Abstract
Significance: Reactive oxygen species (ROS), byproducts of aerobic metabolism, are increased in many types of cancer cells. Increased endogenous ROS lead to adaptive changes and may play pivotal roles in tumorigenesis, metastasis, and resistance to radiation and chemotherapy. In contrast, the ROS generated by xenobiotics disturb the redox balance and may selectively kill cancer cells but spare normal cells. Recent Advances: Cancer stem cells (CSCs) are integral parts of pathophysiological mechanisms of tumor progression, metastasis, and chemo/radio resistance. Currently, intracellular ROS in CSCs is an active field of research. Critical Issues: Normal stem cells such as hematopoietic stem cells reside in niches characterized by hypoxia and low ROS, both of which are critical for maintaining the potential for self-renewal and stemness. However, the roles of ROS in CSCs remain poorly understood. Future Directions: Based on the regulation of ROS levels in normal stem cells and CSCs, future research may evaluate the potential therapeutic application of ROS elevation by exogenous xenobiotics to eliminate CSCs. Antioxid. Redox Signal. 16, 1215–1228.
Introduction
Reactive oxygen species (ROS), including superoxide (O2−), hydrogen peroxide (H2O2), and the hydroxyl free radical (HO•), are formed by the capture of electrons by an oxygen atom (43). Low-to-moderate levels of ROS are essential for cellular proliferation, differentiation, and survival (113). Normal cells regulate the intracellular ROS content within a nontoxic range by balancing the ROS generation and scavenging systems. Chronically increased endogenous ROS lead to adaptive changes that play pivotal roles in tumorigenesis, metastasis, and drug resistance in diverse types of cancer cells. However, ROS generation by xenobiotics may disturb the redox balance and selectively kill cancer cells without significant toxicity to normal cells (113).
Cancer cells are believed to be derived hierarchically from a small subset of malignant cells that have a high capacity of self-renewal and differentiation—namely cancer stem cells (CSCs) or tumor-initiating cells (84). CSCs have the high potential to generate tumors. For instance, CSCs in human acute myeloid leukemia (AML) harbor CD34+/CD38− surface antigens and have the potential to induce leukemia when transplanted into nonobese diabetic severe combined immunodeficiency disease (NOD/SCID) mice, while non-CD34+/CD38− cells do not have this potential (102). In a xenograft model of human breast cancer, as few as 200 CSCs harboring CD44+/CD24− surface antigens were able to form tumors in immunocompromised mice, while more than 500,000 non-CD44+/CD24− cells were not able to do so (6, 107). Similarly, 100 brain tumor CSCs identified as CD133+ cells could form tumors with the same phenotype as the original tumor when transplanted into NOD/SCID mouse brains, while 100,000 CD133− tumor cells could not do so (105). These findings support the characterization that CSCs have a high potential to grow into tumors.
The biological effects of ROS and the biological mechanisms regulating the level of ROS have been studied in cancer cells as a whole, and little is known about these issues specifically in the subpopulation of CSCs. Although evidence has accumulated in normal stem cells, particularly in hematopoietic stem cells (HSCs), a significant gap in knowledge remains in malignant stem cells. This review will focus on the discussion on the regulation of ROS levels in normal stem cells and CSCs, and the potential therapeutic implication of ROS elevation by exogenous xenobiotics to eliminate CSCs.
ROS and Their Regulatory System
ROS are broadly defined as oxygen-containing reactive chemical species. ROS are mainly divided into two classes: free radical ROS and nonradical ROS. The former class, including O2−, nitric oxide (NO•), and HO•, is characterized by containing one or more unpaired electron(s) in their outer molecular orbital. The latter class includes H2O2, ozone, peroxynitrite (ONOO−), and hydroxide, which do not have unpaired electron(s) but are chemically reactive to generate free-radical ROS under certain conditions with or without enzymatic catalysis (Fig. 1) (113). Under physiologic conditions, low-to-moderate levels of ROS interact with macromolecules by reversible oxidative modifications. This process is crucial for cell development because of the wide involvement of ROS in cellular proliferation, differentiation, and trafficking of intracellular vesicles (94, 113). Additionally, ROS elevation is an important mechanism that eliminates pathogens and foreign particles in phagocytic cells (120). However, due to their high chemical reactivity, excessive amounts of ROS, regardless of endogenous or exogenous origin, can lead to cellular senescence, death, or transformation by causing the irreversible peroxidation of lipids, amino acids, nucleic acids, and carbohydrates (94, 114).
FIG. 1.
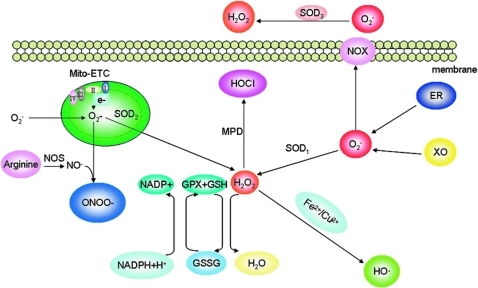
Cells maintain redox homeostasis through a balance of generation and elimination of reactive oxygen species (ROS). Superoxide (O2−), the principal form of ROS, comes from the byproducts of electron leakage of mitochondrial electron transport chain (Mito-ETC), endoplasmic reticulum (ER), and membrane-located NAD(P)H oxidase complex (NOX). O2− can be rapidly converted to hydrogen peroxide (H2O2) by superoxide dismutase (SOD); H2O2 can be catalyzed to release hydroxyl radicals (HO•) in the presence of Fe2+ or Cu2+ ions. H2O2 can be converted by myeloperoxidase (MPD) to hypochlorous acid (HOCl), a stronger oxidant. H2O2 is converted to H2O+O2 by catalase or glutathione peroxidase (GPX). Nitric oxide (NO•), once generated from arginine catalyzed by nitric oxide synthase (NOS), is rapidly converted to peroxynitrite (ONOO−) by reacting with O2−. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Oxidative stress damages both nuclear DNA and mitochondrial DNA and initiates DNA repair signaling pathways (4, 45). Lipid peroxidation is caused by HO•. Lipid oxidation and lipid peroxidation products (e.g., malondialdehyde, 4-hydroxy-2-nonenal) impair biological membrane systems (86). Oxidation in the Fe-S cluster of many proteins may elicit disulfide bond-mediated protein cross-linkage and adduct formation, disabling proteins such as enzymes involved in the mitochondria electron transport chain (ETC). In the case of oxidative modification of 26S and 20S proteasomes, cells may die because of insufficient elimination of damaged proteins (121).
Generation of ROS
The principal source of ROS in mammalian cells comes from the byproducts of electron leakage of mitochondrial ETC. The mutations in nuclear or mitochondrial genes encoding the ETC components can block the electron transfer, leading to electron leakage (Fig. 1). The electrons can then be captured by O2, forming O2−, which is usually converted to H2O2 by manganese (Mn)-containing mitochondrial superoxide dismutase (MnSOD, SOD2), Cu/Zn-containing cytosolic SOD1 or extracellular SOD3 (89). H2O2 diffuses to the nucleus to attack chromosomal DNA. H2O2 can be catalyzed to release HO• in the presence of Fe2+ or Cu2+ ions, which is the Fenton reaction (113). In macrophages and cancer cells, O2− may additionally be generated by a reaction catalyzed by the membrane-located NAD(P)H oxidase complex (NOX) consisting of NOX1, NOX2, NOX3, NOX4, cytochrome c oxidase, cyclo-oxygenase, and endoplasmic reticulum-associated xanthine oxidase (XO) (Fig. 1). NO• is generated from arginine catalyzed by nitric oxide synthase (NOS), which has a diverse tissue distribution with some tissue-specific isoforms: mitochondrial NOS, neuronal NOS, endothelial NOS, and inducible NOS (34). NO• is rapidly converted to ONOO− by reacting with O2−. NO• and ONOO− are also referred to as reactive nitrogen species (RNS). Being oxidative, RNS are often included as ROS. ONOO− can cause the nitration of tyrosine residues, which impairs the activity of the proteins by blocking the phosphorylation and adenylation of these tyrosine residues (22).
Elimination of ROS
The intracellular ROS are maintained at low levels in mammalian cells by powerful scavenger antioxidative enzyme systems, including SOD1, SOD2 and SOD3, catalase, glutathione peroxidase (GPX), and peroxiredoxin. As mentioned earlier, O2−, once formed, is rapidly converted by SODs to H2O2 (Fig. 1). H2O2 is converted to H2O+O2 by peroxisome-located catalase. H2O2 is also converted by GPX to H2O+O2 through coupling with the conversion of reduced glutathione (GSH) to oxidized glutathione (113). The conversion of H2O2 to the highly reactive HO• is catalyzed by Fe2+/Cu2+ (the Fenton reaction) (113). In macrophages, H2O2 can be converted by myeloperoxidase to hypochlorous acid, a strong bacteria-killing oxidant (120).
ROS and Stem Cells
Concept of stem cells
Stem cells are characterized by a high capacity of self-renewal and differentiation (2). Through self-renewal, stem cells maintain the homeostasis of a stem cell pool; through differentiation, stem cells differentiate into terminal cells with diverse morphology and functions. Stem cells are present in at least several types of tissues such as the bone marrow, digestive tract, central nervous system, and epidermis and play an important role in tissue repair and homeostasis (2, 96). Most stem cells in tissues such as bone marrow are in the quiescent state (G0 phase), and they are protected by special microenvironments (niches) (83). The quiescence of stem cells may protect them from the accumulation of DNA replication errors (8). Relatively more cycling stem cells are present in fast-turnover tissues such as intestinal epithelium, mammary epithelium, and hair follicles to maintain homeostasis and regeneration (38). The property of quiescence or slow cycling in stem cells may facilitate resistance to many stressors (e.g., oxidative stress, chemical compounds, and ionizing radiation) (57). Indeed, the quiescent HSC population is resistant to 5-fluorouracil (8).
Microenvironment of stem cells
Compelling evidence has demonstrated that the microenvironments of the HSCs, central nervous stem cells, intestinal epithelium, the hair follicle, and spermatogonial stem cells play a critical role in regulating the characterization of self-renewal and the differentiation of stem cells (38, 83). Two types of niches (osteoblastic and vascular) have been identified (39). The osteoblastic niche is characterized by hypoxia, which may facilitate the maintenance of quiescence of HSCs, while the vascular niche in the bone marrow is characterized by oxygenation that may facilitate the initiation of cycling of HSCs (129). Cipolleschi et al. showed that the colony-forming ability or reconstitution capacity of undifferentiated HSCs was retained after hypoxic culture compared with normoxic culture (26). Hypoxia may prevent the HSC side population (SP) from cycling (95). Similarly, in the hair follicle, the quiescent stem cells reside in the bulge region, while the active proliferating stem cells stay in the bulb region (87). These results suggest that the spatially distinct niches function to maintain stem cells in different states. The niches activate intracellular signaling pathways that induce the quiescent state of HSCs; receptor tyrosine kinase Tie2/Angiopoietin-1 (8), Mpl/Thrombopoietin (130), Wnt/β-catenin (72), and cell adhesion molecules (131) are involved. Additionally, the intracellular ROS level is a critical factor that regulates the quiescent status of HSCs (112). The quiescence of stem cells prevents telomere erosion, accumulation of DNA damage, and loss of the reconstitution capacity (48).
Intracellular ROS and their regulators in stem cells
The low number of stem cells makes it difficult to directly evaluate ROS levels and the redox regulatory mechanism in stem cells. However, interesting evidence has been obtained in HSCs. Similar to the low partial pressure of oxygen, low levels of ROS in niches are of importance for the stemness of HSCs (60). Jang and Sharkis (60) demonstrated that the DCF-DAlow (2,7-dichlorodihydrofluorescein diacetate, the redox-sensitive fluorescent probe) HSC population exhibited more traits of HSCs located within the osteoblastic niche, including high G0 activity and high expressions of the calcium-sensing receptor, N-cadherin, Notch1, Bcrp, telomerase, and p21, which were pivotal in protecting the HSCs from exogenous and endogenous toxins. These markers were lower in the DCF-DAhigh HSC population than in the DCF-DAlow population, and the HSC fraction with DCF-DAhigh was “myeloid shifted” and behaved similar to aged HSCs (60). Several signaling molecules have been reported as being involved in the regulation of ROS in stem cells to maintain the quiescence of HSCs, and are discussed as follows.
Ataxia telangiectasia mutated
Ataxia telangiectasia mutated (ATM) is a critical regulator of the cell cycle and DNA damage repair, particularly for double-strand breaks. Cosentino et al. have shown that ATM reduces the ROS level by activating the glucose-6-phosphate dehydrogenase to promote NADPH production, an essential oxidant cofactor (29). Consistent with the results, ATM knockout mice displayed increased levels of ROS in many organs, and the mechanism may involve the reduced capacity of the major antioxidant enzymes, including catalase, GPX, SOD, and glutathione reductase (58), but the links between ATM and these genes are not clear. This increase of intracellular ROS appears to have deleterious effects (Fig. 2). For instance, the self-renewal capacity of HSCs was found to be decreased in ATM−/− mice compared with wild-type mice, and treatment with an antioxidant, N-acetyl l-cysteine (NAC), dramatically reversed this negative impact on the capability to reconstitute bone marrow hematopoietic cells. These results suggest that ATM may negatively regulate the ROS although the mechanism has not been thoroughly explained.
FIG. 2.
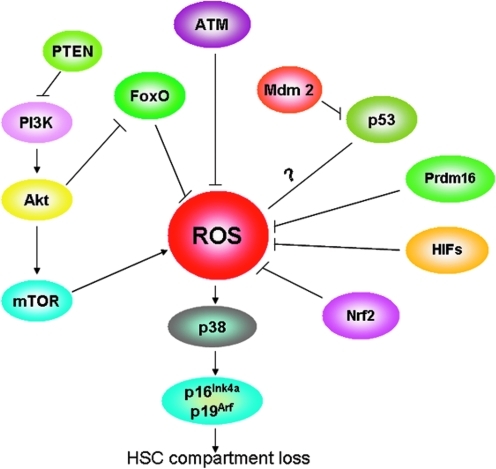
The signal pathways involved in ROS regulation in hematopoietic stem cells (HSCs). The activation of ataxia telangiectasia mutated (ATM) kinase, phosphoinositide 3-kinase (PI3K)/Akt, phosphorylates FoxO transcription factor 3 (FoxO3a), phosphatase and tensin homolog (PTEN), p53, PR domain-containing 16 (Prdm16), hypoxia inducible factor (HIF)-1α, p38 mitogen-activated protein kinase (MAPK), and nuclear factor erythroid-2-related factor 2 (Nrf2) negatively regulate ROS by up-regulating the antioxidant enzymes. PTEN can indirectly decrease ROS by negatively regulating the PI3K/Akt/mammalian target of rapamycin (mTOR), which can up-regulate the ROS levels. The increased ROS level can activate the p38MAPK, which can elevate the expression of the tumor suppressors p16Ink4a and p19Arf, resulting in HSC compartment loss. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Phosphoinositide 3-kinase/Akt
One of the critical downstream signaling pathways of ATM is the phosphoinositide 3-kinase (PI3K)-Akt pathway. HSCs in Akt1- and Akt2-deficient mouse become quiescent and lose the capability of reconstitution (65). These HSCs also have reduced intracellular ROS content, indicating that ROS homeostasis is essential to sustain hematopoiesis and that Akt signaling is involved in the control of ROS levels as well (Fig. 2) (65). In vitro pretreatment of HSCs from Akt1- and Akt2-deficient mice with a low dosage of l-buthionine sulfoximine, an inhibitor of GSH synthesis, can rescue the decreased capability of colony formation (65). Additionally, Chen et al. (21) demonstrated that inhibition of the mammalian target of rapamycin (mTOR) pathway, a key regulator of cellular metabolism and a downstream target of Akt, can drive HSCs from quiescence into rapid cell cycling, with increased mitochondrial biogenesis and elevated levels of ROS.
Phosphorylation of FoxO transcription factor 3a
FoxO, a family of the Forkhead transcription factors, is tightly regulated by PI3K/Akt. The phosphorylation of FoxO transcription factors 3 (FoxO3) by Akt leads to the FoxO3's association with 14-3-3 proteins and the FoxO3's retention in the cytoplasm (16). The sequestration of FoxO3 in the cytoplasm decreases the transcription activity of FoxO3-targeted genes such as SOD2 and catalase, resulting in the elevation of ROS (Fig. 2). Accordingly, FoxO3a knockout mice exhibit increased levels of H2O2 with reduced activity of SOD2 and catalase (82). In an independent study, HSCs in FoxO3a/FoxO1/FoxO4 triple-knockout mice exhibit hyper-proliferation that leads to HSC pool exhaustion in the bone marrow, while treatment with NAC in these mice effectively improved the size of the HSC pool (112). Thus, FoxO proteins seem to be essential for maintaining the low level of intracellular ROS in HSCs.
Phosphatase and tensin homolog
Phosphatase and tensin homolog (PTEN), a negative regulator of PI3K/Akt, has the ability to promote cell differentiation and proliferation, leading to the HSC pool exhaustion (Fig. 2) (128). HSCs in conditional PTEN-deleted mice do not exhibit increased ROS, and the reconstitution capacity of HSCs in these PTEN-negative mice cannot be rescued by NAC treatment (76). Obviously, further exploration about the relationship between the ROS and PTEN is still needed.
p53
Tumor suppressor p53, a transcription factor that transactivates a plethora of genes, is involved in regulating proliferation, differentiation, senescence, and apoptosis in response to DNA damage signals such as ROS (50). In an early report, p53 was shown to elevate ROS by activating ROS-inducing genes such as Pig1, Pig8, and Pig12 (35). In a conflicting report, p53 was shown to protect stem cells from ROS damage through up-regulation of GPX, p53-induce genes (PIGs) and down-regulation of nitric oxide synthase 2 (NOS2), cyclooxygenase 2 (COX2), and led to exhaustion of stem cells (Fig. 2) (1). Mdm2, an E3 ubiquitin ligase responsible for p53 degradation, is essential for cell survival. In vivo data demonstrate that Mdm2 is necessary to control ROS-induced p53 levels for sustainable hematopoiesis later in life (1). By utilizing a rare p53 mutation (p53R172P, i.e., arginine to proline at amino acid 172) that lacks apoptotic activity but with cell-cycle arrest activity, Abbas et al. (1) produced Mdm2−/− p53515C (encoding p53R172P) mice, which have a normal HSC count in fetal livers but a depleted HSC count in postnatal bone marrows. After birth, these mice had elevated ROS and expression of p53R172P, which leads to cell death in the hematopoietic compartment through activating p16Ink4a. This postnatal loss of HSC could partially be antagonized with antioxidant treatment.
PR domain-containing 16
PR domain-containing 16 (Prdm16), a zinc-finger transcription factor and preferentially expressed by stem cells throughout the nervous and hematopoietic systems that control the development of blood cells (e.g., leukocytes, platelets) and brown fat, has also been implicated in the regulation of ROS in HSCs (24). Prdm16 regulates leukemogenesis (24) and promotes neural stem/progenitor cell functions at least in part by promoting hepatocyte growth factor expression and inhibiting ROS generation. Prdm16 deficiency can lead to an increase in ROS levels, increased cell death, altered cell-cycle distribution, and depletion of stem cells (Fig. 2). Administration of anti-oxidant NAC to Prdm16-deficient mice could partially rescue the neural stem/progenitor cell dysfunction, indicating that Prdm16 could function as a modulator of oxidative stress in stem cell maintenance.
Hypoxia inducible factors
Hypoxia inducible factor-1α (HIF-1α) protein, a basic helix-loop-helix-PAS (Per-ARNT-Sim)-type transcriptional regulator under hypoxia, is involved in the maintenance of HSCs. ROS production is paradoxically increased under hypoxia, probably via electron leakage from ubisemiquinone to molecular oxygen (11). The depletion of molecular oxygen stabilizes and activates HIF-1α (20). It is known that HIF-1α activates vascular endothelial growth factor (VEGF), which has a strong correlation with cancer progression and metastasis, by directly binding to the VEGF promoter in response to hypoxia. An increased production of ROS was observed in HIF-1αΔ/Δ CD34−LSK (Lineage− Sca-1+c-Kit+ population) cells, which may account for the loss of HSCs through senescence-associated events (110). The HSCs in HIF-1α deficient mice lose their cell-cycle quiescence, and HSC numbers decreased in a p16Ink4a/p19Arf-dependent manner in various stress settings (110).
p38 mitogen-activated protein kinase
The mitogen-activated protein kinases (MAPKs) family, including extracellular signal-regulated protein kinases, c-Jun N-terminal kinases, and p38, plays an essential role in cell proliferation, differentiation, migration, and apoptosis by modulating gene transcription in the nucleus. The activation of p38MAPK through ROS-induced activation of apoptosis signal-regulated kinase 1 (ASK1) could lead to increased expression of p16Ink4a and p19Arf, which induced the loss of HSCs (Fig. 2) (59). The inhibition of p38MAPK rescued the ROS-induced defects in HSCs' repopulating capacity and maintenance of HSC quiescence, indicating that the ROS-p38MAPK pathway contributed to exhaustion of the stem cell population (69). These data support that p38MAPK activation can induce the loss of HSC self-renewal capacity (Fig. 2).
Nuclear factor erythroid-2-related factor 2
The nuclear factor erythroid-2-related factor 2 (Nrf2), a bZip transcription factor (18, 66), is also involved in the regulation of intracellular ROS. Once activated in response to a range of oxidative and electrophilic stimuli, Nrf2 mediates the expression of a spectrum of cytoprotective genes, including GSH, Trx, heme oxygenase-1, and the members of the glutathione-S-transferase family (111, 118). PhaseIIenzymes, NAD(P)H: quinine oxidoreductase (NQO1) is also regulated by Nrf2. Therefore, Nrf2 may help in maintaining the quiescence of HSCs by maintaining a reduced redox state (Fig. 2). However, Merchant et al. (81) found that the Nrf2−/− hematopoietic stem/progenitor cell compartment had increased rates of apoptosis without elevated levels of ROS, and the administration of NAC did not rescue the apoptosis of Nrf2−/− hematopoietic stem/progenitor cells; these findings suggest that Nrf2 may regulate hematopoiesis and HSCs survival in a ROS-independent manner. The precise mechanism of Nrf2 that maintains HSCs still needs to be elucidated.
In summary, ATM kinase, PI3K/Akt, FoxO3a, PTEN, p53, Prdm16, HIF-1α, p38MAPK, and Nrf2 are important for HSCs to maintain low levels of ROS, which may facilitate chemo/radio resistance and self-renewal of HSCs (Fig. 2). Mice deficiency in ATM, FoxO3a, Prdm16, or p53 exhibits elevated ROS levels in the HSC compartment that lead to a rapid exhaustion of HSCs. In vivo data support the fact that NAC restores the self-renewal capacity of HSCs in the aforementioned genes-deleted mice. These pieces of evidence support a causal effect of ROS on HSC demise.
Although the majority of the current literature supports the concept that low ROS facilitates the quiescent status of stem cells, one notable exception is that proliferative, self-renewing neural stem cells have a high level of ROS, and they are highly responsive to ROS stimulation with ROS serving as a second messenger that regulates normal cellular processes (75).
ROS and Cancer Cells
Cancer cells produce more ROS than normal cells do (109); ROS are involved in each stage of cancer development, including initiation, promotion, and progression (113). The increased levels of intracellular ROS can cause damage to DNA, lipids, and proteins, making cells more vulnerable to injury by further ROS insults induced by exogenous agents. This aspect has been exploited as an effective approach to selectively kill cancer cells without causing significant toxicity to normal cells (94, 113). The increase in intracellular ROS in cancer cells may involve a diversity of mechanisms. The intrinsic mechanism of intracellular ROS increase may result from the activation of oncogenes, inactivation of tumor suppressor genes, high metabolism, and mitochondrial dysfunction (113). It is common in cancers for metabolism to be very active under the drive of oncogenic signals, for example, constitutively active mutant Ras, Bcr-Abl, and c-Myc (54, 100, 115). Irani et al. (54) showed that the Ras-transformed NIH 3T3 fibroblasts produced O2− through the activation of NOX. Ras oncogenic signaling also suppressed the antioxidant molecule sestrin 1 (SESN1) (71). Transformation of the hematopoietic cell lines Ba/F3, 32Dc13, and MO7e with Bcr-Abl resulted in increased intracellular ROS compared with untransformed cells (100). The increase in ROS was directly due to Bcr-Abl, because it could be blocked by the small-molecule tyrosine kinase inhibitor ST1571. The loss-of-function mutation of Bcr-Abl such as Y177F failed to elevate the intracellular ROS levels in contrast with wild-type Bcr-Abl. Further, Bcr-Abl may increase ROS via activation of the PI3K/mTOR pathway, because inhibition of PI3K or mTOR attenuates the Bcr-Abl-induced ROS (70). Similarly, the activation of c-Myc could increase ROS without the induction of apoptosis, while the treatment with antioxidant NAC decreased the number of c-Myc-induced hMre11 signals and improved cell survival after c-Myc activation (115).
Tumor suppressor gene p53 decreases ROS by upregulating the transcription of antioxidant protein TIGAR (p53-induced glycolysis and apoptosis regulator), which inhibits glycolysis and SCO2 that can regulate the mitochondrial ETC (126). Splenocytes and thymocytes of p53−/− mice exhibit elevated ROS in comparison with wild-type mice.
Isocitrate dehydrogenase 1 (IDH1) and isocitrate dehydrogenase 2 (IDH2) are NADP+ dependent enzymes located in the cytoplasm and mitochondria, respectively, that convert isocitrate to α-ketoglutarate and generate NAD(P)H. Genome-wide sequencing studies also indicated somatic mutations in isocitrate dehydrogenase 1/2 (IDH1/2) in AML and central nervous system (CNS) tumors (119, 123). These mutations (e.g., R132 IDH1, R172 IDH2, and R140 IDH2) impair the enzyme's affinity for its substrate and dominantly inhibit wild-type IDH1/2 enzyme activity through the formation of catalytically inactive dimers (133), resulting in the depletion of NAD(P)H and GSH to an increase in ROS. Mutations in IDH1/2 also lead to the accumulation of 2-hydroxyglutarate, which is capable of elevating intracellular ROS levels to induce the differentiation of leukemic blasts (3). Additionally, IDH1/2 mutations decrease α-ketoglutarate to stabilize HIF-1α (78).
Mitochondrial DNA (mtDNA) mutations such as ND1, ND4L, and ND5 (subunits of complex I), cytochrome b (complex III), and COXI, COXII, and COXIII (complex IV) correlate with the increased levels of ROS in solid tumors and leukemia (17, 52, 55). The mutations of mtDNA are likely to impair electron transfer, leading to leakage of electrons and generation of O2− (15). Meanwhile, the active aerobic glycolytic metabolism of cancer cells can also contribute to increase ROS (3).
The extrinsic mechanism of ROS increase may involve the abnormal microenvironment and therapeutic factors. Evidence shows that cancer cells have adaptation for persistently elevated ROS by (i) activating redox-sensitive transcription factors that upregulate ROS scavenging enzymes such as SODs and glutathione synthase, (ii) activating expression of the survival factors, and (iii) inhibiting cell death factors. These adaptive changes prevent the ROS level from reaching the cell-death threshold as well as from raising that threshold to promote cell survival and proliferation (113). Due to the increased ROS level, cancer cells may be more susceptive to oxidative damage induced by exogenous agents (including xenobiotics) and more reliant on the antioxidant system to sustain cell survival. For example, human leukemia cells with intrinsic oxidative stress are highly sensitive to ROS stress induced by 2-methoxyetradiol and arsenic trioxide (51, 134). Wu et al. (122) showed that diallyl disulfide, an oil soluble constituent of garlic (Allium sativum), could induce cell-cycle arrest at the G2/M phase and apoptosis in human A549 lung cancer cells through the production of ROS, which could be completely abrogated by NAC. Trachootham et al. (114) showed that β-phenylethyl isothiocyanate could kill fludarabine-resistant chronic lymphoid leukemia cells through the depletion of glutathione and by raising the ROS, which mediates mitochondria damage and glutathionylation-dependent degradation of Mcl-1. Jin et al. (63) demonstrated that niclosamide, an anti-helminthic agent, could induce apoptosis by increasing the levels of ROS as well as by inhibiting nuclear factor-kappa B (NF-κB) in AML cells. The mechanism of antitumor activity of farnesyltransferase inhibitors was also reported to involve the generation of ROS (86, 104). Increasing ROS by compound piperlongumine kills cancer cells in a p53-independent manner (94).
Recently, microRNAs are also found to regulate ROS. MicroRNAs are a class of short non-coding RNAs that regulate post-transcriptional gene activity by degrading transcripts of target genes (53). Venkataraman et al. (116) found that ectopic expression of miR-128a in medulloblastoma cells increased intracellular ROS (O2−) and inhibited cell growth. The regulatory effect of miR-128a on ROS may be exerted by targeting Bmi-1 (via binding 3′ UTR of Bmi-1), because co-transfection with Bmi-1 and miR-128a did not lead to an increase in ROS when compared with that of miR-128a transfection alone (116). However, the underlying mechanism that Bmi-1 increases ROS remains to be further explored.
ROS in CSCs
Concept of CSCs
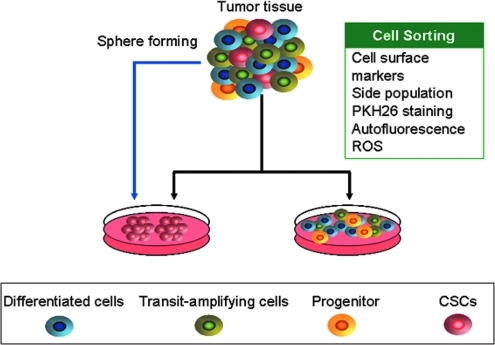
CSCs only constitute a subset of the whole tumor cell population. They share the properties of self-renewal capacity and chemo-/radio resistance with normal stem cells. The study of leukemia stem cells has initiated this CSCs research area. A pioneering study showed that it is possible to use CD34+/CD38− cell surface markers to separate cells into AML CSCs and non-CSCs populations that differ in their self-renewal capacity in vitro and tumorigenicity in vivo (14). Subsequent studies demonstrate that CSCs can be sorted and enriched by cell surface markers in various cancer types (Table 1 and Fig. 3). For instance, ESA+/CD44+/CD24−/Lin− breast cancer cells are capable of generating the phenotypic heterogeneity recapitulating the initial tumor of a patient, and this breast cancer cell sub-population possesses high tumorigenicity and treatment resistance similar to stem cells (14, 78, 90). In the CNS, CD133 is believed to be the marker for CSCs in different types of brain tumor (105). The subpopulation of CD133+ tumor cells is enriched after radiation in gliomas (9). In the absence of known cell surface markers, the SP in flow cytometry seems to be a useful method for isolating the CSCs (11, 12, 14). CSCs are capable of pumping fluorescent dye out of the cells because of the enhanced expression of an ATP-binding cassette (ABC) transporter, ABCG2. Therefore, the cancer cells in the SP fraction possess the properties of stem cells (73). Interestingly, Radovanovic's research group isolated a subpopulation from human glioma tissues and cultures based on intrinsic autofluorescence properties and distinctive cell morphology, and this subpopulation preferentially expressed embryonic stem cell markers, such as octamer-binding transcription factor 4 (Oct4), Nanog, and sRY (sex determining region Y)-box 2 (Sox2), and have the properties of CSCs (27). Of note, PKH26, a lipophilic dye, is becoming a popular tool for isolating CSCs in some types of cancer (25, 88). CSCs can retain PKH26 dye on the basis of asymmetric division. Therefore, CSCs can be isolated by FACS as the PKH26high population (Fig. 3).
Table 1.
Cell Surface Phenotype of Cancer Stem Cells Identified in Human Cancers
| Tumor type | CSC phenotype | References |
|---|---|---|
| AML | CD34+/CD38−, CD44+,CD34+/CD123+, CD47+ | (14, 61, 62, 64, 79) |
| ALL | CD34+/CD10−, CD34+/CD19−; | (30, 74) |
| Breast cancers | CD44+/ESA+/CD24−/Lin−, ALDH1low | (6, 41) |
| Brain tumors | CD133+, CD15+ | (105, 106) |
| Melanoma | ABCB5+, CD271+, JARID1B+, CD133+, CD20+ | (13, 37, 93, 99, 101) |
| Colorectal cancers | CD44+/EpCAM+,CD133+, ALDH1high, Lgr-5+ | (10, 32, 49, 85, 97) |
| Lung cancers | CD133+, CD133+/EpCAM+ | (36, 67) |
| Osteosarcomas | CD44+/CD105+/Stro1+ | (40) |
| Head and neck squamous cell carcinomas | CD44+/ALDHhigh | (23, 92) |
| Liver cancers | CD13+,CD90+/CD44−, CD133+, CD24+ | (68, 77, 108, 124) |
| Pancreatic cancers | CD44+/CD24+/ESA+, CD133+ | (47) |
| Prostatic cancers | CD44+/Integrin α2β1high/CD133+ | (28) |
| Skin squamous cell carcinomas | CD34+, Integrin α5β1high | (80, 103) |
| Ovarian cancers | CD44+/CD117+, CD133+ | (31, 132) |
| Bladder cancers | CD44+ | (19) |
ALDH1, aldehyde dehydrogenase-1; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; EpCAM, epithelial cell adhesion molecule; ESA, epithelial special antigen; JARID1B, the H3K4 demethylase; Lgr-5, leucine-rich-repeat containing G-protein-coupled receptor 5.
FIG. 3.
Isolation of cancer stem cells (CSCs). CSCs can be sorted out on the basis of antigens, functional markers, side population, PKH26 dye intensity, autofluorescence, and ROS levels using flow cytometry (fluorescence-activated cell sorting). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
It is clear that normal stem cells are pluripotent, but how about the differentiation capacity of CSCs? Recent studies showed that CSCs have endothelial and adipocytic lineage potential (5, 91, 98). De Maria et al. showed that a majority of endothelial cells in glioblastoma carry the same mutation as the cancer cells (98). This result suggests that CSCs may at least contribute to the origin of vascular endothelium in the tumor. Therefore, CSCs seem to be capable of trans-differentiating into vascular cells and forming vasculogenic mimicry (7).
On the other hand, increasing evidence indicates that CSCs have an enhanced capacity to initiate and sustain tumor growth, which is important for the progression and relapse of malignant tumors (117). Previous studies speculated that CSCs may differentiate into cancer cells in various stages of maturation by a unidirectional process. However, recent studies tell us another interesting story: the relationship between CSCs and non-CSCs may be bi-directional, and non-CSCs may be reprogrammed into CSCs (42). Furthermore, the kinetic rate of both directions (CSCs to non-CSCs or non-CSCs to CSCs) is not low. This equilibrium may be shifted in either one direction or another based on the contextual signals of the tumor microenvironment (42). Therefore, inter-conversion between the CSCs and non-CSCs compartments seems to be one reason that can explain the variation in the frequency of CSCs in different stages of disease and various types of cancer.
Hypoxia in the solid tumor microenvironment has been shown to be a regulator that maintains the stem-like phenotype of colorectal cancer cell line-derived CSCs and prevents differentiation of enterocytes and goblet cells by regulating CDX1 and Notch1 (127). Given that hypoxia is capable of inducing ROS and the stabilization of HIF1α, more work is needed to elucidate whether there is a linkage between ROS-HIF-1α and CDX1/Notch1 that controls the switch between stemness and differentiation in mature cancer cells.
Regulation of ROS in CSCs
In comparison with normal stem cells or cancer cells as a whole population, relatively little is known in CSCs about ROS levels. Similar to normal stem cells such as HSCs, CSCs also show lower intracellular ROS contents than non-CSCs, which may be due to the increased expression of free radical scavenging systems (34, 125).
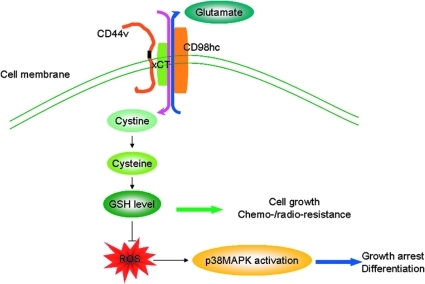
CD44, an adhesion molecule, is expressed in CSCs of various types of cancers. Its variant isoform CD44v as a result of alternative mRNA splicing with insertions in the membrane-proximal extracellular region can protect gastric cancer CSCs from high levels of ROS in the tumor microenvironment (6, 28, 32). Ishimoto et al. (56) demonstrated that CD44v in gastrointestinal CSCs can enhance the intracellular GSH levels by stabilizing xCT, a light-chain subunit of a glutamate-cystine exchange transporter located at the plasma membrane. The function of xCT at the cell surface is to increase the uptake of cystine that is a substrate for GSH synthesis, and, therefore, xCT is critical for controlling the intracellular redox status (Fig. 4). The ablation of CD44v inhibits the activity of xCT, and results in increased ROS and ROS-dependent p38MAPK activation. This is supported by the observation that the ROS levels detected by DCFH-DA staining in CD44low or CD44− tumor cells are much higher than those in CD44high tumor cells. Moreover, the silencing of CD44 by RNA interference leads to increased ROS levels, and the administration of NAC could reverse the oxidative stress phenotype in CD44− cells. Thus, CD44v-targeted therapy that impairs ROS defense may provide a potential approach of killing CSCs.
FIG. 4.
CD44v enhancement of ROS defense by interacting and stabilizing xCT. The CD44v molecule interacts with xCT, a light-chain subunit of cystine-glutamate exchange transporter at the plasma membrane. xCT elevates cystine uptake that facilitates intracellular reduced glutathione (GSH) synthesis, and reduces the ROS levels and ROS-dependent activation of p38MAPK. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Further, CD44+/CD24− breast CSCs have been found to exhibit an enhanced ROS defense system by over-expressing antioxidant enzyme genes and less DNA damage when compared with non-CSCs (34). Lower levels of basal and radiation-induced ROS in CD44+/CD24− breast CSCs have been associated with the tumorigenicity and resistance to radiation (34). Given a link between the management of ROS by CSCs and enhanced tumor radio resistance, a pharmacological increase in ROS in CSCs may decrease their clonogenicity and result in radio sensitization.
CSCs molecule CD13 negatively regulates ROS with a resultant increase of stemness in liver CSCs. Kim et al. (68) showed that increased CD13 expression could reduce ROS and promote the survival of liver CSCs via a transforming growth factor-beta induced epithelial mesenchymal transition-like process. CD13 is also associated with increased ROS scavenger capacity in human liver CSCs. Gclm, encoding the glutamate-cysteine ligase that catalyzes the rate-limiting step in the synthesis of GSH, was over-expressed in the CD13+/CD90− and the CD13+ fractions of cells (HuH7 and PLC/PRF/5), and these cell fractions have lower concentrations of ROS than the CD13− fraction. Due to the suppression of CD13 with the CD13-neutralizing antibody (clone WM15) or ubenimex, a compound that specifically blocks CD13 by antagonizing the zinc-binding site of the aminopeptidase N domain, the ROS concentration was significantly increased in CD13+ cells and reached the level of ROS observed in the CD13− cells (46). Moreover, administration with ubenimex inhibited the self-renewal and the tumor-initiation ability of CD13+ cells in mice xenografted in a CD13+ cell-enriched fraction (46). Targeting CD13 with reagents such as anti-CD13 neutralizing antibody and ubenimex may offer a therapeutic approach for CSCs elimination.
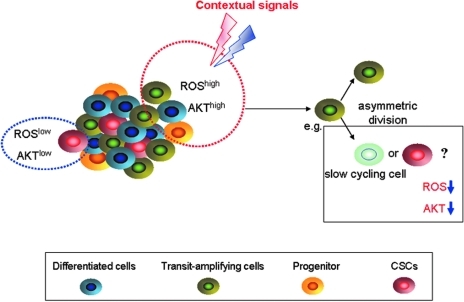
Here, we raise questions for discussion. First, why do CSCs show lower ROS levels than non-CSCs? Second, why do cancer cells keep a subset of ROSlow cells in the whole cell population? The evidence until now is rather limited. Ramaswamy et al. found that rapidly proliferating breast cancer cells can produce “G0-like” progeny by asymmetric division (Fig. 5) (33). The cells in the G0 phase showed lower intracellular ROS contents and decreased Akt activation. Inhibition of the Akt signaling pathway in proliferating cancer cells resulted in an increased ratio of asymmetric division and produced large amounts of ROSlow cells with slow growth (Fig. 6). The stimulators that modulate Akt signaling can shift the dynamics between symmetric and asymmetric division, similar to the equilibrium between CSCs and non-CSCs. We hypothesize here that producing the cells with stem cell characteristics and ROSlow cells during proliferation and passage is the protective and “talented” behavior of cancer cells. Cancer should protect a subset of “tumor seeds” from DNA damage that facilitates tumor propagation. Additionally, ROSlow cells could be enriched after chemotherapy in breast cancer patients (34), indicating that ROSlow cells should be targeted during cancer therapy.
FIG. 5.
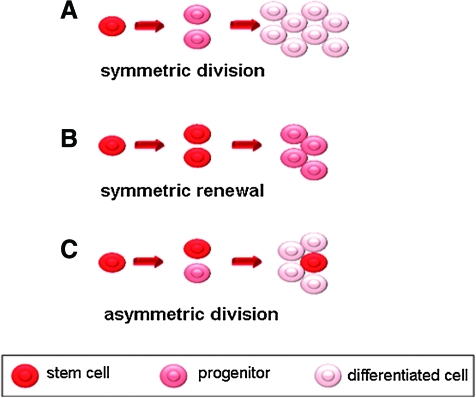
Models for cell division. Stem cells may be divided in the following three possible ways: A, Symmetric division: a stem cell divides into two progenitor cells; B, Symmetric renewal: a stem cell divides into two stem cells. C, Asymmetric division: a stem cell divides into one progenitor cell and one stem cell. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
FIG. 6.
Rapidly proliferating cancer cells produce ROSlow cells by asymmetric division. ROSlow cancer cells with low Akt activity are predominantly seen in the G1/G0 of the cell cycle compared with actively cycling ROShigh cells. The stimulators that modulate Akt signaling can shift the dynamic between symmetric and asymmetric division. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
In addition, is exogenous ROS elevation capable of killing CSCs similar to killing non-CSC cancer cells? The answer may be optimistic. Niclosamide, a potent agent that increases ROS levels, can selectively kill CD34+/CD38− AML stem cells while having minimal cytotoxicity against the progenitor cells in normal bone marrow cells from healthy individuals (63). The antineoplastic mechanism of niclosamide may also involve inactivation of the NF-κB pathway (63). Consistent with this finding, Guzman et al. showed that parthenolide (a natural product) can also induce apoptosis in the CSCs of AML and CML patients at least partially via an increase in ROS (44). The tumor suppressor gene PML (promyelocytic leukemia), which is involved in the t(15;17) chromosomal translocation of acute promyelocytic leukemia, has been demonstrated to be an important factor that maintains the quiescent state of CML CSCs (57). Arsenic trioxide (AS2O3), which can lead to PML degradation and increase ROS, is presumed to eliminate CML stem cells (57). To date, the mechanisms that ROS kill CSCs are elusive. However, at least, high levels of ROS in niches may trigger differentiation (3).
Conclusions
Exogenously increased ROS have been shown to selectively kill cancer cells. Low levels of ROS are associated with the stemness of stem cells and CSCs. However, the underlying mechanism for decreased ROS has not been adequately understood. Intensive research for ROS in CSCs is desperately needed. Oxidative stress exists in cancer cells, and exogenous agents may further increase ROS effectively and selectively kill cancer cells. It will be of interest to elucidate whether increasing ROS levels represent an efficacious strategy to eradicate CSCs.
Abbreviations Used
- ABC
ATP-binding cassette
- ABCB5
a novel human ABC transporter
- ALDH1
aldehyde dehydrogenase-1
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- ASK1
apoptosis signal-regulatory kinase 1
- ATM
ataxia telangiectasia mutated
- COX2
cyclooxygenase 2
- CSCs
cancer stem cells
- DCF-DA
2,7-dichlorodihydrofluorescein diacetate
- EpCAM
epithelial cell adhesion molecule
- ESA
epithelial- special antigen
- ETC
electron transport chain
- FoxO3
phosphorylates FoxO transcription factor 3
- GPX
glutathione peroxidase
- GSH
glutathione
- GSSG
oxidized glutathione
- HIF-1α
hypoxia inducible factor-1α
- HO•
hydroxyl free radical
- H2O2
hydrogen peroxide
- HOCl
hypochlorous acid
- HSCs
hematopoietic stem cells
- IDH
isocitrate dehydrogenase
- JARID1B
the H3K4 demethylase
- Lgr-5
leucine-rich-repeat containing G-protein-coupled receptor 5
- MAPK
mitogen-activated protein kinase
- MPD
myeloperoxidase
- mTOR
mammalian target of rapamycin
- NAC
N-acetyl-cysteine
- NF-κB
nuclear factor-kappa B
- NO•
nitric oxide
- NOD/SCID
nonobese diabetic severe combined immunodeficiency disease
- NOS
nitric oxide synthase
- NOX
NAD(P)H oxidase complex
- Nrf2
nuclear factor erythroid-2-related factor 2
- O2−
superoxide
- Oct4
octamer-binding transcription factor 4
- ONOO−
peroxynitrite
- PI3K
phosphoinositide 3-kinase
- PIGs
p53-induced genes
- PML
promyelocytic leukemia gene
- Prdm 16
PR domain-containing 16
- PTEN
phosphatase and tensin homolog
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- Sox2
sRY (sex determining region Y)-box 2
- SP
side population
- VEGF
vascular endothelial growth factor
Acknowledgments
This work was supported by grants from the National Natural Science Funds of China for Distinguished Young Scholars (81025021) to J.P., the National Basic Research Program of China (973 Program grant 2009CB825506 to J.P., 2009CB94 5400 to Y.Z.), the Research Foundation of Education Bureau of Guangdong Province, China (Grant cxzd1103) to J.P., the Research Foundation of Guangzhou Bureau of Science and Technology, China (Grant to J.P.) the National High Technology Research and Development Program of China (863 Program grant 2008AA02Z420 to J.P., 2008AA092604 to Y.Z.), the Major Research Plan of the National Natural Science Fund of China (90713036) to J.P., and the Fundamental Research Funds for the Central Universities to J.P. The authors thank Dr. S.C.J. Yeung (The University of Texas MD Anderson Cancer Center, Houston, TX) for a critical reading of the article.
Author Disclosure Statement
All the authors declare no commercial associations that might create a conflict of interest in connection with the article submitted.
References
- 1.Abbas HA. Maccio DR. Coskun S. Jackson JG. Hazen AL. Sills TM. You MJ. Hirschi KK. Lozano G. Mdm2 is required for survival of hematopoietic stem cells/progenitors via dampening of ROS-induced p53 activity. Cell Stem Cell. 2010;7:606–617. doi: 10.1016/j.stem.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott A. Stem cells: the cell division. Nature. 2011;480:310–312. doi: 10.1038/480310a. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Wahab O. Levine RL. Metabolism and the leukemic stem cell. J Exp Med. 2010;207:677–680. doi: 10.1084/jem.20100523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achanta G. Sasaki R. Feng L. Carew JS. Lu W. Pelicano H. Keating MJ. Huang P. Novel role of p53 in maintaining mitochondrial genetic stability through interaction with DNA Pol gamma. EMBO J. 2005;24:3482–3492. doi: 10.1038/sj.emboj.7600819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adhikari AS. Agarwal N. Wood BM. Porretta C. Ruiz B. Pochampally RR. Iwakuma T. CD117 and Stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance. Cancer Res. 2010;70:4602–4612. doi: 10.1158/0008-5472.CAN-09-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Hajj M. Wicha MS. Benito-Hernandez A. Morrison SJ. Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvero AB. Fu HH. Holmberg J. Visintin I. Mor L. Marquina CC. Oidtman J. Silasi DA. Mor G. Stem-like ovarian cancer cells can serve as tumor vascular progenitors. Stem Cells. 2009;27:2405–2413. doi: 10.1002/stem.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arai F. Hirao A. Ohmura M. Sato H. Matsuoka S. Takubo K. Ito K. Koh GY. Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Bao S. Wu Q. McLendon RE. Hao Y. Shi Q. Hjelmeland AB. Dewhirst MW. Bigner DD. Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 10.Barker N. Ridgway RA. van Es JH. van de Wetering M. Begthel H. van den Born M. Danenberg E. Clarke AR. Sansom OJ. Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 11.Bell EL. Klimova TA. Eisenbart J. Moraes CT. Murphy MP. Budinger GR. Chandel NS. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilbao PS. Santillan G. Boland R. ATP stimulates the proliferation of MCF-7 cells through the PI3K/Akt signaling pathway. Arch Biochem Biophys. 2010;499:40–48. doi: 10.1016/j.abb.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Boiko AD. Razorenova OV. van de Rijn M. Swetter SM. Johnson DL. Ly DP. Butler PD. Yang GP. Joshua B. Kaplan MJ. Longaker MT. Weissman IL. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnet D. Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 15.Brandon M. Baldi P. Wallace DC. Mitochondrial mutations in cancer. Oncogene. 2006;25:4647–4662. doi: 10.1038/sj.onc.1209607. [DOI] [PubMed] [Google Scholar]
- 16.Brunet A. Bonni A. Zigmond MJ. Lin MZ. Juo P. Hu LS. Anderson MJ. Arden KC. Blenis J. Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 17.Carew JS. Zhou Y. Albitar M. Carew JD. Keating MJ. Huang P. Mitochondrial DNA mutations in primary leukemia cells after chemotherapy: clinical significance and therapeutic implications. Leukemia. 2003;17:1437–1447. doi: 10.1038/sj.leu.2403043. [DOI] [PubMed] [Google Scholar]
- 18.Chan K. Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci U S A. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan KS. Espinosa I. Chao M. Wong D. Ailles L. Diehn M. Gill H. Presti J., Jr Chang HY. van de Rijn M. Shortliffe L. Weissman IL. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandel NS. Maltepe E. Goldwasser E. Mathieu CE. Simon MC. Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C. Liu Y. Liu R. Ikenoue T. Guan KL. Liu Y. Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W. Druhan LJ. Chen CA. Hemann C. Chen YR. Berka V. Tsai AL. Zweier JL. Peroxynitrite induces destruction of the tetrahydrobiopterin and heme in endothelial nitric oxide synthase: transition from reversible to irreversible enzyme inhibition. Biochemistry. 2010;49:3129–3137. doi: 10.1021/bi9016632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen YC. Chen YW. Hsu HS. Tseng LM. Huang PI. Lu KH. Chen DT. Tai LK. Yung MC. Chang SC. Ku HH. Chiou SH. Lo WL. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun. 2009;385:307–313. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 24.Chuikov S. Levi BP. Smith ML. Morrison SJ. Prdm16 promotes stem cell maintenance in multiple tissues, partly by regulating oxidative stress. Nat Cell Biol. 2010;12:999–1006. doi: 10.1038/ncb2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cicalese A. Bonizzi G. Pasi CE. Faretta M. Ronzoni S. Giulini B. Brisken C. Minucci S. Di Fiore PP. Pelicci PG. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138:1083–1095. doi: 10.1016/j.cell.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 26.Cipolleschi MG. Rovida E. Ivanovic Z. Praloran V. Olivotto M. Dello Sbarba P. The expansion of murine bone marrow cells preincubated in hypoxia as an in vitro indicator of their marrow-repopulating ability. Leukemia. 2000;14:735–739. doi: 10.1038/sj.leu.2401744. [DOI] [PubMed] [Google Scholar]
- 27.Clement V. Marino D. Cudalbu C. Hamou MF. Mlynarik V. de Tribolet N. Dietrich PY. Gruetter R. Hegi ME. Radovanovic I. Marker-independent identification of glioma-initiating cells. Nat Methods. 2010;7:224–228. doi: 10.1038/nmeth.1430. [DOI] [PubMed] [Google Scholar]
- 28.Collins AT. Berry PA. Hyde C. Stower MJ. Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 29.Cosentino C. Grieco D. Costanzo V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011;30:546–555. doi: 10.1038/emboj.2010.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox CV. Evely RS. Oakhill A. Pamphilon DH. Goulden NJ. Blair A. Characterization of acute lymphoblastic leukemia progenitor cells. Blood. 2004;104:2919–2925. doi: 10.1182/blood-2004-03-0901. [DOI] [PubMed] [Google Scholar]
- 31.Curley MD. Therrien VA. Cummings CL. Sergent PA. Koulouris CR. Friel AM. Roberts DJ. Seiden MV. Scadden DT. Rueda BR. Foster R. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27:2875–2883. doi: 10.1002/stem.236. [DOI] [PubMed] [Google Scholar]
- 32.Dalerba P. Dylla SJ. Park IK. Liu R. Wang X. Cho RW. Hoey T. Gurney A. Huang EH. Simeone DM. Shelton AA. Parmiani G. Castelli C. Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dey-Guha I. Wolfer A. Yeh AC. J GA. Darp R. Leon E. Wulfkuhle J. Petricoin EF., 3rd Wittner BS. Ramaswamy S. Asymmetric cancer cell division regulated by AKT. Proc Natl Acad Sci U S A. 2011;108:12845–12850. doi: 10.1073/pnas.1109632108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diehn M. Cho RW. Lobo NA. Kalisky T. Dorie MJ. Kulp AN. Qian D. Lam JS. Ailles LE. Wong M. Joshua B. Kaplan MJ. Wapnir I. Dirbas FM. Somlo G. Garberoglio C. Paz B. Shen J. Lau SK. Quake SR. Brown JM. Weissman IL. Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donald SP. Sun XY. Hu CA. Yu J. Mei JM. Valle D. Phang JM. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 2001;61:1810–1815. [PubMed] [Google Scholar]
- 36.Eramo A. Lotti F. Sette G. Pilozzi E. Biffoni M. Di Virgilio A. Conticello C. Ruco L. Peschle C. De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 37.Fang D. Nguyen TK. Leishear K. Finko R. Kulp AN. Hotz S. Van Belle PA. Xu X. Elder DE. Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 38.Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137:811–819. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujisaki J. Wu J. Carlson AL. Silberstein L. Putheti P. Larocca R. Gao W. Saito TI. Lo Celso C. Tsuyuzaki H. Sato T. Cote D. Sykes M. Strom TB. Scadden DT. Lin CP. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibbs CP. Kukekov VG. Reith JD. Tchigrinova O. Suslov ON. Scott EW. Ghivizzani SC. Ignatova TN. Steindler DA. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia. 2005;7:967–976. doi: 10.1593/neo.05394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ginestier C. Hur MH. Charafe-Jauffret E. Monville F. Dutcher J. Brown M. Jacquemier J. Viens P. Kleer CG. Liu S. Schott A. Hayes D. Birnbaum D. Wicha MS. Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta PB. Chaffer CL. Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15:1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 43.Gupta SC. Hevia D. Patchva S. Park B. Koh W. Aggarwal BB. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal. 2012;16:1295–1322. doi: 10.1089/ars.2011.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guzman ML. Rossi RM. Karnischky L. Li X. Peterson DR. Howard DS. Jordan CT. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haghdoost S. Czene S. Naslund I. Skog S. Harms-Ringdahl M. Extracellular 8-oxo-dG as a sensitive parameter for oxidative stress in vivo and in vitro. Free Radic Res. 2005;39:153–162. doi: 10.1080/10715760500043132. [DOI] [PubMed] [Google Scholar]
- 46.Haraguchi N. Ishii H. Mimori K. Tanaka F. Ohkuma M. Kim HM. Akita H. Takiuchi D. Hatano H. Nagano H. Barnard GF. Doki Y. Mori M. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest. 2010;120:3326–3339. doi: 10.1172/JCI42550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hermann PC. Huber SL. Herrler T. Aicher A. Ellwart JW. Guba M. Bruns CJ. Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Hills M. Lucke K. Chavez EA. Eaves CJ. Lansdorp PM. Probing the mitotic history and developmental stage of hematopoietic cells using single telomere length analysis (STELA) Blood. 2009;113:5765–5775. doi: 10.1182/blood-2009-01-198374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang EH. Hynes MJ. Zhang T. Ginestier C. Dontu G. Appelman H. Fields JZ. Wicha MS. Boman BM. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang J. Yang J. Maity B. Mayuzumi D. Fisher RA. Regulator of G protein signaling 6 mediates doxorubicin-induced ATM and p53 activation by a reactive oxygen species-dependent mechanism. Cancer Res. 2011;71:6310–6319. doi: 10.1158/0008-5472.CAN-10-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang P. Feng L. Oldham EA. Keating MJ. Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 52.Indo HP. Davidson M. Yen HC. Suenaga S. Tomita K. Nishii T. Higuchi M. Koga Y. Ozawa T. Majima HJ. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion. 2007;7:106–118. doi: 10.1016/j.mito.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 53.Inui M. Martello G. Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11:252–263. doi: 10.1038/nrm2868. [DOI] [PubMed] [Google Scholar]
- 54.Irani K. Xia Y. Zweier JL. Sollott SJ. Der CJ. Fearon ER. Sundaresan M. Finkel T. Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 55.Ishikawa K. Koshikawa N. Takenaga K. Nakada K. Hayashi J. Reversible regulation of metastasis by ROS-generating mtDNA mutations. Mitochondrion. 2008;8:339–344. doi: 10.1016/j.mito.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Ishimoto T. Nagano O. Yae T. Tamada M. Motohara T. Oshima H. Oshima M. Ikeda T. Asaba R. Yagi H. Masuko T. Shimizu T. Ishikawa T. Kai K. Takahashi E. Imamura Y. Baba Y. Ohmura M. Suematsu M. Baba H. Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 57.Ito K. Bernardi R. Morotti A. Matsuoka S. Saglio G. Ikeda Y. Rosenblatt J. Avigan DE. Teruya-Feldstein J. Pandolfi PP. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453:1072–1078. doi: 10.1038/nature07016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ito K. Hirao A. Arai F. Matsuoka S. Takubo K. Hamaguchi I. Nomiyama K. Hosokawa K. Sakurada K. Nakagata N. Ikeda Y. Mak TW. Suda T. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 59.Ito K. Hirao A. Arai F. Takubo K. Matsuoka S. Miyamoto K. Ohmura M. Naka K. Hosokawa K. Ikeda Y. Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 60.Jang YY. Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin L. Hope KJ. Zhai Q. Smadja-Joffe F. Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 62.Jin L. Lee EM. Ramshaw HS. Busfield SJ. Peoppl AG. Wilkinson L. Guthridge MA. Thomas D. Barry EF. Boyd A. Gearing DP. Vairo G. Lopez AF. Dick JE. Lock RB. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5:31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 63.Jin Y. Lu Z. Ding K. Li J. Du X. Chen C. Sun X. Wu Y. Zhou J. Pan J. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-kappaB pathway and generation of reactive oxygen species. Cancer Res. 2010;70:2516–2527. doi: 10.1158/0008-5472.CAN-09-3950. [DOI] [PubMed] [Google Scholar]
- 64.Jordan CT. Upchurch D. Szilvassy SJ. Guzman ML. Howard DS. Pettigrew AL. Meyerrose T. Rossi R. Grimes B. Rizzieri DA. Luger SM. Phillips GL. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777–1784. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 65.Juntilla MM. Patil VD. Calamito M. Joshi RP. Birnbaum MJ. Koretzky GA. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood. 2010;115:4030–4038. doi: 10.1182/blood-2009-09-241000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kensler TW. Wakabayashi N. Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 67.Kim CF. Jackson EL. Woolfenden AE. Lawrence S. Babar I. Vogel S. Crowley D. Bronson RT. Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 68.Kim HM. Haraguchi N. Ishii H. Ohkuma M. Okano M. Mimori K. Eguchi H. Yamamoto H. Nagano H. Sekimoto M. Doki Y. Mori M. Increased CD13 expression reduces reactive oxygen species, promoting survival of liver cancer stem cells via an epithelial-mesenchymal transition-like phenomenon. Ann Surg Oncol. 2011 doi: 10.1245/s10434-011-2040-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 69.Kim J. Wong PK. Loss of ATM impairs proliferation of neural stem cells through oxidative stress-mediated p38 MAPK signaling. Stem Cells. 2009;27:1987–1998. doi: 10.1002/stem.125. [DOI] [PubMed] [Google Scholar]
- 70.Kim JH. Chu SC. Gramlich JL. Pride YB. Babendreier E. Chauhan D. Salgia R. Podar K. Griffin JD. Sattler M. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood. 2005;105:1717–1723. doi: 10.1182/blood-2004-03-0849. [DOI] [PubMed] [Google Scholar]
- 71.Kopnin PB. Agapova LS. Kopnin BP. Chumakov PM. Repression of sestrin family genes contributes to oncogenic Ras-induced reactive oxygen species up-regulation and genetic instability. Cancer Res. 2007;67:4671–4678. doi: 10.1158/0008-5472.CAN-06-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwon C. Cheng P. King IN. Andersen P. Shenje L. Nigam V. Srivastava D. Notch post-translationally regulates beta-catenin protein in stem and progenitor cells. Nat Cell Biol. 2011;13:1244–1251. doi: 10.1038/ncb2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kyo S. Maida Y. Inoue M. Stem cells in endometrium and endometrial cancer: accumulating evidence and unresolved questions. Cancer Lett. 2011;308:123–133. doi: 10.1016/j.canlet.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 74.Lapidot T. Sirard C. Vormoor J. Murdoch B. Hoang T. Caceres-Cortes J. Minden M. Paterson B. Caligiuri MA. Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 75.Le Belle JE. Orozco NM. Paucar AA. Saxe JP. Mottahedeh J. Pyle AD. Wu H. Kornblum HI. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee JY. Nakada D. Yilmaz OH. Tothova Z. Joseph NM. Lim MS. Gilliland DG. Morrison SJ. mTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion. Cell Stem Cell. 2010;7:593–605. doi: 10.1016/j.stem.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee TK. Castilho A. Cheung VC. Tang KH. Ma S. Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 78.MacKenzie ED. Selak MA. Tennant DA. Payne LJ. Crosby S. Frederiksen CM. Watson DG. Gottlieb E. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol. 2007;27:3282–3289. doi: 10.1128/MCB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Majeti R. Chao MP. Alizadeh AA. Pang WW. Jaiswal S. Gibbs KD., Jr van Rooijen N. Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malanchi I. Peinado H. Kassen D. Hussenet T. Metzger D. Chambon P. Huber M. Hohl D. Cano A. Birchmeier W. Huelsken J. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- 81.Merchant AA. Singh A. Matsui W. Biswal S. The redox-sensitive transcription factor, Nrf2, regulates murine hematopoietic stem cell survival independent of ROS levels. Blood. 2011;118:6572–6579. doi: 10.1182/blood-2011-05-355362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miyamoto K. Araki KY. Naka K. Arai F. Takubo K. Yamazaki S. Matsuoka S. Miyamoto T. Ito K. Ohmura M. Chen C. Hosokawa K. Nakauchi H. Nakayama K. Nakayama KI. Harada M. Motoyama N. Suda T. Hirao A. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 83.Morrison SJ. Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen LV. Vanner R. Dirks P. Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 85.O'Brien CA. Pollett A. Gallinger S. Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 86.Pan J. She M. Xu ZX. Sun L. Yeung SC. Farnesyltransferase inhibitors induce DNA damage via reactive oxygen species in human cancer cells. Cancer Res. 2005;65:3671–3681. doi: 10.1158/0008-5472.CAN-04-2744. [DOI] [PubMed] [Google Scholar]
- 87.Park BS. Kim WS. Choi JS. Kim HK. Won JH. Ohkubo F. Fukuoka H. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed Res. 2010;31:27–34. doi: 10.2220/biomedres.31.27. [DOI] [PubMed] [Google Scholar]
- 88.Pece S. Tosoni D. Confalonieri S. Mazzarol G. Vecchi M. Ronzoni S. Bernard L. Viale G. Pelicci PG. Di Fiore PP. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 89.Pervaiz S. Taneja R. Ghaffari S. Oxidative stress regulation of stem and progenitor cells. Antioxid Redox Signal. 2009;11:2777–2789. doi: 10.1089/ars.2009.2804. [DOI] [PubMed] [Google Scholar]
- 90.Phillips TM. McBride WH. Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 91.Ping YF. Bian XW. Consice review: Contribution of cancer stem cells to neovascularization. Stem Cells. 2011;29:888–894. doi: 10.1002/stem.650. [DOI] [PubMed] [Google Scholar]
- 92.Prince ME. Sivanandan R. Kaczorowski A. Wolf GT. Kaplan MJ. Dalerba P. Weissman IL. Clarke MF. Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quintana E. Shackleton M. Foster HR. Fullen DR. Sabel MS. Johnson TM. Morrison SJ. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell. 2010;18:510–523. doi: 10.1016/j.ccr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raj L. Ide T. Gurkar AU. Foley M. Schenone M. Li X. Tolliday NJ. Golub TR. Carr SA. Shamji AF. Stern AM. Mandinova A. Schreiber SL. Lee SW. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.Rehn M. Olsson A. Reckzeh K. Diffner E. Carmeliet P. Landberg G. Cammenga J. Hypoxic induction of vascular endothelial growth factor regulates murine hematopoietic stem cell function in the low-oxygenic niche. Blood. 2011;118:1534–1543. doi: 10.1182/blood-2011-01-332890. [DOI] [PubMed] [Google Scholar]
- 96.Reya T. Duncan AW. Ailles L. Domen J. Scherer DC. Willert K. Hintz L. Nusse R. Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 97.Ricci-Vitiani L. Lombardi DG. Pilozzi E. Biffoni M. Todaro M. Peschle C. De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 98.Ricci-Vitiani L. Pallini R. Biffoni M. Todaro M. Invernici G. Cenci T. Maira G. Parati EA. Stassi G. Larocca LM. De Maria R. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 99.Roesch A. Fukunaga-Kalabis M. Schmidt EC. Zabierowski SE. Brafford PA. Vultur A. Basu D. Gimotty P. Vogt T. Herlyn M. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sattler M. Verma S. Shrikhande G. Byrne CH. Pride YB. Winkler T. Greenfield EA. Salgia R. Griffin JD. The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. J Biol Chem. 2000;275:24273–24278. doi: 10.1074/jbc.M002094200. [DOI] [PubMed] [Google Scholar]
- 101.Schatton T. Murphy GF. Frank NY. Yamaura K. Waaga-Gasser AM. Gasser M. Zhan Q. Jordan S. Duncan LM. Weishaupt C. Fuhlbrigge RC. Kupper TS. Sayegh MH. Frank MH. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schepers H. van Gosliga D. Wierenga AT. Eggen BJ. Schuringa JJ. Vellenga E. STAT5 is required for long-term maintenance of normal and leukemic human stem/progenitor cells. Blood. 2007;110:2880–2888. doi: 10.1182/blood-2006-08-039073. [DOI] [PubMed] [Google Scholar]
- 103.Schober M. Fuchs E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-beta and integrin/focal adhesion kinase (FAK) signaling. Proc Natl Acad Sci U S A. 2011;108:10544–10549. doi: 10.1073/pnas.1107807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.She M. Yang H. Sun L. Yeung SC. Redox control of manumycin A-induced apoptosis in anaplastic thyroid cancer cells: involvement of the xenobiotic apoptotic pathway. Cancer Biol Ther. 2006;5:275–280. doi: 10.4161/cbt.5.3.2383. [DOI] [PubMed] [Google Scholar]
- 105.Singh SK. Hawkins C. Clarke ID. Squire JA. Bayani J. Hide T. Henkelman RM. Cusimano MD. Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 106.Son MJ. Woolard K. Nam DH. Lee J. Fine HA. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4:440–452. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stingl J. Eirew P. Ricketson I. Shackleton M. Vaillant F. Choi D. Li HI. Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 108.Suetsugu A. Nagaki M. Aoki H. Motohashi T. Kunisada T. Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820–824. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- 109.Szatrowski TP. Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 110.Takubo K. Goda N. Yamada W. Iriuchishima H. Ikeda E. Kubota Y. Shima H. Johnson RS. Hirao A. Suematsu M. Suda T. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 111.Thimmulappa RK. Mai KH. Srisuma S. Kensler TW. Yamamoto M. Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 112.Tothova Z. Kollipara R. Huntly BJ. Lee BH. Castrillon DH. Cullen DE. McDowell EP. Lazo-Kallanian S. Williams IR. Sears C. Armstrong SA. Passegue E. DePinho RA. Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 113.Trachootham D. Alexandre J. Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 114.Trachootham D. Zhang H. Zhang W. Feng L. Du M. Zhou Y. Chen Z. Pelicano H. Plunkett W. Wierda WG. Keating MJ. Huang P. Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood. 2008;112:1912–1922. doi: 10.1182/blood-2008-04-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vafa O. Wade M. Kern S. Beeche M. Pandita TK. Hampton GM. Wahl GM. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol Cell. 2002;9:1031–1044. doi: 10.1016/s1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- 116.Venkataraman S. Alimova I. Fan R. Harris P. Foreman N. Vibhakar R. MicroRNA 128a increases intracellular ROS level by targeting Bmi-1 and inhibits medulloblastoma cancer cell growth by promoting senescence. PLoS One. 2010;5:e10748. doi: 10.1371/journal.pone.0010748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Visvader JE. Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 118.Vollrath V. Wielandt AM. Iruretagoyena M. Chianale J. Role of Nrf2 in the regulation of the Mrp2 (ABCC2) gene. Biochem J. 2006;395:599–609. doi: 10.1042/BJ20051518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ward PS. Patel J. Wise DR. Abdel-Wahab O. Bennett BD. Coller HA. Cross JR. Fantin VR. Hedvat CV. Perl AE. Rabinowitz JD. Carroll M. Su SM. Sharp KA. Levine RL. Thompson CB. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.West AP. Brodsky IE. Rahner C. Woo DK. Erdjument-Bromage H. Tempst P. Walsh MC. Choi Y. Shadel GS. Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wiseman RL. Chin KT. Haynes CM. Stanhill A. Xu CF. Roguev A. Krogan NJ. Neubert TA. Ron D. Thioredoxin-related Protein 32 is an arsenite-regulated Thiol Reductase of the proteasome 19 S particle. J Biol Chem. 2009;284:15233–15245. doi: 10.1074/jbc.M109.002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu XJ. Kassie F. Mersch-Sundermann V. The role of reactive oxygen species (ROS) production on diallyl disulfide (DADS) induced apoptosis and cell cycle arrest in human A549 lung carcinoma cells. Mutat Res. 2005;579:115–124. doi: 10.1016/j.mrfmmm.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 123.Yan H. Parsons DW. Jin G. McLendon R. Rasheed BA. Yuan W. Kos I. Batinic-Haberle I. Jones S. Riggins GJ. Friedman H. Friedman A. Reardon D. Herndon J. Kinzler KW. Velculescu VE. Vogelstein B. Bigner DD. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang ZF. Ho DW. Ng MN. Lau CK. Yu WC. Ngai P. Chu PW. Lam CT. Poon RT. Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 125.Ye XQ. Li Q. Wang GH. Sun FF. Huang GJ. Bian XW. Yu SC. Qian GS. Mitochondrial and energy metabolism-related properties as novel indicators of lung cancer stem cells. Int J Cancer. 2011;129:820–831. doi: 10.1002/ijc.25944. [DOI] [PubMed] [Google Scholar]
- 126.Yeung SJ. Pan J. Lee MH. Roles of p53, MYC and HIF-1 in regulating glycolysis—the seventh hallmark of cancer. Cell Mol Life Sci. 2008;65:3981–3999. doi: 10.1007/s00018-008-8224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yeung TM. Gandhi SC. Wilding JL. Muschel R. Bodmer WF. Cancer stem cells from colorectal cancer-derived cell lines. Proc Natl Acad Sci U S A. 2010;107:3722–3727. doi: 10.1073/pnas.0915135107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yilmaz OH. Valdez R. Theisen BK. Guo W. Ferguson DO. Wu H. Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 129.Yin T. Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yoshihara H. Arai F. Hosokawa K. Hagiwara T. Takubo K. Nakamura Y. Gomei Y. Iwasaki H. Matsuoka S. Miyamoto K. Miyazaki H. Takahashi T. Suda T. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 131.Zhang J. Niu C. Ye L. Huang H. He X. Tong WG. Ross J. Haug J. Johnson T. Feng JQ. Harris S. Wiedemann LM. Mishina Y. Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 132.Zhang S. Balch C. Chan MW. Lai HC. Matei D. Schilder JM. Yan PS. Huang TH. Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao S. Lin Y. Xu W. Jiang W. Zha Z. Wang P. Yu W. Li Z. Gong L. Peng Y. Ding J. Lei Q. Guan KL. Xiong Y. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324:261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou Y. Hileman EO. Plunkett W. Keating MJ. Huang P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood. 2003;101:4098–4104. doi: 10.1182/blood-2002-08-2512. [DOI] [PubMed] [Google Scholar]