodynamic status and angle of incidence. Accordingly, methods based on direct planimetry of the anatomical valve orifice should be more accurate. However, direct planimetry of mitral valve area from 2DE images has two major limitations: i. frequent overestimation of valve orifice area because the orientation of the 2D view used to trace the orifice contour is fixed and seldom orthogonal to the direction of the mitral funnel; ii. there is no anatomical landmark that can be used to ensure that the short-axis view used to trace the orifice is the one with the smallest area. 3DE has overcome these limitations, as the echocardiographer may easily crop the 3D data set to identify the correct orientation and position of the cut plane on which to trace the area of the stenotic valve. 3DE visualization of the actual mitral stenotic orifice can be accomplished either from the LV side or the left atrial side (Fig. 16). Severity of stenosis, extent of leaflet thickening and commissural fusion, as well visualization length and fusion degree of chordae tendineae can be visualized and assessed by 3DE. Compared to all other echo Doppler methods for assessing residual mitral valve orifice area, 3DE has shown the best agreement with invasive methods.64-66) Recently, Anwar et al.67) proposed a new score for assessing mitral stenosis based on 3D evaluation.
Fig. 16.
A: Three-dimensional color flow acquisition using transesophageal approach showing the origin and the three-dimensional shape of the jet. B: A cut-plane at the level of the jet vena contracta (yellow dashed line) allows to display the actual area and shape of the vena contracta for quantification.
Organic mitral regurgitation
Due to the saddle-shaped geometry of the mitral annulus, localization and extent of mitral valve prolapse can sometimes be controversial using 2DE68) and usually requires the acquisition of several views of the valve and subsequent mental reconstruction of its stereoscopic morphology by an experienced echocardiographer. 3DE, by its ability to display the mitral valve en-face from the left atrial perspective ("surgical view") provides an immediate and anatomically sound view of the involved scallops (Fig. 5). Transthoracic 3DE provides the surgeon a unique opportunity to visualize the mitral valve in the beating heart from the same perspective as in the operating room, for a precise identification and sizing of the prolapsing scallops with an accuracy approaching that of transesophageal 2DE.68),69) 3DE assessment has been found to provide a significant added diagnostic yield particularly in cases with complex mitral valve prolapse (commissular lesions, bileaflets lesions, P1 and P3 prolapse).70) This information is mandatory to reliably plan the surgical approach and predict the success rate of valve repair. Annulus dilatation is often coexisting in case of mitral valve prolapse and can be semi-automatically quantitated by 3DE (Fig. 5).71),72) Furthermore, 3D allows to identify factors of propensity to systolic anterior motion developing after valve repair and, consequently, interventional targets in patients with persistent regurgitation despite surgery.73)
Functional mitral regurgitation
Functional mitral regurgitation is defined as an insufficiency of the structurally normal mitral valve, developing as a consequence of a regional or global LV dysfunction. Mitral annulus dilatation, tethering of mitral leaflets secondary to LV dilatation with outward displacement of papillary muscles and reduced transmitral pressure to coapt the leaflets concur to determine the severity of the regurgitation. The extent of mitral valve apparatus distortion in patients with functional mitral regurgitation can be quantitated using 3DE: geometry of the annulus, leaflet surfaces, tethering distance, tenting volume, and the relationship between the mitral valve and the papillary muscles providing new insights into the pathophysiology of this disease, differentiating the mechanisms of the regurgitation in ischemic and dilated cardiomyopathy and assessing its severity.69),74),75)
Severity of mitral regurgitation
In addition to morphology, quantification of the severity of mitral valve regurgitation is mandatory to properly address management, particularly in asymptomatic patients.76) Conventional 2DE and Doppler methods are limited by several assumptions about regurgitant jet shape and orifice area morphology.77)
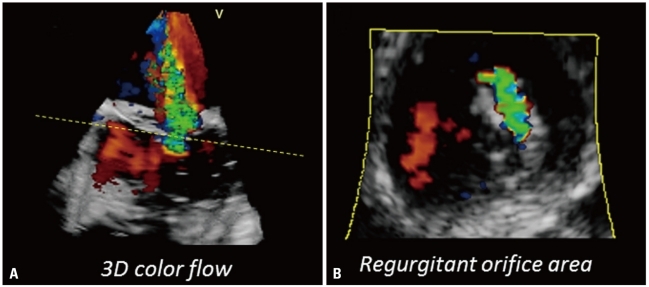
3DE color mode allows the acquisition of the whole regurgitant jet to assess its volume, origin and its extension in relation to adjacent structures (Fig. 17A).56) By cropping the 3DE color data set and using of the tissue/color suppress options, the regurgitant orifice area can be identified and vena contracta planimetered (Fig. 17B). Alternatively, the effective regurgitant orifice area can be measured from a 3D color data set without geometrical assumption about orifice shape and proximal isovelocity surface morphology.78)
Fig. 17.
Normal aortic valve in diastole, full-volume acquisition from transthoracic parasternal approach. Volume rendering display from the aortic (A) and left ventricular outflow tract (B) perspectives.
Thus, 3D allows evaluation of the real anatomic regurgitant orifice area, devoid of flow convergence limitations and distorsions. Correct visualization of regurgitant flow, particularly eccentric ones, improved accuracy in effective regurgitant orifice area quantification.79-81)
Mitral valve
Advantages of 3DE:
3DE offers en-face images of the mitral valve in the beating heart, that are easily interpreted by surgeons and interventional cardiologists
3DE enables a more accurate assessment of mitral stenosis severity by orifice planimetry and a detailed morphologic assessment, even if patients with inadequate parasternal window
En-face "surgical" views of mitral valve allows a reliable identification of the prolapsing scallops and assessment of the extent of valve abnormality in degenerative mitral regurgitation
Automated quantitative assessment of mitral annulus geometry and shape is possible by 3DE, in both degenerative and functional mitral regurgitation
3DE can provide a quantitative assessment of the various mechanisms involved in the development of functional mitral regurgitation and a superior accuracy in the quantitative evaluation of the regurgitation severity
3DE is a safe imaging tool for guiding and monitoring of mitral clipping interventional procedures
More accurate and reproducible quantitation of the chamber volumes and function by 3DE brings benefit in the diagnosis of valve disease severity and timing of corrective interventions
Limitations of 3DE:
Poor acoustic window limits the application of transthoracic 3DE
High temporal and spatial resolution are crucial for a reliable quantitative analysis based on 3D color flow data; significant arrhythmias are, therefore, difficult-to-image scenarious
Technical conditions (image quality, temporal and spatial resolution, gain, compression, dropout artifacts etc.) may significantly impact on valve disease severity assessment
Quantitative analysis is possible only off-line, on dedicated workstations and requires specific training
Aortic valve
3DE is complementary to 2DE for imaging the morphology of the aortic valve (Fig. 14 and 18) and quantitating the aortic root geometry.82) However, obtaining an adequate 3DE imaging of the aortic valve from both transthoracic and transesophageal approaches is sometimes more challenging than of the mitral valve, particularly in normal aortic valves (having very thin cusps that cause dropouts of the leaflet bodies with regular thresholding) or in heavily calcified valve annulus and prostheses (visualization limited by frequent acoustic shadowing due to calcium deposits and prosthesis stents), or when the acoustic window is suboptimal. Once a 3DE data set containing the aortic root is acquired, it can be cropped and rotated for an anatomically sound, dynamic 3D rendering of the aortic valve, which can be visualized both from aortic and ventricular perspectives (Fig. 15), as well as from any desired longitudinal or oblique plane. The visualization of the aortic valve from the aorta (surgical view) is best suited for assessing valve morphology, while the ventricular perspective is more useful to assess aortic tumors/vegetations or subvalvular obstructions.83) In addition, the analysis of 3DE data sets has revealed new insights of valvular dynamics. Veronesi et al.84) demonstrated that mitral and aortic valves are coupled to function in a reciprocal, interdependent way. The expansion of one facilitates contraction of the other. The evidence is observed through decreased mitral regurgitation severity after aortic replacement.
Fig. 18.
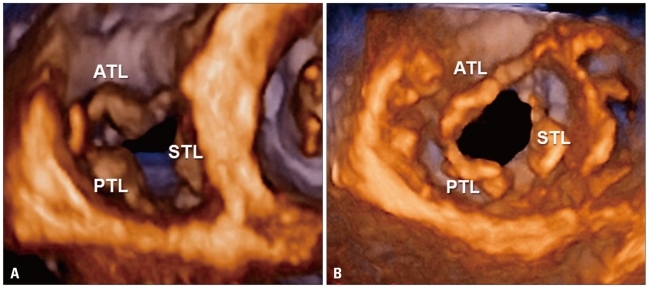
Normal tricuspid valve. Volume rendering display from the right ventricular (A) and atrial (B) perspectives. ATL: anterior tricuspid leaflet, PTL: posterior tricuspid leaflet, STL: septal tricuspid leaflet.
Aortic stenosis
3DE improved accuracy of the echocardiographic assessment of aortic valve area. With conventional 2DE Doppler, continuity equation assume the circularity of LV outflow tract area, but 3DE has shown that the actual cross-section is often elliptical, with the largest diameter being the transversal (Fig. 18B). Therefore, 2DE which derives the LV outflow tract area from the measurement of the antero-posterior diameter may underestimate the actual valve area. Khaw et al.85) have reported that substituting planimetered LV outflow tract area obtained from 3DE data sets enhanced the accuracy of valve area quantification in patients with aortic stenosis.
Gutiérrez-Chico et al.86) proposed another approach to improve accuracy of aortic stenosis severity assessment, by using in the continuity equation direct volumetric measurements of the LV stroke volume from 3D LV data sets. Aortic valve areas obtained with such method were closer correlated to those obtained with invasive measurements and Gorlin formula, than the corresponding values obtained with conventional continuity equation.
Goland et al.87) suggested a direct planimetry of the aortic valve area from the en-face visualization of the valve by 3DE. The method resulted feasible and accurate compared to computerized tomography and CMR.88),89)
Aortic regurgitation
Repair of isolated aortic regurgitation, with or without aortic root pathology, is emerging as a feasible and attractive option to replacement. However, aortic valve repair is technically more demanding than replacement, and careful preoperative echocardiographic assessment of the valve and the aortic root (the so-called "aortic valve complex") is pivotal to identify the mechanism of regurgitation and to provide the surgeon quantitative data about morphology of aortic valve complex for proper patient selection and surgical planning. In a comparative study of transesophageal 2DE and epicardial 3DE against surgical findings, epicardial 3DE was more sensitive than transesophageal 2DE in detecting morphologic abnormalities of aortic valve documented intra-operatively (leaflet deficiency, prolapse/perforation, commissural fusion).90)
3DE is feasible and accurate to precisely describe the mechanism of aortic regurgitation and the complementary use of color 3D enables the quantitation of its severity. 3DE methods, which reconstruct vena contracta region, allow direct measurements of jet cross-sectional area.91) Other 3DE methods for quantification have been evaluated, such as the direct measurement of proximal isovelocity surface volume or the measurement of aortic regurgitant volume by computing the difference between 3DE-determined LV and RV stroke volumes.92)
Furthermore, 3D provides a realistic assessment of aortic root, allowing the measurement of several parameters describing its morphology such as leaflet height, leaflet coaptation height, inter-commissural distance, leaflet edge, coronary ostium to leaflet distance and sinus Valsalva volumes, useful in planning surgical (e.g. aortic valve-sparing operation) or transcatheter aortic valve implantation procedures.83),93)
Aortic valve
Advantages of 3DE:
3DE provides en-face visualizations of the aortic valve in the beating heart, either from aorta or ventricular perspective, that are easily interpreted by surgeons to plan valve repair surgery
Anatomically-corrected measurements of LV outflow tract area by 3DE planimetry are useful for assessing severity of valve stenosis and sizing valve prosthesis
Quantitative assessment of aortic root geometry helps in planning aortic valve-sparing and transcatheter aortic valve implantation interventions
Accurate quantitation of size and function of LV by 3DE is key for clinical decisions
Limitations of 3DE:
Suboptimal acoustic window renders transthoracic acquisitions of aortic valve difficult or at times impossible to interpret
Drop-out artifacts of cusp body is frequent in normal aortic valve
Acoustic shadowing limits the accuracy of 3DE assessment in patients with heavily calcified annuli or with stented or metallic valve prostheses
Tricuspid valve
A complete visualization of tricuspid annulus and all three leaflets in one view is seldom possible by both transthoracic and transesophageal 2DE. 3DE has the unique capability of obtaining a transverse plane encompassing the whole tricuspid valve and provide an en-face visualization of the valve. It allows the simultaneous visualization of all the three leaflets moving during the cardiac cycle, leaflet coaptation and separation of the commissures from both the atrial and ventricular perspective (Fig. 15). Furthermore, tricuspid annulus has been shown to have a complex 3D shape: 3DE is the unique method to provide a reliable assessment of actual annulus size and morphology,94) pivotal to plan surgical management in functional regurgitation.95)
Tricuspidal stenosis
Leaflet thickness, extent of commissural fusion and area planimetry can be readly obtained in rheumatic disease.96-98)
Tricuspidal regurgitation
When a "pathologic" tricuspid regurgitation is detected at color Doppler, a complete understanding of leaflet morphology and of the pathophysiological mechanisms underlying tricuspid regurgitation is mandatory to properly address management. In these cases, a comprehensive assessment of the morphology of tricuspid valve apparatus using transthoracic 3DE provides important clues on the underlying aetiology and mechanisms of valve dysfunction.94),98-100)
Vena contracta and regurgitant orifice area measurements by color 3DE have been reported to provide a reliable quantification of the severity of tricuspid regurgitation.101)
Tricuspid valve
Advantages of 3DE:
3DE offers direct visualization of the valve apparatus and of all 3 leaflets in a single view, which is pivotal to understanding the underlying aetiology and mechanisms of valve dysfunction
3DE provides new opportunities for improving the quantitative assessment of valve disease severity (tricuspid valve stenosis and regurgitation, isolated or associated)
Reliable quantitative assessment of tricuspid annuls size and morphology is possible by semi-automated 3DE softwares
3DE is the most accurate ultrasound method for the quantitation of volumes and function of right heart chambers
Limitations of 3DE:
Suboptimal acoustic window renders transthoracic acquisitions of tricuspid valve difficult or at times impossible to interpret
Pulmonary valve disease
Despite its potential utility, there is no current evidence supporting the routine use of 3DE for pulmonary valve disease assessment.102),103)
Prosthetic valves
Transoesophageal 3DE is the method of choice for an accurate diagnosis of prosthetic valve function. Transoesophageal 3DE enhanced the assessment of prosthetic valves and related complications, such as leaking, dehiscence and endocarditis complications. 3D color Doppler flow mapping is able to qualitatively visualize the size and shape of valvular and paravalvular regurgitations, as well as the underlying pathology, enabling exact definition of jet origin and size which are useful to plan management.104) Transthoracic 3DE is of limited usefulness in assessing mechanical prostheses due to acoustic shadowing and limited spatial resolution, but it may sometimes provide clues on the mechanism of bioprosthesis dysfunction (i.e. cusp prolapse or rupture).
Infective Endocarditis
3D transthoracic and particularly transoesophageal echocardiography can show the 3D configuration and attachment of vegetations, mobility of vegetations with blood flow and potentsial complications, such as valve prolapse and perforation.105) The ability to acquire detailed "en-face" images of the valve allows one to precisely localize the vegetations and assess their size, morphology, attachment points and relationships with anatomical structures.106),107) Despite the high spatial resolution, particularly with transesophageal 3DE, Lang et al.62) underlines that, because of frame rate limitations on 3D, in their experience 2DE remains superior for the identification of small mobile vegetations.
Cardiac Masses
A cardiac mass may have quite variable site of attachment, shape and size, requiring from the echocardiographer to examine it from a series of 2DE images and then to "mentally" reconstruct the tumor to define all morphologic details. To do this accurately, a clinician should understand the relationship of each 2D tomographic image to one another. 3DE eliminates the need for cognitive reconstruction of image planes and use of geometric assumptions about shape of structures for quantitation. This particularly applies to complex shapes such as intracardiac tumors. Once a 3D data set is acquired, it can be cropped and sliced in many different ways. In addition, the possibility of rotating the data sets in the space allows the observer to obtain planes and views and to align structures in ways that were impossible to achieve with conventional 2DE. Thus, additional information about mass location, shape, attaching interface and relationships with adjacent structures can be derived from 3DE data sets.108)
Left and Right Atria
A growing interest has been recently dedicated to atrial cavities due to their prognostic value in various cardiac diseases and the rapid development of cardiac interventional electrophysiology. At present, target structures are mainly localized with post-processing of computer tomography scan sections and with fluoroscopy, but 3DE is emerging as a valuable tool to guide ablation procedures.109) 3D may be a useful tool to identify landmarks, getting images from any perspective and with the possibility to "navigate" through cardiac cavities. 3D has been reported as an accurate and reproducible method to measure left atrial volumes and function (Fig. 19).110-112) Conversely, very limited data is available for right atrial volume assessment by 3DE.113) As for the LV, 3DE corrects for atrial cavity foreshortening and results in less volume underestimation than 2DE, when both methods were compared against magnetic resonance.
Fig. 19.
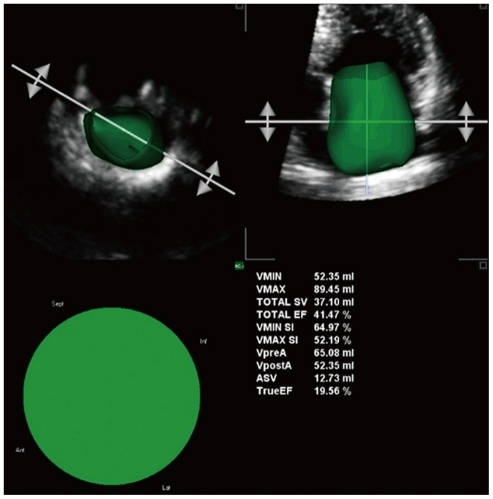
Semi-automated quantification of left atrial volumes (maximal, VMAX; pre A-wave, VpreA; and minimal, VMIN), shape (sphericity indexes at maximal and minimal volumes, VMAX SI and VMIN SI, respectively) and phasic functions (total and active emptying fraction or stroke volumes, Total EF or SV and TrueEF or ASV, respectively) based on three-dimensional echocardiography.
Transesophageal 3DE may also improve assessment of left atrial appendage morphology and size. In addition, orifice area measurements by transesophageal 3DE for device sizing in percutaneous closure has been reported to be accurate when compared to computed tomography measurements.114) The use of transesophageal 3DE monitoring is crucial for guiding percutaneous closure of atrial appendage.
Left and right atria
Advantages of 3DE:
3DE provides a more reproducible assessment of left atrial volumes and less underestimation in comparison with magnetic resonance
A quantitative analysis of left and right atrial phasic functions can be obtained using novel semi-automated 3DE software
Assessment of left atrial appendage size and morphology can be performed by transoesophageal 3DE, improving the accuracy of 2D-based sizing approach
Transoesophageal 3DE is a valuable tool for device sizing, guiding and monitoring interventional procedures involving atrial septum, left atrial appendage, pulmonary veins etc.
Limitations of 3DE:
Atrial fibrillation with highly irregular rhythm prevents multibeat full-volume acquisitions, however single-beat images are feasible
Inadequate apical acoustic window limits the accuracy of atrial volume measurement
Reference values for both left and right atrial volumes and functional parameters derived from large populations are currently lacking
Conclusions
3DE is a novel imaging technique based on acquisition and display of volumetric data sets in the beating heart. This permits a comprehensive evaluation of cardiac anatomy and function from a single acquisition and expands the diagnostic possibilities of non-invasive cardiology. It provides the possibility to quantitate geometry and function of cardiac chambers without pre-established assumptions regarding cardiac chamber shape and allows an echocardiographic assessment of the heart that is less operator-dependent and therefore more reproducible. New visualization and quantitation opportunities have greatly enhanced our understanding of pathophysiology and severity of heart valve diseases and congenital defects.
Further developments and improvements for widespread routine applications include higher spatial and temporal resolution to improve image quality, faster acquisition, processing and reconstruction, and easier approaches to quantitative analysis. At present, 3DE complements routine 2DE in clinical practice, overcoming some of its limitations and offering additional valuable information that has led to recommend its use for routine examination in selected fields. In the future, 3DE may become the standard echocardiographic examination procedure.
References
- 1.Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, Faletra FF, Franke A, Hung J, de Isla LP, Kamp O, Kasprzak JD, Lancellotti P, Marwick TH, McCulloch ML, Monaghan MJ, Nihoyannopoulos P, Pandian NG, Pellikka PA, Pepi M, Roberson DA, Shernan SK, Shirali GS, Sugeng L, Ten Cate FJ, Vannan MA, Zamorano JL, Zoghbi WA. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. 2012;13:1–46. doi: 10.1093/ehjci/jer316. [DOI] [PubMed] [Google Scholar]
- 2.Muraru D, Badano LP, Piccoli G, Gianfagna P, Del Mestre L, Ermacora D, Proclemer A. Validation of a novel automated border-detection algorithm for rapid and accurate quantitation of left ventricular volumes based on three-dimensional echocardiography. Eur J Echocardiogr. 2010;11:359–368. doi: 10.1093/ejechocard/jep217. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins C, Bricknell K, Chan J, Hanekom L, Marwick TH. Comparison of two- and three-dimensional echocardiography with sequential magnetic resonance imaging for evaluating left ventricular volume and ejection fraction over time in patients with healed myocardial infarction. Am J Cardiol. 2007;99:300–306. doi: 10.1016/j.amjcard.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins C, Bricknell K, Hanekom L, Marwick TH. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J Am Coll Cardiol. 2004;44:878–886. doi: 10.1016/j.jacc.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 5.Kühl HP, Schreckenberg M, Rulands D, Katoh M, Schäfer W, Schummers G, Bücker A, Hanrath P, Franke A. High-resolution transthoracic real-time three-dimensional echocardiography: quantitation of cardiac volumes and function using semi-automatic border detection and comparison with cardiac magnetic resonance imaging. J Am Coll Cardiol. 2004;43:2083–2090. doi: 10.1016/j.jacc.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 6.Caiani EG, Corsi C, Zamorano J, Sugeng L, MacEneaney P, Weinert L, Battani R, Gutiérrez-Chico JL, Koch R, Perez de Isla L, Mor-Avi V, Lang RM. Improved semiautomated quantification of left ventricular volumes and ejection fraction using 3-dimensional echocardiography with a full matrix-array transducer: comparison with magnetic resonance imaging. J Am Soc Echocardiogr. 2005;18:779–788. doi: 10.1016/j.echo.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Shiota T, McCarthy PM, White RD, Qin JX, Greenberg NL, Flamm SD, Wong J, Thomas JD. Initial clinical experience of real-time three-dimensional echocardiography in patients with ischemic and idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;84:1068–1073. doi: 10.1016/s0002-9149(99)00500-7. [DOI] [PubMed] [Google Scholar]
- 8.Zeidan Z, Erbel R, Barkhausen J, Hunold P, Bartel T, Buck T. Analysis of global systolic and diastolic left ventricular performance using volume-time curves by real-time three-dimensional echocardiography. J Am Soc Echocardiogr. 2003;16:29–37. doi: 10.1067/mje.2003.40. [DOI] [PubMed] [Google Scholar]
- 9.Chan J, Jenkins C, Khafagi F, Du L, Marwick TH. What is the optimal clinical technique for measurement of left ventricular volume after myocardial infarction? A comparative study of 3-dimensional echocardiography, single photon emission computed tomography, and cardiac magnetic resonance imaging. J Am Soc Echocardiogr. 2006;19:192–201. doi: 10.1016/j.echo.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Corsi C, Lang RM, Veronesi F, Weinert L, Caiani EG, MacEneaney P, Lamberti C, Mor-Avi V. Volumetric quantification of global and regional left ventricular function from real-time three-dimensional echocardiographic images. Circulation. 2005;112:1161–1170. doi: 10.1161/CIRCULATIONAHA.104.513689. [DOI] [PubMed] [Google Scholar]
- 11.Sugeng L, Mor-Avi V, Weinert L, Niel J, Ebner C, Steringer-Mascherbauer R, Schmidt F, Galuschky C, Schummers G, Lang RM, Nesser HJ. Quantitative assessment of left ventricular size and function: side-by-side comparison of real-time three-dimensional echocardiography and computed tomography with magnetic resonance reference. Circulation. 2006;114:654–661. doi: 10.1161/CIRCULATIONAHA.106.626143. [DOI] [PubMed] [Google Scholar]
- 12.Nikitin NP, Constantin C, Loh PH, Ghosh J, Lukaschuk EI, Bennett A, Hurren S, Alamgir F, Clark AL, Cleland JG. New generation 3-dimensional echocardiography for left ventricular volumetric and functional measurements: comparison with cardiac magnetic resonance. Eur J Echocardiogr. 2006;7:365–372. doi: 10.1016/j.euje.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs LD, Salgo IS, Goonewardena S, Weinert L, Coon P, Bardo D, Gerard O, Allain P, Zamorano JL, de Isla LP, Mor-Avi V, Lang RM. Rapid online quantification of left ventricular volume from real-time three-dimensional echocardiographic data. Eur Heart J. 2006;27:460–468. doi: 10.1093/eurheartj/ehi666. [DOI] [PubMed] [Google Scholar]
- 14.Gutiérrez-Chico JL, Zamorano JL, Pérez de Isla L, Orejas M, Almería C, Rodrigo JL, Ferreirós J, Serra V, Macaya C. Comparison of left ventricular volumes and ejection fractions measured by three-dimensional echocardiography versus by two-dimensional echocardiography and cardiac magnetic resonance in patients with various cardiomyopathies. Am J Cardiol. 2005;95:809–813. doi: 10.1016/j.amjcard.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 15.van den Bosch AE, Robbers-Visser D, Krenning BJ, Voormolen MM, McGhie JS, Helbing WA, Roos-Hesselink JW, Simoons ML, Meijboom FJ. Real-time transthoracic three-dimensional echocardiographic assessment of left ventricular volume and ejection fraction in congenital heart disease. J Am Soc Echocardiogr. 2006;19:1–6. doi: 10.1016/j.echo.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Pouleur AC, le Polain de Waroux JB, Pasquet A, Gerber BL, Gérard O, Allain P, Vanoverschelde JL. Assessment of left ventricular mass and volumes by three-dimensional echocardiography in patients with or without wall motion abnormalities: comparison against cine magnetic resonance imaging. Heart. 2008;94:1050–1057. doi: 10.1136/hrt.2007.123711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi X, Cogar B, Hsiung MC, Nanda NC, Miller AP, Yelamanchili P, Baysan O, Wu YS, Lan GY, Ko JS, Cheng CH, Lin CC, Huang CM, Yin WH, Young MS. Live/real time three-dimensional transthoracic echocardiographic assessment of left ventricular volumes, ejection fraction, and mass compared with magnetic resonance imaging. Echocardiography. 2007;24:166–173. doi: 10.1111/j.1540-8175.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 18.Bicudo LS, Tsutsui JM, Shiozaki A, Rochitte CE, Arteaga E, Mady C, Ramires JA, Mathias W., Jr Value of real time three-dimensional echocardiography in patients with hypertrophic cardiomyopathy: comparison with two-dimensional echocardiography and magnetic resonance imaging. Echocardiography. 2008;25:717–726. doi: 10.1111/j.1540-8175.2008.00684.x. [DOI] [PubMed] [Google Scholar]
- 19.Shimada YJ, Shiota T. A meta-analysis and investigation for the source of bias of left ventricular volumes and function by three-dimensional echocardiography in comparison with magnetic resonance imaging. Am J Cardiol. 2011;107:126–138. doi: 10.1016/j.amjcard.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 20.Mor-Avi V, Jenkins C, Kühl HP, Nesser HJ, Marwick T, Franke A, Ebner C, Freed BH, Steringer-Mascherbauer R, Pollard H, Weinert L, Niel J, Sugeng L, Lang RM. Real-time 3-dimensional echocardiographic quantification of left ventricular volumes: multicenter study for validation with magnetic resonance imaging and investigation of sources of error. JACC Cardiovasc Imaging. 2008;1:413–423. doi: 10.1016/j.jcmg.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Tighe DA, Rosetti M, Vinch CS, Chandok D, Muldoon D, Wiggin B, Dahlberg ST, Aurigemma GP. Influence of image quality on the accuracy of real time three-dimensional echocardiography to measure left ventricular volumes in unselected patients: a comparison with gated-SPECT imaging. Echocardiography. 2007;24:1073–1080. doi: 10.1111/j.1540-8175.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 22.Krenning BJ, Kirschbaum SW, Soliman OI, Nemes A, van Geuns RJ, Vletter WB, Veltman CE, Ten Cate FJ, Roelandt JR, Geleijnse ML. Comparison of contrast agent-enhanced versus non-contrast agent-enhanced real-time three-dimensional echocardiography for analysis of left ventricular systolic function. Am J Cardiol. 2007;100:1485–1489. doi: 10.1016/j.amjcard.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins C, Moir S, Chan J, Rakhit D, Haluska B, Marwick TH. Left ventricular volume measurement with echocardiography: a comparison of left ventricular opacification, three-dimensional echocardiography, or both with magnetic resonance imaging. Eur Heart J. 2009;30:98–106. doi: 10.1093/eurheartj/ehn484. [DOI] [PubMed] [Google Scholar]
- 24.Mannaerts HF, van der Heide JA, Kamp O, Stoel MG, Twisk J, Visser CA. Early identification of left ventricular remodelling after myocardial infarction, assessed by transthoracic 3D echocardiography. Eur Heart J. 2004;25:680–687. doi: 10.1016/j.ehj.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Mor-Avi V, Sugeng L, Weinert L, MacEneaney P, Caiani EG, Koch R, Salgo IS, Lang RM. Fast measurement of left ventricular mass with real-time three-dimensional echocardiography: comparison with magnetic resonance imaging. Circulation. 2004;110:1814–1818. doi: 10.1161/01.CIR.0000142670.65971.5F. [DOI] [PubMed] [Google Scholar]
- 26.Caiani EG, Corsi C, Sugeng L, MacEneaney P, Weinert L, Mor-Avi V, Lang RM. Improved quantification of left ventricular mass based on endocardial and epicardial surface detection with real time three dimensional echocardiography. Heart. 2006;92:213–219. doi: 10.1136/hrt.2005.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin JX, Shiota T, Thomas JD. Determination of left ventricular volume, ejection fraction, and myocardial mass by real-time three-dimensional echocardiography. Echocardiography. 2000;17:781–786. doi: 10.1111/j.1540-8175.2000.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 28.Oe H, Hozumi T, Arai K, Matsumura Y, Negishi K, Sugioka K, Ujino K, Takemoto Y, Inoue Y, Yoshikawa J. Comparison of accurate measurement of left ventricular mass in patients with hypertrophied hearts by real-time three-dimensional echocardiography versus magnetic resonance imaging. Am J Cardiol. 2005;95:1263–1267. doi: 10.1016/j.amjcard.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 29.van den Bosch AE, Robbers-Visser D, Krenning BJ, McGhie JS, Helbing WA, Meijboom FJ, Roos-Hesselink JW. Comparison of real-time three-dimensional echocardiography to magnetic resonance imaging for assessment of left ventricular mass. Am J Cardiol. 2006;97:113–117. doi: 10.1016/j.amjcard.2005.07.114. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi M, Nishikage T, Mor-Avi V, Sugeng L, Weinert L, Nakai H, Salgo IS, Gerard O, Lang RM. Measurement of left ventricular mass by real-time three-dimensional echocardiography: validation against magnetic resonance and comparison with two-dimensional and m-mode measurements. J Am Soc Echocardiogr. 2008;21:1001–1005. doi: 10.1016/j.echo.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Galderisi M, Henein MY, D'hooge J, Sicari R, Badano LP, Zamorano JL, Roelandt JR European Association of Echocardiography. Recommendations of the European Association of Echocardiography: how to use echo-Doppler in clinical trials: different modalities for different purposes. Eur J Echocardiogr. 2011;12:339–353. doi: 10.1093/ejechocard/jer051. [DOI] [PubMed] [Google Scholar]
- 32.Jaochim Nesser H, Sugeng L, Corsi C, Weinert L, Niel J, Ebner C, Steringer-Mascherbauer R, Schmidt F, Schummers G, Lang RM, Mor-Avi V. Volumetric analysis of regional left ventricular function with real-time three-dimensional echocardiography: validation by magnetic resonance and clinical utility testing. Heart. 2007;93:572–578. doi: 10.1136/hrt.2006.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo Y, Ishizu T, Enomoto Y, Sugimori H, Yamamoto M, Machino T, Kawamura R, Aonuma K. Validation of 3-dimensional speckle tracking imaging to quantify regional myocardial deformation. Circ Cardiovasc Imaging. 2009;2:451–459. doi: 10.1161/CIRCIMAGING.109.858480. [DOI] [PubMed] [Google Scholar]
- 34.Aggeli C, Giannopoulos G, Misovoulos P, Roussakis G, Christoforatou E, Kokkinakis C, Brili S, Stefanadis C. Real-time three-dimensional dobutamine stress echocardiography for coronary artery disease diagnosis: validation with coronary angiography. Heart. 2007;93:672–675. doi: 10.1136/hrt.2006.101220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Badano LP, Muraru D, Rigo F, Del Mestre L, Ermacora D, Gianfagna P, Proclemer A. High volume-rate three-dimensional stress echocardiography to assess inducible myocardial ischemia: a feasibility study. J Am Soc Echocardiogr. 2010;23:628–635. doi: 10.1016/j.echo.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Kapetanakis S, Kearney MT, Siva A, Gall N, Cooklin M, Monaghan MJ. Real-time three-dimensional echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation. 2005;112:992–1000. doi: 10.1161/CIRCULATIONAHA.104.474445. [DOI] [PubMed] [Google Scholar]
- 37.Kapetanakis S, Bhan A, Murgatroyd F, Kearney MT, Gall N, Zhang Q, Yu CM, Monaghan MJ. Real-time 3D echo in patient selection for cardiac resynchronization therapy. JACC Cardiovasc Imaging. 2011;4:16–26. doi: 10.1016/j.jcmg.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Shimada YJ, Shiota M, Siegel RJ, Shiota T. Accuracy of right ventricular volumes and function determined by three-dimensional echocardiography in comparison with magnetic resonance imaging: a meta-analysis study. J Am Soc Echocardiogr. 2010;23:943–953. doi: 10.1016/j.echo.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 39.Grapsa J, O'Regan DP, Pavlopoulos H, Durighel G, Dawson D, Nihoyannopoulos P. Right ventricular remodelling in pulmonary arterial hypertension with three-dimensional echocardiography: comparison with cardiac magnetic resonance imaging. Eur J Echocardiogr. 2010;11:64–73. doi: 10.1093/ejechocard/jep169. [DOI] [PubMed] [Google Scholar]
- 40.Sugeng L, Mor-Avi V, Weinert L, Niel J, Ebner C, Steringer-Mascherbauer R, Bartolles R, Baumann R, Schummers G, Lang RM, Nesser HJ. Multimodality comparison of quantitative volumetric analysis of the right ventricle. JACC Cardiovasc Imaging. 2010;3:10–18. doi: 10.1016/j.jcmg.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 41.van der Zwaan HB, Helbing WA, McGhie JS, Geleijnse ML, Luijnenburg SE, Roos-Hesselink JW, Meijboom FJ. Clinical value of real-time three-dimensional echocardiography for right ventricular quantification in congenital heart disease: validation with cardiac magnetic resonance imaging. J Am Soc Echocardiogr. 2010;23:134–140. doi: 10.1016/j.echo.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Leibundgut G, Rohner A, Grize L, Bernheim A, Kessel-Schaefer A, Bremerich J, Zellweger M, Buser P, Handke M. Dynamic assessment of right ventricular volumes and function by real-time three-dimensional echocardiography: a comparison study with magnetic resonance imaging in 100 adult patients. J Am Soc Echocardiogr. 2010;23:116–126. doi: 10.1016/j.echo.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 43.Muraru D, Badano LP, Ermacora D, Piccoli G, Iliceto S. Sources of variation and bias in assessing left ventricular volumes and dyssynchrony using three-dimensional echocardiography. Int J Cardiovasc Imaging. 2011 doi: 10.1007/s10554-011-9985-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 44.Monaghan M. Echocardiographic assessment of left ventricular dyssynchrony--is three-dimensional echocardiography just the latest kid on the block? J Am Soc Echocardiogr. 2009;22:240–241. doi: 10.1016/j.echo.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 45.Badano LP, Ginghina C, Easaw J, Muraru D, Grillo MT, Lancellotti P, Pinamonti B, Coghlan G, Marra MP, Popescu BA, De Vita S. Right ventricle in pulmonary arterial hypertension: haemodynamics, structural changes, imaging, and proposal of a study protocol aimed to assess remodelling and treatment effects. Eur J Echocardiogr. 2010;11:27–37. doi: 10.1093/ejechocard/jep152. [DOI] [PubMed] [Google Scholar]
- 46.Tamborini G, Marsan NA, Gripari P, Maffessanti F, Brusoni D, Muratori M, Caiani EG, Fiorentini C, Pepi M. Reference values for right ventricular volumes and ejection fraction with real-time three-dimensional echocardiography: evaluation in a large series of normal subjects. J Am Soc Echocardiogr. 2010;23:109–115. doi: 10.1016/j.echo.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 47.Amaki M, Nakatani S, Kanzaki H, Kyotani S, Nakanishi N, Shigemasa C, Hisatome I, Kitakaze M. Usefulness of three-dimensional echocardiography in assessing right ventricular function in patients with primary pulmonary hypertension. Hypertens Res. 2009;32:419–422. doi: 10.1038/hr.2009.20. [DOI] [PubMed] [Google Scholar]
- 48.Grapsa J, Gibbs JS, Dawson D, Watson G, Patni R, Athanasiou T, Punjabi PP, Howard LS, Nihoyannopoulos P. Morphologic and functional remodeling of the right ventricle in pulmonary hypertension by real time three dimensional echocardiography. Am J Cardiol. 2012;109:906–913. doi: 10.1016/j.amjcard.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 49.Tamborini G, Muratori M, Brusoni D, Celeste F, Maffessanti F, Caiani EG, Alamanni F, Pepi M. Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. Eur J Echocardiogr. 2009;10:630–634. doi: 10.1093/ejechocard/jep015. [DOI] [PubMed] [Google Scholar]
- 50.Giusca S, Dambrauskaite V, Scheurwegs C, D'hooge J, Claus P, Herbots L, Magro M, Rademakers F, Meyns B, Delcroix M, Voigt JU. Deformation imaging describes right ventricular function better than longitudinal displacement of the tricuspid ring. Heart. 2010;96:281–288. doi: 10.1136/hrt.2009.171728. [DOI] [PubMed] [Google Scholar]
- 51.Acar P. [Three-dimensional echocardiography in congenital heart disease] Arch Pediatr. 2006;13:51–56. doi: 10.1016/j.arcped.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 52.Rawlins DB, Austin C, Simpson JM. Live three-dimensional paediatric intraoperative epicardial echocardiography as a guide to surgical repair of atrioventricular valves. Cardiol Young. 2006;16:34–39. doi: 10.1017/S1047951105002064. [DOI] [PubMed] [Google Scholar]
- 53.Seliem MA, Fedec A, Cohen MS, Ewing S, Farrell PE, Jr, Rychik J, Schultz AH, Gaynor JW, Spray TL. Real-time 3-dimensional echocardiographic imaging of congenital heart disease using matrix-array technology: freehand real-time scanning adds instant morphologic details not well delineated by conventional 2-dimensional imaging. J Am Soc Echocardiogr. 2006;19:121–129. doi: 10.1016/j.echo.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 54.Monte I, Grasso S, Licciardi S, Badano LP. Head-to-head comparison of real-time three-dimensional transthoracic echocardiography with transthoracic and transesophageal two-dimensional contrast echocardiography for the detection of patent foramen ovale. Eur J Echocardiogr. 2010;11:245–249. doi: 10.1093/ejechocard/jep195. [DOI] [PubMed] [Google Scholar]
- 55.Cheng TO, Xie MX, Wang XF, Wang Y, Lu Q. Real-time 3-dimensional echocardiography in assessing atrial and ventricular septal defects: an echocardiographic-surgical correlative study. Am Heart J. 2004;148:1091–1095. doi: 10.1016/j.ahj.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 56.Kasliwal RR, Chouhan NS, Sinha A, Gupta P, Tandon S, Trehan N. Real-time three-dimensional transthoracic echocardiography. Indian Heart J. 2005;57:128–137. [PubMed] [Google Scholar]
- 57.Sinha A, Nanda NC, Misra V, Khanna D, Dod HS, Vengala S, Mehmood F, Singh V. Live three-dimensional transthoracic echocardiographic assessment of transcatheter closure of atrial septal defect and patent foramen ovale. Echocardiography. 2004;21:749–753. doi: 10.1111/j.0742-2822.2004.04037.x. [DOI] [PubMed] [Google Scholar]
- 58.van der Zwaan HB, Helbing WA, Boersma E, Geleijnse ML, McGhie JS, Soliman OI, Roos-Hesselink JW, Meijboom FJ. Usefulness of real-time three-dimensional echocardiography to identify right ventricular dysfunction in patients with congenital heart disease. Am J Cardiol. 2010;106:843–850. doi: 10.1016/j.amjcard.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 59.Muraru D, Badano LP, Del Mestre L, Gianfagna P, Proclemer A, Livi U. Real-time three dimensional echocardiography in the postoperative follow-up of type-A aortic dissection--a case report. J Am Soc Echocardiogr. 2010;23:682.e1-4. doi: 10.1016/j.echo.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 60.Pepi M, Tamborini G, Bartorelli AL, Trabattoni D, Maltagliati A, De Vita S, Andreini D, Pontone G. Usefulness of three-dimensional echocardiographic reconstruction of the Amplatzer septal occluder in patients undergoing atrial septal closure. Am J Cardiol. 2004;94:1343–1347. doi: 10.1016/j.amjcard.2004.07.132. [DOI] [PubMed] [Google Scholar]
- 61.Zamorano JL, Badano LP, Bruce C, Chan KL, Gonçalves A, Hahn RT, Keane MG, La Canna G, Monaghan MJ, Nihoyannopoulos P, Silvestry FE, Vanoverschelde JL, Gillam LD, Vahanian A, Di Bello V, Buck T Document Reviewers: European Association of Echocardiography (EAE): American Society of Echocardiography (ASE): The ASE Guidelines and Standards Committee and the ASE Board of Directors. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur J Echocardiogr. 2011;12:557–584. doi: 10.1093/ejechocard/jer086. [DOI] [PubMed] [Google Scholar]
- 62.Lang RM, Tsang W, Weinert L, Mor-Avi V, Chandra S. Valvular heart disease. The value of 3-dimensional echocardiography. J Am Coll Cardiol. 2011;58:1933–1944. doi: 10.1016/j.jacc.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 63.Sugeng L, Coon P, Weinert L, Jolly N, Lammertin G, Bednarz JE, Thiele K, Lang RM. Use of real-time 3-dimensional transthoracic echocardiography in the evaluation of mitral valve disease. J Am Soc Echocardiogr. 2006;19:413–421. doi: 10.1016/j.echo.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 64.Zamorano J, Perez de Isla L, Sugeng L, Cordeiro P, Rodrigo JL, Almeria C, Weinert L, Feldman T, Macaya C, Lang RM, Hernandez Antolin R. Non-invasive assessment of mitral valve area during percutaneous balloon mitral valvuloplasty: role of real-time 3D echocardiography. Eur Heart J. 2004;25:2086–2091. doi: 10.1016/j.ehj.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 65.Zamorano J, Cordeiro P, Sugeng L, Perez de Isla L, Weinert L, Macaya C, Rodríguez E, Lang RM. Real-time three-dimensional echocardiography for rheumatic mitral valve stenosis evaluation: an accurate and novel approach. J Am Coll Cardiol. 2004;43:2091–2096. doi: 10.1016/j.jacc.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 66.Xie MX, Wang XF, Cheng TO, Wang J, Lu Q. Comparison of accuracy of mitral valve area in mitral stenosis by real-time, three-dimensional echocardiography versus two-dimensional echocardiography versus Doppler pressure half-time. Am J Cardiol. 2005;95:1496–1499. doi: 10.1016/j.amjcard.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 67.Anwar AM, Attia WM, Nosir YF, Soliman OI, Mosad MA, Othman M, Geleijnse ML, El-Amin AM, Ten Cate FJ. Validation of a new score for the assessment of mitral stenosis using real-time three-dimensional echocardiography. J Am Soc Echocardiogr. 2010;23:13–22. doi: 10.1016/j.echo.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 68.Pepi M, Tamborini G, Maltagliati A, Galli CA, Sisillo E, Salvi L, Naliato M, Porqueddu M, Parolari A, Zanobini M, Alamanni F. Head-to-head comparison of two- and three-dimensional transthoracic and transesophageal echocardiography in the localization of mitral valve prolapse. J Am Coll Cardiol. 2006;48:2524–2530. doi: 10.1016/j.jacc.2006.02.079. [DOI] [PubMed] [Google Scholar]
- 69.Chandra S, Salgo IS, Sugeng L, Weinert L, Tsang W, Takeuchi M, Spencer KT, O'Connor A, Cardinale M, Settlemier S, Mor-Avi V, Lang RM. Characterization of degenerative mitral valve disease using morphologic analysis of real-time three-dimensional echocardiographic images: objective insight into complexity and planning of mitral valve repair. Circ Cardiovasc Imaging. 2011;4:24–32. doi: 10.1161/CIRCIMAGING.109.924332. [DOI] [PubMed] [Google Scholar]
- 70.Tamborini G, Muratori M, Maltagliati A, Galli CA, Naliato M, Zanobini M, Alamanni F, Salvi L, Sisillo E, Fiorentini C, Pepi M. Pre-operative transthoracic real-time three-dimensional echocardiography in patients undergoing mitral valve repair: accuracy in cases with simple vs. complex prolapse lesions. Eur J Echocardiogr. 2010;11:778–785. doi: 10.1093/ejechocard/jeq066. [DOI] [PubMed] [Google Scholar]
- 71.Park SM, Chai J, Jeon MJ, Lee CK, Kim DH, Park KS. 3D geometry of mitral annulus in mitral valve prolapse in comparison with normal: real-time 3D echocardiography study. J Am Coll Cardiol. 2005;45(Suppl A):281A. [Google Scholar]
- 72.Maffessanti F, Marsan NA, Tamborini G, Sugeng L, Caiani EG, Gripari P, Alamanni F, Jeevanandam V, Lang RM, Pepi M. Quantitative analysis of mitral valve apparatus in mitral valve prolapse before and after annuloplasty: a three-dimensional intraoperative transesophageal study. J Am Soc Echocardiogr. 2011;24:405–413. doi: 10.1016/j.echo.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 73.Kim DH, Handschumacher MD, Levine RA, Choi YS, Kim YJ, Yun SC, Song JM, Kang DH, Song JK. In vivo measurement of mitral leaflet surface area and subvalvular geometry in patients with asymmetrical septal hypertrophy: insights into the mechanism of outflow tract obstruction. Circulation. 2010;122:1298–1307. doi: 10.1161/CIRCULATIONAHA.109.935551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watanabe N, Ogasawara Y, Yamaura Y, Kawamoto T, Toyota E, Akasaka T, Yoshida K. Quantitation of mitral valve tenting in ischemic mitral regurgitation by transthoracic real-time three-dimensional echocardiography. J Am Coll Cardiol. 2005;45:763–769. doi: 10.1016/j.jacc.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 75.Chaput M, Handschumacher MD, Tournoux F, Hua L, Guerrero JL, Vlahakes GJ, Levine RA. Mitral leaflet adaptation to ventricular remodeling: occurrence and adequacy in patients with functional mitral regurgitation. Circulation. 2008;118:845–852. doi: 10.1161/CIRCULATIONAHA.107.749440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, Nataf P, Tornos P, Torracca L, Wenink A Task Force on the Management of Valvular Hearth Disease of the European Society of Cardiology; ESC Committee for Practice Guidelines. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 77.Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, Hagendorff A, Monin JL, Badano L, Zamorano JL European Association of Echocardiography. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease) Eur J Echocardiogr. 2010;11:307–332. doi: 10.1093/ejechocard/jeq031. [DOI] [PubMed] [Google Scholar]
- 78.Chandra S, Salgo IS, Sugeng L, Weinert L, Settlemier SH, Mor-Avi V, Lang RM. A three-dimensional insight into the complexity of flow convergence in mitral regurgitation: adjunctive benefit of anatomic regurgitant orifice area. Am J Physiol Heart Circ Physiol. 2011;301:H1015–H1024. doi: 10.1152/ajpheart.00275.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Little SH, Pirat B, Kumar R, Igo SR, McCulloch M, Hartley CJ, Xu J, Zoghbi WA. Three-dimensional color Doppler echocardiography for direct measurement of vena contracta area in mitral regurgitation: in vitro validation and clinical experience. JACC Cardiovasc Imaging. 2008;1:695–704. doi: 10.1016/j.jcmg.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Skaug TR, Hergum T, Amundsen BH, Skjaerpe T, Torp H, Haugen BO. Quantification of mitral regurgitation using high pulse repetition frequency three-dimensional color Doppler. J Am Soc Echocardiogr. 2010;23:1–8. doi: 10.1016/j.echo.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 81.Song JM, Kim MJ, Kim YJ, Kang SH, Kim JJ, Kang DH, Song JK. Three-dimensional characteristics of functional mitral regurgitation in patients with severe left ventricular dysfunction: a real-time three-dimensional colour Doppler echocardiography study. Heart. 2008;94:590–596. doi: 10.1136/hrt.2007.119123. [DOI] [PubMed] [Google Scholar]
- 82.Otani K, Takeuchi M, Kaku K, Sugeng L, Yoshitani H, Haruki N, Ota T, Mor-Avi V, Lang RM, Otsuji Y. Assessment of the aortic root using real-time 3D transesophageal echocardiography. Circ J. 2010;74:2649–2657. doi: 10.1253/circj.cj-10-0540. [DOI] [PubMed] [Google Scholar]
- 83.Muraru D, Badano LP, Vannan M, Iliceto S. Assessment of aortic valve complex by three-dimensional echocardiography: a framework for its effective application in clinical practice. Eur Heart J Cardiovasc Imaging. 2012 doi: 10.1093/ehjci/jes075. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 84.Veronesi F, Corsi C, Sugeng L, Mor-Avi V, Caiani EG, Weinert L, Lamberti C, Lang RM. A study of functional anatomy of aortic-mitral valve coupling using 3D matrix transesophageal echocardiography. Circ Cardiovasc Imaging. 2009;2:24–31. doi: 10.1161/CIRCIMAGING.108.785907. [DOI] [PubMed] [Google Scholar]
- 85.Khaw AV, von Bardeleben RS, Strasser C, Mohr-Kahaly S, Blankenberg S, Espinola-Klein C, Münzel TF, Schnabel R. Direct measurement of left ventricular outflow tract by transthoracic real-time 3D-echocardiography increases accuracy in assessment of aortic valve stenosis. Int J Cardiol. 2009;136:64–71. doi: 10.1016/j.ijcard.2008.04.070. [DOI] [PubMed] [Google Scholar]
- 86.Gutiérrez-Chico JL, Zamorano JL, Prieto-Moriche E, Hernández-Antolín RA, Bravo-Amaro M, Pérez de Isla L, Sanmartín-Fernández M, Baz-Alonso JA, Iñiguez-Romo A. Real-time three-dimensional echocardiography in aortic stenosis: a novel, simple, and reliable method to improve accuracy in area calculation. Eur Heart J. 2008;29:1296–1306. doi: 10.1093/eurheartj/ehm467. [DOI] [PubMed] [Google Scholar]
- 87.Goland S, Trento A, Iida K, Czer LS, De Robertis M, Naqvi TZ, Tolstrup K, Akima T, Luo H, Siegel RJ. Assessment of aortic stenosis by three-dimensional echocardiography: an accurate and novel approach. Heart. 2007;93:801–807. doi: 10.1136/hrt.2006.110726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Messika-Zeitoun D, Serfaty JM, Brochet E, Ducrocq G, Lepage L, Detaint D, Hyafil F, Himbert D, Pasi N, Laissy JP, Iung B, Vahanian A. Multimodal assessment of the aortic annulus diameter: implications for transcatheter aortic valve implantation. J Am Coll Cardiol. 2010;55:186–194. doi: 10.1016/j.jacc.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 89.Paelinck BP, Van Herck PL, Rodrigus I, Claeys MJ, Laborde JC, Parizel PM, Vrints CJ, Bosmans JM. Comparison of magnetic resonance imaging of aortic valve stenosis and aortic root to multimodality imaging for selection of transcatheter aortic valve implantation candidates. Am J Cardiol. 2011;108:92–98. doi: 10.1016/j.amjcard.2011.02.348. [DOI] [PubMed] [Google Scholar]
- 90.Vida VL, Hoehn R, Larrazabal LA, Gauvreau K, Marx GR, del Nido PJ. Usefulness of intra-operative epicardial three-dimensional echocardiography to guide aortic valve repair in children. Am J Cardiol. 2009;103:852–856. doi: 10.1016/j.amjcard.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 91.Mori Y, Shiota T, Jones M, Wanitkun S, Irvine T, Li X, Delabays A, Pandian NG, Sahn DJ. Three-dimensional reconstruction of the color Doppler-imaged vena contracta for quantifying aortic regurgitation: studies in a chronic animal model. Circulation. 1999;99:1611–1617. doi: 10.1161/01.cir.99.12.1611. [DOI] [PubMed] [Google Scholar]
- 92.Li X, Jones M, Irvine T, Rusk RA, Mori Y, Hashimoto I, Von Ramm OT, Li J, Zetts A, Pemberton J, Sahn DJ. Real-time 3-dimensional echocardiography for quantification of the difference in left ventricular versus right ventricular stroke volume in a chronic animal model study: Improved results using C-scans for quantifying aortic regurgitation. J Am Soc Echocardiogr. 2004;17:870–875. doi: 10.1016/j.echo.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 93.Tamborini G, Fusini L, Gripari P. Feasibility and accuracy of 3D TEE vs computed tomography in the evaluation of aortic valve annulus to left main ostium distance in patients candidates to transcatheter aortic valve implantation. J Am Coll Cardiol Img. 2012 doi: 10.1016/j.jcmg.2012.02.012. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 94.Fukuda S, Saracino G, Matsumura Y, Daimon M, Tran H, Greenberg NL, Hozumi T, Yoshikawa J, Thomas JD, Shiota T. Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation: a real-time, 3-dimensional echocardiographic study. Circulation. 2006;114(1 Suppl):I492–I498. doi: 10.1161/CIRCULATIONAHA.105.000257. [DOI] [PubMed] [Google Scholar]
- 95.Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg. 2005;79:127–132. doi: 10.1016/j.athoracsur.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 96.Anwar AM, Geleijnse ML, Soliman OI, McGhie JS, Nemes A, ten Cate FJ. Evaluation of rheumatic tricuspid valve stenosis by real-time three-dimensional echocardiography. Heart. 2007;93:363–364. doi: 10.1136/hrt.2006.093518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Badano LP, Agricola E, Perez de Isla L, Gianfagna P, Zamorano JL. Evaluation of the tricuspid valve morphology and function by transthoracic real-time three-dimensional echocardiography. Eur J Echocardiogr. 2009;10:477–484. doi: 10.1093/ejechocard/jep044. [DOI] [PubMed] [Google Scholar]
- 98.Muraru D, Badano LP, Sarais C, Soldà E, Iliceto S. Evaluation of tricuspid valve morphology and function by transthoracic three-dimensional echocardiography. Curr Cardiol Rep. 2011;13:242–249. doi: 10.1007/s11886-011-0176-3. [DOI] [PubMed] [Google Scholar]
- 99.Esposito R, Badano LP, Muraru D, Agricolà E, Mele D, Sciomer S, Nistri S, Galderisi M, Mondillo S Gruppo di Studio di Ecocardiografia della Societá Italiana di Cardiologia. [Tricuspid valve morphology and function evaluated by transthoracic real-time three-dimensional echocardiography] G Ital Cardiol (Rome) 2010;11:549–556. [PubMed] [Google Scholar]
- 100.Ton-Nu TT, Levine RA, Handschumacher MD, Dorer DJ, Yosefy C, Fan D, Hua L, Jiang L, Hung J. Geometric determinants of functional tricuspid regurgitation: insights from 3-dimensional echocardiography. Circulation. 2006;114:143–149. doi: 10.1161/CIRCULATIONAHA.106.611889. [DOI] [PubMed] [Google Scholar]
- 101.Song JM, Jang MK, Choi YS, Kim YJ, Min SY, Kim DH, Kang DH, Song JK. The vena contracta in functional tricuspid regurgitation: a real-time three-dimensional color Doppler echocardiography study. J Am Soc Echocardiogr. 2011;24:663–670. doi: 10.1016/j.echo.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 102.Kelly NF, Platts DG, Burstow DJ. Feasibility of pulmonary valve imaging using three-dimensional transthoracic echocardiography. J Am Soc Echocardiogr. 2010;23:1076–1080. doi: 10.1016/j.echo.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 103.Pothineni KR, Wells BJ, Hsiung MC, Nanda NC, Yelamanchili P, Suwanjutah T, Prasad AN, Hansalia S, Lin CC, Yin WH, Young MS. Live/real time three-dimensional transthoracic echocardiographic assessment of pulmonary regurgitation. Echocardiography. 2008;25:911–917. doi: 10.1111/j.1540-8175.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- 104.Kronzon I, Sugeng L, Perk G, Hirsh D, Weinert L, Garcia Fernandez MA, Lang RM. Real-time 3-dimensional transesophageal echocardiography in the evaluation of post-operative mitral annuloplasty ring and prosthetic valve dehiscence. J Am Coll Cardiol. 2009;53:1543–1547. doi: 10.1016/j.jacc.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 105.Thompson KA, Shiota T, Tolstrup K, Gurudevan SV, Siegel RJ. Utility of three-dimensional transesophageal echocardiography in the diagnosis of valvular perforations. Am J Cardiol. 2011;107:100–102. doi: 10.1016/j.amjcard.2010.08.051. [DOI] [PubMed] [Google Scholar]
- 106.Pérez de Isla L, Zamorano J, Malangatana G, Almería C, Rodrigo JL, Cordeiro P, Macaya C. Usefulness of real-time 3-dimensional echocardiography in the assessment of infective endocarditis: initial experience. J Ultrasound Med. 2005;24:231–233. doi: 10.7863/jum.2005.24.2.231. [DOI] [PubMed] [Google Scholar]
- 107.Kort S. Real-time 3-dimensional echocardiography for prosthetic valve endocarditis: initial experience. J Am Soc Echocardiogr. 2006;19:130–139. doi: 10.1016/j.echo.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 108.Plana JC. Three-dimensional echocadiography to assess intra-cardiac masses. In: Badano LP, Lang RM, Zamorano JL, editors. Texbook of real-time three-dimensional echocardiography. London: Springer-Verlag; 2011. pp. 111–119. [Google Scholar]
- 109.Faletra FF, Ho SY, Auricchio A. Anatomy of right atrial structures by real-time 3D transesophageal echocardiography. JACC Cardiovasc Imaging. 2010;3:966–975. doi: 10.1016/j.jcmg.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 110.Miyasaka Y, Tsujimoto S, Maeba H, Yuasa F, Takehana K, Dote K, Iwasaka T. Left atrial volume by real-time three-dimensional echocardiography: validation by 64-slice multidetector computed tomography. J Am Soc Echocardiogr. 2011;24:680–686. doi: 10.1016/j.echo.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 111.Marsan NA, Tops LF, Holman ER, Van de Veire NR, Zeppenfeld K, Boersma E, van der Wall EE, Schalij MJ, Bax JJ. Comparison of left atrial volumes and function by real-time three-dimensional echocardiography in patients having catheter ablation for atrial fibrillation with persistence of sinus rhythm versus recurrent atrial fibrillation three months later. Am J Cardiol. 2008;102:847–853. doi: 10.1016/j.amjcard.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 112.Delgado V, Vidal B, Sitges M, Tamborero D, Mont L, Berruezo A, Azqueta M, Paré C, Brugada J. Fate of left atrial function as determined by real-time three-dimensional echocardiography study after radiofrequency catheter ablation for the treatment of atrial fibrillation. Am J Cardiol. 2008;101:1285–1290. doi: 10.1016/j.amjcard.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 113.Takahashi A, Funabashi N, Kataoka A, Yajima R, Takahashi M, Uehara M, Takaoka H, Saito M, Yamaguchi C, Lee K, Komuro I, Nomura F. Quantitative evaluation of right atrial volume and right atrial emptying fraction by 320-slice computed tomography compared with three-dimensional echocardiography. Int J Cardiol. 2011;146:96–99. doi: 10.1016/j.ijcard.2010.05.069. [DOI] [PubMed] [Google Scholar]
- 114.Nucifora G, Faletra FF, Regoli F, Pasotti E, Pedrazzini G, Moccetti T, Auricchio A. Evaluation of the left atrial appendage with real-time 3-dimensional transesophageal echocardiography: implications for catheter-based left atrial appendage closure. Circ Cardiovasc Imaging. 2011;4:514–523. doi: 10.1161/CIRCIMAGING.111.963892. [DOI] [PubMed] [Google Scholar]