Abstract
The vast majority of materials used in bone tissue engineering and regenerative medicine are based on calcium phosphates due to their similarity with the mineral phase of natural bone. Among them, calcium phosphate cements, which are composed of a powder and a liquid that are mixed to obtain a moldable paste, are widely used. These calcium phosphate cement pastes can be injected using minimally invasive surgery and adapt to the shape of the defect, resulting in an entangled network of calcium phosphate crystals. Adding an organic phase to the calcium phosphate cement formulation is a very powerful strategy to enhance some of the properties of these materials. Adding some water-soluble biocompatible polymers in the calcium phosphate cement liquid or powder phase improves physicochemical and mechanical properties, such as injectability, cohesion, and toughness. Moreover, adding specific polymers can enhance the biological response and the resorption rate of the material. The goal of this study is to overview the most relevant advances in this field, focusing on the different types of polymers that have been used to enhance specific calcium phosphate cement properties.
Keywords: calcium phosphate cement, polymer, hydroxyapatite
Introduction
The search for new synthetic bone grafts is a topic of extensive research. Although autografts are the gold standard for targeted bone regeneration, they present some disadvantages such as pain morbidity, disease transmission, and limited availability.1–7 The development of synthetic materials is an alternative strategy to overcome the limitations associated with these problems. The challenge to material scientists is to produce biomaterials with properties that mimic the natural extracellular matrix of bone tissue, which is mainly composed of hydroxyapatite (HA) and collagen.8 Hence comes the potential for calcium phosphate-based materials, which resemble the bone mineral phase, and more specifically calcium phosphate cements (CPCs). This family of materials allows self-setting HA or brushite (dicalcium phosphate dihydrate (DCPD)) to be obtained by soft chemistry routes. The properties of these two families of cements are quite different. HA CPCs tend to be stronger. Moreover, since DCPD is metastable in physiological conditions, brushite CPCs are much faster resorbable than apatite CPCs, although it has been shown that in vivo DCPD tends to convert into precipitated hydroxylapatite (PHA). CPCs are composed of a powder phase and a liquid phase, which are mixed to form a moldable and injectable paste at a determined liquid to powder (L/P) ratio to obtain a final product different from the initial reagents. The final properties of this end product can be tailored by changing different processing parameters, such as the composition and granulometry of the powder phase, the composition of the liquid phase, or L/P used. The final CPC product arises from a dissolution–precipitation reaction, which produces hydrated compounds with a composition and morphology close to the calcium phosphates found in mineralized tissues.9
An approach that is attracting much attention in the CPC field is to incorporate polymers into the formulation, either as a second solid phase or dissolved in the liquid phase. This appears to be an excellent option to enhance CPC performance and improve not only some properties relevant for the clinical use of these materials, such as injectability, cohesion, or setting time, but also their final performance in terms of resorption rate and cell/tissue response.
The scope of this study is to overview the role of polymers in the design of more efficient CPC formulations. The use of different natural and synthetic polymers is reviewed, and their effects on different CPC properties are analyzed.
Why add a polymer to a CPC?
Incorporating polymers has been a strategy to overcome the intrinsic limitations of an inorganic CPC. Many properties can be improved by adding a polymer phase. Although the effect of adding a polymer depends on the composition of the organic phase, the main trends for some relevant CPC properties are summarized in the following.
Setting time
The setting time is the time when the CPC paste loses its plasticity and starts to harden to form a solid body. Setting times are usually measured by indentation, which is a fast and easy system, although it is imprecise. The most commonly used method consists of two Gilmore needles with different loads that may penetrate into the sample depending on the hardness of the solid paste. Once the needles do not penetrate the sample, the setting time is completed. A CPC must have appropriate setting times of 5–15 min.10 As a general rule, the presence of polymers tends to increase setting time, which may be related to the higher viscosity of the polymer-containing paste, which hinders ion diffusion in the matrix.
Cohesion/washout resistance
Cohesion is the ability of a paste to set in a fluid without disintegrating. Different terms have been used to describe this property, such as nondecay ability, antiwashout, compliance, swelling, or stability, and several studies have been performed on this topic.10–16 Nevertheless, disintegration of the cement paste, in addition to preventing the cement from setting, can provoke an inflammatory response and cell apoptosis.17 For this reason, the cohesion time should be lower than the initial setting time to guarantee the structural integrity of the cement paste.10 In general, adding soluble polymers during the liquid phase tends to enhance CPC cohesion. The mechanism underlying this phenomenon is the increased viscosity of the CPC paste, which prevents penetration of the surrounding fluid.
Injectability
Injectability is a CPC property most appreciated by clinicians, as it allows minimizing the surgery and permits adequate filling of complex-shaped defects. Injectability is the ability of a paste to be extruded through a needle without demixing. Injectability can be increased by increasing the CPC L/P ratio, although this adversely affects mechanical properties.18,19 Some water-soluble polymers, such as polysaccharides, have been extensively used to enhance CPC injectability and to increase cohesion time.
Macroporosity
CPCs are intrinsically porous materials, with pores in the micro- or nanometer range,20 but lack macroporosity, which is an essential feature for tissue colonization and angiogenesis. Two main routes have been explored to introduce macroporosity into CPCs by adding polymers: (a) foaming the liquid phase or the cement paste containing a polymer,21–25 and (b) loading the CPC with biodegradable polymers (e.g. microspheres (MSs) or fibers) that slowly degrade over time, resulting in a macroporous structure.26,27 Actually, even a third method has been proposed in which a collagen and CPC slurry are freeze–dried to produce a macroporous scaffold, although this is no longer injectable.28
Mechanical properties
Poor mechanical performance of CPCs has limited their applicability to nonload-bearing applications.29 Due to the intrinsic porosity of CPCs, their strength is lower than that of calcium phosphate ceramics. Moreover, their toughness, ductility, and fatigue resistance are much less than those of cortical bone. Incorporating a polymer during the CPC liquid phase increases ductility, allowing for a higher deformation before breaking. Moreover, polymer fiber reinforcement has been extensively explored as a strategy to increase toughness and strength of cements.30
Long-term degradation
One of the main drawbacks when working with most CPCs, particularly those resulting in HA as the reaction product, is their slow resorption rate, which impairs healing. In this sense, the strategies mentioned previously aimed at creating macropores in the CPC, namely, incorporation of biodegradable polymers and foaming, also result in an increase in the degradation rate.
Drug eluting properties
The intrinsic porosity of CPCs has been exploited for use in drug delivery applications. The combination of CPC with polymers has been used as a way to tune drug release kinetics.
Biological response
CPCs generally have low cell attachment and low proliferation rates when cells are cultured in vitro, basically due to the spiky crystal morphology that arises from the precipitation of the initial powder.31–36 Therefore, incorporating some polymers may add specific binding domains to permit cell adhesion. The most well-known specific binding domains are those related to cell attachment, such as the RGD sequence found in gelatin.
The different properties that can be enhanced by the addition of the specific polymers in the CPC are listed in Table 1.
Table 1.
Some properties of calcium phosphate cements that can be improved by the incorporation of a polymeric phase and the corresponding polymers
| Property improved | Polymers associated in liquid phase | Polymers associated in powder phase |
|---|---|---|
| Setting time | Alginate | — |
| Chitin | ||
| PEG | ||
| Cohesion | Chitosan | — |
| Alginate | ||
| Silk | ||
| PEG | ||
| Injectability | Hyaluronate | — |
| Cellulose | ||
| Macroporosity | Soybean | Gelatin |
| Albumen | Polyesters | |
| Mechanical properties | Gelatin | Chitosan |
| Chitosan | Polyesters | |
| Chitin | ||
| Polyesters | ||
| PAA | ||
| Fibrin glue | ||
| Long-term degradation | — | Gelatin |
| Chitosan | ||
| Polyesters | ||
| Drug eluting system | Chitosan | Gelatin |
| Polyesters | Polyesters | |
| PAA | ||
| Biological response | Gelatin | Alginate |
| Collagen | Polyesters |
PEG: polyethylene glycol; PAA: polyacrylic acid.
Ways of incorporating polymers to CPCs
CPCs are composed of a powder phase and a liquid phase. Therefore, polymers can be added to CPC, either dissolved in the liquid phase or in a solid state as an additive to the powder phase, as shown schematically in Figure 1. Obviously, only water-soluble polymers can be added to the CPC liquid phase. In this case, the polymer will be present as a continuous phase throughout the entire CPC and, what is more important, the solubilized polymer will be able to interact with the cement setting reaction, namely the dissolution of the original phase and the precipitation of the final product. Depending on the final CPC properties desired, the liquid phase properties may be altered by changing several features of the polymer, such as concentration, molecular weight, and polymer chain length. Conversely, when the polymers are added in solid form, they will act as a second and discontinuous phase in the cement inorganic matrix. Although the extent of chemical interaction with the setting reaction is expected to be lower, the morphology, size, and percentage of this second phase will have significant effects on the handling properties and on the final performance of the material.
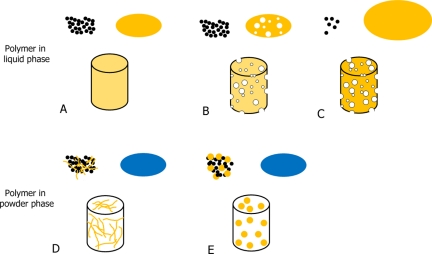
Figure 1.
Different strategies for incorporating polymers in CPCs. The polymer can be incorporated either in the liquid phases (A, B, and C) or in the powder phases (D and E). A represents the mixing of a polymeric solution with the CPC powder to obtain a set CPC, which has the polymer homogeneously distributed in the structure. B represents foaming of the liquid solution, which is then combined with the powder to obtain a set macroporous CPC. C represents the incorporation of a small amount of CPC powder in a big volume of polymer solution, upon which a slurry is formed and is then freeze–dried, resulting in a macroporous polymer–CPC scaffold. D represents the combination of the powder phase with polymer fibers to obtain a fiber-reinforced CPC. Moreover, the fibers may act as pore generators when degraded. E represents the combination of the powder phase with polymer MSs, which can act as controlled drug eluting systems and simultaneously generate macropores in the CPC.
CPC: calcium phosphate cement.
Polymers incorporated into CPCs and their effects on CPCs properties
This section describes the most significant advances in the development of polymer-modified CPC. The results are classified according to the way of adding the polymer within the CPC, and in function of the origin of the polymer, namely, natural or synthetic. Further details of the different formulations are summarized in Table 2.
Table 2.
Description of the different natural and synthetic polymers incorporated into the liquid or the powder phase of the CPC
| Polymer name | % Weight/specifications | Liquid phase | CPC composition | CPC end product | L/P ratio | Main effect | References |
|---|---|---|---|---|---|---|---|
| Liquid phase | |||||||
| Natural polymers | |||||||
| Gelatin | 0%–20% | H2O or Na2HPO4 solution | α-TCP | HA | 0.40–0.80 | Foaming of the gelatin solution results in injectable self-setting gelatin–HA foams | 23, 25 |
| 5% | 10× PBS | α-TCP | HA | 1.2 | Increase in initial cell adhesion and proliferation | 37 | |
| 0%–10% | H2O | CaCO3–MCPM | HA | 0.55 | Increase in setting time. Small effect on cell proliferation. Decrease in mechanical properties | 38 | |
| 15% | H2O | α-TCP–DCPD | HA | 0.3 | Similar proliferation values, but enhanced primary osteoblast activation and ECM mineralization process | 39 | |
| 2%–10% | H2O | CaCO3–MCPM | HA | 0.4 | Increase in setting time. Higher mechanical properties for lowest gelatin concentration (2%) | 40 | |
| 20% | H2O | ACP–DCPD | HA | — | Increase in setting time. Decrease in mechanical properties | 41 | |
| 0%–20% | H2O | α-TCP–DCPD | HA | 0.3 | Faster final production. Increase in mechanical properties with increase in gelatin concentration | 42 | |
| 10% | H2O | α-TCP–DCPD | HA | 0.3–0.4 | Increase in compressive strength | 43 | |
| 0%–20% | H2O | α-TCP | HA | 0.28–0.5 | Increased mechanical properties when gelatin concentration up to 5% | 44 | |
| Collagen | 0%–5%; fibers (∅ = 0.1–3 µm; L = 20–100 µm) | H2O | TTCP–DCPA | HA | 0.25–0.4 | Increase in cell adhesion. Decrease in compressive strength as collagen percentage was increased | 45 |
| 3% | 100–800 mM citric acid | MCPM–β-TCP | DCPD | 0.29 | Increase in cell adhesion. Mechanical properties maintained similar to control | 46 | |
| 0%–2% | H2O/0.2 M N2HPO4 | TTCP–DCPA | HA | 0.29 | Increase in setting times. Decrease in compressive strength as collagen percentage was increased | 47 | |
| Chitosan | 20% | H2O | ACP–DCPD | HA | 0.2 | Increase in setting times. Decrease in compressive strength | 41 |
| 0%–20% | H2O | TTCP–DCPA | HA | 0.5 | Increase in setting time. Increase in flexural strength | 48 | |
| 40% | Glycerol and Ca(OH)2 | TTCP–DCPA | HA | 0.5 | Increase in setting time. Increased antiwashout properties. Increase in diametral tensile strength. No cell cytotoxicity | 49 | |
| 0%–6% | 1 M phosphate buffer | MCPM–CaO | HA | 0.44–1.04 | Increase in compressive strength for low chitosan percentage. Decrease in compressive strength higher than 3% | 50 | |
| 0%–15% | 1 M Na2HPO4 | DCPD–Ca(OH)2 | HA | 0.44–1.04 | Increase in setting times. Increase in compressive strength as chitosan percentage is increased | 50 | |
| 0%–8% | 0.15 g MgCO3 + 0.18 mL 30 wt.% H3PO4 | α-TCP or TTCP | HA | 0.125 | Conversion to HA inhibited by large amounts of chitosan | 51 | |
| 0%–30% | H2O | TTCP–DCPA | HA | 0.5 | Reduction in setting times. Increase in flexural strength up to 20 wt.% chitosan | 52 | |
| 0%–15% | H2O | TTCP–DCPA | HA | 0.22–0.5 | Increase in flexural strength. No significant effect on cell activity | 53–58 | |
| 0%–15% | H2O | TTCP–DCPA | HA | 0.5 | Increase in flexural strength. Significant increase in ALP cell activity | 59, 60 | |
| 0%–15% | H2O | TTCP–DCPA | HA | 0.5 | Increase in flexural strength | 61 | |
| 2% | 1.5% Acetic acid solution | α-TCP | HA | 0.33 | Increase in compressive strength. No cell cytotoxicity. Bigger osteoclastic cell morphology | 62 | |
| 0%–15% | 5% Malic and malonic acid | β-TCP, CaO, MgO, ZnO. TTCP–DCPA | HA | 0.7 | Increase antiwashout properties. No significant effect on injectability | 63–67 | |
| 0%–12% | 1 M Na2HPO4 | MCPM–CaO or DCPD–Ca(OH)2 | HA | 0.96 or 2.29 | Negative effect of chitosan on biodegradation | 68 | |
| 0%–15% | PBS with 0–100 ng/mL protein A solutions | TTCP–DCPA | HA | 0.25–0.5 | Sustained release of gentamicin | 69 | |
| Alginate | 2.2% | 0.2 M neutral phosphate solution | TTCP–DCPA | HA | 0.29 | Increase in setting time. No significant effect of alginate on compressive strength up to 10% concentration | 70 |
| 2% | 0.2 M neutral phosphate solution | TTCP–DCPA | HA | 0.25 | No effect on setting time. Decrease in tensile diametral strength | 12 | |
| 0%–0.5% | 1 M Na2HPO4 | MCPM–CaCO3 incorporation of gentamicin 2.5% or 5% in powder | HA | 0.45 | Slight increase in setting time. Maximum strength unaffected. Extended release of gentamicin | 71 | |
| 20% | H2O | ACP–DCPD | HA | 0.2 | Increase in setting time. Decrease in mechanical properties | 41 | |
| 0%–6% | 2.5% Na2HPO4 | α-TCP | HA | 0.6–0.87 | Increase in setting time. Decrease in diametral tensile strength | 72 | |
| 0%–1% | 1% Na2HPO4 | α-TCP, DCPD, CaCO3, and PHA | HA | 0.35–0.40 | Reduction injectability | 14 | |
| 0%–1% | Chondroitin sulfate and succinic acid | α-TCP–TTCP–DCPD | HA | 0.3 | Increase in cohesion and antiwashout properties | 73 | |
| 0%–2% | 105 mM CaCl2 | ACP–DCPD (α-BSM) | HA | 0.8 | Support cell growth and osteogenesis | 74 | |
| Hyaluronate | 0%–0.5% | 0.5 M citric acid | β-TCP–MCPM | DCPD | 0.4 | Setting times were increased. Mechanical properties unaffected | 75 |
| 0%–8% | 2.5% Na2HPO4 | α-TCP | HA | 0.35 | No effect on mechanical properties | 76 | |
| 0%–1% | 0.2 M PBS | TTCP–DCPD | HA | 0.35 | Increased injectability | 77 | |
| Cellulose | 0%–2.2% | Na2HPO4 | TTCP–DCPA, α-TCP–CaCO3, DCPA–Ca(OH)2 | HA | 0.25 and 0.27 | Increase in setting time. Increase in mechanical properties | 78,79 |
| 0%–3% | 0.2 M sodium phosphate | TTCP–DCPA, TTCP–DCPD | HA | 0.5 | Similar setting times to control. Mechanical properties increased. Increase in injectability | 80 | |
| Silk | 0%–2% | 0.9 NaCl solution | ACP–DCPD (α-BSM) | HA | 0.8 | Decrease in compressive strengths | 74 |
| 0%–2% | 0.25 M NaHPO4/Na2HPO4 | α-TCP | HA | 0.4 | Increase in flexural strength. No difference in setting time or cell viability respect to control | 81 | |
| Chondroitin sulfate | 0%–20% | H2O and 0.5 M citric acid | ACP–DCPDa and β-TCP–MCPMb | HAa and DCPDb | 0.39–0.5 | Slightly higher setting times and mechanical properties | 41, 82 |
| Chitin | 0%–4% | H2O | α-TCP–TTCP–DCPD | HA | 0.43 | Reduction of setting times. Increase in compressive strength | 83 |
| Albumen | 0%–12% | H2O or Na2HPO4 solution | α-TCP | HA | 0.35 | Macroporous self-setting calcium phosphate foams are obtained. Faster resorption in vivo | 21, 22 |
| Soybean- derived hydrogel | 0%–20% | Na2HPO4 solution with or without gelatin | α-TCP | HA | 0.65 | Injectable calcium phosphate foams with an enhanced osteoblast adhesion growth | 24 |
| Synthetic polymers | |||||||
| Polyesters and polyethers | 0%–20% | 2% Alginate in H2O | PCCP–DCPA | HA | 0.31–1 | Scaffold immersed in PLGA solution. Increase in the mechanical properties in the presence of PLGA | 84 |
| 0%–3% | PEG in H2O | TTCP–DCPA | HA | 0.33 | Concentrations higher than 1% decreased mechanical properties | 85 | |
| 1.4% PPF | N-vinyl pyrrolidone | TTCP–DCPA | HA | 0–1 | Decrease in mechanical properties. Prolonged release of protein Rg1 | 86 | |
| 0%–1% Liquid (polysorbate 20) | Glycerol | MCPM–β-TCP | DCPD | 0.21–0.44 | Since the paste is formed with glycerol, no water is contained and reaction does no start until immersed in water. Setting times and compressive strength similar to control | 87 | |
| — | PEG and glycerin | MCPM–β-TCP | DCPD | 0.27–0.4 | Increased setting times. Higher cohesion and antiwashout properties. Higher inflammatory response than control | 88 | |
| 0%–0.5% PEG and glycerin | Na2HPO4 and citric acid | ACP–DCPD | HA | 0.5 | Decrease in setting times. Reduced injectability | 89 | |
| 0%–10% Glycerol | Ca(OH)2, H3PO4, and H2O | α–TCP and TTCP | HA | 0.43 | Increase in setting time. Improvement of injectability and reduction in injecting force | 90 | |
| Polyacrylic acid | 0%–1.45% | 0.0625 g/mL Gentamicin sulfate | MCPM–β-TCP | DCPD | 0.8 | Controls the gentamicin release during prolonged time | 91, 92 |
| 0%–20% Acrylamide | 0.5% MBAM, 0.25% 0.30 mL/g TEMED, 2.5% Na2HPO4, and 1% PA | α-TCP | HA | 0.30–0.32 | Significant increase in the compressive and tensile strength. Reduction of the porosity | 93 | |
| 35% Polymethyl-vinyl ether-maleic acid or 10% polyacrylic acid | H2O | TTCP–DCPD–TCP | HA | 0.25 | Considerable increase in compressive strength, even at short times. Lower cell viability than control after 24 h. After 1 week, similar cell viability to control | 94 | |
| Fibrin glue | — | Fibrin glue (Hualan Biological Engineering, China) | TTCP–DCPA | HA | 0.2–1 | Increase in setting times. Considerable increase in compressive strength. No effect on cell proliferation and differentiation after 14-day culture | 95 |
| Solid phase | |||||||
| Natural polymers | |||||||
| Gelatin MS | 0%–10%; MS size 15.48–8.64 µm; bFGF, TGF-β1 and BMP2 incorporated | 1% Na2HPO4 | α–TCP–DCPA–CaCO3 | HA | 0.91 | Setting time and macroporosity were increased. Compression strength was decreased. Prolonged release of growth factors was obtained | 96–98 |
| Gelatin MS | 10%; 50–150 µm. Gentamicin incorporated in MS (900 mg) | 1% Na2HPO4 | α–TCP–MCPM–CaCO3 | HA | 0.4 | Incorporation of MS increased setting times and porosity. Compressive strength was decreased, but could be enhanced by the incorporation of calcium sulfate hydrate. Release of gentamicin can be controlled depending on cross-link of MS | 99 |
| Gelatin MS | 5%; 20 µg of BMP2 incorporated in implant | 1 M Na2HPO4 | TTCP–DCPA | HA | 0.45 | Release of BMP2 is more prolonged when BMP2 is incorporated in gelatin MS. Can accelerate healing osteoporosis in vivo | 100 |
| Gelatin MS | 0%–5% | 1 M Na2HPO4 | TTCP–DCPA | HA | 0.4 | The mechanical properties of composite initially increased but decrease with degradation. Increased macroporosity. Optimum amount is 2.5% of mass fraction MS. Good biocompatibility in vitro and in vivo | 101 |
| Gelatin MS | 48%–57%; size 37 ± 31 µm | 2% Na2HPO4 | α–TCP–DCPA | HA | 0.35 | Adequate degradation in vivo. Increased macroporosity | 102 |
| Collagen | 0%–5% | H2O/0.2 M neutral phosphate | TTCP–DCPA | HA | 0.29 | Prolonged setting times and reduced mechanical properties | 47 |
| Chitosan | 0%–2% | H2O | ACP–DCPD | HA | 0.5 | Setting times reduced. No effect on compressive strength | 103 |
| Cellulose | 0%–6.4% | 2.5% NaHPO4 | α-TCP | HA | 0.6–0.87 | Increase in injectability | 104 |
| Alginate microbeads | 1.2% sodium alginate; 0%–70% microbeads; size 207 µm | 15% Chitosan in H2O | TTCP–DCPA | HA | 0.25 | Decrease in flexural strength. Cells were able to survive, proliferate, and differentiate | 105–108 |
| Alginate | 0%–1% | H2O | ACP–DCPD | HA | 0.5 | Setting times decreased. Compressive strength decreased as polymer concentration increased. Injectability reduced | 103 |
| Synthetic polymers | |||||||
| PLGA microspheres | 20%; Microspheres 10–110 µm diameter | 2% Na2HPO4 BMP2 adsorbed and entrapped on microparticles | α-TCP, DCPA, and CaCO3 | HA | 0.35–0.5 | Controlled degradation of PLGA allows for a prolonged release of the BMP2. In vitro and in vivo were shown to be biocompatible and the presence of the microparticles allowed to obtain interconnected porosity for tissue ingrowth | 26, 109–114 |
| PLGA MS | 5%; MS size 7–14 µm; gentamicin or BMP2 loading | H2O or 4% Na2HPO4 | TTCP–DCPA | HA | 0.3 | Controlled and prolonged release of growth factor. No change of the setting times or the mechanical performance | 115, 116 |
| PGA fibers | 0%–45%; fraction volume fiber length 8 mm | 15% Chitosan solution or H2O | TTCP–DCPA or TTCP–DCPD | HA | 0.22–0.4 | Material exceeded strength of cancellous bone. Increased flexural strength. Cells presented excellent viability, differentiated, and synthesized bone minerals | 117–120 |
| PCL and PLLA fibers | 0%–7%; fibers 3 mm | 1% Na2HPO4 | α-TCP, DCPA, and CaCO3 | HA | 0.33 | Connective channel-like porous structure was created in the CPC. Toughness was improved. Decreased flexural strength | 121 |
| PGA fibers | 0%–24%; diameter 0.30–0.349 mm | 3.5 M H3PO4 + 100 mM sodium citrate | β-TCP (Plasma Biotal, UK) | DCPD | 0.67 | The yield and ultimate strength increased. Modulus of elasticity also increased in flexural testing. Regular fiber orientation led to higher mechanical properties compared to random fibers | 122 |
| Aramide fibers | 0%–9.5%; fraction volume fiber length 3–200 mm | H2O | TTCP–DCPA | HA | 0.33 | Ultimate strength significantly increased. The longer the fibers, the higher the mechanical properties | 123 |
| Polyamide fibers | 0%–1.6%; diameter 0.1 mm and length 3 mm | 2.5% NaHPO4 | α-TCP | HA | 0.55 | Increase in compression strength, but was not concentration dependent | 124 |
| Polyacrylic acid | 0%–25%; 45- to 75-µm particles | H2O | TTCP–DCPA | HA | 0.4 | Increase in setting time proportional to increase in concentration. Significant increase in the compressive strength | 125 |
CPC: calcium phosphate cement; L/P: liquid to powder; TCP: tricalcium phosphate; HA: hydroxyapatite; PBS: phosphate buffered saline; MCPM: monocalcium phosphate monohydrate; DCPD: dicalcium phosphate dehydrate; ECM: extracellular matrix; ACP: amorphous calcium phosphate; TTCP: tetracalcium phosphate; DCPA: dicalciumphoshphate anhydrous; ALP: alkaline phosphatase; PHA: precipitated hydroxylapatite; PCCP: partially crystallized calcium phosphate; PLGA: poly(lactic-co-glycolic acid); PEG: polyethylene glycol; PPF: poly(propylene fumarate); MBAM: N,N′-methylenebisacrylamide; TEMED: N,N,N′N′-tetramethylethylenediamide; PA: polyacrylate; MS: microsphere; TGF: transforming growth factor; BMP2: bone morphogenetic protein 2; PGA: polyglycolide acid; PCL: poly(ϵ-caprolactone); PLLA: poly(l-lactic acid); bFGF: basic fibroblast growth factor.
Polymer addition in the liquid phase
Natural polymers
Gelatin
Gelatin is a natural polymer derived from collagen, being in fact denatured collagen. Gelatin is soluble in water and shows increased solubility as temperature increases. Gelatin gels at temperatures <38°C–40°C.126 The triple helical structure of collagen is degraded, and uncoiled structures are formed during gelatin processing. This results in exposing the RGD sequence found in the triple helical structure of collagen, which is a specific binding amino acid sequence for cells to attach. Therefore, one of the main reasons to incorporate gelatin into a CPC is to enhance cell adhesion. Some studies have shown a positive effect of incorporating gelatin on initial cell adhesion and proliferation,37,127 although other studies have reported only a small effect on cell proliferation.38,39 Moreover, an increase in the production of bone-related proteins after 3 and 7 days of culture, indicating an increase in osteoblastic activity and differentiation, is observed in gelatin-containing CPCs.39 The same authors showed that gelatin stimulated alkaline phosphatase (ALP) activity as well as collagen and transforming growth factor 31 production.127 Data indicate that gelatin in CPCs favors osteoblast proliferation and activates their metabolism and differentiation.127
Nevertheless, gelatin may negatively affect other parameters, such as setting time, which increases due to the increase in paste viscosity, and subsequent ion diffusion difficulties. Gelatin increases the setting time for a CPC composed of monocalcium phosphate monohydrate (MCPM)-CaCO3; this increase is more pronounced as gelatin concentration is increased.38,40 The same effect is found for a CPC composed of amorphous calcium phosphate (ACP)–DCPD.41 In contrast, the time to completely transform α-tricalcium phosphate (TCP) into calcium-deficient HA was advanced from 7 days in the control CPC to 2 days for the gelatin containing TCP.42
Gelatin also affects CPC mechanical properties, although in different ways depending on the amount of gelatin incorporated. Gelatin increases the compressive strength of an α-TCP cement fourfold, which is related to a decrease in sample porosity.43 Compressive strength increases linearly as a function of gelatin concentration.42 Nevertheless, the general trend is that the highest strengths are obtained with low gelatin concentrations rather than with high gelatin concentrations. Actually, optimum mechanical properties were obtained with a 2 wt.% gelatin solution incorporated into a CPC composed of MCPM–CaCO3.40 CPCs (both ACP–DCPD and MCPM–CaCO3 cements) with gelatin concentrations of 10–20 wt.% clearly showed diminished compressive strength.38,41 In contrast, 5 wt.% gelatin was optimum for a α-TCP CPC.44 Another study showed no difference due to the presence of gelatin in the CPC.128
Gelatin was also used as foaming agent in CPC. Self-setting gelatin–α-TCP foams are obtained by mixing α-TCP with a foamed gelatin solution, which after setting results in a HA solid foam, with high macroporosity and adequate cohesion and injectability.23,25
Collagen
Collagen is a triple helical structure protein and is the most abundant protein found in bone. Collagen is insoluble in water and requires acidic conditions to solubilize. The presence of collagen has a similar effect to that of gelatin in some cases, which is probably expected, as gelatin is denaturized collagen. Nevertheless, as collagen has a triple helical structure, the RGD sequence is not exposed. Instead, other amino acid sequences are exposed such as the glycine–phenylalanine–glutamine–glycine–glutamic acid–arginine sequence, which may also enhance cell adhesion. The effect of adding collagen on the in vitro biological properties of tetracalcium phosphate (TTCP)–dicalciumphoshphate anhydrous (DCPA), MCPM–β-TCP and α-TCP CPCs was assessed in a cell culture study.45,46,129 Initial adhesion was enhanced when the CPC was combined with collagen45,46 and so was the proliferation.129
Collagen also influenced other CPC properties. When the collagen is incorporated during the liquid phase, the setting times are in the range needed for orthopedic applications, although setting times increase as collagen concentration increases.47
Adding collagen decreases the mechanical properties of a TTCP–DCPA CPC, and this decrease is more significant with increasing collagen concentrations.45,47 In contrast, the mechanical properties are slightly improved when collagen is incorporated into a brushite CPC.46
Chitosan
Chitosan is a linear polysaccharide composed of randomly distributed D-glucosamine and N-acetyl-D-glucosamine units. Chitosan can clot blood and has antibacterial properties. Chitosan is insoluble in water and soluble under acidic conditions. When incorporated into different CPCs composed of ACP–DCPD, α–TCP, TTCP, MCPM–CaCO3, or DCPD–Ca(OH)2, chitosan increases setting time and inhibits the setting reaction.41,48–51 Nevertheless, chitosan significantly reduces setting times for TTCP–DCPA cements.52,130
The effect of chitosan on flexural and compressive strength has been widely studied. Chitosan increases the flexural strength of a chitosan–CPC composite composed of TTCP–DCPA considerably, and the highest value was reached when 15–20 wt.% chitosan was incorporated into the CPC,48,53,54,59,61,117 although optimum results also occurred when CPC–chitosan is synergistically combined with Vicryl fibers or alginate microbeads.105,117 Another approach, which actually does not incorporate the polymer in the liquid phase or in the powder phase but in the CPC paste, being the only report that has shown such methodology, also reported an increase in the flexural strength of the composite.131 Even though this last work does not correspond to any of the two sections (polymers incorporated into the liquid phase or in the powder phase), it was incorporated into this section since it is the only case reported and because it shows similar trend to the works in which chitosan was incorporated into the liquid phase. In general, flexural strength decreases when the amount of chitosan increases >20 wt.%.52,55,56 Similarly, compressive strength drastically decreases when chitosan increases to >10 wt.%.41,50 Nevertheless, the compressive strength of CPC composites containing chitosan generally increases.50,62 An interesting property of chitosan is its ability to increase the antiwashout resistance of CPC63–67 but not injectability.63–67
Adding chitosan has a moderate effect on the cell response to CPC. No cytotoxicity38,49,55–58,62,131 is found in chitosan-containing CPCs. Moreover, ALP activity increases considerably in the presence of chitosan when mesenchymal stem cells are cultured on a TTCP–DCPA CPC composite containing 15 wt.% chitosan.59,60 The same authors reported a similar ALP value for the same chitosan–CPC composite compared to the control CPC when culturing MC3T3-E1 cells.53 Cells also survive when encapsulated in sodium alginate droplets and combined with a CPC paste containing chitosan.57,132 When preosteoclastic cells are cultured on a CPC containing chitosan, cell morphology and tartrate-resistant acid phosphatase (TRAP) activity are similar to a control CPC, although the osteoclasts are larger.56,133
Two different chitosan-containing CPCs composed of either MCPM–CaO or DCPD–Ca(OH)2 have shown a lower biodegradation in the presence of chitosan.68 The effect of chitosan on the protein release properties of a CPC loaded with protein A has also been studied. Incorporating chitosan results in sustained release when both the amount of chitosan incorporated and the L/P ratio of the composite are adjusted.69
Alginate
Alginate is an anionic polysaccharide found in brown algae cell walls. It is capable of absorbing 200–300 times its own weight in water and creating a viscous gum. It is known as a biocompatible material, and one of its main features is that it gels through chelation with divalent cations. Alginate has been used for cell immobilization or encapsulation.
When DCPA–TTCP is used as the CPC powder phase, incorporating sodium alginate clearly increases the setting times, and this increase is dose dependent.70 Similar results are found for CPCs composed of MCPM–CaCO3 combined with alginate.71 Nevertheless, this increase in the setting times was only observed when the amount of sodium alginate was >2 wt.%.12 A CPC composed of ACP–DCPD also showed increased setting time in the presence of sodium alginate.41
The compressive strength of a CPC composed of DCPD–ACP containing sodium alginate decreases as the concentration of polymer increases.103 This was also observed for TTCP–DCPA and α-TCP cements, in which the incorporation of low amounts of sodium alginate decreases diametral tensile strength.12,72 Nevertheless, diametral tensile strength is not affected by incorporating sodium alginate at up to 10 wt.% into TTCP–DCPA cement.70 Accordingly, another study reported a decrease in mechanical properties when the amount of alginate incorporated is 20 wt.%.41
Similar to chitosan, sodium alginate hinders the CPC setting reaction and, therefore, delays HA formation. A reduction of injectability has also been reported for sodium alginate-containing cements.14,103 Nevertheless, the presence of sodium alginate generally increases the antiwashout properties of the CPC and their cohesion.73
Sodium alginate has little effect on cell proliferation and differentiation of human bone marrow-derived mesenchymal stem cells.74
Hyaluronate
Hyaluronate is an anionic nonsulfated glycosaminoglycan that is biocompatible and may be cross-linked to produce hydrogels. The molecular weight of the polymer is very important when combining hyaluronate with CPC. The setting times of a CPC composed of β-TCP–MCPM increase with increasing hyaluronate concentration dissolved in the liquid phase, as long as the molecular weight is low (300 and 750 kDa), whereas the values are unaffected in the presence of higher molecular weight hyaluronate (1640 kDa).75
Hyaluronate incorporated into a α-TCP CPC does not significantly affect the mechanical properties of the composite.75,76 In contrast, sodium hyaluronate has high viscosity and creates a network with Ca2+ when incorporated into the CPC liquid phase, which increases injectability of the paste.77
Adding hyaluronic acid slightly delays new bone formation in vivo, although the response is dependent on the initial composition of the CPC solid phase.134
Cellulose
Cellulose is a polysaccharide of several hundreds of β(1→4) linked d-glucose units. Cellulose is found in the cell walls of green plants and algae. Variations in the monomers may change the structure of cellulose, hydroxylation forms hydropropyl methylcellulose (HPMC), and the substitutions with carboxyl groups form carboxy methylcellulose (CMC).
Adding HPMC (0–4 wt.%) to a CPC generally increases setting time78,79 of α-TCP–CaCO3, DCPA–Ca(OH)2, and TTCP–DCPA. Nevertheless, values similar to control CPC values have been reported80 in the compositions of TTCP–DCPA and TTCP–DCPD cements when HPMC was incorporated (0–3 wt.%).80
The mechanical properties (e.g. diametral tensile strength, compressive strength, and elastic modulus) for different CPCs composed of α-TCP–CaCO3, DCPA–Ca(OH)2, TTCP–DCPA, and TTCP–DCPD tend to increase as the amount of HPMC increases.78–80 Nevertheless, opposite results have also been reported, in which the modulus was reduced with added HPMC in a CPC composed of ACP–DCPD.73 HPMC drastically increases the injectability of the CPC even at low concentrations, and the injectability tends to increase as polymer concentration is increased for TTCP–DCPA, TTCP–DCPD, and α-TCP compositions.80
Adding CMC to the CPC does not significantly improve the in vitro biological properties such as cell proliferation or differentiation.74 HPMC has also been used for drug delivery applications. The amount of gentamicin released from a CPC composite made of β-TCP–MCPM and HPMC is reduced, probably due to chemical binding between the polymer and the antibiotic.91
Others
Other natural polymers have also been combined with CPCs, but only a few studies have been reported. For example, silk reduces maximum compressive strength and the elastic modulus compared to those in a control CPC.74 Nevertheless, flexural strength increases significantly in the presence of silk (0.5, 1, and 2 wt.%).81 Setting times do not vary in the presence of silk fibroin.81 Silk can also be used to increase CPC cohesion.135 No differences in terms of cell viability compared to the control CPC were observed when silk was incorporated.81 Another example is incorporating starch and chondroitin sulfate into a CPC, which results in slightly higher setting times and mechanical properties compared to those of a control CPC.41,82,103 Albumen and soybean have also been incorporated into CPCs, with the purpose of creating a liquid phase foam, which enables the production of a macroporous injectable CPC.22,24
Finally, chitin has been incorporated into a CPC composed of α-TCP–TTCP–DCPD at 4 wt.% chitin, resulting in reduced setting times from 32 min in the control to 14 min for the composite CPC.83 Incorporating chitin also increases compressive strength from 23 MPa in the control to 33 MPa in the composite material.83 However, a high chitin content is detrimental to CPC resorption under in vivo conditions.136
Synthetic polymers
Polyesters and polyethers
Polyesters are thermoplastic polymers that contain an ester functional group in their main chain. They are degradable, and the degradation rate is highly dependent on composition. Although hydrolytically degradable, they have far lower water absorption and shrinkage than those of natural polymers. While poly(ϵ-caprolactone) is highly flexible, polylactide acid (PLA) and polyglycolide acid (PGA) have relatively high strength and elastic modulus. Therefore, one of the possible main functions of the polyesters in CPCs is to increase mechanical strength. However, these polymers are not water soluble, and therefore, they cannot be directly incorporated into the liquid phase of the CPC. With this in mind, poly(lactic-co-glycolic acid) (PLGA) dissolved in dichloromethane was infiltrated into the macropores of a alginate/CPC scaffold. Incorporating PLGA in CPC at a concentration of 20 wt.% increased significantly the mechanical strength.84 Opposite results were observed when water-soluble copolymers were obtained by combining polyethylene glycol (PEG) with poly(γ-benzyl l-glutamate), poly(γ-ethyl l-glutamate), and poly(γ-methyl l-glutamate) and incorporated into a CPC. As a result, mechanical strength decreased when polymer concentration was >1 wt.%.85 The incorporation of poly(propylene fumarate) (PPF) also decreases the mechanical strength of a CPC as the amount of PPF is increased in the composite.86 The combination of a CPC composed of TTCP–DCPA with PPF resulted in prolonged release of protein Rg1 with complete release over 20 days without regard to the protein content incorporated.86
PEG is a polyether composed of glycerol monomers. It has been used to obtain premixed CPCs. In fact, when the monomers are combined with CPC, water-free pastes are formed, which can be stored for a long period without reacting. This means that CPC pastes can be prepared at the bench and stored until needed (e.g. operating theater).49,78,87,137 When the premixed CPC pastes are immersed in water for the reaction, they present setting times and compressive strength similar to the conventional CPC.87 However, the mechanical strength decreases in the presence of PEG after a 7-day reaction in water,88 and the injectability of the CPC pastes is reduced with added PEG, glycerol, and glycerin.89,90 Contradictory results were found for setting times; the presence of glycerin and PEG decreases the setting times of ACP–DCPD cement.89 Although PEG increases the setting times of β-TCP–MCPM CPC,88 it is known as an effective antiwashout agent.88
Polyacrylic acid
Polyacrylic acid (PAA) and its derivatives are capable of absorbing water many times their weight. At neutral pH, PAA loses protons and is then negatively charged, facilitating the combination with a range of antibiotics or similar drugs for sustained release. When gentamicin sulfate is incorporated into CPC modified with PAAs, the antibiotic shows quite sustained release from the cement composite.92 The final amount of released gentamicin was thus reduced in the PAA–CPC composites.91 Nevertheless, one of the main problems is that the reaction is hindered, as few reactions occur in the presence of PAA even after 1 month.128
Polyacrylates have considerable effects on mechanical properties. Compressive strength increases substantially to 55 MPa when ammonium PAA is incorporated into the CPC, which is contrasted with 25 MPa for a CPC without PAA.93 The increase in compressive strength can also be deduced from the reduction in composite porosity.93 Furthermore, adding PAA allowed the brittle CPC to become more ductile.138 Within the same family of polymers, a 35 wt.% aqueous solution of poly(methyl vinyl ether-maleic acid) and 10 wt.% PAA were added to a CPC powder composed of 60 wt.% TTCP, 30 wt.% DCPD, and 10 wt.% TCP. As a result, mechanical properties increased considerably with respect to the control CPC, reaching ~70 MPa after 2 weeks compared to ~18 MPa in the control CPC. Moreover, 70 MPa is achieved in as short as 30 min in a CPC containing 10 wt.% PAA.94 TTCP–DCPA CPC also shows a significant increase in strength (diametral tensile strength and compressive strength) with added poly(methyl vinyl ether-maleic acid).139
Incorporating PAA and poly(methyl vinyl ether-maleic acid) results in lower cell viability than that in a control CPC composed of TTCP–DCPD–TCP after the initial 24 h; however, cell viability recovered to a level higher than that of the control CPC after 1 week.94 In general, lower cytotoxicity is achieved when CPCs are combined with PAA and derivatives compared to the acrylic bone cements.140 Composite CPCs have proven in vivo tissue compatibility, suggesting possible clinical applications.141
Fibrin glue
Fibrin glue is produced as a reaction product of fibrinogen with thrombin and is used to create a fibrin clot. Fibrin glue significantly increases setting times of a CPC composed of TTCP–DCPA powder.95 The presence of fibrin glue also increases compressive strength significantly.95 Nevertheless, incorporating fibrin glue into TTCP–DCPA cement does not have a significant effect on the cell proliferation or ALP activity after 14 days of culture.95 Furthermore, no significant difference in bone formation is observed due to the incorporation of fibrin glue.142
Polymer addition as a solid phase
The addition of polymers in solid state, as a second phase in the CPCs, is aimed at achieving two main objectives. On one side, to act as a reinforcing phase that enhances the mechanical properties of the CPC and on the other side to create macroporosity in the CPC after dissolving the polymer, which promotes tissue colonization and eventually enhances CPC resorption. Polymers can be added in the form of powders, MSs, or fibers.
Natural polymers
Adding natural polymers in the form of a liquid phase is preferred, as explained in the previous section, because they dissolve well in water-based liquid. However, the solid forms of natural polymers, such as MS, fibers, and powders, have also been studied. Gelatin MSs have been incorporated into CPC powder to stimulate the degradation and resorption of a CPC.96–102,109 Different amounts and sizes of gelatin MSs were added to the powder phase of the CPC, which was then mixed with the liquid phase. As a result, the MSs degraded in water with time to provide space for cells to penetrate and for new bone ingrowth. Growth factors can be loaded for therapeutic applications.98,100
Lyophilized collagen has also been incorporated as the solid phase. However, this results in significant difficulty in mixing and retards the setting reaction.47 Chitosan has also been incorporated into the solid phase of a ACP–DCPD cement, and setting times are reduced, but no effect on compressive strength was observed.103 The injectability of an α-TCP cement was increased when cellulose was incorporated into the powder phase of the cement.104
Alginate has been added to a CPC (TTCP–DCPA) in the form of microbeads,54 resulting in an increase in the mechanical properties. Moreover, the presence of alginate microbeads helped the formation of macrochannels in the CPC, which stimulate vascularization in vivo and help biodegradation of material.143 In contrast, if alginate is added as smaller particles as the powder phase, setting times decrease, and the compressive strength and injectability also decrease.103
Because of the specific property of sodium alginate for encapsulating tissue cells within gelled microcapsules,54,106–108 cell-encapsulating alginate beads have also been added to CPCs. Cells inside the alginate beads are viable and undergo appropriate cellular processes, such as cell mitosis and tissue differentiation. Therefore, a combined system composed of CPC–alginate with cells is considered a possible tissue-engineered construct. However, concerns may remain as to the mechanical properties of the CPC.
Synthetic polymers
Compared to natural polymers, the synthetic polymers are added more preferably in the form of a solid phase, which is mainly due to the difficulty in dissolving synthetic polymers in water-based liquids. The well-known degradable copolymer PLGA has been widely used as a second solid phase of CPCs to deliver growth factors and antibiotics in a sustained and controllable manner.110,111,115,116 However, even though the growth factor is released from the polymer, this can also be adsorbed on the surface of the CPC due to its high affinity for proteins, this resulting in a reduction of the final release rate.144 As these degradable biopolymers added to CPCs have already proven to be biocompatible,112 more attention has been given to the control of degradation rate and obtaining highly interconnected macroporosity.26,109,113,114
The fiber form of synthetic polymers has been incorporated most widely because of the beneficial mechanical properties of the fibers, such as tensile strength and elastic modulus.30,105,117,118 In general, when polyester fibers were incorporated, the flexural strength and work of fracture increased considerably, and the behaviors were greatly dependent on the fiber length and diameter.27,105,119–122,145 Of special interest is the incorporation of aramide fibers, which have extremely high flexural strength and work of fracture compared to any other types of polymers, including PGA.123,146 In contrast, adding polyamides such as nylon has no significant effect on the mechanical properties of α-TCP cement.124 It was also shown that electrospun submicron fibers enhanced mineralization behavior of cells cultured on the CPCs, which was attributed to the higher surface area and some possible biomimetic features of the fiber morphology.121 The fiber form of degradable polymers also generates pores during degradation within the cement.147 Acrylate derivatives incorporated into CPC also showed increased setting time, and the increase was proportional to the polymer concentration.125 The compressive strength was also considerably increased.125
Conclusion
The combination of polymers with CPC, either solubilized in the liquid phase or as a second solid phase, has proven to be an interesting strategy for the development of bone substitutes with improved performance. Whereas CPCs have outstanding biocompatibility and osteoconductivity, they also have some intrinsic limitations that can be counteracted by the incorporation of a polymer in their formulation. The range of properties that can be modified by the addition of a polymer is broad, covering aspects as diverse as the rheological or the mechanical behavior, the rate of resorption, or the cell and tissue response. The large number of publications on this subject demonstrates, from different perspectives, the feasibility of this approach. However, there are still many areas for further work, especially in terms of understanding and controlling the interactions between the organic and inorganic phases, which may open new avenues for the development of novel self-assembled materials through biomimetic routes. Furthermore, the biological behavior of the CPCs can still be further improved and in this sense, incorporation of other molecules, such as growth factors or genes, can overcome some of the limited biological functionalities. Therefore, the incorporation of these different types of polymers may be a useful tool to be able to control the delivery of the different biologically active molecules.
Footnotes
The research in Dr Kim’s group is supported by Priority Research Centers Program (grant no. 2009-0093829) and World Class University (WCU) program (grant no. R31-10069) through the National Research Foundation (NRF) funded by the Ministry of Education, Science and Technology. The research in Dr Ginebra’s group is funded by the Spanish Ministry of Science and Innovation (MAT 2009-13547 project) and the Generalitat de Catalunya through the prize “ICREA Academia” for excellence in research, and the European Commission through (FP7/2007-2013) under grant agreement no. 241879, REBORNE project.
References
- 1. Arrington ED, Smith WJ, Chambers HG, et al. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res 1996; 329: 300–309 [DOI] [PubMed] [Google Scholar]
- 2. Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity: a statistical evaluation. Spine 1995; 20(9): 1055–1060 [DOI] [PubMed] [Google Scholar]
- 3. Ross N, Tacconi L, Miles JB. Heterotopic bone formation causing recurrent donor site pain following iliac crest bone harvesting. Br J Neurosurg 2000; 14(5): 476–479 [DOI] [PubMed] [Google Scholar]
- 4. Seiler JG, Johnson J. Iliac crest autogenous bone grafting: donor site complications. J South Orthop Assoc 2000; 9(2): 91–97 [PubMed] [Google Scholar]
- 5. Skaggs DL, Samuelson MA, Hale JM, et al. Complications of posterior iliac crest bone grafting in spine surgery in children. Spine 2000; 25(18): 2400–2402 [DOI] [PubMed] [Google Scholar]
- 6. Summers BN, Eisenstein SM. Donor site pain from the ilium: a complication of lumbar spine fusion. J Bone Joint Surg Br 1989; 71(4):677–680 [DOI] [PubMed] [Google Scholar]
- 7. Friedlaender GE, Strong DM, Tomford W, et al. Long-term follow-up of patients with osteochondral allografts. A correlation between immunologic responses and clinical outcome. Orthop Clin North Am 1999; 30(4): 583–588 [DOI] [PubMed] [Google Scholar]
- 8. Dorozhkin SV. Calcium orthophosphates. J Mater Sci Mater Med 2007; 42(4): 1061–1095 [DOI] [PubMed] [Google Scholar]
- 9. Ginebra MP, Fernandez E, De Maeyer EA, et al. Setting reaction and hardening of an apatitic calcium phosphate cement. J Dent Res 1997; 76(4): 905–912 [DOI] [PubMed] [Google Scholar]
- 10. Driessens FC, Planell JA, Boltong MG, et al. Osteotransductive bone cements. Proc Inst Mech Eng H 1998; 212(6): 427–435 [DOI] [PubMed] [Google Scholar]
- 11. Fernandez E, Boltong MG, Ginebra MP, et al. Development of a method to measure the period of swelling of calcium phosphate cements. J Mater Sci Lett 1996; 15(11): 1004–1005 [Google Scholar]
- 12. Ishikawa K, Miyamoto Y, Kon M, et al. Non-decay type fast-setting calcium phosphate cement: composite with sodium alginate. Biomaterials 1995; 16(7): 527–532 [DOI] [PubMed] [Google Scholar]
- 13. Khairoun I, Boltong MG, Driessens FCM, et al. Effect of calcium carbonate on clinical compliance of apatitic calcium phosphate bone cement. J Biomed Mater Res 1997; 38(4): 356–360 [DOI] [PubMed] [Google Scholar]
- 14. Khairoun I, Driessens FCM, Boltong MG, et al. Addition of cohesion promotors to calcium phosphate cements. Biomaterials 1999; 20(4): 393–398 [DOI] [PubMed] [Google Scholar]
- 15. Andrianjatovo H, Lemaitre J. Effect of polysaccharides on the cement properties in the monocalcium phosphate monohydrate/β-tricalcium phosphate system. Innov Tech Biol Med 1995; 16(S1): 140–147 [Google Scholar]
- 16. Miyamoto Y, Ishikawa K, Takechi M, et al. Histological and compositional evaluations of three types of calcium phosphate cements when implanted in subcutaneous tissue immediately after mixing. J Biomed Mater Res A 1999; 48(1): 36–42 [DOI] [PubMed] [Google Scholar]
- 17. Pioletti DP, Takei H, Lin T, et al. The effects of calcium phosphate cement particles on osteoblast functions. Biomaterials 2000; 21(11): 1103–1114 [DOI] [PubMed] [Google Scholar]
- 18. Ishikawa K, Asaoka K. Estimation of ideal mechanical strength and critical porosity of calcium phosphate cement. J Biomed Mater Res A 1995; 29(12): 1537–1543 [DOI] [PubMed] [Google Scholar]
- 19. Khairoun I, Boltong MG, Driessens FCM, et al. Some factors controlling the injectability of calcium phosphate bone cements. J Mater Sci Mater Med 1998; 9(8): 425–428 [DOI] [PubMed] [Google Scholar]
- 20. Espanol M, Perez RA, Montufar EB, et al. Intrinsic porosity of calcium phosphate cements and its significance for drug delivery and tissue engineering applications. Acta Biomater 2009; 5(7): 2752–2762 [DOI] [PubMed] [Google Scholar]
- 21. Ginebra MP, Delgado JA, Harr I, et al. Factors affecting the structure and properties of an injectable self-setting calcium phosphate foam. J Biomed Mater Res A 2007; 80(2): 351–361 [DOI] [PubMed] [Google Scholar]
- 22. Del Valle S, Mino N, Munoz F, et al. In vivo evaluation of an injectable macroporous calcium phosphate cement. J Mater Sci Mater Med 2007; 18(2): 353–361 [DOI] [PubMed] [Google Scholar]
- 23. Montufar EB, Traykova T, Schacht E, et al. Self-hardening calcium deficient hydroxyapatite/gelatin foams for bone regeneration. J Mater Sci Mater Med 2010; 21(3): 863–869 [DOI] [PubMed] [Google Scholar]
- 24. Perut F, Montufar EB, Ciapetti G, et al. Novel soybean/gelatine-based bioactive and injectable hydroxyapatite foam: material properties and cell response. Acta Biomater 2011; 7(4): 1780–1787 [DOI] [PubMed] [Google Scholar]
- 25. Montufar EB, Traykova T, Planell JA, et al. Comparison of a low molecular weight and a macromolecular surfactant foaming agents for injectable self setting hydroxyapatite foams: polysorbate 80 versus gelatine. Mater Sci Eng C 2011; 31: 1498–1504 [Google Scholar]
- 26. Félix Lanao RP, Leeusenburgh SC, Wolke JG, et al. In vitro degradation rate of apatitic calcium phosphate cement with incorporated PLGA microspheres. Acta Biomater 2011; 7(9): 3459–3468 [DOI] [PubMed] [Google Scholar]
- 27. Zuo Y, Yang F, Wolke JG, et al. Incorporation of biodegradable electrospun fibers into calcium phosphate cement for bone regeneration. Acta Biomater 2010; 6(4): 1238–1247 [DOI] [PubMed] [Google Scholar]
- 28. Perez RA, Ginebra MP, Spector M. Cell response to collagen-calcium phosphate cement scaffolds investigated for nonviral gene delivery. J Mater Sci Mater Med 2011; 22(4): 887–897 [DOI] [PubMed] [Google Scholar]
- 29. Ginebra MP. Calcium phosphate bone cements. In: Deb S. (ed.) Orthopaedic bone cements. Cambridge: Woodhead Publishing Limited, 2008, pp.206–230 [Google Scholar]
- 30. Canal C, Ginebra MP. Fibre-reinforced calcium phosphate cements: a review. J Mech Behav Biomed Mater 2011; 4(8): 1658–1671 [DOI] [PubMed] [Google Scholar]
- 31. Engel E, Del Valle S, Aparicio C, et al. Discerning the role of topography and ion exchange in cell response of bioactive tissue engineering scaffolds. Tissue Eng Part A 2008; 14(8): 1341–1351 [DOI] [PubMed] [Google Scholar]
- 32. Deligianni DD, Katsala ND, Koutsoukos PG, et al. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2001; 22(1): 87–96 [DOI] [PubMed] [Google Scholar]
- 33. Knabe C, Driessens FC, Planell JA, et al. Evaluation of calcium phosphates and experimental calcium phosphate bone cements using osteogenic cultures. J Biomed Mater Res 2000; 52(3): 498–508 [DOI] [PubMed] [Google Scholar]
- 34. Link DP, Van den Dolder J, Wolke JG, et al. The cytocompatibility and early osteogenic characteristics of an injectable calcium phosphate cement. Tissue Eng 2007; 13(3): 493–500 [DOI] [PubMed] [Google Scholar]
- 35. Yuasa T, Miyamoto Y, Ishikawa K, et al. Effects of apatite cements on proliferation and differentiation of human osteoblasts in vitro. Biomaterials 2004; 25(7–8): 1159–1166 [DOI] [PubMed] [Google Scholar]
- 36. Oreffo RO, Driessens FCM, Planell JA, et al. Growth and differentiation of human bone marrow osteoprogenitors on novel calcium phosphate cements. Biomaterials 1998; 19(20): 1845–1854 [DOI] [PubMed] [Google Scholar]
- 37. Perez RA, Del Valle S, Altankov G, et al. Porous hydroxyapatite and gelatin/hydroxyapatite microspheres obtained by calcium phosphate cement emulsion. J Biomed Mater Res B 2011; 97(1): 156–166 [DOI] [PubMed] [Google Scholar]
- 38. Chiang TY, Ho CC, Chen DC, et al. Physicochemical properties and biocompatibility of chitosan oligosaccharide/gelatin/calcium phosphate hybrid cements. Mater Chem Phys 2010; 120(2–3): 282–288 [Google Scholar]
- 39. Bigi A, Panzavolta S, Sturba L, et al. Normal and osteopenic bone-derived osteoblast response to a biomimetic gelatin-calcium phosphate bone cement. J Biomed Mater Res A 2006; 78(4): 739–745 [DOI] [PubMed] [Google Scholar]
- 40. Shie MY, Chen DC, Wang CY, et al. Immersion behavior of gelatin containing calcium phosphate cement. Acta Biomater 2008; 4(3): 646–655 [DOI] [PubMed] [Google Scholar]
- 41. Yu T, Ye J, Gao C, et al. Effect of biomedical organic compounds on the setting reaction of calcium phosphates. Colloids Surf B Biointerfaces 2010; 75(1): 363–369 [DOI] [PubMed] [Google Scholar]
- 42. Bigi A, Centelli I, Panzavolta S, et al. Alpha-tricalcium phosphate-gelatin composite cements. J Appl Biomater Biomech 2004; 2(4): 81–87 [PubMed] [Google Scholar]
- 43. Bigi A, Bracci B, Panzavolta S. Effect of added gelatin on the properties of calcium phosphate cement. Biomaterials 2004; 25(14): 2893–2899 [DOI] [PubMed] [Google Scholar]
- 44. Fujishiro Y, Takahashi K, Sato T. Preparation and compressive strength of α-tricalcium phosphate/gelatin gel composite cement. J Biomed Mater Res 2001; 54(4): 525–530 [DOI] [PubMed] [Google Scholar]
- 45. Moreau JL, Weir MD, Xu HH. Self-setting collagen-calcium phosphate bone cement: mechanical and cellular properties. J Biomed Mater Res A 2009; 91(2): 605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tamimi F, Kumarasami B, Doillon C, et al. Brushite-collagen composites for bone regeneration. Acta Biomater 2008; 4(5): 1315–1321 [DOI] [PubMed] [Google Scholar]
- 47. Miyamoto Y, Ishikawa K, Takechi M, et al. Basic properties of calcium phosphate cement containing atelocollagen in its liquid or powder phases. Biomaterials 1998; 19(7–9): 707–715 [DOI] [PubMed] [Google Scholar]
- 48. Xu HH, Quinn JB, Takagi S, et al. Processing and properties of strong and non-rigid calcium phosphate cement. J Dent Res 2002; 81(3): 219–224 [PubMed] [Google Scholar]
- 49. Carey LE, Xu HH, Simon CG, Jr, et al. Premixed rapid-setting calcium phosphate composites for bone repair. Biomaterials 2005; 26(24): 5002–5014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang X, Ma J, Wang Y, et al. Structural characterization of phosphorylated chitosan and their applications as effective additives of calcium phosphate cements. Biomaterials 2001; 22(16): 2247–2255 [DOI] [PubMed] [Google Scholar]
- 51. Rau JV, Generosi A, Smirnov VV, et al. Energy dispersive X-ray diffraction study of phase development during hardening of calcium phosphate bone cements with addition of chitosan. Acta Biomater 2008; 4(4): 1089–1094 [DOI] [PubMed] [Google Scholar]
- 52. Sun L, Xu HH, Tagaki S, et al. Fast setting calcium phosphate cement–chitosan composite: mechanical properties and dissolution rates. J Biomater Appl 2007; 21(3): 299–315 [DOI] [PubMed] [Google Scholar]
- 53. Weir MD, Xu HH. Osteoblastic induction on calcium phosphate cement–chitosan constructs for bone tissue engineering. J Biomed Mater Res A 2010; 94(1): 223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhou H, Weir MD, Xu HH. Effect of cell seeding density on proliferation and osteodifferentiation of umbilical cord stem cells on calcium phosphate cement-fiber scaffold. Tissue Eng Part A 2011; 17(21–22): 2603–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xu HH, Simon CG. Fast setting calcium phosphate–chitosan scaffold: mechanical properties and biocompatibility. Biomaterials 2005; 26(12): 1337–1348 [DOI] [PubMed] [Google Scholar]
- 56. Zhao L, Burguera EF, Xu HH, et al. Fatigue and human umbilical cord stem cell seeding characteristics of calcium phosphate–chitosan–biodegradable fiber scaffolds. Biomaterials 2010; 31(5): 840–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weir MD, Xu HH. Human bone marrow stem cell-encapsulating calcium phosphate scaffolds for bone repair. Acta Biomater 2010; 6(10): 4118–4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weir MD, Xu HH. Culture human mesenchymal stem cells with calcium phosphate cement scaffolds for bone repair. J Biomed Mater Res B 2010; 93(1): 93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moreau JL, Xu HH. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate— chitosan composite scaffold. Biomaterials 2009; 30(14): 2675–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xu HH, Zhao L, Weir MD. Stem cell-calcium phosphate constructs for bone engineering. J Dent Res 2010; 89(12): 1482–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xu HH, Takagi S, Sun L, et al. Development of a nonrigid, durable calcium phosphate cement for use in periodontal bone repair. J Am Dent Assoc 2006; 137(8): 1131–1138 [DOI] [PubMed] [Google Scholar]
- 62. Oh SA, Lee GS, Park JH, et al. Osteoclastic cell behaviors affected by the α-tricalcium phosphate based bone cements. J Mater Sci Mater Med 2010; 21(11): 3019–3027 [DOI] [PubMed] [Google Scholar]
- 63. Maruyama M, Ito M. In vitro properties of a chitosan-bonded self-hardening paste with hydroxyapatite granules. J Biomed Mater Res 1996; 32(4): 527–532 [DOI] [PubMed] [Google Scholar]
- 64. Ito M, Miyazaki A, Yamagishi T, et al. Experimental development of a chitosan-bonded beta tricalcium phosphate bone filling paste. Biomed Mater Eng 1994; 4(6): 439–449 [PubMed] [Google Scholar]
- 65. Ito M, Yamagishi T, Yagasaki H, et al. In vitro properties of a chitosan-bonded bone filling paste studied on the solubility of calcium phosphate compounds. J Biomed Mater Res A 1996; 32(1): 95–98 [DOI] [PubMed] [Google Scholar]
- 66. Takechi M, Miyamoto Y, Ishikawa K, et al. Initial histological evaluation of anti-washout type fast-setting calcium phosphate cement following subcutaneous implantation. Biomaterials 1998; 19(22): 2057–2063 [DOI] [PubMed] [Google Scholar]
- 67. Zhang Y, Zhang M. Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J Biomed Mater Res A 2001; 55(3): 304–312 [DOI] [PubMed] [Google Scholar]
- 68. Wang X, Ma J, Wang Y, et al. Bone repair in radii and tibias of rabbits with phosphorylated chitosan reinforced calcium phosphate cements. Biomaterials 2002; 23(21): 4167–4176 [DOI] [PubMed] [Google Scholar]
- 69. Weir MD, Xu HH. High-strength, in situ-setting calcium phosphate composite with protein release. J Biomed Mater Res A 2008; 85(2): 388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ishikawa K, Miyamoto Y, Takechi M, et al. Non-decay type fast-setting calcium phosphate cement: hydroxyapatite putty containing an increased amount of sodium alginate. J Biomed Mater Res 1997; 36(3): 393–399 [DOI] [PubMed] [Google Scholar]
- 71. Chen CH, Chen CC, Shie MY, et al. Controlled release of gentamicin from calcium phosphate/alginate bone cement. Mater Sci Eng C 2011; 31(2): 334–341 [Google Scholar]
- 72. Dos Santos LA, De Oliveria LC, Rigo EC, et al. Influence of polymeric additives on the mechanical properties of alpha-tricalcium phosphate cement. Bone 1999; 25(2S): 99S–102S [DOI] [PubMed] [Google Scholar]
- 73. Tanaka S, Kishi T, Shimogoryo R, et al. Biopex acquires anti-washout properties by adding sodium alginate into its liquid phase. Dent Mater J 2003; 22(3): 301–312 [DOI] [PubMed] [Google Scholar]
- 74. Park SH, Tofighi A, Wang X, et al. Calcium phosphate combination biomaterials as human mesenchymal stem cell delivery vehicles for bone repair. J Biomed Mater Res B 2011; 97(2): 235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Alkhraisat MH, Rueda C, Marino FT, et al. The effect of hyaluronic acid on brushite cement cohesion. Acta Biomater 2009; 5(8): 3150–3156 [DOI] [PubMed] [Google Scholar]
- 76. Sanginario V, Ginebra MP, Tanner KE, et al. Biodegradable and semi-biodegradable composite hydrogels as bone substitutes: morphology and mechanical characterization. J Mater Sci Mater Med 2006; 17(5): 447–454 [DOI] [PubMed] [Google Scholar]
- 77. Kai D, Li D, Zhu X, et al. Addition of sodium hyaluronate and the effect on performance of the injectable calcium phosphate cement. J Mater Sci Mater Med 2009; 20(8): 1595–1602 [DOI] [PubMed] [Google Scholar]
- 78. Takagi S, Chow LC, Hirayama S, et al. Premixed calcium–phosphate cement pastes. J Biomed Mater Res B 2003; 67(2): 689–696 [DOI] [PubMed] [Google Scholar]
- 79. Cherng A, Takagi S, Chow LC. Effects of hydroxypropyl methylcellulose and other gelling agents on the handling properties of calcium phosphate cement. J Biomed Mater Res A 1997; 35(3): 273–277 [DOI] [PubMed] [Google Scholar]
- 80. Burguera EF, Xu HH, Weir MD. Injectable and rapid-setting calcium phosphate bone cement with dicalcium phosphate dihydrate. J Biomed Mater Res B 2006; 77(1): 126–134 [DOI] [PubMed] [Google Scholar]
- 81. Qu Y, Yang Y, Li J, et al. Preliminary evaluation of a novel strong/osteoinductive calcium phosphate cement. J Biomater Appl 2011; 26(3): 311–325 [DOI] [PubMed] [Google Scholar]
- 82. Tamimi-Marino F, Mastio J, Rueda C, et al. Increase of the final setting time of brushite cements by using chondroitin 4-sulfate and silica gel. J Mater Sci Mater Med 2007; 18(6): 1195–1201 [DOI] [PubMed] [Google Scholar]
- 83. Yokoyama A, Matsuno H, Yamamoto S, et al. Tissue response to a newly developed calcium phosphate cement containing succinic acid and carboxymethyl-chitin. J Biomed Mater Res A 2003; 64(3): 491–501 [DOI] [PubMed] [Google Scholar]
- 84. Qi X, Ye J, Wang Y. Alginate/poly (lactic-co-glycolic acid)/calcium phosphate cement scaffold with oriented pore structure for bone tissue engineering. J Biomed Mater Res A 2009; 89(4): 980–987 [DOI] [PubMed] [Google Scholar]
- 85. Lin J, Zhang S, Chen T, et al. Calcium phosphate cement reinforced by polypeptide copolymers. J Biomed Mater Res B 2006; 76(2): 432–439 [DOI] [PubMed] [Google Scholar]
- 86. Chang CH, Liao TC, Hsu YM, et al. A poly(propylene fumarate)—calcium phosphate based angiogenic injectable bone cement for femoral head osteonecrosis. Biomaterials 2010; 31(14): 4048–4055 [DOI] [PubMed] [Google Scholar]
- 87. Aberg J, Brisby H, Henriksson HB, et al. Premixed acidic calcium phosphate cement: characterization of strength and microstructure. J Biomed Mater Res B 2010; 93(2): 436–441 [DOI] [PubMed] [Google Scholar]
- 88. Han B, Ma PW, Zhang LL, et al. Beta-TCP/MCPM-based premixed calcium phosphate cements. Acta Biomater 2009; 5(8): 3165–3177 [DOI] [PubMed] [Google Scholar]
- 89. Wang X, Ye J, Wang H. Effects of additives on the rheological properties and injectability of a calcium phosphate bone substitute material. J Biomed Mater Res B 2006; 78(2): 259–264 [DOI] [PubMed] [Google Scholar]
- 90. Leroux L, Hatim Z, Freche M, et al. Effects of various adjuvants (lactic acid, glycerol, and chitosan) on the injectability of a calcium phosphate cement. Bone 1999; 25(9): 31S–34S [DOI] [PubMed] [Google Scholar]
- 91. Bohner M, Lemaitre J, Van Landuyt P, et al. Gentamicin-loaded hydraulic calcium phosphate bone cement as antibiotic delivery system. J Pharm Sci 1997; 86(5): 565–572 [DOI] [PubMed] [Google Scholar]
- 92. Bohner M, Lemaître J, Merckle HP, et al. Control of gentamicin release from a calcium phosphate admixed poly(acrylic acid). J Pharm Sci 2000; 89(10): 1262–1270 [DOI] [PubMed] [Google Scholar]
- 93. Dos Santos LA, Carrodeguas RG, Boschi AO, et al. Dual-setting calcium phosphate cement modified with ammonium polyacrylate. Artif Organs 2003; 27(5): 412–418 [DOI] [PubMed] [Google Scholar]
- 94. Khashaba RM, Moussa MM, Mettenburg DJ, et al. Polymeric-calcium phosphate cement composites-material properties: in vitro and in vivo investigations. Int J Biomater 2010; 2010 DOI: 10.1155/2010/691452 10.1155/2010/691452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cui G, Li J, Lei W, et al. The mechanical and biological properties of an injectable calcium phosphate cement-fibrin glue composite for bone regeneration. J Biomed Mater Res B 2010; 92(2): 377–385 [DOI] [PubMed] [Google Scholar]
- 96. Habraken WJ, Wolke JG, Mikos AG, et al. Porcine gelatin microsphere/calcium phosphate cement composites: an in vitro degradation study. J Biomed Mater Res B 2009; 91(2): 555–561 [DOI] [PubMed] [Google Scholar]
- 97. Harbraken WJ, De Jonge LT, Wolke JG, et al. Introduction of gelatin microspheres into an injectable calcium phosphate cement. J Biomed Mater Res A 2008; 87(3): 643–655 [DOI] [PubMed] [Google Scholar]
- 98. Habraken WJ, Boerman OC, Wolke JG, et al. In vitro growth factor release from injectable calcium phosphate cements containing gelatin microspheres. J Biomed Mater Res A 2009; 91(2): 614–622 [DOI] [PubMed] [Google Scholar]
- 99. Cai S, Zhai Y, Xu G, et al. Preparation and properties of calcium phosphate cements incorporated gelatin microspheres and calcium sulfate dihydrate as controlled local drug delivery system. J Mater Sci Mater Med 2011; 22(11): 2487–2496 [DOI] [PubMed] [Google Scholar]
- 100. Li M, Liu X, Liu X, et al. Calcium phosphate cement with BMP-2-loaded gelatin microspheres enhances bone healing in osteoporosis: a pilot study. Clin Orthop Relat Res 2010; 468(7): 1978–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Li M, Liu X, Liu X, et al. Creation of macroporous calcium phosphate cement as bone substitutes by using genipin-crosslinked gelatin microspheres. J Mater Sci Mater Med 2009; 20(4): 925–934 [DOI] [PubMed] [Google Scholar]
- 102. Liao H, Walboomers XF, Habraken WJ, et al. Injectable calcium phosphate cement with PLGA, gelatin and PTMC microspheres in a rabbit femoral defect. Acta Biomater 2011; 7(4): 1752–1759 [DOI] [PubMed] [Google Scholar]
- 103. Wang X, Chen L, Xiang H, et al. Influence of anti-washout agents on the rheological properties and injectability of a calcium phosphate. J Biomed Mater Res B 2007; 81(2): 410–418 [DOI] [PubMed] [Google Scholar]
- 104. Alves HLR, dos Santos LA, Bergmann CP. Injectability evaluation of tricalcium phosphate bone cement. J Mater Sci Mater Med 2008; 19(5): 2241–2246 [DOI] [PubMed] [Google Scholar]
- 105. Xu HH, Quinn JB, Takagi S, et al. Synergistic reinforcement of in situ hardening calcium phosphate composite scaffold for bone tissue engineering. Biomaterials 2004; 25(6): 1029–1037 [DOI] [PubMed] [Google Scholar]
- 106. Simon CG, Guthrie WF, Wang FW. Cell seeding into calcium phosphate cement. J Biomed Mater Res A 2004; 68(4): 628–639 [DOI] [PubMed] [Google Scholar]
- 107. Tang M, Weir MD, Xu HH. Mannitol-containing macroporous calcium phosphate cement encapsulating human umbilical cord stem cells. J Tissue Eng Regen Med. Epub ahead of print 27 March 2011. DOI: 10.1002/term.419 10.1002/term.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhao L, Tang M, Weir MD, et al. Osteogenic media and rhBMP-2-induced differentiation of umbilical cord mesenchymal stem cells encapsulated in alginate microbeads and integrated in an injectable calcium phosphate-chitosan fibrous scaffold. Tissue Eng Part A 2011; 17(7–8): 969–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Habraken WJ, Liao HB, Zhang Z, et al. In vivo degradation of calcium phosphate cement incorporated into biodegradable microspheres. Acta Biomater 2010; 6(6): 2200–2211 [DOI] [PubMed] [Google Scholar]
- 110. Ruhé PQ, Boerman OC, Russel FG, et al. Controlled release of rhBMP-2 loaded poly(dl-lactic-co-glycolic acid)/calcium phosphate cement composites in vivo. J Control Release 2005; 106(1–2): 162–171 [DOI] [PubMed] [Google Scholar]
- 111. Ruhe PQ, Hedberg EL, Padron NT, et al. rhBMP-2 release from injectable poly(dl-lactic-co-glycolic acid)/calcium-phosphate cement composites. J Bone Joint Surg Am 2003; 85(S3): 75–81 [DOI] [PubMed] [Google Scholar]
- 112. Link DP, Van den Dolder J, Van den Beucken JJ, et al. Evaluation of the biocompatibility of calcium phosphate cement/PLGA microparticle composites. J Biomed Mater Res A 2008; 87(3): 760–769 [DOI] [PubMed] [Google Scholar]
- 113. Van de Watering FC, Van den Beucken JJ, Walboomers XF, et al. Calcium phosphate/poly(d,l-lactic-co-glycolic acid) composite bone substitute materials: evaluation of temporal degradation and bone ingrowth in a rat critical-sized cranial defect. Clin Oral Implants Res. Epub ahead of print 2 June 2011 DOI: 10.1111/j.1600-0501.2011.02218.x 10.1111/j.1600-0501.2011.02218.x [DOI] [PubMed] [Google Scholar]
- 114. Ruhé PQ, Hedberg-Dirk EL, Padron NT, et al. Porous poly(Dl-lactic-co-glycolic acid)/calcium phosphate cement composite for reconstruction of bone defects. Tissue Eng 2006; 12(4): 789–800 [DOI] [PubMed] [Google Scholar]
- 115. Schnieders J, Gbureck U, Thull R, et al. Controlled release of gentamicin from calcium phosphate–poly(lactic acid-co-glycolic acid) composite bone cement. Biomaterials 2006; 27(23): 4239–4249 [DOI] [PubMed] [Google Scholar]
- 116. Fei Z, Hu Y, Wu D, et al. Preparation and property of a novel bone graft composite consisting of rhBMP-2 loaded PLGA microspheres and calcium phosphate cement. J Mater Sci Mater Med 2008; 19(3): 1109–1116 [DOI] [PubMed] [Google Scholar]
- 117. Zhang Y, Xu HH. Effects of synergistic reinforcement and absorbable fiber strength on hydroxyapatite bone cement. J Biomed Mater Res A 2005; 75(4): 832–840 [DOI] [PubMed] [Google Scholar]
- 118. Zhao L, Weir MD, Xu HH. Human umbilical cord stem cell encapsulation in calcium phosphate scaffolds for bone engineering. Biomaterials 2010; 31(14): 3848–3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Burguera EF, Xu HH, Takagi S, et al. High early strength calcium phosphate bone cement: effects of dicalcium phosphate dihydrate and absorbable fibers. J Biomed Mater Res A 2005; 75(4): 966–975 [DOI] [PubMed] [Google Scholar]
- 120. Xu HH, Quinn JB. Calcium phosphate cement containing resorbable fibers for short-term reinforcement and macroporosity. Biomaterials 2002; 23(1): 193–202 [DOI] [PubMed] [Google Scholar]
- 121. Bao C, Chen W, Weir MD, et al. Effects of electrospun submicron fibers in calcium phosphate cement scaffold on mechanical properties and osteogenic differentiation of umbilical cord stem cells. Acta Biomater 2011; 7(11): 4037–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gorst NJ, Perrie Y, Gbureck U, et al. Effects of fibre reinforcement on the mechanical properties of brushite cement. Acta Biomater 2006; 2(1): 95–102 [DOI] [PubMed] [Google Scholar]
- 123. Xu HH, Eichmiller FC, Giuseppetti AA. Reinforcement of a self-setting calcium phosphate cement with different fibers. J Biomed Mater Res A 2000; 52(1): 107–114 [DOI] [PubMed] [Google Scholar]
- 124. Dos Santos LA, De Olveira LC, Da Silva Rigo EC, et al. Fiber reinforced calcium phosphate cement. Artif Organs 2000; 24(3): 212–216 [DOI] [PubMed] [Google Scholar]
- 125. Majekodunmi AO, Deb S, Nicholson JW. Effect of molecular weight and concentration of poly(acrylic acid) on the formation of a polymeric calcium phosphate cement. J Mater Sci Mater Med 2003; 14(9): 747–752 [DOI] [PubMed] [Google Scholar]
- 126. Hayashi A, Oh SC. Gelation of gelatin. Agric Biol Chem 1983; 47(6): 1711–1716 [Google Scholar]
- 127. Bigi A, Torricelli P, Fini M, et al. A biomimetic gelatin-calcium phosphate bone cement. Int J Artif Organs 2004; 27(8): 664–673 [DOI] [PubMed] [Google Scholar]
- 128. Miyazaki K, Horibe T, Antonucci JM, et al. Polymeric calcium phosphate cements: analysis of reaction products and properties. Dent Mater 1993; 9(1): 41–45 [DOI] [PubMed] [Google Scholar]
- 129. Perez RA, Altankov G, Jorge-Herrero E, et al. Micro- and nanostructured hydroxyapatite–collagen microcarriers for bone tissue-engineering applications. J Tissue Eng Regen Med. Epub ahead of print 10 February 2012 DOI: 10.1002/term.530 10.1002/term.530 [DOI] [PubMed] [Google Scholar]
- 130. Tagaki S, Chow LC, Hirayama S, et al. Properties of elastomeric calcium phosphate cement-chitosan composites. Dent Mater 2003; 19(8): 797–804 [DOI] [PubMed] [Google Scholar]
- 131. Pan Z, Jiang P, Fan Q, et al. Mechanical and biocompatible influences of chitosan fiber and gelatin on calcium phosphate cement. J Biomed Mater Res B 2007; 82(1): 246–252 [DOI] [PubMed] [Google Scholar]
- 132. Zhao L, Weir MD, Xu HH. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials 2010; 31(25): 6502–6510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Rochet N, Balaguer T, Boukhechba F, et al. Differentiation and activity of human preosteoclasts on chitosan enriched calcium phosphate cement. Biomaterials 2009; 30(26): 4260–4267 [DOI] [PubMed] [Google Scholar]
- 134. Flautre B, Lemaitre J, Maynou C, et al. Influence of polymeric additives on the biological properties of brushite cements: an experimental study in rabbit. J Biomed Mater Res A 2003; 66(2): 214–223 [DOI] [PubMed] [Google Scholar]
- 135. Ding T, Yang H, Maltenfort M, et al. Silk fibroin added to calcium phosphate cement to prevent severe cardiovascular complications. Med Sci Monit 2010; 16(9): HY23–HY26 [PubMed] [Google Scholar]
- 136. Wang X, Ma J, Feng Q, et al. Skeletal repair in rabbits with calcium phosphate cements incorporated phosphorylated chitin. Biomaterials 2002; 23(23): 4591–4600 [DOI] [PubMed] [Google Scholar]
- 137. Xu HH, Carey LE, Simon CG, Jr, et al. Premixed calcium phosphate cements: synthesis, physical properties, and cell cytotoxicity. Dent Mater 2007; 23(4): 433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Chen WC, Ju CP, Wang JC, et al. Brittle and ductile adjustable cement derived from calcium phosphate cement/polyacrylic acid composites. Dent Mater 2008; 24(12): 1616–1622 [DOI] [PubMed] [Google Scholar]
- 139. Matsuya Y, Antonucci JM, Matsuya S, et al. Polymeric calcium phosphate cements derived from poly(methyl vinyl ether-maleic acid). Dent Mater 1996; 12(1): 2–7 [DOI] [PubMed] [Google Scholar]
- 140. Boland EJ, MacDougall M, Carnes DL, et al. In vitro cytotoxicity of a remineralizing resin-based calcium phosphate cement. Dent Mater 2006; 22(4): 338–345 [DOI] [PubMed] [Google Scholar]
- 141. Dickens SH, Schumacher GE, Flaim GM, et al. Pulp capping with a resin-based Ca-PO4 cement. J Dent Res, special issue 740, 2001; 80: Abstract 1710 [Google Scholar]
- 142. Kneser U, Voogd A, Ohnolz J, et al. Fibrin gel-immobilized primary osteoblasts in calcium phosphate bone cement: in vivo evaluation with regard to application as injectable biological bone substitute. Cells Tissues Organs 2005; 179(4): 158–169 [DOI] [PubMed] [Google Scholar]
- 143. Lian Q, Li DC, He JK, et al. Mechanical properties and in-vivo performance of calcium phosphate cement-chitosan fibre composite. Proc Inst Mech Eng H 2008; 222(3): 347–353 [DOI] [PubMed] [Google Scholar]
- 144. Habraken WJ, Wolke JG, Mikos AG, et al. PLGA microsphere/calcium phosphate cement composites for tissue engineering: in vitro release and degradation characteristics. J Biomater Sci Polym Ed 2008; 19(9): 1171–1188 [DOI] [PubMed] [Google Scholar]
- 145. Losquadro WD, Tatum SA, Allen MJ, et al. Archives of facial and plastic surgery. 2009; 11(2): 104–109 [DOI] [PubMed] [Google Scholar]
- 146. Xu HH, Eichmiller FC, Guiseppetti AA. Strong and macroporous calcium phosphate cement: effects of porosity and fiber reinforcement on mechanical properties. J Biomed Mater Res A 2001; 57(1): 457–466 [DOI] [PubMed] [Google Scholar]
- 147. Dunne N, Jack V, O’Hara R, et al. Performance of calcium deficient hydroxyapatite–polyglycolic acid composites: an in vitro study. J Mater Sci Mater Med 2010; 21(8): 2263–2270 [DOI] [PubMed] [Google Scholar]