Abstract
Exposure to toluene diisocyanate (TDI), an industrially important crosslinking agent used in the production of polyurethane products, can cause asthma in sensitive workers. Albumin has been identified as a major reaction target for TDI in vivo, and TDI–albumin reaction products have been proposed to serve as exposure biomarkers and to act as asthmagens, yet they remain incompletely characterized. In the current study, we used a multiplexed tandem mass spectrometry (MS/MS) approach to identify the sites of albumin conjugation by TDI vapors, modeling the air/liquid interface of the lung. Vapor phase TDI was found to react with human albumin in a dose-dependent manner, with up to 18 potential sites of conjugation, the most susceptible being Lys351 and the dilysine site Lys413–414. Sites of vapor TDI conjugation to albumin were quantitatively limited compared with those recently described for liquid phase TDI, especially in domains IIA and IIIB of albumin. We hypothesize that the orientation of albumin at the air/liquid interface plays an important role in vapor TDI conjugation and, thus, could influence biological responses to exposure and the development of in vitro assays for exposure and immune sensitivity.
Keywords: Toluene diisocyanate, Human serum albumin, Tandem mass spectrometry, Allergy
Diisocyanates are highly reactive electrophilic compounds that are industrially useful as crosslinking agents in polyurethane production for diverse products such as flexible and rigid foams, fibers, paints, and varnishes. Global diisocyanate production is dominated by two aromatic diisocyanates, toluene diisocyanate (TDI)1 and methylene diphenyl diisocyanate (MDI), which together account for more than 90% of the diisocyanate market [1]. Exposure to TDI and other diisocyanates is associated with adverse health effects, including asthma, contact dermatitis, and hypersensitivity pneumonitis [2]. Worldwide, diisocyanates are the most commonly reported cause of occupational asthma, with an estimated 5% to 30% of exposed workers at risk for developing disease [2–5].
The mechanistic connections between diisocyanate exposure and health outcomes remain unclear, in large part due to uncertainty regarding diisocyanate reactivity in vivo. The self-protein albumin has been identified as a major reaction target for inhaled diisocyanate, and diisocyanate–albumin reaction products (e.g., conjugates), which accumulate in the circulating blood, may serve as biomarkers of exposure [6–9]. In some workers, diisocyanate–albumin-specific immunoglobulin E (IgE) can be measured in sera and may participate directly in asthma pathogenesis [10,11]. Thus, a better understanding of diisocyanate–albumin reactivity is central to understanding exposure outcomes and to the development of assays for exposure monitoring and disease surveillance.
Human albumin possesses numerous functional groups that could potentially react with diisocyanate; however, diisocyanate–albumin conjugates that form in vivo in exposed workers remain largely uncharacterized due to technical limitations. Studies of diisocyanate–albumin reactivity to date have relied primarily on in vitro modeling and reveal a marked influence of exposure conditions on the conformation and antigenicity of the resulting diisocyanate–albumin reaction products [12,13]. For example, high concentrations of diisocyanate, relative to albumin, result in excessive amounts of diisocyanate conjugation, protein precipitation, and lack of specific antibody recognition [12–18]. Data to date suggest that under occupational exposure conditions (e.g., low diisocyanate concentrations), individual albumin molecules undergo limited conjugation.
The majority of in vitro studies on diisocyanate–albumin reactivity have been performed with liquid phase chemical; however, for volatile diisocyanates such as TDI, the airway microenvironment is exposed to vapor rather than liquid phase chemical [19]. TDI–albumin conjugates that form under such mixed (vapor/liquid) phase exposure conditions differ structurally and conformationally from those formed in liquid phase and have been hypothesized to more closely reflect those that form in vivo, based on immune recognition by IgE from diisocyanate asthma patients [16].
Tandem mass spectrometry (MS/MS) is well-suited to the structural analysis of modified peptides and proteins [20]. Accurate mass measurement of fragment ions can discriminate between iso-mass peptides produced by the enzymatic cleavage of large proteins [20,21]. The current study undertakes a comprehensive MS/MS approach [14,22,23] to unambiguously map the sites on human albumin conjugated by vapor phase TDI. A mixed (vapor/liquid) phase in vitro exposure system, in which vapor dose was titrated by varying the duration of exposure, was used to identify those sites on albumin most susceptible to vapor phase TDI conjugation. Preferential sites of vapor TDI conjugation were compared with those recently identified for liquid TDI and highlight the influence of exposure biophysics on TDI–albumin reactivity [14]. The data are discussed in the context of disease pathogenesis and the development of assays for exposure monitoring and disease surveillance.
Materials and methods
Preparation of vapor TDI–albumin conjugates
Vapor TDI–albumin conjugates were prepared by using a previously described isocyanate vapor phase exposure system [17]. In brief, TDI vapor concentrations in the range of 0.14 to 1.4 mg/m3 (~1–10 μmol/m3) were passively generated inside an exposure chamber monitored with an Autostep monitor (GMD, Pittsburgh, PA, USA). TDI was an 80:20 mixture of 2,4- and 2,6-TDI isomers obtained from Aldrich (St. Louis, MO, USA). Low endotoxin human albumin in phosphate-buffered saline (PBS, pH 7.2) at a concentration of 5 mg/ml (73 nmol/ml) was exposed in open 60-mm Petri dishes (Becton Dickinson, Franklin Lakes, NJ, USA) for 0 min (control), 20 min, 1 h, 4 h, and 24 h. The exposure unit was cleaned with 70% ethanol, and protein solutions were filtered (0.2 μm) before and after exposure.
Quantitation of dissolved TDI after vapor exposures
TDI from 0-min (control), 20-min, 1-h, 4-h, and 24-h exposures was trapped in open 60-mm Petri dishes containing 0.5% H2SO4. Reaction of TDI with dilute sulfuric acid results in rapid hydrolysis of TDI to toluene diamine (TDA), which is stable in solution. Aliquots (1 ml) of the TDA-containing trap solution were selected and buffered back to alkaline pH by adding 1 ml of saturated sodium borate. TDA was subsequently derivatized by adding 50 μl of 1 mg/ml fluorescamine (Fisher Scientific, Pittsburgh, PA, USA) in acetonitrile. Samples were quantified by fluorescence spectroscopy using an LS50B luminescence spectrometer (PerkinElmer, Waltham, MA, USA) controlled by FL WinLab software (version 4.00.02, PerkinElmer) using excitation at 410 nm and observing the emission at 510 nm. A calibration curve was generated using 2,4-TDA (Sigma–Aldrich, St. Louis, MO, USA).
Native gel and anti-TDI Western blot
TDI conjugation to human albumin was detected in native gels based on characteristic changes in electrophoretic mobility, as described previously [17,24]. For native protein analysis, samples were prepared in a 10% glycerol running buffer and then electrophoresed on 10% polyacrylamide gels and stained with Imperial protein stain (Pierce, Rockford, IL, USA). For Western blot analysis, samples were electrophoresed under reducing conditions (for optimal anti-TDI monoclonal antibody [mAb] binding) on precast 4% to 15% gradient gels and transferred to nitrocellulose using an aqueous trans-blot system (Bio-Rad, Hercules, CA, USA). Nitrocellulose membranes were blocked with 3% dry milk in PBS, probed with 1 μg/ml of the anti-TDI mAb 60G2 [25] followed by anti-mouse IgG1 (Pharmingen, San Diego, CA, USA), and developed with ECL reagent (Thermo Fisher Scientific, Rochester, NY, USA).
Trypsin digestion
Aliquots (100 μl) of each conjugate and control were taken for analysis. Disulfide bonds were reduced by reaction with tributylphosphine (5 mM) for 30 min at room temperature, followed by alkylation with iodoacetamide (15 mM) for 1 h at room temperature. Alkylation was quenched by further addition of tributylphosphine (5 mM) for 15 min at room temperature. Samples were twice dialyzed against 3 L of 25 mM NH4HCO3 using 3500-MWCO (molecular weight cutoff) mini dialysis units (Slide-A-Lyzer, Thermo Scientific, Waltham, MA, USA). Porcine trypsin was suspended in 25 mM NH4HCO3 and added to each aliquot at a 40:1 (protein/trypsin) ratio. Samples were incubated overnight at 37 °C with shaking (300 rpm). Samples were centrifuged at 14,100g in a microcentrifuge (MiniSpin, Eppendorf, Hamburg, Germany) to pellet any insoluble material.
Ultra-performance liquid chromatography
Enzymatic peptides were separated on a Waters (Milford, MA, USA) nanoACQUITY ultra-performance liquid chromatography (UPLC) system. Aliquots (1 μl) of the digest mixture were injected and trapped/desalted on a 5-μm Symmetry C18 trapping column (180 μm × 20 mm) with 99.5:0.5 A/B (A: 0.1% formic acid; B: 0.1% formic acid in acetonitrile) at a flow rate of 15 μl/min for 1 min. Separation was performed on a 1.7-μm BEH130 C18 analytical column (100 μm × 100 mm) using gradient elution at a flow rate of 400 nl/min and a gradient of 99:1 to 60:40 A/B over 60 min.
Tandem mass spectrometry
The eluent from the UPLC system was directed to the nanoelectrospray source of a Waters SYNAPT MS quadrupole time-of-flight (qTOF) mass spectrometer. Positive ion nanoelectrospray was performed using 10-μm PicoTip (Waters) emitters held at a potential of +3.5 kV. The cone voltage was held constant at +40 V for all experiments. Dry N2 desolvation gas was supplied to the instrument via a nitrogen generator (NitroFlowLab, Parker Hannifin, Haverhill, MA, USA). [Glu1]-Fibrinopeptide B (100 fmol/μl in 75:25 A/B) was supplied to an orthogonal reference probe, and the [M+2H]2+ ion (m/z = 785.84265 u) was measured as an external calibrant at 30-s intervals. Collision-induced dissociation (CID) was performed using ultra-high-purity (UHP) argon as collision gas. Spectra were acquired in an “MSe” fashion [22]. Briefly, alternating 1-s mass spectra are acquired. The first spectrum acquired at low (6 eV) collision energy allows high mass accuracy precursor ion mass measurement. The second spectrum acquired at high (15–30 eV ramp) collision energy allows high mass accuracy fragment ion mass measurement. The fragment ion spectra may be temporally correlated with precursor spectra postrun. This method of data acquisition allows all precursor ions to be fragmented and analyzed, as opposed to so-called “data-dependent acquisition” methods that require making real-time decisions on which ions to select for fragmentation, which may miss low-abundance precursor ions.
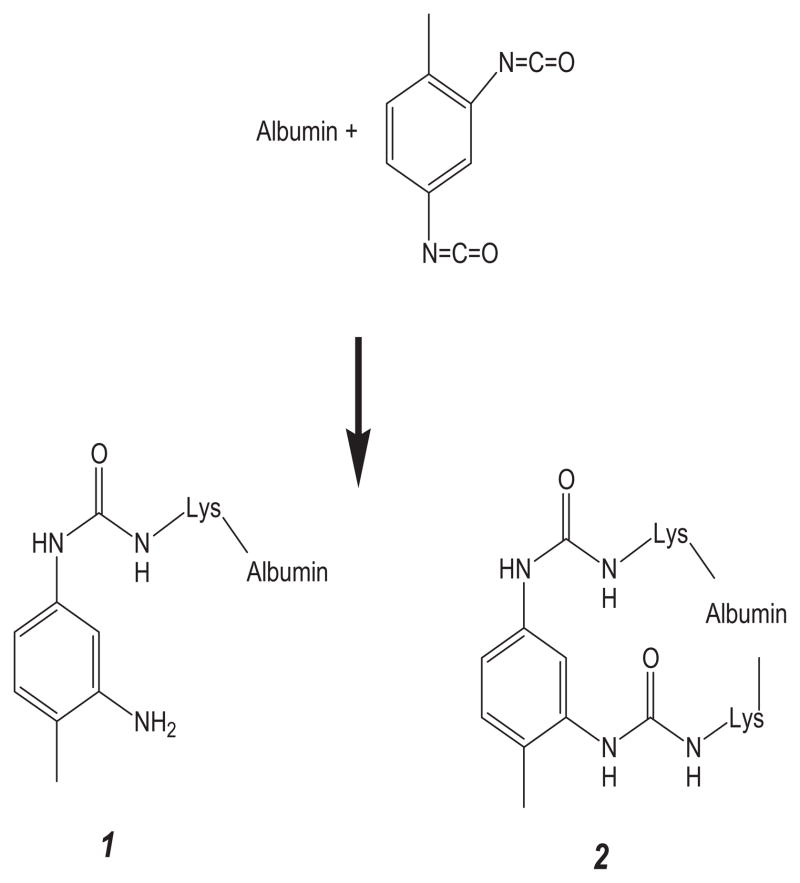
Data analysis
Data were analyzed with BioPharmaLynx (version 1.2, Waters), a software program for analysis of peptide mass maps and identification of sites of modification on known protein sequences. Default peptide mass map analysis criteria of 30 ppm mass error in both low and high collision energy mode were specified. Trypsin was specified as the digestion enzyme, and two missed cleavages were allowed. The submitted protein sequence was taken from P02768, “serum albumin precursor, homo sapiens” (http://www.uniprot.org/uniprot/P02768), and the signal and propeptides (residues 1–24) were removed. Custom modifiers were created for two bound forms of TDI (see Fig. 1). The first (TDI*, C8H8N2O, m/z = 148.0637 u) represents one isocyanate moiety bound to a peptide via a urea bond, whereas the second isocyanate moiety is hydrolyzed to the primary amine. The second (TDI, C9H6N2O2, m/z = 174.0429 u) represents TDI with both isocyanate moieties bound to a peptide via urea bonds. Identification of a potential TDI conjugation site proceeded via a rigorous procedure that involved the following steps. First, a potential peptide–TDI conjugation product with less than 30 ppm m/Δm mass error in the analyte peptide mass map is observed. Second, comparison of analyte and control peptide mass map from unmodified human serum albumin shows that observed m/z and chromatographic retention time are unique to analyte. Third, MS/MS data contain bn- and yn-type ions consistent with the assigned sequence and modifier.
Fig. 1.
Reaction of TDI with albumin. Hydrolysis of one isocyanate moiety to the amine results in adduct 1 (+148.0637 u) is shown. Reaction of both isocyanate moieties results in the intramolecular crosslinked adduct 2 (+174.0429 u).
Results and discussion
Samples of human serum albumin were exposed to increasing doses of TDI vapors, for example, exposure for 0 min, 20 min, 1 h, 4 h, and 24 h. Quantification of TDI exposures by fluorescence spectrometry indicate that these vapor exposures result in diffusion of 0, 0.9, 4.6, 22.2, and 314.6 μg of TDI/ml into the liquid phases, respectively. These exposures correspond to approximate exposure (TDI/albumin) mol ratios of 0, 1:15, 1:3, 1.6:1, and 24:1, respectively. Exposed albumin samples display dose-dependent changes in electrophoretic mobility, consistent with TDI conjugation, as shown in Fig. 2A. The increased migration under native conditions reflects changes in charge and/or conformation of the protein and has been previously observed for diisocyanate-conjugated protein [17,24]. Dose-dependent conjugation of human albumin by TDI vapor was further validated by Western blot with the anti-TDI mAb 60G2 (Fig. 2B).
Fig. 2.
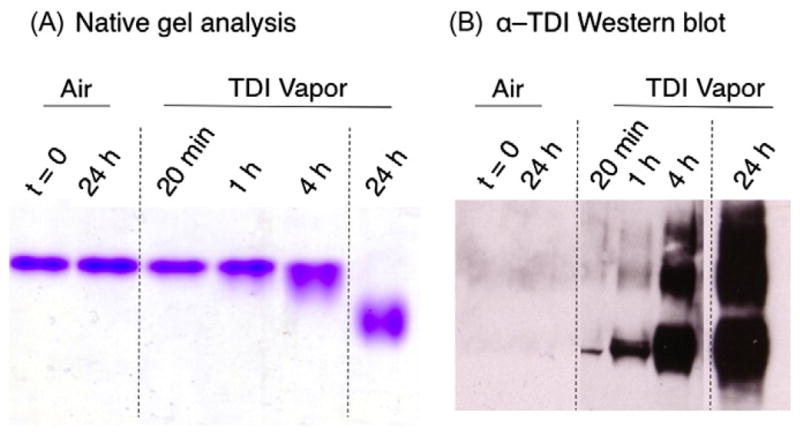
(A) Native gel analysis of TDI–albumin conjugates. Albumin was exposed to TDI vapors or room air for varying time periods as labeled and was stained for protein. (B) Western blot parallel gels were blotted with anti-TDI mAb 60G2.
A comprehensive map of TDI vapor conjugation sites on human albumin was obtained via UPLC–MS/MS analysis of trypsin-digested samples (presented in Fig. 3). The chemistry of vapor TDI conjugation to human serum albumin is similar to that described previously for liquid phase TDI and MDI (Fig. 1) [14,23,24]. TDI is observed to conjugate serum albumin in one of two forms. The first (TDI*, C8H8N2O, m/z = 148.0637 u) results from hydrolysis of one isocyanate to a primary amine, whereas the second isocyanate moiety undergoes nucleophilic addition to the protein. The second (TDI, C9H6N2O2, m/z = 174.0429 u) is the result of both isocyanates undergoing nucleophilic addition to the protein, resulting in an intramolecular crosslinked species. This is often observed when two lysines are located in close proximity, such as is the case for the four dilysine motifs in human albumin. It should be noted that intermolecular crosslinking of two protein molecules via one TDI (e.g., [2 M+TDI+H]+) is possible and is observed in limited amounts. In addition, polymerization of TDI on one protein site (e.g., [M+poly-TDI*+H]+) is also possible. Although we do not discount the possibility that crosslinked or poly-TDI species play a role in the human immune response to TDI in vivo, an in-depth analysis of the thousands of products theoretically formed by such conjugation is beyond the scope of this article.
Fig. 3.
Map of vapor/liquid TDI–albumin conjugation sites. All observed sites are underlined. Favored conjugation sites observed on 20 min of exposure are in bold. UPLC–qTOF MSe analysis resulted in 100% sequence coverage of the protein.
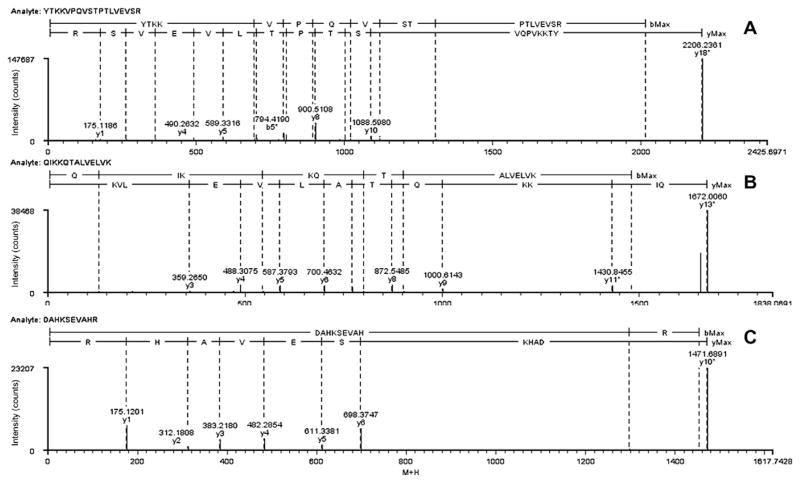
The specific amino acids of human albumin that were conjugated by TDI vapor were identified through CID–MS/MS. Representative fragment ion spectra are presented for serum albumin tryptic fragments 411 to 428 (YTKKVPQVSTPTLVEVSR), 522 to 534 (QIKKQTALVELVK), and 1 to 10 (DAHKSEVAHR) in Figs. 4A, 4B, and 4C, respectively. Peptide fragments are labeled according to a modified Roepstorff–Fohlman nomenclature [26] in which fragment ions containing TDI or the hydrolyzed amine are notated with an asterisk (e.g., bn*, yn*). Both Figs. 4A and 4B represent fragment ion spectra from species of the form [M+TDI+H]+ or intramolecular crosslinked species. In both spectra, TDI reacts with ε-amines on the side chain of each of the dilysine motifs (Lys413–Lys414 and Lys524–Lys525). In Fig. 4A, bn-type ions b4*, b5*, b6*, b7*, b8*, and b10* are observed, each increased in mass by 174.04 u over the theoretical unmodified bn ion. Observation of a b3 or b3* ion would require two bonds to be broken (the peptide bond between lysine residues and one of the urea bonds formed by TDI), a high energy fragmentation channel that is unlikely to be significantly populated (although a very small amount of the analogous b3* ion from fragment 522–524 is observed; see Fig. 4B). Unmodified y1 to y10 ions indicate that TDI is not bound at the C-terminal end of the peptide. TDI can be unambiguously assigned to Lys524 and Lys525 based on the fragment ion spectrum in Fig. 4B given observation of b1, b3*, b5*, and b6* ions. C-terminal ions confirm conjugation of TDI to Lys524 and Lys525 by observation of unmodified y3 through y9 and y11*, coupled with the absence of a y10 or y10* ion. Fig. 4C presents the fragment ion spectrum of the fragment 1 to 10 [M+TDI*+TDI+H]+ ion, which is formed by an intramolecular poly-TDI crosslink between the α-NH2 of the N-terminal Asp1 and side chain ε-NH2 of Lys4. The mass of this peptide is observed to be 322.10 u higher than that of the unmodified peptide. A series of unmodified y1 to y6 ions indicate that neither TDI nor TDI* is bound on the C-terminal residues SEVAHR. This Asp–TDI–TDI*–Lys linkage to the DAHK sequence results in a cyclic peptide; therefore, observation of y7* to y9* ions would require breaking two bonds. No confirmatory bn-type ions are observed in this spectrum because of the higher proton affinity of the C-terminal arginine.
Fig. 4.
MS/MS fragment ion spectra of human serum albumin fragment 411 to 428 (YTKKVPQVSTPTLVEVSR) [M+TDI+H]+ (A), human serum albumin fragment 522 to 534 (QIKKQTALVELVK) [M+TDI+H]+ (B), and human serum albumin fragment 1 to 10 (DAHKSEVAHR) [M+TDI*+TDI+H]+ (C) formed by vapor/liquid TDI exposure.
Table 1 presents the residues of human serum albumin observed to react with TDI as a function of exposure time. Conjugation proceeds in a dose-dependent manner, with more conjugation sites identified as TDI exposure time increases. Three TDI conjugation sites (Lys351, Lys413, and Lys414) were observed for the shortest exposure (20 min) and, therefore, the lowest TDI dose (0.07:1 mol TDI/mol human serum albumin), suggesting that these sites are the favored loci of conjugation for TDI–albumin on vapor/liquid exposure. Lys413 and Lys414 make up one of four dilysine motifs in human serum albumin and have been previously identified as being particularly susceptible to nucleophilic addition with both TDI [14] and MDI [23,24] in liquid/liquid exposures. Interestingly, although Lys351 has been previously identified as a conjugation site for TDI and MDI, liquid/liquid titration of albumin demonstrated that conjugation to Lys351 was not observed until TDI/albumin molar ratios in excess of 10:1 were reached [14]. Similarly, Lys199 is a preferred conjugation site in liquid/liquid exposures (conjugate observed at 1:1 ratio), but this conjugate is not observed in vapor/liquid until a ratio of 24:1 is reached.
Table 1.
Comparison of TDI–albumin conjugation sites for vapor/liquid and liquid/liquid exposures.
| Residue | Vapor/liquid
|
Liquid/liquida |
Domain | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 min(0.07:1) | 1 h(0.35:1) | 4 h(1.6:1) | 24 h(24:1) | 1:1 | 5:1 | 10:1 | 40:1 | ||
| Asp1 | X | X | X | X | X | X | IA | ||
| Lys4 | X | X | X | X | X | X | IA | ||
| Lys12 | X | X | X | X | IA | ||||
| Lys73 | X | X | X | IA | |||||
| Gln104 | X | IA | |||||||
| Lys106 | X | X | IA | ||||||
| Lys136 | X | IB | |||||||
| Lys137 | X | X | X | X | IB | ||||
| Lys159 | X | X | X | IB | |||||
| Lys190 | X | X | X | IB | |||||
| Gln196 | X | X | IIA | ||||||
| Lys199 | X | X | X | X | X | IIA | |||
| Lys205 | X | X | IIA | ||||||
| Lys212 | X | X | IIA | ||||||
| Lys262 | X | IIA | |||||||
| Lys274 | X | X | X | IIA | |||||
| Lys276 | X | X | IIA | ||||||
| Lys281 | X | X | IIA | ||||||
| Lys351 | X | X | X | X | X | X | IIB | ||
| Lys378 | X | X | X | X | IIB | ||||
| Lys402 | X | IIIA | |||||||
| Lys413 | X | X | X | X | X | X | X | X | IIIA |
| Lys414 | X | X | X | X | X | X | X | X | IIIA |
| Lys432 | X | X | X | X | IIIA | ||||
| Lys436 | X | X | X | IIIA | |||||
| Lys439 | X | X | IIIA | ||||||
| Lys444 | X | X | X | X | X | IIIA | |||
| Lys524 | X | X | X | X | X | X | X | IIIB | |
| Lys525 | X | X | X | X | X | X | X | IIIB | |
| Lys534 | X | X | X | IIIB | |||||
| Lys536 | X | X | X | IIIB | |||||
| Lys541 | X | X | X | IIIB | |||||
| Lys545 | X | X | X | IIIB | |||||
| Lys557 | X | X | IIIB | ||||||
| Lys560 | X | X | IIIB | ||||||
| Lys573 | X | X | X | X | IIIB | ||||
| Lys574 | X | X | X | X | IIIB | ||||
TDI–albumin conjugation sites determined for liquid/liquid exposure are from Hettick and Siegel [14].
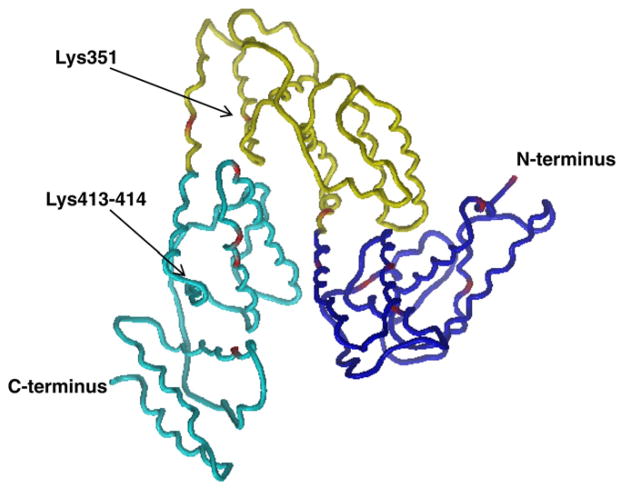
Sites of TDI–albumin conjugation observed for vapor/liquid exposures consist of a subset of those identified previously for liquid/liquid conjugations [14]. Liquid/liquid exposures resulted in conjugation at 37 sites on serum albumin, distributed approximately equally among all domains and subdomains. Vapor/liquid conjugations, however, result in conjugations primarily to domains I and IIIa, with only 3 reactive sites in domain II compared with 10 observed for liquid/liquid exposures. Similarly, only 2 of 10 liquid/liquid conjugation sites in domain IIIB are observed for vapor/liquid conjugation (see Fig. 5).
Fig. 5.
Structure of human serum albumin. Domain I (blue), domain II (yellow), and domain III (cyan) are shown. TDI conjugation sites are identified in red. Chain starts at Lys4; therefore, Asp1 is not shown. (From He and Carter [30].)
Although significant differences between vapor/liquid and liquid/liquid exposures are observed, it should be noted that differences in the experimental methodology exist between the studies. First, liquid/liquid TDI experiments were performed with 2,4- and 2,6-TDI independently, whereas this study used an 80:20 mixture of the two isomers, similar to that used in industrial applications. Although the liquid/liquid study noted no difference in the conjugation specificity of the two isomers, 2,4-TDI did result in ion abundances approximately 2-fold higher than those with 2,6-TDI. Second, the liquid/liquid exposure study used a measured volume of TDI introduced via pipette to a stirred bulk solution, whereas the current study relied on diffusion of the TDI vapors into a nonstirred albumin-containing solution. It can be reasonably suggested, therefore, that the liquid/liquid study results represent a complete list of the 37 sites on the serum albumin protein reactive to TDI at pH 7.4. Because the kinetics of the reaction of TDI with the serum albumin protein are fast relative to the rate of diffusion into the bulk solution, the current pH 7.4 vapor/liquid results suggest that those serum albumin protein molecules nearest the air/liquid interface are oriented in such a way as to present only a subset (18 of 37) of reactive sites. Ultimately, the bound location(s) of TDI to albumin in vapor/liquid and liquid/liquid can be compared over a range of TDI/albumin ratios. Because TDI, once conjugated to albumin, results in a stable, covalently bonded species, TDI–albumin conjugates produced at the lowest exposures, whether from vapor/liquid or liquid/liquid exposures, can be compared.
The TDI conjugation sites identified for vapor/liquid exposures are presented on the ribbon model of human serum albumin in Fig. 5. Reactive lysine residues are generally located on α-helices, which in albumin have a hydrophobic and a hydrophilic side. These α-helices then self-orient on the basis of hydrophobic interactions into the overall three-dimensional arrangement of domains and subdomains. Orientation of proteins at air/liquid surfaces due to hydrophilic/phobic interactions can cause changes in orientation and conformation. Lin and coworkers [27] suggested that proteins spontaneously adsorb from aqueous solution to the air/water interface due to the energetically favorable dehydration of hydrophobic regions of the protein surface. Such behavior can be strongly influenced by the protein concentration, secondary structure of the protein, and solution chemistry. Furthermore, Kudryashova and coworkers determined that although a bulk solution of egg white ovalbumin was not aggregated in the bulk solution, the protein showed anisotropic motion at the surface, indicating a preferential orientation of the protein in at the interface [28]. We hypothesize that the observed differences in TDI conjugation sites between liquid/liquid and vapor/liquid models are due to a more ordered orientation of albumin at the surface than is present in bulk solution. Dockal and coworkers [29] studied the three-dimensional structure of the recombinant domains of human serum albumin and determined, on the basis of ultraviolet circular dichroism spectroscopy, that domain II is significantly more hydrophobic (37% α-helix content) than either domain I or III (46 or 53% α-helix content, respectively). We hypothesize that in this vapor/liquid model system, significant portions of serum albumin domains II and IIIb are blocked from TDI conjugation, most likely by protein aggregation/hydrophobic interactions.
In conclusion, we have used UPLC–MS/MS to determine the conjugation sites of TDI on albumin from vapor/liquid exposures. This exposure may more closely mimic the biophysics of exposure in the lung and shows increased propensity for TDI conjugation to domains I and IIIa of serum albumin than do previous studies using liquid/liquid exposures. Because the orientation of proteins at the air/water interface is strongly influenced by solution concentration and composition, experiments designed to more accurately model the airway, including components such as lung surfactant and glutathione, and ultimately in vivo exposures will be critical to identifying the ultimate bioactive form(s) of isocyanate/protein conjugates formed from airway exposures.
Supplementary Material
Acknowledgments
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institute for Occupational Safety and Health. The work was supported by funding from the National Institute of Environmental Health Sciences (NIEHS, R42ES018021 and R42ES016728) to A.V.W.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ab.2011.12.013.
Footnotes
Abbreviations used: TDI, toluene diisocyanate; MDI, methylene diphenyl diisocyanate; Ig, immunoglobulin; MS/MS, tandem mass spectrometry; PBS, phosphate-buffered saline; TDA, toluene diamine; mAb, monoclonal antibody; UPLC, ultra-performance liquid chromatography; qTOF, quadrupole time-of-flight; CID, collision-induced dissociation.
References
- 1.Allport DC, Gilbert DS, Outterside SM. MDI, TDI, and the polyurethane industry. In: Allport DC, Gilbert DS, Outterside SM, editors. MDI and TDI: Safety, Health, and the Environment. John Wiley; Chichester, UK: 2003. pp. 11–23. [Google Scholar]
- 2.National Institute for Occupational Safety and Health. A Summary of Health Hazard Evaluations: Issues Related to Occupational Exposure to Isocyanates, 1989–2002. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati, OH: 2004. [Google Scholar]
- 3.Porter CV, Higgins RL, Scheel LD. A retrospective study of clinical, physiologic, and immunologic changes in workers exposed to toluene diisocyanate. Am Ind Hyg Assoc J. 1975;36:159–168. doi: 10.1080/0002889758507230. [DOI] [PubMed] [Google Scholar]
- 4.Adams WG. Long-term effects on the health of men engaged in the manufacture of tolylene diisocyanate. Br J Ind Med. 1975;32:72–78. doi: 10.1136/oem.32.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White WG, Morris MJ, Sugden E, Zapata E. Isocyanate-induced asthma in a car factory. Lancet. 1980;1:756–760. doi: 10.1016/s0140-6736(80)91247-7. [DOI] [PubMed] [Google Scholar]
- 6.Jin R, Day BW, Karol MH. Toluene diisocyanate protein adducts in the bronchoalveolar lavage of guinea pigs exposed to vapors of the chemical. Chem Res Toxicol. 1993;6:906–912. doi: 10.1021/tx00036a023. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy AL, Stock MF, Alarie Y, Brown WE. Uptake and distribution of 14C during and following inhalation exposure to radioactive toluene diisocyanate. Toxicol Appl Pharmacol. 1989;100:280–292. doi: 10.1016/0041-008x(89)90314-1. [DOI] [PubMed] [Google Scholar]
- 8.Wisnewski AV, Srivastava R, Herick C, Xu L, Lemus R, Cain H, Magoski NM, Karol MH, Bottomly K, Redlich CA. Identification of human lung and skin proteins conjugated with hexamethylene diisocyanate in vitro and in vivo. Am J Respir Crit Care Med. 2000;162:2330–2336. doi: 10.1164/ajrccm.162.6.2002086. [DOI] [PubMed] [Google Scholar]
- 9.Lind P, Dalene M, Lindstrom V, Grubb A, Skarping G. Albumin adducts in plasma from workers exposed to toluene diisocyanate. Analyst. 1997;122:151–154. doi: 10.1039/a605700d. [DOI] [PubMed] [Google Scholar]
- 10.Cartier A, Grammer L, Malo JL, Lagier F, Ghezzo H, Harris K, Patterson R. Specific serum antibodies against isocyanates: association with occupational asthma. J Allergy Clin Immunol. 1989;84:507–514. doi: 10.1016/0091-6749(89)90364-3. [DOI] [PubMed] [Google Scholar]
- 11.Tee RD, Cullinan P, Welch J, Burge PS, Newman-Taylor AJ. Specific IgE to isocyanates: a useful diagnostic role in occupational asthma. J Allergy Clin Immunol. 1998;101:709–715. doi: 10.1016/S0091-6749(98)70181-2. [DOI] [PubMed] [Google Scholar]
- 12.Wass U, Belin L. Immunologic specificity of isocyanate-induced IgE antibodies in serum from 10 sensitized workers. J Allergy Clin Immunol. 1989;83:126–135. doi: 10.1016/0091-6749(89)90487-9. [DOI] [PubMed] [Google Scholar]
- 13.Campo P, Wisnewski AV, Lummus Z, Cartier A, Malo JL, Boulet LP, Bernstein DI. Diisocyanate conjugate and immunoassay characteristics influence detection of specific antibodies in HDI-exposed workers. Clin Exp Allergy. 2007;37:1095–1102. doi: 10.1111/j.1365-2222.2007.02745.x. [DOI] [PubMed] [Google Scholar]
- 14.Hettick JM, Siegel PD. Determination of the toluene diisocyanate binding sites on human serum albumin by tandem mass spectrometry. Anal Biochem. 2011;414:232–238. doi: 10.1016/j.ab.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Tse CS, Pesce AJ. Chemical characterization of isocyanate–protein conjugates. Toxicol Appl Pharmacol. 1979;51:39–46. doi: 10.1016/0041-008x(79)90006-1. [DOI] [PubMed] [Google Scholar]
- 16.Ye YM, Kim CW, Kim HR, Kim HM, Suh CH, Nahm DH, Park HS, Redlich CA, Wisnewski AV. Biophysical determinants of toluene diisocyanate antigenicity associated with exposure and asthma. J Allergy Clin Immunol. 2006;118:885–891. doi: 10.1016/j.jaci.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Wisnewski AV, Stowe MH, Cartier A, Liu Q, Liu J, Chen L, Redlich CA. Isocyanate vapor-induced antigenicity of human albumin. J Allergy Clin Immunol. 2004;113:1178–11784. doi: 10.1016/j.jaci.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Baur X, Chen Z, Flagge A, Posch A, Raulf-Heimsoth M. EAST and CAP specificity for the evaluation of IgE and IgG antibodies to diisocyanate–HSA conjugates. Int Arch Allergy Immunol. 1996;110:332–338. doi: 10.1159/000237325. [DOI] [PubMed] [Google Scholar]
- 19.Wisnewski AV, Hettick JM, Siegel PD. Toluene diisocyanate reactivity with glutathione across a vapor/liquid interface and subsequent transcarbamoylation of human albumin. Chem Res Toxicol. 2011;24:1686–1693. doi: 10.1021/tx2002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hettick JM, Ruwona TB, Siegel PD. Structural elucidation of isocyanate–peptide adducts using tandem mass spectrometry. J Am Soc Mass Spectrom. 2009;20:1567–1575. doi: 10.1016/j.jasms.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Morgan JW, Hettick JM, Russell DH. Peptide sequencing by MALDI 193-nm photodissociation TOF MS. Methods Enzymol. 2005;402:186–209. doi: 10.1016/S0076-6879(05)02006-9. [DOI] [PubMed] [Google Scholar]
- 22.Chakraborty AB, Berger SJ, Gebler JC. Use of an integrated MS-multiplexed MS/MS data acquisition strategy for high-coverage peptide mapping studies. Rapid Commun Mass Spectrom. 2007;21:730–744. doi: 10.1002/rcm.2888. [DOI] [PubMed] [Google Scholar]
- 23.Hettick JM, Siegel PD. Comparative analysis of aromatic diisocyanate conjugation to human albumin utilizing multiplexed tandem mass spectrometry. Int J Mass Spectrom. 2011 doi: 10.1016/j.ijms.2011.09.015. [DOI] [Google Scholar]
- 24.Wisnewski AV, Liu J, Redlich CA. Antigenic changes in human albumin caused by reactivity with the occupational allergen diphenylmethane diisocyanate. Anal Biochem. 2010;400:251–258. doi: 10.1016/j.ab.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruwona TB, Johnson VJ, Hettick JM, Schmechel D, Beezhold D, Wang W, Simoyi RH, Siegel PD. Production, characterization, and utility of a panel of monoclonal antibodies for the detection of toluene diisocyanate haptenated proteins. J Immunol Methods. 2011;373:127–135. doi: 10.1016/j.jim.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Roepstorff P, Fohlman J. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed Mass Spectrom. 1984;11:601. doi: 10.1002/bms.1200111109. [DOI] [PubMed] [Google Scholar]
- 27.Lin JM, Ang JC, White JW. Resistance of β-casein at the air–water interface to enzymatic cleavage. Langmuir. 2010;26:18985–18991. doi: 10.1021/la103943b. [DOI] [PubMed] [Google Scholar]
- 28.Kudryashova EV, Meinders MB, Visser AJ, van Hoek A, de Jongh HH. Structure and dynamics of egg white ovalbumin adsorbed at the air/water interface. Eur Biophys J. 2003;32:553–562. doi: 10.1007/s00249-003-0301-3. [DOI] [PubMed] [Google Scholar]
- 29.Dockal M, Carter DC, Ruker F. The three recombinant domains of human serum albumin: structural characterization and ligand binding properties. J Biol Chem. 1999;274:29303–29310. doi: 10.1074/jbc.274.41.29303. [DOI] [PubMed] [Google Scholar]
- 30.He XM, Carter DC. Atomic structure and chemistry of human serum albumin. Nature. 1992;358:209–215. doi: 10.1038/358209a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.