Abstract
Despite numerous technological and pharmacological advances and more detailed knowledge of molecular etiologies, cardiovascular diseases remain the leading cause of morbidity and mortality worldwide claiming over 17 million lives a year. Abnormalities in the synthesis, processing and catabolism of lipoprotein particles can result in severe hypercholesterolemia, hypertriglyceridemia or low HDL-C. Although a plethora of antidyslipidemic pharmacological agents are available, these drugs are relatively ineffective in many patients with Mendelian lipid disorders, indicating the need for new and more effective interventions. In vivo somatic gene therapy is one such intervention. This article summarizes current strategies being pursued for the development of clinical gene therapy for dyslipidemias that cannot effectively be treated with existing drugs.
Keywords: dyslipidemia, gene therapy, lipoprotein
Biochemical approaches and classical linkage analysis have helped to identify many of the genes that cause Mendelian monogenic dyslipidemias; these studies have enabled us to understand which proteins act together to regulate LDL, HDL and triglyceride (TG) metabolism [1]. Monogenic disorders comprise rare patient subgroups that are found at the extremes of population-specific lipoprotein distributions. Patients with monogenic disorders that result in complete deficiency of critical proteins are often refractory to pharmacologic intervention [2]. In such cases, somatic cell gene therapy offers an attractive therapeutic solution (Figure 1). Gene therapy is a technique for correcting defective genes responsible for disease development. There are several approaches for correcting faulty genes, including [3]:
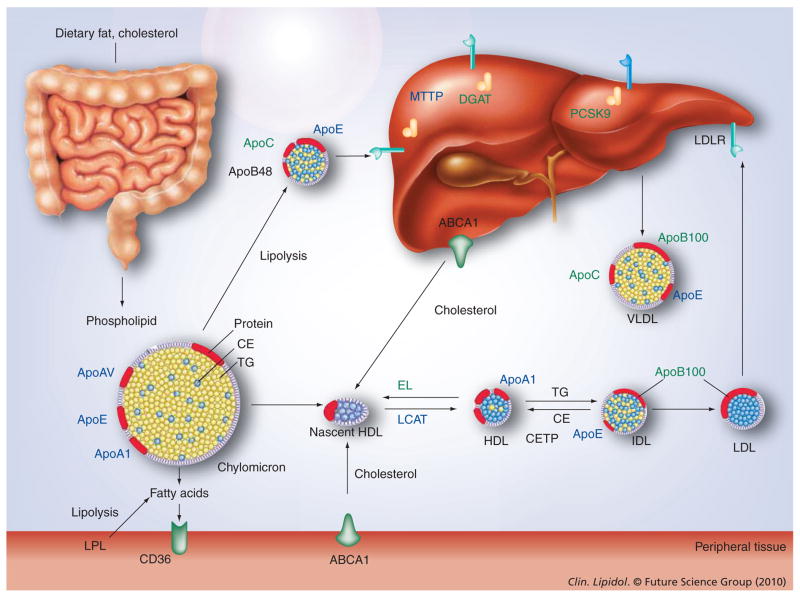
Figure 1. Molecular targets discussed in this review for gene therapy of dyslipidemia.
TG-rich lipoproteins are secreted by the intestine as chylomicrons and the liver as VLDL particles. These particles undergo lipolysis in the circulation, thereby, delivering fatty acids to tissues. Chylomicron remnants and approximately half of VLDL remnants are taken up by the liver. The remainder of the VLDL remnants are further metabolized to cholesterol-rich LDL. HDL is formed in the circulation from lipid-poor apolipoproteins secreted by liver and intestine and from surface components sloughed during lipolysis of TG-rich lipoproteins. Shown in black are candidates for gene replacement, shown in green are candidates for gene inhibition as discussed in this review.
CETP: Cholesterol-ester transfer protein; DGAT: Diacylglycerol acyltransferase; EL: Endothelial lipase; IDL: Intermediate density lipoprotein; LDLR: LDL receptor; TG: Triglyceride.
A normal gene may be inserted into a non-specific location within the genome to replace a nonfunctional gene;
An abnormal gene could be swapped for a normal gene through homologous recombination;
The abnormal gene could be repaired through selective reverse mutation, which returns the gene to its normal function;
The regulation (the degree to which a gene is turned on or off) of a particular gene could be altered.
Targets
The liver plays an important role in most metabolic pathways, and therefore, many metabolic inherited diseases, including lipoporotein disorders, originate from this organ. As the liver is a highly vascularized organ, hepatocytes are readily accessible via the blood stream. Moreover, hepatic sinusoids contain 100 nm wide fenestrations that allow macromolecules such as viral particles to cross the endothelium and reach hepatocytes. Importantly, because hepatic blood flow represents a fifth of cardiac output, any particle injected in the blood circulation can quickly reach the liver [4]. These features make the liver an attractive target for gene therapeutic strategies for lipoprotein disorders. Skeletal muscle has also been proposed as a gene therapy target for some lipoprotein disorders. Experimental evidence has demonstrated that this compartment can serve as a reservoir for production of secreted proteins, such as lipoprotein lipase (LPL) [5] or apoE [6].
Targeted delivery of genes is predicted on the ability of the vector to discriminate between target and nontarget cells via interaction with unique cell- or disease-specific surface markers. The most commonly used platforms for gene delivery are nonviral and viral vectors.
Nonviral vectors
Synthetic or nonviral vectors typically consist of DNA (usually plasmid DNA produced in bacteria) or RNA, which may be delivered to the target cell with the aid of a delivery vehicle. Delivery vehicles may be based around lipids (e.g., cationic liposomes), which fuse with the cell membrane, releasing the nucleic acid into the cytoplasm of the cell. Alternatively, peptides or polymers may be used to form complexes with the nucleic acid, which can protect the therapeutic material as it attempts to reach its target destination. Liposome–polycation–DNA nanoparticles are novel nonviral vectors that use a polymer to condense the nucleic acid and a lipid coat to aid entry to the cell [7]. In theory, synthetic vectors should be nontoxic, nonimmunogenic, stable in plasma and allow for repeated administration; yet, these systems suffer from low transduction efficiency and lack of stable transgene expression. Various biochemical modifications have been developed specifically to enhance synthetic vector targeting of hepatocytes with little success [8]. Another approach, originally developed by Wolff and colleagues, has investigated the potential of naked DNA as a gene transfer vector to the liver [9]. This strategy has been advanced by the development of hydrodynamic tail vein delivery wherein large volumes of DNA-containing solutions (~10% of body weight) are administered over short periods of time (~5–7 s in mouse) [8]. Although this method allows for more efficient transduction of hepatoyctes (up to 40%), its clinical relevance to the treatment of patients remains questionable. A more thorough discussion of nonviral vector platforms can be found here [8] and is beyond the scope of this review.
Viral vectors
Viral vectors are currently the most efficient tools to achieve efficient gene transfer to the liver in vivo [4]. Of all the clinically applicable viral vectors (i.e., oncoretroviruses, lentiviruses, herpes simplex viruses, adeno-associated viruses (AAVs) and adenoviruses [Ads]), the most extensive studies have been performed using Ads and AAVs. Although recombinant adenoviral vectors are extremely efficient in terms of delivering their genetic cargo to the cell nucleus, transgene expression is transient due to the cellular immune responses to the transduced hepatocytes [10]. These immune responses are directed against both adenoviral proteins and the transgene product [11]. Many strategies have been devised to overcome the immune rejection associated with early-generation recombinant adenoviral vectors, including immunomodulatory treatments or modification of the vector itself to reduce immune response [12,13]. However, there remains little justification for the use of adenoviruses for the treatment of lipoprotein disorders.
Helper-dependent Ads (HD-Ads) that are deleted for all viral genes represent an important advance to decrease immunogenicity and prolong transgene expression. Although HD-Ad vectors elicit reduced host-adaptive immune responses and demonstrate improved gene transfer efficiency compared with Ad vectors, it is impossible to avoid innate responses after vector administration in vivo [14]. In fact, it is the innate immune response to the adenoviral capsid that is associated with toxicity in humans, which can be lethal. Furthermore, the large-scale production of these vectors remains a practical problem [4].
Adeno-associated virus vectors represent one of the most promising platforms for transducing the liver and muscle in vivo. AAV is a nonenveloped, icosahedral, single-stranded DNA virus that is 20–26 nm in diameter. Many studies have demonstrated that AAV vectors are able to transduce hepatocytes for prolonged periods of time in animal models of various sizes. These vectors are devoid of all viral genes and therefore induce minimal immune response; moreover, AAV is unique among viral vectors for gene therapy in that the wild-type virus has never been shown to cause human disease. Importantly, AAV vectors are relatively easy to prepare and clinical trials using this vector type have been performed or are still ongoing [4].
AAV serotype 2 (AAV2) is the first AAV that was vectored for gene transfer applications. Several limitations of AAV2 vectors have emerged including low hepatocyte transduction efficiency, high seroprevalence of neutralizing antibodies in humans, and potentially destructive T-cell responses to capsids [15,16]. In an attempt to search for potent AAV vectors with enhanced performance profiles, molecular techniques have been used for the detection and isolation of endogenous AAVs from a variety of human and nonhuman primate tissues. Using this approach, our group was able to isolate novel primate AAVs consisting of over 120 nonredundant species of proviral sequences [17]. Of these, a portfolio of 30 AAV vector candidates based on novel capsids had been established. Based on extensive studies examining toxicity, biodistribution, transgene and capsid T-cell activation, and pre-existing immunity in human populations, AAV8 has emerged as the lead vector for liver-directed gene therapy application [18,19]. AAV1, AAV6 and AAV7 have been shown to perform well in skeletal muscle, demonstrating rapid onset and high levels of transduction [17,20–22].
A major limitation of these vectors is their packaging capacity (~4.5 kb), which precludes the design of vectors for the treatment of diseases associated with larger genes. However, this should not affect gene therapy for dyslipidemia because many of the lipid-related genes (Figure 1), including the lipases and apolipoproteins, are smaller than 4.5 kb. Another practical problem is the relatively long latent period between vector delivery and peak transgene expression (~2–4 weeks). This lag period occurs because conventional recombinant AAV vectors deliver a ssDNA genome, which must be converted to dsDNA before the transgene is expressed [23]. The problem has been solved in part by using self-complementary (sc) vectors, which package an inverted repeat genome that can fold into dsDNA without the requirement for DNA synthesis or base pairing between multiple vector genomes. However, this modification results in the loss of half of the coding capacity of the vector, although small protein-coding genes (up to 2.2 kb), and any currently available RNA-based therapy, can be accommodated [23].
Gene inhibition approaches
Whereas traditional gene therapy is concerned with the replacement of defective genes for inherited and acquired diseases, recent strategies have been designed to specifically and selectively inhibit molecular targets involved in disease pathology. This latter approach makes use of small oligonucleotides, molecules that function in natural cellular processes involving the intermediary metabolism of mRNA, and can be used to induce the suppression of disease-associated gene products. Several recent studies have examined the use of this approach for lipoprotein disorders (reviewed later).
Gene inhibitions strategies can be grouped into two broad categories based on mechanisms of actions – antisense oligonucleotides (ASOs) and RNAi. ASOs are ssDNA molecules, usually 20 nucleotides in length, with a sequence designed to be complementary to an mRNA transcript. Specific base-pairing interactions between the ASO and target mRNA result in the RNase H-mediated degradation of the transcript and a reduction in the protein product of the targeted gene. RNase H is thought to play a role in DNA replication and transcription and is ubiquitously and constitutively active in mammalian cells. As unmodified ASOs, such as natural DNA and RNA, are subject to rapid degradation by ubiquitous cellular nucleases, their utility as therapeutic agents is severely limited. Second-generation ASOs that have been modified to enhance the stability or bioactivity and alter the various physiochemical properties of the molecule represent a viable alternative [24].
RNAi is an endogenous molecular pathway that functions in antiviral defense and gene regulation. This pathway has been exploited as a tool to silence the expression of specific target genes for experimental or therapeutic benefit [25]. Two types of molecules have been extensively used for RNAi applications: chemically synthesized double-stranded siRNA or vector-based shRNA. Although siRNA and shRNA can be applied to achieve similar functional outcomes, they are intrinsically different molecules that employ different mechanisms of action. SiRNAs are loaded directly into the cell’s RNA-induced silencing complex (RISC) where they guide hybridization to the target mRNA. Binding of an activated RISC complex to a target transcript leads to transcript degradation or inhibition of translation. By contrast, expressed shRNA is transcribed in cells from a DNA template as an ssRNA molecule, which are 50–100 bases in length. Complementary regions spaced by a small loop cause the transcript to fold back on itself forming a ‘short hairpin’ in a manner analogous to natural miRNA. The shRNA is subsequently processed by an endoribonuclease known as Dicer and converted into the corresponding siRNA, which is incorporated into the RISC [26].
Antisense oligonucleotides and siRNA molecules face similar pitfalls, including delivery, stability and off-target effects. To achieve systemic and targeted delivery, ASOs and siRNAs are typically delivered within liposomes or conjugated to antibodies, cholesterol, RNA aptamers or peptides [27]. In addition, these approaches require repeated administration of the biological product. Compared with siRNAs and ASOs, shRNAs offer advantages in silencing longevity, delivery options and cost. However, in some cases, constitutive systemic shRNA expression may cause significant complications. In 2006, Grimm and colleagues examined the long-term effects of sustained high-level shRNA expression in livers of adult mice [28]. Using an optimized shRNA delivery vector based on duplex-DNA containing AAV8, the authors were able to achieve robust shRNA expression in hepatocytes after a single intravenous injection. Of the 49 distinct AAV or shRNA vectors that were evaluated in the study, 36 candidate vectors resulted in dose-dependent liver injury and 23 candidate vectors caused death in mice. The authors were able to demonstrate that high-level shRNA expression can saturate and interfere with components of the endogenous miRNA pathway, which may have been the cause of toxicity and morbidity. Subsequent studies have demonstrated that using a tissue-specific promoter to drive shRNA expression, these issues may be overcome [29].
It should be noted that monocolonal antibody strategies, directed against many of the same targets as the gene inhibition approaches are actively being developed for potential clinical application [30].
Disorders associated primarily with altered levels of LDL-C
Gene transfer strategies for reducing LDL-C
Familial hypercholesterolemia
Familial hypercholesterolemia (FH), the most common and most severe form of monogenic hypercholesterolemia, was the first genetic disease of lipid metabolism to be clinically and molecularly characterized [31]. Homozygous FH (HoFH) is a serious life-threatening genetic disease caused by homozygosity or compound heterozygosity for mutations in the LDL receptor (LDLR). This disease formally qualifies as an orphan disease in that it occurs with a frequency of approximately one in a million individuals. Although, it should be noted that HoFH has a much higher incidence in certain populations, such as French–Canadians, Finns, Afrikaners and Christian Lebanese, due to founder effects [32–34].
Patients with HoFH are classified into one of two major groups based on the amount of LDLR activity measured in their skin fibroblasts: patients with less than 2% of normal LDLR activity (receptor-negative), and patients with 2–25% of normal LDLR activity (receptor-defective) [35]. In general, total plasma cholesterol levels are over 500 mg/dl and markedly premature arteriosclerotic cardiovascular disease is the major consequence. Untreated, most patients develop atherosclerosis before 20 years of age [36] and generally do not survive past 30 years. Unfortunately, patients with HoFH are minimally responsive or unresponsive to conventional drug therapy, and thus, there are limited treatment options. Several nonpharmacological options have also been tested. Surgical interventions, such as portacaval shunt and ileal bypass, have resulted only in partial and transient LDL-C lowering [37]. Orthotopic liver transplantation has been demonstrated to substantially reduce LDL-C levels in HoFH patients, but obvious disadvantages and risks are associated with this approach [38]. The current standard of care in HoFH is LDL apheresis, a physical method of purging the plasma of LDL-C, which can transiently reduce LDL-C by more than 50% [39]. However, there is rapid reaccumulation of LDL-C in plasma, and therefore apheresis has to be repeated frequently (every 1–2 weeks) and requires two separate sites for intravenous access. Although anecdotally this procedure may delay the onset of atherosclerosis [40], it is laborious, expensive and not readily available. Furthermore, the procedure needs frequent repetition and intravenous access can be challenging for many patients [39]. Therefore, there is a tremendous unmet medical need for new therapies for this disease.
For these reasons, HoFH is an excellent disease model for the development of liver-directed gene therapy. Initial attempts to treat FH with gene therapy utilized an ex vivo approach wherein autologous hepatocytes transduced with retroviruses containing LDLR cDNA were transplanted into HoFH patients (Table 1) [41,42]. Although this approach was well tolerated by patients, the impact on cholesterol metabolism was modest and variable, due in part to the limited amount of gene transfer achievable [41,42]. More recently, attention has focused on the potential of liver-directed in vivo gene therapy for HoFH. A considerable number of proof-of principle studies using gene transfer of the LDLR have been performed. These efforts have been facilitated by the existence of animal models for HoFH including:
Table 1.
Clinical trials involving gene replacement and gene inhibition for dyslipidemia conducted to date.
| Disease | Platform/name of therapy | Gene target | Primary sponsor | Status of trial/clinical trial number | Ref. |
|---|---|---|---|---|---|
| HoFH | Ex vivo gene therapy | LDLR replacement | University of Pennsylvania | Completed/NCT00004809 | [41,42] |
| HoFH | ASO/ISIS 301012 | ApoB inhibition | Genzyme/ISIS | Completed/NCT00607373 | [58] |
| HetFH | ASO/ISIS 301012 | ApoB inhibition | Genzyme/ISIS | Completed/NCT00281008, NCT 00362180 | [61,62] |
| HoFH | siRNA/PRO-040201 | ApoB inhibition | Tekmira | Completed/NCT00927459 | [65,201] |
| LPL deficiency | AAV1/AMT-011 | LPL replacement | Amsterdam Molecular Therapeutics | Ongoing/NCT00891306 | [145] |
AAV: Adenoassociated viruses; ASO: Antisense oligonucleotide; HetFH: Heterozygous famial hypercholesterolemia; HoFH: Homozygous hypercholesterolemia; LDLR: LDL receptor; LPL: Lipoprotein lipase.
The Watanabe heritable hyperlipidemic rabbit, which is homozygous for a natural mutation in LDLR and develops hypercholesterolemia on a standard chow diet;
The Ldlr−/− mouse, which develops severe hypercholesterolemia when fed a high-fat diet;
Second-generation models such as Ldlr−/−Apo-bec1−/− and Ldlr−/−Human APOB+/+ mice that develop hypercholesterolemia on a standard chow diet [43,44].
In addition to these models of HoFH, the ApoE−/− mouse has also been used for studies of spontaneous hypercholesterolemia [43]. ApoE is a ligand for receptors that clear remnants of chylomicrons and VLDLs. ApoE-deficient mice have five-times higher than normal plasma cholesterol, and develop foam cell-rich depositions in their proximal aortas by 3 months of age. The phenotype of these mutants has made them valuable for investigating genetic and environmental factors that modify the atherogenic process [45].
Injection of a first-generation Ad encoding human LDLR transiently reduced LDL-C levels in fat-fed LDLR-deficient mice [46] and WHHL rabbits [47]. Adenoviral-mediated overexpression of LDLR was transient due in part to immune-mediated elimination of the vector-transduced hepatocytes. Specifically, cytotoxic T lymphocytes were detected against both the LDLR transgene and viral-encoded proteins [46,47]. Subsequent studies with AAV-expressing LDLR under the control of a ubiquitous promoter also resulted in only transient reduction of plasma cholesterol owing to immune activation of the transgene and loss of liver-associated vector DNA [48]. Later studies have demonstrated that expression of LDLR under the control of a liver-specific promoter results in less pronounced immune responses against newly synthesized LDLR and provides long-term reduction in the cholesterol levels of Ldlr−/− [49], Ldlr−/−Apobec1−/− and WHHL rabbits [50]. This has been demonstrated for AAV [49], HD-Ad [51] and lentiviral vector-mediated gene transfer [50]. Several of these vector-based approaches have demonstrated significant reductions in the development of atherosclerosis [49,51,52], with a recent AAV8 study demonstrating dramatic regression of advanced lesions as well [53]. Recently, Hibbitt and colleagues have demonstrated the feasibility of somatic delivery and long-term expression of the genomic DNA LDLR transgene (>100 kb) in vivo following hydrodynamic tail vein injection [54]. The authors postulate that this vector system may provide physiological regulation of LDLR expression [55], although the efficacy of this gene transfer approach has yet to be examined in an animal model of HoFH.
Antisense approaches to decrease LDL-C
More recent strategies have focused on reducing total cholesterol levels by inhibiting LDL synthesis and/or secretion by the liver [56]. The first of these knockdown approaches utilized an ASO specific for apoB-100. Crooke and colleagues found that intraperitoneal injection of murine-specific apoB100 ASO resulted in significant reduction of apoB-100 (>80%), total cholesterol (25–55%) and LDL-C (40–88%) in several models of hyperlipidemia, including fat-fed Apoe−/−-deficient and Ldlr−/− mice [57]. These effects were sustained for up to 6 weeks after cessation of dosing and resulted in no detectable elevations of liver transaminases or hepatic steatosis.
Clinical trials of the human version of apoB-100 ASO, mipomersen (ISIS 30102, see Table 1), have shown significant LDL-C reductions in patients with homozygous [58] and heterozygous FH [59,60] on maximally tolerated lipid-lowering therapy. The Phase III randomized double-blind study in patients with homozygous FH reported a significant reduction in the LDL-C concentrations of patients treated with mipomersen at a dose of 200 mg/week for 26 weeks (−24.7% reduction) compared with those treated with placebo (−3.3% reduction) [58]. A Phase II randomized dose-escalation trial in patients with heterozygous FH reported comparable efficacy [61]. After 6 weeks of treatment with mipomersen, LDL-C was reduced by 21% in those receiving a dose of 200 mg/week and by 34% in those receiving a dose of 300 mg/week [61]. In another Phase II study in patients receiving stable statin therapy, treatment with mipomersen at 200 mg/week for 13 weeks resulted in a 35.8% reduction in LDL-C [62].
Adverse events observed in these clinical trials included injection site reactions and increases in liver transaminases. In the Phase III clinical trial in HoFH, an increase in liver transaminases more than three-times the upper limit of normal occurred in only four patients (12%) in the mipomersen group, of whom one showed a large increase of hepatic fat and reduction in LDL cholesterol [58]. These studies suggest that ASO-mediated inhibition of apo B synthesis with mipomersen is a promising new treatment option for HoFH and severe refractory hypercholesterolemia.
RNAi has also been utilized to silence genes involved in hypercholesterolemia. Inhibition of apoB expression has been observed in mice [63] and NHPs [64] after short-term treatment with an apoB siRNA cholesterol conjugate resulting in significant reductions in apoB protein, serum cholesterol and LDL levels as early as 24 h after treatment. This phenotypic effect lasted for up to 11 days at the highest siRNA dose examined in the NHP studies [64]. To date, one Phase I clinical trial has been conducted using apoB-specific siRNA in hypercholesterolemic patients (Table 1) [65,201]. Seven different dosing levels were examined. Of the two subjects treated at the highest dose level, one subject experienced flu-like symptoms consistent with stimulation of the immune system caused by the apoB siRNA payload. The other subject treated at the highest dose level experienced no side effects. Of the two subjects treated at the highest dose, the average transient reduction of apoB protein and LDL-C was 21.1 and 16.3%, respectively. Based on the potential for the immune stimulation to interfere with further dose escalation, the clinical trial was halted in January 2010 [201].
Another attractive target for gene silencing is proprotein convertase subtililisn-like kexin type-9 (PCSK9) [66]. This protein regulates LDLR protein levels and function by binding to the LDLR and targeting it for lysosomal degradation. Gain-of-function mutations in PCSK9 cause severe hypercholesterolemia resembling HoFH [67], whereas loss-of-function mutations in PCSK9 reduce plasma LDL-C levels and risk of coronary disease [68]. Frank-Kamenetsky and colleagues have shown that intravenous delivery of PCSK9 siRNA formulated in lipidoid nanoparticles leads to liver-specific silencing of PCSK9 in mice and rats associated with up to a 60% reduction in plasma cholesterol concentrations [69]. Likewise, a single intravenous dose of PCSK9-specific siRNA in NHPs resulted in a rapid and reversible lowering of plasma LDL-C (−56%), without measurable effects on either HDL-C or TGs. The authors reported that the effects of PCSK9 silencing lasted for 3 weeks after a single bolus intravenous administration [69].
Homozygous FH will continue to be a model for the development of liver-directed somatic gene therapy. Since many heterozygous FH patients are also relatively refractory to existing drug therapy and remain at very high risk for the development and progression of atherosclerotic cardiovascular disease, a gene therapeutic strategy found to be effective in HoFH may also be extended to clinical trials in severe heterozygous FH [53]. Based on the positive preclinical and clinical data attained for both gene transfer and gene inhibition strategies, it is predicted that gene therapy will be a viable option for HoFH in the not too distant future.
Disorders associated primarily with altered levels of HDL-C
HDL particles transport excess cholesterol from peripheral cells to the liver for excretion into bile; this process is termed reverse cholesterol transport (RCT) and is opposite to the movement of cholesterol from the intestine and liver to peripheral tissues that is mediated by chylomicrons, VLDL, intermediate density lipoprotein and LDL particles. Population studies have demonstrated a highly consistent, inverse correlation between plasma concentrations of HDL-C and atherosclerotic cardiovascular disease risk in humans [70]. The cardioprotective effects of HDL-C have been attributed to its role in RCT, its effect on endothelial cells, its anti-oxidant activity and its anti-inflammatory properties [71]. However, the ‘HDL hypothesis’ – that raising HDL-C levels has beneficial therapeutic effects – remains difficult to prove in humans owing to the lack of interventions that substantially increase HDL-C concentrations. The treatment of low HDL-C remains controversial, in part because the only currently available effective medication, niacin, is poorly tolerated and outcomes studies on cardiovascular disease prevention are still pending [71]. In this respect, gene therapy strategies may be able to provide proof-of-principle evidence in subjects suffering from monogenic disorders of HDL metabolism.
Low levels of HDL-C, or hypoalphalipoproteinemia (HA), includes a variety of conditions, ranging from mild to severe, in which concentrations of HDL-C are reduced. There is no clear-cut definition for HA. An arbitrary cutoff is the tenth percentile of HDL-C levels; a more practical definition derives from the theoretical cardioprotective role of HDL as outlined by the US National Cholesterol Education Program (NCEP) Adult Treatment Panel III [202]. According to NCEP, HDL-C levels below 40 mg/dl constitute a formal coronary heart disease risk factor for men and women. The common, mild forms of HA have no characteristic physical findings, but patients may have premature coronary heart or peripheral vascular disease, as well as a family history of low HDL-C levels and premature CHD [203].
HDL biosynthesis is a complex multistep process that involves the synthesis and secretion of the major protein components of HDL followed by the largely extracellular acquisition of lipid (phospholipids and cholesterol) and the assembly and generation of the mature HDL particle. Mutations in one of three key proteins – APOA-I, ABCA1 and lecithin cholesterol acyltransferase (LCAT) – underlie deficits in HDL synthesis and maturation and account for the majority of monogenic HA disorders [1].
APOA1 gene replacement
The major HDL apolipoprotein, apoA-I, constitutes 70% of HDL protein and is required for normal HDL biosynthesis. Gene deletion of apoA1/APOA1 results in extremely low levels of HDL-C humans and mice [72]. More than a dozen functionally significant mutations of the APOA1 gene have been described, including gene disruptions, nonsense mutations, frame-shifts, missense mutations, chromosomal aberrations or deletions, all of which are typically associated with decreased HDL-C levels [1,72].
ApoA1-knockout mice have extremely low levels of HDL-C and when crossed onto atherosclerosis-susceptible strains develop significantly increased atherosclerosis [73,74]. Although APOA1-deficient patients are rare, gene transfer of APOA1 to liver is expected to reconstitute HDL formation and if sustained may reduce the risk of progression of atherosclerotic disease. Many animal studies have demonstrated the feasibility of this approach. Initial studies using first- and second-generation adenoviral constructs have demonstrated that liver-directed gene transfer of the human APOA1 gene results in the elevation of HDL-C [75], inhibits neointima formation in the common carotid artery of atherosclerosis prone ApoE-knockout mice [76], reduces athero-sclerotic burden [77,78] and regresses pre-existing atherosclerotic lesions in fat-fed Ldlr−/− mice [79]. Although these studies demonstrated robust expression levels of APOA1 in plasma ranging between two- and five-times higher than human physiological levels (i.e., 200–500 mg/dl) in various mouse models, because they were using Ad vectors, expression could only be maintained for a maximum of 4 months, at best [77,80]. More recent studies using an HD-Ad vector based strategy for gene transfer of human apoA-I have demonstrated prolonged production of HDL-C, up to 24 weeks for HD-Ad, with minimal hepatotoxicity and significant reduction of the atherosclerotic burden in both Ldlr−/− and ApoE−/− mice [81,82].
In 2005, Sharifi et al. demonstrated the feasibility of AAV-mediated APOAI gene transfer in Apoa1-deficient mice [83]. Subsequent studies by Kitajima et al. revealed that AAV5 and AAV1 vectors are significantly more effective than AAV2 and result in the sustained production of therapeutic levels of APOA1 in mice for up to 1 year after vector administration [84]. Recently, Vaessen et al. performed head-to-head comparisons of recombinant AAV-1, -2, -6 and -8 administered by different routes with the use of five different liver-specific promoters in addition to cytomegalovirus as single-stranded or as sc AAV vectors. They found that intravenous administration of scAAV8 results in the highest levels of human APOA1 expression in female Apoa1-deficient mice (634 mg/dl), which persisted for the duration of the study (15 weeks) [85]. Cimmino and colleagues have extended upon these findings and have recently demonstrated stable long-term plasma levels of APOA1 in deficient mice after portal vein or muscle injection of AAV8. Both routes of administration lead to similar protein levels of human APOA1 (60 mg/dl) [86].
Patients presenting with the Milano mutation of APOA1, whereby an arginine residue is substituted for a cysteine residue at position 173, have significantly lower plasma HDL levels yet, paradoxically, do not develop premature atherosclerosis [87,88]. Although APOA1 Milano (APOA1-M) has been studied extensively with regard to effects on atherosclerosis, including infusion and genetic expression in animals and even in a clinical trial of repeated intravenous infusion of an a APOA-IM–phospholipid complexes in patients with CHD, the mechanism by which APOA1-M offers atheroprotection remains unclear [89]. A systematic comparison by our group found that liver-directed AAV8-mediated gene transfer of APOA1 or APOA1-M in fat-fed Ldlr−/− mice significantly reduces atherosclerosis progression to a similar extent [90]. Subsequent gene transfer studies comparing APOA1 and APOA1-M found that both isoforms are equally efficient at promoting macrophage RCT, as measured by an in vivo assay [91]. Collectively, these results suggest that if APOA1-M is more atheroprotective than APOA1 it is not attributable to an enhancement of macrophage RCT.
ABCA1 gene replacement
ATP-binding cassette transporter A1 (ABCA1) mediates the efflux of cholesterol from peripheral tissues to plasma APOA1. Mouse studies have shown that both hepatic and extrahepatic ABCA1 contribute to HDL formation. Loss-of-function mutations in both alleles of the ABCA1 gene result in Tangier disease, characterized by profoundly decreased HDL-C, APOA-I and APOA2 levels, reduced total and LDL-C and APOB, and elevated plasma TG levels [92]. Tangier disease patients are characterized by nearly absent HDL, orange tonsils, cloudy corneas, intermittent peripheral neuropathy and an increased risk for atherosclerosis [93]. The prevalence of Tangier disease is approximately one in 120,000,000; approximately 24 cases of Tangier disease have been diagnosed worldwide [94,95].
Modulation of macrophage ABCA1 in mice in vivo has been shown to affect the initiation and progression of atherosclerosis [96,97]. It is generally accepted that ABCA1 promotes the efflux from cells, including macrophages, to lipid-poor APOA1-containing particles, which are HDL precursors present in plasma. In 2003, Wellington and colleagues demonstrated that Ad vector-mediated hepatic ABCA1 expression in C57Bl/6 mice leads to dose-dependent increases in HDL-C [98]. Doses exceeding 5 × 108 plaque forming units did not further increase HDL-C but did unexpectedly lead to increases in total cholesterol, TGs, phospholipids and apoB levels in plasma. Subsequent studies have demonstrated that hepatic overexpression of ABCA1 accelerates the progression of atherosclerosis in Apoe−/− mice [99] and Ldlr−/− mice [100]. Collectively, these results suggest that while ABCA1 overexpression in macrophages exerts a clear atheroprotective effect, overexpression of ABCA1 in the liver may be less desirable and may exacerbate cardiovascular disease. However, it should be noted that there are currently no published studies examining the effects of somatic ABCA1 gene replacement in Abca1-deficient mice. Therefore, it will be important for future studies to examine whether gene transfer of ABCA1 to macrophages or liver within the context of ABCA1 deficiency confers any therapeutic benefit.
LCAT gene replacement
Lecithin cholesterol acyltransferase deficiency and the related fish-eye disease are caused by mutations in the LCAT gene that result, respectively, in complete or partial deficiency of LCAT in plasma. LCAT mediates the esterification of free cholestesterol on plasma lipoporoteins; this important step converts discoidal, nascent HDL into mature spherical HDL particles. Many different LCAT mutations have been reported, although the phenotypes cannot be predicted by the nature of mutation or position in the gene. The prevalence of LCAT deficiency is less than 1/1,000,000 [204]. LCAT deficiency leads to marked reductions in HDL-C levels (5–10% of normal), reduced or normal LDL-C levels and hypertriglyceridemia. Complete LCAT deficiency is characterized clinically by corneal opacities, anemia, progressive proteinuria and renal insufficiency, eventually leading to end-stage renal disease. This is a serious disorder that has no known therapy [101]. A mouse model of this disease has been created and although the lipoprotein phenotype of this animal is similar to LCAT-deficient patients, it does not develop renal disease [102]. Few efforts have been made to develop a gene therapeutic strategy for treating LCAT deficiency. To date, there are no published studies examining the efficacy of LCAT gene transfer in an LCAT-deficient animal model.
It has been suggested that gene transfer to increase plasma LCAT could have therapeutic values in settings other than LCAT deficiency [103]. As LCAT converts unesterified to esterified cholesterol, it is believed to facilitate RCT and is therefore considered to be antiatherogenic. Transgenic overexpression of human LCAT has been shown to increase HDL-C levels in cholesterol-fed transgenic mice, cholesterol-fed rabbits and WHHL rabbits. Using a first-generation Ad construct for LCAT gene transfer in human APOA1 transgenic mice, Seguret-Mace et al. demonstrated that a strong increase in plasma LCAT activity leads to a significant increase in HDL-C with a concomitant rise in human APOA1 [104]. Subsequent studies by Mertens et al. demonstrated that first-generation adenovirus expressing LCAT can reduce the atherosclerotic burden of leptin-deficient (ob/ob) Ldlr−/− mice [105]. Interestingly, the increase of LCAT activity in these studies did not result in changes in HDL-C levels [106]. The authors ascribed the observed protection from atherosclerosis to the prevention of LDL oxidation caused by the increased LCAT activity. Recently, Van Craeyveld et al. examined Ad-mediated gene transfer of rabbit APOA1 or LCAT in cholesterol-fed heterozygous LCLR-deficient rabbits [107]. The authors found that this strategy leads to significant increases in HDL-C, inhibits the progression of atherosclerosis and induces cholesterol unloading in complex lesions in rabbits.
Gene inhibition approaches to increase HDL-C
There are several potentially attractive targets for antisense approaches to increase HDL-C. Among these is cholesterol-ester transfer protein (CETP). This protein mediates the transfer of esterified cholesterol from HDL to VLDL and transfer of triacylglycerol from VLDL particles to HDL. CETP deficiency is typically associated with elevated HDL-C levels and normal or low LDL-C levels [107]. Complete loss of CETP activity due to mutations in the CETP gene can result in up to fivefold the normal HDL-C levels and the accumulation of large cholesterol ester and apoE-rich HDL species. Some Japanese individuals have extremely high concentrations of HDL-C; this result was found to be due to a genetic deficiency in CETP [107]. This finding led to the development of CETP inhibitors. The first CETP inhibitor (torcetrapib) to advance to a Phase III clinical trial was terminated owing to increased mortality and cardiovascular events. The failure of torcetrapib has been attributed to off-target effects [108]. Antisense oligonucleotides or RNAi represent an attractive alternative approach for CETP inhibition, which may be able to minimize untoward off-target effects [109].
Another target that has been proposed for inhibition is endothelial lipase (EL) [107], a member of the extravascular lipase gene family including LPL. EL specifically plays a major role in HDL metabolism. Animal studies have clearly shown that hepatic overexpression of EL in mice results in markedly reduced HDL-C and APOA1 levels [110]. Antibody inhibition or gene deletion of EL in mice results in increased HDL-C levels [111,112]. Loss-of-function mutations in EL in humans are associated with increased HDL-C levels [113], supporting the concept that reduction of EL activity in humans would be expected to raise HDL-C levels. However, no proof-of-principle studies have been reported as to whether this strategy will actually work in vivo.
Disorders associated primarily with altered levels of TGs
Hypertriglyceridemia is a common disorder in the industrialized world, which can result from the overproduction of triglyceride-rich lipoproteins or from attenuated hydrolysis and subsequent clearance of these lipoproteins. Hypertriglyceridemia is defined as severe when the levels of fasting plasma TGs are greater than 500 mg/dl [202]. Severe hypertriglyceridemias are also referred to as hyperchylomicronemia syndromes, owing to plasma accumulation of chylomicrons in the fasting state. Mutations in several genes have been implicated in severe hypertriglyceridemia, including:
LPL gene replacement
Lipoprotein lipase is the major enzyme that hydrolyzes circulating TG-rich lipoprotein such as VLDLs and chylomicrons. A decrease in LPL activity is associated with an increase in plasma TGs and decrease in HDL-C, both of which are major risk factors of coronary heart disease. Complete LPL deficiency is an extremely rare (frequency ~1/1,000,000) autosomal recessive disease resulting from homozygosity or compound heterozygosity for mutant LPL. Many different LPL mutations have been identified to date [117]. Patients with LPL deficiency suffer from the frequent recurrence of acute or chronic pancreatitis that may lead to death. The overall mortality of acute pancreatitis is approximately 5%; rising to 17% in patients with necrotising pancreatitis and increasing to 30% in those with infected necrosis [118]. Strict adherence to a diet severely restricted in fat content is the only means by which patients can limit pancreatitis attacks. Compliance is difficult, as the very-low-fat diet is extremely unpalatable. As current treatment strategies are often insufficient in preventing hospitalization and possible death due to acute hemorrhagic pancreatitis, familial LPL deficiency is a particularly attractive target for gene therapy.
In 1997, Ashbourne and colleagues demonstrated that Ad-mediated gene transfer of LPL in the liver of heterozygous LPL-deficient mice results in the secretion of catalytically active LPL, which leads to a significant decrease in plasma TG for up to 42 days [119]. Liu et al. subsequently demonstrated that Ad-mediated expression of LPL in a naturally occurring animal model, the LPL-deficient cat, leads to a substantial 90% reduction of plasma TG for up to 2 weeks [120]. Unfortunately, a potent antibody response against both the vector and LPL transgene product was mounted, which may have led to the observed transient correction. Subsequent studies by these investigators made use of AAV1 to express a naturally occurring variant of human LPL transgene (LPLS447X, a truncated version of the LPL protein that lacks the C-terminal serine and glycine and is carried by 20% of the general population). The LPLS447X variant has consistantly been associated with lower TG and higher HDL-C levels in candidate gene association studies [121–123]. Intramuscular delivery of this vector construct was shown to partially restore LPL activity levels and resulted in a near-complete normalization of plasma TG, HDL-C, total cholesterol and free fatty acid levels for over 1 year in LPL-deficient mice [5]. This same strategy was effective in the LPL-deficient cat but again was only transient (i.e., up to 2 weeks) [124]. Based on these promising preclinical results, an open-label dose-escalation clinical trial was undertaken (Table 1). AAV1-encoding LPLS447X was administered into the limb muscles of subjects suffering from LPL deficiency [125]. A transient decrease in plasma TG levels was observed in some of the subjects. However, the limited number of subjects that participated in this study coupled with the observed dietary fluctuations precludes definitive conclusions regarding the efficacy of this trial. Importantly, the authors note that none of the subjects developed B- or T-cell responses to the LPL transgene product; four of eight subjects did, however, develop detectable capsid-specific T cells that appeared with dose-dependent kinetics [125]. Further investigation is required to determine whether these capsid-specific responses curtailed long-term transgene expression.
ApoAV gene replacement
Homozygous mutations in APOAV have been reported to cause severe hypertriglyceridemia in both humans and mice [126]. APOAV is naturally expressed in the liver and appears to augment LPL-mediated hydrolysis of VLDL and chylomicron triglycerides by binding these lipoproteins to the proteoglycans on the vascular endothelium, thereby bringing them closer to LPL. APOAV may also directly modulate LPL activity by stabilizing its dimeric structure [127]. Van der Vliet et al. demonstrated that treating APOAV-deficient mice with an Ad expressing human APOAV results in a 70% decrease in plasma TG levels and concomitant cholesterol-lowering in all lipoprotein classes [128]. Subsequent studies by Huang and colleagues demonstrated that Ad-mediated expression of APOAV in Apoe-deficient mice also results in significant reductions of plasma cholesterol (58% decrease) and triglycerides (75% decrease) within 7 days of gene transfer [129].
Antisense approaches to decrease TGs
APOCIII is synthesized in the liver and is thought to inhibit the clearance of TG-rich lipoproteins by noncompetitively inhibiting LPL. APOCIII transgenic mice have high levels of plasma TGs owing to an impaired catabolism of TG-rich lipoproteins; by contrast, APOC-III-deficient mice have reduced levels of plasma TG [130]. Preclinical data suggest that direct inhibition of APOCIII would have beneficial effects. Crooke and colleagues have reported that systemic administration of an APOCIII ASO safely and significantly reduced serum and liver TGs (>80 and >95%, respectively) and was able to ameliorate steatosis in fat-fed C57Bl/6 mice [56].
Another key enzyme involved in the synthesis of triglycerides is acyl-coenzyme A:diacylglycerol acyltransferase (DGAT), which catalyzes the last step in mammalian triglyceride synthesis via the covalent binding of the acyl moiety with diacylglycerol. DGAT is distributed throughout the body and has a high expression in fat, liver and small intestine [131]. Two DGATs encoded by two different gene families have been identified: DGAT1 and DGAT2. In 2005, Yu et al. found that treating high-fat diet-induced obese C57BL/6J mice or ob/ob mice treated with DGAT2-specific ASOs causes a marked reduction in hepatic triglyceride content and improved hepatic steatosis in both models [132]. Subsequent studies by Liu and colleagues found that treatment with DGAT2-specific ASOs results in a dose-dependent decrease in hepatic DGAT2 gene expression, TG secretion, plasma TG, total cholesterol and APOB in ob/ob mice. Moreover, DGAT2 ASO treatment was found to trigger significant decreases in weight gain, adipose weight and hepatic TG content [133].
Other lipid & lipoprotein disorder candidates for gene therapy
Atherosclerosis regression
Gene therapy was the first strategy used to achieve plaque regression in mouse models of atherosclerosis [52]. To date, nearly all gene therapy approaches have depended on overexpressing apolipoproteins with known antiatherogenic properties. In addition to the studies previously discussed, another major strategy involves the hepatic overexpression of apoE. This apolipoprotein is a structural component of TG-rich lipoproteins; it serves as a ligand for lipoprotein receptors and plays an important role in the catabolism of remnant particles. Overexpression of apoE has been found to increase the clearance of plasma atherogenic lipoproteins through receptors in the liver for LDL [134] and post-prandial lipoprotein remnants [135]. There are three common apoE isoforms, apoE4, apoE2 and apoE3, all of which differ by a single amino acid substitution.
Several muscle-directed nonviral gene transfer strategies have been used to deliver apoE. Athanasopoulos et al. demonstrated that intramuscular injection of an apoE2-encoding plasmid in Apoe−/− mice delayed the onset of atherosclerotic and xanthomatous lesions [6]. Subsequent studies revealed that this strategy can be used to reduce severe hypercholesterolemia in newborn Apoe−/− mice [136]. Intramuscular injection of an ApoE3-encoding plasmid in Apoe−/− mice revealed the same overall trend [137]. Although these studies did not demonstrate atherosclerosis regression, they provided important proof-of-principle confirmation that skeletal muscle can serve as an effective secretory platform to express the ApoE transgenes; improved gene transfer vectors will be needed to achieve full therapeutic levels of ApoE in plasma using plasmid DNA [137].
Liver-directed gene transfer of human apoE3 using a first-generation adenovirus to Apoe−/− mice significantly reduced plasma cholesterol for up to 2 weeks [138,139] and for at least 8 weeks with a second-generation adenovirus [140]. apoE3 has been found to reduce the progression of atherosclerotic lesions and significantly induce regression of atherosclerotic lesions. By contrast, the protein products of the other common alleles, apoE2 and apoE4, are not as effective at lowering plasma cholesterol levels, and although they reduced the progression of atherosclerosis, they did not induce regression [141]. A long-term study by Kim et al. found that a single intravenous injection of an HD-Ad vector encoding apoE completely and stably corrected the hypercholesterolemia of Apoe-deficient mice for the natural lifespan of the mice and that the aorta of treated animals was essentially lesion-free compared with control aortas that were covered 100% by atherosclerotic lesions [141]. Interestingly, a study by Tangirala and colleagues found that expression of apoE in fat-fed Ldlr−/− mice resulted in considerable plaque regression, despite having no discernable effect on fasting plasma lipoprotein levels [142]. It was suggested that regression via mechanisms other than cholesterol lowering may occur via the reduction of oxidant stress, such as levels of urinary, LDL-associated and arterial wall isoprotane [142]. Collectively, these data suggest that plaque regression after gene transfer of apoE is a strategy that may be of benefit in the clinic.
Abetalipoproteinemia
Abetalipoproteinemia (ABL) is a rare genetic disease characterized by extremely low/absent LDL-C and TG levels, absent apoB-containing lipoproteins in plasma, fat malabsorption, severe vitamin A and E deficiency, and progressive spinocerebellar and retinal degeneration [143]. Mutations in the microsomal triglyceride transfer protein (MTP) are the genetic cause of abetalipoproteinemia [144]. MTP is responsible for transferring lipids, particularly TG, onto the assembling chylomicron and VLDL particles in the intestine and the liver, respectively. In the absence of functional MTP, chylomicrons and VLDL are not effectively assembled or secreted in the circulation and apoB is likely targeted for degradation. VLDL serves as the metabolic precursor to LDL and the inability to secrete VLDL from the liver results in the absence of LDL and apoB in the blood. The progressive neurodegeneration and degenerative retinopathy seen in ABL is a result of defects in the transport of vitamin E (α-tocopherol) from liver to CNS, a process that requires the hepatic secretion of vitamin E in VLDL, with conversion to LDL and a poorly understood system for transport across the blood–brain barrier and into the CNS. Therapy with extremely high-dose vitamin E may slow the progression of neurologic and retinal degeneration but will not fully prevent the progression of these symptoms [143].
Abetalipoproteinemia is thus an attractive candidate for the development of liver-directed gene therapy, given that it is a serious disabling and life-threatening disease with no truly effective therapy. Successful gene transfer of MTP to liver would be easily detectable and quantifiable in humans with ABL by measurement of serum cholesterol and apoB. Furthermore, even a relatively low level of MTP expression in the liver would be expected to result in some VLDL production and therefore a substantial improvement in vitamin E transport and amelioration or even arrest of the progression of neurologic symptoms. ABL is therefore a model in which a very low level of transgene expression could not only be easily detected through simple assays of serum but could actually be highly efficacious in curing the disease. No gene replacement studies have yet been reported in any MTP-deficient animal model [103].
Conclusion
Somatic gene therapy is a promising approach for the treatment of several dyslipidemias. The results of many proof-of-principle studies are encouraging and have led to several clinical trials (Table 1). Given the positive results of these trials, it is very likely that this treatment modality will become an important addition to the armamentarium available for treatment of severe lipid disorders.
Future perspective
Genome-wide association studies are revealing new gene targets for dyslipidemia. As gene delivery and inhibition technologies continue to evolve, there will be a myriad of opportunities for gene therapy in future clinical applications. The main barriers that must be overcome are:
Improvement of gene transfer or inhibition strategies
Development of clinically relevant animal models
A more thorough understanding of host–vector interactions and their impact on gene transfer or inhibition efficacy
Executive summary.
Gene therapy
Gene therapy is a technique for correcting defective genes responsible for disease development. There are many dyslipidemias that can potentially benefit from this technique.
Tissue targets
Most gene therapy studies for dyslipidemia have targeted muscle and liver. Viral vectors remain the most advanced delivery platform, although innovations in nonviral vector systems are continually evolving and improving.
Clinical trials
At least five clinical gene therapy trials have been conducted to date for dyslipidemias. Most of these trials have been for familial hypercholesterolemia.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
James M Wilson received funding from the National Heart Lung and Blood Institute P01-HL059407 (JMW) and National Institute of Diabetes and Digestive and Kidney Diseases P30-DK047757. The funders had no role in data collection and analysis, decision to publish, or preparation of the manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Hegele RA. Plasma lipoproteins, genetic influences and clinical implications. Nat Rev Genet. 2009;10:109–121. doi: 10.1038/nrg2481. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary, Heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation. 2010;121:E46–E215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Dietz HC. Genomic medicine: new therapeutic approaches to mendelian disorders. N Engl J Med. 2010;363:852–863. doi: 10.1056/NEJMra0907180. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen TH, Ferry N. Liver gene therapy: advances and hurdles. Gene Ther. 2004;11:S76–S84. doi: 10.1038/sj.gt.3302373. [DOI] [PubMed] [Google Scholar]
- 5.Ross CJD, Twisk J, Meulenberg JM, et al. Long-term correction of murine lipoprotein lipase deficiency with AAV1-mediated gene transfer of the naturally occurring LPLS447X beneficial mutation. Hum Gene Ther. 2004;15:906–919. doi: 10.1089/hum.2004.15.906. [DOI] [PubMed] [Google Scholar]
- 6.Athanasopulos T, Owen JS, Hassall DG, et al. Intramuscular injection of a plasmid vector expressing human apolipoprotein E limits progression of xanthoma and aortic atheroma in apoE-deficient mice. Hum Mol Genet. 2000;9:2545–2551. doi: 10.1093/hmg/9.17.2545. [DOI] [PubMed] [Google Scholar]
- 7.Shyh-Dar L, Song L, Leaf H. Lipoplex and LPD nanoparticles for in vivo gene delivery. Cold Spring Harb Protoc. 2006 doi: 10.1101/pdb.prot4448. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 8.Li SD, Huang L. Gene therapy progress and prospects: nonviral gene therapy by systemic delivery. Gene Ther. 2006;13:1313–1319. doi: 10.1038/sj.gt.3302838. [DOI] [PubMed] [Google Scholar]
- 9.Budker V, Zhang G, Knechtle S, Wolff JA. Naked DNA delivered intraportally expresses efficiently in hepatocytes. Gene Ther. 1996;3:593–598. [PubMed] [Google Scholar]
- 10.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 11.Jooss K, Ertl HCJ, Wilson JM. Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. J Virol. 1998;72:2945–2954. doi: 10.1128/jvi.72.4.2945-2954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kay MA, Meuse L, Gown AM, et al. Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA. 1997;94:4686–4691. doi: 10.1073/pnas.94.9.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Su Q, Grewal IS, et al. Transient subversion of CD40 ligand function diminishes immune responses to adenovirus vectors in mouse liver and lung tissues. J Virol. 1996;70:6370–6377. doi: 10.1128/jvi.70.9.6370-6377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seiler MP, Cerullo V, Lee B. Immune response to helper dependent adenoviral mediated liver gene therapy: challenges and prospects. Curr Gene Ther. 2007;7:297–305. doi: 10.2174/156652307782151452. [DOI] [PubMed] [Google Scholar]
- 15.Flotte TR. New AAV serotypes may broaden the therapeutic pipeline to human gene therapy. Mol Ther. 2006;13:1–2. doi: 10.1016/j.ymthe.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- 17.Gao G, Alvira MR, Somanathan S, et al. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci USA. 2003;100:6081–6086. doi: 10.1073/pnas.0937739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Wang H, Bell P, et al. Systematic evaluation of AAV vectors for liver directed gene transfer in murine models. Mol Ther. 2010;18:118–125. doi: 10.1038/mt.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Calcedo R, Wang H, et al. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol Ther. 2010;18:126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao G, Vandenberghe LH, Alvira MR, et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao HJ, Liu YB, Rabinowitz J, et al. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther. 2000;2:619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- 22.Blankinship MJ, Gregorevic P, Allen JM, et al. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 2004;10:671–678. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 23.McCarty DM. Self-complementary AAV vectors: advances and applications. Mol Ther. 2008;16:1648–1656. doi: 10.1038/mt.2008.171. [DOI] [PubMed] [Google Scholar]
- 24.Crooke ST. Progress in antisense technology. Ann Rev Med. 2004:61–95. doi: 10.1146/annurev.med.55.091902.104408. [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, Sinko PJ. SiRNA – getting the message out. Eur J Pharm Sci. 2006;27:401–410. doi: 10.1016/j.ejps.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Sontheimer EJ. Assembly and function of RNA silencing complexes. Nat Rev Mol Cell Biol. 2005;6:127–138. doi: 10.1038/nrm1568. [DOI] [PubMed] [Google Scholar]
- 27.Haasnoot J, Berkhout B. Nucleic acids-based therapeutics in the battle against pathogenic viruses. Handb Exp Pharmacol. 2009;(189):243–263. doi: 10.1007/978-3-540-79086-0_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimm D, Streetz KL, Jopling CL, et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- 29.Giering JC, Grimm D, Storm TA, Kay MA. Expression of shRNA from a tissue-specific pol II promoter is an effective and safe RNAi therapeutic. Mol Ther. 2008;16:1630–1636. doi: 10.1038/mt.2008.144. [DOI] [PubMed] [Google Scholar]
- 30.Ginsburg GS. Regression of atherosclerosis with therapeutic antibodies: pipe cleaner or pipe dream? J Am Coll Cardiol. 2007;50:2319–2321. doi: 10.1016/j.jacc.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 31.Rader DJ, Cohen J, Hobbs HH. Monogenic hypercholesterolemia: new insights in pathogenesis and treatment. J Clin Invest. 2003;111:1795–1803. doi: 10.1172/JCI18925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moorjani S, Roy M, Gagne C, et al. Homozygous familial hypercholesterolemia among French Canadians in Quebec province. Arteriosclerosis. 1989;9:211–216. doi: 10.1161/01.atv.9.2.211. [DOI] [PubMed] [Google Scholar]
- 33.Khachadu Ak, Uthman SM. Experiences with homozygous cases of familial hypercholesterolemia. A report of 52 patients. Nutrition Metab. 1973;15:132–140. doi: 10.1159/000175431. [DOI] [PubMed] [Google Scholar]
- 34.Seftel HC, Baker SG, Sandler MP, et al. A host of hypercholesterolemic homozygotes in South Africa. BMJ. 1980;281:633–636. doi: 10.1136/bmj.281.6241.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein JL, Brown MS. The LDL receptor defect in familial hypercholesterolemia: implications for pathogenesis and therapy. Med Clin N Am. 1982;66:335–362. doi: 10.1016/s0025-7125(16)31424-9. [DOI] [PubMed] [Google Scholar]
- 36.Kolansky DM, Cuchel M, Clark BJ, et al. Longitudinal evaluation and assessment of cardiovascular disease in patients with homozygous familial hypercholesterolemia. Am J Cardiol. 2008;102:1438–1443. doi: 10.1016/j.amjcard.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 37.Moghadasian MH, Frohlich JJ, Saleem M, et al. Surgical management of dyslipidemia: clinical and experimental evidence. J Invest Surg. 2001;14:71–78. doi: 10.1080/08941930152024183. [DOI] [PubMed] [Google Scholar]
- 38.López-Santamaria M, Migliazza L, Gamez M, et al. Liver transplantation in patients with homozygotic familial hypercholesterolemia previously treated by end-to-side portocaval shunt and ileal pass. J Pediatr Surg. 2000;35:630–633. doi: 10.1053/jpsu.2000.0350630. [DOI] [PubMed] [Google Scholar]
- 39.Thompsen J, Thompson PD. A systematic review of LDL apheresis in the treatment of cardiovascular disease. Atherosclerosis. 2006;189:31–38. doi: 10.1016/j.atherosclerosis.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 40.Sachais BS, Katz J, Ross J, Rader DJ. Long-term effects of LDL apheresis in patients with severe hypercholesterolemia. J Clin Apher. 2005;20:252–255. doi: 10.1002/jca.20036. [DOI] [PubMed] [Google Scholar]
- 41.Grossman M, Rader DJ, Muller DWM, et al. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med. 1995;1:1148–1154. doi: 10.1038/nm1195-1148. [DOI] [PubMed] [Google Scholar]
- 42.Grossman M, Raper SE, Kozarsky K, et al. Successful ex vivo gene therapy directed to liver in a patient with familial hypercholesterolaemia. Nat Genet. 1994;6:335–341. doi: 10.1038/ng0494-335. [DOI] [PubMed] [Google Scholar]
- 43.Daugherty A. Mouse models of atherosclerosis. Am J Med Sci. 2002;323:3–10. doi: 10.1097/00000441-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Rader DJ, Tietge UJ. Gene therapy for dyslipidemia: clinical prospects. Curr Atheroscler Rep. 1999;1:58–69. doi: 10.1007/s11883-999-0051-5. [DOI] [PubMed] [Google Scholar]
- 45.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 46.Kozarsky KF, Jooss K, Donahee M, Strauss JF, III, Wilson JM. Effective treatment of familial hypercholesterolaemia in the mouse model using adenovirus-mediated transfer of the VLDL receptor gene. Nat Genet. 1996;13:54–62. doi: 10.1038/ng0596-54. [DOI] [PubMed] [Google Scholar]
- 47.Kozarsky KF, McKinley DR, Austin LL, et al. In vivo correction of low density lipoprotein receptor deficiency in the Watanabe heritable hyperlipidemic rabbit with recombinant adenoviruses. J Biol Chem. 1994;269:13695–13702. [PubMed] [Google Scholar]
- 48.Chen SJ, Rader DJ, Tazelaar J, et al. Prolonged correction of hyperlipidemia in mice with familial hypercholesterolemia using an adeno-associated viral vector expressing very-low-density lipoprotein receptor. Mol Ther. 2000;2:256–261. doi: 10.1006/mthe.2000.0122. [DOI] [PubMed] [Google Scholar]
- 49.Lebherz C, Gao G, Louboutin JP, et al. Gene therapy with novel adeno-associated virus vectors substantially diminishes atherosclerosis in a murine model of familial hypercholesterolemia. J Gene Med. 2004;6:663–672. doi: 10.1002/jgm.554. [DOI] [PubMed] [Google Scholar]
- 50.Kankkonen HM, Vähäkangas E, Marr RA, et al. Long-term lowering of plasma cholesterol levels in LDL-receptor-deficient WHHL rabbits by gene therapy. Mol Ther. 2004;9:548–556. doi: 10.1016/j.ymthe.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 51.Nomura S, Merched A, Nour E, et al. Low-density lipoprotein receptor gene therapy using helper-dependent adenovirus produces long-term protection against atherosclerosis in a mouse model of familial hypercholesterolemia. Gene Ther. 2004;11:1540–1548. doi: 10.1038/sj.gt.3302310. [DOI] [PubMed] [Google Scholar]
- 52.Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5:91–102. doi: 10.1038/ncpcardio1086. [DOI] [PubMed] [Google Scholar]
- 53.Sadik H, Kassim HL, Luk H, et al. Rader gene therapy in a humanized mouse model of familial hypercholesterolemia leads to marked regression of atherosclerosis. PLoS One. 2010;5(10):E13424. doi: 10.1371/journal.pone.0013424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hibbitt OC, Harbottle RP, Waddington SN, et al. Delivery and long-term expression of a 135 kb LDLR genomic DNA locus in vivo by hydrodynamic tail vein injection. J Gene Med. 2007;9:488–497. doi: 10.1002/jgm.1041. [DOI] [PubMed] [Google Scholar]
- 55.Hibbitt OC, McNeil E, Lufino MMP, et al. Long-term physiologically regulated expression of the low-density lipoprotein receptor in vivo using genomic DNA mini-gene constructs. Mol Ther. 2010;18:317–326. doi: 10.1038/mt.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crooke RM. Antisense oligonucleotides as therapeutics for hyperlipidaemias. Exp Opin Biol Ther. 2005;5:907–917. doi: 10.1517/14712598.5.7.907. [DOI] [PubMed] [Google Scholar]
- 57.Crooke RM, Graham MJ, Lemonidis KM, et al. An apolipoprotein B antisense oligonucleotide lowers LDL cholesterol in hyperlipidemic mice without causing hepatic steatosis. J Lipid Res. 2005;46:872–884. doi: 10.1194/jlr.M400492-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Raal FJ, Santos RD, Blom DJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia, a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
- 59.Visser ME, Akdim F, Tribble DL, et al. Effect of apolipoprotein-B synthesis inhibition on liver triglyceride content in patients with familial hypercholesterolemia. J Lipid Res. 2010;51:1057–1062. doi: 10.1194/jlr.M002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Visser ME, Kastelein JJP, Stroes ESG. Apolipoprotein B synthesis inhibition: results from clinical trials. Curr Opin Lipidol. 2010 doi: 10.1097/MOL.0b013e32833af4c1. [DOI] [PubMed] [Google Scholar]
- 61.Akdim F, Visser ME, Tribble DL, et al. Effect of mipomersen, an apolipoprotein B synthesis inhibitor, on low-density lipoprotein cholesterol in patients with familial hypercholesterolemia. Am J Cardiol. 2010;105:1413–1419. doi: 10.1016/j.amjcard.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Akdim F, Stroes ES, Sijbrands EJ, et al. Efficacy and safety of mipomersen, an antisense inhibitor of apolipoprotein B, in hypercholesterolemic subjects receiving stable statin therapy. J Am Coll Cardiol. 2010;55(15):1611–1618. doi: 10.1016/j.jacc.2009.11.069. [DOI] [PubMed] [Google Scholar]
- 63.Soutschek J, Akinc A, Bramlage B, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 64.Zimmermann TS, Lee ACH, Akinc A, et al. RNAi-mediated gene silencing in nonhuman primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 65.MacLachlan I. A Phase I study to evaluate the safety, tolerability, and pharmacokinetics of lipid nanoparticle ApoB siRNA (ApoB-SNALP) in hypercholesterolemic subjects. Presented at: American Society of Gene & Cell Therapy 13th Annual Conference; Washington, DC, USA. 17–22 May 2010. [Google Scholar]
- 66.Kotowski IK, Pertsemlidis A, Luke A, et al. A spectrum of PCSK9 alleles contributes to plasma levels of low-density lipoprotein cholesterol. Am J Hum Genet. 2006;78:410–422. doi: 10.1086/500615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abifadel M, Varret M, Rabès JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 68.Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 69.Frank-Kamenetsky M, Grefhorst A, Anderson NN, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci USA. 2008;105:11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest. 2006;116:3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alwaili K, Awan Z, Alshahrani A, Genest J. High-density lipoproteins and cardiovascular disease: 2010 update. Expert Rev Cardiovasc Ther. 2010;8:413–423. doi: 10.1586/erc.10.4. [DOI] [PubMed] [Google Scholar]
- 72.Sviridov D, Nestel PJ. Genetic factors affecting HDL levels, structure, metabolism and function. Curr Opin Lipidol. 2007;18:157–163. doi: 10.1097/MOL.0b013e32803dbdd6. [DOI] [PubMed] [Google Scholar]
- 73.Moore RE, Kawashiri MA, Kitajima K, et al. Apolipoprotein A-I deficiency results in markedly increased atherosclerosis in mice lacking the LDL receptor. Arterioscler Thromb Vasc Biol. 2003;23:1914–1920. doi: 10.1161/01.ATV.0000092328.66882.F5. [DOI] [PubMed] [Google Scholar]
- 74.Moore RE, Navab M, Millar JS, et al. Increased atherosclerosis in mice lacking apolipoprotein A-I attributable to both impaired reverse cholesterol transport and increased inflammation. Circulation Res. 2005;97:763–771. doi: 10.1161/01.RES.0000185320.82962.F7. [DOI] [PubMed] [Google Scholar]
- 75.Kopfler WP, Willard M, Betz T, et al. Adenovirus-mediated transfer of a gene encoding human apolipoprotein A-I into normal mice increases circulating high-density lipoprotein cholesterol. Circulation. 1994;90:1319–1327. doi: 10.1161/01.cir.90.3.1319. [DOI] [PubMed] [Google Scholar]
- 76.De Geest B, Zhao Z, Collen D, Holvoet P. Effects of adenovirus-mediated human apoA-I gene transfer on neointima formation after endothelial denudation in apoE-deficient mice. Circulation. 1997;96:4349–4356. doi: 10.1161/01.cir.96.12.4349. [DOI] [PubMed] [Google Scholar]
- 77.Tsukamoto K, Hiester KG, Smith P, et al. Comparison of human apoA-I expression in mouse models of atherosclerosis after gene transfer using a second generation adenovirus. J Lipid Res. 1997;38:1869–1876. [PubMed] [Google Scholar]
- 78.Benoit P, Emmanuel F, Caillaud JM, et al. Somatic gene transfer of human apoA-I inhibits atherosclerosis progression in mouse models. Circulation. 1999;99:105–110. doi: 10.1161/01.cir.99.1.105. [DOI] [PubMed] [Google Scholar]
- 79.Tangirala RK, Tsukamoto K, Chun SH, et al. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 80.De Geest B, Van Linthout S, Lox M, Collen D, Holvoet P. Sustained expression of human apolipoprotein A-I after adenoviral gene transfer in C57BL/6 mice, Role of apolipoprotein A-I promoter, apolipoprotein A-I introns, and human apolipoprotein E enhancer. Hum Gene Ther. 2000;11:101–112. doi: 10.1089/10430340050016193. [DOI] [PubMed] [Google Scholar]
- 81.Belalcazar LM, Merched A, Carr B, et al. Long-term stable expression of human apolipoprotein A-I mediated by helper-dependent adenovirus gene transfer inhibits atherosclerosis progression and remodels atherosclerotic plaques in a mouse model of familial hypercholesterolemia. Circulation. 2003;107:2726–2732. doi: 10.1161/01.CIR.0000066913.69844.B2. [DOI] [PubMed] [Google Scholar]
- 82.Pastore L, Belalcazar LM, Oka K, et al. Helper-dependent adenoviral vector-mediated long-term expression of human apolipoprotein A-I reduces atherosclerosis in apo E-deficient mice. Gene. 2004;327:153–160. doi: 10.1016/j.gene.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 83.Sharifi BG, Wu K, Wang L, et al. AAV serotype-dependent apolipoprotein A-IMilano gene expression. Atherosclerosis. 2005;181:261–269. doi: 10.1016/j.atherosclerosis.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 84.Kitajima K, Marchadier DHL, Burstein H, Rader DJ. Persistent liver expression of murine apoA-l using vectors based on adeno-associated viral vectors serotypes 5 and 1. Atherosclerosis. 2006;186:65–73. doi: 10.1016/j.atherosclerosis.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 85.Vaessen SFC, Veldman RJ, Cornijn EM, et al. AAV gene therapy as a means to increase apolipoprotein (Apo) A-I and high-density lipoprotein-cholesterol levels: correction of murine ApoA-I deficiency. J Gene Med. 2009;11:697–707. doi: 10.1002/jgm.1344. [DOI] [PubMed] [Google Scholar]
- 86.Cimmino G, Chen W, Speidl WS, et al. Safe and sustained overexpression of functional apolipoprotein-AI/high-density lipoprotein in apolipoprotein-AI-null mice by muscular adeno-associated viral serotype 8 vector gene transfer. J Cardiovasc Pharmacol. 2009;54:405–411. doi: 10.1097/FJC.0b013e3181bad264. [DOI] [PubMed] [Google Scholar]
- 87.Weisgraber KH, Bersot TP, Mahley RW. A-I(Milano) apoprotein. Isolation and characterization of a cysteine-containing variant of the A-I apoprotein from human high density lipoproteins. J Clin Invest. 1980;66:901–907. doi: 10.1172/JCI109957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gualandri V, Franceschini G, Sirtori CR. AI(Milano) apoprotein identification of the complete kindred and evidence of a dominant genetic transmission. Am J Hum Genet. 1985;37:1083–1097. [PMC free article] [PubMed] [Google Scholar]
- 89.Chiesa G, Sirtori CR. Recombinant apolipoprotein A-IMilano: a novel agent for the induction of regression of atherosclerotic plaques. Ann Med. 2003;35:267–273. doi: 10.1080/07853890310005281. [DOI] [PubMed] [Google Scholar]
- 90.Lebherz C, Sanmiguel J, Wilson JM, Rader DJ. Gene transfer of wild-type apoA-I and apoA-I Milano reduce atherosclerosis to a similar extent. Cardiovasc Diabetol. 2007;6:15. doi: 10.1186/1475-2840-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alexander ET, Weibel GL, Joshi MR, et al. Macrophage reverse cholesterol transport in mice expressing ApoA-I milano. Arterioscler Thromb Vasc Biol. 2009;29:1496–1501. doi: 10.1161/ATVBAHA.109.191379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Santamarina-Fojo S, Peterson K, Knapper C, et al. Complete genomic sequence of the human ABCA1 gene. Analysis of the human and mouse ATP-binding cassette A promoter. Proc Natl Acad Sci USA. 2000;97:7987–7992. doi: 10.1073/pnas.97.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iatan I, Alrasadi K, Ruel I, Alwaili K, Genest J. Effect of ABCA1 mutations on risk for myocardial infarction. Curr Atheroscler Rep. 2008;10:413–426. doi: 10.1007/s11883-008-0064-5. [DOI] [PubMed] [Google Scholar]
- 94.Schaefer EJ, Zech LA, Schwartz DE, Brewer HB. Coronary heart-disease prevalence and other clinical-features in familial high-density lipoprotein deficiency (tangier disease) Ann Intern Med. 1980;93:261–266. doi: 10.7326/0003-4819-93-2-261. [DOI] [PubMed] [Google Scholar]
- 95.Tall AR, Wang N. Tangier disease as a test of the reverse cholesterol transport hypothesis. J Clin Invest. 2000;106:1205–1207. doi: 10.1172/JCI11538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang X, Collins HL, Ranalletta M, et al. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aiello RJ, Brees D, Bourassa PA, et al. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler Thromb Vasc Biol. 2002;22:630–637. doi: 10.1161/01.atv.0000014804.35824.da. [DOI] [PubMed] [Google Scholar]
- 98.Wellington CL, Brunham LR, Zhou S, et al. Alterations of plasma lipids in mice via adenoviral-mediated hepatic overexpression of human ABCA1. J Lipid Res. 2003;44:1470–1480. doi: 10.1194/jlr.M300110-JLR200. [DOI] [PubMed] [Google Scholar]
- 99.Feng Y, Lievens J, Jacobs F, et al. Hepatocyte-specific ABCA1 transfer increases HDL cholesterol but impairs HDL function and accelerates atherosclerosis. Cardiovasc Res. 2010;88(2):376–385. doi: 10.1093/cvr/cvq204. [DOI] [PubMed] [Google Scholar]
- 100.Joyce CW, Wagner EM, Basso F, et al. ABCA1 overexpression in the liver of LDLr-KO mice leads to accumulation of pro-atherogenic lipoproteins and enhanced atherosclerosis. J Biol Chem. 2006;281:33053–33065. doi: 10.1074/jbc.M604526200. [DOI] [PubMed] [Google Scholar]
- 101.Rousset X, Vaisman B, Amar M, Sethi AA, Remaley AT. Lecithin: cholesterol acyltransferase – from biochemistry to role in cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. 2009;16:163–171. doi: 10.1097/med.0b013e328329233b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sakai N, Vaisman BL, Koch CA, et al. Targeted disruption of the mouse lecithin: cholesterol acyltransferase (LCAT) gene: generation of a new animal model for human LCAT deficiency. J Biol Chem. 1997;272:7506–7510. doi: 10.1074/jbc.272.11.7506. [DOI] [PubMed] [Google Scholar]
- 103.Broedl UC, Rader DJ. Gene therapy for lipoprotein disorders. Exp Opin Biol Ther. 2005;5:1029–1038. doi: 10.1517/14712598.5.8.1029. [DOI] [PubMed] [Google Scholar]
- 104.Séguret-Macé S, Latta-Mahieu M, Castro G, et al. Potential gene therapy for lecithin-cholesterol acyltransferase (LCAT)-deficient and hypoalphalipoproteinemic patients with adenovirus-mediated transfer of human LCAT gene. Circulation. 1996;94:2177–2184. doi: 10.1161/01.cir.94.9.2177. [DOI] [PubMed] [Google Scholar]
- 105.Mertens A, Verhamme P, Bielicki JK, et al. Increased low-density lipoprotein oxidation and impaired high-density lipoprotein antioxidant defense are associated with increased macrophage homing and atherosclerosis in dyslipidemic obese mice, LCAT gene transfer decreases atherosclerosis. Circulation. 2003;107:1640–1646. doi: 10.1161/01.CIR.0000056523.08033.9F. [DOI] [PubMed] [Google Scholar]
- 106.Van Craeyveld E, Lievens J, Jacobs F, et al. Apolipoprotein A-I and lecithin, Cholesterol acyltransferase transfer induce cholesterol unloading in complex atherosclerotic lesions. Gene Ther. 2009;16:757–765. doi: 10.1038/gt.2009.8. [DOI] [PubMed] [Google Scholar]
- 107.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 108.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 109.Bhatt KN, Wells BJ, Sperling LS, Baer JT. High-density lipoprotein therapy: is there hope? Curr Treat Options Cardiovasc Med. 2010:1–14. doi: 10.1007/s11936-010-0081-x. [DOI] [PubMed] [Google Scholar]
- 110.Tanaka H, Ishida T, Johnston TP, et al. Role of endothelial lipase in plasma HDL levels in a murine model of hypertriglyceridemia. J Atheroscler Thromb. 2009;16:327–338. doi: 10.5551/jat.no844. [DOI] [PubMed] [Google Scholar]
- 111.Jin W, Millar JS, Broedl U, Glick JM, Rader DJ. Inhibition of endothelial lipase causes increased HDL cholesterol levels in vivo. J Clin Invest. 2003;111:357–362. doi: 10.1172/JCI16146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ishida T, Choi S, Kundu RK, et al. Endothelial lipase is a major determinant of HDL level. J Clin Invest. 2003;111:347–355. doi: 10.1172/JCI16306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Edmondson AC, Brown RJ, Kathiresan S, et al. Loss-of-function variants in endothelial lipase are a cause of elevated HDL cholesterol in humans. J Clin Invest. 2009;119:1042–1050. doi: 10.1172/JCI37176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garg A, Simha V. Update: update on dyslipidemia. J Clin Endocrinol Metab. 2007;92:1581–1589. doi: 10.1210/jc.2007-0275. [DOI] [PubMed] [Google Scholar]
- 115.Beigneux AP, Davies BSJ, Gin P, et al. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007;5:279–291. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peterfy M, Ben-Zeev O, Mao HZ, et al. Mutations in LMF1 cause combined lipase deficiency and severe hypertriglyceridemia. Nat Genet. 2007;39:1483–1487. doi: 10.1038/ng.2007.24. [DOI] [PubMed] [Google Scholar]
- 117.Rahalkar AR, Giffen F, Har B, et al. Novel LPL mutations associated with lipoprotein lipase deficiency: two case reports and a literature review. Can J Physiol Pharmacol. 2009;87:151–160. doi: 10.1139/y09-005. [DOI] [PubMed] [Google Scholar]
- 118.Banks PA. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 1997;92:377–386. [PubMed] [Google Scholar]
- 119.Ashbourne Excoffon KJD, Liu G, Miao L, et al. Correction of hypertriglyceridemia and impaired fat tolerance in lipoprotein lipase-deficient mice by adenovirus-mediated expression of human lipoprotein lipase. Arterioscler Thromb Vasc Biol. 1997;17:2532–2539. doi: 10.1161/01.atv.17.11.2532. [DOI] [PubMed] [Google Scholar]
- 120.Liu G, Ashbourne Excoffon KJD, Wilson JE, et al. Phenotypic correction of feline lipoprotein lipase deficiency by adenoviral gene transfer. Hum Gene Ther. 2000;11:21–32. doi: 10.1089/10430340050016120. [DOI] [PubMed] [Google Scholar]
- 121.Groenemeijer BE, Hallman MD, Reymer PW, et al. Genetic variant showing a positive interaction with β-blocking agents with a beneficial influence on lipoprotein lipase activity, HDL cholesterol, and triglyceride levels in coronary artery disease patients. The Ser447-stop substitution in the lipoprotein lipase gene REGRESS Study Group. Circulation. 1997;95(12):2628–2635. doi: 10.1161/01.cir.95.12.2628. [DOI] [PubMed] [Google Scholar]
- 122.Gagné SE, Larson MG, Pimstone SN, et al. A common truncation variant of lipoprotein lipase (Ser447X) confers protection against coronary heart disease: the Framingham Offspring Study. Clin Genet. 1999;55(6):450–454. doi: 10.1034/j.1399-0004.1999.550609.x. [DOI] [PubMed] [Google Scholar]
- 123.Wittrup HH, Tybjaerg-Hansen A, Steffensen R, et al. Mutations in the lipoprotein lipase gene associated with ischemic heart disease in men, The Copenhagen city heart study. Arterioscler Thromb Vasc Biol. 1999;19:1535–1540. doi: 10.1161/01.atv.19.6.1535. [DOI] [PubMed] [Google Scholar]
- 124.Ross CJD, Twisk J, Bakker AC, et al. Correction of feline lipoprotein lipase deficiency with adeno-associated virus serotype 1-mediated gene transfer of the lipoprotein lipase S447X beneficial mutation. Hum Gene Ther. 2006;17:487–499. doi: 10.1089/hum.2006.17.487. [DOI] [PubMed] [Google Scholar]
- 125.Mingozzi F, Meulenberg JJ, Hui DJ, et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114:2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pennacchio LA, Olivier M, Hubacek JA, et al. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294:169–173. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 127.Tai ES, Ordovas JM. Clinical significance of apolipoprotein A5. Curr Opin Lipidol. 2008;19:349–354. doi: 10.1097/MOL.0b013e328304b681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Van der Vliet HN, Schaap FG, Levels JHM, et al. Adenoviral overexpression of apolipoprotein A-V reduces serum levels of triglycerides and cholesterol in mice. Biochem Biophys Res Comm. 2002;295:1156–1159. doi: 10.1016/s0006-291x(02)00808-2. [DOI] [PubMed] [Google Scholar]
- 129.Huang W, Bi N, Zhang X, et al. Overexpression of apolipoprotein AV in the liver reduces plasma triglyceride and cholesterol but not HDL in ApoE deficient mice. Biochem Biophys Res Comm. 2006;346:14–18. doi: 10.1016/j.bbrc.2006.05.072. [DOI] [PubMed] [Google Scholar]
- 130.Hofker MH. APOC3 null mutation affects lipoprotein profile APOC3 deficiency, from mice to man. Eur J Hum Genet. 2010;18:1–2. doi: 10.1038/ejhg.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smith SJ, Cases S, Jensen DR, et al. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking DGAT. Nat Genet. 2000;25:87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- 132.Yu XX, Murray SF, Pandey SK, et al. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology. 2005;42:362–371. doi: 10.1002/hep.20783. [DOI] [PubMed] [Google Scholar]
- 133.Liu Y, Millar JS, Cromley DA, et al. Knockdown of Acyl-CoA: diacylglycerol acyltransferase 2 with antisense oligonucleotide reduces VLDL TG and ApoB secretion in mice. Biochim Biophys Acta. 2008;1781:97–104. doi: 10.1016/j.bbalip.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 134.Ishibashi S, Brown MS, Goldstein JL, et al. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Williams KJ, Fless GM, Petrie KA, et al. Mechanisms by which lipoprotein lipase alters cellular metabolism of lipoprotein(a), low density lipoprotein, and nascent lipoproteins. Roles for low density lipoprotein receptors and heparan sulfate proteoglycans. J Biol Chem. 1992;267:13284–13292. [PubMed] [Google Scholar]
- 136.Signori E, Rinaldi M, Fioretti D, et al. ApoE gene delivery inhibits severe hypercholesterolemia in newborn ApoE-KO mice. Biochem Biophys Res Comm. 2007;361:543–548. doi: 10.1016/j.bbrc.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 137.Evans V, Foster H, Graham IR, et al. Human apolipoprotein E expression from mouse skeletal muscle by electrotransfer of nonviral DNA (plasmid) and pseudotyped recombinant adeno-associated virus (AAV2/7) Hum Gene Ther. 2008;19:569–578. doi: 10.1089/hum.2007.169. [DOI] [PubMed] [Google Scholar]
- 138.Stevenson SC, Marshall-Neff J, Teng B, et al. Phenotypic correction of hypercholesterolemia in ApoE-deficient mice by adenovirus-mediated in vivo gene transfer. Arterioscler Thromb Vasc Biol. 1995;15:479–484. doi: 10.1161/01.atv.15.4.479. [DOI] [PubMed] [Google Scholar]
- 139.Kashyap VS, Santamarina-Fojo S, Brown DR, et al. Apolipoprotein E deficiency in mice: gene replacement and prevention of atherosclerosis using adenovirus vectors. J Clin Invest. 1995;96:1612–1620. doi: 10.1172/JCI118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tsukamoto K, Smith P, Glick JM, Rader DJ. Liver-directed gene transfer and prolonged expression of three major human apoE isoforms in apoE-deficient mice. J Clin Invest. 1997;100:107–114. doi: 10.1172/JCI119501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim IH, Józkowicz A, Piedra PA, Oka K, Chan L. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci USA. 2001;98:13282–13287. doi: 10.1073/pnas.241506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tangirala RK, Praticó D, FitzGerald GA, et al. Reduction of isoprostanes and regression of advanced atherosclerosis by apolipoprotein E. J Biol Chem. 2001;276:261–266. doi: 10.1074/jbc.M003324200. [DOI] [PubMed] [Google Scholar]
- 143.Zamel R, Khan R, Pollex RL, Hegele RA. Abetalipoproteinemia: two case reports and literature review. Orphanet J Rare Dis. 2008;3:19. doi: 10.1186/1750-1172-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sharp D, Blinderman L, Combs KA, et al. Cloning and gene defects in microsomal triglyceride transfer protein associated with abetalipoproteinaemia. Nature. 1993;365:65–69. doi: 10.1038/365065a0. [DOI] [PubMed] [Google Scholar]
- 145.Stroes ES, Nierman MC, Meulenberg JJ, et al. Intramuscular administration of AAV1-lipoprotein lipaseS447X lowers triglycerides in lipoprotein lipase-deficient patients. Arterioscler Thromb Vasc Biol. 2008;28:2303–2304. doi: 10.1161/ATVBAHA.108.175620. [DOI] [PubMed] [Google Scholar]
Websites
- 201.Tekmira Pharmaceuticals Completes ApoB SNALP Phase I Clinical Trial. Tekmira Pharmaceuticals. www.tekmirapharm.com/News_and_Events/News_Releases/Tekmira01071001.aspx.
- 202.National Institutes of Health; 2002. Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III Final Report) www.nhlbi.nih.gov/guidelines/cholesterol/profmats.htm. [Google Scholar]
- 203.Singh VN, Citkowitz E. Low HDL Cholesterol (Hypoalphalipoproteinemia) Medscape. 2009 http://emedicine.medscape.com/article/127943-overview.
- 204.Carrié A. LCAT deficiency. 2006 www.orphanet.net/consor/cgi-bin/Disease_Search.php?lng=EN&data_id=924&Disease_Disease_Search_diseaseGroup=LCAT&Disease_Disease_Search_diseaseType=Pat&Disease%28s%29/group%20of%20diseases=LCAT-deficiency&title=LCAT-deficiency&search=Disease_Search_Simple.