Abstract
The ACI rat is a unique model of human breast cancer in that mammary cancers are induced by estrogen without carcinogens, irradiation, xenografts or transgenic manipulations. We sought to characterize mammary cancers in a congenic variant of the ACI rat, the ACI.COP-Ept2. All rats with estradiol implants developed mammary cancers in 5–7 months. Rats bearing estradiol-induced mammary cancers were treated with tamoxifen for three weeks. Tamoxifen reduced tumor mass, measured by magnetic resonance imaging, by 89%. Tumors expressed estrogen receptors (ER), progesterone receptor (PR), and Erbb2. ERα and PR were overexpressed in tumor compared to adjacent non-tumor mammary gland. Thus, this model is highly relevant to hormone responsive human breast cancers.
Keywords: ACI rat, breast cancer etiology, breast cancer prevention, tamoxifen, estrogen receptor, rat model
Introduction
Cumulative exposure to estrogens through early menarche, late menopause (reviewed by [1]), or hormone replacement [2, 3] increases breast cancer risk. The selective estrogen receptor modulator tamoxifen prevents approximately 50% of breast cancers in women at increased breast cancer risk [4]. At this time, the molecular mechanisms through which estrogens contribute to breast cancer development remain poorly defined.
The inbred ACI rat provides a valuable model for defining the mechanisms through which estrogens contribute to breast cancer development. Ovary intact, but not ovariectomized, ACI rats exhibit a unique, genetically conferred propensity to develop mammary cancer when treated continuously with physiological levels of 17β-estradiol (E2) [5–10]. Induction by E2 of mammary cancer development in ACI rats is blocked by simultaneous treatment with tamoxifen, indicating that E2-induced mammary carcinogenesis is mediated through estrogen receptors [11]. It has recently been demonstrated that simultaneous treatment of ovariectomized ACI rats with E2 and progesterone have a mammary cancer susceptibility to a degree similar to that exhibited by ovary intact ACI rats, illustrating the importance of progesterone for mammary cancer development in this rat model [12]. Thus, estrogens and progestins both are likely to contribute to mammary cancer development in the ACI rat and breast cancer in humans.
The genetic bases of susceptibility to E2-induced mammary cancer have been evaluated in crosses between the highly susceptible ACI rat strain and resistant Copenhagen (COP) or Brown Norway rat strains [7–10]. These studies revealed the existence of multiple quantitative trait loci, designated Emca1 (Estrogen-induced mammary cancer) through Emca9, each of which harbors one or more genetic determinants of breast cancer susceptibility. Four single nucleotide polymorphisms associated with breast cancer risk in a multi-stage genome-wide association study reside in regions of the human genome that are orthologous to peak LOD regions for different Emca loci [13]. The data from these comparative genetic studies in the rat model and humans strongly suggest that the rat and human share specific genetic determinants of breast cancer susceptibility.
Mammary cancers that develop in E2-treated ACI rats are in many respects similar to human breast cancers. In addition to being dependent upon estrogen for their development, most E2-induced mammary cancers exhibit genome instability [8, 11, 14–16]. Loss of rat chromosome 5 (RNO5), the distal half of which harbors Emca1 and Emca8, was the most commonly observed somatic aberration in a large panel of E2-induced mammary cancers evaluated by comparative genomic hybridization (CGH) [16]. The distal half of RNO5 is orthologous to human chromosomes 9p and 1p, both of which are deleted in a large fraction of human breast cancers evaluated by CGH. Together, these data suggest yet another genetic commonality between the ACI rat model of E2-induced mammary cancer and breast cancer in humans.
In addition to providing novel insights into the genetics of breast cancer susceptibility, the ACI rat model of E2-induced mammary cancer is useful to study breast cancer prevention. For example, a 40% restriction of dietary energy consumption dramatically inhibited mammary cancer development [17], and dietary supplementation with black raspberries or ellagic acid reduced cancer multiplicity [18]. One shortcoming of the ACI rat for mammary cancer prevention studies is that continuous E2 treatment induces pituitary lactotroph hyperplasia resulting in dramatic pituitary gland enlargement, with potential morbidity and mortality prior to reaching desired experimental endpoint. To alleviate pituitary associated morbidities, a novel rat strain was generated, designated ACI.COP-Ept2, which retains the unique susceptibility of the ACI rat to E2-induced mammary cancer while exhibiting reduced pituitary hyperplasia in response to estrogens [19–21].
Estrogen action in responsive tissues is mediated in large part through estrogen receptor (ER) α and ERβ. ERα serves as a valuable marker for predicting clinical outcomes for breast cancer patients. Expression of progesterone receptor (PR) is induced by estrogens, and PR, ike ERα, serves as an important breast cancer prognostic biomarker. The purpose of this study was to characterize the mammary cancers that develop in E2 treated ACI.COP-Ept2 rats with respect to mammary gland histopathology and expression of ERα, ERβ, progesterone receptor (PR) and additional clinically proven or promising prognostic protein biomarkers. We also demonstrate for the first time that tamoxifen treatment results in rapid regression of estrogen-induced mammary cancers in a rat model. Together, our data illustrate the usefulness of the ACI.COP-Ept2 rat strain as a model for mammary cancer prevention and treatment studies.
Methods
Treatment of animals
ACI.COP-Ept2 rat colony was established from breeder pairs provided by James Shull. Rats were housed in polycarbonate cages on wood chip bedding, 23 °C, 12 hr light/dark cycle, and fed Purina 5008 (Purina Mills, St. Louis, MO). The University of Missouri Animal Care and Use Committee approved all animal procedures which conform to the NIH Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources 1996).
Silastic capsules were prepared as described with minor modifications [5]. Silastic medical tubing (0.078 in. id, 0.125 in. od, Dow Corning, Midland, MI) was cut into 14 mm lengths. One end was capped with 1–2 mm silastic adhesive (Silastic Type A, NuSil Technology, Carpinteria, CA) and cured overnight. Crystalline estradiol (Sigma, St. Louis, MO) was packed into the tube to a 9 mm height. The open end was then capped with silastic adhesive and cured overnight. Tamoxifen (catalog #T5648, Sigma, St. Louis, MO) capsules were prepared the same way. Capsules were soaked in phosphate buffered saline (PBS) 12 hours before being implanted subcutaneously (s.c.) in the rats. To determine estradiol release, a capsule was placed in 5 ml (PBS). At monthly intervals the PBS was removed and stored for analysis, and the capsule placed in fresh PBS. Estradiol concentration in PBS was determined by radioimmunoassay (Diagnostic Systems Laboratories, Webster, TX).
Rats were palpated twice weekly, and tumors monitored until total tumor mass reached 2.5% of the pre-tumor body weight, at which time rats were sacrificed per IACUC recommendations. To determine tumor mass % of body weight, palpable tumors were measured with digital calipers, and the diameter used to calculate spherical volume. To determine if tumors responded to tamoxifen, rats with easily palpable tumors (greater than 11 mm in one dimension) were implanted with 5 silastic capsules containing tamoxifen. Twice weekly rats were anesthetized under 2% isoflurane and tumors measured in two dimensions with digital calipers.
Imaging
Magnetic Resonance Imaging (MRI) was performed using a 7T/210mm Varian Unity Inova MRI system equipped with a gradient insert (400 mT/m, 115 mm I.D.) and a quadrature driven birdcage coil (63 mm I.D.) (Varian Inc., Palo Alto, CA). Rats were anesthetized with 1–2% isoflurane in oxygen via a nose cone during the entire imaging period. A respiratory sensor was placed on the abdomen for respiratory gating and to monitor vital signs. Body temperature was maintained at 37°C with warm air circulating in the magnet bore. Physiological monitoring was performed using a Physiological Monitoring System (SA Instruments, Inc.; Stony Brook NY). Two rats were sequentially imaged pre- and post-treatment to screen for mammary tumor growth and treatment response to tamoxifen. Upper and lower body imaging was acquired in coronal and axial planes using multislice fast-spin-echo (FSEMS) and T2-weighted spin-echo sequences. Acquisition was performed with data collection synchronized with the respiration of the animal to minimize breathing motion artifact. The following parameters were used: FSEMS: repetition time (TR)/echo time (TE) = 2250 msec/ 10 msec, 21 slices, slice thickness =1.5 mm with no gap, number of averages=2, field of view (FOV) = 90 mm × 50 mm, and in-plane resolution = 176 μm × 98 μm. T2-weighted: TR/TE = 2600 msec /37.2 msec, 21 slices, 1.2mm slice thickness, matrix number of averages=2, FOV = 90 mm × 52 mm for coronal or 5.5 cm × 4.5 cm for axial imaging, and in-plane resolution = 176μm × 102 μm for coronal and 107 μm × 176 μm for axial imaging. Tumor volume measurements were performed using FSEMS and T2-weighted coronal and axial image stacks. The tumors were manually segmented using VnmrJ software (Varian Inc.) to obtain the tumor volume in mm3.
Euthanasia and tissue collection
The number, location, and diameters in three dimensions of gross tumors were recorded. Half the rats were perfused and tissues fixed, sectioned and stained. The other half were sacrificed, trunk blood collected, and tissues snap frozen and stored at −80° C. Blood was allowed to clot overnight at 4° C, centrifuged at 1000× g, and serum stored at −80° C. Serum estradiol and progesterone were measured by radioimmunoassay (Diagnostic Systems Laboratories, Webster, TX).
Immunohistochemistry
From three rats, one section from each gland or gland pair was stained for ERα, ERβ, PR, Erbb2 and Cox-2. Rats were perfused with 4% paraformaldehyde (PFA). Mammary tissue and tumor were placed in 4% PFA overnight, transferred to 70% ethanol, processed and embedded in paraffin. Five-micron sections were stained with hematoxylin and eosin and evaluated by light microscopy. ERα, ERβ, PR, Erbb2, and Cox-2 were identified immunohistochemically. Sections were deparaffinized, rehydrated to water, heated in 0.1M citrate buffer (pH 6.0) for 20 min and cooled for 20 min. Sections were incubated for 20 min at room temperature (RT) in 3% hydrogen peroxide, 20 min at RT in 5% bovine serum albumin, and 60 min at RT with the following primary polyclonal or monoclonal antibodies (pAb or mAb): ERα, (SC-542 pAb 1:300), Erbb2 (SC-284 pAb 1:250), and Cox-2 (SC-1746 pAb 1:100) from Santa Cruz Biotechnology (Santa Cruz, CA), ERβ (MCA1974S mAb: 1:50) from Serotec (Raleigh, NC), PR (A0098 pAb:1:50) from DAKO (Carpinteria, CA). Positive controls were rat uterus (ERα, PR), rat prostate (ERβ), breast cancer cell line T47D (Erbb2), and neutrophils in abcessed rat lung (Cox-2). Negative controls were analyzed without primary antibody. For ERβ and Cox-2, a secondary antibody (BRbX Mus, DAKO) for 30 min was used, followed by streptavidin for 30 min and visualized by 3,3'-diaminobenzidine tetrahydrochloride (DAB, DAKO). ERα, PR, and Erbb2 were detected with a horseradish peroxidase-labeled polymer conjugated with anti-rabbit antibodies (Envision+System-Hrp,DAKO, Carpinteria, CA) for 30 min incubation and visualized by high sensitivity DAB for 5 minutes. Sections were counterstained with Mayer's hematoxylin for 1 minute and examined by bright field microscopy.
To determine percent positive cells, positive and negative cells in images captured using a Zeiss Axiophot microscope (40× objective) with Olympus DP70 digital camera were either counted manually with the Count Tool in Adobe CS2 (Adobe Systems Incorporated, San Jose, CA), or automatically with Adobe CS3 (Adobe Systems Incorporated) and Fovea Pro (Reindeer Graphics, Asheville, NC). A representative cancer and non-cancer field was selected from each of three rats. All images for each IHC assay were counted with identical techniques. Automatic counting was done by selecting brown or blue (after equalization) cells with the magic wand tool (tolerance = 50), setting the bilevel threshold, EDM morphology (erode), and watershed segmentation. Objects <50 pixels or touching the top or left edge were rejected.
Statistics
Data were analyzed by ANOVA considering the effect of treatment in the model using the Statistical Analysis System. If the main effect was significant, least squared difference was used for means separation. A P-value of less than 0.05 was considered statistically significant. All data are expressed as mean ± SE from at least three experiments. Tumor distribution was analyzed by t-test.
Results
E2 induces mammary cancer in ovary intact ACI.COP-Ept2 rats
Continuous E2 treatment induced mammary cancer in ovary intact ACI.COP-Ept2 rats. Mean latency to palpating mammary cancer was 183 ± 6 days, with a range of 132–253 days (Figure 1). Tumors were distributed equally among the abdominal/inguinal (49%) and cervical/thoracic (51%) mammary glands. Mammary cancer did not develop in untreated, ovary-intact, control ACI.COP-Ept2 rats.
Figure 1.
Latency of ACI.COP-Ept2 mammary tumors induced by 9 mg E2 capsules (squares). Circles indicate sacrifice dates of untreated rats.
Tamoxifen shrinks E2 induced mammary cancer in ACI.COP-Ept2 rats
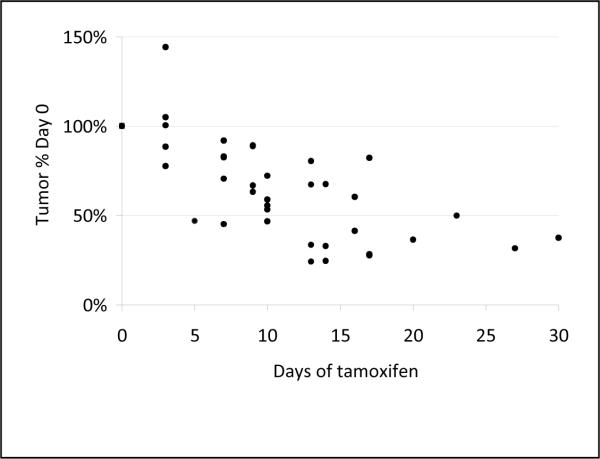
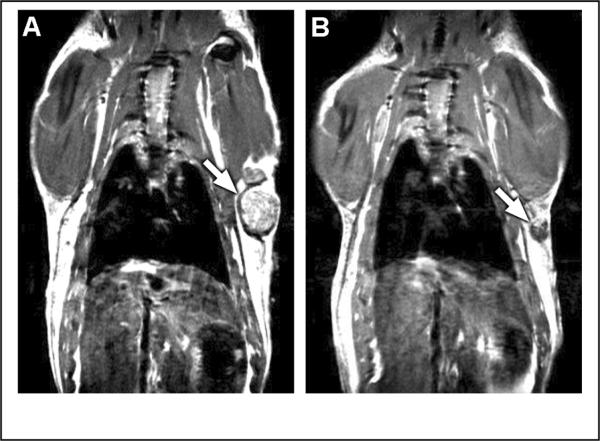
The antiestrogen tamoxifen is clinically proven to treat ER positive human breast cancer. E2 treated rats bearing mammary tumors were treated with tamoxifen and tumor burden was evaluated by measuring tumor size and by MRI. Treatment resulted in a rapid reduction in tumor burden (Figure 2). At baseline, eleven rats had 21 palpable tumors. After two and three weeks of tamoxifen, only five and two tumors, respectively, were palpable. Tumors from two tamoxifen-treated rats were further evaluated by MRI (Figure 3). Following three weeks of tamoxifen treatment, mammary tumor burden was reduced on average by 89% in 11 tumors in the two rats. Similarly, at necropsy, mammary tumor mass was reduced 86% in rats treated simultaneously with E2 and tamoxifen relative to rats treated with E2 alone. The difference in tumor burden between rats treated with E2 plus tamoxifen (899 ± 267 mm3, n = 13) and those treated with E2 alone (7554 ± 1523 mm3, n = 19) was statistically significant (p < 0.01).
Figure 2.
Tumor regression after tamoxifen treatment of tumors measured manually in two dimensions.
Figure 3.
MRI of rat A) before and B) after 3 weeks of tamoxifen treatment. Arrow points to tumor.
Histopathology
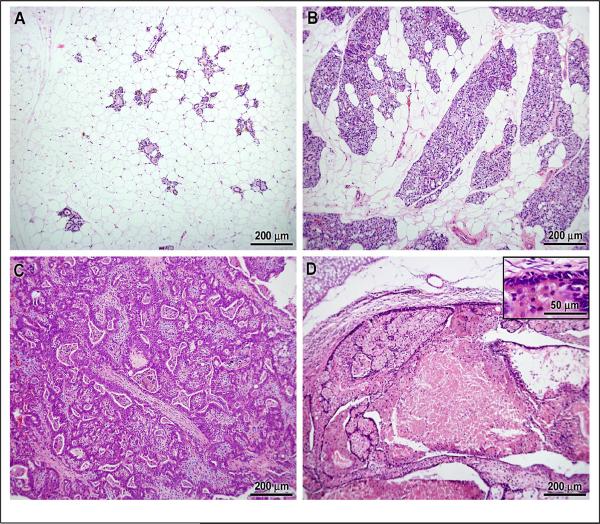
The mammary glands of untreated ACI.COP-Ept2 rats were comprised of isolated ductal structures within an adipose stroma (Figure 4A). Treatment with E2 induced a robust hyperplastic response within the mammary epithelium (Figure 4B) and development of mammary carcinoma (Figure 4C). Mammary cancers in tamoxifen treated rats exhibited necrosis and restoration of a normal ductal architecture comprised of a single layer of epithelial cells (Figure 4D).
Figure 4.
Histological appearance of A) normal mammary tissue from rat not treated with E2, B) benign, hyperplastic mammary tissue, C) mammary carcinoma from same rat as in B, D) mammary carcinoma from rat treated with tamoxifen. Inset in D shows epithelium returning to single layer.
ERα, ERβ, PR, Erbb2, and COX-2 expression in normal and neoplastic mammary tissue
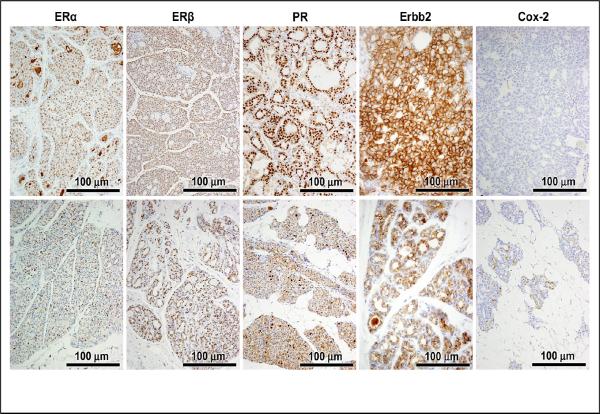
We first evaluated expression of ERα, ERβ, and PR in non-tumor and neoplastic mammary tissues from E2 treated ACI.COP-Ept2 rats. Approximately 50% of the cells in non-tumor mammary gland from E2 treated rats expressed a detectable level of ERα (Figure 5 and Table 1). By contrast, approximately 80% of the cells in E2-induced mammary cancers were ERα positive. ERβ was expressed at a similar level in normal (72% of cells) and neoplastic (83% of cells) mammary tissues. PR was expressed at a detectable level in 7% of normal mammary cells and 81% of mammary tumor cells. Thus, the expression patterns of these nuclear receptors are consistent with the observation that the tumors are estrogen dependent.
Figure 5.
Immunohistochemical stains of ERα, ERβ, PR, Her2, and COX-2 in tumor (upper panels) and adjacent non-tumor hyperplasia (lower panels).
Table 1.
Percent positive cells.
P<0.01 relative to adjacent non-tumor
The protein product of the ERBB2 gene (also known as Her2 or Neu) is over expressed in a subset of breast cancers, and ERBB2 over expression is a predictor of poor clinical outcome. Erbb2 protein was expressed in a similar number of epithelial cells in normal (80%) and neoplastic (90%) mammary cells (Figure 5 and Table 1).
Production of prostaglandin E2, an important mediator of inflammation and cancer [22, 23], is mediated by cyclooxygenase (COX)-2. Elevated COX-2 expression is associated with poor prognosis [24]. Constitutive, low level Cox-2 expression was present in both cancer and non-cancer mammary epithelial cells (Figure 5).
Circulating E2 levels are related to dose
The release rate of silastic capsules is proportional to capsule surface area. We chose 9 mm rather than 30 mm capsules used in previous studies from the Shull lab to reduce the estradiol dose. Two 9 mm capsules each delivered about 1.5 ng estradiol per day for up to four months. The use of 9 mm capsules resulted in a mean serum estradiol concentration of 36±4 pg/ml (Figure 6) compared to 185 pg/ml for the 30 mm capsules [5] Circulating E2 levels in intact rats reach up to 75 pg/ml [5, 15], and levels in human premenopausal females range from 50–200 pg/ml [25]. Tamoxifen treatment did not alter serum estradiol (34±5 pg/ml, n=5). Two untreated rats had serum estradiol of 23 and 8 pg/ml. Progesterone, which may play an important role in tumor initiation, was 35±5 ng/ml in rats implanted with estradiol capsules. Two untreated rats had serum progesterone of 19 and 39 ng/ml. Serum progesterone in tamoxifen treated rats was 23±9 ng/ml (n=5), consistent with a previous finding of 26 ng/ml [17].
Figure 6.
Serum hormones in rats treated with E2 or E2 + Tam. Mean ± SE.
Discussion
Estradiol-induced mammary cancers in ACI.COP-Ept2 rats respond to the antiestrogen tamoxifen and express hormone receptors, confirming prior reports suggesting that tumors in this model are hormone responsive. The ACI rat and the ACI.COP-Ept2 rat have similar susceptibility to mammary cancers induced by 30-mm E2 capsules, with reduced pituitary hyperplasia in the ACI.COP-Ept2 rat [21]. We report lower circulating estradiol and prolonged tumor latency compared to previous reports, likely the result of using the smaller, 9-mm E2 capsules [21]. Both the ACI.COP-Ept2 rat and the lower E2 dose were chosen to minimize pituitary hyperplasia reported in prior studies [17, 20, 26]. Our observations are consistent with an earlier report which observed a delayed latency in ACI rats treated with 9 mg E2 capsules compared to 27 mg, and which also reported a reduction in pituitary weight and in circulating prolactin [27]. In rats treated with 3-mm E2 capsules, we have observed a further prolonged latency to tumor (unpublished observation). Although our observed mean serum estradiol was within observed physiological parameters of untreated rats with functioning ovaries, tumors are not seen in intact rats not treated with supplemental E2. Continuous estradiol exposure in conjunction with progesterone seems to be key, and is likely more critical than E2 dose [12].
It has previously been reported that estradiol-induced tumors in ACI rats regressed after the estradiol capsule was removed [14], and that tamoxifen prevented induction of tumors by estradiol in ACI rats [28]. We show here that tamoxifen treatment leads to tumor regression in a rat model of estrogen-induced mammary cancer. These findings demonstrate that both tumor initiation and progression in this model is estrogen dependent and is mediated through estrogen receptor-dependent mechanisms. We found that both ERα and ERβ were expressed in cancers, with ERα levels higher in cancer tissue than in surrounding non-cancer tissue. Whether ERα or ERβ, or both, are critical for mammary cancer development is unknown.
Recent advances in imaging technology including MRI make possible non-invasive, sensitive and accurate monitoring of tumor growth or regression in small animals [29–32]. Tumors as small as 0.5 mm diameter can be detected by MRI, while palpable tumors are at least 5 mm diameter (unpublished observation). Furthermore, imaged tumors can be measured in three dimensions, while only two dimensions can easily be obtained manually. Thus, MRI is a highly sensitive, accurate, non-invasive method to monitor tumor regression.
In both the ACI and ACI.COP-Ept2 rats, PR is upregulated in mammary tumors compared to adjacent non-tumor tissue [17, 28]. PR is upregulated in early mammary lesions as well as cancers [14]. Thus, PR may contribute to the development of mammary cancers in these models. Alternatively, since PR is an estrogen responsive gene, the cancer cells may have a heightened response to estrogen, evidenced by increased PR. The upregulation of ER in tumors compared to adjacent non-tumor tissue, and increased mitotic index of tumor cells [14], suggest that the cancer cells are more responsive to estrogen. On the other hand, we and others have established that ovariectomized rats treated with estradiol do not develop mammary cancers [5], while replacing progesterone restores tumor development in ovariectomized rats [12]. These suggest that estrogen and PR are important factors in this model, as in human breast cancer.
In humans, lifetime estrogen exposure is a known risk factor for breast cancer, with early menarche, late menopause, and hormone replacement therapy (HRT) increasing risk [33]. Combination HRT, containing both estrogen and progesterone, increases breast cancer risk more than estrogen alone [33]. In the Women's Health Initiative study, the combination HRT treatment arm was halted after a median treatment of 5.2 years because of increased breast cancer risk, while the estrogen-alone arm ended after a median 6.9 years of treatment due to increased stroke, not breast cancer risk [3]. The Million Women Study also found a greater increase in breast cancer risk for combination HRT users than in estrogen-only users [34]. In the ACI rat model, removal of ovaries in the presence of exogenous estrogen does not lead to tumor formation, whereas adding back progesterone and estrogen led to mammary tumor formation [12]. The similarity to human disease suggests that our model is well suited to study hormone responsive breast cancer.
ERBB2 overexpression is associated with tumor relapse and poor survival [35]. While Erbb2 was expressed in both benign and malignant mammary tissue, we did not find upregulation in cancer tissue. Erbb2 is encoded on rat chromosome 10, which is duplicated in 15% of ACI mammary tumors [16]. Thus, we cannot exclude the possibility that Erbb2 overexpression might have been observed if more tumors were analyzed, and/or if mRNA or protein expression were quantified, rather than assessing expression by measuring percent Erbb2-positive cells.
The anti-inflammatory drug celecoxib, a selective COX-2 inhibitor, reduces breast cancer risk [36]. COX-2 breast cancer expression is associated with unfavorable outcome, and is more common in breast cancers with poor prognosis [24]. We observed a low degree of Cox-2 expression in cancer and non-cancer mammary tissue, consistent with the idea that ours is a model of breast cancer with good prognosis.
In summary, we demonstrate by physical examination and by MRI imaging that a lower dose than generally used of E2 leads to mammary tumor formation in 100% of ACI.COP-Ept2 rats, although with a prolonged latency compared to the higher dose. Tamoxifen induces rapid regression of E2-induced mammary cancers in the rats. The tumors overexpress ERα and PR, but not Erbb2 nor Cox-2. Based on biochemical and morphological characteristics, this model is most representative of hormone responsive breast cancers.
Acknowledgements
The authors thank Howard Wilson for technical assistance with images, the Harry S Truman VA Hospital for small animal MRI facilities, and the Research Animal Diagnostic Laboratory for IHC and histology services. RLR is supported by a Susan G. Komen Postdoctoral Fellowship.
References
- 1.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. New England Journal of Medicine. 2006;354(3):270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 2.Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer.[see comment][erratum appears in Lancet 1997 Nov 15;350(9089):1484] Lancet. 1997;350(9084):1047–1059. Anonymous: [PubMed] [Google Scholar]
- 3.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study.[see comment] J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 5.Shull JD, Spady TJ, Snyder MC, et al. Ovary-intact, but not ovariectomized female ACI rats treated with 17beta-estradiol rapidly develop mammary carcinoma. Carcinogenesis. 1997;18(8):1595–1601. doi: 10.1093/carcin/18.8.1595. [DOI] [PubMed] [Google Scholar]
- 6.Spady TJ, Harvell DM, Snyder MC, et al. Estrogen-induced tumorigenesis in the Copenhagen rat: disparate susceptibilities to development of prolactin-producing pituitary tumors and mammary carcinomas. Cancer Lett. 1998;124(1):95–103. doi: 10.1016/s0304-3835(97)00455-2. [DOI] [PubMed] [Google Scholar]
- 7.Shull JD, Pennington KL, Reindl TM, et al. Susceptibility to estrogen-induced mammary cancer segregates as an incompletely dominant phenotype in reciprocal crosses between the ACI and Copenhagen rat strains. Endocrinology. 2001;142(12):5124–5130. doi: 10.1210/endo.142.12.8530. [DOI] [PubMed] [Google Scholar]
- 8.Gould KA, Tochacek M, Schaffer BS, et al. Genetic determination of susceptibility to estrogen-induced mammary cancer in the ACI rat: mapping of Emca1 and Emca2 to chromosomes 5 and 18. Genetics. 2004;168(4):2113–2125. doi: 10.1534/genetics.104.033878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaffer BS, Lachel CM, Pennington KL, et al. Genetic bases of estrogen-induced tumorigenesis in the rat: mapping of loci controlling susceptibility to mammary cancer in a Brown Norway x ACI intercross. Cancer Research. 2006;66(15):7793–7800. doi: 10.1158/0008-5472.CAN-06-0143. [DOI] [PubMed] [Google Scholar]
- 10.Shull JD. The rat oncogenome: comparative genetics and genomics of rat models of mammary carcinogenesis. Breast Dis. 2007;28:69–86. doi: 10.3233/bd-2007-28108. [DOI] [PubMed] [Google Scholar]
- 11.Li JJ, Papa D, Davis MF, et al. Ploidy differences between hormone- and chemical carcinogen-induced rat mammary neoplasms: comparison to invasive human ductal breast cancer. Mol Carcinog. 2002;33(1):56–65. doi: 10.1002/mc.10022. [DOI] [PubMed] [Google Scholar]
- 12.Blank EW, Wong P-Y, Lakshamanaswamy R, et al. Both ovarian hormones estrogen and progesterone are necessary for hormonal mammary carcinogenesis in ovariectomized ACI rats. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(9):3527–3532. doi: 10.1073/pnas.0710535105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Easton DF, Pooley KA, Dunning AM, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447(7148):1087–1093. doi: 10.1038/nature05887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvell DM, Strecker TE, Tochacek M, et al. Rat strain-specific actions of 17beta-estradiol in the mammary gland: correlation between estrogen-induced lobuloalveolar hyperplasia and susceptibility to estrogen-induced mammary cancers. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(6):2779–2784. doi: 10.1073/pnas.050569097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li JJ, Weroha SJ, Lingle WL, et al. Estrogen mediates Aurora-A overexpression, centrosome amplification, chromosomal instability, and breast cancer in female ACI rats. Proc Natl Acad Sci U S A. 2004;101(52):18123–18128. doi: 10.1073/pnas.0408273101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adamovic T, Roshani L, Chen L, et al. Nonrandom pattern of chromosome aberrations in 17beta-estradiol-induced rat mammary tumors: indications of distinct pathways for tumor development. Genes Chromosomes Cancer. 2007;46(5):459–469. doi: 10.1002/gcc.20428. [DOI] [PubMed] [Google Scholar]
- 17.Harvell DM, Strecker TE, Xie B, et al. Dietary energy restriction inhibits estrogen-induced mammary, but not pituitary, tumorigenesis in the ACI rat. Carcinogenesis. 2002;23(1):161–169. doi: 10.1093/carcin/23.1.161. [DOI] [PubMed] [Google Scholar]
- 18.Aiyer HS, Srinivasan C, Gupta RC. Dietary Berries and Ellagic Acid Diminish Estrogen-Mediated Mammary Tumorigenesis â^^ ACI rats. Nutrition & Cancer. 2008;60(2):227. doi: 10.1080/01635580701624712. [DOI] [PubMed] [Google Scholar]
- 19.Spady TJ, Harvell DM, Lemus-Wilson A, et al. Modulation of estrogen action in the rat pituitary and mammary glands by dietary energy consumption. J Nutr. 1999;129(2S Suppl):587S–590S. doi: 10.1093/jn/129.2.587S. [DOI] [PubMed] [Google Scholar]
- 20.Strecker TE, Spady TJ, Schaffer BS, et al. Genetic bases of estrogen-induced pituitary tumorigenesis: identification of genetic loci determining estrogen-induced pituitary growth in reciprocal crosses between the ACI and Copenhagen rat strains. Genetics. 2005;169(4):2189–2197. doi: 10.1534/genetics.104.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurz SG, Hansen KK, McLaughlin MT, et al. Tissue Specific Actions of the Ept1, Ept2, Ept6 and Ept9 Genetic Determinants of Responsiveness to Estrogens in the Female Rat. Endocrinology. 2008 doi: 10.1210/en.2008-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masferrer JL, Leahy KM, Koki AT, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60(5):1306–1311. [PubMed] [Google Scholar]
- 23.Fulton AM, Gimotty P, Alonsozana E, et al. Elevated prostaglandin E2 (PGE2) levels in human breast cancer are associated with poor long-term survival. Proc Am Assn Cancer Res. 2000;41:3660A. [Google Scholar]
- 24.Ristimaki A, Sivula A, Lundin J, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Research. 2002;62(3):632–635. [PubMed] [Google Scholar]
- 25.Norman A, Litwack G. Hormones. 2nd edn Academic Press; San Diego, CA: 1997. [Google Scholar]
- 26.Spady TJ, Pennington KL, McComb RD, et al. Estrogen-induced pituitary tumor development in the ACI rat not inhibited by dietary energy restriction. Mol Carcinog. 1999;26(4):239–253. [PubMed] [Google Scholar]
- 27.Ravoori S, Vadhanam MV, Sahoo S, et al. Mammary tumor induction in ACI rats exposed to low levels of 17beta-estradiol. Int J Oncol. 2007;31(1):113–120. [PubMed] [Google Scholar]
- 28.Li SA, Weroha SJ, Tawfik O, et al. Prevention of solely estrogen-induced mammary tumors in female aci rats by tamoxifen: evidence for estrogen receptor mediation. J Endocrinol. 2002;175(2):297–305. doi: 10.1677/joe.0.1750297. [DOI] [PubMed] [Google Scholar]
- 29.Yankeelov TE, DeBusk LM, Billheimer DD, et al. Repeatability of a reference region model for analysis of murine DCE-MRI data at 7T. J Magn Reson Imaging. 2006;24(5):1140–1147. doi: 10.1002/jmri.20729. [DOI] [PubMed] [Google Scholar]
- 30.Rowland DJ, Garbow JR, Laforest R, et al. Registration of [18F]FDG microPET and small-animal MRI. Nuclear Medicine & Biology. 2005;32(6):567–572. doi: 10.1016/j.nucmedbio.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Turetschek K, Preda A, Floyd E, et al. MRI monitoring of tumor response following angiogenesis inhibition in an experimental human breast cancer model. Eur J Nucl Med Mol Imaging. 2003;30(3):448–455. doi: 10.1007/s00259-002-1000-5. [DOI] [PubMed] [Google Scholar]
- 32.Marzola P, Ramponi S, Nicolato E, et al. Effect of tamoxifen in an experimental model of breast tumor studied by dynamic contrast-enhanced magnetic resonance imaging and different contrast agents. Invest Radiol. 2005;40(7):421–429. doi: 10.1097/01.rli.0000167124.89782.db. [DOI] [PubMed] [Google Scholar]
- 33.Dunn BK, Wickerham DL, Ford LG. Prevention of hormone-related cancers: breast cancer. Journal of Clinical Oncology. 2005;23(2):357–367. doi: 10.1200/JCO.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 34.Banks E, Beral V, Bull D, et al. Breast cancer and hormone-replacement therapy in the Million Women Study. The Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 35.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 36.Harris RE, Beebe-Donk J, Alshafie GA. Reduction in the risk of human breast cancer by selective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer. 2006;6:27. doi: 10.1186/1471-2407-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]