Abstract
Objective
The goal of this study was to examine the effects of thyroid hormone status on the ability of serum to accept cellular cholesterol.
Methods and Results
Sera from hypophysectomized rats treated ± T3 was used to evaluate the role of thyroid hormone on serum efflux capacity. 2D-DIGE analysis of serum proteins showed that T3 treated rats had increased ApoA-I, ApoA-IV and fetuin A levels with decreased Apo E levels. Microarray and real-time RT-PCR analysis of rat liver revealed large increases in ApoA-I, ApoA-IV, ABCG5, and ABCG8 in response to T3. J774 macrophages, BHK cells, and Fu5AH rat hepatoma cells were used to measure cholesterol efflux mediated by ABCA1, ABCG1 transporters or SR-BI. Sera from T3-treated rats stimulated efflux via ABCA1 but not by ABCG1 or SR-BI. Gel filtration chromatography revealed that T3 treatment caused a decrease in HDL particle size accompanied by higher levels of lipid-poor ApoA-I.
Conclusions
Thyroid hormone enhances the ability of serum to accept cellular cholesterol via the ABCA1 transporter. This effect is most likely attributable to increases in small HDL and lipid poor ApoA-I in response to T3.
Keywords: Thyroid hormone, atherosclerosis, Cholesterol transport, ABCA1 transporter
Introduction
It is well established that thyroid hormone acts to lower serum cholesterol levels1, 2. The mechanism by which this occurs appears to involve the modulation of several key processes in cholesterol metabolism. These likely include: increased elimination of neutral sterols and bile acids from the body, decreased rates of intestinal absorption of cholesterol, and increased removal of LDL cholesterol from the circulation by the liver3, 4. These findings have lead to the investigation of thyroid hormone mimetic compounds, thyromimetics, as a treatment for hypercholesterolemia5, 6. Recent clinical trials have found that thyromimetics are capable of lowering plasma LDL cholesterol and increasing bile acid synthesis in the absence of negative cardiac side effects7. Furthermore, animal studies utilizing the liver-selective thyromimetic T-0681 have shown that treatment with T-0681 promotes reverse cholesterol transport as measured by fecal sterol excretion8. Additional knowledge on the role of thyroid hormone in foam cell formation and cholesterol efflux will be beneficial to the future clinical use of thyromimetics in the treatment of hypercholesterolemia and its associated diseases9. However until now, there has been very little information regarding the effects of thyroid hormone on cholesterol efflux capacity - the quintessential anti-atherogenic property of HDL.
Low HDL levels are a strong independent risk factor for death from cardiovascular disease, however HDL cholesterol levels may be an inadequate indicator of HDL function. Strategies that enhance the anti-atherogenic properties of HDL, particularly its ability to promote cholesterol efflux from the macrophage and thereby drive reverse cholesterol transport, are being actively pursued10. The contributions of the ABCA1 and ABCG1 transporters in macrophage cholesterol transport to HDL and ApoA-I are well-documented11, 12. In this study, we sought to examine the effects of thyroid hormone status on the ability of HDL to promote cholesterol efflux from the macrophage. Using two hypothyroid rat models, we investigated the effect of thyroid hormone on ABCA1, ABCG1, and SR-BI-mediated cholesterol efflux ex vivo. We report that serum from thyroid hormone treated animals promotes cholesterol efflux via the ABCA1 transporter but not ABCG1.
Methods
Experimental Animals
Hypophysectomized, thyroidectomized and normal male Sprague- Dawley rats weighing 125 to 150 g were purchased from Harlan (Indianapolis, IN). Hypophysectomized rats received Tekland Iodine Deficient chow and water ad libitum and were housed in a reverse-cycle light controlled room with a 12-hour light period followed by a 12-hour dark period. The animals were cared for according to the NIH guidelines set forth in the “Guide for the Care and Use of Laboratory Animals” and specifically in accord with protocol 35 71 approved by the University of South Florida Institutional Animal Care and Use Committee. Thyroidectomized rats received 1% calcium gluconate as their drinking water in addition to the Iodine deficient chow. The rats were maintained on the Iodine deficient diet for at least 14 days prior to being used in experiments in order to achieve sufficient turnover of T4. The normal rats were fed Tekland 22/5 rodent chow ad libitum. Hypophysectomized and thyroidectomized rats were given an initial injection of 1.0 mg/kg T3 72 hours prior to harvest of tissue and an additional injection of 0.25 mg/kg T3 24 hours prior to harvest of tissue. In both hypothyroid animal models, administration of T3 alone restored cholesterol efflux via ABCA1 transporters, indicating that other pituitary regulated hormones are not required.
Cholesterol, LDL/VLDL, HDL and T3 Assays
Blood was collected and centrifuged at 16,000×g for 5 min. The supernatant (serum) was collected and used for these determinations. HDL levels were measured using the HDL and LDL/VLDL Cholesterol Quantification Kit from BioVision (Cat# K613-100; Mountain View, CA). T3 levels were measured using the Free T3 ELISA from Calbiotech (Cat# F3106T; Spring Valley, CA).
RNA Isolation
A portion of liver (about 200 mg) was quickly excised and immediately homogenized in 4 ml of Tri-Reagent from Molecular Research Center (Cincinnati, OH) using a Polytron homogenizer at room temperature. The remainder of the isolation steps was carried out using volumes corresponding to 4x the manufacturer’s recommendations.
Microarray Analysis
Isolated RNA was further purified using the RNeasy kit from Qiagen. RNA integrity was confirmed and microarray analysis was performed by the Moffitt Core Facility (Tampa, FL) using the Affymetrix GeneChip instrument system following the protocol established by Affymetrix, Inc. Ten µg each of RNA from the livers of 3 Hx and 3 Hx+T3 rats was used in the analysis. An Affymetrix GeneChip Rat Genome 230 Plus 2.0 arrays which detects about 28,000 genes was used for the analysis. Multiple oligos are used for each gene with the data averaged. Scanned chip images were analyzed using GeneChip algorithms.
Real Time PCR
To validate the microarray results, we assessed the expression of a subset of genes using real-time PCR. RNA was DNAse treated using the TURBO DNA-Free Kit (Ambion, Austin, TX). cDNA was prepared using the Reverse Transcription System (Promega) per the manufacturer’s protocol. The primer sequences are listed in the Supplementary Materials. Hepatic mRNA was quantified under the following reaction conditions: 95°C for 5 minutes, followed by 40 cycle of 95°C for 15 seconds, 61°C for 1 minute and melt curve 55°C + 0.5°C each 10 seconds, × 80. All samples were run in duplicate on a Bio-Rad Chromo4 DNA Engine thermal cycler using SYBR green chemistry. Relative mRNA was calculated as a function of the internal control 18s using ΔΔ Ct.
Cell Culture and Efflux Studies
Efflux studies were performed as previously described13 using either J774 macrophages, Fu5AH rat hepatoma cells, or BHK cells expressing ABCG1 (gift from Dr. Jack Oram, University of Washington School of Medicine). J774 cells were grown in 20%FBS-RPMI, Fu5AH cells in 10%CS-EMEM and BHK-GI cells in 10% FBS-DMEM in the presence of antibiotics. For efflux, cells were plated in 24-well plates (J774 and Fu5AH: 70.000cells/well; BHK:125,000 cells/well) and radiolabeled using [1,2-3H] cholesterol (Perkin Elmer. J774: 2mci/ml in 5%FBS-RPMI; Fu5AH: 2mci/ml in 5%CS-EMEM and BHK:1mci/ml in 2.5% FBS-DMEM) in the presence of an ACAT inhibitor (2 µg/ml, CP 113,818, a gift from Pfizer), also present during efflux, to prevent accumulation of cholesteryl esters. J774 cells were treated with 0.3 mM c-AMP (cpt-AMP, Sigma) in 0.2% BSA for 16–18 hours to upregulate ABCA1. BHK cells were treated with 10 nM mifepristone for 16–18 hours to upregulate ABCG1. Fu5AH were pretreated for 2h with MEM-2% BSA + 1µM BLT to inhibit SR-BI. All efflux medium was prepared using serum from ≥4 Tx or Hx rats treated ±T3 diluted to 2% in MEM-HEPES and sera were assayed in triplicate. Efflux was measured as the fraction of total radiolabeled cell cholesterol released to the medium in 4h and is corrected for the small amount released to MEM. In all experiments we also measured efflux to a pool of human serum diluted to 2%. ABCA1 efflux is considered to be the difference in efflux between control and c-AMP treated J774 cells and ABCA1 expression was verified by increased efflux to 20 µg/ml ApoA-I from c-AMP treated cells. SR-BI efflux is the BLT-1 sensitive efflux calculated from cells treated ± BLT and the inhibitor effect was monitored as loss of efflux to 25 µg/ml HDL3. ABCG1 efflux is considered to be the difference in efflux between control and mifepistrone treated BHK cells and ABCG1 expression was verified by increased efflux to 25 µg/ml HDL3 from mifepristone-treated cells.
2D-DIGE
Two dimensional differential in-gel electrophoresis was performed by Applied Biomics (Hayward, CA). Briefly, equal volumes of serum from 3 Hx rats or 3 Hx + T3 rats was combined to form a pool for each treatment. A pool of serum from 2 normal rats was included as a control. Each pool was fluorescently labeled with different CyDye (Hx+T3 - red, Hx-green, normal-blue) for downstream visualization. The samples were run on first dimension isoelectric focusing and second dimension SDS-PAGE. Fluorescent images were captured using a Typhoon image scanner and analyzed using ImageQuant software. Differentially expressed proteins were quantified using DeCyder software analysis, cut out, and subjected to in-gel trypsin digestion followed by protein identification by MALDI-TOF mass spectrometry.
Fast performance liquid chromatography (FPLC) separation of lipoproteins
Pooled rat sera (150 µL) was diluted in running buffer (150 mM NaCl, 1 mM EDTA pH 8.0) and run over a two superpose 6 10/300 GL columns arranged in tandem (Pharmacia Biotech). A total of forty-six fractions (500 µL each) were collected and assayed for total cholesterol using the Cholesterol E kit from WAKO. The following antibodies were used for western blotting of FPLC fractions, serum and livers: Rabbit anti-mouse Apolipoprotein A-I Biodesign K23500R, Rabbit anti-ApoE Biodesign K23100R, a polyclonal antibody to the LDL receptor4, and mouse monoclonal anti-ABCA1 (Abcam ab18180).
Statistical Analyses
Data are expressed as means ± standard error for a minimum of four animals per group. Experimental treatments included hypophysectomized or thyroidectomized Sprague–Dawley and hypophysectomized Sprague–Dawley rats treated with T3. Treated animals were only compared to their non-treated matching surgical counterparts. In comparisons of multiple groups, a one-way between groups analysis of variance (ANOVA) was conducted initially to determine if differences existed. Tukey’s post test was then used to compare between the relevant treatment and control groups.
Results
Thyroid Hormone Treatment Dramatically Changes Levels of Serum Apolipoproteins
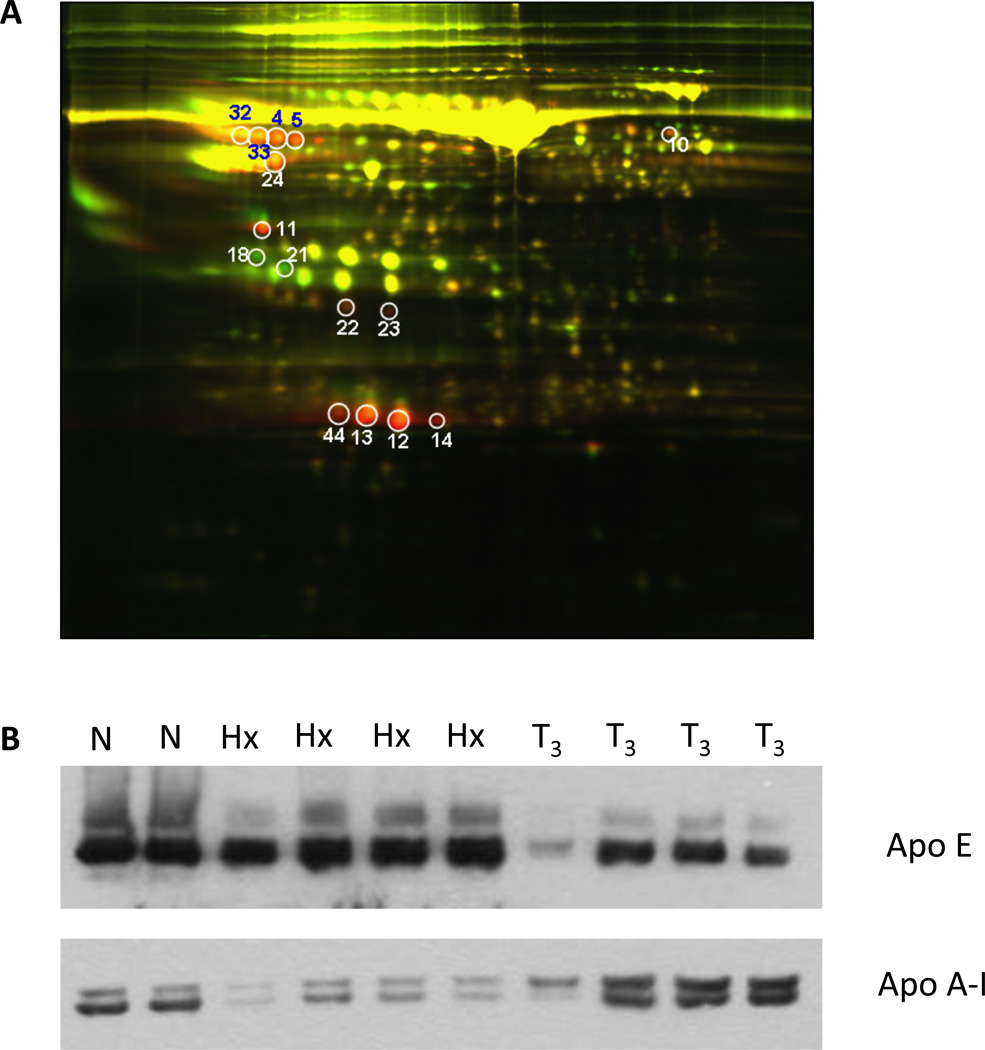
We sought to examine the effects of thyroid hormone treatment on the serum proteome in an unbiased manner. To accomplish this we used hypophysectomized (Hx) rats, a surgically modified animal model in which the pituitary gland has been removed to render the animal hypothyroid. To look specifically at the effects of thyroid hormone, these animals were also injected with T3. Serum proteins from Hx rats and those replenished with thyroid hormone (Hx + T3) were labeled and subjected to two dimensional differential in-gel electrophoresis (2D-DIGE) (Fig. 1A). Differentially expressed proteins were excised from the gel, digested with trypsin, and identified by MALDI-TOF mass spectrometry. The determined protein sequences were compared to the database and those with high confidence (95–100%) are listed in Table 1. ApoA-I was identified in four separate spots, where its levels were increased 5 to 12 fold by T3. Serum ApoA-IV was also increased 2.4-fold by T3. Fetuin-A, serine protease inhibitor alpha 1, fibrinogen alpha subunit, and Clu protein were also increased. Serum Apo E was decreased 3 to 8-fold in response to T3 treatment, consistent with the known effects of T3 on LDL receptor expression14. To confirm the changes in apolipoprotein levels, we examined the serum of these animals by western blot analysis. ApoA-I was substantially reduced in Hx rats relative to normal animals. T3 treatment fully restored ApoA-I levels to those seen in normal rats. In contrast, ApoE levels were markedly decreased by T3 treatment (Fig. 1B).
Figure 1.
A. 2D DIGE of Rat Serum. Serum was pooled from 3 hypophysectomized rats treated ± T3 and fluorescently labeled for analysis by 2D DIGE. This image shows gel analysis of pooled serum from Hx+T3 rats (red) overlayed with pooled serum from Hx rats (green). Red spots indicate proteins expressed in the Hx+T3 sera and green spots indicate proteins expressed in the Hx sera. Yellow spots are the result of similar protein expression in both conditions. The fold-change of differentially expressed proteins was quantified using ImageQuant software analysis. Proteins were then cut out, subjected to in-gel trypsin digestion, and identified by MALDI-TOF mass spectrometry. B. ApoA-I western blot analysis. Sera from the individual animals pooled for 2D DIGE analysis in Figure 1A was analyzed by western blot for ApoA-I and ApoE expression. Normal serum is shown as a control.
Table 1.
List of serum proteins altered by thyroid hormone
| Spot | Protein | Fold Change |
|---|---|---|
| 12 | ApoA-I | +12.5 |
| 5 | Fetuin-A | +10.8 |
| 44 | ApoA-I | +8.54 |
| 14 | PreproapoA-I | +7.80 |
| 4 | Fetuin-A | +6.12 |
| 13 | ApoA-I | +5.02 |
| 33 | Fetuin-A | +4.57 |
| 32 | Fetuin-A | +4.12 |
| 24 | Serine protease inhibitor α1 | +3.61 |
| 10 | Fibrinogen a subunit | +3.48 |
| 23 | Clu protein | +3.29 |
| 22 | Clu protein | +2.66 |
| 11 | ApoA-IV | +2.38 |
| 18 | ApoE | −3.45 |
| 21 | ApoE | −8.62 |
The differentially expressed proteins, by spot number in Fig. 1, were quantified. The fold-increase or decrease in response to T3 is presented. The spots were cut out and subjected to in-gel trypsin digestion followed by protein identification by MALDI-TOF ass spectrometry.
Hepatic ApoA-I and ApoA-IV are Induced by Thyroid Hormone
We then tested whether thyroid hormone acts to increase serum ApoA-I and ApoA-IV by increasing hepatic transcription of these genes. A microarray analysis of liver RNA isolated from Hx rats treated ± T3 was performed. Supplementary Table 2 shows a list of selected genes that were found to positively or negatively respond to T3 treatment. The cholesterol transporter ABCG5 and ApoA-IV showed the greatest activations by T3. Phospholipase A2 showed the greatest decrease, 142-fold, in response to T3. This protein plays a crucial role in vascular inflammation and may contribute to atherosclerotic development and progression15. We did not detect ApoA-I due to limitations of the array. We confirmed the microarray findings using to real-time RT-PCR analysis. As shown in Table 2, ApoA-I and ApoA-IV were both induced about 10-fold in response to T3 treatment. As controls, we observed 10-fold and 90-fold inductions by T3 for ABCG5 and ABCG8 respectively. These proteins mediate transport of cholesterol from liver into bile and are known to be induced by T33.
Table 2.
RT-PCR analysis of hepatic genes that may contribute to cholesterol efflux
| Gene | Hx | Hx + T3 |
|---|---|---|
| ApoA-I | 1.02 ±0.20 | 12.17 ±6.85* |
| ApoA-IV | 1.19 ±0.80 | 13.29 ±8.79* |
| ApoE | 2.11 ±0.95 | 1.04 ±0.16 |
| ABCG5 | 1.16 ±0.66 | 13.86 ±1.93* |
| ABCG8 | 1.73 ±2.19 | 166.1 ±44.8* |
Relative RNA levels are presented as means ±SD for ≥4 rats per group.
p < 0.01 statistical significance relative to Hx.
Thyroid Hormone Promotes Macrophage Cholesterol Efflux via ABCAI
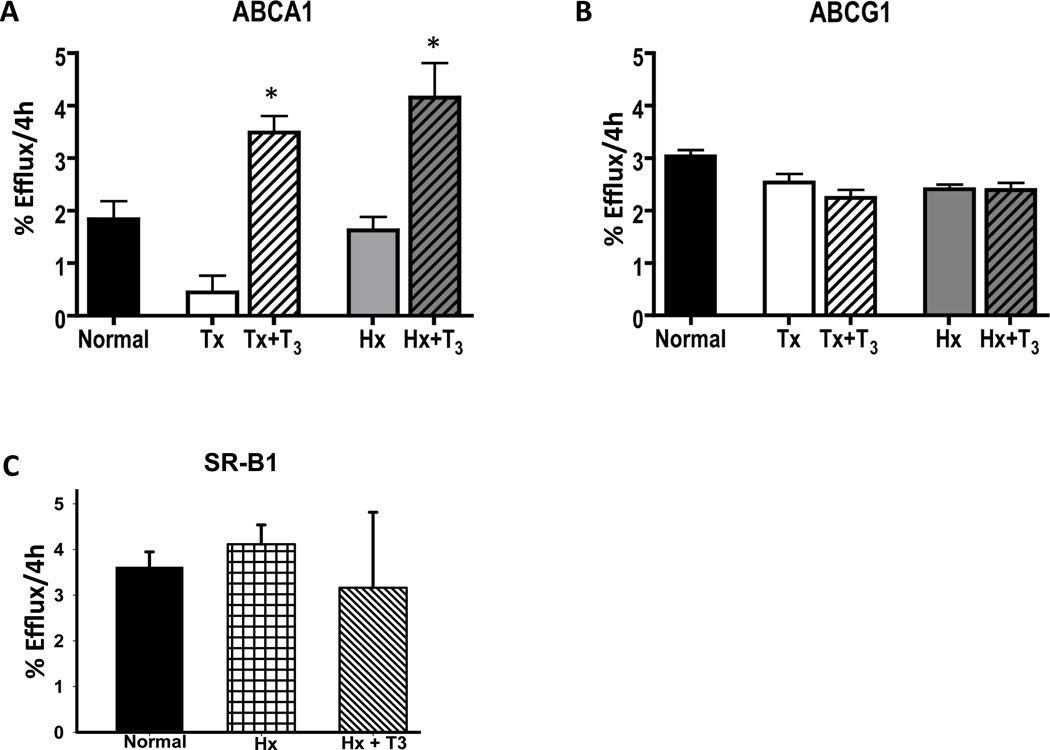
In order to determine if thyroid hormone affects the functionality of HDL in these animals, we performed cholesterol efflux assays. To rule out possible effects by alterations in other hormones in the hypophysectomized rats, we also used the thyroidectomized (Tx) rat for these studies. In our hands, hypophysectomy more reliably ensured a hypothyroid state, which is why most of our studies were performed in this model. Serum was obtained from Hx and Tx hypothyroid animals, as well as Hx and Tx rats replenished with T3. The sera from these animals was tested for its ability to promote cholesterol efflux from cells. Different cell lines were used to specifically evaluate efflux via ABCA1, ABCG1 and SR-BI as described in the Materials and Methods section. As shown in Fig. 2A, serum from Hx rats treated with T3 increased ABCA1-mediated efflux over 2-fold as compared to serum from control or Hx rats. Compared to Tx rats, serum from Tx rats treated with T3 caused a 7-fold increase in efflux mediated by ABCA1 (Fig. 2A). The greater fold increase in Tx rats likely relates to the lower T3 levels in the Tx rats (Supplementary Table 3). The effect of T3- treated sera on ABCA1 mediated efflux is almost certainly not due to T3 itself, or other small molecules, as dialysis had no effect on efflux capacity (Supplementary Figure 1).
Figure 2.
Effects of Thyroid Hormone Deficiency and Administration on Cholesterol Efflux. Serum (2% v/v) from normal, hypophysectomized and thyroidectomized rats treated ± T3 was used to measure transporter-mediated cholesterol efflux capacity in J774 (ABCA1), BHK (ABCG1), and Fu5AH (SR-BI) cells as described in the Methods (*p<0.05 by ANOVA and Tukey’s Post-test).
We also measured cholesterol efflux via ABCG1 using BHK cells harboring an inducible ABCG1 expression cassette (Fig. 2B). ABCG1 is known to promote cholesterol efflux to a variety of lipoprotein acceptors by a mechanism consistent with driving aqueous diffusion16. Despite the much lower LDL and VLDL levels in the T3 treated sera, ABCG1-mediated efflux was unaffected. Recent work has shown that in addition to their known role in promoting ABCA1-mediated efflux, pre-beta HDL can also promote ABCG1-mediated efflux17. To test whether increased ABCG1-mediated efflux to pre-beta was compensating for the lack of efflux to ApoB lipoproteins (ApoB Lp), we performed additional efflux studies with PEG-precipitated sera. We found no statistically significant difference in the ABCG1-mediated efflux to the PEG supernatants from T3 treated sera (data not shown), suggesting the specific efflux capacity of the ApoB Lp may be affected. Lastly, we also tested the ability of T3 treated sera to promote SR-BI mediated efflux using Fu5AH cells treated with and without the SR-BI inhibitor BLT-I. We found that SR-BI mediated efflux to T3 treated sera was unaffected in this cell system (Fig. 2C).
Thyroid Hormone Replacement Increases Small HDL and Lipid Poor ApoA-I
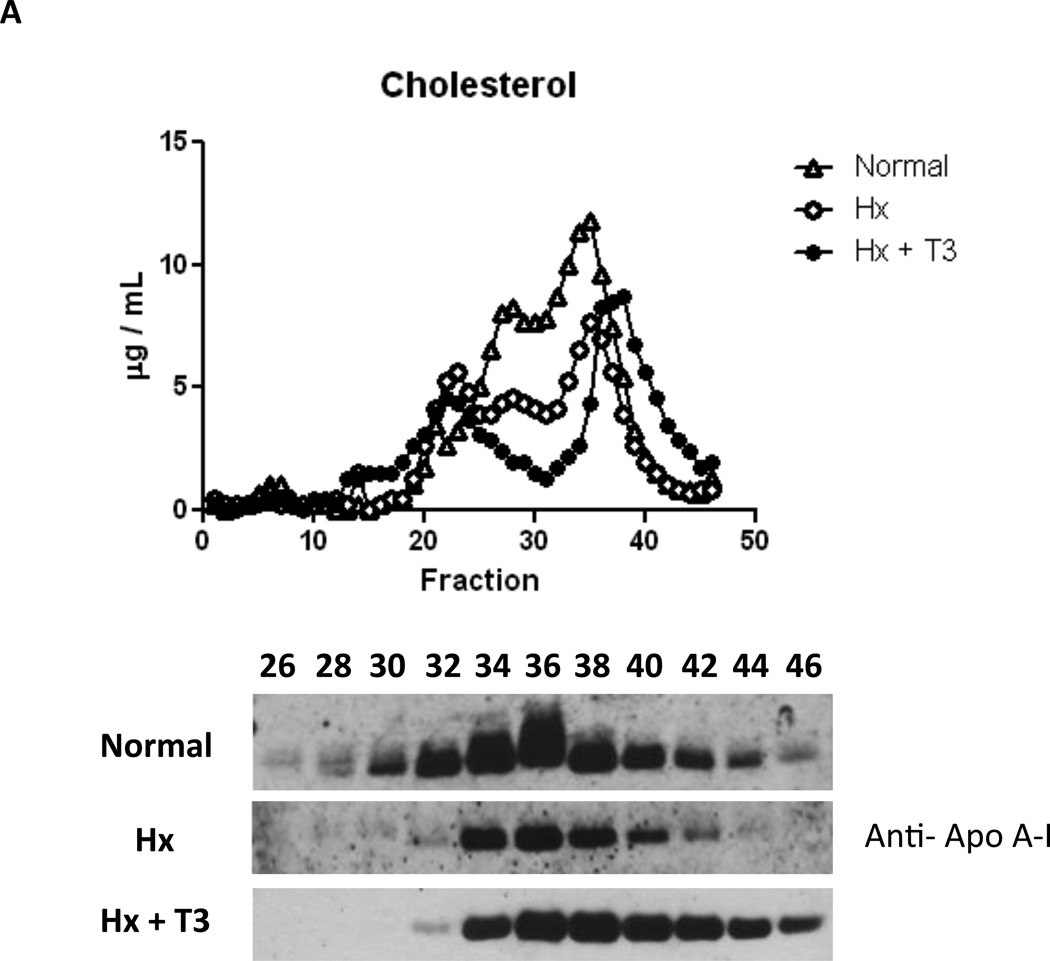
We then investigated whether changes in HDL levels could explain the large increase in ABCA1-mediated efflux capacity in the serum from T3 treated animals. Total cholesterol and VLDL/LDL cholesterol levels were substantially lower in animals receiving T3, consistent with the known actions of this hormone on LDL receptor activity (Supplementary Table 3). Interestingly, despite the large changes in ApoA-I and ApoA-IV protein levels, HDL was not increased with T3 treatment. We hypothesized that the increase in ABCA1-mediated efflux could be explained by a change in HDL size or lipid poor ApoA-I levels. To address this, we separated serum lipoproteins by gel filtration chromatography to examine the lipoprotein profile (Fig. 3). Interestingly, T3 treatment reduced LDL cholesterol levels and dramatically shifted the HDL cholesterol peak to the right, indicating an increase in smaller HDL particles at the expense of larger HDL. We also observed considerably higher ApoA-I levels in the “lipid free fractions” (normally fractions 42–46), likely indicating a substantial increase in pre-beta HDL/ lipid poor ApoA-I. Western blotting of livers from these animals revealed increased LDLR and ApoA-I protein levels, strongly supportive of greater LDL clearance and increased ApoA-I production (Supplementary Figure 2).
Figure 3.
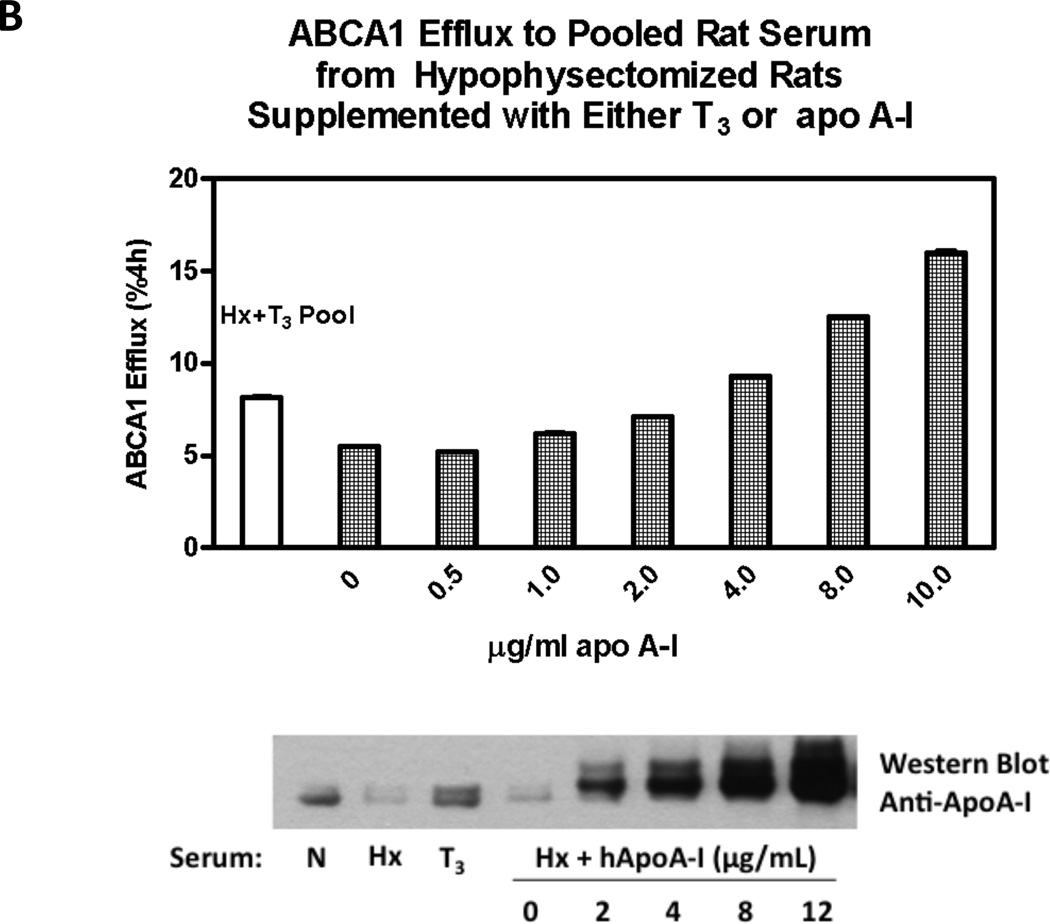
A. FPLC Analysis of Rat Serum Serum from normal, hypophysectomized, and hypophysectomized rats treated with T3 were fractionated and assayed for cholesterol in the upper panel. The lower panel displays Western blots for ApoA-I of selected fractions from 26 to 46. B. Supplementation of Hypothyroid Serum with ApoA-I. Efflux assays using serum from Hyphysectomized rats (Hx) supplemented with varying amounts of ApoA-I.
ApoA-I Promotes ABCA1-mediated Efflux to Serum from Hyphysectomized rats
In order to test whether changes in ApoA-I levels can explain the increased efflux to serum from thyroid hormone treated animals, we performed additional efflux assays using serum from hyphysectomized rats (Hx) supplemented with varying amounts of ApoA-I. Addition of human ApoA-I more than tripled ABCA1-mediated efflux at the highest dose tested (10 ug/mL), indicating that ApoA-I is capable of rescuing the defective efflux seen in hypothyroid sera. However, when added back at lower doses of ApoA-I (2 ug/mL) to the levels seen in T3 treated rats, efflux was not completely rescued (Figure 3B). This suggests that changes in lipid poor ApoA-I alone may be insufficient to fully explain the effects of T3 on serum efflux capacity. Alterations in HDL size, lipid and protein composition are likely involved as well.
Discussion
Hypothyroidism has been shown to be associated with hypertriglyceridemia as well as hypercholesterolemia18, 19. Thyroid hormone and thyromimetics are known to exert several actions that lead to lowered serum cholesterol levels, and an improved lipid profile, which is associated with a decreased risk of atherosclerosis2, 5, 7, 14. However, the role of thyroid hormone in promoting cholesterol efflux has not been previously investigated. This study was performed to test the effects of thyroid hormone treatment on the cholesterol efflux capacity of serum.
The contributions of ABCA1 and ABCG1 in macrophage cholesterol transport to HDL and ApoA-I are well-documented11, 12. SR-BI mediates selective uptake of cholesterol and cholesteryl esters from HDL and has been shown to promote efflux of 3H-cholesterol out of the cell20, 21. The present study demonstrates that thyroid hormone (T3) enhances the ability of serum to accept cellular cholesterol via the ABCA1 transporter, but not the ABCG1 or SR-BI transporters. The enhanced efflux was not solely related to total serum HDL cholesterol levels, supporting the concept that particle size and composition are important factors influencing the cholesterol efflux capacity of HDL.
Analysis of serum lipoproteins by gel filtration chromatography allowed us to examine the effect of thyroid hormone on the lipoprotein profile. We observed a shift to the right in the HDL cholesterol peak in response to T3, suggesting an increase in small HDL particles as well as lipid poor ApoA-I. Small discoidal pre-beta HDL particles are better cholesterol acceptors than mature lipid-rich HDL particles via the ABCA1-mediated pathway22, 23. In fact, treatment of mice with ApoA-I mimetic peptides was shown to increase the formation of pre-beta high-density lipoprotein and thereby increase cholesterol efflux24. Delipidation of lipid-rich HDL to pre-beta HDL was shown to increase the efficacy of plasma to stimulate ABCA1-mediated cholesterol transfer from monocytic cells to HDL and improved aortic atherosclerosis in African Green Monkeys25. We were surprised to see that despite lower T3 levels accompanied by lower HDL in the Hx rat sera, ABCA1-mediated efflux was not significantly lower than that for normal sera. While we do not have an obvious explanation for this, it may be due to alterations in the protein or lipid composition of the HDL. Nonetheless, this study presents the first clear evidence that thyroid hormone replacement substantially improves the ability of serum lipoproteins to accept cholesterol via an ABCA1-dependent mechanism.
We also tested whether the efflux we observed was solely attributable to increased ApoA-I. To accomplish this, we performed additional efflux assays in which serum was reconstituted with purified human ApoA-I. Addition of purified human ApoA-I dramatically increased efflux to Hx sera. However, at doses determined to be equivalent to sera from Hx + T3 animals, ApoA-I could not fully restore the ABCA1-mediated efflux (Figure 3B). Taken together this indicates that while ApoA-I contributes to the T3-stimulated efflux capacity, the quality and quantity of the HDL are probably also very important. Supporting this idea, it was recently found that HDL efflux capacity is in fact a better predictor of coronary artery disease status than HDL levels alone26. Future studies are needed to test the effects of thyroid hormone on the cholesterol efflux capacity of human serum, as well as the corresponding alterations in the protein and lipid composition of the HDL.
We discovered a number of effects of thyroid hormone that were of significance, but unfortunately out of the scope of this study. Of interest, is the induction of serum fetuin A by thyroid hormone. Fetuin A has been suggested to play a role in the pathophysiology of coronary artery disease27 and is implicated in an increased risk for cardiovascular disease28. On the other hand, fetuin A inhibits pathologic calcification in both the soft tissue and vasculature, even in the setting of atherosclerosis29. The examination of the role of fetuin A in cardiovascular disease has began relatively recently and certainly requires further investigation.
Also of interest is the profound decrease of hepatic phospholipase A2 mRNA in response to T3 treatment. PLA2 is a small lipolytic enzyme that releases fatty acids from the second carbon of glycerol phospholipids generating lysophosphatidylcholine, a proinflammatory mediator15. Inflammation has been shown to impair reverse cholesterol transport in vivo30. PLA2 is up-regulated in hepatocytes in response to stress stimuli and is secreted from liver cells. More importantly, overexpression of PLA2 in mice increased the incidence of atherosclerotic lesions, decreased plasma HDL, and increased plasma LDL31. PLA2 has been identified in atherosclerotic lesions and has been suggested to aid in the transformation of macrophages into foam-cells by promoting macrophage LDL uptake32, 33. The striking reduction in hepatic expression of this gene in response to thyroid hormone suggests an additional anti-atherogenic property of T3.
Conclusions
We have provided the first report of the ability of thyroid hormone to enhance the cholesterol-accepting capacity of serum via the ABCAI transporter. Based on an analysis of serum proteins, is seems that increases in the amounts of ApoA-I in serum from the T3- treated animals may be responsible for the enhanced cholesterol accepting capacity of the associated lipoproteins. These data suggest that T3 promotes the first step in reverse cholesterol transport in addition to increasing hepatic LDL receptor expression, bile acid synthesis and transport of cholesterol into the bile by ABCG5 and ABCG82, 3. We also found that thyroid hormone acts to markedly decrease expression of hepatic PLA2 and to increase serum fetuin A levels. These actions suggest protective anti-inflammatory and anti-calcification roles for the thyroid hormone.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ness GC. Thyroid hormone. Basis for its hypocholesterolemic effect. J Fla Med Assoc. 1991;78:383–385. [PubMed] [Google Scholar]
- 2.Martinez-Triguero ML, Hernandez-Mijares A, Nguyen TT, Munoz ML, Pena H, Morillas C, Lorente D, Lluch I, Molina E. Effect of thyroid hormone replacement on lipoprotein(a), lipids, and apolipoproteins in subjects with hypothyroidism. Mayo Clin Proc. 1998;73:837–841. doi: 10.4065/73.9.837. [DOI] [PubMed] [Google Scholar]
- 3.Galman C, Bonde Y, Matasconi M, Angelin B, Rudling M. Dramatically increased intestinal absorption of cholesterol following hypophysectomy is normalized by thyroid hormone. Gastroenterology. 2008;134:1127–1136. doi: 10.1053/j.gastro.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 4.Ness GC, Zhao Z. Thyroid hormone rapidly induces hepatic LDL receptor mRNA levels in hypophysectomized rats. Arch Biochem Biophys. 1994;315:199–202. doi: 10.1006/abbi.1994.1490. [DOI] [PubMed] [Google Scholar]
- 5.Erion MD, Cable EE, Ito BR, Jiang H, Fujitaki JM, Finn PD, Zhang BH, Hou J, Boyer SH, van Poelje PD, Linemeyer DL. Targeting thyroid hormone receptor-beta agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc Natl Acad Sci U S A. 2007;104:15490–15495. doi: 10.1073/pnas.0702759104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tancevski I, Wehinger A, Demetz E, Hoefer J, Eller P, Huber E, Stanzl U, Duwensee K, Auer K, Schgoer W, Kuhn V, Fievet C, Stellaard F, Rudling M, Foeger B, Patsch JR, Ritsch A. The thyromimetic T-0681 protects from atherosclerosis. Journal of lipid research. 2009;50:938–944. doi: 10.1194/jlr.M800553-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkenstam A, Kristensen J, Mellstrom K, Carlsson B, Malm J, Rehnmark S, Garg N, Andersson CM, Rudling M, Sjoberg F, Angelin B, Baxter JD. The thyroid hormone mimetic compound KB2115 lowers plasma LDL cholesterol and stimulates bile acid synthesis without cardiac effects in humans. Proc Natl Acad Sci U S A. 2008;105:663–667. doi: 10.1073/pnas.0705286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tancevski I, Demetz E, Eller P, Duwensee K, Hoefer J, Heim C, Stanzl U, Wehinger A, Auer K, Karer R, Huber J, Schgoer W, Van Eck M, Vanhoutte J, Fievet C, Stellaard F, Rudling M, Patsch JR, Ritsch A. The liver-selective thyromimetic T-0681 influences reverse cholesterol transport and atherosclerosis development in mice. PLoS One. 2010;5:e8722. doi: 10.1371/journal.pone.0008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagor WR, Millar JS. Overview of the LDL receptor: relevance to cholesterol metabolism and future approaches for the treatment of coronary heart disease. Journal of Receptor, Ligand, and Channel Research. 2010:1–14. [Google Scholar]
- 10.Eriksson M, Carlson LA, Miettinen TA, Angelin B. Stimulation of fecal steroid excretion after infusion of recombinant proapolipoprotein A-I. Potential reverse cholesterol transport in humans. Circulation. 1999;100:594–598. doi: 10.1161/01.cir.100.6.594. [DOI] [PubMed] [Google Scholar]
- 11.Duong PT, Weibel GL, Lund-Katz S, Rothblat GH, Phillips MC. Characterization and properties of pre beta-HDL particles formed by ABCA1-mediated cellular lipid efflux to apoA-I. Journal of lipid research. 2008;49:1006–1014. doi: 10.1194/jlr.M700506-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Out R, Hoekstra M, Habets K, Meurs I, de Waard V, Hildebrand RB, Wang Y, Chimini G, Kuiper J, Van Berkel TJ, Van Eck M. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler Thromb Vasc Biol. 2008;28:258–264. doi: 10.1161/ATVBAHA.107.156935. [DOI] [PubMed] [Google Scholar]
- 13.Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH. The roles of different pathways in the release of cholesterol from macrophages. Journal of lipid research. 2007;48:2453–2462. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Ness GC, Lopez D, Chambers CM, Newsome WP, Cornelius P, Long CA, Harwood HJ., Jr Effects of L-triiodothyronine and the thyromimetic L-94901 on serum lipoprotein levels and hepatic low-density lipoprotein receptor, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and apo A-I gene expression. Biochem Pharmacol. 1998;56:121–129. doi: 10.1016/s0006-2952(98)00119-1. [DOI] [PubMed] [Google Scholar]
- 15.Wilensky RL, Shi Y, Mohler ER, 3rd, Hamamdzic D, Burgert ME, Li J, Postle A, Fenning RS, Bollinger JG, Hoffman BE, Pelchovitz DJ, Yang J, Mirabile RC, Webb CL, Zhang L, Zhang P, Gelb MH, Walker MC, Zalewski A, Macphee CH. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat Med. 2008;14:1059–1066. doi: 10.1038/nm.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sankaranarayanan S, Oram JF, Asztalos BF, Vaughan AM, Lund-Katz S, Adorni MP, Phillips MC, Rothblat GH. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J Lipid Res. 2009;50:275–284. doi: 10.1194/jlr.M800362-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favari E, Calabresi L, Adorni MP, Jessup W, Simonelli S, Franceschini G, Bernini F. Small discoidal pre-beta1 HDL particles are efficient acceptors of cell cholesterol via ABCA1 and ABCG1. Biochemistry. 2009;48:11067–11074. doi: 10.1021/bi901564g. [DOI] [PubMed] [Google Scholar]
- 18.Santi A, Duarte MM, Moresco RN, Menezes C, Bagatini MD, Schetinger MR, Loro VL. Association between thyroid hormones, lipids and oxidative stress biomarkers in overt hypothyroidism. Clin Chem Lab Med. 2010 doi: 10.1515/CCLM.2010.309. [DOI] [PubMed] [Google Scholar]
- 19.Kvetny J, Heldgaard PE, Bladbjerg EM, Gram J. Subclinical hypothyroidism is associated with a low-grade inflammation, increased triglyceride levels and predicts cardiovascular disease in males below 50 years. Clin Endocrinol (Oxf) 2004;61:232–238. doi: 10.1111/j.1365-2265.2004.02088.x. [DOI] [PubMed] [Google Scholar]
- 20.Ji Y, Wang N, Ramakrishnan R, Sehayek E, Huszar D, Breslow JL, Tall AR. Hepatic scavenger receptor BI promotes rapid clearance of high density lipoprotein free cholesterol and its transport into bile. J Biol Chem. 1999;274:33398–33402. doi: 10.1074/jbc.274.47.33398. [DOI] [PubMed] [Google Scholar]
- 21.Ji Y, Jian B, Wang N, Sun Y, Moya ML, Phillips MC, Rothblat GH, Swaney JB, Tall AR. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J Biol Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- 22.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol. 2010;21:229–238. doi: 10.1097/mol.0b013e328338472d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Lenten BJ, Wagner AC, Anantharamaiah GM, Navab M, Reddy ST, Buga GM, Fogelman AM. Apolipoprotein A-I mimetic peptides. Curr Atheroscler Rep. 2009;11:52–57. doi: 10.1007/s11883-009-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacks FM, Rudel LL, Conner A, Akeefe H, Kostner G, Baki T, Rothblat G, de la Llera-Moya M, Asztalos B, Perlman T, Zheng C, Alaupovic P, Maltais JA, Brewer HB. Selective delipidation of plasma HDL enhances reverse cholesterol transport in vivo. Journal of lipid research. 2009;50:894–907. doi: 10.1194/jlr.M800622-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bilgir O, Kebapcilar L, Bilgir F, Bozkaya G, Yildiz Y, Pinar P, Tastan A. Decreased serum fetuin-A levels are associated with coronary artery diseases. Intern Med. 2010;49:1281–1285. doi: 10.2169/internalmedicine.49.3223. [DOI] [PubMed] [Google Scholar]
- 28.Weikert C, Stefan N, Schulze MB, Pischon T, Berger K, Joost HG, Haring HU, Boeing H, Fritsche A. Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke. Circulation. 2008;118:2555–2562. doi: 10.1161/CIRCULATIONAHA.108.814418. [DOI] [PubMed] [Google Scholar]
- 29.Westenfeld R, Schafer C, Kruger T, Haarmann C, Schurgers LJ, Reutelingsperger C, Ivanovski O, Drueke T, Massy ZA, Ketteler M, Floege J, Jahnen-Dechent W. Fetuin-A protects against atherosclerotic calcification in CKD. J Am Soc Nephrol. 2009;20:1264–1274. doi: 10.1681/ASN.2008060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivandic B, Castellani LW, Wang XP, Qiao JH, Mehrabian M, Navab M, Fogelman AM, Grass DS, Swanson ME, de Beer MC, de Beer F, Lusis AJ. Role of group II secretory phospholipase A2 in atherosclerosis, 1. Increased atherogenesis and altered lipoproteins in transgenic mice expressing group IIa phospholipase A2. Arterioscler Thromb Vasc Biol. 1999;19:1284–1290. doi: 10.1161/01.atv.19.5.1284. [DOI] [PubMed] [Google Scholar]
- 32.Divchev D, Schieffer B. The secretory phospholipase A2 group IIA: a missing link between inflammation, activated renin-angiotensin system, and atherogenesis? Vasc Health Risk Manag. 2008;4:597–604. doi: 10.2147/vhrm.s2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menschikowski M, Lattke P, Bergmann S, Jaross W. Exposure of macrophages to PLA2-modified lipoproteins leads to cellular lipid accumulations. Anal Cell Pathol. 1995;9:113–121. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.