Abstract
GB virus C/hepatitis G (GBV-C) is an RNA virus of the family Flaviviridae. Despite replicating with an RNA-dependent RNA polymerase, some previous estimates of rates of evolutionary change in GBV-C suggest that it fixes mutations at the anomalously low rate of ~10−7 nucleotide substitution per site, per year. However, these estimates were largely based on the assumption that GBV-C and its close relative GBV-A (New World monkey GB viruses) codiverged with their primate hosts over millions of years. Herein, we estimated the substitution rate of GBV-C using the largest set of dated GBV-C isolates compiled to date and a Bayesian coalescent approach that utilizes the year of sampling and so is independent of the assumption of codivergence. This revealed a rate of evolutionary change approximately four orders of magnitude higher than that estimated previously, in the range of 10−2 to 10−3 sub/site/year, and hence in line with those previously determined for RNA viruses in general and the Flaviviridae in particular. In addition, we tested the assumption of host-virus codivergence in GBV-A by performing a reconciliation analysis of host and virus phylogenies. Strikingly, we found no statistical evidence for host-virus codivergence in GBV-A, indicating that substitution rates in the GB viruses should not be estimated from host divergence times.
Keywords: GB virus C, Molecular clock, Substitution rate, Codivergence, Phylogeny, Coalescent theory
Introduction
GB virus C (GBV-C), also known as hepatitis G virus (HGV), is a member of the family Flaviviridae, that was first identified in 1995 (Simons et al. 1995). This single-strand RNA virus comprises a positive-sense genome of a 9733 nucleotides arranged as a single open reading frame (Leary et al. 1996; Linnen et al. 1996). GBV-C is particularly notable in that it establishes a persistent, asymptomatic infection within hosts and is widely distributed in the healthy human population. The prevalence of GBV-C ranges between 1% and 14% in healthy blood donors (Moaven et al. 1996; Nakai et al. 2001; Abe 2001). GBV-C has also been isolated from chimpanzees (Adams et al. 1998; Birkenmeyer et al. 1998), and the related GBV-A and –B viruses from New World monkeys (Simons et al. 1995; Bukh and Apgar 1997). Some phylogenetic studies suggest that these viruses have codiverged with their primate hosts over a period of several million years (Charrel et al. 1999; Simmonds 2001). In particular, the phylogenetic relationships among a small sample of human GBV-C, chimpanzee GBV-C, and monkey GBV-A sequences matched those of their hosts (Charrel et al. 1999), and the distribution of current human GBV-C genotypes was proposed as being consistent with patterns of ancient human migration (Katayama et al. 1997; Pavesi 2001).
Initial estimates of the rate of evolutionary change (nucleotide substitution) of GBV-C were obtained by simply dividing the amount of divergence in sequences isolated from a single patient at different times by the sampling period (Nakao et al. 1997). From this, estimates of 3.9 × 10−4 nucleotide substitutions (subs) per site per year and 4.6 × 10−4 sub/site/year were obtained for the envelope (E) gene and the entire genome, respectively (Nakao et al. 1997). These are similar to those seen in a broad range of RNA viruses (Jenkins et al. 2002). In a more comprehensive survey, Sarrazin et al (2000) analyzed intrahost variability in 42 GBV-C-infected patients sampled over 2 years and inferred a similar rate of ~10−3 sub/site/year. In marked contrast, far lower evolutionary rates, always less than 10−5 sub/site/year, were estimated in an analysis of two pairs of complete sequences isolated from single patients at different times (Suzuki et al. 1999). This low rate is in general accordance with that estimated assuming codivergence between GBV-A/C and their primates hosts (~10−7 sub/site/year [Hanada et al. 2004]). If corroborated, this would make GBV-A/C the slowest evolving of all viruses that replicate using an RNA-dependent RNA polymerase.
Several explanations have been put forward to reconcile the apparent conflict between high short-term rates and low long-term rates. For example, Charrel et al. (1999) suggested that this discrepancy could be due to differences in selection pressures within and between hosts; some mutations may be neutral within a host yet deleterious at transmission. Similarly, Simmonds and Smith (1999) proposed that among-site rate heterogeneity due to constraints imposed by RNA secondary may greatly affect rate estimates. In this scenario, unpaired nucleotide sites would evolve rapidly during short time periods, but over longer time periods, these sites would become saturated so that further changes would only occur at paired sites. Finally, it has been suggested that the anomalously low substitution rate in GBV-C may be because this virus is effectively latent, with very low rates of replication resulting in little mutation accumulation (Hanada et al. 2004).
Critically, much of the evidence for the anomalously low rate of evolutionary change in GBV-A/C rests on assumption that these viruses codiverged with their primate hosts over millions of years (Hanada et al. 2004; Charrel et al. 1999). Herein, we rigorously test this assumption for the first time. To estimate substitution rates with as much accuracy as possible we employed a Bayesian coalescent method that analyzes the distribution of mutational differences among viruses sampled at different times and that provides a natural measure of statistical uncertainty.
Methods
Sequence Data
All available GBV-C sequences were downloaded from GenBank. Where possible, we collected the sampling date (year) either from the GenBank record or following direct contact with the relevant authors. This resulted in four data sets: 5′-UTR (108 sequences, 223 nt; sampled between 1993 and 2002), envelope E1 (19 sequences, 297 nt; sampled between 1993 and 1997), envelope E2 (49 sequences, 1266 nt; sampled between 1996 and 1999), and NS5b (40 sequences, 354 nt; data sampled between 1993 and 1997) (Table 1). All sequences were aligned using ClustalW (Thompson et al. 1994).
Table 1.
Estimates of evolutionary rates and numbers of nonsynonymous and synonymous substitutions per site (dN/dS) for GBV-C
| Region | No. | Date range | dN/dS (SLAC) | Molecular clock | Nucleotide substitution per site per year (95% HPD)
|
||
|---|---|---|---|---|---|---|---|
| Constant | Logistic | Exponential | |||||
| 5′ UTR | 108 | 1993–2002 | NA | Relaxed exponential | 4.13 × 10−3 (1.59 × 10−3–6.96 × 10−3) | 4.45 × 10−3 (2.11 × 10−3–7.01 × 10−3) | 5.03 × 10−3 (4.98 × 10−3–7.8 × 10−3) |
| Relaxed lognormal | 4.15 × 10−3 (1.64 × 10−3–7.37 × 10−3) | 4.13 × 10−3 (1.73 × 10−3–6.72 × 10−3) | 4.99 × 10−3 (1.79 × 10−3–7.95 × 10−3) | ||||
| Strict | 2.1 × 10−3 (1.32 × 10−3–2.96 × 10−3) | 2.09 × 10−3 (1.32 × 10−3–2.97 × 10−3) | 1.85 × 10−3 (1.06 × 10−3–2.6 × 10−3) | ||||
| E1 | 19 | 1993–1997 | 0.039 | Relaxed exponential | 5.43 × 10−2 (1.41 × 10−2–9.92 × 10−2) | 1.94 × 10−2 (6.11 × 10−3–3.64 × 10−2) | 2.49 × 10−2 (1.11 × 10−2–3.79 × 10−2) |
| Relaxed lognormal | 4.33 × 10−2 (1.39 × 10−2–7.37 × 10−2) | 3.95 × 10−2 (9.44 × 10−3–7.06 × 10−2) | 4.3 × 10−2 (1.48 × 10−2–7.58 × 10−2) | ||||
| Strict | 3.28 × 10−2 (1.3 × 10−2–5.4 × 10−2) | 3.95 × 10−2 (9.44 × 10−3–7.06 × 10−2) | 3.16 × 10−2 (1.34 × 10−2–5.21 × 10−2) | ||||
| E2 | 49 | 1996–1999 | 0.046 | Relaxed exponential | 2.19 × 10−2 (7.33 × 10−3–3.791 × 10−2) 2.74 × 10−2 (1.22 × 10−2–4.3 × 10−2) | 2.89 × 10−2 (1.68 × 10−2–4.19 × 10−2) | |
| Relaxed lognormal | 1.46 × 10−2 (5.19 × 10−3–2.32 × 10−2) | 7.92 × 10−3 (5.52 × 10−6–1.65 × 10−2) | 8.67 × 10−3 (1.49 × 10−3–1.12 × 10−2) | ||||
| Strict | 3.53 × 10−3 (3.73 × 10−6–9.1 × 10−3) | 2.61 × 10−3 (1.69 × 10−5–6.27 × 10−3) | 3.34 × 10−3 (3.25 × 10−4–6.91 × 10−3) | ||||
| NS5b | 40 | 1993–1997 | 0.047 | Relaxed exponential | 3.55 × 10−2 (1.36 × 10−2–6.13 × 10−2) | 3.58 × 10−2 (1.0 × 10−2–6.3 × 10−2) | 3.24 × 10−2 (9.99 × 10−3–5.46 × 10−2) |
| Relaxed lognormal | 2.12 × 10−2 (7.74 × 10−3–3.55 × 10−2) | 8.49 × 10−3 (1.88 × 10−5–1.94 × 10−2) | 1.14 × 10−2 (2.56 × 10−3–2.14 × 10−2) | ||||
| Strict | 1.52 × 10−2 (4.96 × 10−3–2.57 × 10−2) | 6.99 × 10−3 (4.03 × 10−5–1.43 × 10−2) | 1.02 × 10−2 (2.74 × 10−3–1.84 × 10−2) | ||||
Analysis of Substitution Dynamics in GBV-C
Overall rates of evolutionary change, measured as the number of nucleotide substitutions per site, per year were estimated using the Bayesian Markov chain Monte Carlo (MCMC) method implemented in the BEAST package (version 1.4.6 [Drummond and Rambaut 2007]). In all cases, we employed the most general GTR+I+Γ4 model of nucleotide substitution, as this was the best-fit to all four data sets by MODELTEST (Posada and Crandall 1998), and used a range of prior values for the substitution rate. We employed demographic models of (i) constant population size, (ii) exponential population growth, and (iii) logistic population growth, under both strict and relaxed (uncorrelated exponential and uncorrelated lognormal) molecular clocks. In all cases the convergence of parameters was assessed using TRACER (Drummond and Rambaut 2007), with statistical uncertainty reflected in intervals of the 95% highest probability (HPD).
Analysis of Selection Pressures
To assess the selection pressures acting on these GBV-C sequence data (excluding the 5′-UTR, which does not encode amino acids), we estimated the overall ratio of nonsynonymous (dN)-to-synonymous substitutions (dS) per site (dN/dS ratio). This was achieved using the maximum likelihood (ML)-based single likelihood ancestor counting (SLAC), fixed effects likelihood (FEL), internal branches fixed effects likelihood (IFEL), and random effects likelihood (REL) methods available at the online Datamonkey facility (http://www.datamonkey.org/; Kosakovsky Pond and Frost 2005). In all cases we utilized the GTR substitution model and input neighbor-joining trees. Datamonkey was also used as a resource to test for recombination in GBV-C using the GARD (Genetic Algorithms for Recombination Detection) method.
Analysis of Codivergence
To determine whether GB viruses have codiverged with their hosts, we inferred a phylogeny of GBV-A sequences from New World monkeys utilizing 45 sequences of 206 nt from the 5′-UTR region (as this contained the widest species coverage). This phylogeny was inferred using the ML method available in PAUP* (Swofford et al. 2003), again using GTR+I+Γ4 model of nucleotide substitution as well as TBR branch-swapping. This data set includes GBV-A isolates from six species of New World monkey Saguinus mystax (SM), Saguinus labiatus (SL), Saguinus oedipus (SO), Saguinus nigricollis (SN), and Callithrix jacchus (CJ), with Aotus trivirgatus (AT) used as an out-group (accession numbers given by Charrel et al. [1999]). This virus tree was then compared with the host phylogeny using the previously reported phylogenetic relationships among the New World monkeys (Jacobs et al. 1995; Canavez et al. 1998) using the reconciled trees method implemented in the TREEMAP program (version 2; Charleston and Page 2002). This method assumes, a priori, that host and parasite trees are congruent. If these trees are incongruent, various evolutionary events are then hypothesized (duplication, loss, and switching on parasite tree) creating the (optimal) cophylogenies necessary to make them congruent (Charleston 1998). One thousand randomized parasite trees were then mapped onto the host tree and compared with the optimal cophylogenies. This allows a test of the significance of phylogenetic similarity and is given as a p-value that represents the mean percentage of times that random trees were recovered with the same or a greater number of codivergences as observed in the optimal cophylogenies.
Results and Discussion
Rates of Evolutionary Change in GBV-C
Our Bayesian coalescent analysis revealed that the mean rate of nucleotide substitution in GBV-C is in the range of 10−2 to 10−3 sub/site/year (Table 1) and hence equivalent to that expected for RNA viruses, including other members of the Flaviviridae (Jenkins et al. 2002; Hanada et al. 2004). Notably, this rate estimate is four orders of magnitude higher than those based on the assumption of host-virus codivergence, and in no case did the lowest 95% HPD value encompass a value of ~10−7 sub/site/year. The highest mean rate was observed in the E2 region, at 2.2 × 10−2 sub/site/year, and the lowest in the 5′-UTR, at 4 × 10−3 sub/site/year. The far lower substitution rates observed in some previous studies are therefore likely to reflect very small sample sizes. Further, there was little variation among parameter estimates under any demographic or clock model (Table 1), or according to the prior distribution of the substitution rate (results available from the authors upon request), illustrating the robustness of these estimates. Such internal consistency also suggests that recombination, which has been reported in GBV-C (Worobey and Holmes 2001), has had little impact on these rate estimates. Indeed, we found no significant evidence of recombination in any of the four data sets under the GARD method. However, because of the short time span of sampling used, it is possible that these rates have to some extent been inflated by the inclusion of deleterious mutations yet to be removed by purifying selection, including synonymous sites involved in RNA secondary structure (Simmonds and Smith 1999). Indeed, the lowest rates are observed in the data set (5′-UTR) that covers the longest sampling period.
Our analysis of selection pressures also revealed an extremely low rate of nonsynonymous-to-synonymous substitutions per site, with a mean dN/dS ratio of 0.04 across all coding regions (Table 1). This is in agreement with previous observations of extremely conserved amino acid sequences in this virus (Seipp et al. 1996; Shao et al. 2000). Further, although there are likely to be constraints against synonymous substitution in GBV-C (Simmonds and Smith 1999), the low dN/dS values indicate that these constraints are still far weaker than those acting on amino acid sites. Also of note was that one codon, position 188 in E2, was found to be under positive selection in both the FEL and IFEL methods (p = 0.1). In addition, two other codons—positions 110 and 223 in E2—were found to be positively selected with IFEL (p = 0.1). Although the cause of this adaptive evolution is unclear, that the E2 region is a target for immune neutralization (Tacke et al. 1997) hints that these mutations may have been fixed because of their ability to confer immune escape. Whatever the cause, the occurrence of positive selection over this short sampling period is incompatible with the notion that GBV-C is a latent virus.
Analysis of GBV-A-Primate Codivergence
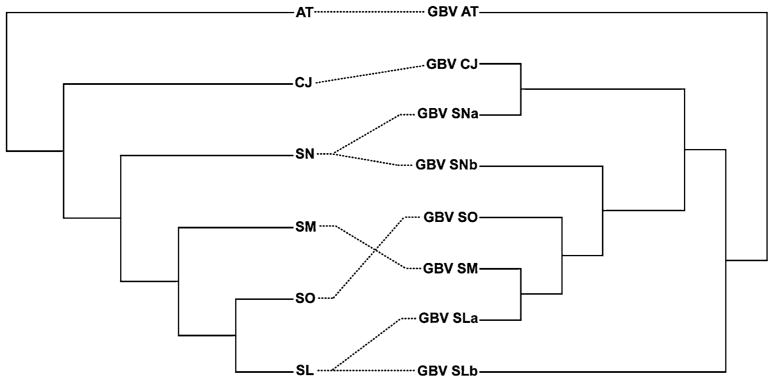
The topological differences between the virus and the host phylogenies are depicted in the tanglegram (Fig. 1; the ML tree for GBV-C is available from the authors upon request). On these data, the null hypothesis of codivergence was rejected (p = 0.363) in 1000 randomized trees. Indeed, some clear mismatches between the virus and the host phylogenies were observed. First, the GBV-A sequences isolated from Saguinus (S.) labiatus (SL) and S. nigricollis (SN) are not monophyletic, with the two component lineages within each species (designated ‘a’ and ‘b’ in both cases) occupying disparate phylogenetic positions. Second, although in the host phylogeny S. labiatus is most closely related to S. oedipus, GBV-A sequences from S. labiatus are more closely related to S. mystax than to S. oedipus. Notably, S. labiatus and S. mystax are sympatric (they both inhabit the Amazonian region of Brazil, Bolivia, and Peru) but are allopatric with respect to S. oedipus, which is found in northern Columbia (Hershkovitz 1977). Such geographical overlap clearly makes host-jumping of GBV-A between S. labiatus and S. mystax a plausible scenario. Further, while previous studies supported codivergence in GBV-A/C, they utilized a far smaller sample of taxa. For example, the study by Charrel et al. (1999) used only three GBV-A (from S. midas, Callitrix jacchus, and Aotus azarai) and two GBV-C (from human and chimpanzee), such that congruence could easily arise by chance alone.
Fig. 1.
Tanglegram of phylogenies of GBV-A virus (right) with the New World monkeys species from which they were isolated (left). Monkey species: SM, Saguinus mystax; SL, Saguinus labiatus; SO, Saguinus oedipus; SN, Saguinus nigricollis; CJ, Callithrix jacchus; AT, Aotus trivirgatus. In the case of SL and SN two phylogenetically distinct lineages were observed in each species data set, denoted ‘a’ and ‘b,’ respectively
More generally, a match between the host and the parasite phylogenies does not, in itself, prove long-term codivergence. Such matching could also be obtained by host-jumping if the probabilities of such events are higher among more closely related hosts, which may represent an important generality in the biology of cross-species transmission (Kuiken et al. 2006), or occurs more frequently among those species that inhabit overlapping (sympatric) geographical distributions. Indeed, successful host-jumping of viruses has been previously documented among primates (Charleston and Robertson 2002) including the simian immunodeficiency viruses (SIVs) (Wertheim and Worobey 2007). Our observation of phylogenetic incongruence in GBV-A indicates that rates of nucleotide substitution in the GB viruses should not be estimated utilizing the divergence times of their primate hosts.
Acknowledgments
We thank Dr. H. Hotta, Dr. A. Sathar, Dr. S. Muerhoff, Dr. J. Stapleton, Dr. S. Ross, Dr. K. Abe, Dr. P. Gobau, Dr. A. M. Bisceglie, and Dr. M. Tacácks for kindly providing additional information concerning the sequences used in this study. The research undertaken in this study was funded in part by NIH Grant GM080533-01.
Contributor Information
Camila M. Romano, Laboratory of Molecular Evolution and Bioinformatics, Department of Microbiology, Biomedical Sciences Institute–ICBII, University of São Paulo, São Paulo, Brazil. Center for Infectious Disease Dynamics, Department of Biology, The Pennsylvania State University, Mueller Laboratory, University Park, PA 16802, USA
Paolo M. de A. Zanotto, Laboratory of Molecular Evolution and Bioinformatics, Department of Microbiology, Biomedical Sciences Institute–ICBII, University of São Paulo, São Paulo, Brazil
Edward C. Holmes, Email: ech15@psu.edu, Center for Infectious Disease Dynamics, Department of Biology, The Pennsylvania State University, Mueller Laboratory, University Park, PA 16802, USA. Fogarty International Center, National Institutes of Health, Bethesda, MD 20892, USA
References
- Abe K. GB virus-C/hepatiitis G virus. Japan J Infect Dis. 2001;54:55–63. [PubMed] [Google Scholar]
- Adams NJ, Prescott LE, Jarvis LM, Lewis JC, McClure MO, Smith DB, Simmonds P. Detection in chimpanzees of a novel flavivirus related to GB virus-C/hepatitis G virus. J Gen Virol. 1998;79:1871–1877. doi: 10.1099/0022-1317-79-8-1871. [DOI] [PubMed] [Google Scholar]
- Birkenmeyer LG, Desai SM, Muerhoff AS, Leary TP, Simons JN, Montes CC, Mushahwar IK. Isolation of a GB virus-related genome from a chimpanzee. J Med Virol. 1998;56:44–51. doi: 10.1002/(sici)1096-9071(199809)56:1<44::aid-jmv8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Bukh J, Apgar CL. Five new or recently discovered (GBV–A) virus species are indigenous to New World monkeys and may constitute a separate genus of the Flaviviridae. Virology. 1997;17:429–436. doi: 10.1006/viro.1997.8461. [DOI] [PubMed] [Google Scholar]
- Canavez FC, Moreira MAM, Ladasky JJ, Pissinatti A, Parham P, Seuanez HN. Molecular phylogeny of New World primates (Platyrrhini) based on β2-microglobulin DNA sequences. Mol Phylogenet Evol. 1999;12:74–82. doi: 10.1006/mpev.1998.0589. [DOI] [PubMed] [Google Scholar]
- Charleston MA. Jungles: a new solution to the host/parasite phylogeny reconciliation problem. Math Biosci. 1998;149:191–223. doi: 10.1016/s0025-5564(97)10012-8. [DOI] [PubMed] [Google Scholar]
- Charleston MA, Page RDM. Treemap, version 20. 2002 Available at: http://www.taxonomy.zoology.gla.ac.uk/mac/treemap/index.html.
- Charleston MA, Robertson DL. Preferential host switching by primate lentiviruses can account for phylogenetic similarity with the primate phylogeny. Syst Biol. 2002;51:528–535. doi: 10.1080/10635150290069940. [DOI] [PubMed] [Google Scholar]
- Charrel RM, De Micco P, de Lamballerie X. Phylogenetic analysis of GB viruses A and C: evidence for codivergence between virus isolates and their primate hosts. J Gen Virol. 1999;80:2329–2335. doi: 10.1099/0022-1317-80-9-2329. [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Suzuki Y, Gojobori T. A large variation in the rates of synonymous substitution for RNA viruses and its relationship to a diversity of viral infection and transmission modes. Mol Biol Evol. 2004;21:1074–1080. doi: 10.1093/molbev/msh109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershkovitz P. Living New World monkeys (Platyrrhini) University of Chicago Press; Chicago: 1977. [Google Scholar]
- Jacobs SC, Larson A, Cheverud JM. Phylogenetic relationships and orthogenetic evolution of coat color among tamarins (genus Saguinus) Syst Biol. 1995;44:515–532. [Google Scholar]
- Jenkins GM, Rambaut A, Pybus OG, Holmes EC. Rates of molecular evolution in RNA viruses: a quantitative phylogenetic analysis. J Mol Evol. 2002;54:152–161. doi: 10.1007/s00239-001-0064-3. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Apichartpiyakul C, Handajani R, Ishido S, Hotta H. GB virus C/hepatitis G virus (GBV-C/HGV) infection in Chiang Mai, Thailand, and identification of variants on the basis of 5′-untranslated region sequences. Arch Virol. 1997;142:2433–2445. doi: 10.1007/s007050050253. [DOI] [PubMed] [Google Scholar]
- Kosakovsky Pond SL, Frost SD. Datamonkey: rapid detection of selective pressure on individual sites of codon alignments. Bioinformatics. 2005;21:2531–2533. doi: 10.1093/bioinformatics/bti320. [DOI] [PubMed] [Google Scholar]
- Kuiken T, Holmes EC, McCauley J, Rimmelzwaan GF, Williams CS, Grenfell BT. Host species barriers to influenza virus infections. Science. 2006;312:394–397. doi: 10.1126/science.1122818. [DOI] [PubMed] [Google Scholar]
- Leary TP, Muerhoff AS, Simons JN, Pilot-Matias TJ, Erker JC, Chalmers ML, Schlauder GG, Dawson GJ, Desai SM, Mushahwar IK. Sequence and genomic organization of GBV-C: a novel member of the flaviviridae associated with human non–A-E hepatitis. J Med Virol. 1996;48:60–67. doi: 10.1002/(SICI)1096-9071(199601)48:1<60::AID-JMV10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Linnen J, Wages J, Jr, Zhang-Keck ZY, Fry KE, Krawczynski KZ, Alter H, Koonin E, Gallagher M, Alter M, Hadziyannis S, Karayiannis P, Fung K, Nakatsuji Y, Shih JW, Young L, Piatak M, Jr, Hoover C, Fernandez J, Chen S, Zou JC, Morris T, Hyams KC, Ismay S, Lifson JD, Hess G, Foung SK, Thomas H, Bradley D, Margolis H, Kim JP. Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science. 1996;26:505–508. doi: 10.1126/science.271.5248.505. [DOI] [PubMed] [Google Scholar]
- Moaven LD, Hyland CA, Young IF, Bowden DS, McCaw R, Mison L, Locarnini SA. Prevalence of hepatitis G virus in Queensland blood donors. Med J Aust. 1996;7:369–371. doi: 10.5694/j.1326-5377.1996.tb125019.x. [DOI] [PubMed] [Google Scholar]
- Nakai K, Win KM, Oo SS, Arakawa Y, Abe K. Molecular characteristic-based epidemiology of hepatitis B, C, and E viruses and GB virus C/hepatitis G virus in Myanmar. J Clin Microbiol. 2001;39:1536–1539. doi: 10.1128/JCM.39.4.1536-1539.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao H, Okomoto H, Fukuda M, Tsuda F, Mitsui T, Masuko K, Iizuka H, Miyakawa Y, Mayumi M. Mutation rate of GB virus C/hepatitis G virus over the entire genome and in subgenomic regions. Virology. 1997;233:43–50. doi: 10.1006/viro.1997.8615. [DOI] [PubMed] [Google Scholar]
- Pavesi A. Origin and evolution of GBV-C/hepatitis G virus and relationships with ancient human migrations. J Mol Evol. 2001;53:104–113. doi: 10.1007/s002390010198. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Sarrazin C, Rüster B, Lee JH, Kronenberger B, Roth WK, Zeuzem S. Prospective follow-up of patients with GBV-C/HGV infection: specific mutational patterns, clinical outcome, and genetic diversity. J Med Virol. 2000;62:191–198. doi: 10.1002/1096-9071(200010)62:2<191::aid-jmv10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Seipp S, Wahl R, Mueller H, Stremmel W, Theilmann L, Goeser T. Sequence analysis of hepatitis GB virus C (GBV-C) isolates from 14 patients. Virus Res. 1996;46:81–88. doi: 10.1016/s0168-1702(96)01377-9. [DOI] [PubMed] [Google Scholar]
- Shao L, Shinzawa H, Zhang X, Smith DB, Watanabe H, Mitsuhashi H, Saito K, Saito T, Togashi H, Takahashi T. Diversity of hepatitis G virus within a single infected individual. Virus Genes. 2000;21:215–221. doi: 10.1023/a:1008195631870. [DOI] [PubMed] [Google Scholar]
- Simmonds P. 2000 Fleming Lecture. The origin and evolution of hepatitis viruses in humans. J Gen Virol. 2001;82:693–712. doi: 10.1099/0022-1317-82-4-693. [DOI] [PubMed] [Google Scholar]
- Simmonds P, Smith DB. Structural constraints on RNA virus evolution. J Virol. 1999;73:5787–5794. doi: 10.1128/jvi.73.7.5787-5794.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JN, Leary TP, Dawson GJ, Pilot-Matias TJ, Muerhoff AS, Schlauder GG, Desai SM, Mushahwar IK. Isolation of novel virus-like sequences associated with human hepatitis. Nat Med. 1995;1:564–569. doi: 10.1038/nm0695-564. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Katayama K, Fukushi S, Kageyama T, Oya A, Okamura H, Tanaka Y, Mizokami Y, Gojobori T. Slow evolutionary rate of GB virus C/hepatitis G virus. J Mol Evol. 1999;48:383–389. doi: 10.1007/pl00006482. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods) Version 4. Sinauer Associates; Sunderland, MA: 2003. [Google Scholar]
- Tacke M, Kiyosawa K, Stark K, Schlueter V, Ofenloch-Haehnle B, Hess G, Engel AM. Detection of antibodies to a putative hepatitis G virus envelope protein. Lancet. 1997;1:318–320. doi: 10.1016/S0140-6736(96)06461-6. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheim JO, Worobey M. A challenge to the ancient origin of SIVagm based on African green monkey mitochondrial genomes. PLoS Pathog. 2007;3:e95. doi: 10.1371/journal.ppat.0030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worobey M, Holmes EC. Homolgous recombination in GB virus C/hepatitis G virus. Mol Biol Evol. 2001;18:254–261. doi: 10.1093/oxfordjournals.molbev.a003799. [DOI] [PubMed] [Google Scholar]