Abstract
Background
In 1997, guidelines were developed for the management of high-level ventilator-dependent patients with spinal cord injury who had little or no ventilator-free breathing ability (VFBA). This article describes the three categories of patients, the decannulation criteria, and the successful decannulation of four patients with no VFBA and electrophrenic/diaphragm pacing, using these criteria.
Method
Case series.
Conclusion
Lack of VFBA in patients with high-level spinal cord injury does not mandate tracheostomy or electrophrenic/diaphragm pacing.
Keywords: Glossopharyngeal breathing, Assisted cough, Mechanical insufflation–exsufflation, Spinal cord injuries, Tetraplegia, Respiratory therapy, Noninvasive mechanical ventilation, Electrophrenic pacing, Diaphragm pacing
Introduction
In 1997, we suggested guidelines for the management of ventilator-dependent patients with high-level spinal cord injury who have little or no ventilator-free breathing ability (VFBA).1 Three categories were described: (1) Patients with lesions extending into the brainstem who have severe bulbar-innervated muscle (bulbar) impairment and inability to protect the airways require tracheostomy tubes for airway protection. These patients may benefit from electrophrenic/diaphragm pacing (EPP/DP) for respiratory support. (2) Patients with high-level injuries who have sufficiently functional bulbar musculature to protect the airways, but without sufficient neck rotation to grab a mouthpiece for noninvasive ventilatory support (NVS). These patients can use mechanically assisted coughing (MAC) to clear the airways and EPP/DP around-the-clock or, in the event of pacer-associated sleep apneas, EPP/DP during daytime hours, NVS during sleep, and be safely decannulated. (3) Patients with or without VFBA who can grab a mouthpiece fixed adjacent to the mouth. These patients can be decannulated and use an intermittent abdominal pressure ventilator2 or NVS via a 15-mm angled mouthpiece during the day and nasal or oro-nasal interface overnight. Although many category (3) patients have been successfully decannulated,3–7 until now no one has described decannulation of ventilator-dependent patients with electrophrenic or diaphragm pacemakers.
The following cases satisfied our decannulation criteria, which included being fully alert and cooperative, having an oxyhemoglobin saturation baseline (SpO2) ≥95%, and manually assisted cough peak flows (CPF) exceeding 2.7 l/s (liters per second) with a capped tracheostomy tube or the tube out and the stoma covered (Table 1). In addition, all patients spoke clearly and received all nutrition by mouth. Following decannulation, all four patients had ostomy pressure dressings to permit use of NSV without air leaking out of the ostomy.8 All four stomas closed without complication and all maintained normal EtCO2 and SpO2 while using NVS.
Table 1.
Decannulation criteria for unweanable patients with SCI
| • Fully alert and cooperative, receiving no sedating medications |
| • Afebrile with normal white blood cell count |
| • PaCO2 40 mmHg or less at peak inspiratory pressures <30 cmH2O on up to full ventilatory support as needed |
| • Oxyhemoglobin saturation baseline (SpO2) ≥95% over 12 hours in ambient air |
| • All oxyhemoglobin desaturations below 95% reversed by MAC and suctioning via translaryngeal tube |
| • CPF, unassisted or assisted, exceed 2.7 l/s with the fenestrated tracheostomy tube capped or the tube out and the stoma covered |
Case 1
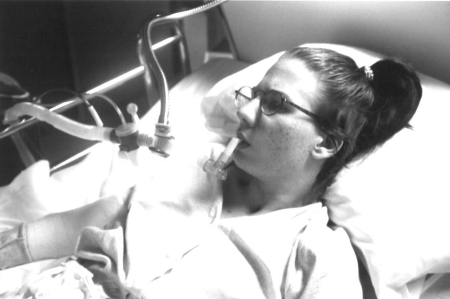
A 35-year old woman who at age 17 years sustained a complete ASIA A C1-C2 cervical spine injury in a motor vehicle collision. Following injury, she had no VFBA and a vital capacity (VC) just under 200 ml but intact bulbar musculature. She was trained in mouthpiece and nasal NVS with her fenestrated tracheostomy tube capped, then decannulated to NIV during a rehabilitation stay 4 months after her injury. She was initially continuously NVS dependent, but achieved 20–30 minutes of VFBA by using accessory muscles and glossopharyngeal breathing (GPB). She used NVS via a 15-mm angled mouth piece during daytime hours (Fig. 1) and via Lipseal (Phillips Respironics International Inc., Murraysville, PA, USA) or nasal interface overnight. Over the next 9 years, her VC slowly increased to 540 ml. She used air stacking to facilitate assisted coughing, maintain pulmonary compliance, and increase voice volume.9
Figure 1.
A 35-year-old woman with no autonomous breathing ability was decannulated, transitioned to mouth piece ventilation (15-mm piece for daytime use seen here and Lipseal for sleep), and ostomy closure. She had used noninvasive ventilation continuously for 17 years before undergoing tracheotomy, then 5 months later electrophrenic pacemaker placement, then subsequent re-decannulation and ostomy closure.
At age 34, she became septic from an unknown source, was hospitalized in an obtunded state, intubated, and placed on antibiotic and narcotic medication, and supplemental oxygen. After failing extubation and while heavily sedated on narcotic medication, her parents consented to her undergoing tracheotomy. Following tracheotomy she lost all VFBA and felt that her VC had decreased. Five months later, a phrenic pacemaker (EPP) was placed. She was able to use it for continuous ventilatory support for 6 months, but lost all ability to breathe when her pacer was turned off and her clinicians refused to decannulate her. Her loss of sustainable respiration was at least in part due to her pacer delivering 520-ml volumes at a set rate of 20/minute for >10 l/m of minute ventilation, which lowered her end-tidal CO2 to 21 mmHg.
She presented for possible re-decannulation. On examination, her injury was classified as C2 ASIA A; she had intact cognition but no neck rotation or pacer/VFBA. Lung fields were clear on auscultation; VC was 340 ml. Assisted CPF (abdominal thrust following air stacking to a maximum insufflation capacity or MIC)9 was 3.7 l/m with 2.6 l/m being the indication for safe extubation/decannulation and she satisfied all other decannulation criteria (Table 1).8
While using EPP she was re-decannulated in the outpatient clinic with only trace bleeding. A pressure dressing was placed.8 Oxygen saturation remained above 94% and she was discharged home 2 hours later. She resumed routine air stacking.9
Three months post-decannulation, VC had increased to 420 ml through use of accessory muscles and she re-developed pacer/VFBA. To cough effectively she switches off the pacer to air stack using mouthpiece NSV to 1980 ml for CPF of 2.7 l/s, whereas maximal paced breath supplemented by her own tidal volume is 920 ml for a CPF of only 1.2 l/s. Manually assisted CPF from an air stacked volume is 5 l/s. She reports that purulent secretions, previously cleared by suctioning 15 times per day, have completely resolved and that she ‘feel[s] so much better not having that tube in [her] neck’ and can once again breathe autonomously for 20–30 minutes.
Case 2
A 61-year-old man who at age 17, fell from a gym horse and sustained a C1 on C2 fracture dislocation and complete C2 tetraplegia 44 years ago. Following injury, he was apneic, was resuscitated, intubated, and underwent tracheostomy, but remained permanently institutionalized with no VFBA. He subsequently developed severe trachiectasis, chronic bronchitis, a respiratory arrest from airway mucus plugging that caused partial cortical blindness, and two near arrests at age 19 years before undergoing EPP, which was never adequate for continuous use. One month later, after practicing NVS, he was decannulated, transitioned to continuous NVS, and his pacemaker was removed. Although his supine VC remained unmeasurable, following decannulation his VC using his accessory muscles increased to 420 ml and he could use GPB for VFBA most of the day. He used NVS for 32 years of essentially continuous use when, depressed from long-term institutionalization and severe disability, he was euthanized.
Case 3
A 48 year old, who as a 28-year-old Greek soldier, sustained a C4 complete SCI requiring 20 hours per day of tracheostomy ventilation. He was transferred from Greece to New Jersey 20 years ago for EPP placement despite having VC of 750 ml and not requiring continuous support. Following pacer placement he was transferred to our rehabilitation unit to practice EPP, but it remained ineffective. Once taught NVS, he discontinued EPP trials, was decannulated, and had the pacemaker removed 6 months later. He used mouthpiece NVS during the day and nasal NVS at night. At 3-year followup, VC had increased to 880 ml, but he still required mouthpiece NVS for periods during daytime hours as well as nocturnal nasal NVS, which he continues to use.
Case 4
A 22-year-old man sustained ASIA A C1-C2 tetraplegia due to a fall 5 years ago. He was managed by continuous tracheostomy ventilation with no VFBA for almost 2 years when EPP (ATROTECH Atrostim Phrenic Nerve Stimulator) was placed. It provided adequate ventilation 5 hours per day.
Two and half years later, he was transferred for decannulation with a VC of 650 ml. A fenestrated, cuffless tube was placed. He practiced NVS, was able to air stack to 1300 ml,9 and combined stacking with abdominal thrusts to achieve assisted CPF of 3.7 l/s. He satisfied all Table 1 criteria and was decannulated to volume-cycled ventilation on volumes of 1400 ml and at a rate of 15/minute via a 15-mm angled mouthpiece during the day and a Lipseal during sleep. The EPP facilitated post-decannulation stomal closure, which occurred in 4 days.
After 3 days of continuous NVS and MAC to clear secretions, VC increased to 1100 ml, MIC to 1800 ml, assisted CPF to 4.7 l/s; he required fewer and fewer mouthpiece-assisted breaths and weaned to nocturnal-only NSV. He also learned and used GPB for independent air stacking to 1800 ml. He was discharged home 5 days post-decannulation. Due to patient preference, he stopped EPP except for occasion brief periods and used NSV for sleep via a Lipseal interface, maintaining a mean SpO2 of 96% during sleep.
Discussion
Cases 1 and 4 demonstrated decannulation facilitated by EPP but EPP/DP was indicated only for Case 1 in accordance with our published criteria.10 Case 2 also satisfied criteria for EPP/DP and decannulation but, although EPP was ineffective, he was decannulated anyway to NVS. Cases 3 and 4 did not satisfy criteria for EPP/DP and for both it was inadequate for continuous support. For Cases 1–3, the pacemakers were placed more than 10 years ago, whereas EPP success rates have subsequently improved.
For Cases 1 and 4, as well as for any patients with no VFBA or ability to grab a mouth piece for NVS, EPP can simplify decannulation by eliminating the need for a pressure dressing to permit NSV before the ostomy has closed. Even without EPP/DP however, numerous patients with high-level SCI or neuromuscular disease and no VFBA have been successfully decannulated to NVS.3–6 Despite this, no EPP/DP publications, including a recent general survey by Onders et al.,11 have described decannulation of paced patients with or without autonomous breathing ability.
Likewise, no EPP/DP publications have reported patients’ respiratory function to improve following pacer placement nor decreased hospitalization rates post-EPP/DP placement by comparison to NSV. Complications of EPP include myopathic changes of the diaphragm, phrenic compression neuropathies, and infection,10 and DP is too recent an innovation to rule out comparable long-term complications. Both are expensive. Thus, Bach et al. recommended that EPP/DP be indicated only for patients with SCI who have no neck rotation and no VFBA or VC to lose.1 Patients with VFBA, especially those who can autonomously sustain their breathing for more than 10-minute periods, should be offered decannulation to NVS rather than EPP/DP. Besides eliminating the unnecessary expense of EPP/DP, noninvasive management decreases nursing requirements, permits GPB mastery for VFBA and security, and, like EPP and DP, facilitates transition to the community. Patients 1–3 of this report used continuous NVS for a total of 68 years without a single acute hospitalization for respiratory complications.
Published criteria for intubation include ‘atelectasis,’ blood gas derangement ‘unresponsive to continuous (CPAP) or bi-level positive airway pressure (BiPAP),’ which are not substitutes for ventilatory support and are only used to ‘decrease atelectasis,’ and when ‘noninvasive ventilation is not adequate.’12 However, no such publications indicate how to use full NVS and MAC to avoid intubation.13–17 Once intubated, no publications discuss extubation of ‘unweanable’ patients to NVS.3,4,6,8,12 The general recommendation is that following three or more failed spontaneous breathing trials the patient be considered a ‘prolonged weaning patient’ and undergo tracheotomy.13–16 This is true despite the fact that even ‘unweanable’ patients with functioning bulbar musculature can usually be extubated to NVS and may subsequently wean themselves.17 Further, without explaining NVS, convention has it that ‘…it is usually best to proceed with a tracheostomy if noninvasive means of ventilation are not an option.’12 This occurs despite the cost and quality of life benefits of using long-term NVS rather than invasive management18 and that noninvasive management permits patients to master GPB for security in the event of ventilator/device failure.3,4 In addition, there is evidence of a greater risk of airway complications for intubated patients undergoing tracheostomy than for those intubated for 3 or 4 weeks and then extubated successfully to NVS.19 Likewise, other than for this center,3,4 there are no publications that suggest that long-term ventilator users be offered decannulation. Books dedicated to SCI do not even broach the subject.12,20
Reasons why decannulation of EPP/DP users has not been reported can only be speculated. First, there is a widespread notion that patients with no pacer/VFBA are safer with tracheostomy tubes irrespective of method of ventilatory support and despite evidence to the contrary.3–6,21 Second, our indication for EPP/DP is when VFBA is completely absent and the patient cannot grab a mouthpiece for daytime NVS. However, no clinicians placing EPP/DP have ever reported using NVS or even considering bulbar muscle function, i.e. CPF ≥ 2.7 l/s, as a criterion for decannulation. Consequently, EPP/DP is marketed not only to patients with high-level SCI who have no VFBA (for whom they can at times be ideal), but also to patients with VFBA who could be managed noninvasively and less expensively. Further, despite the enormous expense of EPP and DP and potential complications, there are no efficacy studies comparing it to NVS with or without decannulation. Indeed, there are few publications on EPP and DPT in general.22–25
These cases also demonstrate that VC can increase post-decannulation and during the 8 years or so post-SCI, as previously reported.3–7,26 Additionally, Case 1 demonstrated that undergoing tracheotomy with or without EPP can result in loss of VC and VFBA because of some combination of diaphragm deconditioning, tube-induced airway secretions, hyperventilation causing hypocapnia (bypassing upper airway sensation), impaired ability to cough, and loss of glottic valving.27 Indeed, for Case 1 recovery of some VFBA occurred within 1 month following both decannulations despite being perfectly medically stable for 4 months prior to the first one and at home and stable for 14 months prior to the second. Thus, her re-development of VFBA cannot be attributed to natural recovery. Cases 3 and 4 also demonstrated improved VC and respiratory function following decannulation. No recovery of EPP/DP-free breathing ability has been reported.
Conclusion
Thus, lack of all VFBA in SCI does not mandate tracheostomy or EPP/DP. Only severe glottis dysfunction that results in aspiration of saliva and oxyhemoglobin saturation below 95%, as it can in amyotrophic lateral sclerosis, may mandate the need for tracheostomy for survival for SCI.28
References
- 1.Bach JR. Noninvasive alternatives to tracheostomy for managing respiratory muscle dysfunction in spinal cord injury. Top Spinal Cord Inj Rehabil 1997;2(3):49–58 [Google Scholar]
- 2.Bach JR, Alba AS. Intermittent abdominal pressure ventilator in a regimen of noninvasive ventilatory support. Chest 1991;99(3):630–6 [DOI] [PubMed] [Google Scholar]
- 3.Bach JR, Alba AS. Noninvasive options for ventilatory support of the traumatic high level quadriplegic patient. Chest 1990;98(3):613–19 [DOI] [PubMed] [Google Scholar]
- 4.Bach JR. New approaches in the rehabilitation of the traumatic high level quadriplegic. Am J Phys Med Rehabil 1991;70(1):13–20 [DOI] [PubMed] [Google Scholar]
- 5.Viroslav J, Rosenblatt R, Morris-Tomazevic S. Respiratory management, life expectancy, and quality of life of patients with high-level traumatic tetraplegia. Respir Care Clin North Am 1996;2:313–22 [PubMed] [Google Scholar]
- 6.Gonçalves MR, Bach JR, Ishikawa Y, DeVito EL, Prado F, Salinas P, et al. Evolution of noninvasive management of end-stage respiratory muscle failure in neuromuscular diseases. Report to the: 69th Congress of the Mexican Society of Respirologist and Thoracic Surgeons, 1st International Consensus on Diagnosis and Management of Respiratory Complications of Neuromuscular Disease, 2010 Apr 6–9;, Guadalajara, Jalisco, Mexico. [Google Scholar]
- 7.Bach JR. The management of patients with neuromuscular disease. Philadelphia, PA: Elsevier; 2004 [Google Scholar]
- 8.Bach JR, Saporito LR. Criteria for extubation and tracheostomy tube removal for patients with ventilatory failure: a different approach to weaning. Chest 1996;110(6):1566–71 [DOI] [PubMed] [Google Scholar]
- 9.Kang SW, Bach JR. Maximum insufflation capacity: vital capacity and cough flows in neuromuscular disease. Am J Phys Med Rehabil 2000;79(3):222–7 [DOI] [PubMed] [Google Scholar]
- 10.Bach JR, O'Connor K. Electrophrenic ventilation: a different perspective. J Am Paraplegia Soc 1991;14(1):9–17 [DOI] [PubMed] [Google Scholar]
- 11.Onders RP, Elmo M, Khansarinia S, Bowman B, Yee J, Road J, et al. Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg Endosc (Germany) 2009;23(7):1433–40 [DOI] [PubMed] [Google Scholar]
- 12.Paterson WP, Kirshblum S. Pulmonary management of SCI. In: Kirshblum S, Campagnolo DI, DeLisa JA.(eds.) Spinal cord medicine. Philadelphia, PA: Lippincott, Williams & Wilkens; 2012. p. 135–54 [Google Scholar]
- 13.Berney S, Bragge P, Granger C, Opdam H, Denehy L. The acute respiratory management of cervical spinal cord injury in the first 6 weeks after injury: a systematic review. Spinal Cord 2011;49(1):17–29 [DOI] [PubMed] [Google Scholar]
- 14.Harrop JS, Sharan AD, Scheid EH, Jr, Vaccaro AR, Przybylski GJ. Tracheostomy placement in patients with complete cervical spinal cord injuries: American Spinal Injury Association grade A. J Neurosurg 2004;100(1 Suppl Spine:20–3 [DOI] [PubMed] [Google Scholar]
- 15.Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J 2007;29(5):1033–56 [DOI] [PubMed] [Google Scholar]
- 16.Romero J, Vari A, Gambarrutta C, Oliviero A. Tracheostomy timing in traumatic spinal cord injury. Eur Spine J 2009;18(10):1452–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bach JR, Gonçalves MR, Hamdani I, Winck JC. Extubation of unweanable patients with neuromuscular weakness: a new management paradigm. Chest 2010;137(5):1033–9 [DOI] [PubMed] [Google Scholar]
- 18.Bach JR. A comparison of long-term ventilatory support alternatives from the perspective of the patient and care giver. Chest 1993;104(6):1702–6 [DOI] [PubMed] [Google Scholar]
- 19.Fishburn MJ, Marino RJ, Ditunno JF. Atelectasis and pneumonia in acute spinal cord injury. Arch Phys Med Rehabil 1990;71:197–200 [PubMed] [Google Scholar]
- 20.Stover SL, DeLisa JA, Whiteneck GG. Spinal cord injury: clinical outcomes from model systems. Gaithersberg, MD: Aspen Publishers; 1995 [Google Scholar]
- 21.Bach JR, Rajaraman R, Ballanger F, Tzeng AC, Ischikawa Y, Kulessa R, et al. Neuromuscular ventilatory insufficiency: the effect of home mechanical ventilator use vs. oxygen therapy on pneumonia and hospitalization rates. Am J Phys Med Rehabil 1998;77(1):8–19 [DOI] [PubMed] [Google Scholar]
- 22.Morgan JA, Morales DL, John R, Ginsburg ME, Kherani AR, Vigilance DW, et al. Endoscopic, robotically assisted implantation of phrenic pacemakers. J Thorac Cardiovasc Surg 2003;126(2):582–3 [DOI] [PubMed] [Google Scholar]
- 23.Biering-Sørensen F, Jacobsen E, Hjelms E, Fodstad H, Trojaborg W. [Diaphragm pacing by electric stimulation of the phrenic nerves] (Danish). Ugeskr Laeger 1990;152(16):1143–5 [PubMed] [Google Scholar]
- 24.Chiodo AE, Scelza W, Forchheimer M. Predictors of ventilator weaning in individuals with high cervical spinal cord injury. J Spinal Cord Med 2008;31(1):72–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Marco AF. Phrenic nerve stimulation in patients with spinal cord injury. Respir Physiol Neurobiol (Netherlands) 2009;169(2):200–9 [DOI] [PubMed] [Google Scholar]
- 26.Bach JR. Inappropriate weaning and late onset ventilatory failure of individuals with traumatic quadriplegia. Paraplegia 1993;31(7):430–8 [DOI] [PubMed] [Google Scholar]
- 27.Bach JR. Home mechanical ventilation for neuromuscular ventilatory failure: conventional approaches and their outcomes. In: Bach JR. (ed.)Noninvasive mechanical ventilation. Philadelphia, PA: Hanley & Belfus; 2002 [Google Scholar]
- 28.Bach JR, Bianchi C, Aufiero E. Oximetry and indications for tracheotomy in amyotrophic lateral sclerosis. Chest 2004;126(5):1502–7 [DOI] [PubMed] [Google Scholar]