Abstract
Cancer development, progression, and metastasis are multistep processes. Accumulating evidence suggests that reactive oxygen species (ROS) are critically involved in cancer cell functions. This Forum reviews our current understanding of the important and paradoxical role of ROS in the regulation of tumor-associated cell properties, genes, and signaling pathways. The six reviews in this Forum showcase the up-to-date knowledge on how ROS modulate or interact with the p53 protein, epithelial–mesenchymal transition, tumor stromal cells, angiogenesis, and cancer stem cells, which are essential factors in cancer development and metastasis. The contributions demonstrate that ROS levels in cancer cells are tightly controlled, which brings promises and challenges in the development of novel ROS-targeted anticancer therapies. Further understanding of the biological mechanisms underlying the effects of oxidative stress on tumor growth and metastasis will contribute to the advancement of cancer biology and cancer treatment. Antioxid. Redox Signal. 16, 1212–1214.
Introduction
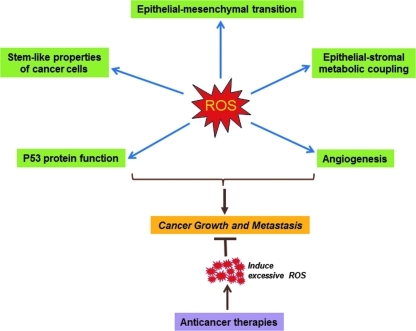
Oxidative modification of DNA, proteins, and lipids by reactive oxygen species (ROS) plays a role in a wide range of common diseases and degenerative conditions. ROS can be generated by both endogenous and exogenous sources, some of which are known to be carcinogenic. While ROS have been shown to be deleterious to cells, they can also function as signaling molecules to induce cellular adaptive responses to stress or adverse microenvironment. In light of a large body of evidence indicating that ROS are key players in tumor formation and progression and the increasing complexity of ROS-related biology, there is a compelling reason to review and summarize current data on this field and disseminate fresh ideas that may evolve to new research paradigms. This Forum attempts to provide thought-provoking overviews of existing concepts, novel findings, controversies, and challenges in regard to the role of ROS in cancer cell growth and metastasis and the prospects of targeting ROS signaling in cancer treatment (Fig. 1).
FIG. 1.
The role of reactive oxygen species (ROS) in cancer cell growth and metastasis. The schematic diagram provides an overview of ROS-regulated cellular processes involved in tumor growth and progression, as referred to in the Forum contributions.
ROS and p53
Mutations of the p53 gene are found in more than 50% of malignancies and are the single most common molecular abnormality in human cancer (6). Loss of p53 function is associated with loss of cell-cycle control, decreased apoptosis, and genomic instability. The p53 protein can be regulated by different post-translational modifications such as phosphorylation of serine and/or threonine residues, acetylation, ubiquitylation, or sumoylation of lysines residues. The review article by Maillet and Pervaiz (7) summarizes recent findings in another critical regulatory mechanism—the redox modifications of p53. It is documented that ROS can function upstream of p53 and regulate p53 activity and that ROS production can also be a downstream effect of p53 activation. The redox status and consequently the function of p53 can be affected by redox molecules such as glutathione and thioredoxin/thioredoxin reductase. For example, S-glutathionylation or oxidation of p53 cysteine residues under oxidative stress was associated with a loss of p53 protein function. The article further describes that as a transcription factor, p53 protein can influence cellular ROS levels and oxidative stress by regulating the expression of pro- or antioxidant gene. In addition, the modulation of mitochondrial respiration by p53 and the resulting ROS production are also discussed. It is concluded that crosstalk between p53 and ROS signaling networks plays an important role in cell cycle and apoptosis regulation.
Regulation of Epithelial–Mesenchymal Transition by ROS
The review by Giannoni et al. (3) is devoted to the role of ROS in epithelial–mesenchymal transition (EMT), which is a complex process associated with alterations in epithelial cell junctions, changes in cell morphology, reorganization of cell cytoskeleton, expression of fibroblastic markers, and enhancement of cell migration and invasion (5). EMT promotes tumor progression, enabling cancer cells to evade from their homeland and to colonize foreign tissues. The signals able to induce EMT have been extensively studied. This review focuses on signaling mechanisms underlying the redox control of EMT and the importance of a pro-oxidant microenvironment in driving tumor progression. One of the well-established pivotal regulators of EMT is transforming growth factor-beta (TGF-β). Interestingly, TGF-β-induced EMT is dependent on ROS production. The article also describes that cancer-associated fibroblasts (CAFs) may exert their known effect on EMT programming by eliciting a pro-oxidant and proinflammatory signature in cancer cells. In addition, ROS-mediated regulation of Snail, Src, and matrix metalloproteinases as well as the reverse effect of these EMT inducers on ROS production is discussed. The article raises an interesting question. As is known, stem-like and chemo- or radioresistant cancer cells normally possess low ROS content (2). On the other hand, EMT is associated with cancer stem cells (CSCs). The seeming contradiction between these two notions might reside in the dynamic and temporal regulation of ROS levels required for execution of a cellular process in stem cells.
Metabolic Coupling of Stromal and Epithelial Cells by ROS
In another review related to CAFs (8), Pavlides et al. delineate a new paradigm termed “reverse Warburg effect,” in which cancer cells secrete ROS such as hydrogen peroxide. As a consequence, elevated oxidative stress in CAFs drives autophagy, mitophagy, and aerobic glycolysis. This parasitic metabolic-coupling converts the stroma into a factory for the local production of recycled and high-energy nutrients (such as lactate)—to fuel oxidative mitochondrial metabolism in cancer cells. The Caveolin-1 (Cav-1) gene, a glycolysis regulator, is proposed as a key mediator in the tumor-stroma coevolution promoted by oxidative stress. Mitochondrial dys-function and autophagy in CAFs, triggered by ROS from cancer cells, lead to Cav-1 protein degradation and thus aerobic glycolysis in CAFs. Ultimately, high-energy lactate and glutamine are shuttled from CAFs to cancer cells. Further understanding of this ROS- and Cav-1-mediated interplay between cancer cells and CAFs may provide novel targets for the development of more potent anticancer therapies by blocking energy transfer between cancer cells and CAFs, thereby metabolically uncoupling tumor cells from their surrounding stroma.
Regulation of Angiogenesis by NADPH Oxidases
Angiogenesis is another important factor for tumor growth and metastasis. ROS has emerged as a critical regulator of angiogenesis. This link is reviewed in this Forum by Coso et al. (1). Their article focuses on the role of NADPH oxidases in angiogenesis. It highlights that the NADPH oxidase family of ROS-generating enzymes are a key source of ROS and thus play an important role in redox signaling within tumor, endothelial, and immune cells to promote tumor angiogenesis. Of note, NADPH oxidases are the only enzymes whose primary function is to generate superoxide/ROS, while other enzymes produce superoxide as a by-product. The article summarizes the key structural features, expression patterns, activity regulations, and localizations of each of the NADPH oxidases and their respective regulatory subunits. The signaling pathways induced by NADPH oxidase-derived ROS are also outlined. Knowledge of intricate ROS signaling pathways, identification of the culprit NADPH oxidases in tumor development and progression, and development of specific inhibitors of these NADPH oxidases and associated redox signaling components could provide useful therapeutic strategies for preventing tumor-associated angiogenesis and the revascularization following current antiangiogenic treatment.
ROS and CSCs
Recently, much effort has gone into understanding the potential role of CSCs in cancer progression and metastasis. CSCs are integral parts of pathophysiologic mechanisms of tumor progression, metastasis, and chemo/radioresistance. To date, molecular events that govern the survival and self-renewal of therapy-resistant CSCs are poorly defined. Nevertheless, as a rapidly evolving research field, studies on ROS are increasingly attracting much attention. The review by Shi et al. (9) summarizes the current research of the function and regulation of ROS in normal stem cells and CSCs, which are known to reside in niches characterized by low ROS, a critical factor in maintaining stem cell properties such as self-renewal. This review describes in detail the regulation of ROS levels by multiple signaling pathways in normal stem cells and CSCs, and the prospects of utilizing ROS elevation by exogenous xenobiotics to eliminate CSCs. Currently, little is known about whether ROS regulate different signaling pathways in stem cells and differentiated cells and whether ROS play a different role in these cells. Elucidation of ROS function in CSCs will enrich our knowledge of cancer development and metastasis.
Promises and Challenges of Exploiting ROS Signaling in Anticancer Therapies
Finally, Gupta et al. (4), in a comprehensive review, discuss current progress in identifying the in-depth biochemical mechanisms underlying ROS-mediated inflammation, cellular transformation, tumor cell survival, tumor cell proliferation, and invasion, angiogenesis, and metastasis. The article also brings to attention the dual role of ROS in cancer cell function. For example, ROS have been shown to inhibit or enhance cancer cell proliferation in different cancer cells. Thus, both pro-oxidant- and antioxidant-based agents have been developed for cancer prevention and therapy. Pro-oxidant-based anticancer agents can increase ROS production and decrease the antioxidant capacity of cancer cells, while the antioxidant-based agents can scavenge intracellular ROS. A combination of these approaches is very effective in some occasions. The article presents a panoramic view of the status of development and effectiveness of novel anticancer therapies targeting ROS signaling—a potential Achilles' heel of cancer cell survival. The outstanding questions and future directions raised by our current understanding of ROS in cancer are discussed in detail.
Summary
This Forum illustrates the progress and critical issues in the understanding of ROS in cancer development and metastasis. Current insight, although far from being comprehensive and complete, justifies a closer look at the molecular mechanisms of how cancer-associated genes and pathways are regulated by specific ROS. Cancer development and treatment involves ROS. On the other hand, increase or decrease of ROS can affect tumor properties and treatment effect. It is hoped that this Forum can stimulate substantial interest in more mechanistic studies on the biology of ROS in cancer and in the development of effective ROS-targeted cancer treatment modalities.
Abbreviations Used
- CAF
cancer-associated fibroblast
- Cav-1
Caveolin-1
- CSC
cancer stem cells
- EMT
epithelial–mesenchymal transition
- ROS
reactive oxygen species
- TGF-β
transforming growth factor-beta
Acknowledgments
I thank National Institutes of Health (CA151610), QVC and the Fashion Footwear Association of New York Charitable Foundation, and the Avon Foundation (02-2010-068) for the support.
References
- 1.Coso S. Harrison I. Harrison CB. Vinh A. Sobey C. Drummond GR. Williams E. Selemidis S. NADPH oxidases as regulators of tumor angiogenesis: current and emerging concepts. Antioxid Redox Signal. 2012;16:1229–1247. doi: 10.1089/ars.2011.4489. [DOI] [PubMed] [Google Scholar]
- 2.Diehn M. Cho RW. Lobo NA. Kalisky T. Dorie MJ. Kulp AN. Qian D. Lam JS. Ailles LE. Wong M. Joshua B. Kaplan MJ. Wapnir I. Dirbas FM. Somlo G. Garberoglio C. Paz B. Shen J. Lau SK. Quake SR. Brown JM. Weissman IL. Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giannoni E. Parri M. Chiarugi P. EMT and oxidative stress: a bidirectional interplay affecting tumor malignancy. Antioxid Redox Signal. 2012;16:1248–1263. doi: 10.1089/ars.2011.4280. [DOI] [PubMed] [Google Scholar]
- 4.Gupta SC. Hevia D. Patchva S. Park B. Koh W. Aggarwal BB. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal. 2012;16:1295–1322. doi: 10.1089/ars.2011.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalluri R. Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 7.Maillet A. Pervaiz S. Redox regulation of p53, redox effectors regulated by p53: a subtle balance. Antioxid Redox Signal. 2012;16:1285–1294. doi: 10.1089/ars.2011.4434. [DOI] [PubMed] [Google Scholar]
- 8.Pavlides S. Vera I. Gandara R. Sneddon S. Pestell RG. Mercier I. Martinez-Outschoorn UE. Whitaker-Menezes D. Howell A. Sotgia F. Lisanti MP. Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxid Redox Signal. 2012;16:1264–1284. doi: 10.1089/ars.2011.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi X. Zhang Y. Zheng J. Pan J. Reactive oxygen species in cancer stem cells. Antioxid Redox Signal. 2012;16:1215–1228. doi: 10.1089/ars.2012.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]