Abstract
Significance: Extensive research during the last quarter century has revealed that reactive oxygen species (ROS) produced in the body, primarily by the mitochondria, play a major role in various cell-signaling pathways. Most risk factors associated with chronic diseases (e.g., cancer), such as stress, tobacco, environmental pollutants, radiation, viral infection, diet, and bacterial infection, interact with cells through the generation of ROS. Recent Advances: ROS, in turn, activate various transcription factors (e.g., nuclear factor kappa-light-chain-enhancer of activated B cells [NF-κB], activator protein-1, hypoxia-inducible factor-1α, and signal transducer and activator of transcription 3), resulting in the expression of proteins that control inflammation, cellular transformation, tumor cell survival, tumor cell proliferation and invasion, angiogenesis, and metastasis. Paradoxically, ROS also control the expression of various tumor suppressor genes (p53, Rb, and PTEN). Similarly, γ-radiation and various chemotherapeutic agents used to treat cancer mediate their effects through the production of ROS. Interestingly, ROS have also been implicated in the chemopreventive and anti-tumor action of nutraceuticals derived from fruits, vegetables, spices, and other natural products used in traditional medicine. Critical Issues: These statements suggest both “upside” (cancer-suppressing) and “downside” (cancer-promoting) actions of the ROS. Thus, similar to tumor necrosis factor-α, inflammation, and NF-κB, ROS act as a double-edged sword. This paradox provides a great challenge for researchers whose aim is to exploit ROS stress for the development of cancer therapies. Future Directions: The various mechanisms by which ROS mediate paradoxical effects are discussed in this article. The outstanding questions and future directions raised by our current understanding are discussed. Antioxid. Redox Signal. 16, 1295–1322.
Introduction
Whether hydrogen peroxide (H2O2), a hydroxyl radical, or superoxide, all are constantly generated and eliminated in the biological system and are required to drive regulatory pathways (75). Under normal physiologic conditions, cells control reactive oxygen species (ROS) levels by balancing the generation of ROS with their elimination by a scavenging system (328). However, under oxidative stress conditions, excessive ROS can damage cellular proteins, lipids, and DNA, giving rise to fatal lesions in cells that, in turn, contribute to many human diseases, including cancer (14, 29, 67, 90, 314).
The association of ROS with cancer has been difficult to understand for numerous reasons. First, ROS play an important role in the initiation and progression of cancer (38, 45, 268, 332). Second, cancer cells exhibit greater ROS stress than normal cells do, owing in part to oncogenic stimulation, increased metabolic activity, and mitochondrial malfunction (27, 119, 299). Third, cell-cycle progression by growth factors and receptor tyrosine kinases require ROS (138). Fourth, chronic inflammation, one of the major mediators of cancer, is regulated by ROS (132, 259). Fifth, ROS controls the expression of various tumor suppressor genes, including p53 (47, 190, 275). Sixth, a high level of ROS can suppress tumor growth through the sustained activation of the cell-cycle inhibitor (256, 296). Seventh, most of the chemotherapeutic and radiotherapeutic agents kill cancer cells by augmenting ROS stress (258, 298). These contradictory statements imply that cancer cells die by the same mechanism which facilitates their survival. This paradox provides a great challenge for researchers whose aim is to exploit ROS stress for the development of cancer therapies.
Over the past several years, researchers have noticed that the role of ROS depends on their level. While a modest amount of ROS is required for tumor promotion, an excessive level serves to suppress tumors (113, 334). However, ROS effects cannot be regarded as a general phenomenon, as ROS constitute several molecular entities, each of which might have a differential effect, if examined separately. Both ROS-elevating and ROS-eliminating strategies have been developed; the former have been predominantly used (134, 135, 237, 272). ROS-elevating strategies are based on the fact that cancer cells with elevated ROS levels depend heavily on the antioxidant defense system. A further increase in the ROS stress level, either by ROS-generating agents or by agents that abrogate the inherent antioxidant system, should result in an overall increase in endogenous ROS, which when above a cellular tolerability threshold may induce cell death. This point is the so-called “threshold concept for cancer therapy” (168, 271). On the other hand, normal cells appear to have, under lower basal stress and reserve, a higher capacity to cope with additional ROS-generating insults than cancer cells do (271, 300). Therefore, it should be possible to preferentially accumulate ROS in cancer cells and kill them selectively. Kong and colleagues were the first to prove the idea of inducing death preferentially in cancer cells by an ROS-mediated mechanism (168, 169). ROS-depleting strategies are based on the use of antioxidants to scavenge ROS, thereby abrogating ROS signaling and suppressing tumor growth (63, 273). A number of pro-oxidant- and antioxidant-based anticancer agents have been developed, some of which have been approved by the U.S. Food and Drug Administration. For instance, procarbazine, motexafin gadolinium, elesclomol, 2-methoxyestradiol, and imexon are used to increase ROS content, and minodronate and histamine are used to eliminate ROS.
Although redox-based cancer therapy seems promising, it is likely that the biochemical and molecular changes caused by ROS stress may contribute to the emergence of drug-resistant machinery during disease progression. Under persistent intrinsic ROS stress, many cancer cells become highly adapted to such stress and become resistant to exogenous stress, partly due to the activation of redox-sensitive transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), nuclear factor (erythroid-derived 2)-like factor 2, cellular Ju-nanna (c-Jun), and hypoxia-inducible factor-1α (HIF-1α) (243, 289, 297). The activation of these transcription factors, in turn, leads to enhanced activation of the antioxidant defense system and promotes the expression of cell survival proteins. For example, increased resistance of multi-drug resistant leukemia cells to the cytotoxic effects of H2O2 was found to be due mainly to elevated levels of catalase (182). Similarly, the resistance of bladder cancer cells to arsenic trioxide (As2O3) was associated with elevated superoxide dismutase (SOD) activity and reduced glutathione (GSH) content (125). Therefore, a combination approach based on the modulation of ROS stress and the breaking of signaling molecules associated with redox adaptation might be required to effectively eliminate cancer cells. In this context, nutraceuticals seem highly promising not only because they have the potential to generate ROS but also because of their ability to modulate signaling molecules associated with drug resistance.
In this article, we discuss how ROS modulate different stages of tumorigenesis, and the signaling molecules downstream of ROS and upstream of cancer, and contribute to chronic inflammation. Pro-oxidant- and antioxidant-based anticancer drugs are discussed. We argue that nutraceuticals derived from Mother Nature serve as excellent sources of anticancer agents. The outstanding questions raised by our current understanding are also discussed.
Sources of Biologically Relevant ROS
Broadly, there are two types of ROS: the free oxygen radical and the nonradical. While the free oxygen radical ROS contain one or more unpaired electron in their outer molecular orbital, the nonradical ROS lack unpaired electrons but are chemically reactive and can be converted to radical ROS (Table 1). Superoxide, H2O2, and hydroxyl radicals are the most well studied and common ROS in cancer.
Table 1.
A List of Major Reactive Oxygen Species and Antioxidant Systems in Living Organisms
|
ROS |
Symbol |
Antioxidant system |
Symbol |
|---|---|---|---|
| Radical ROS | Enzymatic | ||
| Superoxide | O2•− | Superoxide dismutase | SOD |
| Hydroxyl radical | •OH | Catalase | CAT |
| Nitric oxidea | NO• | Glutathione peroxidase | GPx |
| Organic radical | R• | Glutathione reductase | GR |
| Peroxyl radical | ROO• | Glutathione-S-transferase | GST |
| Alkoxyl radical | RO• | Thioredoxin peroxidase | TrxPx |
| Thiyl radical | RS• | Thioredoxin reductase | TrxR |
| Sulphonyl radical | ROS• | ||
| Thiyl peroxyl radical | RSOO• |
| Nonradical ROS/RNS | Nonenzymatic | ||
|---|---|---|---|
| Hydrogen peroxide | H2O2 | Glutathione | GSH |
| Singlet oxygen | 1O2 | Glutaredoxin | Grx |
| Ozone (trioxygen) | O3 | Thioredoxin | Trx |
| Organic hydroperoxide | ROOH | Peroxiredoxin | Prx |
| Hypochlorous acid | HOCl | Sulfiredoxin | Srx |
| Peroxynitritea | ONOO− | Phytochemicals | |
| Vitamins A, C, E | |||
| Ceruloplasmin |
Actually a reactive nitrogen species.
ROS, reactive oxygen species; RNS, reactive nitrogen species.
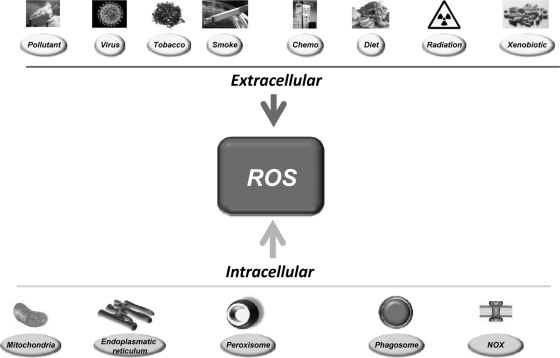
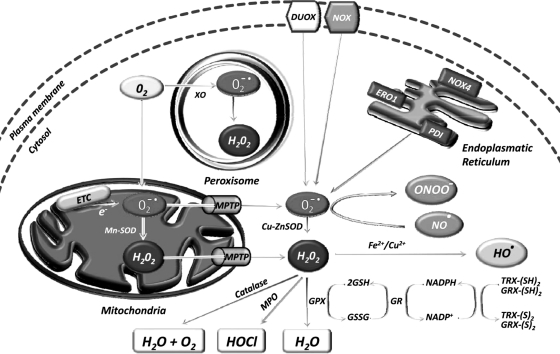
The sources of ROS are both extracellular and intracellular (Fig. 1). Extracellular ROS can be found as pollutants, tobacco, smoke, drugs, xenobiotics, or radiation. ROS are produced intracellularly through multiple mechanisms, the major sources being mitochondria, peroxisomes, endoplasmic reticulum, and the NADPH oxidase (NOX) complex in cell membranes (71, 137). Mitochondria house the electron transport chain, which transfers electrons from NADPH and succinate during respiratory ATP synthesis. The leakage of electrons from the electron transport chain during ATP synthesis results in the reduction of molecular oxygen to superoxide (100, 223). The mitochondrial permeability transition pore in the outer membrane of the mitochondria allows the leakage of superoxide into the cytoplasm (65, 287). Superoxide is dismutated to H2O2, either in the mitochondrial matrix (by Mn-SOD) or in the cytosol (by Cu-ZnSOD) (260). Peroxisomes are other major sites for superoxide and H2O2 production through the action of xanthine oxidase (37, 71, 284). H2O2, which is a highly diffusible oxygen species (219), can be converted to water by catalase, or in the presence of transition metals, it can be converted to highly reactive hydroxyl radicals. Superoxide can also react with the reactive nitric oxide (NO·) to form peroxynitrite (ONOO−) (295). Another major source of ROS, in the form of superoxide or H2O2, is NOX and its dual oxidase relatives, which are localized to various cellular membranes (26, 96, 174, 288, 291). NOX consists of NOX1, NOX2, NOX4, NOX5, p22phox, p47phox, and the small G protein Rac1. ROS are also generated in the endoplasmic reticulum during the process of protein folding and disulfide bond formation. The glycoprotein endoplasmic reticulum oxidoreductin 1, the protein disulfide isomerase, and NOX4 are the major sources of ROS in the endoplasmic reticulum (Fig. 2).
FIG. 1.
Risk factors for ROS in cancer. ROS can be generated by numerous external sources as well as intracellularly by various organs and enzymes. ROS, reactive oxygen species.
FIG. 2.
Major sources of ROS inside a cancer cell. The main sources are mitochondria, peroxisomes, endoplasmic reticulum, and the NOX complex in cell membranes. Under normoxic conditions, excess ROS is scavenged by the antioxidant defense system of the cell. DUOX, dual oxidase; ERO1, endoplasmic reticulum oxidoreductin 1; ETC, electron transport chain; GPx, glutathione peroxidase; GR, glutathione reductase; GRX-(S)2, glutaredoxin oxidized; GRX-(SH)2, glutaredoxin reduced; GSH, glutathione; GSSG, glutathione oxidized; H2O2, hydrogen peroxide; HO ·, hydroxyl radical; HOCl, hypochlorous acid; MPO, myeloperoxidase; MPTP, mitochondrial permeability transition pore; NADP+, nicotinamide adenine dinucleotide phosphate oxidized; NADPH, nicotinamide adenine dinucleotide phosphate reduced; NO ·, nitric oxide; NOX, NADPH oxidase; O2− ·, superoxide radical; ONOO−, peroxynitrite; PDI, protein disulfide isomerase; SOD, superoxide dismutase; TRX-(S)2, thioredoxin oxidized; TRX-(SH)2, thioredoxin reduced; XO, xanthine oxidase.
Under normoxic conditions, intracellular levels of ROS are maintained to protect cells from damage. Scavenging of ROS is facilitated by a dedicated set of antioxidants that may be both enzymatic and nonenzymatic in nature (Table 1).
Role of ROS in Tumorigenesis
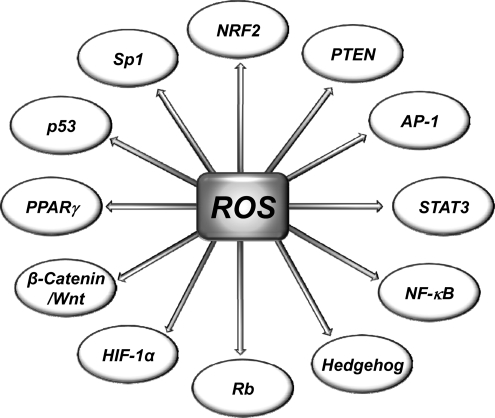
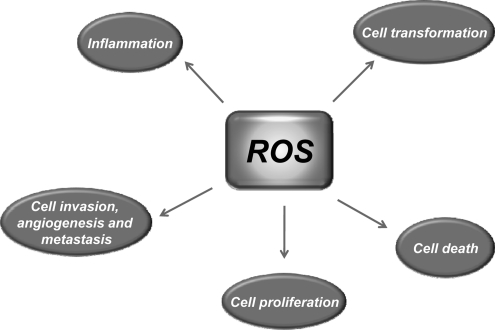
Most risk factors associated with cancer interact with cells through the generation of ROS. ROS, in turn, activate the transcription factors NF-κB, activator protein-1 (AP-1), HIF-1α, signal transducer and activator of transcription 3 (STAT3), and others (Fig. 3). These ROS-mediated transcription factors control the expression of genes involved in inflammation; cell transformation; and tumor cell death or survival, proliferation, invasion, angiogenesis, and metastasis (Fig. 4).
FIG. 3.
Molecular targets of ROS linked with cancer. ROS can target both transcription factors and tumor suppressor genes. AP-1, activator protein-1; HIF-1α, hypoxia-inducible factor-1 alpha; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf2, nuclear factor (erythroid-derived 2)-like factor 2; PPARγ, peroxisome proliferator-activated receptor gamma; PTEN, phosphatase and tensin homolog deleted on chromosome 10; Rb, retinoblastoma; Sp1, specificity protein 1; STAT3, signal transducer and activator of transcription 3.
FIG. 4.
Targets of ROS in tumorigenesis. ROS can both suppress and promote the transformation, survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells. In addition, ROS can regulate inflammation, one of the major mediators of cancer.
Role of ROS in cellular transformation
Cellular transformation in cancer biology is a process whereby normal cells acquire properties of malignant cells. The underlying causes of malignant transformation are the gain-of-function mutations in oncogenes and the loss-of-function mutations in tumor suppressor genes (319). The mutations lead to perturbations of a number of signaling molecules, including p53, Raf, retinoblastoma (Rb), protein phosphatase 2A, telomerase, Ral-GEFs, phosphatidylinositol 3-kinase (PI3K), Ras, Rac, cellular v-myc myelocytomatosis viral oncogene homolog (c-Myc), STAT3, NF-κB, and HIF-1α. Chemicals, viruses, radiation, hypoxia, and nutrient deprivation can also induce mutations in these genes, thereby giving rise to cancer cells (255).
Evidence accumulated over the past several years has indicated an association between ROS and malignant transformation (141, 311, 318). How elevated ROS levels lead to oncogene activation remains poorly understood, but DNA damage is known to play a role. For instance, the oncogenic transformation of ovarian epithelial cells with H-RasV12 or tyrosine kinase Bcr-Abl in hematopoietic cells was associated with an increase in ROS (301). In another study, transformation of fibroblasts with constitutively active isoforms of Rac and Ras was associated with production of superoxide; further study revealed that transformation could be suppressed by treatment with antioxidants (138). Mox1 is a phagocytic NOX, the over-expression of which has been shown to increase superoxide generation in mouse fibroblasts (288). The cells expressing Mox1 exhibited a transformed appearance and produced tumors in athymic mice (288).
In a recent study, cells genetically transformed to express the cancer phenotype were able to generate ROS in response to the small-molecule piperlongumine; normal cells, on the other hand, could rarely be induced to generate ROS (252). Wang et al. observed that chronic exposure of normal human lung epithelial cells to hexavalent chromium resulted in enhanced ROS production that correlated with an increase in NOX activity. Chromium exposure was also associated with malignant transformation that was suppressed by the over-expression of SOD1, SOD2, or CAT (321). In another study, sub-toxic doses of chromium transformed nontumorigenic lung epithelial cells into malignant cells (21). Exposure also led to an increase in NO production, which mediated S-nitrosylation and stabilization of the cell survival B-cell lymphoma-2 (Bcl-2) protein. Stabilization of the Bcl-2 was proposed to be a primary mechanism of malignant transformation (21).
The inflammatory cytokine tumor necrosis factor-α (TNF-α) has been shown to play a role in the transformation of mouse fibroblasts into malignant cells; this effect was partially suppressed by antioxidants (338). Apurinic/apyrimidinic endonuclease/redox effector factor-1 (APE/Ref-1) is a multifunctional protein involved in both DNA repair and redox regulation. Ref-1 was shown to induce malignant transformation in JB6 mouse epithelial cells through the mediation of ROS (342). Matrix metalloproteinase (MMP)-3, a stromal enzyme that is up-regulated in many breast tumors, has been shown to induce ROS, DNA damage, genomic instability, and the transformation of mouse mammary epithelial cells into malignant cells (251).
In summary, ROS seem to play a role in the transformation of normal cells into cancer cells. The major conclusion to be drawn is that transformed cells appear to have greater ROS levels than normal cells do. However, how ROS transform normal cells is not precisely known. Further work in this direction is needed to fully elucidate the mechanism involved in ROS-mediated malignant transformation.
Role of ROS in tumor cell death
One of the chief characteristics of cancer cells is their inherent capacity to survive. Therefore, the major goal of cancer therapy is to selectively kill cancer cells without harming normal cells. There are three major ways by which a cancer cell can die: apoptosis, necrosis, and autophagy (283, 285, 329).
ROS and apoptosis
Apoptosis is a tightly controlled form of cell death and can be initiated by death receptors (extrinsic pathway) or through mitochondria (intrinsic pathway). Both extrinsic and intrinsic pathways of apoptosis depend on ROS (237). In the extrinsic pathway of apoptosis, ROS are generated by Fas ligand as an upstream event for Fas activation. In turn, ROS are required for Fas phosphorylation at the tyrosine residue, which is a signal for subsequent recruitment of Fas-associated protein with death domain and caspase 8 and for apoptosis induction (72, 216, 257, 305). In addition, ROS are required for the ubiquitination and subsequent degradation of the FLICE inhibitory protein to further enhance Fas activation (322). In contrast, the intrinsic pathway of apoptosis is characterized by the opening of the permeability transition pore complex on the mitochondrial membrane, which results in cytochrome c release, apoptosome formation, and caspase activation. Opposing effects of pro-apoptotic and anti-apoptotic Bcl-2 family proteins are required for opening of the permeability transition pore. ROS function to open the pore by both activating pore-destabilizing proteins (Bcl-2-associated X protein, Bcl-2 homologous antagonist/killer) and inhibiting pore-stabilizing proteins (Bcl-2 and Bcl-xL) (212).
Extensive research over the past several years from both cell culture and animal models has demonstrated the potential of ROS in inducing apoptosis in cancer cells. As of August 2011, a search on PubMed database (www.ncbi.nlm.nih.gov/pubmed) generates >2000 publications on this subject. While in some cases ROS has been shown to target oncogenes (301), in other cases, ROS target nononcogene (252) that induce apoptosis in cancer cells. In this section, we discuss some of these studies (Table 2).
Table 2.
Role of Reactive Oxygen Species in Tumor Cell Death
| ROS | Source | Cancer type | Molecular target | Reference |
|---|---|---|---|---|
| Induction of cell death | ||||
| In vitro studies | ||||
| H2O2 | Exogenous | Lymphoma | Capase-3 ↑a | (114) |
| Exogenous | Bladder | MEK1/2 ↑a, ERK1/2 ↑a | (59) | |
| Caspase-3 ↑a, Caspase-7 ↑a | ||||
| Apigenin | Hepatoma | Catalase ↓ | (310) | |
| PDT | Leukemia | Caspase 3 ↑a | (248) | |
| Melatonin | Leukemia | Caspase-3 ↑a, Caspase-8 ↑a | (28) | |
| Caspase-9 ↑a | ||||
| PL | Osteosarcoma, breast, bladder, lung | Bcl-2 ↓, Survivin ↓, XIAP ↓ | (252) | |
| NO | PL | Osteosarcoma, Breast, Bladder, Lung | Bcl-2 ↓, Survivin ↓, XIAP ↓ | (252) |
| ROS | Bortezomib | Gastric | NF-κB ↓b, JNK ↑a | (228) |
| Orthovanadate | Thyroid | mTOR ↑a, PI3K/AKT ↑a, Caspase-3 ↑a | (102) | |
| Bufalin | Colon | JNK ↑a, ATG5 ↑, Beclin-1 ↑ | (336) | |
| Capsaicin | Colon | Caspase-3 ↑a, Caspase-8 ↑a, | (198) | |
| Caspase-9 ↑a, Bax ↑, Bcl-2 ↓ | ||||
| Eugenol | Colon | p53 ↑, Caspase-3 ↑ | (142) | |
| Cantharidin | Bladder | Caspase-3 ↑a, Caspase-8 ↑a, | (172) | |
| Caspase-9 ↑a, Bax ↑, Bcl-2 ↓ | ||||
| GA | Bladder | p-p53 ↑ | (188) | |
| Cajanol | Breast | Caspase-3 ↑a, Caspase-9 ↑a | (202) | |
| Bax ↑, Bcl-2 ↓ | ||||
| Ginseng | Breast | COX-2 ↓, PGE-2 ↓ | (155) | |
| BITC | Osteosarcoma | Caspase-3 ↑a, Caspase-9 ↑a | (331) | |
| PEITC | Osteosarcoma | Caspase-3 ↑a, Caspase-9 ↑a | (331) | |
| Selenite | Osteosarcoma | Caspase-3 ↑a, Bcl-2 ↓ | (52) | |
| p53 ↑, PTEN ↑ | ||||
| ABITC | Endometrial | Caspase-8 ↑a, JNK ↑a, SAPK ↑a | (123) | |
| Garcinol | Hepatocellular | Bax ↑, Bcl-2 ↓, Caspase-3 ↑a | (55) | |
| Caspase-8 ↑a, Caspase-9 ↑a | ||||
| Casticin | Cervical | Caspase-3 ↑a, Caspase-9 ↑a, Bax ↑ | (49) | |
| Bcl-xL ↓, XIAP ↓ | ||||
| Deltonin | Multiple | Bcl-2 ↓; Bax ↑, Caspase-3 ↑a | (282) | |
| Caspase-9 ↑a, AKT ↑a, MAPK ↑a | ||||
| Pipoxolan | Leukemia | Bcl-2 ↓, Bcl-xL ↓, MiMP ↓, Bax ↑ | (54) | |
| ESB | Leukemia | Caspase-3 ↑a, Caspase-9 ↑a | (6) | |
| UDCA | Gastric | DR5 ↑, PKC-δ ↑a, Caspase-3 ↑a | (185) | |
| Caspase-6 ↑a, Caspase-8 ↑a | ||||
| Carnosic Acid | Neuroblastoma | Bcl-2 ↓, Caspase-3 ↑a, Caspase-9 ↑a | (303) | |
| Withaferin A | Melanoma | Bcl-2 ↓, Bax↑, Bim↑, Caspase-3 ↑a | (215) | |
| Caspase-9 ↑a | ||||
| EGCG | Chondrosarcoma | Bax ↑, Bak ↑, Bcl-2 ↓, Bcl-xL ↓ | (343) | |
| Thymoquinone | Lymphoma | Caspase-3 ↑a, Caspase-9 ↑a, DR5 ↑ | (130) | |
| Progesterone | Ovarian/Endometrial | p53 ↑, Bax ↑, Bcl-2 ↓ | (231) | |
| Danthron | Gastric | Caspase-3 ↑a, Caspase-8 ↑a | (58) | |
| Caspase-9 ↑a, Bax ↑, Bcl-2 ↓ | ||||
| Tricetin | Liver | DR5 ↑ | (126) | |
| In vivo studies | ||||
| H2O2 | PL | Breast, Bladder, Lung | Bcl-2 ↓, Survivin ↓, XIAP ↓ | (252) |
| NO | PL | Breast | Bcl-2 ↓, Survivin ↓, XIAP ↓ | (252) |
| ROS | CHL | Leukemia | Caspase-8 ↑a, Caspase-9 ↑a, MiMP ↓ | (254) |
| AGL | Lymphoma | Caspase-3 ↑a, Caspase-8 ↑a | (341) | |
| Caspase-9 ↑a | ||||
| DMAPT | Prostate | NF-κB ↓, JNK ↑, TRAF2 ↓, | (276) | |
| Caspase-8 ↑a, XIAP ↓ | ||||
| Inhibition of cell death | ||||
| ROS | Endogenous | Prostate | ND | (41) |
| IGF-I | Pancreatic | JAK2 ↑a | (178) | |
ND, not determined; ↑a, activation; ↓b, inactivation; ↓, down-regulation; ↑, up-regulation.
ABITC, abietyl isothiocyanate; AGL, andrographolide; AKT, AKT8 virus oncogene cellular homolog; ATG5, autophagy protein 5; Bak, Bcl-2 homologous antagonist/killer; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2; Bcl-xL, B-cell lymphoma-extra large; Bim, Bcl-2-interacting mediator; BITC, benzyl isothiocyanate; CHL, chlorogenic acid; COX-2, cyclooxygenase-2; DMAPT, dimethylaminoparthenolide; DR5, death receptor 5; EGCG, epigallocatechin gallate; ERK1/2, extracellular signal-regulated kinase 1/2; ESB, erythrina suberosa stem bark; GA, 18 β-glycyrrhetinic acid; H2O2, hydrogen peroxide; IGF-I, insulin-like growth factor-1; JAK2, janus kinase 2; JNK, c-jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MEK1/2, MAPK/ERK kinase 1/2; MiMP, mitochondrial membrane potential; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NO, nitric oxide; PDT, photodynamic therapy; PEITC, β-phenylethylisothiocyanate; PGE-2, prostaglandin E2; PI3K, phosphoinositide 3-kinase; PKC-δ, protein kinase C-delta; PL, piperlongumine; PTEN, phosphatase and tensin homolog deleted on chromosome 10; SAPK, stress-activated protein kinase; TRAF2, TNF receptor-associated factor 2; UDCA, ursodeoxycholic acid; XIAP, X-linked inhibitor of apoptosis protein.
Exogenous administration of H2O2 has been shown to induce apoptosis in lymphoma cells through activation of caspase-3 (114). H2O2 has also been shown to activate MAPK/ERK kinase1/2, extracellular signal-regulated kinase 1/2 (ERK1/2), and caspase, and to induce cell death in human bladder cancer cells (59). H2O2 generated by external sources has the potential to induce apoptosis in hepatoma cells (310), leukemia cells (28, 248), and osteosarcoma, breast, bladder, and lung cancer cells (252). NO generated by the small-molecule piperlongumine has also been shown to induce apoptosis in osteosarcoma cells and in breast, bladder, and lung cancer cells but not in normal cells (252). The authors of this study concluded that increased dependence of cancer cells on the ROS stress-response pathway could be a basis for the selectivity of piperlongumine-induced apoptosis in cancer cells (252). ROS has been shown to induce apoptosis in cancer cells in a caspase-independent manner as well. For instance, in human lung endothelial cells, ROS was shown to induce apoptosis in a caspase-independent manner but involved mitochondrial-to-nuclear translocation of apoptosis-inducing factor and endonuclease G (193).
Numerous agents have been shown to induce ROS and apoptosis in various cancer types. The most common signaling molecules modulated by ROS in these cell models are kinases, pro-inflammatory transcription factors such as NF-κB, caspases, cell survival proteins, pro-apoptotic proteins, and phosphatase, and tensin homolog deleted on chromosome 10 (PTEN). For example, the proteasome inhibitor bortezomib induces apoptosis in gastric cancer cells by inactivating NF-κB, activating c-jun N-terminal kinase (JNK), and inducing ROS generation (228). Some other common cancers for which ROS have demonstrated potential are listed in Table 2.
The potential of ROS in inducing apoptosis is evident from animal studies as well. Using mouse models of breast, bladder, and lung cancer, Raj et al. recently demonstrated that H2O2 generated by piperlongumine can selectively kill cancer cells (252). Chlorogenic acid has been shown to induce apoptosis in cells from chronic myeloid leukemia patients and also in nude mice bearing K562 xenografts in a ROS-dependent manner (254). In another study, andrographolide, a diterpenoid lactone, induced apoptosis in patient-derived lymphoma cells in an ROS-dependent manner; ROS induced apoptosis in these cells through activation of caspases-3, −8, and −9 that was inhibited by an ROS scavenger (341). Dimethylaminoparthenolide, a water-soluble parthenolide analog, has also been shown to induce apoptosis in prostate cancer cells in vivo by targeting NF-κB and generating ROS (276).
Some of the agents have been shown to generate ROS but they lack pro-apototic potential. Mi et al. found that the pro-apototic activities of benzyl isothiocyanate (BITC) and β-phenylethylisothiocyanate (PEITC) are not due to their ROS-inducing potential but to their ability to inhibit proteasomes and to bind covalently with target proteins (218).
Apart from their ability to kill cells, ROS are also required for cancer cell survival. In fact, the ability of cancer cells to distinguish between ROS as a survival or apoptotic signal is controlled by the dosage, duration, type, and site of ROS production. However, modest levels of ROS are required for cancer cells to survive, whereas excessive levels kill them (168, 268). Similarly, NOX-derived ROS in the cytoplasm in response to TNF-α play a protective role, whereas mitochondria-derived ROS promote apoptosis (73). Low levels of ROS have been shown to promote the survival of serum-deprived anaplastic large cell lymphoma cells (339). In prostate cancer cells, inhibition of ROS by antioxidants or NOX inhibitors was associated with an increase in apoptosis (41). ROS produced by NOX4 has also been shown to act as a mediator of cell survival (85, 220, 313). Similarly, in pancreatic cancer cells, ROS produced by NOX was shown to promote survival by inhibiting tyrosine phosphatase-mediated dephosphorylation of janus kinase 2 (178).
ROS and necrosis
Although an excess level of ROS is known to induce apoptosis, massive levels may lead to necrotic cell death. In some cases, ROS can induce both apoptosis and necrosis in cancer cells. For example, in Jurkat T-lymphocytes, H2O2 was found to have dual effects: At low H2O2 concentrations, the cells were found to undergo apoptosis by caspase activation, but at higher H2O2 concentrations, no detectable caspase activity was observed and the cells died of necrosis (114). In multiple myeloma cells, ROS generated in response to a peptide have been shown to induce necrosis (226). A switch from apoptotic to necrotic cell death has also been shown to be dependent on the ROS content in prostate cancer cells (93) and hepatoma cells (160). Similarly, 8-nitrocaffeine and its analog, which are candidate radiosensitizers for cancer therapy, were found to induce necrotic cell death in leukemia cells in an ROS-dependent manner (227).
ROS and autophagy
Autophagy is a self-catabolic process that involves sequestration of exhausted organelles and protein aggregates from the cytoplasm and their delivery into lysosomes for degradation. Autophagy is involved in both cell survival and cell death pathways, and the process is altered in cancer cells (120). Studies during the past 5 years have indicated a role for ROS as a signaling molecule in inducing autophagic cell death in cancer cells (20, 97, 139). For example, H2O2 production in human colon cancer cells has been associated with autophagic cell death (64). In a resistant pancreatic cancer model, gemcitabine and cannabinoid combinations triggered autophagic cell death through a ROS-mediated mechanism (77). Bufalin, which is isolated from a traditional Chinese medicine, was unable to induce apoptosis in colon cancer cells, contrary to its well-documented apoptosis-promoting activity in other cancer cells (336). Instead, bufalin activated an autophagy pathway, as characterized by the accumulation of LC3-II and the stimulation of autophagic flux. The induction of autophagy by bufalin was linked to ROS generation. ROS activated autophagy via JNK activation, which, in turn, increased the expression of autophagy protein 5 and Beclin-1. Further, bufalin-induced autophagy was attenuated by an ROS scavenger (336). Some other cancer types for which ROS have been shown to effectively induce autophagic cell death are breast cancer (281), nonsmall cell lung cancer (NSCLC) (184), glioma (239, 317), neuroblastoma (317), glioblastoma (53), and cervical cancer (53, 108).
In summary, ROS have dual roles: They can not only kill cancer cells but they can also promote tumor survival. The great challenge for cancer researchers is determining how to exploit this dual property of ROS for therapeutic development.
Role of ROS in tumor cell proliferation
Uncontrolled proliferation is one of the chief characteristics of tumor cells (115, 116). A precise set of cell cycle regulators such as cyclins and cyclin-dependent kinases (CDKs) control the progression of cell-cycle events. CDK activity is controlled by the opposing effects of cyclins and CDK inhibitors. CDK inhibitors such as p21 and p27 negatively regulate CDK activity, whereas cyclins are required for CDK activity and cell cycle progression. Another protein, c-Myc, is required for the G1-to-S-phase transition (118). The expression of c-Myc, in turn, is regulated by cdc25, a phosphatase that activates CDKs.
Intracellular ROS produced by exogenous stimuli as well as exogenous administration of ROS have been shown to enhance the proliferation of numerous cancer types (Table 3). For example, exogenous administration of H2O2 was shown to enhance the proliferation of hepatoma cells by increasing protein kinase B and extracellular signal-regulated kinase (ERK) activities (195). In another study, transformed bladder urothelial cells were found to be hyper-proliferative and produced elevated ROS levels in the presence of monomethylarsonous acid; the up-regulation in cyclooxygenase-2 (COX-2) expression observed in these cells was found to be ROS dependent (84). ROS produced by low concentrations of arsenite has been shown to enhance the proliferation of breast cancer cells by recruiting cells into the S phase of the cell cycle, enhancing the expression of c-Myc and heme oxygenase-1, and increasing NF-κB activity (262).
Table 3.
Role of Reactive Oxygen Species in Tumor Cell Proliferation
| ROS | Source | Cancer type | Molecular target | Reference |
|---|---|---|---|---|
| Induction of cell proliferation | ||||
| In vitro studies | ||||
| H2O2 | Exogenous | Hepatoma | PKB ↑a, ERK ↑a | (195) |
| ROS | MMA | Bladder | COX-2 ↑ | (84) |
| Arsenite | Breast | c-Myc ↑, HO-1 ↑, NF-κB ↑a | (262) | |
| Endogenous | Lung | ERK ↑a | (224) | |
| Superoxide | Endogenous | Hepatoma | PI3K/AKT ↑ | (78) |
| ROS | Endogenous | Breast | PTEN ↓, PI3K ↑a | (70) |
| Superoxide | SOD silencing | Ovarian | ND | (127) |
| ROS | Endogenous | NSCLC | G2/M arrest ↓ | (196) |
| Endogenous | Liver | pAKT ↑, pRb ↑, Cyclin D1 ↑ | (249) | |
| Cyclin E ↑, CDK2 ↑, p27 ↓ | ||||
| H2O2 | Endogenous | Multiple | ND | (245) |
| ROS | Endogenous | Lymphoma | NF-κB ↑ | (69) |
| Endogenous | Glioma | AKT ↑a, ERK1/2 ↑a, NF-κB ↑a | (211) | |
| LPA | Ovarian | pERK ↑, pAKT ↑, NF-κB ↑a | (267) | |
| H2O2 | Endogenous | Ovarian | MKP-3 ↓, ERK1/2 ↑ | (46) |
| ROS | DEN | Liver | JNK ↑ | (205) |
| Endogenous | Melanoma | NF-κB ↑a | (42) | |
| Superoxide | Endogenous | Melanoma | NF-κB ↑a | (43) |
| ROS | Tobacco | Lung | EGFR ↑a, PKC ↑a | (181) |
| In vivo studies | ||||
| ROS | Endogenous | Breast | PTEN ↓, PI3K ↑a | (70) |
| Superoxide | SOD silencing | Ovarian | ND | (127) |
| Inhibition of cell proliferation | ||||
| ROS | Endogenous | Breast | NF-κB ↓b | (250) |
| Butein | Hepatoma | G2/M arrest ↑, pATM ↑, pChk ↑ | (221) | |
| pChk2 ↑, Cdc25c ↓ | ||||
| Gemcitabine | Pancreatic | ND | (76) | |
| Thymoquinone | Prostate | GSH ↓ | (167) | |
ATM, ataxia telangiectasia mutated; Cdc25c, cell division cycle 25 homolog c (S. pombe); CDK2, cyclin-dependent kinase 2; Chk, checkpoint kinase; c-Myc, cellular v-myc myelocytomatosis viral oncogene homolog (avian); DEN, diethylnitrosamine; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; GSH, glutathione; HO-1, heme oxygenase-1; LPA, lysophosphatidic acid; MKP-3, mitogen-activated protein kinase phosphatase-3; MMA, monomethylarsonous acid; NSCLC, nonsmall cell lung cancer; PI3K, phosphatidylinositol 3-kinase; PKB, protein kinase B; Rb, retinoblastoma; SOD, superoxide dismutase.
ROS produced by endogenous sources can also enhance cancer cell proliferation. For example, ROS produced by Romo1, a mitochondria-localized protein (61, 133), was shown to be indispensable to the proliferation of lung cancer cells (224). Such an induction in cell proliferation was found to be ERK dependent (224). Endogenous production of superoxide has also been shown to enhance tumor proliferation in hepatoma cells that was mediated through AKT8 virus oncogene cellular homolog (AKT) phosphorylation (78). Similarly, an increase in endogenous ROS due to reduction in the antioxidant defense system has been correlated with an increase in the proliferation of breast (70) and ovarian (127) cancer cells. In breast cancer cells, ROS-mediated tumor proliferation was found to be dependent on activation of PI3K pathway and reduction of PTEN activity (70).
The role of ROS in promoting tumor proliferation is further supported by observations that agents with the potential to inhibit ROS generation can also inhibit tumor cell proliferation. For instance, N-ethoxymethyl-3-amino-1,2,4-benzotriazine-1,4-dioxide, a novel N-ethoxymethyl-3-amino-1,2,4-benzotriazine-1,4-dioxide derivative, was found to inhibit the growth of NSCLC cells by inducing cell cycle arrest at the G2/M phase and suppressing ROS generation (196). Similarly, attenuation of ROS by a squamosamide derivative was associated with inhibition of the proliferation of liver cancer cells; decreased phosphorylation of AKT and Rb protein; down-regulated expression of cyclin D1, cyclin E, and CDK2; and enhanced expression of p27 (249). In another study, exogenous catalase inhibited the proliferation of numerous cancer types (245). Consistent with these observations, stable expression of human catalase in MCF-7 cells inhibited proliferation and reverted malignant features (245). Curcumin has been shown to inhibit the proliferation of lymphoma cells by increasing endogenous antioxidant enzyme activity and by inhibiting NF-κB activity (69). Inhibition of ROS generation by N-acetyl-L-cysteine (NAC), one of the most widely used ROS scavengers, has been correlated with decreased proliferation of cancer cells. For example, treatment of glioma cells with NAC inhibited cell proliferation by arresting cells in the G1 phase; this inhibition was correlated with a decrease in the activities of AKT, ERK1/2, and NF-κB (211). In another study, NAC was shown to reduce the proliferation of ovarian cancer cells (267).
Some other common cancers for which ROS has been shown to enhance proliferation are listed in Table 3. The ability of ROS in promoting tumor cell proliferation is supported by animal studies as well (70, 127).
Although ROS promote tumor cell proliferation in general, an increase in the level of ROS has also been correlated with reduced tumor proliferation (Table 3). For example, silencing of the redox protein thioredoxin-like 2 (TXNL-2) in human breast cancer cells was associated with an increased ROS level, reduced NF-κB activity, and inhibited tumor proliferation (250). Recently, butein was shown to inhibit the growth of hepatoma cells, which was correlated with ROS content (221). The increase in ROS and the inhibition in growth were further correlated with the induction of G2/M cell cycle arrest; increased phosphorylation of ataxia telangiectasia mutated, checkpoint kinase (Chk) 1, and Chk2; and reduced cell division cycle 25 homolog c levels. Further, an antioxidant pretreatment abrogated butein's inhibitory effect on cell growth (221). ROS generated by gemcitabine and by thymoquinone have also been shown to inhibit the growth of pancreatic (76) and prostate (167) cancer cells, respectively.
Role of ROS in tumor cell invasion, angiogenesis, and metastasis
Tumor cell invasion, angiogenesis, and metastasis are inter-related processes that represent the final, most devastating stage of malignancy. The process involves cell growth, adhesion, and migration; proteolytic degradation of tissue barriers; and formation of new blood vessels (86). Several proteolytic enzymes, such as matrix metalloproteinases (MMPs) (146, 286) and the intercellular adhesion molecule, participate in the degradation of these barriers (9, 165). Other molecules involved in this process are serine proteases such as urokinase-type plasminogen activator and its receptor, vascular endothelial growth factor (VEGF) and its receptors, platelet-derived growth factor, fibroblast growth factors, epidermal growth factor (EGF), ephrins, angiopoietins, endothelins, integrins, cadherins, and transcription factors (e.g., AP-1, NF-κB) (3, 5, 103, 183, 229, 323) (Table 4).
Table 4.
Role of Reactive Oxygen Species in Tumor Angiogenesis and Metastasis
| ROS | Source | Cancer type | Molecular target | Reference |
|---|---|---|---|---|
| Induction of angiogenesis and metastasis | ||||
| In vitro studies | ||||
| H2O2 | Exogenous | Colorectal | JNK ↑a, ERK ↑a, p38 ↑a, MMP-7 ↑, AP-1 ↑a | (121) |
| Exogenous | Prostate | CXCR4 ↑, pAKT ↑, PTEN ↓b | (57) | |
| Exogenous | Lung | Cav-1 stabilization | (263) | |
| Exogenous | EC | p38 ↑, MMP-9 ↑ | (253) | |
| Exogenous | BAEC | Ets-1 ↑ | (344) | |
| Exogenous | HNSCC | CXCL14 ↓, IL-8 ↑ | (206) | |
| EGF | Ovarian | VEGF ↑ | (192) | |
| Endogenous | Prostate | VEGF ↑, VEGFR1 ↑, VEGFR2 ↑, MMP ↑ | (18) | |
| ROS | Endogenous | Colorectal | ITGB3 ↑, STMN1 ↑ | (180) |
| Endogenous | Ovarian | HIF-1 ↑, VEGF ↑ | (335) | |
| Endogenous | Prostate | HIF-1 ↑, VEGF ↑ | (335) | |
| Endogenous | Colon | VEGF ↑, HIF-1α ↑ | (154) | |
| Endogenous | Liver | HIF-1α ↑, VEGF ↑, IL-8 ↑, u-PA ↑ | (186) | |
| TPA | Hepatocellular | ERK ↑a, JNK ↑a, p38 ↑a, NF-κB ↑a, AP-1 ↑a | (177) | |
| COX-2 ↑, iNOS ↑, MMP-9 ↑ | ||||
| LPA | Breast | PI3K ↑a, PAK1 ↑a, ERK ↑a | (80) | |
| LTB4 | Bladder | NF-κB ↑ | (157) | |
| EGF | Pancreatic | MMP-2 ↑ | (36) | |
| PMS | Gastric | u-PAR ↑, AP-1 ↑a, ERK1/2 ↑a | (161) | |
| PCBs | Breast | ROCK ↑a | (194) | |
| TGF-β1 | Pancreatic | MMP-2 ↑a, NF-κB ↑a, IL-6 ↑ | (35) | |
| PKC-δ activator | Prostate | HIF-1α ↑, VEGF ↑ | (159) | |
| Hydroxyl | Donor | Lung | Cav-1 ↑ | (200) |
| Exogenous | HNSCC | CXCL14 ↓, IL-8 ↑ | (206) | |
| In vivo studies | ||||
| ROS | Endogenous | Breast | p38 ↑a | (101) |
| Endogenous | Bladder | NF-κB ↑a | (157) | |
| Endogenous | Lung | MMP-9 ↑, NF-κB ↑ | (156) | |
| Endogenous | Lung | VEGF ↑, HIF-1α ↑ | (140) | |
| Endogenous | Melanoma | c-Met ↑, c-Met ↑a | (89) | |
| Endogenous | Sarcoma | VEGF ↑, HIF-1α ↑ | (203) | |
| Endogenous | Colon | VEGF ↑, HIF-1α ↑ | (154) | |
| PKC-δ activator | Prostate | HIF-1α ↑, VEGF ↑ | (159) | |
| Surgery | Melanoma | EGFR ↑ | (135) | |
| Reduction of Angiogenesis and Metastasis | ||||
| ROS | Endogenous | Breast | NF-κB ↓ | (250) |
| Theaflavin | Breast | p38 ↑a, NF-κB ↓, MMP-2 ↓, MMP-9 ↓ | (1) | |
| BITC | NSCLC | AKT ↓b, NF-κB ↓b, MMP-2 ↓, Twist ↓ | (333) | |
| PEITC | NSCLC | AKT ↓b, NF-κB ↓b, MMP-2 ↓, Twist ↓ | (333) | |
| GT-094 | Colon | VEGF ↓, VEGFR1 ↓, VEGFR2 ↓ | (241) | |
| Endogenous | Pancreatic | ND | (270) | |
| Superoxide | Donor | Lung | Cav-1 ↓ | (200) |
| H2O2 | Donor | Lung | Cav-1 ↓ | (200) |
AP-1, activator protein-1; BAEC, bovine aortic endothelial cell; Cav-1, caveolin-1; c-Met, hepatocyte growth factor receptor; CXCL14, CXC chemokine ligand 14; CXCR4, CXC chemokine receptor 4; EC, endothelial cell; EGF, epidermal growth factor; Ets-1 v-ets erythroblastosis virus E26 oncogene homolog 1; GT094, ethyl 2-((2,3-bis(nitrooxy)propyl)disulfanyl)benzoate; HIF-1, hypoxia-inducible factor-1; HNSCC, head and neck squamous cell carcinoma; IL, interleukin; iNOS, inducible nitric oxide synthase; ITGB3, integrin beta 3; LTB4, leukotriene B4; MMP, matrix metalloproteinase; PAK1, p21 activated kinase 1; PCBs, polychlorinated biphenyls; PMS, phenazine methosulfate; ROCK, rho-associated kinase; STMN1, stathmin 1; TGF-β1, transforming growth factor beta 1; TPA, 12-O-tetradecanoylphorbol-13-acetate; u-PA, urokinase-plasminogen activator; u-PAR, urokinase-plasminogen activator surface receptor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Accumulating evidence over the past several years from both in vitro and in vivo studies has indicated a role for ROS as a signaling mediator of angiogenesis and metastasis (306–308). ROS has been shown to mediate these effects through induction of transcription factors and genes involved in angiogenesis and metastasis. However, the role of ROS in modulating tumor cell metastasis and angiogenesis has seemed paradoxical: High ROS levels suppress tumor angiogenesis and metastasis by destroying cancer cells, whereas sub-optimal concentrations assist cancer cells in metastasizing (232).
Exogenous administration of H2O2 enhances metastasis by modulating multiple signaling molecules. For example, in colorectal cancer cells, H2O2 induced metastasis in a JNK- and mitogen-activated protein kinase (MAPK) mediated activation of AP-1 and MMP-7 up-regulation (121). H2O2 has also been shown to promote metastasis by up-regulating CXC chemokine receptor 4 (CXCR4) and pAKT and inactivating PTEN in prostate cancer cells (57); while in lung cancer cells, it stabilized caveolin-1 (263). Exogenous H2O2 can also induce angiogenesis of endothelial cells (253), bovine aortic endothelial cells (344), head and neck squamous cell carcinoma cells (206), and ovarian cancer cells (192).
In a few cases, endogenous H2O2 has been found to induce tumor angiogenesis. For example, Arbiser et al. demonstrated the potential of Nox-expressing prostate tumors to up-regulate VEGF, VEGF receptors 1 and 2, and MMP (18). These up-regulations were associated with vascularization and rapid expansion of the tumors. Further, induction of VEGF was eliminated by co-expression of catalase, indicating that H2O2 was required for induction of the angiogenic phenotype (18).
In one study, higher levels of ROS were observed in a colorectal cancer-derived metastatic cell line that correlated with an up-regulation in integrin beta 3 and stathmin 1 (180). Endogenous production of ROS has also been shown to induce angiogenesis and metastasis in ovarian (335), prostate (335), colon (154), and liver (186) cancer cells. ROS-generating agents can also induce angiogenesis and metastasis in cancer cells. Common agents under this category are 12-O-tetradecanoylphorbol-13-acetate in hepatocellular (177), lysophosphatidic acid in breast (80), leukotriene B4 in bladder (157), EGF in pancreatic (36), phenazine methosulfate in gastric (161), polychlorinated biphenyls in breast (194), transforming growth factor beta 1 in pancreatic (35), and protein kinase C-delta (PKC-δ) activator in prostate (159) cancer cells. The most common signaling molecules modulated by these agents through ROS production are MAPK, JNK, NF-κB, AP-1, MMPs, inducible NO synthase, cytokines, PI3K, p21 activated kinase 1, rho-associated kinase, VEGF, and the urokinase-type plasminogen activator receptor.
The potential of ROS in promoting tumor cell angiogenesis and metastasis has also been demonstrated in animal models of breast cancer (101), bladder cancer (157), lung cancer (140, 156, 162), melanoma (89), sarcoma (203), colon cancer (154), and prostate cancer (159) cells. In a transgenic mouse model that develops metastatic breast cancer (MMTV-PyMT), the invasive behavior of tumor cells was significantly reduced by catalase (101). In a mouse model of bladder cancer, ROS were shown to play a role in inducing metastasis through the stimulation of NF-κB (157). Ras-evoked lung metastasis was also recently shown to be induced through the generation of ROS and the up-regulation of NF-κB and MMP-9 in a mouse model (156). Of note is that the surgical procedure used to remove tumors has been shown to induce ROS generation and to enhance the growth of metastatic tumors in a mouse model of melanoma (135).
One study provided direct evidence for the causative relationship between ROS generation and tumor metastasis (140). After replacement of mitochondrial DNA derived from a highly metastatic mouse tumor cell line, a poorly metastatic cell line acquired the metastatic potential in mice. The transferred mitochondrial DNA contained mutations with deficiency in respiratory complex I activity and was associated with enhanced ROS production. Further, pretreatment of the highly metastatic tumor cells with ROS scavengers suppressed the metastatic potential in the mice (140).
In most cases, ROS have been demonstrated to induce angiogenesis and metastasis, but in a few studies, an increase in ROS levels has been shown to play a negative role. For instance, an increase in ROS after TXNL-2 silencing has been associated with reduction in NF-κB activity and metastasis of breast cancer (250). In another study, theaflavin, the bioactive flavonoid of black tea, suppressed breast cancer metastasis by activating the p53-ROS-p38MAPK pathway and inhibiting NF-κB activation and MMP-2 and MMP-9 expression (1). Similarly, the anti-metastatic potential of BITC and PEITC in human NSCLC cells has been associated with an increase in ROS generation and depletion in GSH content (333). Pathi et al. found that treatment of colon cancer cells with GT-094, a nonsteroidal anti-inflammatory drug, was associated with an increase in ROS and decreases in VEGF and VEGF receptors 1 and 2 (241). The combination of tyrosine kinase inhibitor dasatinib with oxaliplatin has also been shown to reduce angiogenesis in colon cancer cells in association with an increase in ROS generation (170). Fibulin-5 is a matricellular protein that has been shown to regulate angiogenesis (12, 13). In a recent study, the angiogenesis of pancreatic tumors was found to be suppressed in Fibulin-5–null (Fbln5−/−) mice compared with in wild-type littermates; this suppression was associated with an increase in ROS in these tumors (270).
Interestingly in one study, modulation of lung cancer metastasis was dependent on ROS type. The hydroxyl radical up-regulated caveolin-1 expression and promoted metastasis, whereas superoxide and H2O2 down-regulated caveolin-1 and inhibited metastasis (200).
ROS, Chronic Inflammation, and Cancer
Inflammation is a part of the body's defense system to counteract an insult incurred by internal or external stimuli. Acute inflammation is therapeutic, whereas chronic inflammation is a culprit for numerous chronic diseases, including cancer. It was Virchow in the nineteenth century who first noticed the presence of inflammatory cells within tumors and found that tumors arise at sites of chronic inflammation (24, 269). Experimental and epidemiologic research over the past several years has indicated close associations between ROS, chronic inflammation, and cancer (62, 105, 106, 132, 209, 210, 259, 269, 326). How ROS induce inflammation has also been investigated over the years. Induction of COX-2, inflammatory cytokines (TNF-α, interleukin [IL]-1, IL-6), chemokines (IL-8, CXCR4), and pro-inflammatory transcription factors (e.g., NF-κB)—all well-known mediators of inflammation and tumorigenesis—is regulated by ROS (131, 222). Further, mitochondrial ROS play a major role in inducing chronic inflammation and cancer (4, 149, 225, 324).
The involvement of chemokines and chemokine receptors in the invasion and metastasis of various tumors has been reported (236, 345). The metastatic potential of chemokines has been attributed to their ability to induce the expression of MMPs, which facilitate tumor invasion (197, 345). The silencing of endogenous chemokine receptors has been shown to inhibit the proliferation, adhesion, and invasion of salivary gland mucoepidermoid carcinoma cells (327). One study found a close association between the expression of IL-8 by human melanoma and ovarian cancer cells and their metastatic potential (129, 201, 337). In another study, serum IL-6 and IL-8 were associated with the incidence of lung cancer (244). Pro-inflammatory cytokines have been demonstrated to be predictors of prognosis for esophageal adenocarcinoma (230). Pro-inflammatory molecules have also been shown to be predictors of multiple myeloma (164), non-Hodgkin's lymphoma (316), colorectal cancer (176), bladder cancer (235), lung cancer (244), esophageal cancer (230), and renal cell carcinoma (17).
Although the examples just presented indicate a positive role of ROS-mediated inflammatory cytokines in cancer development, in a few cases, the suppression of inflammatory pathways has been found to be detrimental. For example, administration of TNF blockers to patients with rheumatoid arthritis was found to increase the risk for developing lymphomas (95). Similarly, suppression or deletion of NF-κB has been associated with progression to carcinogenesis (68, 92, 261, 274, 312). Thus, it can be concluded that the effects of ROS on inflammation may be either beneficial or detrimental depending on the cell type, species involved, and physiologic conditions.
Role of ROS in Cancer Prevention and Therapy: Lessons from Clinical Studies
Due to the dual role of ROS in cancer development, both pro-oxidant- and antioxidant-based agents have been developed for cancer prevention and therapy (15, 90, 271, 300, 318). Pro-oxidant-based anticancer agents can not only directly increase ROS production but also decrease the antioxidant defense system of cancer cells. The antioxidant-based agents can directly scavenge intracellular ROS, enhance ROS-scavenging enzyme activities, and inhibit NOX activity. In some cases, a combination of these approaches has been found to be very successful.
Role of nutraceuticals and antioxidants in cancer prevention
According to one report, 90%–95% of cancers are caused by life style factors and only 5%–10% are caused by genetic defects (16). These proportions indicate that cancer is a disease which can be prevented largely by life style changes. Since ROS are involved in the transformation of nonmalignant cells to malignant cells, a potential approach to cancer prevention might be to control ROS production at the transformative stage.
Due to their effect on multiple targets as well as their cost-effectiveness, efficacy, safety, and immediate availability, plant-derived nutraceuticals and antioxidants have attracted the attention of clinicians and researchers during the past two decades. Nutraceuticals can act as either a pro-oxidant or an antioxidant on the basis of the concentration and cancer type. Although they have been proved beneficial for both cancer prevention and treatment, in this section, we discuss the role of nutraceuticals for prevention.
Curcumin is one of the most widely studied nutraceuticals that has potential against numerous cancers. Depending on the concentration and cancer type, curcumin can exhibit both antioxidant and pro-oxidant activities (8, 19, 33, 91, 280). For instance, in one study, curcumin at 12.5 μM reduced ROS formation in human myeloid leukemia cells but at higher concentrations, curcumin elevated ROS levels (50). A number of clinical trials have evaluated the potential of curcumin for cancer prevention. For example, curcumin was found to benefit patients with ulcerative proctitis and Crohn's disease (122). In one study, the regimen of curcumin (8 g/day) given for 3 months to patients with high-risk or premalignant lesions was found to be safe and effective (56). Curcumin also seems a promising and safe medication for maintaining remission in patients with quiescent ulcerative colitis (117). One study investigated the effect of oral curcumin in combination with piperine on pain and on the markers of oxidative stress in patients with tropical pancreatitis (83). Twenty patients were randomly allocated to receive 500 mg of curcumin with 5 mg of piperine thrice a day, or placebo for 6 weeks. The effects on the pattern of pain and on the malondialdehyde and GSH content in red blood cells were assessed. Curcumin in combination with piperine was correlated with a significant reduction in the erythrocyte malondialdehyde content and a significant increase in GSH levels in patients with tropical pancreatitis (83). Curcumin in combination with quercetin has been found to reduce the number and size of ileal and rectal adenomas in patients with familial adenomatous polyposis, an autosomal-dominant disorder characterized by the development of colorectal adenomas and eventually colorectal cancer, without appreciable toxicity (66). In addition, curcumin in combination with isoflavones has been found to suppress the production of prostate-specific antigen, which is a biomarker of prostate cancer (136).
Vitamins, selenium, carotenoids, pomegranate, green tea, and soy have also been effective in human clinical trials for cancer prevention (175, 302). Lycopene is one of the main carotenoids in the regional Mediterranean diet and can account for 50% of the carotenoids in human serum. Lycopene is present in fruits, including watermelon, apricots, pink guava, grapefruit, rose hip, and tomatoes. Scavenging of ROS is one of the mechanisms for the anticancer effects of lycopene. In one study, consumption of tomato sauce before prostatectomy decreased the serum prostate-specific antigen level and oxidative DNA damage and increased the lycopene concentration in prostate tissue (51). In another study, tomato sauce suppressed the progression of disease in patients diagnosed with prostate carcinoma (158). In a study conducted in Japan, 244 subjects with atrophic gastritis were randomly allocated to receive vitamin C (50 or 500 mg) for 5 years. Vitamin C was found to reduce oxidative stress among subjects with atrophic gastritis (266). An inverse correlation between vitamin C and cancer risk has been demonstrated by other studies as well (44, 153).
Green tea is popular for its epigallocatechin gallate, a polyphenolic compound that contributes to the potential health benefits associated with green tea consumption (152). In a study conducted in China, the risk of prostate cancer declined with increasing frequency, duration, and quantity of green tea consumption (145). Pomegranate has been used for centuries for medicinal purposes. The fruit is known for its isoflavonoid contents, such as quercetin, kaempferol, and luteolin (98). A phase II clinical trial evaluated the effects of pomegranate juice consumption in men with a rising prostate-specific antigen level after surgery or radiotherapy for prostate cancer. The mean prostate-specific antigen doubling time significantly increased after treatment with pomegranate juice from a mean of 15 months at baseline to 54 months post-treatment. Further, a decrease in cell proliferation and an increase in apoptosis were observed in pomegranate-consuming patients (238). Selenium supplementation has also been found to reduce the incidence of prostate, colorectal, and lung cancers (82).
In addition to the reports just cited supporting the clinical efficacy of antioxidants, numerous preclinical studies have demonstrated the efficacy of antioxidants against cancer (39, 272). For instance, over-expression of Mn-SOD retarded the growth of prostate cancer cells both in vitro and in vivo (315). Over-expression of glutathione peroxidase has been associated with a decrease in pancreatic cancer growth in mice (191). Delivery of PEG-conjugated antioxidant enzymes has also shown promise in preventing tumor growth in a mouse model of melanoma (134, 233).
In summary, the use of nutraceuticals and antioxidants seems promising for reducing the risk of cancer. In addition, antioxidants have been shown to enhance the effectiveness of cancer chemotherapy by minimizing the associated side effects (10, 63). However, antioxidant-based cancer therapy has two caveats. First, the use of antioxidants can disturb the ROS-dependent normal cell function and promote tumor growth, especially when ROS are required for apoptotic cell death of precancerous and transformed cells. Second, the use of antioxidants would interfere with radiotherapy and chemotherapy, which are largely dependent on ROS that induce cytotoxicity in tumors (63, 273).
Role of ROS in cancer therapy
Chemotherapy
Sydney Farber and colleagues were the first that introduced the concept of chemotherapy for cancer treatment in 1948 (87, 88). The group found that an injection of a synthetic folic acid antagonist might be of value in the treatment of acute leukemia. Since then, a number of chemotherapeutic agents have been developed. Most of these agents work through ROS generation (330). Some have already been approved by the U.S. Food and Drug Administration (Tables 5 and 6), while others are still in clinical trials (Table 7).
Table 5.
A List of U.S. Food and Drug Administration-Approved Nontargeted Anticancer Drugs That Work Through Generation of Reactive Oxygen Species
| Drug | Year | Cancer type |
|---|---|---|
| Cell cycle specific anti cancer drugs | ||
| S-phase (Antimetabolites) | ||
| Leucovorin | 1952 | Colorectal |
| Cytarabine | 1969 | Meningeal leukemia, ALL, AML, CML |
| Methotrexate | 1988 | Osteosarcoma, Breast, ALL, GTD, HL |
| Fludarabine | 1991 | CLL |
| Gemcitabine | 1996 | Pancreatic, Ovarian, Breast, Lung |
| Cytarabine | 1999 | Meningeal leukemia, ALL, AML, CML |
| Capecitabine | 2001 | Breast, Colorectal |
| 5-Fluorouracil | 2002 | Breast, Gastric, Pancreatic, Colorectal, Basal cell carcinoma |
| Clofarabine | 2004 | ALL |
| Azacitidine | 2004 | Myelodysplastic syndrome |
| Nelarabine | 2005 | ALL |
| Decitabine | 2006 | Myelodysplastic syndrome |
| Pralatrexate | 2009 | Peripheral T-cell lymphoma |
| Pemetrexed | 2009 | Mesothelioma, Lung |
| G1/S phase (Topoisomerase II inhibitors) | ||
| Etoposide | 1994 | Ewing's sarcoma, Testicular, Lung |
| M phase | ||
| Docetaxel | 1996 | Breast, Gastric, Lung, Prostate, Head and neck |
| Paclitaxel | 2005 | Breast |
| Ixabepilone | 2007 | Breast |
| Cabazitaxel | 2010 | Prostate |
| Eribulin mesylate | 2010 | Breast |
| Vincristine | 1963 | Wilm's tumor, Rhabdomyosarcoma, NHL, ALL, HL |
| Vinblastine | 1964 | Breast, Lung, Head and neck, HL |
| G2/M phase (Antitumor antibiotic) | ||
| Bleomycin | 1973 | Lung, Testicular, Cervical, Vulva, NHL, HL, MPE |
| Cell cycle nonspecific | ||
| Alkylating agents | ||
| Chlorambucil | 1957 | HL, CLL, NHL |
| Procarbazine | 1969 | HL |
| Dacarbazine | 1975 | Metastatic melanoma, HL |
| Ifosfamide | 1988 | Testicular, Ovarian, Breast, Lung, Osteosarcoma, Lymphoma |
| Temozolomide | 2000 | Anaplastic astrocytoma, Glioblastoma multiforme |
| Oxaliplatin | 2002 | Colorectal |
| Bendamustine | 2008 | Multiple myeloma, Lung, CLL, NHL, HL |
| Anthracyclines | ||
| Daunorubicin | 1979 | AML, ALL |
| Epirubicin | 1999 | Breast |
| Doxorubicin | 1999 | Neuroblastoma, Wilm's tumor, Thyroid, Gastric, Breast |
| Ovarian, Bone, Bladder, ALL, AML, NHL, HL, | ||
| Topoisomerase I inhibitors | ||
| Irinotecan | 1998 | Colorectal |
| Topotecan | 2007 | Ovarian, Cervical, Lung |
| Platinum Analogues | ||
| Cisplatin | 1978 | Lung, Ovarian, Mesothelioma |
| Miscellaneous | ||
| Pegaspargase | 1994 | Acute lymphoblastic leukemia |
| Arsenic trioxide | 2000 | Acute promyelocytic leukemia |
| Lenalidomide | 2005 | Multiple myeloma, Myelodysplastic syndrome |
| Plerixafor | 2008 | NHL, Multiple myeloma |
ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; GTD, gestational trophoblastic disease; HL, hodgkin's lymphoma; MPE, malignant pleural effusion; NHL, non-Hodgkin's lymphoma.
Table 6.
A List of U.S. Food and Drug Administration-Approved Targeted Anticancer Drugs That Work Through Generation of Reactive Oxygen Species
| Target | Drug | Year | Cancer type |
|---|---|---|---|
| CD 20 ↓ | Rituximab | 1997 | Non-Hodgkin's lymphoma, CLL |
| Ibritumomab tiuxetan | 2002 | Non-Hodgkin's lymphoma | |
| Tositumomab and I 131 | 2003 | Non-Hodgkin's lymphoma | |
| Ofatumumab | 2009 | CLL | |
| CD 33 ↓ | Gemtuzumab ozagamicin | 2000 | Acute myelogenous leukemia |
| CD 52 ↓ | Alemtuzumab | 2001 | CLL |
| CD 117 ↓ | Imatinib | 2001 | Gastrointestinal, CML |
| Interleukin-2 ↓ | Aldesleukin | 1998 | Melanoma, Renal |
| Denileukin diftitox | 1999 | Cutaneous T-cell lymphoma | |
| EGFR ↓ | Gefitinib | 2003 | Lung |
| Cetuximab | 2004 | Colorectal, Head and neck | |
| Erlotinib | 2004 | Prostate, Lung | |
| Panitumumab | 2006 | Colorectal | |
| HER2 /neu ↓ | Trastuzumab | 2010 | Breast, Gastric |
| VEGFR ↓ | Bevacizumab | 2004 | Colorectal, Renal, Lung, Glioblastoma |
| Pazopanib | 2009 | Renal | |
| HER2 and EGFR ↓ | Lapatinib ditosylate | 2007 | Breast |
| EGFR and VEGFR ↓ | Vandetanib | 2011 | Thyroid |
| PDGFR, VEGFR and | Sorafenib tosylate | 2005 | Renal, Liver |
| CD 117 ↓ | Sunitinib malate | 2006 | Renal, Gastrointestinal |
| PDGFR, BCR-ABL and CD 117 ↓ | Nilotinib | 2007 | CML |
| PDGFR, BCR-ABL, Src and CD 117 ↓ | Dasatinib | 2006 | CML, ALL |
| RANKL ↓ | Denosumab | 2010 | MM, Bone |
| HDAC ↓ | Vorinostat | 2006 | Cutaneous T-cell lymphoma |
| Romidepsin | 2009 | Cutaneous T-cell lymphoma | |
| mTOR ↓ | Temsirolimus | 2007 | Renal |
| Everolimus | 2009 | Renal, Astrocytoma | |
| Proteasome ↓ | Bortezomib | 2003 | Mantle cell lymphoma, MM |
| CTLA 4 ↓ | Ipilimumab | 2011 | Melanoma |
| CXCR4 ↓ | Plerixafor acetate | 2008 | Non-Hodgkin's lymphoma, MM |
| GnRH ↑ | Leuprolide acetate | 2000 | Prostate |
| GnRH ↓ | Abarelix | 2003 | Prostate |
| Degarelix | 2009 | Prostate | |
| Aromatase ↓ | Anastrozole | 1996 | Breast |
| Exemestane | 1999 | Breast | |
| Letrozole | 2001 | Breast | |
| Estrogen receptor ↓ | Tamoxifen citrate | 1977 | Breast |
| SERM | Toremifene | 1997 | Breast |
| Raloxifene | 2007 | Breast | |
| SERD | Fulvestrant | 2002 | Breast |
| Retinoid X receptor ↑ | Bexarotene | 2000 | Cutaneous T-cell lymphoma |
BCR-ABL, breakpoint cluster region gene on chromosome22 and Abelson murine leukemia viral oncogene homologue; CTLA 4, cytotoxic T-lymphocyte-associated antigen 4; ER, estrogen receptor; GnRH, gonadotrophin releasing hormone; HDAC, histone deacetylase; HER2, human epidermal receptor 2; MM, multiple myeloma; PDGFR, platelet derived growth factor receptor; RANKL, receptor activated NF-κB ligand; SERD, selective estrogen receptor down regulator; SERM, selective estrogen receptor modulator; Src, sarcoma; TLR, Toll like receptor.
Table 7.
A List of Reactive Oxygen Species-Modulating Anti-cancer Agents in Clinical Trial
| Agent | Mechanism | Cancer type | Clinical use | Reference |
|---|---|---|---|---|
| ROS elevators | ||||
| Motexafin gadolinium | ROS ↑, TrxR ↓ | Lymphocytic leukemia, lung, brain | Phase III trial, exhibited activity | (189, 207, 208) |
| Elesclomol (STA-4783) | ROS ↑ | Melanoma | Phase II trial, enhanced paclitaxel activity | (163, 304) |
| ATN-224 | SOD ↓ | Prostate | Phase II trial, exhibited activity | (187) |
| 2-ME | SOD ↓, superoxide ↑ | Prostate, breast | Phase II trial, well tolerated, exhibited activity | (128, 294) |
| BSO | GSH ↓, glutamyl synthetase ↓ | Random | Phase I trial, safe with melphalan | (23) |
| Imexon | GSH ↓, ROS ↑, apoptosis ↑, mitochondria function ↓ | Non-Hodgkin's lymphoma, melanoma | Phase I trial, well tolerated with dacarbazine | (79, 325) |
| ROS scavengers | ||||
| Minodronate | NOX ↓ | Multiple myeloma | Phase I trial | (279) |
| Histamine | NOX ↓ | Melanoma | Phase III trial, prolongs survival | (2) |
↓, down-regulation; ↑, up-regulation.
2-ME, 2-methoxyestradiol; ATN-224, choline tetrathiomolybdate; BSO, buthionine sulfoximine; GSH, glutathione reduced; NOX, NADPH oxidase; ROS, reactive oxygen species; SOD, superoxide dismutase; TrxR, thioredoxin reductase.
The cancer drugs approved by the U.S. Food and Drug Administration may be basically classified into two categories: nontargeted and targeted (265). The nontargeted drugs may be cell-cycle specific or cell-cycle nonspecific. The cell-cycle specific drugs act at specific phases during the cell-cycle progression, whereas the nonspecific drugs may act at any point (Table 5). Targeted cancer drugs block the growth and spread of cancer by interfering with signaling molecules, growth factors, and receptors associated with tumor growth and progression. Some of these targeted drugs are monoclonal antibodies such as rituximab, ibritumomab tiuxetan, ofatumumab, and alemtuzumab (Table 6). Procarbazine was one of the first drugs developed based on its ROS-generating properties (258). Procarbazine undergoes oxidation in aqueous solution and results in H2O2 production that is believed to be essential for the cytotoxic effects of the drug (30, 31). The drug is now approved for the treatment of Hodgkin's lymphoma, non-Hodgkin's lymphoma, and primary brain tumors (40, 112, 213, 214). As2O3 is another approved anti-cancer agent that has shown potential against acute promyelocytic leukemia. As2O3 has the ability to induce superoxide production in cancer cells (147, 234, 242, 277). As2O3 has also been shown to irreversibly inhibit mammalian thioredoxin reductase (TrxR) and impair mitochondrial functions (199).
Some of the anti-cancer agents that work through ROS generation and in development process are listed in Table 7. Motexafin gadolinium is an anticancer drug that selectively localizes in tumors. The molecular mechanism for ROS production by this drug appears to be inhibition of TrxR (207, 208). The drug exhibited modest anti-tumor activity in patients with chronic lymphocytic leukemia in a phase II trial (189). The efficacy of motexafin was demonstrated in another phase III trial with NSCLC patients who had brain metastases (217). Some compounds have exhibited anticancer activity in clinical trials through ROS generation, but their mechanism of ROS production is unknown. Elesclomol (STA-4783) is one such compound that has shown therapeutic activity against malignant melanoma; it was shown to prolong the progression-free survival of patients in a phase II clinical trial (163, 304). The progression-free survival induced by elesclomol in melanoma patients was further increased when given in combination with paclitaxel (304).
Anticancer agents have also been shown to enhance ROS stress in cancer cells by inhibiting the antioxidant defense system. SOD has emerged as one of the important targets under this category. For example, in a phase II clinical trial, a low dose of a SOD inhibitor (ATN-224) exhibited activity in patients with biochemically recurrent prostate cancer (187). 2-Methoxyestradiol is another known inhibitor of SOD that has the potential to increase superoxide radical levels (128). In a phase I clinical trial of patients with metastatic breast cancer, 2-methoxyestradiol, alone and in combination with docetaxel, was well tolerated (143). Another phase II randomized clinical trial evaluated the safety and efficacy of this drug for patients with prostate cancer; it was well tolerated and exhibited some anticancer activity (294).
Some of the anticancer agents target the GSH system. Examples include PEITC (301) and buthionine sulfoximine (BSO) (258). Although to our knowledge no clinical data on the efficacy and safety of PEITC with cancer patients are available, this drug has been shown to deplete cellular levels of GSH and to inhibit the activity of glutathione peroxidase (301). BSO is a specific inhibitor of glutamylsynthetase and, thus, can inhibit GSH synthesis. In a phase I study, administration of BSO and melphalan was found to be safe and significantly reduced the GSH content in cancer patients (23). Maeda et al. recently reported that the combination of BSO and As2O3 resulted in the effective treatment of advanced solid tumors (204). Imexon also has GSH-depleting, ROS-accumulating, and apoptosis-inducing potential, as revealed from a phase I study of patients with non-Hodgkin's lymphoma (79) and melanoma (325).
Two anticancer agents that are based on the modulation of NOX activity are minodronate and histamine (Table 7). Minodronate was found to be safe in a 60-year-old man with multiple myeloma in a phase I trial (279). In a phase III clinical trial, histamine was used as an adjunct to IL-2 therapy in melanoma patients. This agent was safe, well tolerated, and associated with a statistically significant prolongation of survival compared with IL-2 alone (2).
Radiotherapy
Similar to chemotherapy, radiotherapy employs ROS to eradicate cancer cells (22, 246). Radiotherapy uses X-rays, γ-rays, and, to a lesser extent, heavy particle radiation, such as with protons and neutrons. Radiation kills cancer cells by inducing apoptosis and mitotic failure and by inhibiting their proliferation (25, 175).
The role of ROS in mediating radiation-induced cancer cell killing is evident from a number of preclinical and clinical studies. For example, in a recent study, HIF-2α inhibition was found to enhance the response of lung cancer cells to radiation treatment that was associated with an accumulation of ROS and increased p53 activity (32). In another study, radiation induced death in prostate and breast cancer cells (74). Some other cancer types for which ROS have been shown to play a role in radiation-induced cancer cell death are lung adenocarcinoma (171), nonsmall-cell-lung cancer (290), prostate cancer (166), and breast cancer (7, 173). The role of ROS in mediating the anti-tumorigenic response of radiotherapy is evident from animal studies as well (278).
Clinical studies also support the role of ROS in mediating radiation-induced cancer cell death. For example, an elevated level of cellular damage induced by radiation was associated with increased ROS stress in patients with head and neck squamous cell carcinoma (109). ROS have been shown to play a role in the radiation-induced death of cells from cervical cancer patients as well (34). Other clinical studies for which ROS have been shown to play a role in radiation-induced therapy include patients with prostate cancer (148), NSCLC (104, 107, 144, 290), rectal cancer (81), and breast cancer (309).
Role of ROS in Eliminating Chemoresistance and Radioresistance
One of the major hurdles in treating cancer is that tumor cells, although initially sensitive, gradually develop resistance to chemotherapy and radiotherapy, in part owing to the induction of multidrug resistance proteins. Extensive research over the past several years has indicated that ROS-generating anticancer agents can reduce the chemoresistance and radioresistance of cancer cells. In this regard, nutraceuticals have shown promise in sensitizing tumor cells to chemotherapeutic and radiotherapeutic agents.
Curcumin has been shown to eliminate chemoresistant cells by sensitizing them to chemotherapy, in part by inhibiting pathways that lead to treatment resistance (94, 99). For example, curcumin treatment in conjunction with 5-fluorouracil (5-FU) or with both 5-FU and oxaliplatin resulted in significantly greater growth inhibition and more apoptosis in HCT116 and HT29 colon cancer cells than that caused by curcumin alone or 5-FU alone (240). In another study, curcumin given with tamoxifen resulted in synergistically induced apoptosis and autophagy in chemoresistant melanoma cells that correlated with an increase in ROS generation (48). An interesting finding from that study was that noncancerous cells were unaffected by the combination treatment (48). A number of other in vitro and in vivo studies have provided evidence for curcumin's use singly or as an adjunct to current chemotherapeutic drugs (99).
Other neutraceuticals have demonstrated usefulness in reducing tumor cell resistance to chemotherapy or radiotherapy. Emodin, an active component of Chinese medicinal herbs, was shown to enhance the sensitivity of gallbladder cancer cells to cisplatin in an ROS-dependent manner (320). Resveratrol is another nutraceutical that has shown potential in overcoming the chemoresistance of tumor cells (110). Our laboratory has identified a number of nutraceuticals over the past 5 years that can sensitize cancer cells to TNF-related apoptosis inducing ligand through an ROS-dependent mechanism. Some of these agents are nimbolide (111), ursolic acid (247), gossypol (293), γ-tocotrienol (151), and celastrol (292).
In addition to its role as a potent chemosensitizer, curcumin shows promise as a radiosensitizer in a wide variety of cancer cells (99). A sesquiterpene lactone was shown to sensitize prostate cancer cells to radiation by increasing ROS stress (166). 2-Methoxyestradiol has been shown to sensitize radioresistant breast cancer cells to γ radiation by generating ROS (264). The rare sugar D-allose was recently shown to enhance the efficacy of radiation against human head and neck cancer cells through ROS generation (124), and the natural compound Withaferin A sensitized renal cancer cells to radiation, also through ROS generation (340). As2O3 enhances the radiation response of cervical cancer cells (60, 150). Some other common cancers for which ROS has been shown to play a role in eliminating radioresistance are colon cancer (346), nasopharyngeal cancer (11), lung cancer (179), hepatoma (179), and leukemia (179).
Summary, Conclusion, and Future Perspectives
ROS are integral components of cell signaling pathways and have been shown to regulate cell transformation, survival, proliferation, invasion, angiogenesis, and metastasis. Paradoxically, ROS can also suppress tumor progression, and most chemotherapeutic and radiotherapeutic agents work by augmenting ROS stress in cancer cells. Due to the dual role of ROS, both pro-oxidant- and antioxidant-based anticancer agents have been developed. However, modulation of ROS signaling alone seems not to be an ideal approach, because some cancers are highly adapted to ROS stress, the redundant pathways supporting cancer growth are complex, and some ROS-generating anticancer drugs are associated with toxic side effects. Combinations of ROS-generating agents with agents that can break the redox adaptation could be a better strategy for enhancing cancer cell cytotoxicity. Due to their ROS-generating and multi-targeting properties, nutraceuticals might offer an advantage in selectively killing cancer cells. However, only a limited number of nutraceuticals have shown clinical efficacy, and none has been approved for human use. Future attempts in this direction will hopefully lead to the development of novel drugs.
Abbreviations Used
- 2-ME
2-methoxyestradiol
- 5-FU
5-fluorouracil
- ABITC
abietyl isothiocyanate
- AGL
andrographolide
- AKT
AKT8 virus oncogene cellular homolog
- ALL
acute lymphoblastic leukemia
- AML
acute myelogenous leukemia
- AP-1
activator protein-1
- As2O3
arsenic trioxide
- ATG5
autophagy protein 5
- ATM
ataxia telangiectasia mutated
- ATN-224
choline tetrathiomolybdate
- BAEC
bovine aortic endothelial cell
- Bak
Bcl-2 homologous antagonist/killer
- Bax
Bcl-2-associated X protein
- Bcl-2
B-cell lymphoma-2
- Bcl-xL
B-cell lymphoma-extra large
- BCR-ABL
breakpoint cluster region gene on chromosome22 and Abelson murine leukemia viral oncogene homologue
- BITC
benzyl isothiocyanate
- BSO
buthionine sulfoximine
- CAT
catalase
- Cav-1
caveolin-1
- Cdc25c
cell division cycle 25 homolog c (S. pombe)
- CDK
cyclin-dependent kinase
- Chk
checkpoint kinase
- CHL
chlorogenic acid
- c-Jun
cellular Ju-nanna
- CLL
chronic lymphocytic leukemia
- c-Met
hepatocyte growth factor receptor
- CML
chronic myelogenous leukemia
- c-Myc
cellular v-myc myelocytomatosis viral oncogene homolog (avian)
- COX-2
cyclooxygenase-2
- CTLA 4
cytotoxic T-lymphocyte-associated antigen 4
- CXCL14
CXC chemokine ligand 14
- CXCR4
CXC chemokine receptor 4
- DEN
diethylnitrosamine
- DMAPT
dimethylaminoparthenolide
- DR5
death receptor 5
- DUOX
dual oxidase
- EC
endothelial cell
- EGCG
epigallocatechin gallate
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ER
estrogen receptor
- ERK
extracellular signal-regulated kinase
- ERK1/2
extracellular signal-regulated kinase 1/2
- ERO1
endoplasmic reticulum oxidoreductin 1
- ESB
erythrina suberosa stem bark
- Ets-1
v-ets erythroblastosis virus E26 oncogene homolog 1
- GA
18 β-glycyrrhetinic acid
- GnRH
gonadotrophin releasing hormone
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GRX-(S)2
glutaredoxin oxidized
- GRX-(SH)2
glutaredoxin reduced
- GSH
glutathione
- GSSG
glutathione oxidized
- GT094
ethyl 2-((2,3-bis(nitrooxy)propyl)disulfanyl)benzoate
- GTD
gestational trophoblastic disease
- HDAC
histone deacetylase
- HER2
human epidermal receptor 2
- HIF
hypoxia-inducible factor
- HL
hodgkin's lymphoma
- HNSCC
head and neck squamous cell carcinoma
- HO-1
heme oxygenase-1
- H2O2
hydrogen peroxide
- IGF-I
insulin-like growth factor-1
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- ITGB3
integrin beta 3
- JAK
janus kinase
- JNK
c-jun N-terminal kinase
- LPA
lysophosphatidic acid
- LTB4
leukotriene B4
- MAPK
mitogen-activated protein kinase
- MEK
MAPK/ERK kinase
- MiMP
mitochondrial membrane potential
- MM
multiple myeloma
- MMA
monomethylarsonous acid
- MMP
matrix metalloproteinase
- MPE
malignant pleural effusion
- mTOR
mammalian target of rapamycin
- NAC
N-acetyl-L-cysteine
- NADPH
nicotinamide adenine dinucleotide phosphate reduced
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NHL
non-Hodgkin's lymphoma
- NO
nitric oxide
- NOX
NADPH oxidase
- Nrf2
nuclear factor (erythroid-derived 2)-like factor 2
- NSCLC
nonsmall cell lung cancer
- ONOO−
peroxynitrite
- PAK1
p21 activated kinase 1
- PCBs
polychlorinated biphenyls
- PDGFR
platelet derived growth factor receptor
- PDI
protein disulfide isomerase
- PDT
photodynamic therapy
- PEITC
β-phenylethylisothiocyanate
- PGE-2
prostaglandin E2
- PI3K
phosphatidylinositol 3-kinase
- PKB
protein kinase B
- PKC-δ
protein kinase C-delta
- PL
piperlongumine
- PMS
phenazine methosulfate
- PPARγ
peroxisome proliferator-activated receptor gamma
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- RANKL
receptor activated NF-κB ligand
- Rb
retinoblastoma
- RNS
reactive nitrogen species
- ROCK
rho-associated kinase
- ROS
reactive oxygen species
- SAPK
stress-activated protein kinase
- SERD
selective estrogen receptor down regulator
- SERM
selective estrogen receptor modulator
- SOD
superoxide dismutase
- Sp1
specificity protein 1
- Src
sarcoma
- STAT3
signal transducer and activator of transcription 3
- STMN1
stathmin 1
- TGF-β1
transforming growth factor beta 1
- TLR
Toll like receptor
- TNF-α
tumor necrosis factor-α
- TPA
12-O-tetradecanoylphorbol-13-acetate
- TRAF2
TNF receptor-associated factor 2
- TRX-(S)2
thioredoxin oxidized
- TRX-(SH)2
thioredoxin reduced
- TrxR
thioredoxin reductase
- TXNL-2
thioredoxin-like 2
- UDCA
ursodeoxycholic acid
- u-PA
urokinase-plasminogen activator
- u-PAR
urokinase-plasminogen activator surface receptor
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- XIAP
X-linked inhibitor of apoptosis protein
- XO
xanthine oxidase
Acknowledgments
The authors thank Elizabeth L. Hess of The University of Texas MD Anderson Cancer Center's Department of Scientific Publications for carefully editing the article and providing valuable comments. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a core grant from the National Institutes of Health (CA16672), a program project grant from the National Institutes of Health (NIH CA124787-01A2), and a grant from the Center for Targeted Therapy at the MD Anderson Cancer Center.
References
- 1.Adhikary A. Mohanty S. Lahiry L. Hossain DM. Chakraborty S. Das T. Theaflavins retard human breast cancer cell migration by inhibiting NF-kappaB via p53-ROS cross-talk. FEBS Lett. 2010;584:7–14. doi: 10.1016/j.febslet.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 2.Agarwala SS. Glaspy J. O'Day SJ. Mitchell M. Gutheil J. Whitman E. Gonzalez R. Hersh E. Feun L. Belt R. Meyskens F. Hellstrand K. Wood D. Kirkwood JM. Gehlsen KR. Naredi P. Results from a randomized phase III study comparing combined treatment with histamine dihydrochloride plus interleukin-2 versus interleukin-2 alone in patients with metastatic melanoma. J Clin Oncol. 2002;20:125–133. doi: 10.1200/JCO.2002.20.1.125. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal BB. Kunnumakkara AB. Harikumar KB. Gupta SR. Tharakan ST. Koca C. Dey S. Sung B. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aggarwal BB. Sung B. The relationship between inflammation and cancer is analogous to that between fuel and fire. Oncology (Williston Park) 2011;25:414–418. [PubMed] [Google Scholar]
- 5.Aggarwal BB. Vijayalekshmi RV. Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal SK. Agrawal M. Sharma PR. Gupta BD. Arora S. Saxena AK. Induction of apoptosis in human promyelocytic leukemia Hl60 cells by an extract From Erythrina suberosa stem bark. Nutr Cancer. 2011;63:802–813. doi: 10.1080/01635581.2011.573900. [DOI] [PubMed] [Google Scholar]
- 7.Ahn J. Ambrosone CB. Kanetsky PA. Tian C. Lehman TA. Kropp S. Helmbold I. von Fournier D. Haase W. Sautter-Bihl ML. Wenz F. Chang-Claude J. Polymorphisms in genes related to oxidative stress (CAT, MnSOD, MPO, and eNOS) and acute toxicities from radiation therapy following lumpectomy for breast cancer. Clin Cancer Res. 2006;12:7063–7070. doi: 10.1158/1078-0432.CCR-06-0039. [DOI] [PubMed] [Google Scholar]
- 8.Ahsan H. Parveen N. Khan NU. Hadi SM. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chem Biol Interact. 1999;121:161–175. doi: 10.1016/s0009-2797(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 9.Aimes RT. Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995;270:5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- 10.Akbas HS. Timur M. Ozben T. Concurrent use of antioxidants in cancer therapy: an update. Expert Rev Clin Immunol. 2006;2:931–939. doi: 10.1586/1744666X.2.6.931. [DOI] [PubMed] [Google Scholar]
- 11.Alajez NM. Shi W. Hui AB. Yue S. Ng R. Lo KW. Bastianutto C. O'Sullivan B. Gullane P. Liu FF. Targeted depletion of BMI1 sensitizes tumor cells to P53-mediated apoptosis in response to radiation therapy. Cell Death Differ. 2009;16:1469–1479. doi: 10.1038/cdd.2009.85. [DOI] [PubMed] [Google Scholar]
- 12.Albig AR. Neil JR. Schiemann WP. Fibulins 3 and 5 antagonize tumor angiogenesis in vivo. Cancer Res. 2006;66:2621–2629. doi: 10.1158/0008-5472.CAN-04-4096. [DOI] [PubMed] [Google Scholar]
- 13.Albig AR. Schiemann WP. Fibulin-5 antagonizes vascular endothelial growth factor (VEGF) signaling and angiogenic sprouting by endothelial cells. DNA Cell Biol. 2004;23:367–379. doi: 10.1089/104454904323145254. [DOI] [PubMed] [Google Scholar]
- 14.Alexander A. Cai SL. Kim J. Nanez A. Sahin M. MacLean KH. Inoki K. Guan KL. Shen J. Person MD. Kusewitt D. Mills GB. Kastan MB. Walker CL. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci U S A. 2010;107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ames BN. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983;221:1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- 16.Anand P. Kunnumakkara AB. Sundaram C. Harikumar KB. Tharakan ST. Lai OS. Sung B. Aggarwal BB. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angelo LS. Talpaz M. Kurzrock R. Autocrine interleukin-6 production in renal cell carcinoma: evidence for the involvement of p53. Cancer Res. 2002;62:932–940. [PubMed] [Google Scholar]
- 18.Arbiser JL. Petros J. Klafter R. Govindajaran B. McLaughlin ER. Brown LF. Cohen C. Moses M. Kilroy S. Arnold RS. Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci U S A. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atsumi T. Fujisawa S. Tonosaki K. Relationship between intracellular ROS production and membrane mobility in curcumin- and tetrahydrocurcumin-treated human gingival fibroblasts and human submandibular gland carcinoma cells. Oral Dis. 2005;11:236–242. doi: 10.1111/j.1601-0825.2005.01067.x. [DOI] [PubMed] [Google Scholar]
- 20.Azad MB. Chen Y. Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2009;11:777–790. doi: 10.1089/ars.2008.2270. [DOI] [PubMed] [Google Scholar]
- 21.Azad N. Iyer AK. Wang L. Lu Y. Medan D. Castranova V. Rojanasakul Y. Nitric oxide-mediated bcl-2 stabilization potentiates malignant transformation of human lung epithelial cells. Am J Respir Cell Mol Biol. 2010;42:578–585. doi: 10.1165/rcmb.2009-0094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azzam EI. de Toledo SM. Little JB. Stress signaling from irradiated to non-irradiated cells. Curr Cancer Drug Targets. 2004;4:53–64. doi: 10.2174/1568009043481641. [DOI] [PubMed] [Google Scholar]
- 23.Bailey HH. Ripple G. Tutsch KD. Arzoomanian RZ. Alberti D. Feierabend C. Mahvi D. Schink J. Pomplun M. Mulcahy RT. Wilding G. Phase I study of continuous-infusion L-S,R-buthionine sulfoximine with intravenous melphalan. J Natl Cancer Inst. 1997;89:1789–1796. doi: 10.1093/jnci/89.23.1789. [DOI] [PubMed] [Google Scholar]
- 24.Balkwill F. Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 25.Bauer G. Low dose radiation and intercellular induction of apoptosis: potential implications for the control of oncogenesis. Int J Radiat Biol. 2007;83:873–888. doi: 10.1080/09553000701727523. [DOI] [PubMed] [Google Scholar]
- 26.Bedard K. Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 27.Behrend L. Henderson G. Zwacka RM. Reactive oxygen species in oncogenic transformation. Biochem Soc Trans. 2003;31:1441–1444. doi: 10.1042/bst0311441. [DOI] [PubMed] [Google Scholar]
- 28.Bejarano I. Espino J. Marchena AM. Barriga C. Paredes SD. Rodriguez AB. Pariente JA. Melatonin enhances hydrogen peroxide-induced apoptosis in human promyelocytic leukaemia HL-60 cells. Mol Cell Biochem. 2011;353:167–176. doi: 10.1007/s11010-011-0783-8. [DOI] [PubMed] [Google Scholar]
- 29.Benhar M. Engelberg D. Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–425. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berneis K. Bollag W. Kofler M. Luthy H. The enhancement of the after effect of ionizing radiation by a cytotoxic methylhydrazine derivative. Eur J Cancer. 1966;2:43–49. doi: 10.1016/0014-2964(66)90088-0. [DOI] [PubMed] [Google Scholar]
- 31.Berneis K. Kofler M. Bollag W. Kaiser A. Langemann A. The degradation of deoxyribonucleic acid by new tumour inhibiting compounds: the intermediate formation of hydrogen peroxide. Experientia. 1963;19:132–133. doi: 10.1007/BF02171591. [DOI] [PubMed] [Google Scholar]
- 32.Bertout JA. Majmundar AJ. Gordan JD. Lam JC. Ditsworth D. Keith B. Brown EJ. Nathanson KL. Simon MC. HIF2alpha inhibition promotes p53 pathway activity, tumor cell death, and radiation responses. Proc Natl Acad Sci U S A. 2009;106:14391–14396. doi: 10.1073/pnas.0907357106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhaumik S. Anjum R. Rangaraj N. Pardhasaradhi BV. Khar A. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999;456:311–314. doi: 10.1016/s0014-5793(99)00969-2. [DOI] [PubMed] [Google Scholar]
- 34.Bhosle SM. Pandey BN. Huilgol NG. Mishra KP. Membrane oxidative damage and apoptosis in cervical carcinoma cells of patients after radiation therapy. Methods Cell Sci. 2002;24:65–68. [PubMed] [Google Scholar]
- 35.Binker MG. Binker-Cosen AA. Gaisano HY. de Cosen RH. Cosen-Binker LI. TGF-beta1 increases invasiveness of SW1990 cells through Rac1/ROS/NF-kappaB/IL-6/MMP-2. Biochem Biophys Res Commun. 2011;405:140–145. doi: 10.1016/j.bbrc.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 36.Binker MG. Binker-Cosen AA. Richards D. Oliver B. Cosen-Binker LI. EGF promotes invasion by PANC-1 cells through Rac1/ROS-dependent secretion and activation of MMP-2. Biochem Biophys Res Commun. 2009;379:445–450. doi: 10.1016/j.bbrc.2008.12.080. [DOI] [PubMed] [Google Scholar]
- 37.Bonekamp NA. Volkl A. Fahimi HD. Schrader M. Reactive oxygen species and peroxisomes: struggling for balance. Biofactors. 2009;35:346–355. doi: 10.1002/biof.48. [DOI] [PubMed] [Google Scholar]
- 38.Boonstra J. Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337:1–13. doi: 10.1016/j.gene.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 39.Borek C. Dietary antioxidants and human cancer. Integr Cancer Ther. 2004;3:333–341. doi: 10.1177/1534735404270578. [DOI] [PubMed] [Google Scholar]
- 40.Bouffet E. Jouvet A. Thiesse P. Sindou M. Chemotherapy for aggressive or anaplastic high grade oligodendrogliomas and oligoastrocytomas: better than a salvage treatment. Br J Neurosurg. 1998;12:217–222. doi: 10.1080/02688699845023. [DOI] [PubMed] [Google Scholar]
- 41.Brar SS. Corbin Z. Kennedy TP. Hemendinger R. Thornton L. Bommarius B. Arnold RS. Whorton AR. Sturrock AB. Huecksteadt TP. Quinn MT. Krenitsky K. Ardie KG. Lambeth JD. Hoidal JR. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003;285:C353–C369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 42.Brar SS. Kennedy TP. Sturrock AB. Huecksteadt TP. Quinn MT. Whorton AR. Hoidal JR. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am J Physiol Cell Physiol. 2002;282:C1212–C1224. doi: 10.1152/ajpcell.00496.2001. [DOI] [PubMed] [Google Scholar]
- 43.Brar SS. Kennedy TP. Whorton AR. Sturrock AB. Huecksteadt TP. Ghio AJ. Hoidal JR. Reactive oxygen species from NAD(P)H:quinone oxidoreductase constitutively activate NF-kappaB in malignant melanoma cells. Am J Physiol Cell Physiol. 2001;280:C659–C676. doi: 10.1152/ajpcell.2001.280.3.C659. [DOI] [PubMed] [Google Scholar]
- 44.Burr M. Appleby P. Key T. Thorogood M. Plasma ascorbic acid and risk of heart disease and cancer. Lancet. 2001;357:2135–2136. doi: 10.1016/S0140-6736(00)05207-7. [DOI] [PubMed] [Google Scholar]
- 45.Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227:375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 46.Chan DW. Liu VW. Tsao GS. Yao KM. Furukawa T. Chan KK. Ngan HY. Loss of MKP3 mediated by oxidative stress enhances tumorigenicity and chemoresistance of ovarian cancer cells. Carcinogenesis. 2008;29:1742–1750. doi: 10.1093/carcin/bgn167. [DOI] [PubMed] [Google Scholar]
- 47.Chandel NS. Vander Heiden MG. Thompson CB. Schumacker PT. Redox regulation of p53 during hypoxia. Oncogene. 2000;19:3840–3848. doi: 10.1038/sj.onc.1203727. [DOI] [PubMed] [Google Scholar]
- 48.Chatterjee SJ. Pandey S. Chemo-resistant melanoma sensitized by tamoxifen to low dose curcumin treatment through induction of apoptosis and autophagy. Cancer Biol Ther. 2011;11:216–228. doi: 10.4161/cbt.11.2.13798. [DOI] [PubMed] [Google Scholar]
- 49.Chen D. Cao J. Tian L. Liu F. Sheng X. Induction of apoptosis by casticin in cervical cancer cells through reactive oxygen species-mediated mitochondrial signaling pathways. Oncol Rep. 2011;26:1287–1294. doi: 10.3892/or.2011.1367. [DOI] [PubMed] [Google Scholar]
- 50.Chen J. Wanming D. Zhang D. Liu Q. Kang J. Water-soluble antioxidants improve the antioxidant and anticancer activity of low concentrations of curcumin in human leukemia cells. Pharmazie. 2005;60:57–61. [PubMed] [Google Scholar]
- 51.Chen L. Stacewicz-Sapuntzakis M. Duncan C. Sharifi R. Ghosh L. van Breemen R. Ashton D. Bowen PE. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entrees as a whole-food intervention. J Natl Cancer Inst. 2001;93:1872–1879. doi: 10.1093/jnci/93.24.1872. [DOI] [PubMed] [Google Scholar]
- 52.Chen XJ. Duan FD. Zhang HH. Xiong Y. Wang J. Sodium selenite-induced apoptosis mediated by ROS attack in human osteosarcoma U2OS cells. Biol Trace Elem Res. 2011 doi: 10.1007/s12011-011-9154-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Chen Y. McMillan-Ward E. Kong J. Israels SJ. Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15:171–182. doi: 10.1038/sj.cdd.4402233. [DOI] [PubMed] [Google Scholar]
- 54.Chen YF. Yang JS. Huang WW. Tsai HY. Novel anti-leukemia activities of pipoxolan operate via the mitochondria-related pathway in human leukemia U937 cells and attenuate U937 cell growth in an animal model. Mol Med Report. 2010;3:851–856. doi: 10.3892/mmr.2010.330. [DOI] [PubMed] [Google Scholar]
- 55.Cheng AC. Tsai ML. Liu CM. Lee MF. Nagabhushanam K. Ho CT. Pan MH. Garcinol inhibits cell growth in hepatocellular carcinoma Hep3B cells through induction of ROS-dependent apoptosis. Food Funct. 2010;1:301–307. doi: 10.1039/c0fo00134a. [DOI] [PubMed] [Google Scholar]
- 56.Cheng AL. Hsu CH. Lin JK. Hsu MM. Ho YF. Shen TS. Ko JY. Lin JT. Lin BR. Ming-Shiang W. Yu HS. Jee SH. Chen GS. Chen TM. Chen CA. Lai MK. Pu YS. Pan MH. Wang YJ. Tsai CC. Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 57.Chetram MA. Don-Salu-Hewage AS. Hinton CV. ROS enhances CXCR4-mediated functions through inactivation of PTEN in prostate cancer cells. Biochem Biophys Res Commun. 2011;410:195–200. doi: 10.1016/j.bbrc.2011.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiang JH. Yang JS. Ma CY. Yang MD. Huang HY. Hsia TC. Kuo HM. Wu PP. Lee TH. Chung JG. Danthron, an anthraquinone derivative, induces DNA damage and caspase cascades-mediated apoptosis in SNU-1 human gastric cancer cells through mitochondrial permeability transition pores and Bax-triggered pathways. Chem Res Toxicol. 2011;24:20–29. doi: 10.1021/tx100248s. [DOI] [PubMed] [Google Scholar]
- 59.Choudhary S. Wang KK. Wang HC. Oncogenic H-Ras, FK228, and exogenous H2O2 cooperatively activated the ERK pathway in selective induction of human urinary bladder cancer J82 cell death. Mol Carcinog. 2011;50:215–219. doi: 10.1002/mc.20708. [DOI] [PubMed] [Google Scholar]
- 60.Chun YJ. Park IC. Park MJ. Woo SH. Hong SI. Chung HY. Kim TH. Lee YS. Rhee CH. Lee SJ. Enhancement of radiation response in human cervical cancer cells in vitro and in vivo by arsenic trioxide (As2O3) FEBS Lett. 2002;519:195–200. doi: 10.1016/s0014-5793(02)02765-5. [DOI] [PubMed] [Google Scholar]
- 61.Chung YM. Kim JS. Yoo YD. A novel protein, Romo1, induces ROS production in the mitochondria. Biochem Biophys Res Commun. 2006;347:649–655. doi: 10.1016/j.bbrc.2006.06.140. [DOI] [PubMed] [Google Scholar]
- 62.Colotta F. Allavena P. Sica A. Garlanda C. Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 63.Conklin KA. Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effects. Nutr Cancer. 2000;37:1–18. doi: 10.1207/S15327914NC3701_1. [DOI] [PubMed] [Google Scholar]
- 64.Coriat R. Marut W. Leconte M. Ba LB. Vienne A. Chereau C. Alexandre J. Weill B. Doering M. Jacob C. Nicco C. Batteux F. The organotelluride catalyst LAB027 prevents colon cancer growth in the mice. Cell Death Dis. 2011;2:e191. doi: 10.1038/cddis.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341(Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- 66.Cruz-Correa M. Shoskes DA. Sanchez P. Zhao R. Hylind LM. Wexner SD. Giardiello FM. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 67.D'Autreaux B. Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 68.Dajee M. Lazarov M. Zhang JY. Cai T. Green CL. Russell AJ. Marinkovich MP. Tao S. Lin Q. Kubo Y. Khavari PA. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639–643. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- 69.Das L. Vinayak M. Anticarcinogenic action of curcumin by activation of antioxidant defence system and inhibition of NF-kappaB signaling in lymphoma bearing mice. Biosci Rep. 2012;32:161–170. doi: 10.1042/BSR20110043. [DOI] [PubMed] [Google Scholar]
- 70.De Luca A. Sanna F. Sallese M. Ruggiero C. Grossi M. Sacchetta P. Rossi C. De Laurenzi V. Di Ilio C. Favaloro B. Methionine sulfoxide reductase A down-regulation in human breast cancer cells results in a more aggressive phenotype. Proc Natl Acad Sci U S A. 2010;107:18628–18633. doi: 10.1073/pnas.1010171107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.del Rio LA. Sandalio LM. Palma JM. Bueno P. Corpas FJ. Metabolism of oxygen radicals in peroxisomes and cellular implications. Free Radic Biol Med. 1992;13:557–580. doi: 10.1016/0891-5849(92)90150-f. [DOI] [PubMed] [Google Scholar]
- 72.Denning TL. Takaishi H. Crowe SE. Boldogh I. Jevnikar A. Ernst PB. Oxidative stress induces the expression of Fas and Fas ligand and apoptosis in murine intestinal epithelial cells. Free Radic Biol Med. 2002;33:1641–1650. doi: 10.1016/s0891-5849(02)01141-3. [DOI] [PubMed] [Google Scholar]
- 73.Deshpande SS. Angkeow P. Huang J. Ozaki M. Irani K. Rac1 inhibits TNF-alpha-induced endothelial cell apoptosis: dual regulation by reactive oxygen species. FASEB J. 2000;14:1705–1714. doi: 10.1096/fj.99-0910com. [DOI] [PubMed] [Google Scholar]
- 74.Di Pietro C. Piro S. Tabbi G. Ragusa M. Di Pietro V. Zimmitti V. Cuda F. Anello M. Consoli U. Salinaro ET. Caruso M. Vancheri C. Crimi N. Sabini MG. Cirrone GA. Raffaele L. Privitera G. Pulvirenti A. Giugno R. Ferro A. Cuttone G. Lo Nigro S. Purrello R. Purrello F. Purrello M. Cellular and molecular effects of protons: apoptosis induction and potential implications for cancer therapy. Apoptosis. 2006;11:57–66. doi: 10.1007/s10495-005-3346-1. [DOI] [PubMed] [Google Scholar]
- 75.Dickinson BC. Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011;7:504–511. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Donadelli M. Costanzo C. Beghelli S. Scupoli MT. Dandrea M. Bonora A. Piacentini P. Budillon A. Caraglia M. Scarpa A. Palmieri M. Synergistic inhibition of pancreatic adenocarcinoma cell growth by trichostatin A and gemcitabine. Biochim Biophys Acta. 2007;1773:1095–1106. doi: 10.1016/j.bbamcr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 77.Donadelli M. Dando I. Zaniboni T. Costanzo C. Dalla Pozza E. Scupoli MT. Scarpa A. Zappavigna S. Marra M. Abbruzzese A. Bifulco M. Caraglia M. Palmieri M. Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis. 2011;2:e152. doi: 10.1038/cddis.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dong-Yun S. Yu-Ru D. Shan-Lin L. Ya-Dong Z. Lian W. Redox stress regulates cell proliferation and apoptosis of human hepatoma through Akt protein phosphorylation. FEBS Lett. 2003;542:60–64. doi: 10.1016/s0014-5793(03)00338-7. [DOI] [PubMed] [Google Scholar]
- 79.Dragovich T. Gordon M. Mendelson D. Wong L. Modiano M. Chow HH. Samulitis B. O'Day S. Grenier K. Hersh E. Dorr R. Phase I trial of imexon in patients with advanced malignancy. J Clin Oncol. 2007;25:1779–1784. doi: 10.1200/JCO.2006.08.9672. [DOI] [PubMed] [Google Scholar]
- 80.Du J. Sun C. Hu Z. Yang Y. Zhu Y. Zheng D. Gu L. Lu X. Lysophosphatidic acid induces MDA-MB-231 breast cancer cells migration through activation of PI3K/PAK1/ERK signaling. PLoS One. 2010;5:e15940. doi: 10.1371/journal.pone.0015940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dubois JB. Bussieres E. Richaud P. Rouanet P. Becouarn Y. Mathoulin-Pelissier S. Saint-Aubert B. Ychou M. Intra-operative radiotherapy of rectal cancer: results of the French multi-institutional randomized study. Radiother Oncol. 2011;98:298–303. doi: 10.1016/j.radonc.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 82.Duffield-Lillico AJ. Reid ME. Turnbull BW. Combs GF., Jr. Slate EH. Fischbach LA. Marshall JR. Clark LC. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- 83.Durgaprasad S. Pai CG. Vasanthkumar Alvres JF. Namitha S. A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J Med Res. 2005;122:315–318. [PubMed] [Google Scholar]
- 84.Eblin KE. Jensen TJ. Wnek SM. Buffington SE. Futscher BW. Gandolfi AJ. Reactive oxygen species regulate properties of transformation in UROtsa cells exposed to monomethylarsonous acid by modulating MAPK signaling. Toxicology. 2009;255:107–114. doi: 10.1016/j.tox.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Edderkaoui M. Hong P. Vaquero EC. Lee JK. Fischer L. Friess H. Buchler MW. Lerch MM. Pandol SJ. Gukovskaya AS. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1137–G1147. doi: 10.1152/ajpgi.00197.2005. [DOI] [PubMed] [Google Scholar]
- 86.Fan TP. Yeh JC. Leung KW. Yue PY. Wong RN. Angiogenesis: from plants to blood vessels. Trends Pharmacol Sci. 2006;27:297–309. doi: 10.1016/j.tips.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 87.Farber S. Cutler EC. Hawkins JW. Harrison JH. Peirce EC., 2nd Lenz GG. The action of pteroylglutamic conjugates on man. Science. 1947;106:619–621. doi: 10.1126/science.106.2764.619. [DOI] [PubMed] [Google Scholar]
- 88.Farber S. Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med. 1948;238:787–793. doi: 10.1056/NEJM194806032382301. [DOI] [PubMed] [Google Scholar]
- 89.Ferraro D. Corso S. Fasano E. Panieri E. Santangelo R. Borrello S. Giordano S. Pani G. Galeotti T. Pro-metastatic signaling by c-Met through RAC-1 and reactive oxygen species (ROS) Oncogene. 2006;25:3689–3698. doi: 10.1038/sj.onc.1209409. [DOI] [PubMed] [Google Scholar]
- 90.Fruehauf JP. Meyskens FL., Jr. Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 91.Fujisawa S. Atsumi T. Ishihara M. Kadoma Y. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer Res. 2004;24:563–569. [PubMed] [Google Scholar]
- 92.Gapuzan ME. Yufit PV. Gilmore TD. Immortalized embryonic mouse fibroblasts lacking the RelA subunit of transcription factor NF-kappaB have a malignantly transformed phenotype. Oncogene. 2002;21:2484–2492. doi: 10.1038/sj.onc.1205333. [DOI] [PubMed] [Google Scholar]
- 93.Garbarino JA. Cardile V. Lombardo L. Chamy MC. Piovano M. Russo A. Demalonyl thyrsiflorin A, a semisynthetic labdane-derived diterpenoid, induces apoptosis and necrosis in human epithelial cancer cells. Chem Biol Interact. 2007;169:198–206. doi: 10.1016/j.cbi.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 94.Garg AK. Buchholz TA. Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal. 2005;7:1630–1647. doi: 10.1089/ars.2005.7.1630. [DOI] [PubMed] [Google Scholar]
- 95.Geborek P. Nitelius E. Noltorp S. Petri H. Jacobsson L. Larsson L. Saxne T. Leden I. Population based studies of biological antirheumatic drug use in southern Sweden: comparison with pharmaceutical sales. Ann Rheum Dis. 2005;64:1805–1807. doi: 10.1136/ard.2005.036715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Geiszt M. Kopp JB. Varnai P. Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gibson SB. A matter of balance between life and death: targeting reactive oxygen species (ROS)-induced autophagy for cancer therapy. Autophagy. 2010;6:835–837. doi: 10.4161/auto.6.7.13335. [DOI] [PubMed] [Google Scholar]
- 98.Gil MI. Tomas-Barberan FA. Hess-Pierce B. Holcroft DM. Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 99.Goel A. Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62:919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- 100.Gogvadze V. Orrenius S. Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol. 2008;18:165–173. doi: 10.1016/j.tcb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 101.Goh J. Enns L. Fatemie S. Hopkins H. Morton J. Pettan-Brewer C. Ladiges W. Mitochondrial targeted catalase suppresses invasive breast cancer in mice. BMC Cancer. 2011;11:191. doi: 10.1186/1471-2407-11-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goncalves AP. Videira A. Soares P. Maximo V. Orthovanadate-induced cell death in RET/PTC1-harboring cancer cells involves the activation of caspases and altered signaling through PI3K/Akt/mTOR. Life Sci. 2011;89:371–377. doi: 10.1016/j.lfs.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 103.Gordon MS. Mendelson DS. Kato G. Tumor angiogenesis and novel antiangiogenic strategies. Int J Cancer. 2010;126:1777–1787. doi: 10.1002/ijc.25026. [DOI] [PubMed] [Google Scholar]
- 104.Gore EM. Bae K. Wong SJ. Sun A. Bonner JA. Schild SE. Gaspar LE. Bogart JA. Werner-Wasik M. Choy H. Phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: primary analysis of radiation therapy oncology group study RTOG 0214. J Clin Oncol. 2011;29:272–278. doi: 10.1200/JCO.2010.29.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grivennikov SI. Greten FR. Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grivennikov SI. Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guckenberger M. Wilbert J. Richter A. Baier K. Flentje M. Potential of adaptive radiotherapy to escalate the radiation dose in combined radiochemotherapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79:901–908. doi: 10.1016/j.ijrobp.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 108.Guo WJ. Ye SS. Cao N. Huang J. Gao J. Chen QY. ROS-mediated autophagy was involved in cancer cell death induced by novel copper(II) complex. Exp Toxicol Pathol. 2010;62:577–582. doi: 10.1016/j.etp.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 109.Gupta A. Bhatt ML. Misra MK. Assessment of free radical-mediated damage in head and neck squamous cell carcinoma patients and after treatment with radiotherapy. Indian J Biochem Biophys. 2010;47:96–99. [PubMed] [Google Scholar]
- 110.Gupta SC. Kannappan R. Reuter S. Kim JH. Aggarwal BB. Chemosensitization of tumors by resveratrol. Ann N Y Acad Sci. 2011;1215:150–160. doi: 10.1111/j.1749-6632.2010.05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gupta SC. Reuter S. Phromnoi K. Park B. Hema PS. Nair M. Aggarwal BB. Nimbolide sensitizes human colon cancer cells to TRAIL through reactive oxygen species- and ERK-dependent up-regulation of death receptors, p53, and Bax. J Biol Chem. 2011;286:1134–1146. doi: 10.1074/jbc.M110.191379. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 112.Gutin PH. Wilson CB. Kumar AR. Boldrey EB. Levin V. Powell M. Enot KJ. Phase II study of procarbazine, CCNU, and vincristine combination chemotherapy in the treatment of malignant brain tumors. Cancer. 1975;35:1398–1404. doi: 10.1002/1097-0142(197505)35:5<1398::aid-cncr2820350524>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 113.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 114.Hampton MB. Orrenius S. Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett. 1997;414:552–556. doi: 10.1016/s0014-5793(97)01068-5. [DOI] [PubMed] [Google Scholar]
- 115.Hanahan D. Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 116.Hanahan D. Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 117.Hanai H. Iida T. Takeuchi K. Watanabe F. Maruyama Y. Andoh A. Tsujikawa T. Fujiyama Y. Mitsuyama K. Sata M. Yamada M. Iwaoka Y. Kanke K. Hiraishi H. Hirayama K. Arai H. Yoshii S. Uchijima M. Nagata T. Koide Y. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:1502–1506. doi: 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 118.Heikkila R. Schwab G. Wickstrom E. Loke SL. Pluznik DH. Watt R. Neckers LM. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. Nature. 1987;328:445–449. doi: 10.1038/328445a0. [DOI] [PubMed] [Google Scholar]
- 119.Hileman EO. Liu J. Albitar M. Keating MJ. Huang P. Intrinsic oxidative stress in cancer cells: a biochemical basis for therapeutic selectivity. Cancer Chemother Pharmacol. 2004;53:209–219. doi: 10.1007/s00280-003-0726-5. [DOI] [PubMed] [Google Scholar]
- 120.Hippert MM. O'Toole PS. Thorburn A. Autophagy in cancer: good, bad, or both? Cancer Res. 2006;66:9349–9351. doi: 10.1158/0008-5472.CAN-06-1597. [DOI] [PubMed] [Google Scholar]
- 121.Ho BY. Wu YM. Chang KJ. Pan TM. Dimerumic acid inhibits SW620 cell invasion by attenuating H(2)O(2)-mediated MMP-7 expression via JNK/C-Jun and ERK/C-Fos activation in an AP-1-dependent manner. Int J Biol Sci. 2011;7:869–880. doi: 10.7150/ijbs.7.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Holt PR. Katz S. Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50:2191–2193. doi: 10.1007/s10620-005-3032-8. [DOI] [PubMed] [Google Scholar]
- 123.Horan TC. Zompa MA. Seto CT. Kim KK. Moore RG. Lange TS. Description of the cytotoxic effect of a novel drug Abietyl-Isothiocyanate on endometrial cancer cell lines. Invest New Drugs. 2011 doi: 10.1007/s10637-011-97282. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 124.Hoshikawa H. Indo K. Mori T. Mori N. Enhancement of the radiation effects by D-allose in head and neck cancer cells. Cancer Lett. 2011;306:60–66. doi: 10.1016/j.canlet.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 125.Hour TC. Huang CY. Lin CC. Chen J. Guan JY. Lee JM. Pu YS. Characterization of molecular events in a series of bladder urothelial carcinoma cell lines with progressive resistance to arsenic trioxide. Anticancer Drugs. 2004;15:779–785. doi: 10.1097/00001813-200409000-00007. [DOI] [PubMed] [Google Scholar]
- 126.Hsu YL. Hou MF. Tsai EM. Kuo PL. Tricetin, a dietary flavonoid, induces apoptosis through the reactive oxygen species/c-Jun NH(2)-terminal kinase pathway in human liver cancer cells. J Agric Food Chem. 2010;2010;58:12547–12556. doi: 10.1021/jf103159r. [DOI] [PubMed] [Google Scholar]
- 127.Hu Y. Rosen DG. Zhou Y. Feng L. Yang G. Liu J. Huang P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: role in cell proliferation and response to oxidative stress. J Biol Chem. 2005;280:39485–39492. doi: 10.1074/jbc.M503296200. [DOI] [PubMed] [Google Scholar]
- 128.Huang P. Feng L. Oldham EA. Keating MJ. Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 129.Huang S. Mills L. Mian B. Tellez C. McCarty M. Yang XD. Gudas JM. Bar-Eli M. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol. 2002;161:125–134. doi: 10.1016/S0002-9440(10)64164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hussain AR. Ahmed M. Ahmed S. Manogaran P. Platanias LC. Alvi SN. Al-Kuraya KS. Uddin S. Thymoquinone suppresses growth and induces apoptosis via generation of reactive oxygen species in primary effusion lymphoma. Free Radic Biol Med. 2011;50:978–987. doi: 10.1016/j.freeradbiomed.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 131.Hussain SP. Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 132.Hussain SP. Hofseth LJ. Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 133.Hwang IT. Chung YM. Kim JJ. Chung JS. Kim BS. Kim HJ. Kim JS. Yoo YD. Drug resistance to 5-FU linked to reactive oxygen species modulator 1. Biochem Biophys Res Commun. 2007;359:304–310. doi: 10.1016/j.bbrc.2007.05.088. [DOI] [PubMed] [Google Scholar]
- 134.Hyoudou K. Nishikawa M. Kobayashi Y. Ikemura M. Yamashita F. Hashida M. SOD derivatives prevent metastatic tumor growth aggravated by tumor removal. Clin Exp Metastasis. 2008;25:531–536. doi: 10.1007/s10585-008-9165-3. [DOI] [PubMed] [Google Scholar]
- 135.Hyoudou K. Nishikawa M. Kobayashi Y. Umeyama Y. Yamashita F. Hashida M. PEGylated catalase prevents metastatic tumor growth aggravated by tumor removal. Free Radic Biol Med. 2006;41:1449–1458. doi: 10.1016/j.freeradbiomed.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 136.Ide H. Tokiwa S. Sakamaki K. Nishio K. Isotani S. Muto S. Hama T. Masuda H. Horie S. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate. 2010;70:1127–1133. doi: 10.1002/pros.21147. [DOI] [PubMed] [Google Scholar]
- 137.Inoue M. Sato EF. Nishikawa M. Park AM. Kira Y. Imada I. Utsumi K. Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr Med Chem. 2003;10:2495–2505. doi: 10.2174/0929867033456477. [DOI] [PubMed] [Google Scholar]
- 138.Irani K. Xia Y. Zweier JL. Sollott SJ. Der CJ. Fearon ER. Sundaresan M. Finkel T. Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 139.Ishdorj G. Li L. Gibson SB. Regulation of autophagy in hematological malignancies: role of ROS. Leuk Lymphoma. 2011 doi: 10.3109/10428194.2011.604752. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 140.Ishikawa K. Takenaga K. Akimoto M. Koshikawa N. Yamaguchi A. Imanishi H. Nakada K. Honma Y. Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 141.Jackson AL. Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001;477:7–21. doi: 10.1016/s0027-5107(01)00091-4. [DOI] [PubMed] [Google Scholar]
- 142.Jaganathan SK. Mazumdar A. Mondhe D. Mandal M. Apoptotic effect of eugenol in human colon cancer cell lines. Cell Biol Int. 2011;35:607–615. doi: 10.1042/CBI20100118. [DOI] [PubMed] [Google Scholar]
- 143.James J. Murry DJ. Treston AM. Storniolo AM. Sledge GW. Sidor C. Miller KD. Phase I safety, pharmacokinetic and pharmacodynamic studies of 2-methoxyestradiol alone or in combination with docetaxel in patients with locally recurrent or metastatic breast cancer. Invest New Drugs. 2007;25:41–48. doi: 10.1007/s10637-006-9008-5. [DOI] [PubMed] [Google Scholar]
- 144.Jeremic B. Milicic B. Milisavljevic S. Clinical prognostic factors in patients with locally advanced (stage III) nonsmall cell lung cancer treated with hyperfractionated radiation therapy with and without concurrent chemotherapy: single-Institution Experience in 600 Patients. Cancer. 2011;117:2995–3003. doi: 10.1002/cncr.25910. [DOI] [PubMed] [Google Scholar]
- 145.Jian L. Xie LP. Lee AH. Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. Int J Cancer. 2004;108:130–135. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- 146.Jiang MC. Liao CF. Lee PH. Aspirin inhibits matrix metalloproteinase-2 activity, increases E-cadherin production, and inhibits in vitro invasion of tumor cells. Biochem Biophys Res Commun. 2001;282:671–677. doi: 10.1006/bbrc.2001.4637. [DOI] [PubMed] [Google Scholar]
- 147.Jing Y. Dai J. Chalmers-Redman RM. Tatton WG. Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–2111. [PubMed] [Google Scholar]
- 148.Jones CU. Hunt D. McGowan DG. Amin MB. Chetner MP. Bruner DW. Leibenhaut MH. Husain SM. Rotman M. Souhami L. Sandler HM. Shipley WU. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 149.Kamp DW. Shacter E. Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology (Williston Park) 2011;25:400–410. 413. [PubMed] [Google Scholar]
- 150.Kang YH. Lee SJ. Role of p38 MAPK and JNK in enhanced cervical cancer cell killing by the combination of arsenic trioxide and ionizing radiation. Oncol Rep. 2008;20:637–643. [PubMed] [Google Scholar]
- 151.Kannappan R. Ravindran J. Prasad S. Sung B. Yadav VR. Reuter S. Chaturvedi MM. Aggarwal BB. Gamma-tocotrienol promotes TRAIL-induced apoptosis through reactive oxygen species/extracellular signal-regulated kinase/p53-mediated upregulation of death receptors. Mol Cancer Ther. 2010;9:2196–2207. doi: 10.1158/1535-7163.MCT-10-0277. [DOI] [PubMed] [Google Scholar]
- 152.Khan N. Afaq F. Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 153.Khaw KT. Bingham S. Welch A. Luben R. Wareham N. Oakes S. Day N. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet. 2001;357:657–663. doi: 10.1016/s0140-6736(00)04128-3. [DOI] [PubMed] [Google Scholar]
- 154.Khromova NV. Kopnin PB. Stepanova EV. Agapova LS. Kopnin BP. p53 hot-spot mutants increase tumor vascularization via ROS-mediated activation of the HIF1/VEGF-A pathway. Cancer Lett. 2009;276:143–151. doi: 10.1016/j.canlet.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 155.Kim AD. Kang KA. Zhang R. Lim CM. Kim HS. Kim DH. Jeon YJ. Lee CH. Park J. Chang WY. Hyun JW. Ginseng saponin metabolite induces apoptosis in MCF-7 breast cancer cells through the modulation of AMP-activated protein kinase. Environ Toxicol Pharmacol. 2010;30:134–140. doi: 10.1016/j.etap.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 156.Kim EY. Seo JM. Cho KJ. Kim JH. Ras-induced invasion and metastasis are regulated by a leukotriene B4 receptor BLT2-linked pathway. Oncogene. 2010;29:1167–1178. doi: 10.1038/onc.2009.412. [DOI] [PubMed] [Google Scholar]
- 157.Kim EY. Seo JM. Kim C. Lee JE. Lee KM. Kim JH. BLT2 promotes the invasion and metastasis of aggressive bladder cancer cells through a reactive oxygen species-linked pathway. Free Radic Biol Med. 2010;49:1072–1081. doi: 10.1016/j.freeradbiomed.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 158.Kim HS. Bowen P. Chen L. Duncan C. Ghosh L. Sharifi R. Christov K. Effects of tomato sauce consumption on apoptotic cell death in prostate benign hyperplasia and carcinoma. Nutr Cancer. 2003;47:40–47. doi: 10.1207/s15327914nc4701_5. [DOI] [PubMed] [Google Scholar]
- 159.Kim J. Koyanagi T. Mochly-Rosen D. PKCdelta activation mediates angiogenesis via NADPH oxidase activity in PC-3 prostate cancer cells. Prostate. 2011;71:946–954. doi: 10.1002/pros.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim JS. Ahn KJ. Kim JA. Kim HM. Lee JD. Lee JM. Kim SJ. Park JH. Role of reactive oxygen species-mediated mitochondrial dysregulation in 3-bromopyruvate induced cell death in hepatoma cells: ROS-mediated cell death by 3-BrPA. J Bioenerg Biomembr. 2008;40:607–618. doi: 10.1007/s10863-008-9188-0. [DOI] [PubMed] [Google Scholar]
- 161.Kim MH. Cho HS. Jung M. Hong MH. Lee SK. Shin BA. Ahn BW. Jung YD. Extracellular signal-regulated kinase and AP-1 pathways are involved in reactive oxygen species-induced urokinase plasminogen activator receptor expression in human gastric cancer cells. Int J Oncol. 2005;26:1669–1674. [PubMed] [Google Scholar]
- 162.Kim SR. Lee KS. Park SJ. Min KH. Lee KY. Choe YH. Hong SH. Koh GY. Lee YC. Angiopoietin-1 variant, COMP-Ang1 attenuates hydrogen peroxide-induced acute lung injury. Exp Mol Med. 2008;40:320–331. doi: 10.3858/emm.2008.40.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kirshner JR. He S. Balasubramanyam V. Kepros J. Yang CY. Zhang M. Du Z. Barsoum J. Bertin J. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol Cancer Ther. 2008;7:2319–2327. doi: 10.1158/1535-7163.MCT-08-0298. [DOI] [PubMed] [Google Scholar]
- 164.Klein B. Zhang XG. Jourdan M. Content J. Houssiau F. Aarden L. Piechaczyk M. Bataille R. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood. 1989;73:517–526. [PubMed] [Google Scholar]
- 165.Kleiner DE., Jr. Stetler-Stevenson WG. Structural biochemistry and activation of matrix metalloproteases. Curr Opin Cell Biol. 1993;5:891–897. doi: 10.1016/0955-0674(93)90040-w. [DOI] [PubMed] [Google Scholar]
- 166.Koh EK. Ryu BK. Jeong DY. Bang IS. Nam MH. Chae KS. A 60-Hz sinusoidal magnetic field induces apoptosis of prostate cancer cells through reactive oxygen species. Int J Radiat Biol. 2008;84:945–955. doi: 10.1080/09553000802460206. [DOI] [PubMed] [Google Scholar]
- 167.Koka PS. Mondal D. Schultz M. Abdel-Mageed AB. Agrawal KC. Studies on molecular mechanisms of growth inhibitory effects of thymoquinone against prostate cancer cells: role of reactive oxygen species. Exp Biol Med (Maywood) 2010;235:751–760. doi: 10.1258/ebm.2010.009369. [DOI] [PubMed] [Google Scholar]
- 168.Kong Q. Beel JA. Lillehei KO. A threshold concept for cancer therapy. Med Hypotheses. 2000;55:29–35. doi: 10.1054/mehy.1999.0982. [DOI] [PubMed] [Google Scholar]
- 169.Kong Q. Lillehei KO. Antioxidant inhibitors for cancer therapy. Med Hypotheses. 1998;51:405–409. doi: 10.1016/s0306-9877(98)90036-6. [DOI] [PubMed] [Google Scholar]
- 170.Kopetz S. Lesslie DP. Dallas NA. Park SI. Johnson M. Parikh NU. Kim MP. Abbruzzese JL. Ellis LM. Chandra J. Gallick GE. Synergistic activity of the SRC family kinase inhibitor dasatinib and oxaliplatin in colon carcinoma cells is mediated by oxidative stress. Cancer Res. 2009;69:3842–3849. doi: 10.1158/0008-5472.CAN-08-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Korinkova G. Cwiertka K. Paprskarova M. Dzubak P. Hajduch M. The radiosensitising effect of olomoucine derived synthetic cyclin-dependent kinase inhibitors. Neoplasma. 2010;57:161–169. doi: 10.4149/neo_2010_02_161. [DOI] [PubMed] [Google Scholar]
- 172.Kuo JH. Chu YL. Yang JS. Lin JP. Lai KC. Kuo HM. Hsia TC. Chung JG. Cantharidin induces apoptosis in human bladder cancer TSGH 8301 cells through mitochondria-dependent signal pathways. Int J Oncol. 2010;37:1243–1250. doi: 10.3892/ijo_00000775. [DOI] [PubMed] [Google Scholar]
- 173.Kuptsova N. Chang-Claude J. Kropp S. Helmbold I. Schmezer P. von Fournier D. Haase W. Sautter-Bihl ML. Wenz F. Onel K. Ambrosone CB. Genetic predictors of long-term toxicities after radiation therapy for breast cancer. Int J Cancer. 2008;122:1333–1339. doi: 10.1002/ijc.23138. [DOI] [PubMed] [Google Scholar]
- 174.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 175.Lamson DW. Brignall MS. Antioxidants in cancer therapy; their actions and interactions with oncologic therapies. Altern Med Rev. 1999;4:304–329. [PubMed] [Google Scholar]
- 176.Landi S. Moreno V. Gioia-Patricola L. Guino E. Navarro M. de Oca J. Capella G. Canzian F. Association of common polymorphisms in inflammatory genes interleukin (IL)6, IL8, tumor necrosis factor alpha, NFKB1, and peroxisome proliferator-activated receptor gamma with colorectal cancer. Cancer Res. 2003;63:3560–3566. [PubMed] [Google Scholar]
- 177.Lee J. Lim KT. Inhibitory effect of phytoglycoprotein (38 kDa) on expression of matrix metalloproteinase-9 in 12-O-tetradecanoylphorbol-13-acetate-treated HepG2cells. Naunyn Schmiedebergs Arch Pharmacol. 2011;384:185–196. doi: 10.1007/s00210-011-0663-5. [DOI] [PubMed] [Google Scholar]
- 178.Lee JK. Edderkaoui M. Truong P. Ohno I. Jang KT. Berti A. Pandol SJ. Gukovskaya AS. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007;133:1637–1648. doi: 10.1053/j.gastro.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 179.Lee KB. Kim KR. Huh TL. Lee YM. Proton induces apoptosis of hypoxic tumor cells by the p53-dependent and p38/JNK MAPK signaling pathways. Int J Oncol. 2008;33:1247–1256. [PubMed] [Google Scholar]
- 180.Lei Y. Huang K. Gao C. Lau QC. Pan H. Xie K. Li J. Liu R. Zhang T. Xie N. Nai HS. Wu H. Zhao X. Nice EC. Huang C. Wei Y. Proteomics identification of ITGB3 as a key regulator in ROS-induced migration and invasion of colorectal cancer cells. Mol Cell Proteomics. 2011;10:M110.005397. doi: 10.1074/mcp.M110.005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lemjabbar-Alaoui H. Sidhu SS. Mengistab A. Gallup M. Basbaum C. TACE/ADAM-17 phosphorylation by PKC-epsilon mediates premalignant changes in tobacco smoke-exposed lung cells. PLoS One. 2011;6:e17489. doi: 10.1371/journal.pone.0017489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lenehan PF. Gutierrez PL. Wagner JL. Milak N. Fisher GR. Ross DD. Resistance to oxidants associated with elevated catalase activity in HL-60 leukemia cells that overexpress multidrug-resistance protein does not contribute to the resistance to daunorubicin manifested by these cells. Cancer Chemother Pharmacol. 1995;35:377–386. doi: 10.1007/s002800050250. [DOI] [PubMed] [Google Scholar]
- 183.Lengyel E. Gum R. Stepp E. Juarez J. Wang H. Boyd D. Regulation of urokinase-type plasminogen activator expression by an ERK1-dependent signaling pathway in a squamous cell carcinoma cell line. J Cell Biochem. 1996;61:430–443. doi: 10.1002/(sici)1097-4644(19960601)61:3<430::aid-jcb10>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 184.Li J. Wang XL. Fang YC. Wang CY. Tephrosin-induced autophagic cell death in A549 non-small cell lung cancer cells. J Asian Nat Prod Res. 2010;12:992–1000. doi: 10.1080/10286020.2010.513034. [DOI] [PubMed] [Google Scholar]
- 185.Lim SC. Duong HQ. Choi JE. Lee TB. Kang JH. Oh SH. Han SI. Lipid raft-dependent death receptor 5 (DR5) expression and activation are critical for ursodeoxycholic acid-induced apoptosis in gastric cancer cells. Carcinogenesis. 2011;32:723–731. doi: 10.1093/carcin/bgr038. [DOI] [PubMed] [Google Scholar]
- 186.Lin CC. Huang CY. Mong MC. Chan CY. Yin MC. Antiangiogenic potential of three triterpenic acids in human liver cancer cells. J Agric Food Chem. 2011;59:755–762. doi: 10.1021/jf103904b. [DOI] [PubMed] [Google Scholar]
- 187.Lin J. Zahurak M. Beer TM. Ryan CJ. Wilding G. Mathew P. Morris M. Callahan JA. Gordon G. Reich SD. Carducci MA. Antonarakis ES. A non-comparative randomized phase II study of 2 doses of ATN-224, a copper/zinc superoxide dismutase inhibitor, in patients with biochemically recurrent hormone-naive prostate cancer. Urol Oncol. 2011 doi: 10.1016/j.urolonc.2011.04.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lin KW. Huang AM. Hour TC. Yang SC. Pu YS. Lin CN. 18beta-Glycyrrhetinic acid derivatives induced mitochondrial-mediated apoptosis through reactive oxygen species-mediated p53 activation in NTUB1 cells. Bioorg Med Chem. 2011;19:4274–4285. doi: 10.1016/j.bmc.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 189.Lin TS. Naumovski L. Lecane PS. Lucas MS. Moran ME. Cheney C. Lucas DM. Phan SC. Miller RA. Byrd JC. Effects of motexafin gadolinium in a phase II trial in refractory chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50:1977–1982. doi: 10.3109/10428190903288464. [DOI] [PubMed] [Google Scholar]
- 190.Liu B. Chen Y. St. Clair DK. ROS and p53: a versatile partnership. Free Radic Biol Med. 2008;44:1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liu J. Du J. Zhang Y. Sun W. Smith BJ. Oberley LW. Cullen JJ. Suppression of the malignant phenotype in pancreatic cancer by overexpression of phospholipid hydroperoxide glutathione peroxidase. Hum Gene Ther. 2006;17:105–116. doi: 10.1089/hum.2006.17.105. [DOI] [PubMed] [Google Scholar]
- 192.Liu LZ. Hu XW. Xia C. He J. Zhou Q. Shi X. Fang J. Jiang BH. Reactive oxygen species regulate epidermal growth factor-induced vascular endothelial growth factor and hypoxia-inducible factor-1alpha expression through activation of AKT and P70S6K1 in human ovarian cancer cells. Free Radic Biol Med. 2006;41:1521–1533. doi: 10.1016/j.freeradbiomed.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 193.Liu PL. Chen YL. Chen YH. Lin SJ. Kou YR. Wood smoke extract induces oxidative stress-mediated caspase-independent apoptosis in human lung endothelial cells: role of AIF and EndoG. Am J Physiol Lung Cell Mol Physiol. 2005;289:L739–L749. doi: 10.1152/ajplung.00099.2005. [DOI] [PubMed] [Google Scholar]
- 194.Liu S. Li S. Du Y. Polychlorinated biphenyls (PCBs) enhance metastatic properties of breast cancer cells by activating Rho-associated kinase (ROCK) PLoS One. 2010;5:e11272. doi: 10.1371/journal.pone.0011272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liu SL. Lin X. Shi DY. Cheng J. Wu CQ. Zhang YD. Reactive oxygen species stimulated human hepatoma cell proliferation via cross-talk between PI3-K/PKB and JNK signaling pathways. Arch Biochem Biophys. 2002;406:173–182. doi: 10.1016/s0003-9861(02)00430-7. [DOI] [PubMed] [Google Scholar]
- 196.Lou J. Zhou X. Weng Q. Wang DD. Xia Q. Hu Y. He Q. Yang B. Luo P. XQ2, a novel TPZ derivative, induced G2/M phase arrest and apoptosis under hypoxia in non-small cell lung cancer cells. Biosci Biotechnol Biochem. 2010;74:1181–1187. doi: 10.1271/bbb.90889. [DOI] [PubMed] [Google Scholar]
- 197.Lu H. Ouyang W. Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 198.Lu HF. Chen YL. Yang JS. Yang YY. Liu JY. Hsu SC. Lai KC. Chung JG. Antitumor activity of capsaicin on human colon cancer cells in vitro and colo 205 tumor xenografts in vivo. J Agric Food Chem. 2010;58:12999–13005. doi: 10.1021/jf103335w. [DOI] [PubMed] [Google Scholar]
- 199.Lu J. Chew EH. Holmgren A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc Natl Acad Sci U S A. 2007;104:12288–12293. doi: 10.1073/pnas.0701549104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Luanpitpong S. Talbott SJ. Rojanasakul Y. Nimmannit U. Pongrakhananon V. Wang L. Chanvorachote P. Regulation of lung cancer cell migration and invasion by reactive oxygen species and caveolin-1. J Biol Chem. 2010;285:38832–38840. doi: 10.1074/jbc.M110.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Luca M. Huang S. Gershenwald JE. Singh RK. Reich R. Bar-Eli M. Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol. 1997;151:1105–1113. [PMC free article] [PubMed] [Google Scholar]
- 202.Luo M. Liu X. Zu Y. Fu Y. Zhang S. Yao L. Efferth T. Cajanol, a novel anticancer agent from Pigeonpea [Cajanus cajan (L.) Millsp.] roots, induces apoptosis in human breast cancer cells through a ROS-mediated mitochondrial pathway. Chem Biol Interact. 2010;188:151–160. doi: 10.1016/j.cbi.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 203.Ma Q. Cavallin LE. Yan B. Zhu S. Duran EM. Wang H. Hale LP. Dong C. Cesarman E. Mesri EA. Goldschmidt-Clermont PJ. Antitumorigenesis of antioxidants in a transgenic Rac1 model of Kaposi's sarcoma. Proc Natl Acad Sci U S A. 2009;106:8683–8688. doi: 10.1073/pnas.0812688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Maeda H. Hori S. Ohizumi H. Segawa T. Kakehi Y. Ogawa O. Kakizuka A. Effective treatment of advanced solid tumors by the combination of arsenic trioxide and L-buthionine-sulfoximine. Cell Death Differ. 2004;11:737–746. doi: 10.1038/sj.cdd.4401389. [DOI] [PubMed] [Google Scholar]
- 205.Maeda S. Kamata H. Luo JL. Leffert H. Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 206.Maehata Y. Ozawa S. Kobayashi K. Kato Y. Yoshino F. Miyamoto C. Izukuri K. Kubota E. Hata R. Lee MC. Reactive oxygen species (ROS) reduce the expression of BRAK/CXCL14 in human head and neck squamous cell carcinoma cells. Free Radic Res. 2010;44:913–924. doi: 10.3109/10715762.2010.490836. [DOI] [PubMed] [Google Scholar]
- 207.Magda D. Lepp C. Gerasimchuk N. Lee I. Sessler JL. Lin A. Biaglow JE. Miller RA. Redox cycling by motexafin gadolinium enhances cellular response to ionizing radiation by forming reactive oxygen species. Int J Radiat Oncol Biol Phys. 2001;51:1025–1036. doi: 10.1016/s0360-3016(01)01810-7. [DOI] [PubMed] [Google Scholar]
- 208.Magda D. Miller RA. Motexafin gadolinium: a novel redox active drug for cancer therapy. Semin Cancer Biol. 2006;16:466–476. doi: 10.1016/j.semcancer.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 209.Mantovani A. Cancer: inflammation by remote control. Nature. 2005;435:752–753. doi: 10.1038/435752a. [DOI] [PubMed] [Google Scholar]
- 210.Mantovani A. Allavena P. Sica A. Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 211.Martin V. Herrera F. Garcia-Santos G. Antolin I. Rodriguez-Blanco J. Rodriguez C. Signaling pathways involved in antioxidant control of glioma cell proliferation. Free Radic Biol Med. 2007;42:1715–1722. doi: 10.1016/j.freeradbiomed.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 212.Martindale JL. Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 213.Martz G. D Alessandri A. Keel HJ. Bollag W. Preliminary clinical results with a new antitumor agent Ro 4–6467 (Nsc-77213) Cancer Chemother Rep. 1963;33:5–14. [PubMed] [Google Scholar]
- 214.Mathe G. Schweisguth O. Schneider M. Amiel JL. Berumen L. Brule G. Cattan A. Schwarzenberg L. Methyl-Hydrazine in treatment of Hodgkin's disease and various forms of haematosarcoma and leukaemia. Lancet. 1963;2:1077–1080. doi: 10.1016/s0140-6736(63)92854-x. [DOI] [PubMed] [Google Scholar]
- 215.Mayola E. Gallerne C. Esposti DD. Martel C. Pervaiz S. Larue L. Debuire B. Lemoine A. Brenner C. Lemaire C. Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2. Apoptosis. 2011;16:1014–1027. doi: 10.1007/s10495-011-0625-x. [DOI] [PubMed] [Google Scholar]
- 216.Medan D. Wang L. Toledo D. Lu B. Stehlik C. Jiang BH. Shi X. Rojanasakul Y. Regulation of Fas (CD95)-induced apoptotic and necrotic cell death by reactive oxygen species in macrophages. J Cell Physiol. 2005;203:78–84. doi: 10.1002/jcp.20201. [DOI] [PubMed] [Google Scholar]
- 217.Mehta MP. Shapiro WR. Phan SC. Gervais R. Carrie C. Chabot P. Patchell RA. Glantz MJ. Recht L. Langer C. Sur RK. Roa WH. Mahe MA. Fortin A. Nieder C. Meyers CA. Smith JA. Miller RA. Renschler MF. Motexafin gadolinium combined with prompt whole brain radiotherapy prolongs time to neurologic progression in non-small-cell lung cancer patients with brain metastases: results of a phase III trial. Int J Radiat Oncol Biol Phys. 2009;73:1069–1076. doi: 10.1016/j.ijrobp.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 218.Mi L. Gan N. Chung FL. Isothiocyanates inhibit proteasome activity and proliferation of multiple myeloma cells. Carcinogenesis. 2011;32:216–223. doi: 10.1093/carcin/bgq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mishina NM. Tyurin-Kuzmin PA. Markvicheva KN. Vorotnikov AV. Tkachuk VA. Laketa V. Schultz C. Lukyanov S. Belousov VV. Does cellular hydrogen peroxide diffuse or act locally? Antioxid Redox Signal. 2011;14:1–7. doi: 10.1089/ars.2010.3539. [DOI] [PubMed] [Google Scholar]
- 220.Mochizuki T. Furuta S. Mitsushita J. Shang WH. Ito M. Yokoo Y. Yamaura M. Ishizone S. Nakayama J. Konagai A. Hirose K. Kiyosawa K. Kamata T. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene. 2006;25:3699–3707. doi: 10.1038/sj.onc.1209406. [DOI] [PubMed] [Google Scholar]
- 221.Moon DO. Kim MO. Choi YH. Hyun JW. Chang WY. Kim GY. Butein induces G(2)/M phase arrest and apoptosis in human hepatoma cancer cells through ROS generation. Cancer Lett. 2010;288:204–213. doi: 10.1016/j.canlet.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 222.Morgan MJ. Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Na AR. Chung YM. Lee SB. Park SH. Lee MS. Yoo YD. A critical role for Romo1-derived ROS in cell proliferation. Biochem Biophys Res Commun. 2008;369:672–678. doi: 10.1016/j.bbrc.2008.02.098. [DOI] [PubMed] [Google Scholar]
- 225.Naik E. Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208:417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nair RR. Emmons MF. Cress AE. Argilagos RF. Lam K. Kerr WT. Wang HG. Dalton WS. Hazlehurst LA. HYD1-induced increase in reactive oxygen species leads to autophagy and necrotic cell death in multiple myeloma cells. Mol Cancer Ther. 2009;8:2441–2451. doi: 10.1158/1535-7163.MCT-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Naito M. Hashimoto C. Masui S. Tsuruo T. Caspase-independent necrotic cell death induced by a radiosensitizer, 8-nitrocaffeine. Cancer Sci. 2004;95:361–366. doi: 10.1111/j.1349-7006.2004.tb03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Nakata W. Hayakawa Y. Nakagawa H. Sakamoto K. Kinoshita H. Takahashi R. Hirata Y. Maeda S. Koike K. Anti-tumor activity of the proteasome inhibitor bortezomib in gastric cancer. Int J Oncol. 2011;39:1529–1536. doi: 10.3892/ijo.2011.1141. [DOI] [PubMed] [Google Scholar]
- 229.Nerlov C. Rorth P. Blasi F. Johnsen M. Essential AP-1 and PEA3 binding elements in the human urokinase enhancer display cell type-specific activity. Oncogene. 1991;6:1583–1592. [PubMed] [Google Scholar]
- 230.Nguyen GH. Schetter AJ. Chou DB. Bowman ED. Zhao R. Hawkes JE. Mathe EA. Kumamoto K. Zhao Y. Budhu A. Hagiwara N. Wang XW. Miyashita M. Casson AG. Harris CC. Inflammatory and microRNA gene expression as prognostic classifier of Barrett's-associated esophageal adenocarcinoma. Clin Cancer Res. 2010;16:5824–5834. doi: 10.1158/1078-0432.CCR-10-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nguyen H. Syed V. Progesterone inhibits growth and induces apoptosis in cancer cells through modulation of reactive oxygen species. Gynecol Endocrinol. 2011;27:830–836. doi: 10.3109/09513590.2010.538100. [DOI] [PubMed] [Google Scholar]
- 232.Nishikawa M. Reactive oxygen species in tumor metastasis. Cancer Lett. 2008;266:53–59. doi: 10.1016/j.canlet.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 233.Nishikawa M. Hyoudou K. Kobayashi Y. Umeyama Y. Takakura Y. Hashida M. Inhibition of metastatic tumor growth by targeted delivery of antioxidant enzymes. J Control Release. 2005;109:101–107. doi: 10.1016/j.jconrel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 234.Niu C. Yan H. Yu T. Sun HP. Liu JX. Li XS. Wu W. Zhang FQ. Chen Y. Zhou L. Li JM. Zeng XY. Yang RR. Yuan MM. Ren MY. Gu FY. Cao Q. Gu BW. Su XY. Chen GQ. Xiong SM. Zhang TD. Waxman S. Wang ZY. Chen Z. Hu J. Shen ZX. Chen SJ. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94:3315–3324. [PubMed] [Google Scholar]
- 235.Okamoto M. Kawamata H. Kawai K. Oyasu R. Enhancement of transformation in vitro of a nontumorigenic rat urothelial cell line by interleukin 6. Cancer Res. 1995;55:4581–4585. [PubMed] [Google Scholar]
- 236.Owen JD. Strieter R. Burdick M. Haghnegahdar H. Nanney L. Shattuck-Brandt R. Richmond A. Enhanced tumor-forming capacity for immortalized melanocytes expressing melanoma growth stimulatory activity/growth-regulated cytokine beta and gamma proteins. Int J Cancer. 1997;73:94–103. doi: 10.1002/(sici)1097-0215(19970926)73:1<94::aid-ijc15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 237.Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci. 2007;96:2181–2196. doi: 10.1002/jps.20874. [DOI] [PubMed] [Google Scholar]
- 238.Pantuck AJ. Leppert JT. Zomorodian N. Aronson W. Hong J. Barnard RJ. Seeram N. Liker H. Wang H. Elashoff R. Heber D. Aviram M. Ignarro L. Belldegrun A. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res. 2006;12:4018–4026. doi: 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- 239.Park EJ. Choi KS. Kwon TK. Beta-Lapachone-induced reactive oxygen species (ROS) generation mediates autophagic cell death in glioma U87 MG cells. Chem Biol Interact. 2011;189:37–44. doi: 10.1016/j.cbi.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 240.Patel BB. Sengupta R. Qazi S. Vachhani H. Yu Y. Rishi AK. Majumdar AP. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122:267–273. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- 241.Pathi SS. Jutooru I. Chadalapaka G. Sreevalsan S. Anand S. Thatcher GR. Safe S. GT-094, a NO-NSAID, inhibits colon cancer cell growth by activation of a reactive oxygen species-microRNA-27a: ZBTB10-specificity protein pathway. Mol Cancer Res. 2011;9:195–202. doi: 10.1158/1541-7786.MCR-10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pelicano H. Feng L. Zhou Y. Carew JS. Hileman EO. Plunkett W. Keating MJ. Huang P. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J Biol Chem. 2003;278:37832–37839. doi: 10.1074/jbc.M301546200. [DOI] [PubMed] [Google Scholar]
- 243.Pervaiz S. Clement MV. Tumor intracellular redox status and drug resistance—serendipity or a causal relationship? Curr Pharm Des. 2004;10:1969–1977. doi: 10.2174/1381612043384411. [DOI] [PubMed] [Google Scholar]
- 244.Pine SR. Mechanic LE. Enewold L. Chaturvedi AK. Katki HA. Zheng YL. Bowman ED. Engels EA. Caporaso NE. Harris CC. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst. 2011;103:1112–1122. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Policastro L. Molinari B. Larcher F. Blanco P. Podhajcer OL. Costa CS. Rojas P. Duran H. Imbalance of antioxidant enzymes in tumor cells and inhibition of proliferation and malignant features by scavenging hydrogen peroxide. Mol Carcinog. 2004;39:103–113. doi: 10.1002/mc.20001. [DOI] [PubMed] [Google Scholar]
- 246.Pollycove M. Radiobiological basis of low-dose irradiation in prevention and therapy of cancer. Dose Response. 2007;5:26–38. doi: 10.2203/dose-response.06-112.Pollycove. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Prasad S. Yadav VR. Kannappan R. Aggarwal BB. Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors: evidence for the role of reactive oxygen species and JNK. J Biol Chem. 2011;286:5546–5557. doi: 10.1074/jbc.M110.183699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 248.Price M. Terlecky SR. Kessel D. A role for hydrogen peroxide in the pro-apoptotic effects of photodynamic therapy. Photochem Photobiol. 2009;85:1491–1496. doi: 10.1111/j.1751-1097.2009.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Qin Y. Pan X. Tang TT. Zhou L. Gong XG. Anti-proliferative effects of the novel squamosamide derivative (FLZ) on HepG2 human hepatoma cells by regulating the cell cycle-related proteins are associated with decreased Ca(2+)/ROS levels. Chem Biol Interact. 2011;193:246–253. doi: 10.1016/j.cbi.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 250.Qu Y. Wang J. Ray PS. Guo H. Huang J. Shin-Sim M. Bukoye BA. Liu B. Lee AV. Lin X. Huang P. Martens JW. Giuliano AE. Zhang N. Cheng NH. Cui X. Thioredoxin-like 2 regulates human cancer cell growth and metastasis via redox homeostasis and NF-kappaB signaling. J Clin Invest. 2011;121:212–225. doi: 10.1172/JCI43144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Radisky DC. Levy DD. Littlepage LE. Liu H. Nelson CM. Fata JE. Leake D. Godden EL. Albertson DG. Nieto MA. Werb Z. Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Raj L. Ide T. Gurkar AU. Foley M. Schenone M. Li X. Tolliday NJ. Golub TR. Carr SA. Shamji AF. Stern AM. Mandinova A. Schreiber SL. Lee SW. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 253.Rajashekhar G. Kamocka M. Marin A. Suckow MA. Wolter WR. Badve S. Sanjeevaiah AR. Pumiglia K. Rosen E. Clauss M. Pro-inflammatory angiogenesis is mediated by p38 MAP kinase. J Cell Physiol. 2011;226:800–808. doi: 10.1002/jcp.22404. [DOI] [PubMed] [Google Scholar]
- 254.Rakshit S. Mandal L. Pal BC. Bagchi J. Biswas N. Chaudhuri J. Chowdhury AA. Manna A. Chaudhuri U. Konar A. Mukherjee T. Jaisankar P. Bandyopadhyay S. Involvement of ROS in chlorogenic acid-induced apoptosis of Bcr-Abl+ CML cells. Biochem Pharmacol. 2010;80:1662–1675. doi: 10.1016/j.bcp.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 255.Ralph SJ. Rodriguez-Enriquez S. Neuzil J. Saavedra E. Moreno-Sanchez R. The causes of cancer revisited: “mitochondrial malignancy” and ROS-induced oncogenic transformation—why mitochondria are targets for cancer therapy. Mol Aspects Med. 2010;31:145–170. doi: 10.1016/j.mam.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 256.Ramsey MR. Sharpless NE. ROS as a tumour suppressor? Nat Cell Biol. 2006;8:1213–1215. doi: 10.1038/ncb1106-1213. [DOI] [PubMed] [Google Scholar]
- 257.Reinehr R. Becker S. Eberle A. Grether-Beck S. Haussinger D. Involvement of NADPH oxidase isoforms and Src family kinases in CD95-dependent hepatocyte apoptosis. J Biol Chem. 2005;280:27179–27194. doi: 10.1074/jbc.M414361200. [DOI] [PubMed] [Google Scholar]
- 258.Renschler MF. The emerging role of reactive oxygen species in cancer therapy. Eur J Cancer. 2004;40:1934–1940. doi: 10.1016/j.ejca.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 259.Reuter S. Gupta SC. Chaturvedi MM. Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 261.Ricca A. Biroccio A. Trisciuoglio D. Cippitelli M. Zupi G. Del Bufalo D. relA over-expression reduces tumorigenicity and activates apoptosis in human cancer cells. Br J Cancer. 2001;85:1914–1921. doi: 10.1054/bjoc.2001.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ruiz-Ramos R. Lopez-Carrillo L. Rios-Perez AD. De Vizcaya-Ruiz A. Cebrian ME. Sodium arsenite induces ROS generation, DNA oxidative damage, HO-1 and c-Myc proteins, NF-kappaB activation and cell proliferation in human breast cancer MCF-7 cells. Mutat Res. 2009;674:109–115. doi: 10.1016/j.mrgentox.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 263.Rungtabnapa P. Nimmannit U. Halim H. Rojanasakul Y. Chanvorachote P. Hydrogen peroxide inhibits non-small cell lung cancer cell anoikis through the inhibition of caveolin-1 degradation. Am J Physiol Cell Physiol. 2011;300:C235–C245. doi: 10.1152/ajpcell.00249.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Salama S. Diaz-Arrastia C. Patel D. Botting S. Hatch S. 2-Methoxyestradiol, an endogenous estrogen metabolite, sensitizes radioresistant MCF-7/FIR breast cancer cells through multiple mechanisms. Int J Radiat Oncol Biol Phys. 2011;80:231–239. doi: 10.1016/j.ijrobp.2010.10.080. [DOI] [PubMed] [Google Scholar]
- 265.Salmon SE. Sartorelli AC. In: Cancer Chemotherapy in Basic and Clinical Pharmacology. Katzung BG, editor. Appleton: Lange; 1998. pp. 881–911. [Google Scholar]
- 266.Sasazuki S. Hayashi T. Nakachi K. Sasaki S. Tsubono Y. Okubo S. Hayashi M. Tsugane S. Protective effect of vitamin C on oxidative stress: a randomized controlled trial. Int J Vitam Nutr Res. 2008;78:121–128. doi: 10.1024/0300-9831.78.3.121. [DOI] [PubMed] [Google Scholar]
- 267.Saunders JA. Rogers LC. Klomsiri C. Poole LB. Daniel LW. Reactive oxygen species mediate lysophosphatidic acid induced signaling in ovarian cancer cells. Free Radic Biol Med. 2010;49:2058–2067. doi: 10.1016/j.freeradbiomed.2010.10.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Schafer FQ. Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 269.Schetter AJ. Heegaard NH. Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Schluterman MK. Chapman SL. Korpanty G. Ozumi K. Fukai T. Yanagisawa H. Brekken RA. Loss of fibulin-5 binding to beta1 integrins inhibits tumor growth by increasing the level of ROS. Dis Model Mech. 2010;3:333–342. doi: 10.1242/dmm.003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 272.Seifried HE. Anderson DE. Fisher EI. Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18:567–579. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 273.Seifried HE. McDonald SS. Anderson DE. Greenwald P. Milner JA. The antioxidant conundrum in cancer. Cancer Res. 2003;63:4295–4298. [PubMed] [Google Scholar]
- 274.Seitz CS. Lin Q. Deng H. Khavari PA. Alterations in NF-kappaB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF-kappaB. Proc Natl Acad Sci U S A. 1998;95:2307–2312. doi: 10.1073/pnas.95.5.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Seo YR. Kelley MR. Smith ML. Selenomethionine regulation of p53 by a ref1-dependent redox mechanism. Proc Natl Acad Sci U S A. 2002;99:14548–14553. doi: 10.1073/pnas.212319799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Shanmugam R. Kusumanchi P. Cheng L. Crooks P. Neelakantan S. Matthews W. Nakshatri H. Sweeney CJ. A water-soluble parthenolide analogue suppresses in vivo prostate cancer growth by targeting NFkappaB and generating reactive oxygen species. Prostate. 2010;70:1074–1086. doi: 10.1002/pros.21141. [DOI] [PubMed] [Google Scholar]
- 277.Shen ZX. Chen GQ. Ni JH. Li XS. Xiong SM. Qiu QY. Zhu J. Tang W. Sun GL. Yang KQ. Chen Y. Zhou L. Fang ZW. Wang YT. Ma J. Zhang P. Zhang TD. Chen SJ. Chen Z. Wang ZY. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 278.Shil P. Sanghvi SH. Vidyasagar PB. Mishra KP. Enhancement of radiation cytotoxicity in murine cancer cells by electroporation: in vitro and in vivo studies. J Environ Pathol Toxicol Oncol. 2005;24:291–298. doi: 10.1615/jenvironpatholtoxicoloncol.v24.i4.60. [DOI] [PubMed] [Google Scholar]
- 279.Shimura K. Shimazaki C. Taniguchi K. Akamatsu S. Okamoto M. Uchida R. Nomura K. Inaba T. Horiike S. Kanamura N. Taniwaki M. Hyperbaric oxygen in addition to antibiotic therapy is effective for bisphosphonate-induced osteonecrosis of the jaw in a patient with multiple myeloma. Int J Hematol. 2006;84:343–345. doi: 10.1532/IJH97.06110. [DOI] [PubMed] [Google Scholar]
- 280.Shishodia S. Chaturvedi MM. Aggarwal BB. Role of curcumin in cancer therapy. Curr Probl Cancer. 2007;31:243–305. doi: 10.1016/j.currproblcancer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 281.Shrivastava A. Kuzontkoski PM. Groopman JE. Prasad A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol Cancer Ther. 2011;10:1161–1172. doi: 10.1158/1535-7163.MCT-10-1100. [DOI] [PubMed] [Google Scholar]
- 282.Shu D. Qing Y. Tong Q. He Y. Xing Z. Zhao Y. Li Y. Wei Y. Huang W. Wu X. Deltonin isolated from Dioscorea zingiberensis inhibits cancer cell growth through inducing mitochondrial apoptosis and suppressing Akt and mitogen activated protein kinase signals. Biol Pharm Bull. 2011;34:1231–1239. doi: 10.1248/bpb.34.1231. [DOI] [PubMed] [Google Scholar]
- 283.Simon HU. Haj-Yehia A. Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 284.Singh I. Mammalian peroxisomes: metabolism of oxygen and reactive oxygen species. Ann N Y Acad Sci. 1996;804:612–627. doi: 10.1111/j.1749-6632.1996.tb18648.x. [DOI] [PubMed] [Google Scholar]
- 285.Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 286.Sternlicht MD. Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Storz P. Reactive oxygen species-mediated mitochondria-to-nucleus signaling: a key to aging and radical-caused diseases. Sci STKE. 2006;2006:re3. doi: 10.1126/stke.3322006re3. [DOI] [PubMed] [Google Scholar]
- 288.Suh YA. Arnold RS. Lassegue B. Shi J. Xu X. Sorescu D. Chung AB. Griendling KK. Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 289.Sullivan R. Graham CH. Chemosensitization of cancer by nitric oxide. Curr Pharm Des. 2008;14:1113–1123. doi: 10.2174/138161208784246225. [DOI] [PubMed] [Google Scholar]
- 290.Sun A. Bae K. Gore EM. Movsas B. Wong SJ. Meyers CA. Bonner JA. Schild SE. Gaspar LE. Bogart JA. Werner-Wasik M. Choy H. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. J Clin Oncol. 2011;29:279–286. doi: 10.1200/JCO.2010.29.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Sundaresan M. Yu ZX. Ferrans VJ. Irani K. Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 292.Sung B. Park B. Yadav VR. Aggarwal BB. Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the down-regulation of cell survival proteins and up-regulation of death receptors. J Biol Chem. 2010;285:11498–11507. doi: 10.1074/jbc.M109.090209. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 293.Sung B. Ravindran J. Prasad S. Pandey MK. Aggarwal BB. Gossypol induces death receptor-5 through activation of the ROS-ERK-CHOP pathway and sensitizes colon cancer cells to TRAIL. J Biol Chem. 2010;285:35418–35427. doi: 10.1074/jbc.M110.172767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 294.Sweeney C. Liu G. Yiannoutsos C. Kolesar J. Horvath D. Staab MJ. Fife K. Armstrong V. Treston A. Sidor C. Wilding G. A phase II multicenter, randomized, double-blind, safety trial assessing the pharmacokinetics, pharmacodynamics, and efficacy of oral 2-methoxyestradiol capsules in hormone-refractory prostate cancer. Clin Cancer Res. 2005;11:6625–6633. doi: 10.1158/1078-0432.CCR-05-0440. [DOI] [PubMed] [Google Scholar]
- 295.Szabo C. Ischiropoulos H. Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 296.Takahashi A. Ohtani N. Yamakoshi K. Iida S. Tahara H. Nakayama K. Nakayama KI. Ide T. Saya H. Hara E. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat Cell Biol. 2006;8:1291–1297. doi: 10.1038/ncb1491. [DOI] [PubMed] [Google Scholar]
- 297.Tiligada E. Chemotherapy: induction of stress responses. Endocr Relat Cancer. 2006;13(Suppl 1):S115–S124. doi: 10.1677/erc.1.01272. [DOI] [PubMed] [Google Scholar]
- 298.Toler SM. Noe D. Sharma A. Selective enhancement of cellular oxidative stress by chloroquine: implications for the treatment of glioblastoma multiforme. Neurosurg Focus. 2006;21:E10. doi: 10.3171/foc.2006.21.6.1. [DOI] [PubMed] [Google Scholar]
- 299.Toyokuni S. Okamoto K. Yodoi J. Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- 300.Trachootham D. Alexandre J. Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 301.Trachootham D. Zhou Y. Zhang H. Demizu Y. Chen Z. Pelicano H. Chiao PJ. Achanta G. Arlinghaus RB. Liu J. Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 302.Trottier G. Bostrom PJ. Lawrentschuk N. Fleshner NE. Nutraceuticals and prostate cancer prevention: a current review. Nat Rev Urol. 2010;7:21–30. doi: 10.1038/nrurol.2009.234. [DOI] [PubMed] [Google Scholar]
- 303.Tsai CW. Lin CY. Lin HH. Chen JH. Carnosic acid, a Rosemary phenolic compound, induces apoptosis through reactive oxygen species-mediated p38 activation in human neuroblastoma IMR-32 cells. Neurochem Res. 2011;36:2442–2451. doi: 10.1007/s11064-011-0573-4. [DOI] [PubMed] [Google Scholar]
- 304.Tuma RS. Reactive oxygen species may have antitumor activity in metastatic melanoma. J Natl Cancer Inst. 2008;100:11–12. doi: 10.1093/jnci/djm299. [DOI] [PubMed] [Google Scholar]
- 305.Uchikura K. Wada T. Hoshino S. Nagakawa Y. Aiko T. Bulkley GB. Klein AS. Sun Z. Lipopolysaccharides induced increases in Fas ligand expression by Kupffer cells via mechanisms dependent on reactive oxygen species. Am J Physiol Gastrointest Liver Physiol. 2004;287:G620–G626. doi: 10.1152/ajpgi.00314.2003. [DOI] [PubMed] [Google Scholar]
- 306.Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res. 2006;71:226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 307.Ushio-Fukai M. Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 308.Ushio-Fukai M. Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008;266:37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Vaidya JS. Joseph DJ. Tobias JS. Bulsara M. Wenz F. Saunders C. Alvarado M. Flyger HL. Massarut S. Eiermann W. Keshtgar M. Dewar J. Kraus-Tiefenbacher U. Sutterlin M. Esserman L. Holtveg HM. Roncadin M. Pigorsch S. Metaxas M. Falzon M. Matthews A. Corica T. Williams NR. Baum M. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376:91–102. doi: 10.1016/S0140-6736(10)60837-9. [DOI] [PubMed] [Google Scholar]
- 310.Valdameri G. Trombetta-Lima M. Worfel PR. Pires AR. Martinez GR. Noleto GR. Cadena SM. Sogayar MC. Winnischofer SM. Rocha ME. Involvement of catalase in the apoptotic mechanism induced by apigenin in HepG2 human hepatoma cells. Chem Biol Interact. 2011;193:180–189. doi: 10.1016/j.cbi.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 311.Valko M. Rhodes CJ. Moncol J. Izakovic M. Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 312.van Hogerlinden M. Rozell BL. Ahrlund-Richter L. Toftgard R. Squamous cell carcinomas and increased apoptosis in skin with inhibited Rel/nuclear factor-kappaB signaling. Cancer Res. 1999;59:3299–3303. [PubMed] [Google Scholar]
- 313.Vaquero EC. Edderkaoui M. Pandol SJ. Gukovsky I. Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279:34643–34654. doi: 10.1074/jbc.M400078200. [DOI] [PubMed] [Google Scholar]
- 314.Veal EA. Day AM. Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 315.Venkataraman S. Jiang X. Weydert C. Zhang Y. Zhang HJ. Goswami PC. Ritchie JM. Oberley LW. Buettner GR. Manganese superoxide dismutase overexpression inhibits the growth of androgen-independent prostate cancer cells. Oncogene. 2005;24:77–89. doi: 10.1038/sj.onc.1208145. [DOI] [PubMed] [Google Scholar]
- 316.Voorzanger N. Touitou R. Garcia E. Delecluse HJ. Rousset F. Joab I. Favrot MC. Blay JY. Interleukin (IL)-10 and IL-6 are produced in vivo by non-Hodgkin's lymphoma cells and act as cooperative growth factors. Cancer Res. 1996;56:5499–5505. [PubMed] [Google Scholar]
- 317.Wang H. Ma J. Tan Y. Wang Z. Sheng C. Chen S. Ding J. Amyloid-beta1-42 induces reactive oxygen species-mediated autophagic cell death in U87 and SH-SY5Y cells. J Alzheimers Dis. 2010;21:597–610. doi: 10.3233/JAD-2010-091207. [DOI] [PubMed] [Google Scholar]
- 318.Wang J. Yi J. Cancer cell killing via ROS: to increase or decrease, that is the question. Cancer Biol Ther. 2008;7:1875–1884. doi: 10.4161/cbt.7.12.7067. [DOI] [PubMed] [Google Scholar]
- 319.Wang JC. Good cells gone bad: the cellular origins of cancer. Trends Mol Med. 2010;16:145–151. doi: 10.1016/j.molmed.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 320.Wang W. Sun Y. Li X. Li H. Chen Y. Tian Y. Yi J. Wang J. Emodin potentiates the anticancer effect of cisplatin on gallbladder cancer cells through the generation of reactive oxygen species and the inhibition of survivin expression. Oncol Rep. 2011;26:1143–1148. doi: 10.3892/or.2011.1390. [DOI] [PubMed] [Google Scholar]
- 321.Wang X. Son YO. Chang Q. Sun L. Hitron JA. Budhraja A. Zhang Z. Ke Z. Chen F. Luo J. Shi X. NADPH oxidase activation is required in reactive oxygen species generation and cell transformation induced by hexavalent chromium. Toxicol Sci. 2011;123:399–410. doi: 10.1093/toxsci/kfr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Wang X. Zhang J. Xu T. Cyclophosphamide as a potent inhibitor of tumor thioredoxin reductase in vivo. Toxicol Appl Pharmacol. 2007;218:88–95. doi: 10.1016/j.taap.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 323.Wang Y. The role and regulation of urokinase-type plasminogen activator receptor gene expression in cancer invasion and metastasis. Med Res Rev. 2001;21:146–170. doi: 10.1002/1098-1128(200103)21:2<146::aid-med1004>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 324.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 325.Weber JS. Samlowski WE. Gonzalez R. Ribas A. Stephenson J. O'Day S. Sato T. Dorr R. Grenier K. Hersh E. A phase 1–2 study of imexon plus dacarbazine in patients with unresectable metastatic melanoma. Cancer. 2010;116:3683–3691. doi: 10.1002/cncr.25119. [DOI] [PubMed] [Google Scholar]
- 326.Weitzman SA. Gordon LI. Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood. 1990;76:655–663. [PubMed] [Google Scholar]
- 327.Wen DS. Zhu XL. Guan SM. Wu YM. Yu LL. Wu JZ. Silencing of CXCR4 inhibits the proliferation, adhesion, chemotaxis and invasion of salivary gland mucoepidermoid carcinoma Mc3 cells in vitro. Oral Oncol. 2008;44:545–554. doi: 10.1016/j.oraloncology.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 328.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 329.Wochna A. Niemczyk E. Kurono C. Masaoka M. Kedzior J. Slominska E. Lipinski M. Wakabayashi T. A possible role of oxidative stress in the switch mechanism of the cell death mode from apoptosis to necrosis—studies on rho0 cells. Mitochondrion. 2007;7:119–124. doi: 10.1016/j.mito.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 330.Wondrak GT. Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxid Redox Signal. 2009;11:3013–3069. doi: 10.1089/ars.2009.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Wu CL. Huang AC. Yang JS. Liao CL. Lu HF. Chou ST. Ma CY. Hsia TC. Ko YC. Chung JG. Benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC)-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of caspase-3, mitochondria dysfunction and nitric oxide (NO) in human osteogenic sarcoma U-2 OS cells. J Orthop Res. 2011;29:1199–1209. doi: 10.1002/jor.21350. [DOI] [PubMed] [Google Scholar]
- 332.Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 333.Wu X. Zhu Y. Yan H. Liu B. Li Y. Zhou Q. Xu K. Isothiocyanates induce oxidative stress and suppress the metastasis potential of human non-small cell lung cancer cells. BMC Cancer. 2010;10:269. doi: 10.1186/1471-2407-10-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Wu XJ. Hua X. Targeting ROS: selective killing of cancer cells by a cruciferous vegetable derived pro-oxidant compound. Cancer Biol Ther. 2007;6:646–647. doi: 10.4161/cbt.6.5.4092. [DOI] [PubMed] [Google Scholar]
- 335.Xia C. Meng Q. Liu LZ. Rojanasakul Y. Wang XR. Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 336.Xie CM. Chan WY. Yu S. Zhao J. Cheng CH. Bufalin induces autophagy-mediated cell death in human colon cancer cells through reactive oxygen species generation and JNK activation. Free Radic Biol Med. 2011;51:1365–1375. doi: 10.1016/j.freeradbiomed.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 337.Xu L. Fidler IJ. Acidic pH-induced elevation in interleukin 8 expression by human ovarian carcinoma cells. Cancer Res. 2000;60:4610–4616. [PubMed] [Google Scholar]
- 338.Yan B. Peng Y. Li CY. Molecular analysis of genetic instability caused by chronic inflammation. Methods Mol Biol. 2009;512:15–28. doi: 10.1007/978-1-60327-530-9_2. [DOI] [PubMed] [Google Scholar]
- 339.Yang C. Jo SH. Csernus B. Hyjek E. Liu Y. Chadburn A. Wang YL. Activation of peroxisome proliferator-activated receptor gamma contributes to the survival of T lymphoma cells by affecting cellular metabolism. Am J Pathol. 2007;170:722–732. doi: 10.2353/ajpath.2007.060651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Yang ES. Choi MJ. Kim JH. Choi KS. Kwon TK. Withaferin A enhances radiation-induced apoptosis in Caki cells through induction of reactive oxygen species, Bcl-2 downregulation and Akt inhibition. Chem Biol Interact. 2011;190:9–15. doi: 10.1016/j.cbi.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 341.Yang S. Evens AM. Prachand S. Singh AT. Bhalla S. David K. Gordon LI. Mitochondrial-mediated apoptosis in lymphoma cells by the diterpenoid lactone andrographolide, the active component of Andrographis paniculata. Clin Cancer Res. 2010;16:4755–4768. doi: 10.1158/1078-0432.CCR-10-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Yang S. Misner BJ. Chiu RJ. Meyskens FL., Jr. Redox effector factor-1, combined with reactive oxygen species, plays an important role in the transformation of JB6 cells. Carcinogenesis. 2007;28:2382–2390. doi: 10.1093/carcin/bgm128. [DOI] [PubMed] [Google Scholar]
- 343.Yang WH. Fong YC. Lee CY. Jin TR. Tzen JT. Li TM. Tang CH. Epigallocatechin-3-gallate induces cell apoptosis of human chondrosarcoma cells through apoptosis signal-regulating kinase 1 pathway. J Cell Biochem. 2011;112:1601–1611. doi: 10.1002/jcb.23072. [DOI] [PubMed] [Google Scholar]
- 344.Yasuda M. Ohzeki Y. Shimizu S. Naito S. Ohtsuru A. Yamamoto T. Kuroiwa Y. Stimulation of in vitro angiogenesis by hydrogen peroxide and the relation with ETS-1 in endothelial cells. Life Sci. 1999;64:249–258. doi: 10.1016/s0024-3205(98)00560-8. [DOI] [PubMed] [Google Scholar]
- 345.Yuecheng Y. Xiaoyan X. Stromal-cell derived factor-1 regulates epithelial ovarian cancer cell invasion by activating matrix metalloproteinase-9 and matrix metalloproteinase-2. Eur J Cancer Prev. 2007;16:430–435. doi: 10.1097/01.cej.0000236259.88146.a4. [DOI] [PubMed] [Google Scholar]
- 346.Yuk JM. Shin DM. Song KS. Lim K. Kim KH. Lee SH. Kim JM. Lee JS. Paik TH. Kim JS. Jo EK. Bacillus calmette-guerin cell wall cytoskeleton enhances colon cancer radiosensitivity through autophagy. Autophagy. 2010;6:46–60. doi: 10.4161/auto.6.1.10325. [DOI] [PubMed] [Google Scholar]