Preface
The brain continuously adapts its processing machinery to behavioural demands. To achieve this it rapidly modulates the operating mode of cortical circuits, controlling the way information is transformed and routed. This article will focus on two experimental approaches by which the control of cortical information processing has been investigated: the study of state-dependent cortical processing in rodents, and attention in the primate visual system. Both processes involve a modulation of low-frequency activity fluctuations and spiking correlation, and are mediated by common receptor systems. We suggest that selective attention involves processes similar to state change, operating at a local columnar level to enhance the representation of otherwise nonsalient features while suppressing internally generated activity patterns.
Introduction
Cortical activity, even in primary sensory areas, is not strictly determined by sensory input, but reflects an interaction of external stimuli with spontaneous patterns produced endogenously1. The form of this spontaneous activity — and the way it shapes sensory responses — is determined by cortical state. Cortical states were first studied as patterns of electroencephalogram (EEG) activity, and have more recently been shown to determine patterns of population spiking, neuronal correlation, and intracellular potentials. Because cortical spiking patterns can depend as much on state as on sensory inputs, an understanding of state is essential to study how information is processed by neuronal populations.
A classical view holds that cortical state is a function of the sleep cycle: during slow-wave sleep the cortex operates in a “synchronized” state, characterized by strong low-frequency fluctuations in cortical activity; whereas during waking and REM it operates in a “desynchronized” state in which low-frequency fluctuations are suppressed2. Recent experiments in rodents have indicated a more complex picture, in which cortical state also varies within wakefulness. Although alert or actively behaving animals exhibit a highly desynchronized state, awake quiescent animals can show spontaneous fluctuations in cortical activity which are prominent although smaller than those observed during slow-wave sleep3–11. Thus, the classical synchronized and desynchronized states are likely to represent two extremes of a continuum of states corresponding to varying levels of spontaneous fluctuations in neural population activity. As we argue below, the continuum is also likely to be multidimensional: there are several different behavioural and experimental conditions that all cause desynchronization, but that can have diverse effects on other variables, such as gamma frequency power and activity of different cortical cell classes.
Attention refers to an animal’s ability to selectively enhance the detection of and response to certain stimuli, at the expense of others. In this Review, we use the word attention specifically to mean “top-down” attention, in which enhanced responses are caused by a prior expectation of which stimuli will be important, rather than “bottom-up” attention, in which the physical properties of an intrinsically salient stimulus itself directs the animal’s attention. Primate studies have shown that in multiple cortical regions, attended stimuli produce larger spiking responses than unattended stimuli12–17. But this is not the only effect of attention on cortical activity. Intriguingly, many of attention’s other effects — such as decreased low-frequency fluctuations, trial-to-trial variability, and correlations — resemble cortical desynchronization, occuring specifically in parts of cortex that represent the attended stimulus18–23.
In this Review, we first discuss the nature and mechanisms of state-dependent cortical processing in rodents, then discuss how attention modulates processing in primate visual cortex. We argue that attention involves similar processes to those causing cortical desynchronization, but operating at a local level. We suggest that this local desynchronization arises from a combination of diffuse neuromodulatory inputs, and tonic glutamatergic drive focused on the cortical populations representing the attended stimulus.
State-dependent organization of cortical activity
Modern multielectrode and optical techniques have revealed how the spiking of cortical populations is organized both within individual columns and across the cortical surface. This has provided an understanding of cortical state at the neuronal level. The organization of cortical population activity is best understood for the case of spontaneous activity. Spontaneous activity has been studied with a number of experimental techniques, including optical imaging3, 6, 24–28, extracellular population recording8, 9, 29–34 and intracellular recording5, 7, 11, 35–37, all of which paint a broadly consistent picture. Although the largest fluctuations in cortical population activity are seen in sleeping and anaesthetized animals, studies from many labs have now shown that spontaneous fluctuations in firing rates and intracellular potentials can also occur during quiet wakefulness3–11. Spontaneous cortical population activity in an awake animal does not simply switch between discrete synchronized and desynchronized states, but forms a continuum of states characterized by variations in fluctuation depth that correlate at least partially with ongoing behaviours, such as whisking and locomotion5–7, 38.
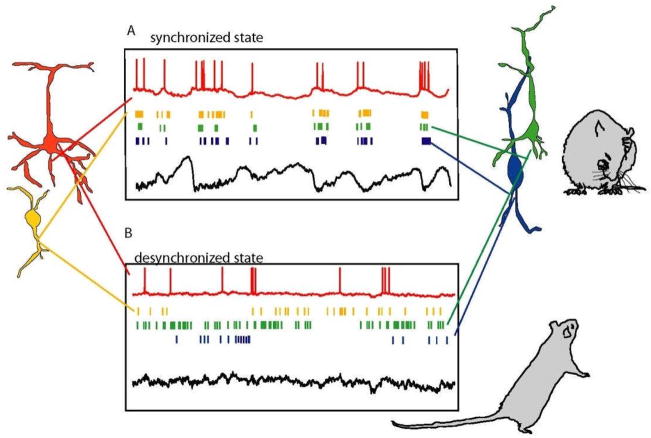
Figure 1 shows a cartoon illustration of neuronal population activity in synchronized and desynchronized states. The defining characteristic of cortical state, for our purposes, is the amount of common fluctuation in population spiking activity (as we argue below, other measurements such as low-frequency LFP power, spiking correlations, and variability also relate to this definition). Fig. 1a illustrates activity typical of a synchronized state, where activity fluctuates between up phases characterized by firing in multiple neuronal classes, and down phases in which the whole network is quiet (See Box 1 for a discussion of terminology). Fig. 1b illustrates activity typical of a desynchronized state, where spontaneous fluctuations are weaker. Cortical state is not bimodal; these two examples illustrate the extreme ends of a continuum of cortical state seen in awake rodents. Strong variations in cortical state are also seen during sleep (most notably between slow-wave and REM), and also under certain anaesthetics31, 39–41. Urethane anaesthesia, where a continuum of cortical states is seen, ranging from states more synchronized than slow-wave sleep, to transient desynchronized epochs similar to REM or waking31, 32, 41, is a frequently used experimental model to investigate how state affects cortical processing.
Figure 1.
Cartoon Illustration of how population activity patterns vary with cortical state. The two panels illustrate two extremes of a continuum of states seen in awake rodents. A. In synchronized states, cortical populations show spontaneous common fluctuations in firing rate. During the active phase, all neuronal classes show a propensity to fire (coloured rasters), whereas during the silent phase, spiking is reduced or absent. These phases are accompanied by corresponding depolarization and hyperpolarization in intracellular potentials (red, top). Deep-layer cortical local field potential (black, bottom) shows slow negative waves accompanied by high-frequency activity in the active phase, and smooth dome-shaped positive waves in the silent phase. This type of activity is seen in drowsy or quiescent animals (right). B. In the desynchronized state, coordinated slow fluctuations in population activity are not seen, and low-frequency fluctuations in the local field potential and membrane potentials are suppressed. This type of activity is seen in actively behaving, alert animals (right).
Box 1. What’s in a name?
The study of cortical state suffers from confusing terminology, with multiple terms being used to refer to a single phenomenon, as well single terms being used with different meanings by different authors. We here provide definitions of the terminology as used in this Review.
Cortical state
Our definition of cortical state refers to the dynamics of network activity on a time scale of seconds or more. For our purposes, the defining characteristic of cortical state is the amount of slow fluctuation in the summed activity of a set of local neurons. Cortical state is not bimodal; however for linguistic convenience we will refer to synchronized and desynchronized states to describe relative positions along a continuum.
Desynchronized state
We use the term desynchronized state to mean a situation in which the population rate in a cortical column fluctuates only weakly. In such a state, low-frequency LFP power is also comparatively small. Note however that neuronal coherence at high (gamma) frequencies often actually increases in the desynchronized state52, leading some authors to question the use of this term. The terms asynchronous state and activated state are largely synonymous with desynchronized state, although one should remember that the term activated does not refer to an increase in firing rates, as firing rates of some neurons may go down in desynchronized states.
Synchronized state
We use the term synchronized state to mean a situation in which the average population firing rate in a cortical column fluctuates strongly at a timescale of ~100 ms or slower. In such a state, low-frequency LFP power is high, although power at gamma frequencies may decrease. Other terms used for this state include deactivated and inactivated; once again one should remember this does not necessarily imply lower firing rates, or ion channel inactivation.
Up phase and down phase
The terms “Up State” and “Down State” were originally used to refer to the two modes of the bimodal distribution of membrane potentials seen in intracellular recordings of striatal and cortical neurons in vivo198. Subsequently, however, the usage of these terms has widened to include any periods of spontaneous depolarization and hyperpolarization, even when the histogram of membrane potentials is not bimodal. Furthermore, as intracellular Up and Down states occur during phases of strong local network spiking and silence, respectively, the terms are now also used to refer to spiking and silent phases of population activity, even in the absence of intracellular recordings. Spontaneous depolarizations and spiking periods also go by other names such as population bursts 199 or bumps4.
To add further confusion, the Up State and Down State are not, in our usage, actually cortical states. A cortical state (as the term is used in this Review) refers to a global pattern of cortical dynamics — such as the desynchronized or synchronized state — that changes over a time course of seconds or more and is defined by fluctuation magnitudes or power spectra that can only be computed from several seconds of data. Up and down states occur at timescales of ~100 ms, and are thus not states, but phases of an ongoing fluctuation. For the sake of clarity, we therefore use the terms up phase and down phase in this Review.
Spontaneous cortical activity during sleep can display slow, regular, rhythmic patterns42. By contrast, the spontaneous fluctuations seen in cortex of awake rodents typically have an irregular structure, and the length and depth of the up and down phases varies from one period to the next. These waking patterns are therefore not well described as ‘oscillations’. Recordings of different cell types have shown that the firing and membrane potential of most, if not all, cortical neuronal classes increase in the up phase, although differences in the precise timing of neuronal firing relative to the onset of the up phase exist both between and within cortical neuronal classes and layers10, 37, 43–45.
Relation of cortical state to pairwise correlations
When the firing rate of a neuronal population is modulated by global fluctuations in activity, the activity of neuronal pairs is generally positively correlated. This idea can be mathematically expressed by describing the relationship between the (weighted) mean of pairwise correlation coefficients and the variance of the population rate (see Box 2). If more pairs are positively than negatively correlated, the mean pairwise correlation is positive, and so the population rate has large variance, indicative of coordinated global fluctuations that are typical of a synchronized state. If no neuronal pairs are correlated, the variance of population activity is small, and the neuronal population is in a desynchronized state. However, a mean correlation of zero does not require every single pair to be uncorrelated; it is also possible to have a desynchronized state in which positive correlations between neurons exist, but are counterbalanced by an equal number of negative correlations32 (See Box 2).
Box 2. Mathematical relationship between fluctuation and correlation.
Intuitively, one would expect that if a set of neurons show a common fluctuation in firing rate, their activity will in general be positively correlated. To make this idea precise, consider a population of N neurons, and let xi refer to the instantaneous firing rate of the ith neuron, as could be measured by counting spikes in some timebin. Then is the population rate in the corresponding timebin. For any set of random variables xi it holds that
where .
This equation therefore implies that the variance of the population is dominated by a (weighted) mean of the correlation coefficient of all cell pairs. If the population rate does not fluctuate and has small variance, one must therefore have a mean correlation close to zero. This could happen either when all neurons fire independently, or when positively correlated cell pairs are counterbalanced by an equal number of negative correlations. Conversely, when mean correlations are non-zero, this implies large fluctuations in population rate even if the correlations are themselves of moderate order (e.g. <0.1).
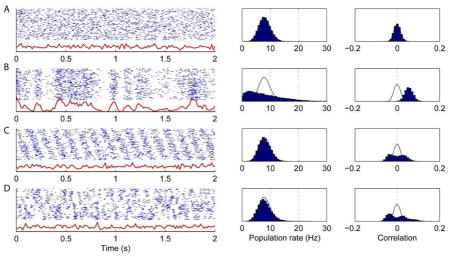
The figure shows four illustrations of this relationship, for simulated spike trains of 128 neurons of equal firing rate. In each row, the left panel shows 2 s of simulated data, with the blue raster representing the spikes of all cells, and the red trace representing the instantaneous population rate; the middle panel shows a histogram of population rates over 50 s of simulated data; and the right panel shows the histogram of correlation coefficients over all cell pairs. Black curves in the right two histograms correspond to outlines of the histograms for independent Poisson neurons.
The neurons fire as independent Poisson processes. The population rate has low variance and the mean correlation is zero. The width of the correlation histogram reflects the expected size of statistically insignificant correlation coefficients arising from random fluctuations.
The neurons fire as inhomogeneous Poisson processes, modulated by a single fluctuating rate function. The population rate has high variance, and the mean correlation is positive though of modest magnitude. This data resembles cortical activity in a synchronized state.
The neurons are modulated by a common sinusoidal oscillation, but with phases distributed evenly across the population. As for the independent cells of (A), the population rate has low variance and the mean correlation is close to zero. In this case, however, the correlation histogram is wider than in (A), indicating an equal number of significantly positively and negatively correlated pairs. This pattern illustrates how it is possible for a population to be modulated by a common oscillation (such as hippocampal theta), while remaining in a desynchronized state.
The neurons display a more complex pattern of cell assembly activity. However, population activity has low variance, as positive correlations between neurons frequently joining in one assembly are counterbalanced by negative correlations amongst neurons that only rarely do so (c.f. Ref. 200). As in (C), the population rate has low variance, and the mean correlation is close to zero, but the distribution of correlations is wide, indicating an equal number of significantly positively and negatively correlated pairs.
Relation of cortical state to local field and intracellular potentials
Fluctuations in cortical population activity are strongly correlated with local field potential (LFP) patterns. The LFP can show large differences between sleeping, quiescent awake, and actively exploring animals. Although the physics underlying local field potentials is complex, periods of strong firing in a column are generally accompanied by depth-negative waves generated by local excitatory synapses46–48. The down phases of the synchronized state are accompanied by depth-positive LFP waves of smooth appearance, reflecting a lack of spiking and synaptic activity. In synchronized states, alternations between up and down phases in fluctuating population activity therefore give rise to local field potential patterns of strong low-frequency power (Fig. 1a). In desynchronized states, smaller fluctuations in global firing rates are mirrored by less LFP power at low frequencies (Fig. 1b).
Fluctuations in population activity correlate strongly with fluctuations in intracellular voltages of multiple neuronal classes7, 45, 49, 50. The strength of membrane potential fluctuations thus also varies with cortical state, with periods of strong low-frequency LFP showing large intracellular fluctuations, and periods of LFP desynchronization showing more steady intracellular potentials5, 7, 11. Intracellular recordings in awake rodents again reveal a continuum of states, rather than a switch between discrete synchronized and desynchronized states11.
Relation of cortical state to gamma oscillations
Cortical desynchronization has often been linked with increased power of gamma frequency oscillations (25–100 Hz), which have in turn been proposed to assemble neurons into synchronous groups that are capable of strongly driving their targets51. Gamma power increases when cortical desynchronization occurs during active behaviour in rodents38, or after electrical stimulation of basal forebrain, brainstem cholinergic, and nonspecific thalamic nuclei52–55. However, the correlation between desynchronization and gamma power is not absolute. For example, in rats, stimulation of dorsal Raphe nuclei causes a decrease in low-frequency LFP power and a simultaneous decrease in gamma power56. Moreover, selective attention in primates uniformly decreases low-frequency power, but can either raise or lower gamma power, depending on cortical area and task18, 21, 22. Finally, although local gamma power in visual cortex is typically increased by visual stimuli, the correlation between gamma power and firing rate is also complex, with certain stimuli being able to increase gamma power without changing, or even decreasing neuronal firing rates57, 58.
Coordination of spontaneous population activity across areas
Electrical recordings have shown that neuronal correlations and LFP coherence decay as the lateral distance between recording sites increases33, 34, 59, 60. Recent results have revealed a spatiotemporal picture of cortical activity that can explain these findings. In synchronized states, cortical activity often takes the form of spatially extended travelling waves6, 9, 27, 61–65. Despite variations in trajectory from one wave to the next, these waves share a number of characteristic features, such as timescales of the order 10–100ms and remarkable bilateral symmetry66. The passage of a wave through a cortical column initiates a local spiking pattern that is independent of the wave direction9. The correlated fluctuations seen in any one column during synchronized states are thus part of much larger patterns that spread out across the cortical surface.
Fluctuating spontaneous activity is not restricted to cortex, but is also found, among other areas, in thalamus67, 68, basal ganglia69, 70, cerebellum71, and hippocampus. Hippocampal spontaneous activity has been particularly well studied, and is similar to that in neocortex, with a few key differences. The hippocampal equivalent of the synchronized state — the “large irregular activity” (LIA) seen during immobility and sleep — consists of periods of near silence punctuated by “sharp waves” of strong population activity whose occurrence is correlated with the timing of cortical up phases72, 73. Unlike in cortex, however, hippocampal sharp waves are accompanied by a 150–200Hz “ripple” oscillation74. In the hippocampal equivalent of desynchronization, known as the “theta state,” sharp-wave fluctuations are suppressed, leading to a steadier spiking pattern; but unlike in the neocortex, this relatively steady activity is superimposed with a rhythmic 6–10 Hz theta oscillation. The character of the hippocampal theta oscillation is also fundamentally different to the fluctuations of cortex in the synchronized state. In the latter, all neurons show increases in firing in the up phase and decreases in the down phase, resulting in a strongly fluctuating population rate during the synchronized state. By contrast, individual hippocampal pyramidal cells show phase relationships to the theta rhythm that vary between neurons, and even within individual cells from moment to moment75–77. This results in a pyramidal cell population whose summed firing rate is only weakly modulated by theta phase78 (see Box 2).
How does state correlate with ongoing behaviour?
The behaviour dependence of brain state has been best studied in the hippocampus. In the rat hippocampus, the theta state occurs during behaviours such as walking, running, rearing and exploratory sniffing, whereas LIA occurs during behaviours such as waking immobility, eating, grooming, and defecation79. Although various terms have been used to characterize the circumstances under which these states are seen in rats (e.g. “voluntary” or “exploratory” for theta, “automatic” or “consummatory” for LIA), the precise list of behaviours for each state is species-dependent80, 81. The mechanisms and significance of these species differences are still poorly understood.
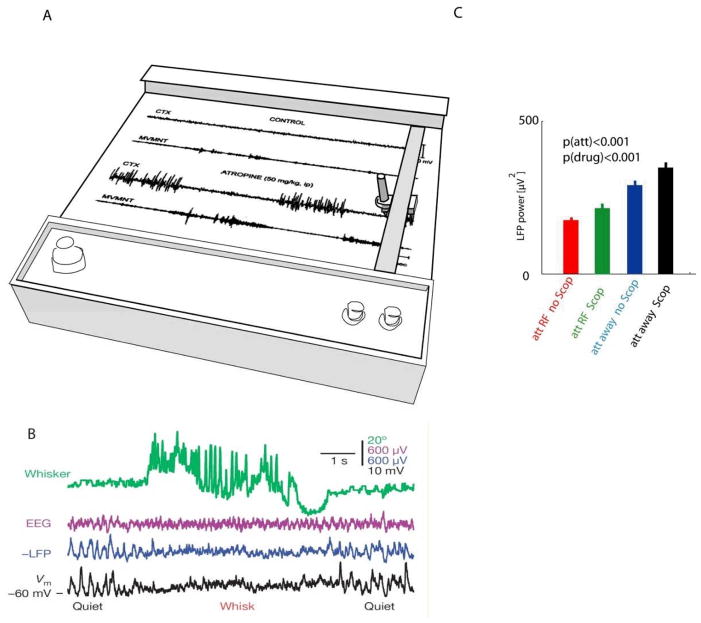
In neocortex, variations in state during waking are more subtle than in the hippocampus, and were not detected in early studies based on visual inspection of pen-chart EEG recordings82(Figure 2a). However, spectral analysis later revealed a suppression of low-frequency LFP power in actively behaving awake rats compared to awake but immobile rats83. In head-fixed mice, cortical activity becomes desynchronized during behaviours such as whisking or ball-running5, 7, 38 (Figure 2b). The effect of behaviour on cortical state can be greatly amplified by pharmacological treatments: for example, after delivery of the muscarinic antagonist atropine, large, slow, sleep-like cortical activity occurs during quiet rest but this activity is suppressed during locomotion84(Figure 2a, 2c).
Figure 2.
Cortical LFP and behaviour. A, In this classical pen-chart recording, the correlation between behaviour and cortical EEG is difficult to detect visually under control conditions (top two traces, showing EEG and movement activity), but is greatly amplified by application of the muscarinic antagonist atropine (bottom two traces). B, A recent study showing a reduction of spontaneous fluctuations during whisking behaviour, clearly visible in intracranial LFP and membrane potential, but more difficult to detect visually in the surface EEG. C, In monkey V1, low-frequency (2–10Hz) power is reduced when attention is directed into the receptive field corresponding to the electrode site (att RF: red, green), and is increased by application of the muscarinic antagonist scopolamine (Scop: green, black) or when attention is directed to a different location (att away: blue, black). Part A is modified from Ref. 82, Part B from Ref. 7, Part C from recordings in the Thiele lab (Herrero, Delicato, Thiele: methods details in Ref. 183).
Mechanisms controlling cortical state
What are the mechanisms responsible for control and maintenance of cortical states? We will divide this question into three: how can a cortical area maintain a desynchronized state; what produces the fluctuations of the synchronized state; and what causes a cortical area to switch between states.
How can cortex maintain a desynchronized state?
As discussed earlier, the level of fluctuation exhibited by a neuronal population is related to the mean pairwise correlation of the constituent neurons. It has long been recognized that two neurons can be correlated without having a direct synaptic connection, and that a correlation can also arise from excitatory or inhibitory inputs shared between the two cells85. Conversely, one might expect that any two neurons that share a large number of inputs – such as neighbouring pyramidal cells of cortex – will necessarily display correlated activity. This is indeed seen in feed-forward network models, where even uncorrelated inputs can lead to synchronous spiking output86–88. Furthermore, neuronal pairs in primates frequently have noise correlations of the order 0.133, 89–93, numerically close to that predicted on the basis of anatomically shared connections86. It was thus suggested that such correlations are an inevitable property of cortical shared connections, and result in a reduction in coding capacity as shared noise cannot be averaged out efficiently86, 92.
Although for any two neurons a correlation of 0.1 is small, this level in fact indicates prominent global fluctuations in population firing (Box 2). If it really were the case that such correlations were inevitable, a truly desynchronized state would be impossible. However, recent studies have shown that mean correlations very close to zero can occur in V1 of behaving primates94 and in somatosensory and auditory cortices of rats in the desynchronized state under urethane anaesthesia32. The cortex must therefore have some mechanism to enforce decorrelation even in the face of multiple shared inputs.
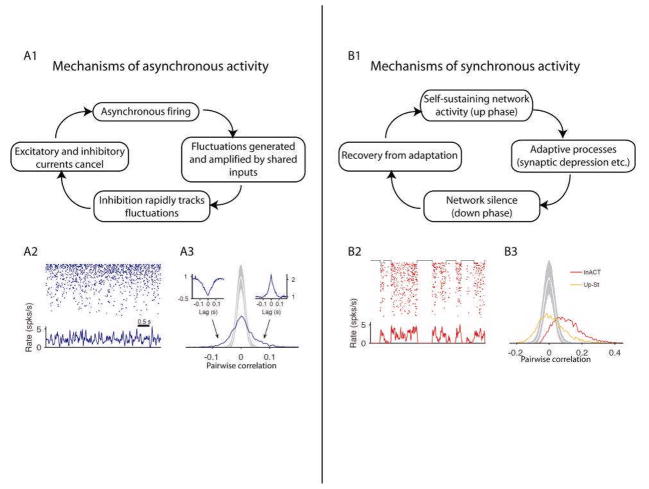
The answer to this conundrum may lie with inhibition (Figure 3a). Although shared inhibitory inputs lead to positive correlations95, this effect can be cancelled if the excitatory and inhibitory inputs to a pair of neurons are themselves correlated. Theoretical analysis suggests that if inhibition is fast and strong enough, recurrent networks self-organize into a state in which the fluctuations produced by shared connections are rapidly ‘tracked’ by inhibitory interneurons, leading to extremely small mean correlations in large networks32. This analysis also predicts that despite a mean correlation close to zero, a substantial number of neuronal pairs should remain correlated, but with approximately equal numbers of positively and negatively correlated cell pairs. This prediction is borne out in rat cortex, both in the desynchronized state and within up phases of the synchronized state32.
Figure 3.
Possible mechanisms of asynchronous and synchronous activity. A1, Correlations generated by shared excitatory input may be cancelled by rapid recurrent inhibition. A2, Raster showing spontaneous activity of simultaneously recorded population from rat somatosensory cortex in a desynchronized state. Bottom trace shows population rate as a function of time, showing a small degree of fluctuation. A3, Histogram of pairwise correlations in this population, with a mean close to zero but long tails indicating an approximately equal number of significant positively and negatively correlated pairs (insets). The gray curve indicates the distribution of correlations that would be expected by chance. B1, Excitable system model of slow fluctuations in cortical activity where up phases are generated and sustained by recurrent synaptic activity before being overcome by adaptive processes. B2, Raster showing spontaneous activity of same population as A2, but now in a synchronized state. B3, Histogram of pairwise correlations in this population, showing positive mean for the whole data set (red) but a mean close to zero when considering active phases only (yellow). Modified from Ref. 32.
What causes fluctuations during synchronized states?
If positive correlations do not inevitably arise from shared inputs, why are they so frequently observed? The relationship between mean pairwise correlation and the variance of population rate allows us to ask the same question in a different way: why do neural populations activity exhibit spontaneous fluctuations in population rate? And why do sensory stimuli induce responses whose population rate varies from one presentation to the next? We address spontaneous fluctuations now, and will discuss sensory-evoked correlations in the next section.
The fact that fluctuating spontaneous activity occurs even in isolated cortical slices and slabs96, 97 suggests that intracortical mechanisms may have a primary role in generating them. Many cellular and synaptic processes have been implicated in the generation of these fluctuations, but all these processes can be understood within a single conceptual framework, known as the theory of excitable systems (Fig. 3b). In this framework, spiking in the up phase is sustained by recurrent synaptic activity96, 98. But after a period of prolonged firing, a number of adaptive processes occur that steadily reduce the excitability of the network, such as synaptic depression99, 100, a build-up of afterhyperpolarizing K+ conductances96, and decreased ATP levels101. After enough adaptation, the network’s ability to sustain firing fades, leading to a period of network silence. Subsequently, after enough “rest,” the synapses and cells recover, and the network can again sustain recurrent activity. Computational models based on these principles are able to produce data very similar to those obtained in vivo and in vitro31, 102–105. A key feature of this mechanism is that the down phase is caused not by synaptic inhibition but by disfacilitation, i.e. the temporary absence of synaptic drive49, 106. Both in vitro and in vivo studies suggest that within a cortical column, the up phase is generated in layer V, from where it can, but does not always spread to superficial layers10, 96.
This scenario also explains why spontaneous cortical fluctuations spread as travelling waves. Once a certain region of cortex has entered an up phase, lateral excitation from this region can spark an activity in neighbouring cortex, but a second wave cannot pass until sufficient time for recovery of synaptic and cellular adaptation. Models of spatially coupled excitable systems are known as an excitable media, and have been used to describe diverse phenomena including forest fires, wave propagation in cardiac tissue, and stadium waves at sporting events107.
What causes changes in cortical state?
Several mechanisms have been implicated in cortical state shifts, including increased activity of subcortical cholinergic and monoaminergic nuclei, as well as sustained glutamatergic inputs from thalamus and potentially other cortical regions82, 108. Much of the work we review here is classical but, despite its relevance, it is rarely discussed in the modern literature.
The cholinergic system plays an important, but not exclusive role in controlling cortical state. Lesions of the basal forebrain – the primary source of cholinergic input to cortex – increases low-frequency LFP power83, whereas electrical or pharmacological stimulation of basal forebrain or cholinergic brainstem nuclei causes cortical desynchronization that is blocked by systemic or cortically applied muscarinic antagonists such as atropine52, 53, 83, 109. Cholinergic basal forebrain and brainstem neurons show increased firing during cortical desynchronization109–112. Nevertheless, cholinergic input is not necessary for cortical desynchronization: although atropine causes a strongly synchronized state in awake immobile rats, actively behaving rats show atropine-resistant desynchronization82, 84 (Figure 2a). Moreover, selective lesions of cholinergic neurons in BF are not sufficient to abolish cortical desynchronization113.
What might be responsible for ACh-independent desynchronization? Stimulation of other neuromodulatory systems can cause desynchronization, although in some cases this occurs via their effects on the basal forebrain114–117. Serotonin may play an important part in ACh-independent desynchronization, as suggested by the atropine resistance of desynchronization induced by dorsal Raphe stimulation, and by the ability of serotonin depletors combined with atropine to block desynchronization even during active behaviour115, 118, 119. Cortical norephinephrine release plays an important role in reducing spontaneous fluctuations as rats wake from anaesthesia, at least in layer 4 of primary somatosensory cortex120. Neurons in cholinergic and other neuromodulatory nuclei exhibit diverse and rapid modulation of firing rate by salient sensory stimuli, behavioural events, and cognitive factors such as attention and expected reward, which is consistent with control of cortical state on a moment-to-moment basis121–129.
An additional mechanism implicated in cortical desynchronization involves increased tonic firing of glutamatergic afferents from the thalamus and perhaps elsewhere. Tonic thalamic firing increases under several conditions that are associated with cortical desynchronization130–134. This may in turn reflect thalamic neuromodulation; the thalamus receives strong cholinergic input from the brainstem, which depolarizes thalamic relay cells and can shift them to a mode of steady tonic firing, both by direct excitation of relay cells and by disinhibition resulting from cholinergic inhibition of thalamic reticular neurons135–137. Increasing tonic relay cell firing (by microdialysis of the unspecific cholinergic agonist carbachol into somatosensory thalamus) causes desynchronization of barrel cortex138, suggesting that tonic glutamatergic input from thalamus is sufficient to desynchronize barrel cortex.
How could neuromodulatory and tonic glutamatergic inputs suppress fluctuations in cortical activity? Neuromodulatory systems and metabotropic glutamate receptors have diverse effects on different classes of cortical neurons and synapses, in a manner that may further vary as a function of cortical area and age139–150. These multiple receptor systems probably allow fine-tuning in a high-dimensional space of cortical operating modes. Nevertheless, their effects also have several similarities, which paint a basic picture of how these systems can reduce cortical fluctuations. First, multiple neuromodulatory systems and metabotropic glutamate receptors affect the firing mode of pyramidal cells, reducing bursting, afterhyperpolarization and adaptation, and promoting tonic firing141, 143, 148, 150–158. Second, multiple neuromodulatory systems cause a reduction in the strength of recurrent excitatory synapses within cortex, with a concomitant reduction in synaptic depression146, 159–161. Third, metabotropic glutamate and multiple neuromodulatory systems cause tonic depolarization of cortical layer V pyramidal cells, where cortical fluctuations are thought to be generated150, 162, 163. These three processes should switch the cortex from a mode in which bursting and recurrent excitation lead to rapid increases in population rate that are then dampened by adaptation, to a mode in which tonic depolarization causes neurons to maintain relatively steady activity. Simulations based on both detailed biophysical models and simple dynamical systems support this picture31, 102, 104, 105.
Recent results suggest that cortical state is in fact a multidimensional continuum, with different forms of desynchronization reflecting non-identical changes in cortical operating mode. As mentioned above, gamma power may either increase or decrease during desynchronization. Furthermore, the firing rates of individual neuronal classes can differ between desynchronizing conditions: putative fast-spiking interneurons fire faster during locomotion38, in more difficult discrimination tasks164, and after artificial elevation of thalamic firing138; but during rodent whisking, fast-spiking interneurons fire slower whereas non-fast-spiking interneurons fire faster45. Many non-fast-spiking interneurons are excited ionotropically by acetylcholine and serotonin receptors165 and inhibit fast-spiking interneurons166, whereas fast-spiking interneurons receive strong glutamatergic drive from thalamus167. One might therefore speculate that the difference between these types of desynchronization reflects a different balance in neuromodulatory versus tonic glutamatergic drive. Modern techniques such as optogenetics and enzyme-linked electrochemistry168 may help to resolve the full space of cortical states and the roles of different afferent systems on state and information processing.
Cortical state and sensory responses
Cortical sensory responses correlate strongly with state. However, the way state shapes sensory processing is complex: one cannot simply ask whether responses are larger or smaller in one state or another; instead the effect of cortical state on neuronal responses appears to depend on the specific type of stimulus the system is confronted with. Nevertheless, some commonalities are beginning to emerge from studies of multiple sensory areas. In particular, it appears that isolated punctuate stimuli (which could also be labelled as salient, unexpected, brief stimuli) are able to generate substantial responses regardless of cortical state, whereas temporally extended or rapidly repeated stimuli are “filtered out” in synchronized states, but efficiently processed in the desynchronized state (Fig. 4).
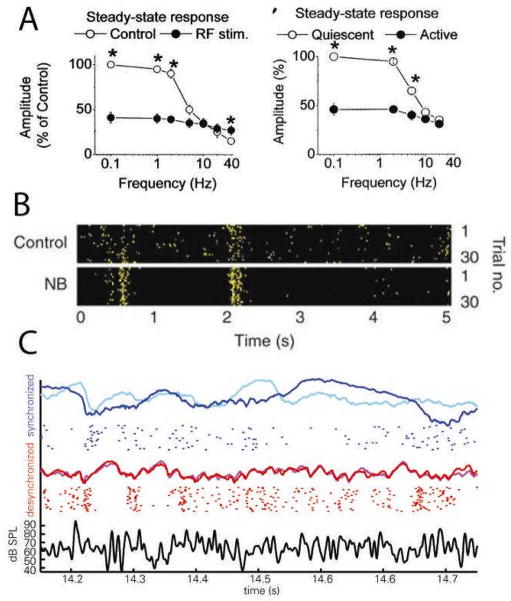
Figure 4.
Desynchronization suppresses responses to punctuate stimuli, but decreases adaptation and enhances representation of temporally extended stimuli. A, State-dependent responses to stimulus trains in barrel cortex of anesthetized (left) or awake rats (right). In synchronized states under anaesthesia and in quiescent awake animals (open circles), responses to rare punctuate stimuli are large, but responses adapt strongly at high repetition frequencies. After electrical stimulation of reticular formation (RF) or during active behaviour (solid circles), responses to rare stimuli are smaller but adaptation is reduced. B, Raster representation of a visual cortical unit response to repeated presentations of a temporally extended natural scene movie. Stimulation of nucleus basalis (NB) increases reliability of responses from trial-to-trial. C, Response in auditory cortex to repeated presentations of a temporally extended amplitude-modulated noise stimulus. Evoked LFPs (coloured curves) from two presentations of the same stimulus in synchronized (blue and cyan) and desynchronized (red and magenta) states, as well as raster representation of spikes from one cell in response to repeated presentations in each state. Black curve (bottom) shows stimulus envelope. LFP and spiking responses are more reliable in desynchronized state. Part A is modified from Ref. 170, Part B from Ref. 132, Part C from Ref. 176.
Responses to simple punctuate stimuli (for example, whisker deflections and auditory clicks) have been well studied in barrel and auditory cortices in rodents. When discussing how a sensory response is modulated by state, we must consider both how the response differs between synchronized or desynchronized states, and between the up and down phases within the synchronized state. In rat auditory and somatosensory cortices, the initial response (up to ~50ms) to a punctuate stimulus is larger in synchronized states and quiescent animals, than in desynchronized states and actively engaged animals130, 169, 170; larger auditory onset responses are also seen in passive compared to behaviourally engaged ferrets, with smaller onset responses for more difficult detection tasks171. Within the synchronized state, the initial response does not depend strongly on phase, with stimuli arriving in the up and down phase evoking initial responses of approximately equal magnitude31, 172, a phenomenon that may related to suppression of firing variability by stimulus onsets173. The later stimulus response period (after ~50ms) shows more complex dependence on state and phase31, 172. In synchronized states, punctuate stimuli can trigger long-lasting up phases that can spread over the cortical surface6, 61 — a phenomenon that may be related to observations that receptive fields of sensory cortex neurons are often wider in synchronized states than in desynchronized states40, 174. In the desynchronized state, the response to a single punctuate stimulus is typically simpler, consisting of a brief response followed by a transient ~100ms suppression below baseline and a depth-positive LFP wave in all but the most desynchronized conditions31, 175. Despite the apparent complexity of these results, such state-dependent sensory responses can be quantitatively predicted on a trial-by-trial basis by a simple excitable system model, using parameters derived from spontaneous activity preceding the stimulus31.
Presentation of repeated or temporally extended stimuli reveals another aspect of state-dependence. Although the response to the first in a train of rapidly repeated punctuate stimuli is larger in synchronized than in desynchronized states, response adaptation is stronger in the synchronized state, so by the end of the train responses that are equal between states or smaller in the synchronized state130, 170 (Fig. 4a). In rat visual cortex, temporally extended movies of natural scenes are more faithfully represented after basal forebrain stimulation132 (Fig 4b), and responses to continuously drifting gratings are larger in visual cortex of running compared to stationary mice38. These data might appear to conflict with the smaller responses to isolated punctuate stimuli in desynchronized somatosensory and auditory cortex. However, it seems unlikely that this is due to a difference between sensory modalities, as the representation of temporally extended amplitude-modulated noise stimuli is also more faithful in desynchronized auditory cortex176 (Fig 4c). We therefore suggest that the effects of desynchronization on neuronal responses to sensory stimuli are similar across modalities: onset responses are smaller, but adaptation is reduced, leading to enhanced representation of repeated or temporally extended stimuli.
We emphasize that state-dependent changes in cortical representations do not necessarily arise from changes in cortical processing, but could also reflect changes in lower structures. Indeed, in synchronized states thalamic relay neurons show an enhanced propensity to fire in “burst mode,” which may emphasize the response to the onset of a stimulus train but may interfere with linear representation of temporally extended stimuli177. During desynchronized states, increased baseline firing of thalamic relay cells may cause depression of thalamocortical synapses, which would reduce the response to the first stimulus in a train, but reduce the potential for further adaptation, as these synapses are already close to fully depressed170.
Why would it be beneficial for an animal to enhance responses to sudden punctuate stimuli, but suppress responses to temporally extended stimuli in the synchronized state? One suggestion relates to the different behavioural needs of active versus quiescent animals. The fine details of ongoing continuous sensory stimuli may be of little relevance to a resting animal, so nothing is lost by filtering them out and allowing cortex to exhibit the endogenously generated patterns typical of a synchronized state. However, a punctuate stimulus such as a sudden unexpected sound or touch, may signal the need for an immediate behavioural response. Larger responses to punctuate stimuli in the synchronized state might thus serve as a “wake up call,” enabling appropriate motor responses to unexpected events in passive animals6, 178.
Cortical state and attention in primates
The classical view of cortical states is that they are global, synchronizing or desynchronizing all cortical areas simultaneously. With regard to the sleep cycle this is generally the case (with certain exceptions such as cetaceans179). However, a growing body of evidence suggests that in awake primates, selective attention also affects the level of cortical desynchronization at a local level, at a reduced scale compared to differences between sleep and waking. In this interpretation, the maximum desynchronization is restricted to a small patch of cortical tissue that represents the attended stimulus while cortical tissue representing non-attended parts of the sensory world would be in a more synchronized state. We argue that such local changes of state can explain results from multiple labs on attention-related changes in changes in and LFP power, response variability, and correlation (Table 1).
Table 1.
Similarities between desynchronization in rodent and attention in primate
| Measurement | Effect of desynchronization or active behaviour in rodent | Effect of attention in primate |
|---|---|---|
| Low frequency LFP power | Reduced7, 38, 53, 83 | Reduced18, 21, 22 |
| Gamma LFP power | Increased (running, cholinergic stimulation, thalamic stimulation) 38, 52–55; Decreased (dorsal Raphe stimulation) 56 | Increased (V4)18; Decreased (V1)21 |
| Trial-to-trial variability | Reduced31, 132, 176 | Reduced19, 20 |
| Noise correlation | Reduced32, 132 | Reduced19, 20 |
| Response size | Reduced (sudden punctuate stimuli)130, 170; Unchanged or enhanced (rapidly repeated or temporally extended stimuli)132, 170, 176 | Reduced (unattended stimuli)208; enhanced (attended stimuli)12, 13, 16 |
One of the hallmarks of cortical desynchronization is a decrease in low-frequency LFP power. Studies in multiple visual areas have shown that attention directed to the receptive field of the recorded neurons near the recording electrode results in decreased low-frequency LFP power, compared to attention directed outside the receptive field18, 21, 22 (Figure 2c). This result implies that attention modulates the size of low-frequency fluctuations at a local level: if attention simply caused desynchronization uniformly across the cortical surface, this would not change LFP power depending on receptive field location. Surprisingly, attention-associated low-frequency desynchronization can be accompanied by either increased or decreased gamma LFP power, a finding that is reminiscent of the differential effects on gamma power after stimulating different subcortical structures in rats (discussed above). The circuit mechanisms underlying these dissociations between low-frequency desynchronization and gamma power are as yet unknown.
Also consistent with local desynchronization, attention reduces trial-to-trial variability and firing rate correlations between neurons. Responses to repeated presentations of an identical stimulus vary from trial to trial, and variations in the responses of neighbouring neurons are typically correlated in cortex of an awake primate90. These “noise correlations” typically reduce the information that can be encoded in populations92, 180, 181. Recent studies have shown that in area V4, both variability and noise correlations are reduced when attention is directed to the receptive field of the recorded neurons19, 20, 23. Coherence analysis suggests these correlations arise from low-frequency (<5 Hz) fluctuations in rate correlated across the neuronal population19, resembling a more synchronized state, which attention desynchronizes. Reduced noise correlations are also found in rodent auditory cortex after spontaneous desynchronization31 and in visual cortex after basal forebrain stimulation132, where reduced correlations were mediated by cortical muscarinic mechanisms.
The effects of attention in primates vary throughout the response time course, similarly to the effects of cortical state in rats. Immediately after a stimulus appears in the receptive field, spike count variability and correlation in V4 are suppressed regardless of whether the stimulus was attended to. During the sustained response period, however, variability and correlation increase in unattended conditions19, 20. This effect is similar to the effects of state on responses to punctuate stimuli in rat auditory and barrel cortex31, 172, where the initial response is largely independent of cortical state and phase and thus not highly variable from trial to trial, but the later response shows strong variability in the synchronized state. Also similarly to desynchronization in rodents, attention reduces the adaptation of V4 responses to repeatedly presented visual stimuli182.
Circuit mechanisms of attention in primates
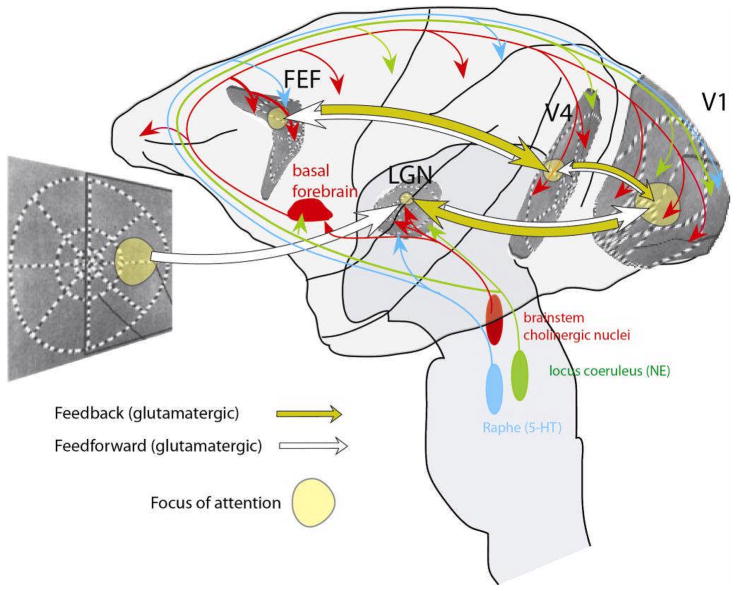
Although the circuit mechanisms of top-down attention are still largely mysterious, recent primate experiments have begun to cast light on the question, in particular the contribution of different receptor systems. Once again, strong parallels to cortical state are indicated, in particular an important role for the cholinergic and glutamatergic systems. In behaving primates, iontophoresis of ACh or muscarinic antagonists respectively increases or decreases the effects of attention on spiking patterns in V1183 and V4184. It appears unlikely, however, that cholinergic input could be solely responsible for attentional modulation within cortex: despite topographic organization of basal forebrain projections at the level of cortical lobes and regions185, it seems unlikely that cholinergic afferents have the spatial specificity to target a small patch of cortex representing an attended stimulus. Thus, although cholinergic mechanisms are involved in attentional modulation in visual cortex, they probably work in interaction with other systems. It may be that cholinergic drive is critical for setting the network to be maximally responsive to localized glutamatergic feedback from higher cortical areas (Figure 5; Ref. 186).
Figure 5.
Suggested mechanisms underlying widespread and focused desynchronization during state changes and attention. Increased activity of cortical neuromodulatory afferents (red [cholinergic], blue [serotonergic] and green [noradrenergic] arrows) causes a general desynchronization and reduction in spontaneous fluctuation, but may lack the spatial selectivity to desynchronize the patch of cortex representing the attended stimulus. Focused glutamatergic inputs arising from feedback connections could provide this specificity (yellow arrows), causing enhanced desynchronization and sensory responses in the regions of cortex that represent the attended stimulus. The yellow circle in the visual display indicates the focus of attention, which affects processing in thalamic and cortical areas at specific locations (indicated by the yellow patches). The distorted replication of the visual world in the different areas illustrates (approximately) the known retinotopic organization of these different areas.
A role for glutamatergic feedback projections in producing the local effects of attention has been demonstrated by studies of the frontal eye fields (FEF). FEF projects to extrastriate visual cortices, these feedback projections are glutamatergic, and spiking activity in FEF correlates with attention187. Elegant studies have shown that microstimulation of appropriate parts of FEF shift the focus of attention as measured at the behavioural level188, and produce a modulation of neuronal coding in V4 similar to that seen with attention189. As described above, a combination of cholinergic and tonic glutamatergic input is likely to place cortex in a strongly desynchronized state.
The mechanisms by which glutamatergic feedback could increase the gain of visual cortical responses are unknown. However, one intriguing suggestion involves the NMDA receptor. Because of the voltage-dependent nature of NMDAR-mediated glutamate responses, postsynaptic depolarization might multiplicatively increase the excitatory currents induced by other inputs190, a proposal supported by recent results on the effects of NMDA antagonists on responses in macaque V1 during a visual task191. Additionally, it is possible that focused glutamatergic input could cause localized ACh release, either through presynaptic glutamate receptors on cholinergic fibres192, 193 or by activating cholinergic cortical interneurons194.
Summary and outlook
Large changes in cortical state are seen between waking and sleep. These changes are controlled by alterations in neuromodulatory and tonic glutamatergic input, which change the dynamics of cortical networks and alter their propensity to generate fluctuations. Recent research suggests that similar mechanisms control the more subtle variations in state seen in awake animals. Furthermore, it appears that cortical state can be controlled at a local level, with the strongest desynchronization seen in patches of cortex that represent attended stimuli. Computational, in vitro, and in vivo research in anaesthetized animals suggests that these fluctuations are generated by excitable system dynamics, reflecting the interaction of fast recurrent excitation and slower adaptive processes; further research is required to verify that excitable system dynamics also underpin the more subtle spontaneous fluctuations seen in quiescent or inattentive awake animals.
Cortical state appears to be a multidimensional continuum, with variables such as gamma power and interneuron firing rate showing diverse changes depending on how desynchronization was induced. Future work with modern techniques such as optogenetics and enzyme-linked electrochemistry may soon reveal how multiple neuromodulatory systems shift cortical operating mode in a multidimensional space of states.
The effects of cortical state on sensory responses are complex. However, a theme emerging from work in multiple modalities is that sudden, punctate stimuli are able to generate large cortical responses in both states, whereas temporally extended stimuli are only faithfully represented in cortex during desynchronized states. This could be a manifestation of a more general phenomenon in which stimuli of strong bottom-up salience can produce large responses regardless of state, whereas desynchronization enhances responses to stimuli that are of themselves more subtle while decreasing the response to unattended and bottom-up salient stimuli. Future work is required to test this hypothesis.
Cortical state does not simply affect sensory responses, but also changes the character of cortical spontaneous activity. More synchronized states are defined by larger low-frequency fluctuations in population firing and local field potentials, as well as higher spontaneous and noise correlations. Similar effects are found locally within cortical columns that represent unattended stimuli. What function might these spontaneous fluctuations have, and why would they be preferentially expressed in cortical areas that are not involved in representing attended stimuli? We suggest two, non-exclusive, possibilities. The first is that synchronized states represent a “power save” mode. Synaptic activity uses a great deal of energy, and if fluctuating activity means a column is electrically active less of the time, this presumably releases more energy for other functions. The second possibility is that the fluctuations of the synchronized state are themselves a signature of non-sensory information processing. In the absence of sensory input, the brain is still active, engaged in processes such as mental imagery and memory recall, which presumably arise from structured cortical activity independent of external stimuli. Human fMRI studies have revealed complex large-scale fluctuations in BOLD activity, which are at least partially related to the underlying anatomical connectivity matrix195, 196. Understanding the relationship between BOLD and cortical state is an important topic for future research (Box 3).
Box 3. Cortical state and resting state networks.
A complementary view on spontaneous cortical activity comes from studies in humans using functional magnetic resonance imaging (fMRI)195, 196. As with electrical recordings, these studies suggest that spontaneous activity is not simply noise, but a structured signal related to behavioural and cognitive factors. Spontaneous BOLD activity shows slow (<0.1 Hz) spatiotemporally patterned fluctuations, with positive correlations within a number of “resting state networks” but weaker or negative correlations between networks201, 202. The resting state networks consist of groups of functionally and anatomically related cortical areas, for example sets of regions involved in visual or auditory processing, as well as a “default mode network” whose activity is greatest in resting subjects. Fluctuations in the spontaneous activity of these networks correlate with natural fluctuations in task performance203.
How might these observations relate to the phenomena described in this Review? It seems unlikely that spontaneous fluctuations in BOLD correspond to up and down phases, as their timecourse is at least an order of magnitude slower. Instead, a number of observations suggest that the BOLD signal may be related to local cortical state. BOLD correlates with LFP power both spontaneously and in response to sensory stimuli, with the strongest correlations seen in the gamma band204, 205; visual cortical BOLD increases with visual attention in a topographic manner, with larger increases for more difficult detection tasks206; and cortical blood flow can be controlled by neuromodulatory activity207. These findings would seem to suggest that BOLD should increase during cortical desynchronization. Nevertheless the exact relationship of BOLD to cortical state is not yet fully clear. Although BOLD reliably correlates with gamma power, the correlation between spontaneous BOLD and lower-frequency LFP power varies between animals and can be positive204. This result might appear puzzling, as gamma and low-frequency power are themselves typically anticorrelated. One explanation may be that the space of cortical states is high dimensional. Under some conditions, gamma and low frequency power exhibit a positive correlation21, indicating that different LFP power bands may relate differently to a high dimensional space of states; understanding the relationship of the BOLD signal to this space is an important topic of future work.
Although there are many similarities in the structure of spontaneous and sensory-evoked cortical activity patterns, they differ in their laminar profile, with spontaneous up phases initiated not in the thalamorecipient cortical layers but in the deep cortical layers, which receive feedback projections from higher order cortices10, 197. In addition, unlike sensory-evoked activity, spontaneous up phases typically cover large areas of cortex and could for example reflect coordinated recapitulation of recalled memories, including modality-specific reactivation of appropriate sensory areas.
From this perspective, suppression of spontaneous fluctuations could be interpreted as a form of attention — not between competing sensory stimuli, but between the internal and external worlds. Subjective experience suggests that when we need to detect a subtle stimulus such as a quiet sound, internally generated distractors (such as a tune running through one’s head) can be as much of a hindrance as competing external stimuli. Accurate detection of subtle stimuli might thus require suppression of structured spontaneous activity as well as nonattended sensory inputs. Systems to control the state of cortical processing in a column-specific manner may provide animals with a “toolkit” with which to adaptively route sensory and nonsensory information through the brain, to help them survive in a complex and changing world.
Acknowledgments
We thank J. Reynolds, H. Dringenberg, R. Malach, S. David and M. Okun for helpful conversations and G. Buzsaki, M. Carandini, and T. Mrsic-Flogel for comments on the manuscript. Research in the Harris lab is supported by NIH (MH073245 and DC009947), NSF (SBE-0542013 to the Temporal Dynamics of Learning Center), EPSRC (EP/I005102), and a Royal Society Wolfson Research Merit award. Research in the Thiele lab is supported by supported by the BBSRC (BBS/B/09325) and the Wellcome Trust (070380/Z/03/Z).
Glossary
- Electroencephalogram
an electrical recording made from the scalp, which reflects the global structure of cortical synaptic activity
- Population rate
the mean of the firing rates of all neurons in a population. Population rate does not denote an average over multiple presentations of a stimulus, but rather the averaged activity of multiple neurons at a single moment in time
- Local field potential (LFP)
An electrical potential measured from extracellular space. LFP reflects primarily synaptic activity rather than action potential waveforms
- Depth-negative/Depth-positive
LFP waves where a negativity/positivity is seen in the subgranular layers. This laminar specification of polarity of LFP waves is needed because cortical local field potentials typically show a reversal at or around the middle layers
- Excitable systems
a class of dynamical system models used to describe various physical, chemical, and biological phenomena including lasers, chemical oscillations, heartbeat, and action potential generation. These systems reflect a combination of fast positive feedback that amplifies small perturbations, with slower negative feedback that brings the system back to baseline once fluctuations become large
References
- 1.Ringach DL. Spontaneous and driven cortical activity: implications for computation. Curr Opin Neurobiol. 2009;19:439–44. doi: 10.1016/j.conb.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steriade M, McCarley RW. Brainstem control of wakefulness and sleep. Plenum Press; New York: 2005. [Google Scholar]
- 3.Greenberg DS, Houweling AR, Kerr JN. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat Neurosci. 2008;11:749–51. doi: 10.1038/nn.2140. [DOI] [PubMed] [Google Scholar]
- 4.DeWeese MR, Zador AM. Non-Gaussian membrane potential dynamics imply sparse, synchronous activity in auditory cortex. J Neurosci. 2006;26:12206–12218. doi: 10.1523/JNEUROSCI.2813-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat Neurosci. 2006;9:608–10. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- 6.Ferezou I, et al. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56:907–923. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–5. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- 8.Luczak A, Bartho P, Harris KD. Spontaneous events outline the realm of possible sensory responses in neocortical populations. Neuron. 2009;62:413–25. doi: 10.1016/j.neuron.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luczak A, Bartho P, Marguet SL, Buzsaki G, Harris KD. Sequential structure of neocortical spontaneous activity in vivo. Proc Natl Acad Sci US A. 2007;104:347–352. doi: 10.1073/pnas.0605643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakata S, Harris KD. Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron. 2009;64:404–18. doi: 10.1016/j.neuron.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okun M, Naim A, Lampl I. The subthreshold relation between cortical local field potential and neuronal firing unveiled by intracellular recordings in awake rats. J Neurosci. 2010;30:4440–8. doi: 10.1523/JNEUROSCI.5062-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–4. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 13.Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol. 1993;70:909–19. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J Neurosci. 1999;19:1736–53. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts M, Delicato LS, Herrero J, Gieselmann MA, Thiele A. Attention alters spatial integration in macaque V1 in an eccentricity-dependent manner. Nat Neurosci. 2007;10:1483–91. doi: 10.1038/nn1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roelfsema PR, Lamme VA, Spekreijse H. Object-based attention in the primary visual cortex of the macaque monkey. Nature. 1998;395:376–81. doi: 10.1038/26475. [DOI] [PubMed] [Google Scholar]
- 17.Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–41. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- 18.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–88. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009;12:1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chalk M, et al. Attention reduces stimulus-driven gamma frequency oscillations and spike field coherence in V1. Neuron. 2010;66:114–25. doi: 10.1016/j.neuron.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khayat PS, Niebergall R, Martinez-Trujillo JC. Frequency-dependent attentional modulation of local field potential signals in macaque area MT. J Neurosci. 2010;30:7037–48. doi: 10.1523/JNEUROSCI.0404-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007;55:131–41. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–71. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- 25.Tsodyks M, Kenet T, Grinvald A, Arieli A. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science. 1999;286:1943–1946. doi: 10.1126/science.286.5446.1943. [DOI] [PubMed] [Google Scholar]
- 26.Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. Spontaneously emerging cortical representations of visual attributes. Nature. 2003;425:954–956. doi: 10.1038/nature02078. [DOI] [PubMed] [Google Scholar]
- 27.Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci US A. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerr JN, Greenberg D, Helmchen F. Imaging input and output of neocortical networks in vivo. Proc Natl Acad Sci US A. 2005;102:14063–14068. doi: 10.1073/pnas.0506029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiser J, Chiu C, Weliky M. Small modulation of ongoing cortical dynamics by sensory input during natural vision. Nature. 2004;431:573–578. doi: 10.1038/nature02907. [DOI] [PubMed] [Google Scholar]
- 30.Berkes P, Orban G, Lengyel M, Fiser J. Spontaneous cortical activity reveals hallmarks of an optimal internal model of the environment. Science. 2011;331:83–7. doi: 10.1126/science.1195870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curto C, Sakata S, Marguet S, Itskov V, Harris KD. A simple model of cortical dynamics explains variability and state dependence of sensory responses in urethane-anesthetized auditory cortex. J Neurosci. 2009;29:10600–12. doi: 10.1523/JNEUROSCI.2053-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renart A, et al. The asynchronous state in cortical circuits. Science. 2010;327:587–90. doi: 10.1126/science.1179850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith MA, Kohn A. Spatial and temporal scales of neuronal correlation in primary visual cortex. J Neurosci. 2008;28:12591–603. doi: 10.1523/JNEUROSCI.2929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nauhaus I, Busse L, Carandini M, Ringach DL. Stimulus contrast modulates functional connectivity in visual cortex. Nat Neurosci. 2009;12:70–6. doi: 10.1038/nn.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993;13:3266–83. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 37.Okun M, Lampl I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat Neurosci. 2008;11:535–7. doi: 10.1038/nn.2105. [DOI] [PubMed] [Google Scholar]
- 38.Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–9. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanderwolf CH. Are neocortical gamma waves related to consciousness? Brain Res. 2000;855:217–24. doi: 10.1016/s0006-8993(99)02351-3. [DOI] [PubMed] [Google Scholar]
- 40.Worgotter F, et al. State-dependent receptive-field restructuring in the visual cortex. Nature. 1998;396:165–8. doi: 10.1038/24157. [DOI] [PubMed] [Google Scholar]
- 41.Clement EA, et al. Cyclic and sleep-like spontaneous alternations of brain state under urethane anaesthesia. PLoS ONE. 2008;3:e2004. doi: 10.1371/journal.pone.0002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–85. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 43.Volgushev M, Chauvette S, Mukovski M, Timofeev I. Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave oscillations [corrected] J Neurosci. 2006;26:5665–72. doi: 10.1523/JNEUROSCI.0279-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fanselow EE, Connors BW. The roles of somatostatin-expressing (GIN) and fast-spiking inhibitory interneurons in UP-DOWN states of mouse neocortex. J Neurophysiol. 2010;104:596–606. doi: 10.1152/jn.00206.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CC. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron. 2010;65:422–35. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- 47.Katzner S, et al. Local origin of field potentials in visual cortex. Neuron. 2009;61:35–41. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Einevoll GT, et al. Laminar population analysis: estimating firing rates and evoked synaptic activity from multielectrode recordings in rat barrel cortex. J Neurophysiol. 2007;97:2174–2190. doi: 10.1152/jn.00845.2006. [DOI] [PubMed] [Google Scholar]
- 49.Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26:4535–45. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saleem AB, Chadderton P, Apergis-Schoute J, Harris KD, Schultz SR. Methods for predicting cortical UP and DOWN states from the phase of deep layer local field potentials. J Comput Neurosci. 2010;29:49–62. doi: 10.1007/s10827-010-0228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–24. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 52.Munk MH, Roelfsema PR, Konig P, Engel AK, Singer W. Role of reticular activation in the modulation of intracortical synchronization [see comments] Science. 1996;272:271–4. doi: 10.1126/science.272.5259.271. [DOI] [PubMed] [Google Scholar]
- 53.Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brett B, Barth DS. Subcortical modulation of high-frequency (gamma band) oscillating potentials in auditory cortex. J Neurophysiol. 1997;78:573–81. doi: 10.1152/jn.1997.78.2.573. [DOI] [PubMed] [Google Scholar]
- 55.Steriade M, Dossi RC, Pare D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Natl Acad Sci U S A. 1991;88:4396–400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puig MV, Watakabe A, Ushimaru M, Yamamori T, Kawaguchi Y. Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J Neurosci. 2010;30:2211–22. doi: 10.1523/JNEUROSCI.3335-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henrie JA, Shapley R. LFP power spectra in V1 cortex: the graded effect of stimulus contrast. J Neurophysiol. 2005;94:479–90. doi: 10.1152/jn.00919.2004. [DOI] [PubMed] [Google Scholar]
- 58.Gieselmann MA, Thiele A. Comparison of spatial integration and surround suppression characteristics in spiking activity and the local field potential in macaque V1. Eur J Neurosci. 2008;28:447–59. doi: 10.1111/j.1460-9568.2008.06358.x. [DOI] [PubMed] [Google Scholar]
- 59.Leopold DA, Murayama Y, Logothetis NK. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex. 2003;13:422–33. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- 60.Destexhe A, Contreras D, Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J Neurosci. 1999;19:4595–4608. doi: 10.1523/JNEUROSCI.19-11-04595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferezou I, Bolea S, Petersen CC. Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron. 2006;50:617–29. doi: 10.1016/j.neuron.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 62.Han F, Caporale N, Dan Y. Reverberation of recent visual experience in spontaneous cortical waves. Neuron. 2008;60:321–7. doi: 10.1016/j.neuron.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benucci A, Frazor RA, Carandini M. Standing waves and traveling waves distinguish two circuits in visual cortex. Neuron. 2007;55:103–17. doi: 10.1016/j.neuron.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song WJ, et al. Cortical intrinsic circuits can support activity propagation through an isofrequency strip of the guinea pig primary auditory cortex. Cereb Cortex. 2006;16:718–729. doi: 10.1093/cercor/bhj018. [DOI] [PubMed] [Google Scholar]
- 66.Mohajerani MH, McVea DA, Fingas M, Murphy TH. Mirrored bilateral slow-wave cortical activity within local circuits revealed by fast bihemispheric voltage-sensitive dye imaging in anesthetized and awake mice. J Neurosci. 2010;30:3745–51. doi: 10.1523/JNEUROSCI.6437-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13:3284–99. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slezia A, Hangya B, Ulbert I, Acsady L. Phase advancement and nucleus-specific timing of thalamocortical activity during slow cortical oscillation. J Neurosci. 2011;31:607–17. doi: 10.1523/JNEUROSCI.3375-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stern EA, Kincaid AE, Wilson CJ. Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. J Neurophysiol. 1997;77:1697–715. doi: 10.1152/jn.1997.77.4.1697. [DOI] [PubMed] [Google Scholar]
- 70.Mahon S, et al. Distinct patterns of striatal medium spiny neuron activity during the natural sleep-wake cycle. J Neurosci. 2006;26:12587–95. doi: 10.1523/JNEUROSCI.3987-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ros H, Sachdev RN, Yu Y, Sestan N, McCormick DA. Neocortical networks entrain neuronal circuits in cerebellar cortex. J Neurosci. 2009;29:10309–20. doi: 10.1523/JNEUROSCI.2327-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Battaglia FP, Sutherland GR, McNaughton BL. Hippocampal sharp wave bursts coincide with neocortical “up-state” transitions. Learn Mem. 2004;11:697–704. doi: 10.1101/lm.73504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–7. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- 75.O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–30. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 76.Harris KD, et al. Spike train dynamics predicts theta-related phase precession in hippocampal pyramidal cells. Nature. 2002;417:738–741. doi: 10.1038/nature00808. [DOI] [PubMed] [Google Scholar]
- 77.Mehta MR, Lee AK, Wilson MA. Role of experience and oscillations in transforming a rate code into a temporal code. Nature. 2002;417:741–746. doi: 10.1038/nature00807. [DOI] [PubMed] [Google Scholar]
- 78.Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci. 1999;19:274–87. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 80.Ulanovsky N, Moss CF. Hippocampal cellular and network activity in freely moving echolocating bats. Nat Neurosci. 2007;10:224–33. doi: 10.1038/nn1829. [DOI] [PubMed] [Google Scholar]
- 81.Winson J. Interspecies differences in the occurrence of theta. Behav Biol. 1972;7:479–87. doi: 10.1016/s0091-6773(72)80210-4. [DOI] [PubMed] [Google Scholar]
- 82.Vanderwolf CH. An odyssey through the brain, behavior, and the mind. Kluwer Academic Pub; Boston: 2003. [Google Scholar]
- 83.Buzsaki G, et al. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–26. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vanderwolf CH, Pappas BA. Reserpine abolishes movement-correlated atropine-resistant neocortical low voltage fast activity. Brain Res. 1980;202:79–94. [PubMed] [Google Scholar]
- 85.Gerstein GL, Perkel DH. Simultaneously recorded trains of action potentials: analysis and functional interpretation. Science. 1969;164:828–30. doi: 10.1126/science.164.3881.828. [DOI] [PubMed] [Google Scholar]
- 86.Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci. 1998;18:3870–96. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diesmann M, Gewaltig MO, Aertsen A. Stable propagation of synchronous spiking in cortical neural networks. Nature. 1999;402:529–533. doi: 10.1038/990101. [DOI] [PubMed] [Google Scholar]
- 88.Reyes AD. Synchrony-dependent propagation of firing rate in iteratively constructed networks in vitro. Nat Neurosci. 2003;6:593–9. doi: 10.1038/nn1056. [DOI] [PubMed] [Google Scholar]
- 89.Thiele A, Hoffmann KP. Neuronal firing rate, inter-neuron correlation and synchrony in area MT are correlated with directional choices during stimulus and reward expectation. Exp Brain Res. 2008;188:559–77. doi: 10.1007/s00221-008-1391-z. [DOI] [PubMed] [Google Scholar]
- 90.Gawne TJ, Richmond BJ. How independent are the messages carried by adjacent inferior temporal cortical neurons? J Neurosci. 1993;13:2758–71. doi: 10.1523/JNEUROSCI.13-07-02758.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bair W, Zohary E, Newsome WT. Correlated firing in macaque visual area MT: time scales and relationship to behavior. J Neurosci. 2001;21:1676–1697. doi: 10.1523/JNEUROSCI.21-05-01676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zohary E, Shadlen MN, Newsome WT. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature. 1994;370:140–3. doi: 10.1038/370140a0. [DOI] [PubMed] [Google Scholar]
- 93.Kohn A, Smith MA. Stimulus dependence of neuronal correlation in primary visual cortex of the macaque. J Neurosci. 2005;25:3661–73. doi: 10.1523/JNEUROSCI.5106-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ecker AS, et al. Decorrelated neuronal firing in cortical microcircuits. Science. 2010;327:584–7. doi: 10.1126/science.1179867. [DOI] [PubMed] [Google Scholar]
- 95.Moore GP, Segundo JP, Perkel DH, Levitan H. Statistical signs of synaptic interaction in neurons. Biophys J. 1970;10:876–900. doi: 10.1016/S0006-3495(70)86341-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- 97.Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- 98.Shu YS, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- 99.Crochet S, Chauvette S, Boucetta S, Timofeev I. Modulation of synaptic transmission in neocortex by network activities. Eur J Neurosci. 2005;21:1030–44. doi: 10.1111/j.1460-9568.2005.03932.x. [DOI] [PubMed] [Google Scholar]
- 100.Rigas P, Castro-Alamancos MA. Impact of persistent cortical activity (up States) on intracortical and thalamocortical synaptic inputs. J Neurophysiol. 2009;102:119–31. doi: 10.1152/jn.00126.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cunningham MO, et al. Neuronal metabolism governs cortical network response state. Proc Natl Acad Sci U S A. 2006;103:5597–601. doi: 10.1073/pnas.0600604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Compte A, Sanchez-Vives MV, McCormick DA, Wang XJ. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J Neurophysiol. 2003;89:2707–25. doi: 10.1152/jn.00845.2002. [DOI] [PubMed] [Google Scholar]
- 103.Holcman D, Tsodyks M. The emergence of Up and Down states in cortical networks. PLoS Comput Biol. 2006;2:e23. doi: 10.1371/journal.pcbi.0020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Model of thalamocortical slow-wave sleep oscillations and transitions to activated States. J Neurosci. 2002;22:8691–704. doi: 10.1523/JNEUROSCI.22-19-08691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hill S, Tononi G. Modeling sleep and wakefulness in the thalamocortical system. J Neurophysiol. 2005;93:1671–98. doi: 10.1152/jn.00915.2004. [DOI] [PubMed] [Google Scholar]
- 106.Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: an intracellular study. Proc Natl Acad Sci U S A. 2001;98:1924–9. doi: 10.1073/pnas.041430398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Farkas I, Helbing D, Vicsek T. Mexican waves in an excitable medium. Nature. 2002;419:131–2. doi: 10.1038/419131a. [DOI] [PubMed] [Google Scholar]
- 108.Steriade M, Buzsaki G. In: Brain Cholinergic Systems. Steriade M, Biesold D, editors. Oxford University Press; Oxford: 1990. pp. 3–62. [Google Scholar]
- 109.Mena-Segovia J, Sims HM, Magill PJ, Bolam JP. Cholinergic brainstem neurons modulate cortical gamma activity during slow oscillations. J Physiol. 2008;586:2947–60. doi: 10.1113/jphysiol.2008.153874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boucetta S, Jones BE. Activity profiles of cholinergic and intermingled GABAergic and putative glutamatergic neurons in the pontomesencephalic tegmentum of urethane-anesthetized rats. J Neurosci. 2009;29:4664–74. doi: 10.1523/JNEUROSCI.5502-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Manns ID, Alonso A, Jones BE. Discharge properties of juxtacellularly labeled and immunohistochemically identified cholinergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000;20:1505–18. doi: 10.1523/JNEUROSCI.20-04-01505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Duque A, Balatoni B, Detari L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. J Neurophysiol. 2000;84:1627–1635. doi: 10.1152/jn.2000.84.3.1627. [DOI] [PubMed] [Google Scholar]
- 113.Kaur S, Junek A, Black MA, Semba K. Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats. J Neurosci. 2008;28:491–504. doi: 10.1523/JNEUROSCI.1585-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci. 1991;11:3135–45. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dringenberg HC, Vanderwolf CH. Neocortical activation: modulation by multiple pathways acting on central cholinergic and serotonergic systems. Exp Brain Res. 1997;116:160–74. doi: 10.1007/pl00005736. [DOI] [PubMed] [Google Scholar]
- 116.Berridge CW, Bolen SJ, Manley MS, Foote SL. Modulation of forebrain electroencephalographic activity in halothane-anesthetized rat via actions of noradrenergic beta-receptors within the medial septal region. J Neurosci. 1996;16:7010–20. doi: 10.1523/JNEUROSCI.16-21-07010.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berridge CW, Foote SL. Enhancement of behavioral and electroencephalographic indices of waking following stimulation of noradrenergic beta-receptors within the medial septal region of the basal forebrain. J Neurosci. 1996;16:6999–7009. doi: 10.1523/JNEUROSCI.16-21-06999.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vanderwolf CH, Baker GB. Evidence that serotonin mediates non-cholinergic neocortical low voltage fast activity, non-cholinergic hippocampal rhythmical slow activity and contributes to intelligent behavior. Brain Res. 1986;374:342–56. doi: 10.1016/0006-8993(86)90428-2. [DOI] [PubMed] [Google Scholar]
- 119.Vanderwolf CH, Leung LW, Baker GB, Stewart DJ. The role of serotonin in the control of cerebral activity: studies with intracerebral 5,7-dihydroxytryptamine. Brain Res. 1989;504:181–91. doi: 10.1016/0006-8993(89)91355-3. [DOI] [PubMed] [Google Scholar]
- 120.Constantinople CM, Bruno RM. Effects and mechanisms of wakefulness on local cortical networks. Neuron. 2011;69:1061–8. doi: 10.1016/j.neuron.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rajkowski J, Majczynski H, Clayton E, Aston-Jones G. Activation of monkey locus coeruleus neurons varies with difficulty and performance in a target detection task. J Neurophysiol. 2004;92:361–71. doi: 10.1152/jn.00673.2003. [DOI] [PubMed] [Google Scholar]
- 122.Clayton EC, Rajkowski J, Cohen JD, Aston-Jones G. Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. J Neurosci. 2004;24:9914–20. doi: 10.1523/JNEUROSCI.2446-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koyama Y, Jodo E, Kayama Y. Sensory responsiveness of “broad-spike” neurons in the laterodorsal tegmental nucleus, locus coeruleus and dorsal raphe of awake rats: implications for cholinergic and monoaminergic neuron-specific responses. Neuroscience. 1994;63:1021–31. doi: 10.1016/0306-4522(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 124.Lin SC, Nicolelis MA. Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence. Neuron. 2008;59:138–49. doi: 10.1016/j.neuron.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Richardson RT, DeLong MR. Context-dependent responses of primate nucleus basalis neurons in a go/no-go task. J Neurosci. 1990;10:2528–40. doi: 10.1523/JNEUROSCI.10-08-02528.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nakamura K, Matsumoto M, Hikosaka O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J Neurosci. 2008;28:5331–43. doi: 10.1523/JNEUROSCI.0021-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bromberg-Martin ES, Hikosaka O, Nakamura K. Coding of task reward value in the dorsal raphe nucleus. J Neurosci. 2010;30:6262–72. doi: 10.1523/JNEUROSCI.0015-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ranade SP, Mainen ZF. Transient firing of dorsal raphe neurons encodes diverse and specific sensory, motor, and reward events. J Neurophysiol. 2009;102:3026–37. doi: 10.1152/jn.00507.2009. [DOI] [PubMed] [Google Scholar]
- 129.Okada K, Toyama K, Inoue Y, Isa T, Kobayashi Y. Different pedunculopontine tegmental neurons signal predicted and actual task rewards. J Neurosci. 2009;29:4858–70. doi: 10.1523/JNEUROSCI.4415-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Otazu GH, Tai LH, Yang Y, Zador AM. Engaging in an auditory task suppresses responses in auditory cortex. Nat Neurosci. 2009;12:646–54. doi: 10.1038/nn.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Castro-Alamancos MA. Different temporal processing of sensory inputs in the rat thalamus during quiescent and information processing states in vivo. J Physiol. 2002;539:567–578. doi: 10.1113/jphysiol.2001.013283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009;12:1444–9. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bezdudnaya T, et al. Thalamic burst mode and inattention in the awake LGNd. Neuron. 2006;49:421–32. doi: 10.1016/j.neuron.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 134.Fanselow EE, Sameshima K, Baccala LA, Nicolelis MA. Thalamic bursting in rats during different awake behavioral states. Proc Natl Acad Sci U S A. 2001;98:15330–5. doi: 10.1073/pnas.261273898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McCormick DA, Prince DA. Acetylcholine induces burst firing in thalamic reticular neurones by activating a potassium conductance. Nature. 1986;319:402–5. doi: 10.1038/319402a0. [DOI] [PubMed] [Google Scholar]
- 136.McCormick DA, Prince DA. Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. J Physiol. 1987;392:147–65. doi: 10.1113/jphysiol.1987.sp016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hirata A, Aguilar J, Castro-Alamancos MA. Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. J Neurosci. 2006;26:4426–36. doi: 10.1523/JNEUROSCI.5298-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hirata A, Castro-Alamancos MA. Neocortex network activation and deactivation states controlled by the thalamus. J Neurophysiol. 2010;103:1147–57. doi: 10.1152/jn.00955.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kawaguchi Y, Shindou T. Noradrenergic excitation and inhibition of GABAergic cell types in rat frontal cortex. J Neurosci. 1998;18:6963–76. doi: 10.1523/JNEUROSCI.18-17-06963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kawaguchi Y. Selective cholinergic modulation of cortical GABAergic cell subtypes. J Neurophysiol. 1997;78:1743–7. doi: 10.1152/jn.1997.78.3.1743. [DOI] [PubMed] [Google Scholar]
- 141.Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–8. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- 142.Beique JC, et al. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci. 2004;24:4807–17. doi: 10.1523/JNEUROSCI.5113-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Villalobos C, Beique JC, Gingrich JA, Andrade R. Serotonergic regulation of calcium-activated potassium currents in rodent prefrontal cortex. Eur J Neurosci. 2005;22:1120–6. doi: 10.1111/j.1460-9568.2005.04307.x. [DOI] [PubMed] [Google Scholar]
- 144.Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron. 2008;58:911–24. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lawrence JJ. Cholinergic control of GABA release: emerging parallels between neocortex and hippocampus. Trends Neurosci. 2008;31:317–27. doi: 10.1016/j.tins.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 146.Dinh L, Nguyen T, Salgado H, Atzori M. Norepinephrine homogeneously inhibits alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate- (AMPAR-) mediated currents in all layers of the temporal cortex of the rat. Neurochem Res. 2009;34:1896–906. doi: 10.1007/s11064-009-9966-z. [DOI] [PubMed] [Google Scholar]
- 147.Eggermann E, Feldmeyer D. Cholinergic filtering in the recurrent excitatory microcircuit of cortical layer 4. Proc Natl Acad Sci U S A. 2009;106:11753–8. doi: 10.1073/pnas.0810062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dembrow NC, Chitwood RA, Johnston D. Projection-specific neuromodulation of medial prefrontal cortex neurons. J Neurosci. 2010;30:16922–37. doi: 10.1523/JNEUROSCI.3644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Giocomo LM, Hasselmo ME. Neuromodulation by glutamate and acetylcholine can change circuit dynamics by regulating the relative influence of afferent input and excitatory feedback. Mol Neurobiol. 2007;36:184–200. doi: 10.1007/s12035-007-0032-z. [DOI] [PubMed] [Google Scholar]
- 150.Wang Z, McCormick DA. Control of firing mode of corticotectal and corticopontine layer V burst-generating neurons by norepinephrine, acetylcholine, and 1S,3R- ACPD. J Neurosci. 1993;13:2199–216. doi: 10.1523/JNEUROSCI.13-05-02199.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Roberts MJ, et al. Acetylcholine dynamically controls spatial integration in marmoset primary visual cortex. J Neurophysiol. 2005;93:2062–72. doi: 10.1152/jn.00911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zinke W, et al. Cholinergic modulation of response properties and orientation tuning of neurons in primary visual cortex of anaesthetized Marmoset monkeys. Eur J Neurosci. 2006;24:314–28. doi: 10.1111/j.1460-9568.2006.04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schwindt PC, Spain WJ, Foehring RC, Chubb MC, Crill WE. Slow conductances in neurons from cat sensorimotor cortex in vitro and their role in slow excitability changes. J Neurophysiol. 1988;59:450–67. doi: 10.1152/jn.1988.59.2.450. [DOI] [PubMed] [Google Scholar]
- 154.Foehring RC, Schwindt PC, Crill WE. Norepinephrine selectively reduces slow Ca2+-and Na+-mediated K+ currents in cat neocortical neurons. J Neurophysiol. 1989;61:245–56. doi: 10.1152/jn.1989.61.2.245. [DOI] [PubMed] [Google Scholar]
- 155.Sourdet V, Russier M, Daoudal G, Ankri N, Debanne D. Long-term enhancement of neuronal excitability and temporal fidelity mediated by metabotropic glutamate receptor subtype 5. J Neurosci. 2003;23:10238–48. doi: 10.1523/JNEUROSCI.23-32-10238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McCormick DA, Wang Z, Huguenard J. Neurotransmitter control of neocortical neuronal activity and excitability. Cereb Cortex. 1993;3:387–98. doi: 10.1093/cercor/3.5.387. [DOI] [PubMed] [Google Scholar]
- 157.Nicoll RA. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988;241:545–51. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- 158.Sidiropoulou K, et al. Dopamine modulates an mGluR5-mediated depolarization underlying prefrontal persistent activity. Nat Neurosci. 2009;12:190–9. doi: 10.1038/nn.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron. 1997;19:679–86. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- 160.Hasselmo ME. Neuromodulation and cortical function: modeling the physiological basis of behavior. Behav Brain Res. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- 161.Hasselmo ME, Linster C, Patil M, Ma D, Cekic M. Noradrenergic suppression of synaptic transmission may influence cortical signal-to-noise ratio. J Neurophysiol. 1997;77:3326–39. doi: 10.1152/jn.1997.77.6.3326. [DOI] [PubMed] [Google Scholar]
- 162.Hagenston AM, Fitzpatrick JS, Yeckel MF. MGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons. Cereb Cortex. 2008;18:407–23. doi: 10.1093/cercor/bhm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Carr DB, Surmeier DJ. M1 muscarinic receptor modulation of Kir2 channels enhances temporal summation of excitatory synaptic potentials in prefrontal cortex pyramidal neurons. J Neurophysiol. 2007;97:3432–8. doi: 10.1152/jn.00828.2006. [DOI] [PubMed] [Google Scholar]
- 164.Chen Y, et al. Task difficulty modulates the activity of specific neuronal populations in primary visual cortex. Nat Neurosci. 2008;11:974–82. doi: 10.1038/nn.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30:16796–808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.David C, Schleicher A, Zuschratter W, Staiger JF. The innervation of parvalbumin-containing interneurons by VIP-immunopositive interneurons in the primary somatosensory cortex of the adult rat. Eur J Neurosci. 2007;25:2329–40. doi: 10.1111/j.1460-9568.2007.05496.x. [DOI] [PubMed] [Google Scholar]
- 167.Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nature Neuroscience. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- 168.Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–54. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fanselow EE, Nicolelis MA. Behavioral modulation of tactile responses in the rat somatosensory system. J Neurosci. 1999;19:7603–16. doi: 10.1523/JNEUROSCI.19-17-07603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Castro-Alamancos MA. Absence of rapid sensory adaptation in neocortex during information processing states. Neuron. 2004;41:455–64. doi: 10.1016/s0896-6273(03)00853-5. [DOI] [PubMed] [Google Scholar]
- 171.Atiani S, Elhilali M, David SV, Fritz JB, Shamma SA. Task difficulty and performance induce diverse adaptive patterns in gain and shape of primary auditory cortical receptive fields. Neuron. 2009;61:467–80. doi: 10.1016/j.neuron.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hasenstaub A, Sachdev RN, McCormick DA. State changes rapidly modulate cortical neuronal responsiveness. J Neurosci. 2007;27:9607–9622. doi: 10.1523/JNEUROSCI.2184-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Churchland MM, et al. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat Neurosci. 2010;13:369–78. doi: 10.1038/nn.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Castro-Alamancos MA. Role of thalamocortical sensory suppression during arousal: focusing sensory inputs in neocortex. J Neurosci. 2002;22:9651–5. doi: 10.1523/JNEUROSCI.22-22-09651.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dringenberg HC, Vanderwolf CH. Transcallosal evoked potentials: behavior-dependent modulation by muscarinic and serotonergic receptors. Brain Res Bull. 1994;34:555–62. doi: 10.1016/0361-9230(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 176.Marguet SL, Harris KD. State-dependent representation of amplitude-modulated noise stimuli in rat auditory cortex. J Neurosci. 2011;31:6414–20. doi: 10.1523/JNEUROSCI.5773-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci. 2001;24:122–6. doi: 10.1016/s0166-2236(00)01714-8. [DOI] [PubMed] [Google Scholar]
- 178.Cohen JD, Castro-Alamancos MA. Behavioral state dependency of neural activity and sensory (whisker) responses in superior colliculus. J Neurophysiol. 2010;104:1661–72. doi: 10.1152/jn.00340.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lyamin OI, Manger PR, Ridgway SH, Mukhametov LM, Siegel JM. Cetacean sleep: an unusual form of mammalian sleep. Neurosci Biobehav Rev. 2008;32:1451–84. doi: 10.1016/j.neubiorev.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pooresmaeili A, Poort J, Thiele A, Roelfsema PR. Separable codes for attention and luminance contrast in the primary visual cortex. J Neurosci. 2010;30:12701–11. doi: 10.1523/JNEUROSCI.1388-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Abbott LF, Dayan P. The effect of correlated variability on the accuracy of a population code. Neural Comput. 1999;11:91–101. doi: 10.1162/089976699300016827. [DOI] [PubMed] [Google Scholar]
- 182.Hudson AE, Schiff ND, Victor JD, Purpura KP. Attentional modulation of adaptation in V4. Eur J Neurosci. 2009;30:151–71. doi: 10.1111/j.1460-9568.2009.06803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Herrero JL, et al. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–4. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gieselmann MA, Thiele A. Society for Neuroscience Abstracts #429.7. 2010. [Google Scholar]
- 185.Zaborszky L. The modular organization of brain systems. Basal forebrain: the last frontier. Prog Brain Res. 2002;136:359–72. doi: 10.1016/s0079-6123(02)36030-8. [DOI] [PubMed] [Google Scholar]
- 186.Deco G, Thiele A. Cholinergic control of cortical network interactions enables feedback-mediated attentional modulation. Eur J Neurosci. 2011 doi: 10.1111/j.1460-9568.2011.07749.x. [DOI] [PubMed] [Google Scholar]
- 187.Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–10. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol. 2004;91:152–62. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- 189.Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–3. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- 190.Kepecs A, Raghavachari S. Gating information by two-state membrane potential fluctuations. J Neurophysiol. 2007;97:3015–23. doi: 10.1152/jn.01242.2006. [DOI] [PubMed] [Google Scholar]
- 191.Self MW, Super H, Roelfsema PR. Society for Neuroscience Abstracts #769.14. 2008. [Google Scholar]
- 192.Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2010;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Parikh V, Man K, Decker MW, Sarter M. Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex. J Neurosci. 2008;28:3769–80. doi: 10.1523/JNEUROSCI.5251-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.von Engelhardt J, Eliava M, Meyer AH, Rozov A, Monyer H. Functional characterization of intrinsic cholinergic interneurons in the cortex. J Neurosci. 2007;27:5633–42. doi: 10.1523/JNEUROSCI.4647-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- 196.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 197.Takeuchi D, Hirabayashi T, Tamura K, Miyashita Y. Reversal of interlaminar signal between sensory and memory processing in monkey temporal cortex. Science. 2011;331:1443–7. doi: 10.1126/science.1199967. [DOI] [PubMed] [Google Scholar]
- 198.Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kisley MA, Gerstein GL. Trial-to-trial variability and state-dependent modulation of auditory-evoked responses in cortex. J Neurosci. 1999;19:10451–60. doi: 10.1523/JNEUROSCI.19-23-10451.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsaki G. Organization of cell assemblies in the hippocampus. Nature. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- 201.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–83. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–84. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 204.Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci U S A. 2010;107:10238–43. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Goense JB, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol. 2008;18:631–40. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 206.Ress D, Backus BT, Heeger DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci. 2000;3:940–5. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- 207.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–64. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 208.Martinez-Trujillo JC, Treue S. Feature-based attention increases the selectivity of population responses in primate visual cortex. Curr Biol. 2004;14:744–51. doi: 10.1016/j.cub.2004.04.028. [DOI] [PubMed] [Google Scholar]