Abstract
Objective
To estimate the burden and age-specific rates of influenza-associated hospitalization in rural western Kenya.
Methods
All 3924 patients with respiratory illness (defined as acute cough, difficulty in breathing or pleuritic chest pain) who were hospitalized between June 2007 and May 2009 in any inpatient health facility in the Kenyan district of Bondo were enrolled. Nasopharyngeal and oropharyngeal swabs were collected and tested for influenza viruses using real-time reverse transcriptase polymerase chain reaction (RT–PCR). In the calculation of annual rates, adjustments were made for enrolled patients who did not have swabs tested for influenza virus.
Findings
Of the 2079 patients with tested swabs, infection with influenza virus was confirmed in 204 (10%); 176, 27 and 1 were found to be RT–PCR-positive for influenza A virus only, influenza B virus only, and both influenza A and B viruses, respectively. Among those tested for influenza virus, 6.8% of the children aged < 5 years and 14.0% of the patients aged ≥ 5 years were found positive. The case-fatality rate among admitted patients with PCR-confirmed infection with influenza virus was 2.0%. The annual rate of hospitalization (per 100 000 population) was 699.8 among patients with respiratory illness and 56.2 among patients with influenza (with 143.7, 18.8, 55.2, 65.1 and 57.3 hospitalized patients with influenza virus per 100 000 people aged < 5, 5–19, 20–34, 35–49 and ≥ 50 years, respectively).
Conclusion
In a rural district of western Kenya, the rate of influenza-associated hospitalization was highest among children aged less than 5 years.
Résumé
Objectif
Estimer les taux spécifiques par âge et par charge de l'hospitalisation liée à la grippe dans les régions rurales de l'ouest du Kenya.
Méthodes
Tous les 3924 patients souffrant de maladie respiratoire (définies comme toux aiguë, difficulté respiratoire ou douleur de poitrine pleurétique) qui ont été hospitalisés entre juin 2007 et mai 2009 dans tous les établissements hospitaliers du district du Bondo, au Kenya, ont été inscrits. Des prélèvements nasopharyngés et oropharyngés ont été rassemblés et testés pour les virus de la grippe au moyen d'une transcription inverse et amplification en chaîne par polymérase en temps réel (RT-PCR). Dans le calcul des taux annuels, des ajustements ont été faits pour les patients inscrits qui n'ont pas eu de prélèvements testés pour le virus de la grippe.
Résultats
Des 2079 patients avec prélèvements testés, l'infection au virus de la grippe a été confirmée dans 204 (10%) des cas; 176, 27 et 1 cas se sont révélés RT-PCR positifs pour, respectivement, le virus de la grippe A uniquement, le virus de la grippe B uniquement, les virus des deux grippes A et B. Parmi les personnes testées pour le virus de la grippe, 6,8% des enfants âgés de <5 ans et 14,0% des patients âgés de 5 ans ou plus se sont révélés positifs. Le taux de létalité parmi les patients admis avec infection confirmée PCR au virus de la grippe était de 2.0%. Le taux annuel d'hospitalisation (par 100 000 habitants) était de 699,8 parmi les patients souffrant de maladie respiratoire et de 56,2 parmi les patients souffrant de la grippe (avec 143,7, 18,8, 55,2, 65,1 et 57,3 des patients hospitalisés avec le virus de la grippe par 100 000 personnes âgés de respectivement <5, 5-19, 20-34, 35-49 et ≥50 ans).
Conclusion
Dans une région rurale de l'ouest du Kenya, le taux d'hospitalisation liée à la grippe était plus élevé parmi les enfants âgés de moins de 5 ans.
Resumen
Objetivo
Calcular la carga y las tasas por edades de las hospitalizaciones asociadas a la gripe en el oeste rural de Kenya.
Métodos
Se inscribieron los 3924 pacientes con enfermedades respiratorias (definidas como tos aguda, dificultad para respirar o dolor torácico pleurítico) hospitalizados entre junio de 2007 y mayo de 2009 en cualquier centro sanitario hospitalario del distrito keniata de Bodo. Se recogieron frotis nasofaríngeos y bucofaríngeos y se comprobó la presencia de virus de la gripe mediante una reacción en cadena de la polimerasa dirigida por ARN (transcriptasa inversa) (RT-PCR). Se llevaron a cabo ajustes para aquellos pacientes inscritos para cuyos frotis no se había realizado la prueba del virus de la gripe para realizar el cálculo de las tasas anuales.
Resultados
Se confirmó la presencia de una infección por virus de la gripe en 204 (10%) de los 2079 pacientes cuyos frotis se sometieron a prueba; 176, 27 y 1 casos dieron positivo en la prueba de la RT-PCR sólo para el virus gripal A, sólo para el virus gripal B y para ambos virus gripales A y B, respectivamente. El 6,8% de los niños menores de 5 años y el 14,0% de los pacientes mayores de 5 años entre los que se realizó la prueba para el virus gripal dio positivo. La tasa de letalidad entre los pacientes admitidos con una infección por el virus gripal confirmada por la prueba de la PCR fue del 2,0% La tasa anual de hospitalizaciones (por cada 100 000 habitantes) fue de 699,8 entre pacientes con enfermedades respiratorias y de 56,2 entre los pacientes con gripe (con 143,7, 18,8, 55,2, 65,1 y 57,3 pacientes hospitalizados con virus gripal por cada 100 000 habitantes con edades menores de 5, comprendidas entre 5-19, 20-34, 35-49 y más de 50 años, respectivamente).
Conclusión
En un distrito rural del oeste de Kenya, la tasa de hospitalizaciones asociadas a la gripe fue más alta entre los niños menores de 5 años.
ملخص
الغرض
تقدير العبء والمعدلات الخاصة بالعمر لدخول المستشفى المرتبط بالأنفلونزا في المنطقة الريفية غرب كينيا.
الطريقة
تم تسجيل جميع المرضى المصابين بأمراض الجهاز التنفسي البالغ عددهم 3924 مريضًا (التي تم تحديدها على أنها سعال حاد أو صعوبة في التنفس أو ألم صدري جنبي) الذين دخلوا المستشفى فيما بين يونيو 2007 ومايو 2009 في أي مرفق صحي للمرضى الداخليين في مقاطعة بوندو الكينية. وتم جمع مسحات بلعومية أنفية وفموية بلعومية وفحصها للبحث عن فيروسات الأنفلونزا باستخدام تفاعل البوليمريز المتسلسل للنسخ العكسي في الوقت الحقيقي (RT–PCR). وعند حساب المعدلات السنوية، تم إجراء تعديلات على المرضى المسجلين الذين لم يتم فحص مسحات خاصة بهم لفيروس الأنفلونزا.
النتائج
من إجمالي 2079 مريضًا تم فحص مسحات خاصة بهم، تم تأكيد الإصابة بفيروس الأنفلونزا لدى 204 (10 %)، وتم اكتشاف 176 و27 وحالة واحدة إيجابية لتفاعل البوليمريز المتسلسل للنسخ العكسي في الوقت الحقيقي (RT–PCR) بالنسبة لفيروس الأنفلونزا A فقط وفيروس الأنفلونزا B فقط وكلٍ من فيروس الأنفلونزا A وB، على التوالي. ومن بين الذين تم فحصهم للبحث عن فيروس الأنفلونزا، كانت نتيجة الفحص إيجابية لنسبة 6.8 % من الأطفال ممن يزيد أعمارهم عن 5 أعوام و و14 % من المرضى الذين تزيد أعمارهم عن – أو تساوي - 5 أعوام. وكانت نسبة معدل وفيات الحالات بين المرضى النزلاء المصابين بعدوى فيروس الأنفلونزا التي تم تأكيدها باستخدام تفاعل البوليمريز المتسلسل 2.0 %. وبلغ المعدل السنوي لدخول المستشفى (لكل 100000 نسمة) 699.8 بين المرضى المصابين بالأمراض التنفسية و56.2 بين مرضى الأنفلونزا (بمعدل 143.7 و18.8, و55.2 و65.1 و57.3 مريضًا ممن دخلوا المستشفى مصابين بفيروس الأنفلونزا لكل 100000 شخص يقل عمره عن 5 و5 – 19 و20 – 34 و35 – 49 ويزيد عمره أو يساوي 50 عامًا، على التوالي).
الاستنتاج
في المنطقة الريفية غرب كينيا، كان معدل دخول المستشفى المرتبط بالأنفلونزا الأعلى بين الأطفال الذين تقل أعمارهم عن 5 أعوام.
摘要
目的
评估在肯尼亚西部农村流感相关的住院负担和特定年龄比率。
方法
登记在 2007 年 6 月至 2009 年 5 月期间在肯尼亚朋多地区任何住院医疗机构住院的所有 3924 名呼吸道疾病(定义为急性咳嗽、呼吸困难或肋膜炎胸痛)病人。收集鼻咽和口咽化验标本,并使用实时逆转录酶聚合酶链反应(RT-PCR)检验流感病毒。在计算年率中,针对没有检验其化验标本流感病毒的已登记病人进行调整。
结果
在检验了化验标本的 2079 名病人中,确认感染流感病毒的人数为 204(10%)人;发现仅流感 A 病毒 RT–PCR 呈阳性、仅流感 B 病毒 RT–PCR 呈阳性,和流感 A 和 B 病毒 RT–PCR 均呈阳性的人数分别为 176、27 和 1 人。在经过流感病毒检验的人群中,发现 6.8% 不到 5 岁的儿童和 14.0% 的 5 岁或以上的病人呈阳性。在 PCR 证实感染流感病毒的确认病人中的病死率为 2.0%。住院的年率(每 10 万人口):呼吸道疾病病人中为 699.8 人,流行性感冒病人中为 56.2 人(每 10 万在 < 5、5–19、20–34、35–49 和 ≥ 50 年龄区间人口中的流感病毒住院病人分别为 143.7、18.8、55.2、65.1 和 57.3 人)
结论
在肯尼亚西部农村地区,不到 5 岁的儿童的流感相关住院率最高。
Резюме
Цель
Произвести оценку уровня госпитализации, связанного с заболеванием гриппом в сельской местности западной Кении, а также его распределение по возрастным категориям.
Методы
Исследование было проведено среди всех 3924 пациентов с респираторными заболеваниями (с такими симптомами как сильный кашель, затрудненное дыхание или боль в грудной клетке), которые были госпитализированы в период с июня 2007 г. по май 2009 г. во всех стационарах лечебных заведениях округа Бондо в Кении. Был произведен сбор мазков из носоглотки и ротоглотки, после чего полученный материал был протестирован на наличие вирусов гриппа с использованием полимеразной цепной реакции с обратной транскриптазой в режиме реального времени (ПЦР РВ). При расчете годичных показателей были внесены коррективы для включенных в исследование пациентов, мазки которых не проверялись на наличие вируса гриппа.
Результаты
Из 2079 пациентов, у которых были взяты мазки, заражение вирусом гриппа было подтверждено в 204 случаях (10%); результаты теста ПРЦ РВ дали следующий результат: 176 заражений вирусом гриппа A, 27 заражений вирусом гриппа B и 1 заражение вирусами гриппа A и B одновременно. Среди протестированных на наличие вируса гриппа у 6,8% детей в возрасте до 5 лет и у 14,0% пациентов от 5 лет и старше был выявлен вирус гриппа. Количество случаев заболевания со смертельным исходом среди пациентов с положительным результатом ПЦР-тест, подтверждающий наличие заражения гриппом, составило 2,0%. Уровень госпитализации за год (на 100 000 человек) составил 699,8 человек среди пациентов с респираторными заболеваниями и 56,2 человек среди пациентов, больных гриппом (среди которых 143,7, 18,8, 55,2, 65,1 и 57,3 госпитализированных пациентов, зараженных вирусом гриппа, на 100 000 человек были в возрасте < 5, 5–19, 20–34, 35–49 и ≥ 50 лет соответственно).
Вывод
В сельской местности западной Кении наибольшее количество случаев госпитализации по причине заболевания гриппом было зарегистрировано среди детей в возрасте до 5 лет.
Introduction
Although influenza has been widely studied in developed countries, little is known about its epidemiology, seasonality and burden in developing countries.1–6 The epidemiology of influenza and the hospitalization rates associated with the illness may be different in sub-Saharan Africa and more developed areas for several reasons. In temperate regions influenza is highly seasonal, with transmission peaking during winter, whereas in the tropics influenza viruses are thought to circulate year round.7 In Africa, the risk factors for severe outcomes from influenza, such as chronic heart disease, chronic lung disease, infection with human immunodeficiency virus (HIV) and smoking, differ from those found in Europe and North America.4,8–10 Regional differences in the age distribution of human populations and their use of health services may also affect the rate of hospitalization for influenza.
In Kenya, people hospitalized with respiratory illnesses are rarely tested for viral pathogens. Influenza vaccine and drugs active against influenza viruses are not readily available, and little is known about the relative contribution of influenza to severe respiratory disease. In any country, data on influenza can help guide clinical management and allow the health ministry to estimate the influenza burden so that the potential impact of targeted interventions, such as immunization, can be assessed.
Following the 2006 outbreak of avian influenza in Nigerian poultry, Kenya established prospective surveillance for human influenza in several hospitals, with particular emphasis on the detection of the H5N1 subtype of the influenza A virus.11 We describe here the results of population-based, district-wide surveillance in western Kenya for hospitalization with influenza virus in the two years before A(H1N1)pdm09 reached Kenya.12
Methods
Study site
The study was based in the Bondo district of western Kenya, which had a population of 238 780 at the time of the 1999 national census. The district’s population is culturally homogenous and almost entirely rural and poor, as it consists mainly of individuals of Luo ethnicity who live by subsistence farming and fishing.13 Most roads in the district are unpaved. The area is holoendemic for malaria and has a high child mortality rate (212 deaths per 1000 live births in 2008).13 It also has a high prevalence of HIV infection in young adults. In 2003–2004, for example, HIV prevalence was 15.4%, or twice the corresponding national value, among those aged 13–34 years who lived in Bondo district.8
Bondo district has seven inpatient facilities, including one district hospital, as well as 45 outpatient health facilities. The residents of Bondo district seek care in these facilities and also from private local providers, traditional healers, drug sellers and community health workers.14 The hospital in the neighbouring district of Siaya and the provincial hospital in Kisumu are 27 and 57 km from Bondo town, respectively, and rarely admit patients from Bondo district.15 In one part of Bondo district, 25 000 people are offered free, high-quality care at Lwak Mission Hospital, including admission, as part of population-based infectious disease surveillance.16,17 Influenza vaccination is not available in the district in either the public or the private sector.
Data collection
In May 2007, the United States Centers for Disease Control and Prevention (CDC) and the Kenya Medical Research Institute (KEMRI) established prospective population-based surveillance for influenza in all seven inpatient facilities in Bondo district. Trained surveillance officers, mostly nurses, enrolled all consenting patients who were hospitalized for respiratory illness, defined as cough, difficulty breathing or (for patients aged ≥ 5 years) pleuritic chest pain. Patients who had had a cough for more than 2 weeks when they presented were excluded because the study’s focus was on acute respiratory illness. Also excluded were those presenting within 3 days of a previous hospitalization for the same illness. A structured questionnaire and scannable forms (Cardiff Teleform, Vista, United States of America) were used to collect the data, which included demographic characteristics, clinical symptoms and signs, treatment history, avian influenza risk factors and medications. Data were stored in Access (Microsoft, Redmond, USA) databases and regularly checked for errors using cleaning programs; incorrectly completed forms were returned to the field for correction.
Specimens and laboratory methods
Dacron-tipped swabs were used to collect a nasopharyngeal and an oropharyngeal sample from each enrolled subject, and then the pair of swabs was placed, together, in a vial containing 1 ml of virus transport medium (VTM) without antibiotics. In four of the health facilities these specimens were transported on the day of their collection, at 2−8 °C, to the KEMRI/CDC laboratories in Kisumu, approximately 60 km from the collection sites. In the other three health facilities, which were relatively far, the specimens were rapidly placed in tanks of liquid nitrogen and these tanks were taken to the KEMRI/CDC laboratories in Kisumu every 2 weeks. Once at Kisumu, the VTM in each specimen (after thawing, if frozen) was divided into four aliquots and stored at −70 °C. About once a month, the frozen aliquots were transported, on dry ice, to the KEMRI/CDC laboratory in Nairobi (a distance of about 350 km), where one aliquot per enrolled patient was tested for influenza virus ribonucleic acid (RNA) by means of real-time reverse transcriptase polymerase chain reaction (RT–PCR).
The total RNA from a 100-µl subsample of an aliquot was extracted using a QIAamp viral RNA minikit (Qiagen Inc., Valencia, USA), in accordance with the manufacturer’s instructions. One-step RT–PCR was then carried out using the AgPath kit (Applied Biosystems, Foster City, USA) and a protocol developed by the CDC’s Influenza Division in Atlanta (S Lindstrom, unpublished data, 2010). The protocol is available upon request, under a material transfer agreement, from the CDC in Atlanta. Specimens yielding crossover threshold (CT) values of ≤ 39.9 were considered positive, whereas those giving higher CT values or no CT values were considered negative. Specimens found positive for influenza A virus RNA were subtyped for seasonal H1 and H3 using another real-time RT–PCR, with primers, probes and positive control samples of influenza viruses provided by the CDC in Atlanta.
Blood smears from all febrile patients (≥ 38.0 °C) admitted to Lwak Mission Hospital and from all children admitted to Bondo District Hospital were prepared, Giemsa-stained and checked for malarial parasites by light microscopy. At the five other study facilities, such blood smears were prepared at the clinician’s discretion. Although there was no routine testing of inpatients for HIV, some enrolled patients were checked for HIV as part of a case–control study of influenza risk factors.10
Data analysis
Rates were calculated using age-specific population projections for Bondo district from the 1999 census, assuming an annual growth rate of 1.8%.18 Adjustments were made for the proportion of eligible patients who did not have a swab taken (because the patient refused, or was admitted when surveillance staff were not available, or left the hospital or died before specimens could be collected) and for the proportion of patients from whom swabs were taken but not tested for influenza virus. These adjustments were made separately for each health facility and included in the study, each age group considered and by season (high in May–October, low in November–April), each time assuming that the proportion of the enrolled but untested patients who were infected with influenza virus was the same as the corresponding proportion of tested patients who were found positive for influenza virus RNA. The binomial distribution method was used to calculate 95% confidence intervals (CIs) around each rate.19 Case-fatality rates (CFRs) were estimated, with similar adjustments for the proportion of eligible patients who had swabs tested, age group, season and health facility. The categorical variables were compared by means of χ2 tests. Stata version 11 (StataCorp. LP, College Station, USA) was used for all data analyses.
Ethical review
This surveillance project was approved by the KEMRI National Ethical Review Committee and exempted, as non-research, by the CDC Institutional Review Board. Written informed consent was obtained from all adult participants and from the parents or legally authorized representatives of the enrolled children.
Results
Between June 2007 and May 2009, 3924 patients were hospitalized with respiratory illness and enrolled in the present study (Table 1). Most (58.6%) were children aged < 5 years. The median length of stay was 2 days. Although nasopharyngeal and oropharyngeal swabs were collected from 2389 (60.9%) of the patients with respiratory illness, only the swabs from 2079 (87.0%) of these patients were tested (Table 1); the swabs from the other 310 patients were not of adequate quality to be tested or were mislaid. When the enrolled patients from whom swabs were collected were compared with the other enrolled patients, no marked differences were seen in terms of demographic characteristics or median length of hospital stay. Although those who had swabs collected had a slightly higher CFR, the difference was not statistically significant (3.6% versus 2.7%; P = 0.12). The proportion of eligible patients who were swabbed was much smaller in the Lwak Mission Hospital than in the other six study facilities (20.3% versus 89.2–98.8%; P < 0.001 for each pair-wise comparison; Table 2).
Table 1. Patients hospitalized for respiratory illness and patients positive for influenza virus, by age and type of virus, Bondo district, Kenya, June 2007–May 2009.
| Characteristic | No. (%) of enrolled patients |
||
|---|---|---|---|
| Aged < 5 years | Aged ≥ 5 years | Total | |
| With respiratory illness | 2301 (100) | 1623 (100) | 3924 (100) |
| With collected swabs | 1382 (60.1) | 1007 (62.0) | 2389 (60.9) |
| With tested swabs | 1213 (87.8)a | 866 (86.0)a | 2079 (87.0)a |
| PCR-positive for viral RNAb | 167 (13.8)c | 242 (28.0)c | 409 (20.0)c |
| Influenza, A or B virus | 83 (6.8)c | 121 (14.0)c | 204 (9.8)c |
| Influenza, A virus | 81 (6.7)c | 96 (11.1)c | 177 (8.5)c |
| Influenza, B virus | 3 (0.2)c | 25 (2.9)c | 28 (1.3)c |
| Influenza, A and B viruses | 1 (0.1)c | 0 (0.0)c | 1 (0.05)c |
PCR, polymerase chain reaction; RNA, ribonucleic acid.
a Denominator: patients with swabs collected.
b One patient, aged < 5 years, was positive for both influenza A virus and influenza B virus.
c Denominator: patients with swabs tested.
Table 2. Admissions for respiratory illness in inpatient health facilities, Bondo district, Kenya, June 2007–May 2009.
| Health facility | No. (%) of patients with respiratory illness |
No. of influenza-associated deaths | Influenza CFR | ||||
|---|---|---|---|---|---|---|---|
| Enrolled | Deceased | With swabs |
|||||
| Collected | Testeda | Positive for viral RNAb | |||||
| Bondo District Hospital | 667 | 52 (7.8) | 649 (97.3) | 554 (85.4) | 37 (6.7) | 1 | 2.7 |
| Lwak Mission Hospital | 1820 | 40 (2.2) | 370 (20.3) | 330 (89.2) | 19 (5.8) | 1 | 5.3 |
| Madiany Sub-District Hospital | 372 | 17 (4.6) | 353 (94.9) | 301 (85.3) | 40 (13.3) | 0 | 0.0 |
| Matangwe Health Centre | 233 | 4 (1.7) | 224 (96.1) | 188 (83.9) | 15 (8.0) | 0 | 0.0 |
| Got Agulu Health Centre | 464 | 7 (1.5) | 440 (94.8) | 396 (90.0) | 72 (18.2) | 1 | 1.4 |
| Abidha Health Centre | 257 | 8 (3.1) | 254 (98.8) | 217 (85.4) | 13 (6.0) | 1 | 7.7 |
| St Anne’s Health Centre | 111 | 1 (0.9) | 99 (89.2) | 93 (93.9) | 8 (8.6) | 0 | 0.0 |
| Total | 3924 | 129 (3.3) | 2389 (60.9) | 2079 (87.0) | 204 (9.8) | 4 | 2.0 |
CFR, case-fatality rate; RNA, ribonucleic acid.
a The denominator for each percentage is the number of patients with swabs collected.
b The denominator for each percentage is the number of patients with swabs tested.
Influenza virus RNA was detected in 204 (9.8%) of the 2079 swabs tested; 177 (8.5%) and 28 (1.3%) of the swabs tested were PCR-positive for influenza A virus and influenza B virus RNA, respectively. One patient’s swab was positive for both influenza A and B virus RNA (Table 1). Influenza virus RNA was detected in 6.8% and 14.0% of the swabs from patients aged < 5 and ≥ 5 years, respectively. Four patients with PCR-confirmed influenza – three children aged < 5 years and one older patient (giving a CFR of 3.6% and 0.8% for the two age groups, respectively) – died. The overall CFR was similar for patients who tested positive and negative for influenza virus (2.0% versus 3.7%; P = 0.24). The subtypes of influenza virus were successfully identified for 25 patients infected with influenza A virus: 18 harboured H3N2 and 7 carried seasonal H1N1 (although the first case of A(H1N1)pdm09 in Kenya was not identified until late June 2009, after surveillance for the present study had ended)12.
Females accounted for slightly more than half of the patients with respiratory illness (54.3%), influenza A virus (59.3%) or influenza B virus (67.9%). The median age of patients admitted with respiratory illness was 3 years (range: 2 days to 99 years; (Table 3). The patients found infected with influenza A virus were generally younger than those found infected with influenza B virus, with median ages of 6 years (range: 1 month to 77 years) and 26.5 years (range: 6 months–80 years), respectively. Overall, 53 patients aged > 12 years were found PCR-positive for influenza virus and were tested for HIV. Of these, 13 patients (24.5%) – all from the 22 patients aged ≥ 18 years – were found to be HIV-infected. The cases found infected with influenza virus had been ill for a median of 4 days when their swabs were collected. Such cases were less likely to have presented with fever (i.e. a body temperature of at least 38.0 °C) than the patients found negative for influenza virus (17.2% versus 27.6%; P < 0.01).
Table 3. Patients hospitalized for respiratory illness and laboratory-confirmed influenza, by age and type of influenza virus, Bondo district, Kenya, June 2007–May 2009.
| Characteristic | No. (%) of patients hospitalized with: |
||
|---|---|---|---|
| Respiratory illness (n = 3 924) | Influenza, A virus (n = 177) | Influenza, B virus (n = 28) | |
| Female | 2132 (54.3) | 105 (59.3a) | 19 (67.9)b |
| Age (years) | |||
| < 5 | 2301 (58.6) | 81 (45.8)a | 3 (10.7)b |
| 5–19 | 529 (13.5) | 27 (15.3)a | 5 (17.9)b |
| 20–34 | 499 (12.7) | 37 (20.9)a | 10 (35.7)b |
| 35–49 | 317 (8.1) | 20 (11.3)a | 5 (17.9)b |
| ≥ 50 | 278 (7.1) | 12 (6.8)a | 5 (17.9)b |
a The denominator for this percentage is all hospitalized patients with influenza A virus.
b The denominator for this percentage is all hospitalized patients with influenza B virus.
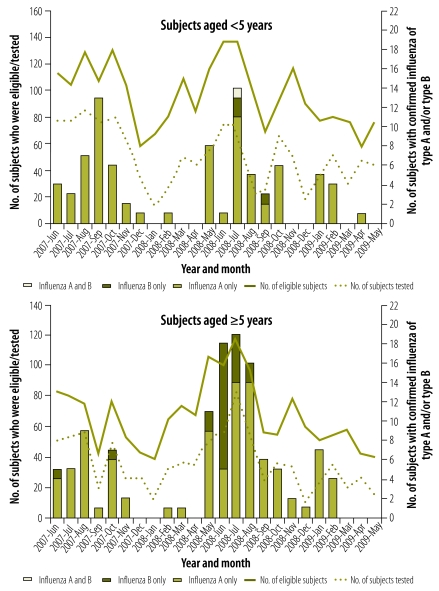
Blood samples for the preparation of smears were collected from 2623 (67%) of the inpatients with respiratory illness. The influenza-virus-positive cases investigated were just as likely to be found smear-positive for malaria as the influenza-virus-negative cases (36.2% versus 33.8%; P = 0.62). Only one patient found infected with influenza virus had a positive blood culture, with Group B Salmonella isolated. Although more swabs were tested in the first year of surveillance than in the second, the proportion of swabs found positive for influenza virus was lower in the first year (7.7% versus 12.3%; P < 0.01; Table 4). In both years, most influenza-virus-positive cases were detected between May and October, with the monthly numbers of cases peaking in July and August (Fig. 1) – a time when western Kenya is usually dry and relatively cool (21–23 °C). In the second year, but not the first, there was a second smaller peak of cases in January and February – a period that is also usually dry in western Kenya but warmer (24–25 °C).
Table 4. Hospital admissions associated with respiratory illness and influenza in two time periods, Bondo district, Kenya, June 2007–May 2009.
| Characteristic | No. (%) of inpatients with respiratory illness |
||
|---|---|---|---|
| Year 1a | Year 2b | Both years | |
| Recorded | 2007 (100) | 1917 (100) | 3924 (100) |
| With swabs collected | 1205 (60.0) | 1184 (61.8) | 2389 (60.9) |
| With swabs tested | 1129 (93.7)c | 950 (80.2)c | 2079 (87.0)c |
| With influenza, A virus | 83 (7.4)d | 94 (9.9)d | 177 (8.5)d |
| With influenza, B virus | 4 (0.4)d | 24 (2.5)d | 28 (1.3)d |
a June 2007–May 2008.
b June 2008–May 2009.
c The denominator for this percentage is the number of patients with swabs collected in the period indicated.
d The denominator for this percentage is the number of patients with swabs tested in the period indicated.
Fig. 1.
Laboratory-confirmed influenza among patients hospitalized with respiratory illness, Bondo district, Kenya, June 2007–May 2009
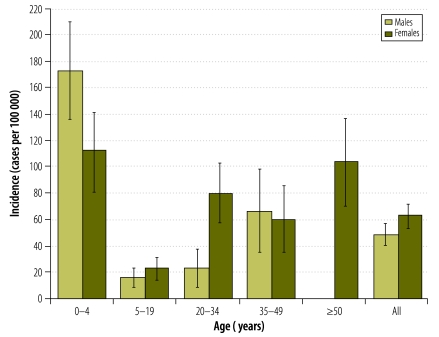
Over the two-year study period, the mean annual rate (per 100 000 population) of hospitalization for respiratory illness was 699.8, whereas the corresponding rates of influenza-associated hospitalizations were 56.2 for influenza virus infection of any type, 50.3 for influenza A virus and 6.1 for influenza B virus (Table 5, available at: http://www.who.int/bulletin/volumes/90/4/11-094326). Compared with older children and adults, children aged < 5 years had a much higher rate of influenza-associated hospitalization: 143.7 cases (95% CI: 119.6–167.8) per 100 000 versus 36.7 cases (95% CI: 33.0–44.4) per 100 000. Overall, the rate of influenza-associated hospitalization was also higher among females than males (Fig. 2). There was a significantly higher rate of influenza-associated hospitalization among females aged 20–34 or ≥ 50 years than among males of the same age groups.
Table 5. Annual hospitalizations (per 100 000 population) associated with respiratory illness and influenza by age group, Bondo district, Kenya, June 2007–May 2009.
| Illness and age group | Year 1a |
Year 2b |
Both years |
|||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjustedc |
Unadjusted |
Adjustedc |
Unadjusted |
Adjustedc |
|||
| No. (95% CI) | No. (95% CI) | No. (95% CI) | No. (95% CI) | No. (95% CI) | No. (95% CI) | |||
| All age groups | ||||||||
| Respiratory illness | 722.3 | NA | 677.6 | NA | 699.8 | NA | ||
| (690.8–753.8) | (647.4–707.9) | (678.0–721.6) | ||||||
| Influenza, A virus | 29.9 | 53.5 | 33.2 | 47.1 | 31.6 | 50.3 | ||
| (23.4–36.3) | (44.9–62.1) | (26.5–39.9) | (39.1–55.1) | (26.9–36.2) | (44.4–56.1) | |||
| Influenza, B virus | 1.4 | 2.4 | 8.5 | 9.8 | 5.0 | 6.1 | ||
| (0.0–2.9) | (0.6–4.2) | (5.1–11.9) | (6.2–13.4) | (3.1–6.8) | (4.1–8.2) | |||
| Influenza, A virus, B virus or both | 31.3 | 55.8 | 41.4 | 56.5 | 36.4 | 56.2 | ||
| (24.7–37.9) | (47.0–64.6) | (33.9–48.9) | (47.7–65.3) | (31.4–41.4) | (50.0–62.4) | |||
| Influenza-associated hospitalizations (A, B or both virus types) | ||||||||
| < 1 year | 93.8 | 105.5 | 122.8 | 169.5 | 108.4 | 137.8 | ||
| (40.7–146.8) | (49.3–161.8) | (62.7–182.9) | (98.9–240.1) | (68.3–148.5) | (92.6–183.0) | |||
| 1 year | 204.0 | 406.3 | 100.2 | 122.5 | 151.6 | 263.1 | ||
| (109.9–298.1) | (273.6–539.0) | (34.8–165.6) | (50.1–194.8) | (94.5–208.8) | (187.9–338.3) | |||
| 2 years | 58.5 | 145.3 | 68.9 | 84.7 | 63.8 | 114.7 | ||
| (7.2–109.8) | (64.5–226.0) | (13.8–124.1) | (23.6–145.9) | (26.1–101.4) | (64.2–165.2) | |||
| 3 years | 60.6 | 163.9 | 59.5 | 67.0 | 60.1 | 115.0 | ||
| (7.5–113.7) | (76.6–251.2) | (7.4–111.7) | (11.6–122.3) | (22.9–97.3) | (63.5–166.5) | |||
| 4 years | 58.2 | 124.2 | 22.9 | 29.1 | 40.4 | 76.2 | ||
| (7.2–109.3) | (49.7–198.7) | (0.0–54.6) | (0.0–64.8) | (10.5–70.3) | (35.1–117.3) | |||
| < 5 years | 95.7 | 182.4 | 79.4 | 105.6 | 87.5 | 143.7 | ||
| (67.8–123.7) | (143.8–221.0) | (54.2–104.6) | (76.5–134.7) | (68.7–106.3) | (119.6–167.8) | |||
| 5–19 years | 9.7 | 16.8 | 18.1 | 20.7 | 13.9 | 18.8 | ||
| (4.0–15.4) | (9.3–24.4) | (10.4–25.9) | (12.5–29.0) | (9.1–18.7) | (13.1–24.4) | |||
| 20–34 years | 28.1 | 33.6 | 58.9 | 76.5 | 43.6 | 55.2 | ||
| (13.9–42.3) | (18.0–49.1) | (38.5–79.3) | (53.2–99.7) | (31.2–56.1) | (41.2–69.2) | |||
| 35–49 years | 33.0 | 49.7 | 48.6 | 80.3 | 40.9 | 65.1 | ||
| (12.6–53.5) | (24.6–74.8) | (24.0–73.2) | (48.7–111.9) | (24.9–56.9) | (44.9–85.3) | |||
| ≥ 50 years | 19.2 | 55.0 | 34.6 | 59.5 | 27.0 | 57.3 | ||
| (3.8–34.6) | (29.0–81.1) | (14.2–55.0) | (32.7–86.3) | (14.2–39.8) | (38.6–76.0) | |||
CI, confidence interval; NA, not applicable.
a June 2007–May 2008.
b June 2008–May 2009.
c Adjusted for the number of hospitalized patients meeting case definitions who did not have swabs tested. Adjustment was made separately for each age group, health facility and influenza season.
Fig. 2.
Age- and gender-specific annual influenza-associated hospital admissions (per 100 000 people), Bondo district, Kenya, June 2007–May 2009
Note: The vertical lines indicate 95% confidence intervals.
The mean annual rate of influenza-associated inpatient mortality was 1.5 deaths (95% CI: 0.5–2.5) per 100 000 population. There were 7.6 influenza-associated deaths (95% CI: 2.1–13.2) per 100 000 children aged < 5 years but only 0.2 (95% CI: 0.0–0.6) per 100 000 individuals aged ≥ 5 years.
No avian influenza virus (H5N1) was detected, but 19.1% of the enrolled participants reported having heard rumours of sick or dead birds in their neighbourhood in the past two weeks; 9.3% said that they had been within 1 m of a sick or dead bird, and 4.8% that they had touched a dead bird.20
Discussion
Our prospective surveillance in a district in Kenya is one of the first to report directly-measured rates of influenza-associated hospitalization in African adults and children.4 In developing countries, sentinel hospital surveillance can provide data for describing influenza epidemiology and seasonality, characterizing the circulating strains of influenza virus (to guide vaccinations) and monitoring influenza pandemics. Such surveillance can seldom be used to define the burden of influenza, however, because it often occurs in referral hospitals, where the denominator population is difficult to define and the health-seeking patterns may not be representative of the norm.21,22
The rate of influenza-associated hospitalization that we report in children of Bondo district who were aged < 5 years (i.e. 143.7 cases per 100 000 child-years) is lower than the corresponding values reported in South Africa, Thailand and the United States.23–25 In urban Soweto, South Africa, for example, 309 annual cases of hospitalized influenza were recorded per 100 000 HIV-uninfected children and 1444 such cases were recorded per 100 000 HIV-infected children.25 The lower rate of influenza-associated hospitalization seen in Bondo district might only reflect the local epidemiology of influenza, as related to the patient population and/or climate. However, it can probably be attributed, at least in part, to the relatively low levels of health-seeking for severe illness in this region of Kenya, probably attributable to cultural beliefs, the costs of health care and the large distances to the nearest health-care facilities.15,26–28 In a survey of health utilization in Bondo district in 2005, only 26% of children aged < 5 years and 16% of older children and adults were hospitalized for a reported episode of pneumonia in the previous year.26 The observation that health-care utilization for severe illness is low in this part of Kenya is reinforced by the finding that most deaths in the area occur at home.13 If hospital utilization for severe influenza-associated illness in Bondo district is as low as for pneumonia, the incidence of severe influenza-related respiratory illness in this area could be up to fourfold higher for children and sixfold higher for adults than those reported in this article.
Low levels of hospital utilization despite severe illness may also explain why, in the present study, the elderly had some of the lowest rates of hospitalization for respiratory illness, whereas in developed countries the elderly have some of the highest rates.15,29,30 In rural Kenya, it is generally the elderly who find it hardest to travel the often long distances to their nearest hospital and it is generally also they who are least comfortable with the non-traditional medicine practised in hospitals. The elderly of Bondo district are probably also less likely to suffer from an underlying cardiopulmonary medical condition than their counterparts in developed countries,31 partly because the prevalence of smoking in Kenya is still very low. Most influenza-associated hospitalizations of adults in the United States result from exacerbation of underlying medical conditions.29
Unlike developed areas, Bondo district had an influenza-associated hospitalization rate in young adults that was similar to – not lower than – the rate among the elderly.30,32 Although this could, again, result from the relatively low levels of care-seeking among the elderly, we believe it may also reflect the high prevalence of HIV infection among young adults in this area of Kenya.8 HIV infection may also explain why influenza-associated hospitalizations were more common among the young women of Bondo district than among the young men, since the young women have the higher prevalence of HIV infection.8 In an earlier study in the same district, HIV infection was associated with a 3.5-fold risk of influenza-associated hospitalization among adults.10 Among South African children infected with influenza virus, HIV infection was associated with an estimated eightfold higher risk of hospitalization.33
Although influenza virus activity in temperate climates peaks during winter seasons, influenza virus in Bondo district circulates throughout the year – as seen in other tropical and subtropical climates, such as those of Indonesia, Singapore, Thailand and Viet Nam4–6 – but tends to have a broad peak of activity coincident with winter in the southern hemisphere. Bondo town is about 11 km south of the Equator.
The proportion of swabs found positive for influenza virus RNA and the CFRs for influenza-associated hospitalizations varied among the seven health facilities included in the present study, although the total number of deaths was small. Lwak Mission Hospital admitted many more patients with respiratory illness than any other of the health facilities, and almost three times more than the district hospital, probably because the hospital has a programme for the population-based surveillance of infectious diseases. This programme offers free, high-quality health care to participants, which promotes greater health care utilization.16,17 The variability in findings between health facilities highlights the limitations of using one sentinel hospital to draw conclusions about the epidemiology and burden of influenza in a given district.
Our study had several limitations. First, we used only molecular methods to detect influenza virus, and some infected cases may not have been detected. In Thailand, serological testing with haemagglutinin inhibition tests identified influenza cases not detected by RT–PCR.34 Second, some of the patients found swab-positive for influenza virus may not have been hospitalized for influenza but for another cause, such as bacterial pneumonia or malaria. Influenza virus might have been present from a recent or concurrent upper respiratory tract infection. Influenza virus has been detected in healthy children used as controls.35 Third, only about 60% of the enrolled patients with respiratory illness had a swab collected. If patients who were not swabbed had a different risk of developing influenza than patients who were swabbed, our adjusted rates could be inaccurate. There were, however, no significant differences in age, length of hospitalization or CFR between those who had and those who did not have swabs taken, and the extrapolation to include untested patients therefore seems appropriate. Excluding untested hospitalized patients with respiratory illness from the hospitalization rate calculation would have led to a clear underestimate of the true rate. Fourth, to define seasonality and annual fluctuations in the rates of influenza-associated hospitalization clearly, surveillance for a period longer than 2 years is required. Fifth, by excluding those who had had a cough for more than 2 weeks at presentation, we may have underestimated the burden of influenza by excluding patients hospitalized with tuberculosis or chronic lung diseases, both of which have been identified as risk factors for influenza-associated hospitalization.10
In conclusion, our population-based study highlights the disproportionately high burden of influenza among children aged < 5 years and young adults in western Kenya. The findings suggest that influenza prevention activities could substantially reduce severe respiratory disease in other developing countries, especially in young children and other people at particular risk of severe respiratory illness, such as those living with HIV.10,25
Acknowledgements
This study was conducted as a collaborative activity between the Kenya Medical Research Institute and the United States Centers for Disease Control and Prevention. We thank the District Medical Office of Health of Bondo district. This paper is published with the permission of the director of the Kenya Medical Research Institute.
The findings and conclusions are those of the authors and do not necessarily represent the views of the United States Centers for Disease Control and Prevention.
Competing interests:
None declared.
References
- 1.Li CK, Choi BC, Wong TW. Influenza-related deaths and hospitalizations in Hong Kong: a subtropical area. Public Health. 2006;120:517–24. doi: 10.1016/j.puhe.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Viboud C, Alonso WJ, Simonsen L. Influenza in tropical regions. PLoS Med. 2006;3:e89. doi: 10.1371/journal.pmed.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harper SA, Bradley JS, Englund JA, File TM, Gravenstein S, Hayden FG, et al. Seasonal influenza in adults and children – diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1003–32. doi: 10.1086/598513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gessner BD, Shindo N, Briand S. Seasonal influenza epidemiology in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2011;11:223–35. doi: 10.1016/S1473-3099(11)70008-1. [DOI] [PubMed] [Google Scholar]
- 5.Leo YS, Lye DC, Chow A. Influenza in the tropics. Lancet Infect Dis. 2009;9:457–8. doi: 10.1016/S1473-3099(09)70182-3. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen HL, Saito R, Ngiem HK, Nishikawa M, Shobugawa Y, Nguyen DC, et al. Epidemiology of influenza in Hanoi, Vietnam, from 2001 to 2003. J Infect. 2007;55:58–63. doi: 10.1016/j.jinf.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Monto AS. Epidemiology of influenza. Vaccine. 2008;26(Suppl 4):D45–8. doi: 10.1016/j.vaccine.2008.07.066. [DOI] [PubMed] [Google Scholar]
- 8.Amornkul PN, Vandenhoudt H, Nasokho P, Odhiambo F, Mwaengo D, Hightower A, et al. HIV prevalence and associated risk factors among individuals aged 13–34 years in rural Western Kenya. PLoS ONE. 2009;4:e6470. doi: 10.1371/journal.pone.0006470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuzil KM, Coffey CS, Mitchel EF, Jr, Griffin MR. Cardiopulmonary hospitalizations during influenza season in adults and adolescents with advanced HIV infection. J Acquir Immune Defic Syndr. 2003;34:304–7. doi: 10.1097/00126334-200311010-00008. [DOI] [PubMed] [Google Scholar]
- 10.Ope MO, Katz MA, Aura B, Gikunju S, Njenga MK, Ng’ang’a Z, et al. Risk factors for hospitalized seasonal influenza in rural western Kenya. PLoS ONE 20116e20111Epub 2011 May 26 10.1371/journal.pone.0020111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortiz JR, Katz MA, Mahmoud MN, Ahmed S, Bawa SI, Farnon EC, et al. Lack of evidence of avian-to-human transmission of avian influenza A (H5N1) virus among poultry workers, Kano, Nigeria, 2006. J Infect Dis. 2007;196:1685–91. doi: 10.1086/522158. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention Introduction and transmission of 2009 pandemic influenza A (H1N1) virus – Kenya, June–July 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1143–6. [PubMed] [Google Scholar]
- 13.Hamel MJ, Adazu K, Obor D, Sewe M, Vulule J, Williamson JM, et al. A reversal in reductions in child mortality in western Kenya, 2003–2009. Am J Trop Med Hyg. 2011;85:597–605. doi: 10.4269/ajtmh.2011.10-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bigogo G, Audi A, Aura B, Aol G, Breiman RF, Feikin DR. Health-seeking patterns among participants of population-based morbidity surveillance in rural western Kenya: implications for calculating disease rates. Int J Infect Dis. 2010;14:e967–73. doi: 10.1016/j.ijid.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Tornheim JA, Manya AS, Oyando N, Kabaka S, Breiman RF, Feikin DR. The epidemiology of hospitalized pneumonia in rural Kenya: the potential of surveillance data in setting public health priorities. Int J Infect Dis. 2007;11:536–43. doi: 10.1016/j.ijid.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Burgert CR, Bigogo G, Adazu K, Odhiambo F, Buehler J, Breiman RF, et al. Impact of implementation of free high-quality health care on health facility attendance by sick children in rural western Kenya. Trop Med Int Health. 2011;16:711–20. doi: 10.1111/j.1365-3156.2011.02752.x. [DOI] [PubMed] [Google Scholar]
- 17.Feikin DR, Audi A, Olack B, Bigogo GM, Polyak C, Burke H, et al. Evaluation of the optimal recall period for disease symptoms in home-based morbidity surveillance in rural and urban Kenya. Int J Epidemiol. 2010;39:450–8. doi: 10.1093/ije/dyp374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Analytical report on population projections. In: Kenya 1999 population and housing census, VII Nairobi: Central Bureau of Statistics, Ministry of Planning and National Development; 2002. p. 126. [Google Scholar]
- 19.Bickel PJ, Doksum KA. Mathematical statistics: basic ideas and selected topics Upper Saddle River: Prentice Hall; 2001. [Google Scholar]
- 20.WHO case definitions for human infections with influenza A(H5N1) virus [Internet]. Geneva: World Health Organization; 2006. Available from: http://www.who.int/influenza/resources/documents/case_definition2006_08_29/en/ [accessed 9 June 2010].
- 21.Ortiz JR, Sotomayor V, Uez OC, Oliva O, Bettels D, McCarron M, et al. Strategy to enhance influenza surveillance worldwide. Emerg Infect Dis. 2009;15:1271–8. doi: 10.3201/eid1508.081422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipsitch M, Hayden FG, Cowling BJ, Leung GM. How to maintain surveillance for novel influenza A H1N1 when there are too many cases to count. Lancet. 2009;374:1209–11. doi: 10.1016/S0140-6736(09)61377-5. [DOI] [PubMed] [Google Scholar]
- 23.Bourgeois FT, Valim C, McAdam AJ, Mandl KD. Relative impact of influenza and respiratory syncytial virus in young children. Pediatrics. 2009;124:e1072–80. doi: 10.1542/peds.2008-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmerman JM, Chittaganpitch M, Levy J, Chantra S, Maloney S, Uyeki T, et al. Incidence, seasonality and mortality associated with influenza pneumonia in Thailand: 2005-2008. PLoS ONE. 2009;4:e7776. doi: 10.1371/journal.pone.0007776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madhi SA, Ramasamy N, Bessellar TG, Saloojee H, Klugman KP. Lower respiratory tract infections associated with influenza A and B viruses in an area with a high prevalence of pediatric human immunodeficiency type 1 infection. Pediatr Infect Dis J. 2002;21:291–7. doi: 10.1097/00006454-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Burton DC, Flannery B, Onyango B, Larson C, Alaii J, Zhang X, et al. Healthcare-seeking behaviour for common infectious disease-related illnesses in rural Kenya: a community-based house-to-house survey. J Health Popul Nutr. 2011;29:61–70. doi: 10.3329/jhpn.v29i1.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bigogo G, Audi A, Aura B, Aol G, Breiman RF, Feikin DR. Health-seeking patterns among participants of population-based morbidity surveillance in rural western Kenya: implications for calculating disease rates. Int J Infect Dis. 2010;14:e967–73. doi: 10.1016/j.ijid.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Källander K, Hildenwall H, Waiswa P, Galiwango E, Peterson S, Pariyo G. Delayed care seeking for fatal pneumonia in children aged under five years in Uganda: a case-series study. Bull World Health Organ. 2008;86:332–8. doi: 10.2471/BLT.07.049353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 30.Proff R, Gershman K, Lezotte D, Nyquist AC. Case-based surveillance of influenza hospitalizations during 2004–2008, Colorado, USA. Emerg Infect Dis. 2009;15:892–8. doi: 10.3201/eid1506.081645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ntusi NB, Mayosi BM. Epidemiology of heart failure in sub-Saharan Africa. Expert Rev Cardiovasc Ther. 2009;7:169–80. doi: 10.1586/14779072.7.2.169. [DOI] [PubMed] [Google Scholar]
- 32.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 33.Madhi SA, Schoub B, Simmank K, Blackburn N, Klugman KP. Increased burden of respiratory viral associated severe lower respiratory tract infections in children infected with human immunodeficiency virus type-1. J Pediatr. 2000;137:78–84. doi: 10.1067/mpd.2000.105350. [DOI] [PubMed] [Google Scholar]
- 34.Katz MA, Tharmaphornpilas P, Chantra S, Dowell SF, Uyeki T, Lindstrom S, et al. Who gets hospitalized for influenza pneumonia in Thailand? Implications for vaccine policy. Vaccine. 2007;25:3827–33. doi: 10.1016/j.vaccine.2007.01.109. [DOI] [PubMed] [Google Scholar]
- 35.Singleton RJ, Bulkow LR, Miernyk K, DeByle C, Pruitt L, Hummel KB, et al. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol. 2010;82:1282–90. doi: 10.1002/jmv.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]