Abstract
Influenza infection represents a major socio-economic burden worldwide. Novel delivery methods can render influenza vaccination easier and more acceptable by the public, and importantly confer protection equal or superior to that induced by conventional systemic administration. An attractive target for vaccine delivery is the skin. Recent studies have demonstrated improved immune responses after transdermal delivery of inactivated influenza virus with microneedle patches. Here we show that immunization with a licensed influenza subunit vaccine coated on metal microneedles can activate both humoral and cellular arms of the immune response and confer improved long-term protection in the mouse model when compared to the conventional systemic route of delivery. These results demonstrate the promising potential of microneedle delivery of licensed influenza subunit vaccines, that could be beneficial in increasing vaccine coverage and protection and reducing influenza-related mortality worldwide.
Influenza virus is one of the most common pathogens responsible for serious respiratory illness. Typically, the virus causes seasonal epidemics of influenza with outbreaks in the U.S. ranging from October through April. During a typical season, the CDC estimates that more than 200,000 hospitalizations, and 40,000 deaths are associated with influenza infection and related complications1, with up to 1.5 million deaths worldwide2. Seasonal influenza infection affects all age groups with the greatest disease severity occurring in the elderly3,4,5, persons with underlying chronic diseases6,7, infants and young children who have not been previously exposed to the virus8, pregnant women9,10, and health care workers11.
There are three distinct serotypes of influenza viruses, designated A, B, and C, with types A12 and B13,14 playing the major role in human infection. It is well established that the best way of prophylaxis and control of influenza infection is vaccination, which reduces morbidity and mortality of the disease15. Currently there are several types of licensed trivalent influenza vaccines containing 15 µg of hemagglutinin of each H1N1 and H3N2 influenza A subtypes and of type B influenza virus: formalin inactivated whole virus (WV), ether-treated hemagglutinin (HA) and neuraminidase (NA)-enriched split virus (SV) and subunit virus (SU) vaccine which are the most widely utilized vaccines and are usually administered intramuscularly16,17.
The immunogenicity of different influenza vaccine formulations was studied in the 1970s; despite the fact that whole virus was found to be more immunogenic compared to other formulations, its use was discontinued in most countries and it was replaced with the less reactogenic split or subunit influenza vaccine18,19,20,21. Although the latter have been proven to be safe, several studies have demonstrated decreased magnitude and longevity of the immune responses after intramuscular administration especially in high risk groups such as the elderly, immunocompromised individuals and children22,23,24. These facts strongly suggest the necessity for new vaccine formulations or new delivery methods that could improve the immune responses, thus reducing morbidity and mortality from influenza infection worldwide.
The current target for influenza vaccination is the deltoid muscle. However, the muscle tissue contains low numbers of antigen presenting cells (APCs) such as dendritic cells and macrophages, and lacks MHC class II expressing cells leading to poor antigen-dependent T cell activation and reduced humoral and cellular immune responses25. Other tissue targets for delivery of influenza vaccine have been proposed in recent years including the skin, which represents an ideal target for vaccine delivery. It contains a large network of immunologically active cells and APCs that take up the antigen and migrate to the proximal lymph nodes where naïve T and B cells will be activated to initiate the adaptive immune responses26. We and others have previously reported that delivery of whole inactivated influenza virus via the skin using antigen-coated metal microneedle patches elicits strong humoral and cellular immune responses, serological memory and improved long term protection compared to the conventional routes of vaccine delivery25,27,28.
In this study we investigate for the first time the efficacy of a single-dose skin vaccination with a licensed influenza subunit vaccine using antigen-coated metal microneedles and the longevity of immune responses induced upon skin delivery.
Results
Humoral immune responses after microneedle or IM delivery of influenza subunit vaccine
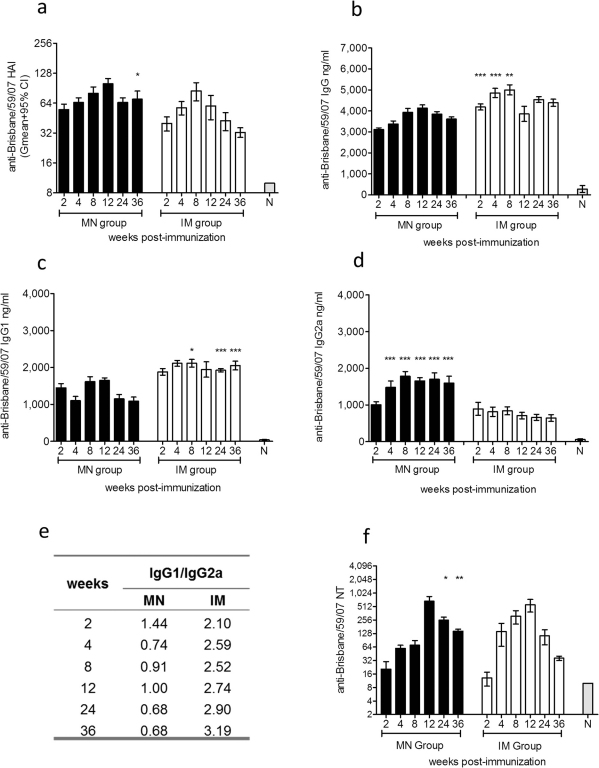
To compare the efficacy of immunization via the skin or intramuscularly, we first measured the levels of functional antibody titers against the hemagglutinin antigen (HA) of influenza virus induced after immunization. The Centers for Disease Control and Prevention (CDC) state that a titer of ≥1∶40 is indicative of protection in humans. In our experience titers of ≥1∶40 are also indicative or protection in mice. As shown in Fig. 1a, microneedle delivery of the subunit vaccine elicited levels of functional antibody titers indicative of protection (HAI>40) as early as week 4. By week 8 the IM immunized group exhibited the peak of protective HAI titers whereas the microneedle group reached the highest levels by week 12. Thirty six weeks post-immunization, we observed that 62% of the IM vaccinated animals exhibited protective levels (HAI>40) while 100% of the microneedle group maintained protective levels of functional antibody titers and two-fold higher titers when compared to the IM immunized group (p<0.0262). These results demonstrate that delivery of subunit influenza vaccine through the skin using microneedles elicits and sustains higher functional antibody titers, indicative of better duration of protection when compared to IM immunization.
Figure 1. Evaluation of humoral immune responses and neutralizing antibody titers.
Serum samples from mice were collected 2, 4, 8, 12, 24, and 36 weeks post immunization and analyzed for the levels of functional antibody titers against A/Brisbane/59/2007 by HAI (a), total serum IgG titers (b), and the IgG isotypes, IgG1 (c) and IgG2a (d) by quantitative ELISA, isotype profile ratio (e) and neutralizing antibody titers by microneutralization assay (f). MN: microneedle immunized group, IM: intramuscularly immunized group. ELISA antibody data represent the mean ± SEM. HAI data represent the geometric mean ± 95%CI. *: p<0.05, **: p<0.005, ***: p<0.0001. Statistics for the MN and IM group were performed with two-tailed unpaired Student's t-test.
We additionally determined the levels of influenza-specific circulating IgG antibodies in sera of vaccinated mice. Up to 8 weeks post-immunization the IM group demonstrated significantly higher antibody titers than the microneedle group (p<0.0018). From week 12 until week 36 there were no significant differences between the immunized groups (Fig. 1b). In our previous studies we reported that the isotype profile of the immune responses is affected by the route of immunization25,26,27. Mice that received the subunit vaccine through the IM route of delivery exhibited approximately two-fold greater levels of IgG1 circulating antibodies when compared to microneedle immunized mice (p<0.0001) (Fig. 1c). In contrast, microneedle immunization induced a more potent IgG2a response with a two-fold greater difference when compared to IM injection (p<0.0001) (Fig. 1d). We found a more balanced IgG1/IgG2a ratio in the microneedle group, which skewed towards IgG2a responses at later time points (Fig. 1e). In contrast the IM immunized group demonstrated a predominant IgG1 response. These results indicate that microneedle-based delivery of influenza subunit vaccine induces stronger, long-lasting IgG2a titers indicative of a Th1 response when compared to IM immunization.
Delivery of subunit influenza vaccine through the skin using microneedle or through IM injection induced high levels of neutralizing antibody titers in both vaccinated groups. These responses in the microneedle group were significantly higher than the IM group at 24 (p = 0.0426) and 36 weeks post-vaccination (p<0.0002) (Fig. 1f).These results further demonstrate the stronger immune responses induced after microneedle delivery of influenza subunit vaccine in the skin.
Evaluation of systemic recall and mucosal immune responses and assessment of viral replication after challenge
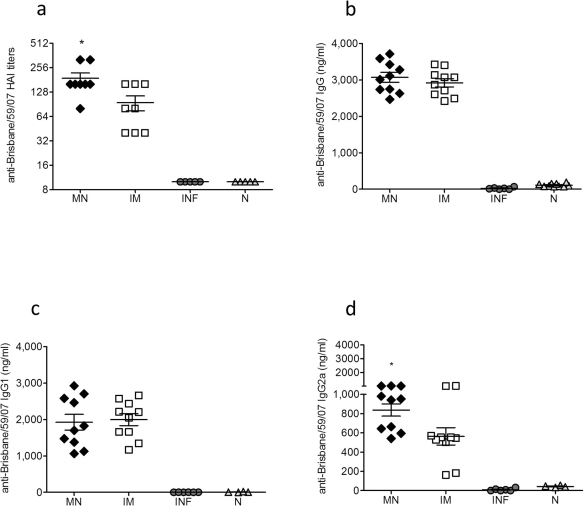
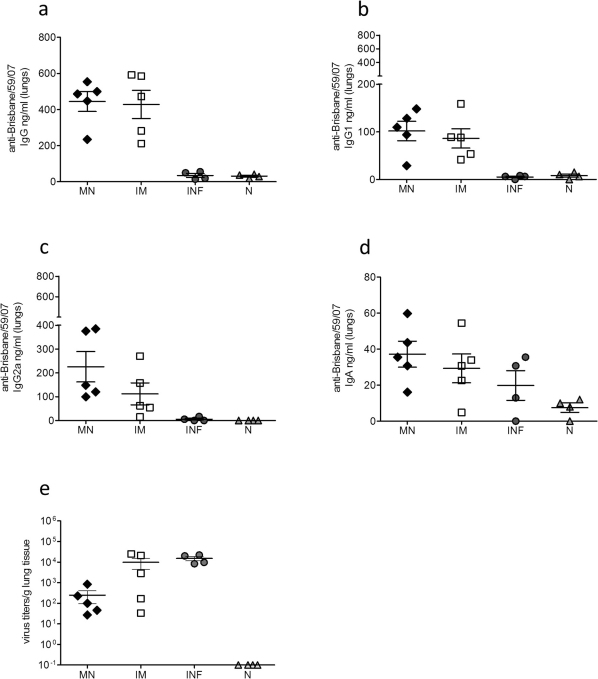
Twelve weeks after delivery of a single dose of subunit influenza vaccine, mice from all groups were challenged with 5xLD50 of mouse-adapted A/Brisbane/59/2007 virus and 4 days later sera were collected for the evaluation of recall responses. We observed that the HAI titers were two-fold higher in the microneedle immunized group than the IM group (p<0.0195) (Fig. 2a), although there were no significant differences in the levels of IgG and IgG1 influenza-specific antibody titers between the vaccinated groups (Fig. 2b, 2c). In contrast, the levels of influenza-specific IgG2a antibody titers were significantly higher in the microneedle group when compared to the IM group (p<0.0223) (Fig. 2d) further indicating a stronger Th1 response induced after skin vaccination. Evaluation of mucosal immune responses (IgG, IgG1, IgG2a and IgA) in the lungs of microneedle and IM immunized animals 12 weeks post-vaccination and 4 days post-challenge did not reveal significant differences between the two immunized groups (Fig. 3a–d).
Figure 2. Recall systemic immune responses.
The recall humoral immune responses in serum and lung suspensions were determined in MN and IM immunized mice 12 weeks post immunization and 4 days post-challenge. Naïve infected (Inf) and naïve uninfected (N) mice were used as control groups. Serum HAI titers (a), total serum IgG titers (b), and isotype IgG1 and IgG2a profile (c, d). INF: infected mice, N: naïve mice. ELISA antibody data represent the mean ± SEM and the HAI data represent the geometric mean ± 95% CI. *: p<0.05. Statistics for the MN and IM group were performed with two-tailed unpaired Student's t-test.
Figure 3. Recall mucosal immune responses and lung viral titers.
Lung IgG (a), IgG1 (b) IgG2a (c) and IgA titers (d). Titers and isotype profiles were determined by ELISA. Lung viral titers as determined by plaque assay (e). INF: infected mice, N: naïve mice. Data represent the mean ± SEM.
In order to evaluate the protective capacity of the immune response induced after microneedle or IM delivery of influenza subunit vaccine, we determined the lung virus titers after challenge at three months post-vaccination. We were able to detect a high replication rate of the virus in the IM immunized group with a 1.5-fold decrease when compared to unimmunized infected animals (Fig. 3e). In contrast, mice that received the subunit vaccine through the microneedle route of delivery exhibited 40-fold lower lung virus titers when compared to the IM group. These results demonstrate the induction of more effective immune responses after microneedle delivery of subunit influenza vaccine in the skin resulting in greater inhibition of virus replication and more rapid clearance from the lungs of infected animals when compared to IM delivery.
Cellular immune responses in the lungs of immunized animals after lethal challenge
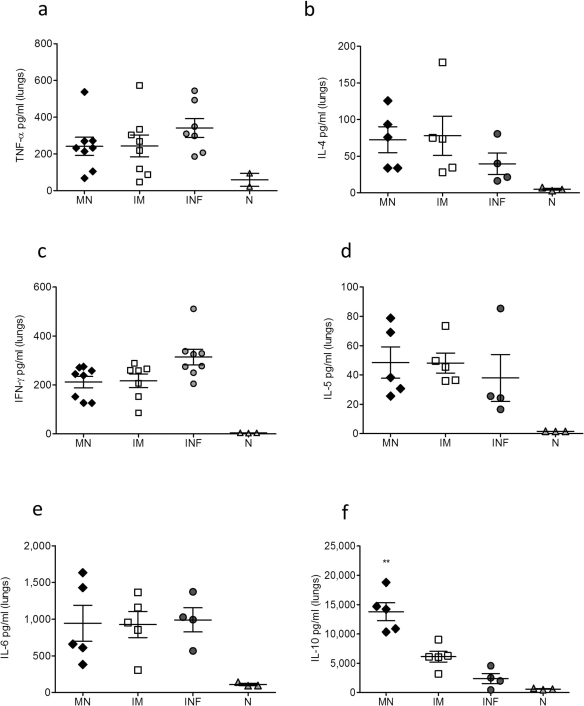
The levels of TNF-α, IL-4, IFN-γ, IL-5, IL-6 and IL-10 were measured as indicators of cellular immune responses in the lung suspensions of mice challenged 12 weeks after delivery of subunit influenza vaccine via microneedle administration or IM injection. We observed higher TNF-α levels in the unimmunized infected mice (Fig. 4a) when compared to either group of vaccinated animals, representative of the inflammatory process and cell death in their lungs25. We measured similar levels of IL-4 in both microneedle and IM vaccinated groups (Fig. 4b), but these were elevated when compared to the levels detected in the unimmunized infected mice. Interleukin-4 inhibits the production of different pro-inflammatory cytokines including TNF-α. Evaluation of IFN-γ, IL-5 and IL-6 levels (Fig. 4c, 4d, 4e) did not reveal any significant differences between the microneedle and IM vaccinated groups. The most notable difference in cytokine production was observed in the levels of IL-10 in the microneedle vaccinated group (Fig. 4f). Interleukin-10 is an important anti-inflammatory molecule recognized for blocking pulmonary inflammation and lung injury29. We observed a 2-fold higher level in IL-10 production levels in microneedle immunized animals when compared to the IM group (p<0.0026). These results demonstrate that delivery of subunit influenza vaccine through the skin using microneedles induces robust cellular immune responses and increased production of IL-10, correlating with reduced lung inflammation after lethal challenge.
Figure 4. Cellular immune responses in lung suspensions after virus challenge.
The levels of TNF-α (a), IL-4 (b), IFN-γ (c), IL-5 (d), IL-6 (e) and IL-10 (f) cytokines were measured in lung suspensions of MN and IM immunized animals 12 weeks post vaccination and 4 days post-challenge with 5xLD50 of live virus. INF: infected mice, N: naïve mice. Data represent the mean ± SEM. **: p<0.005. Statistics for the MN and IM group were performed with two-tailed unpaired Student's t-test.
Evaluation of long-lived bone marrow plasma cells
In order to further investigate the basis for high serum functional antibody titers and improved long-lived protective immune responses after microneedle delivery of influenza subunit vaccine when compared to IM immunization, we determined the numbers of influenza virus-specific IgG long-lived plasma cells in the bone marrow 12 weeks after a single immunization, a sufficient period for the establishment of memory30. We detected elevated numbers of-influenza virus-specific long-lived plasma cells in the bone marrow of both immunized groups when compared to the naïve group, and a significant increase in the numbers of long lived plasma cells in the microneedle group when compared to IM vaccinated animals (p<0.0051) (Fig. 5a). No significant difference was detected in the number of anti-influenza specific IgA antibody secreting cells in the bone marrow of microneedle or IM vaccinated groups (Fig. 5b). The higher numbers of influenza virus-specific long-lived plasma cells in the bone marrow of microneedle vaccinated animals may be responsible for the maintenance of high levels of influenza-specific functional antibody titers in the serum and improved protective humoral responses when compared to IM delivery of the vaccine.
Figure 5. Evaluation of bone marrow plasma cells by ELISPOT.
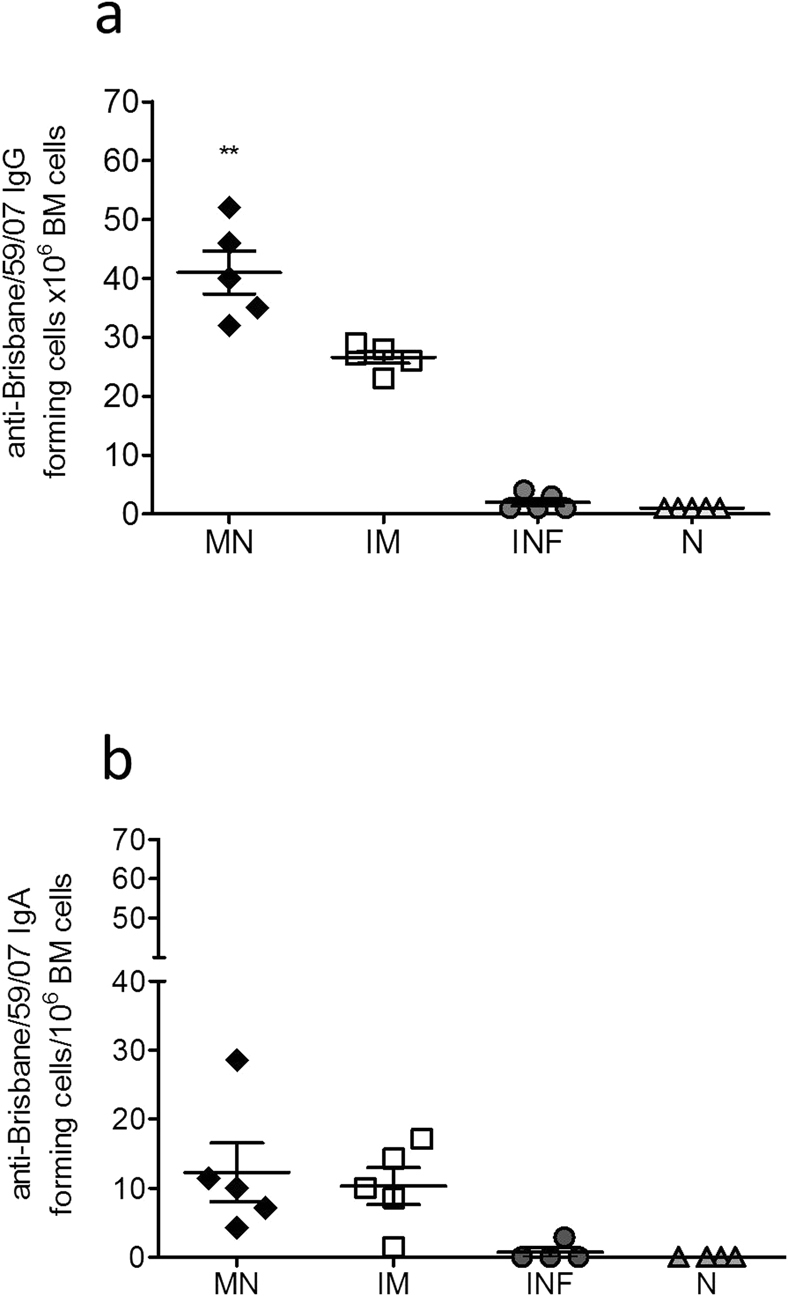
The numbers of influenza-specific IgG (a) and IgA (b) plasma cells in the bone marrow were determined 12 weeks after a single immunization. The cells were cultured in the presence of 4 µg/ml purified inactivated A/Brisbane/59/2007 virus and 18 h later the numbers of plasma cells in the bone marrow from MN and IM vaccinated mice and unvaccinated infected and non-infected mice were determined. Plasma cell numbers of vaccinated mice were considered positive if the numbers of spots were higher than the sum of naïve infected group spots + 3xSDev. INF: infected mice, N: naïve mice. Data represent the mean ± SEM. **: p<0.005. Statistics for the MN and IM group were performed with two-tailed unpaired Student's t-test.
Determination of IFN-γ and IL-4 secreting cells in the spleen
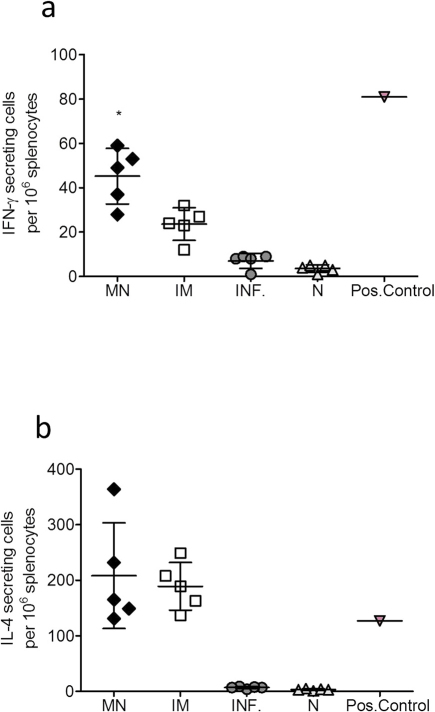
To compare the cellular immune responses induced after microneedle or IM delivery of subunit influenza vaccine, we determined the numbers of IFN-γ and IL-4 secreting cells in the spleens of mice 12 weeks after immunization. We detected significantly higher numbers of IFN-γ secreting cells in the microneedle immunized group when compared to IM vaccinated animals (p = 0.0105) (Fig. 6a). However we did not find any differences between the two immunized groups in the numbers of IL-4 secreting cells (Fig. 6b). These results indicate improved cellular immune responses after microneedle delivery of subunit influenza vaccine compared to IM delivery.
Figure 6. Cellular immune responses in spleen cells.
The numbers of IFN-γ (a) and IL-4 (b) secreting cells from the spleen were determined by ELISPOT. Spleen cells from MN and IM immunized mice and unvaccinated infected and non-infected mice were cultured in the presence of 4 µg/ml purified inactivated A/Brisbane/59/2007 virus for 36 h. Splenocytes from naïve mice were stimulated with a typical bacterial super antigen (SAB) as positive control. INF: infected mice, N: naïve mice. Data represent the mean ± SEM. *: p<0.05. Statistics for the MN and IM group were performed with two-tailed unpaired Student's t-test.
Duration of protective immunity
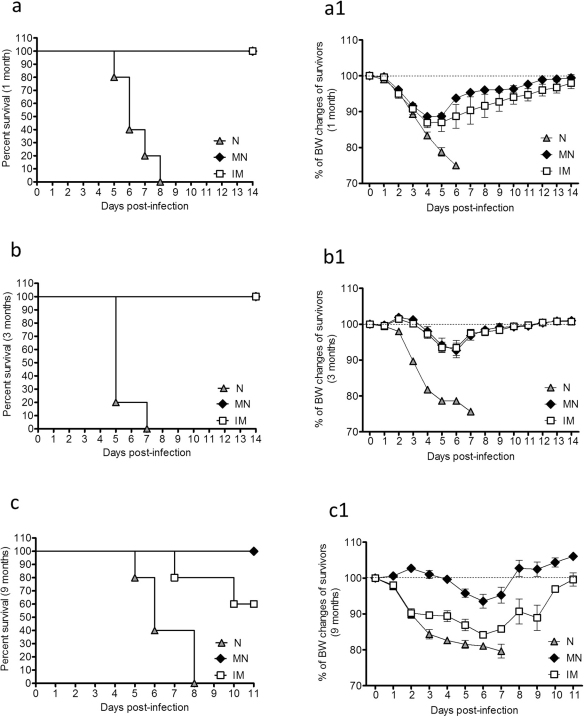
To evaluate the longevity and efficacy of subunit influenza vaccine in conferring protection after a single immunization through the skin or the muscle, cohorts of vaccinated mice were challenged with 5xLD50 of mouse-adapted virus at 4, 12 and 36 weeks post-vaccination. Body weight changes and survival rates were registered daily as previously described25. Four weeks post-immunization both vaccinated groups were fully protected against lethal challenge with an average body weight loss of 10–12% (Fig. 7a–a1). Twelve weeks post-immunization both groups again exhibited full protection against lethal challenge and an average body weight loss of 5–6% (Fig. 7b–b1). At week 36 post-immunization, the microneedle group was still fully protected against the virus challenge, exhibiting an average body weight loss of 5% and 100% survival. In contrast, the IM group was only partially protected with a mortality rate of 40% and an average body weight loss of 15% for the mice that survived the challenge (Fig. 7c–c1). These findings demonstrate that skin immunization with microneedles coated with an influenza subunit vaccine induces long-lived immunity capable of conferring full protection and survival against lethal challenge with the homologous virus at least up to thirty six weeks after a single immunization.
Figure 7. Protective efficacy against H1N1 A/Brisbane/59/2007 lethal infection.
Body weight changes and survival rates were recorded after lethal challenge with 5xLD50 of live A/Brisbane/59/2007 virus in MN and IM immunized and naïve mice 1 month (a–a1), 3 months (b–b1) and nine months (c–c1) post vaccination. N: naïve mice. Data represent the mean ± SEM.
Discussion
In the present study we report for the first time that microneedle delivery of a single dose of a licensed influenza subunit vaccine to BALB/c mice confers complete protection 36 weeks post-vaccination whereas only partial protection was induced after the conventional IM route of delivery. At the same period microneedle immunization also induces higher functional antibody titers and higher influenza specific IgG2a antibodies than the IM immunized group, indicative of stronger cellular immune responses25. In our experimental mouse model, both microneedle and IM immunized groups exhibited similar levels of hemagglutination inhibition titers (HAI) at early time points after immunization. Thirty six weeks later, though, we found that 38% of the IM immunized animals demonstrated a significant drop of HAI titers below protective levels (HAI<40), while the microneedle vaccinated group in its entity retained HAI titers above protective levels (HAI>40). The maintenance of these high levels of functional antibody titers throughout a period of nine months can be attributed to the higher number of influenza-specific antibody secreting cells (long-lived bone marrow plasma cells) detected in the microneedle immunized group when compared to the ASC numbers found in the IM cohort25,30.
We have previously reported that skin immunization with whole inactivated virus-coated metal microneedles was equal or superior to systemic vaccination. In those studies we found a high anti-influenza virus IgG1/IgG2a antibody ratio in the microneedle group that received 10 µg of vaccine indicating a skewed Th2 response. This response resulted in a two-fold increase of IL-4 production when compared to the intramuscularly delivered vaccine26 whereas the IFN-γ production was higher in the IM group than the microneedle cohort. The long-lived antibody secreting cells and the memory B cells were at similar levels in the bone marrow of both immunized cohorts. Those data could not clearly favor either route of vaccination because whole inactivated virus vaccine displays stronger immunogenicity which can activate both arms of immune responses, humoral and cellular. In the present study we tested a licensed influenza subunit vaccine as an antigen by metal microneedle delivery through the skin and compared it to IM delivery. Several studies have previously demonstrated that IM delivery of subunit influenza vaccines induces poor cellular immune responses in humans18. Here we show that microneedle delivery of a subunit influenza vaccine elicits higher numbers of IFN-γ secreting cells in the spleen of immunized animals when compared to IM immunization. IFN-γ has been reported to play a major role in the control of viral infections leading to faster viral clearance and apoptosis of infected cells25. Furthermore, while microneedle delivery of whole inactivated virus demonstrated a robust IgG1 response, and similar numbers of ASC in the bone marrow, delivery of the subunit vaccine induced a balanced IgG1 and IgG2a response (ratio = 1) and higher numbers of ASC in the bone marrow when compared to IM delivery. These data are similar to these obtained with polymer microneedle delivery of whole inactivated virus27.
Activation of both arms of the adaptive immune response contributes to enhanced protection and rapid virus clearance from the lungs after infection, as demonstrated by the 40-fold lower virus titers in the lungs of microneedle immunized mice after lethal challenge, compared to the titers measured in the IM immunized mice. This finding can also be significant for influenza transmission. Influenza vaccines in 90% of vaccinees are delivered by the intramuscular route. Although vaccinated individuals are protected from the disease, they are still able to transmit the virus for a 24–48 hour window31,32,33. The greater inhibition of viral replication and more rapid clearance of the virus observed in the microneedle immunized group could further reduce the infectious potential of vaccinated individuals minimizing transmission form direct contact.
Influenza represents a significant burden to public health34,35,36,37. The World Health Organization estimates that there are more than 1.2 billion people at high risk for severe influenza outcomes worldwide: 385 million elderly, 140 million infants and 700 million adults and children with underlying health conditions including pregnant women38. Additionally the WHO estimates that there are approximately 24 million health care workers at high risk for influenza infection who pose a risk for transmission of influenza virus to high risk populations39. Besides increased health care costs, influenza infection poses a major socio-economic burden leading to high levels of worker absenteeism, disruption in work and productivity losses. It is estimated that in the United States, annual influenza epidemics result in an average of 3.1 million hospitalization days and 31.4 million outpatient visits with an average direct medical cost of 10.4 billion dollars annually40. Projected lost earnings due to illness and loss of life averages to 16.3 billion dollars annually, while the total direct and indirect economic burden of annual influenza epidemics amount to 87.1 billion dollars in the US alone40.
Furthermore, the CDC reports that in randomized control trials among different age groups and individuals with medical conditions, the inactivated influenza vaccine effectiveness was estimated to be between 48%–70% during seasons when the vaccine was well matched with the circulating influenza strains41. These data indicate that better influenza vaccines and more effective vaccination methods are needed that will increase vaccine coverage and reduce morbidity and mortality rates.
The skin represents an attractive target for vaccine delivery. Skin-based vaccination has received more attention recently due to encouraging results demonstrating improved immune responses after antigen delivery25,42,43,44. Additionally there is evidence for dose sparing using transdermal immunization45,46. Several studies have demonstrated improved immune responses after intradermal delivery of influenza vaccines25,44,47,48. We and others have previously demonstrated that administration of whole inactivated influenza virus through the skin using solid metal microneedles was capable of inducing improved immune responses, serological memory and long-term protection when compared to the conventional routes of influenza immunization25,28,49. However the effectiveness of this route for immunization using a subunit vaccine has not been investigated previously and the longevity of the immune responses has received only limited attention.
Here, we used microneedles to simply and reliably deliver influenza subunit vaccine targeted to the skin and we demonstrated enhanced immune responses when compared to the conventional systemic route of delivery. Microneedles, therefore, have the potential to make skin-based vaccination a clinically viable alternative which, besides the immunologic advantages demonstrated in this study, offers several logistical advantages including inexpensive manufacturing, small size for easy storage and distribution, a simple administration process that might enable self-vaccination to increase patient coverage, and overall better acceptance of this route of vaccination by the general public (Kim Y.C. et al Advanced Drug Delivery Reviews, 2012 in press). Overall, our study demonstrates that microneedle-based delivery has the potential to be used with currently approved vaccines and formulations, and for improving results when compared to the current conventional routes, especially to generate long-lived protective immunity.
Methods
Microneedle fabrication and coating
As previously described25, metal microneedles were fabricated by etching stainless steel sheets (McMaster-Carr, Atlanta, GA). Each microneedle measured 700 µm tall, with a cross sectional area of 170 µm by 55 µm at the base and tapering to a sharp tip, with five microneedles per row. Microneedles were dip-coated using a coating solution formulated with 1% (w/v) carboxymethylcellulose (Carbo-Mer, San Diego, CA), 0.5% (w/v) Lutrol F-68NF (BASF, Mt. Olive, NJ), 15% (w/v) D-(+)-trehalose dihydrate (Sigma, St. Louis, MO) and H1N1 A/Brisbane/59/2007 subunit vaccine solution. The vaccine was kindly provided by Novartis Vaccines and Diagnostics (Cambridge, MA).
Cells and viruses
Madin-Darby canine kidney (MDCK) cells (ATCC CCL 34, American Type Culture Collection, Manassas, VA) were maintained in Dulbecco's Modified Eagle's Medium (DMEM) (Mediatech, Herndon, VA) containing 10% fetal bovine serum (Hyclone, ThermoFisher Scientific, Rockford, IL). Influenza virus stocks (A/Brisbane/59/2007) were prepared, purified and inactivated as previously described27. Hemagglutination (HA) activity was determined using turkey red blood cells (LAMPIRE Biological Laboratories, Pipersville, PA) as previously described50. For inactivation, the purified virus was treated with formalin at a final concentration of 0.1% (vol/vol) for 48 h at 4°C and then dialyzed against PBS buffer. Inactivation of the virus was confirmed by plaque assay in MDCK cells51. The mouse-adapted virus was prepared by serial passage 8 times in lungs of BALB/c mice. The LD50 was calculated by the method of Reed-Muench52, and viral titers were determined by plaque assay.
Immunizations, lethal challenge and sample collection
Female BALB/c mice (Charles River Laboratory, Wilmington, MA) (50 mice per group, 6–8 weeks old) received one dose (3 µg) of the subunit A/Brisbane/59/2007 vaccine through the skin using microneedle arrays coated with the antigen or by IM injection. For microneedle delivery the mice were vaccinated by manual insertion of the microneedles into the skin on the dorsal surface for 5 min while for IM immunization mice received an equal dose of the vaccine in both legs25. Unimmunized mice were used as an additional negative control. Animals were bled retro-orbitally 2, 4, 8, 12, 24 and 36 weeks post-immunization under systemic anesthesia. Four, 12 and 36 weeks following immunization, mice (n = 5) were challenged with 5xLD50 dose of live mouse-adapted H1N1 A/Brisbane/59/2007 virus and were monitored for 14 days for signs of morbidity (body weight changes, fever and hunched posture) and mortality. A weight loss exceeding 25% was used as the experimental end point, at which mice were euthanized according to IACUC guidelines. All animal studies had approval of Emory University's Institutional Animal Care and Use Committee. Twelve weeks post-immunization and four days post-challenge of an independent cohort of microneedle and IM vaccinated groups, blood was collected by retro-orbital bleeding with non-heparinized microcapillary tubes (Fisher Scientific, Pittsburg, PA). Lungs were collected in DMEM at 12 weeks, 4 days post-lethal challenge and processed as previously described27. All serum samples and lung homogenates were individually processed to determine humoral immune responses26. Lung supernatants were mixed with the protease inhibitor phenylmethylsulfonyl fluoride (1 mM) (Sigma, St. Louis, MO) and stored at −80°C until assayed for viral titers or cytokine levels with cytokine ELISA. Spleens were collected for the evaluation of cellular immune responses and processed into single cell suspensions in complete RPMI 1640 for cytokine determination53. Bone marrow was collected and processed in single cell suspension for the determination of influenza-specific IgG and IgA antibody secreting cells (ASC) as previously described25.
Evaluation of humoral immune responses
All sera and lung suspensions were individually processed, and anti-influenza specific IgG, IgG1, IgG2a and IgA antibody levels were determined quantitatively by enzyme-linked immunosorbent assay (ELISA) as previously described53. Purified mouse IgG, IgG1, IgG2a, IgA and goat anti-mouse-HRP for ELISA were purchased from Southern Biotechnology Associates (Birmingham, AL). Optical density was read at 490 nm.
Hemagglutination inhibition (HAI) titers were determined based on the WHO protocol54 as described previously51. Sera were pre-treated with receptor-destroying neuraminidase (RDE) (Roche Diagnostics, Indianapolis, IN). The HAI titer was read as the reciprocal of the highest dilution of serum that conferred inhibition of hemagglutination. The values were expressed as the geometric mean +/− standard error of the mean. Virus microneutralization assay was performed as described elsewhere [CDC Influenza manual Rev 06FEB98] with modifications. Briefly, 50 µl of virus diluent containing 100 xTCID50 of each virus was incubated with 50 µl of serially diluted heat-inactivated sera at 37°C for 1 h, and then mixed with 100 µl of freshly trypsinized low-passage MDCK cell suspension (ATCC # CRL-2936) containing 5×104 cells in 96-well tissue plates. The controls wells contained cells alone (negative control) and cells infected with virus (positive control). Following an 18 – 22 h incubation period the cells were fixed with acetone and the infected wells were detected by ELISA. Mouse monoclonal biotinylated anti-NP antibody MAB8257B (Millipore, Temecula, CA), dilution 1∶3,000, and HRP-streptavidin conjugate (BD # 51-9000209, BD Biosciences, San Jose, CA), dilution 1∶4,000 were used in the ELISA. Color was developed using OPD substrate (#002003 Invitrogen, Frederick, MD) and optical density was measured at 490 nm in plate reader (model 680 BioRad, Hercules, CA). The highest serum dilution that generated >50% specific signal was considered to be the neutralization titer; 50% specific signal = (OD490 virus control -OD490 cell control)/2 +OD490 cell control.
Measurement of lung viral titers after challenge
To determine the titers of influenza virus in the lungs of immunized mice after challenge, the animals of each group were infected with 5xLD50 3 months after vaccination and were sacrificed on day 4 post-infection. Naïve mice and unimmunized mice infected with 5xLD50 were used as control groups. Lung homogenates were prepared in DMEM serum-free medium to assess the viral titers per gram of tissue by plaque assay as described25 and the LD50 was determined in the same mouse strain and calculated with the Reed-Muench formula.
Evaluation of cellular immune responses
Cellular immune responses were estimated using ELISPOT assays in the spleens and cytokine ELISA in the lung suspensions. Freshly isolated splenocytes (1.0×106/200 µl cRPMI) were cultured for 48 h in the presence of 4 µg/ml whole inactivated influenza virus in cRPMI 1640 medium26. IL-4 and IFN-γ ELISPOT reagents were purchased from BD-PharMingen (San Jose, CA). Colored spots were visualized and counted using an ELISPOT reader and counter (Cellular Technologies, USA). Lung supernatants in DMEM prepared from immunized mice on day 4 post-challenge were assayed for TNF-α, IFN-γ, IL-4, IL-5, IL-6 and IL-10 according to the manufacturer's instructions (eBioscience, San Diego, CA)27.
Quantification of anti-Brisbane/59/07 antibody secreting cells
Virus-specific antibody-secreting cells (ASC) in the bone marrow were determined by a modification of the ELISPOT assay30,55. Briefly, 96-well plates were coated overnight at 4°C with purified inactivated A/Brisbane/59/07 at a final concentration 4 µg/ml. The plates were washed three times with RPMI and blocked for 2 h with 10% fetal calf serum prior to sample addition. Bone marrow single cell suspensions collected at 4 days after challenge (1×106/well) in cRPMI were plated directly on coated blocked plates and were incubated at 37°C for 18 h26. Samples were enumerated in an ELISPOT reader (Cellular Technology, Shaker Heights, OH) and the results shown as the number of ASC per 106 cells. Plasma cell numbers of vaccinated mice were considered positive if the numbers of spots were higher than the sum of naïve infected group spots + 3xSDev.
Statistics
The statistical significance of the differences was calculated by two-tailed unpaired Student's t-test and one-way ANOVA (one–way analysis of variance including Bonferroni's multiple comparison test) or two-way ANOVA. For statistical designations in the figures, * denotes p<0.01; ** denotes p<0.005 and ***denotes p<0.0001. Unless otherwise stated, independent experiments were run at least in triplicate.
Author Contributions
DGK and IS conceived and designed the project. DGK, and IS prepared the figures and did the statistics. DGK executed the majority of the experiments. EVV, AS, VGZ, ESE, MT and IS assisted in the execution of the experiments. DGK and IS wrote the manuscript. RWC and MRP edited the manuscript. All authors reviewed the final version of manuscript.
Acknowledgments
We thank Derek O'Hagan, Gene Palmer and others at Novartis Vaccines and Diagnostics for providing us with the H1N1 A/Brisbane/59/2007 influenza subunit vaccine. This study was carried out at the Emory Vaccine Center and the Georgia Tech Center for Drug Design, Development and Delivery and Institute for Bioengineering and Biosciences. The work was supported in part by U.S. National Institutes of Health grants EB006369 and AI074579. Dimitrios G. Koutsonanos is a trainee of IPIRC/CEIRS (Influenza Pathogenesis and Immunology Research Center/Centers of Excellence for Influenza Research and Surveillance) and supported by contract 5 HHSN266200700006C from NIH/NIAID.
Footnotes
Mark R. Prausnitz serves as a consultant to companies, is a founding share-holder of companies and is an inventor on patents licensed to companies developing microneedle-based products. This possible conflict of interest has been disclosed and is being managed by Georgia Institute of Technology and Emory University.
References
- Fiore A. E. et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 57, 1–60 (2008). [PubMed] [Google Scholar]
- Osterholm M. T. Preparing for the next pandemic. N Engl J Med 352, 1839–1842 (2005). [DOI] [PubMed] [Google Scholar]
- Camilloni B. et al. An influenza B outbreak during the 2007/2008 winter among appropriately immunized elderly people living in a nursing home. Vaccine 28, 7536–7541 (2010). [DOI] [PubMed] [Google Scholar]
- Iorio A. M. et al. An influenza A/H3 outbreak during the 2004/2005 winter in elderly vaccinated people living in a nursing home. Vaccine 24, 6615–6619 (2006). [DOI] [PubMed] [Google Scholar]
- Baldo V. et al. Immunogenicity of three different influenza vaccines against homologous and heterologous strains in nursing home elderly residents. Clin Dev Immunol 2010, 517198 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel M. et al. Low rate of seroconversion after vaccination with a split virion, adjuvanted pandemic H1N1 influenza vaccine in HIV-1-infected patients. AIDS 24, F31–35 (2010). [DOI] [PubMed] [Google Scholar]
- Cooper C. et al. Immunogenicity is not improved by increased antigen dose or booster dosing of seasonal influenza vaccine in a randomized trial of HIV infected adults. PloS one 6, e17758 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan T. et al. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA : the journal of the American Medical Association 303, 37–46 (2010). [DOI] [PubMed] [Google Scholar]
- Myers E. R., Misurski D. A. & Swamy G. K. Influence of timing of seasonal influenza vaccination on effectiveness and cost-effectiveness in pregnancy. Am J Obstet Gynecol 204, S128–140 (2011). [DOI] [PubMed] [Google Scholar]
- Blanchard-Rohner G. & Siegrist C. A. Vaccination during pregnancy to protect infants against influenza: why and why not? Vaccine 29, 7542–7550 (2011). [DOI] [PubMed] [Google Scholar]
- Clarke C. E. & McComas K. Seeking and Processing Influenza Vaccine Information: A Study of Health Care Workers at a Large Urban Hospital. Health Commun (2011). [DOI] [PubMed] [Google Scholar]
- Medina R. A. & Garcia-Sastre A. Influenza A viruses: new research developments. Nat Rev Microbiol 9, 590–603 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R. et al. Structural basis for the sequence-specific recognition of human ISG15 by the NS1 protein of influenza B virus. Proceedings of the National Academy of Sciences of the United States of America 108, 13468–13473 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan H., Zhao C. & Krug R. M. Species specificity of the NS1 protein of influenza B virus: NS1 binds only human and non-human primate ubiquitin-like ISG15 proteins. The Journal of biological chemistry 285, 7852–7856 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol K. L. & Treanor J. J. Vaccines for seasonal and pandemic influenza. J Infect Dis 194 Suppl 2, S111–118 (2006). [DOI] [PubMed] [Google Scholar]
- Fiore A. E. et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 59, 1–62 (2010). [PubMed] [Google Scholar]
- Beyer W. E., Nauta J. J., Palache A. M., Giezeman K. M. & Osterhaus A. D. Immunogenicity and safety of inactivated influenza vaccines in primed populations: a systematic literature review and meta-analysis. Vaccine 29, 5785–5792 (2011). [DOI] [PubMed] [Google Scholar]
- Koyama S. et al. Plasmacytoid dendritic cells delineate immunogenicity of influenza vaccine subtypes. Sci Transl Med 2, 25ra24 (2010). [DOI] [PubMed] [Google Scholar]
- Gross P. A. Reactogenicity and immunogenicity of bivalent influenza vaccine in one- and two-dose trials in children: a summary. J Infect Dis 136 Suppl, S616–625 (1977). [DOI] [PubMed] [Google Scholar]
- Gross P. A. et al. A controlled double-blind comparison of reactogenicity, immunogenicity, and protective efficacy of whole-virus and split-product influenza vaccines in children. J Infect Dis 136, 623–632 (1977). [DOI] [PubMed] [Google Scholar]
- Gross P. A. & Ennis F. A. Influenza vaccine: split-product versus whole-virus types--How do they differ. N Engl J Med 296, 567–568 (1977). [DOI] [PubMed] [Google Scholar]
- McElhaney J. E. Influenza vaccine responses in older adults. Ageing Res Rev 10, 379–388 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellebedy A. H. & Webby R. J. Influenza vaccines. Vaccine 27 Suppl 4, D65–68 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelin G. et al. Inactivated seasonal influenza vaccines increase serum antibodies to the neuraminidase of pandemic influenza A(H1N1) 2009 virus in an age-dependent manner. J Infect Dis 202, 1634–1638 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsonanos D. G. et al. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. J Infect Dis 204, 582–591 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsonanos D. G. et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PloS one 4, e4773 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S. P. et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med 16, 915–920 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q. et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proceedings of the National Academy of Sciences of the United States of America 106, 7968–7973 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Madan R., Karp C. L. & Braciale T. J. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med 15, 277–284 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka M. K. Immunological memory to viral infection. Curr Opin Immunol 16, 443–450 (2004). [DOI] [PubMed] [Google Scholar]
- Maltezou H. C. & Tsakris A. Vaccination of health-care workers against influenza: our obligation to protect patients. Influenza and other respiratory viruses 5, 382–388 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenah E., Chao D. L., Matrajt L., Halloran M. E. & Longini I. M. Jr The global transmission and control of influenza. PloS one 6, e19515 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmelzwaan G. F., Bodewes R. & Osterhaus A. D. Vaccination strategies to protect children against seasonal and pandemic influenza. Vaccine 29, 7551–7553 (2011). [DOI] [PubMed] [Google Scholar]
- Nair H. et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 378, 1917–1930 (2011). [DOI] [PubMed] [Google Scholar]
- Zambon M. Assessment of the burden of influenza in children. Lancet 378, 1897–1898 (2011). [DOI] [PubMed] [Google Scholar]
- Palache A. Seasonal influenza vaccine provision in 157 countries (2004–2009) and the potential influence of national public health policies. Vaccine 29, 9459–9466 (2011). [DOI] [PubMed] [Google Scholar]
- Osterhaus A., Fouchier R. & Rimmelzwaan G. Towards universal influenza vaccines? Philos Trans R Soc Lond B Biol Sci 366, 2766–2773 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Public Health Research Agenda for Influenza. (World Health Organization, 2009).
- Collin N. & de Radigues X. Vaccine production capacity for seasonal and pandemic (H1N1) 2009 influenza. Vaccine 27, 5184–5186 (2009). [DOI] [PubMed] [Google Scholar]
- Molinari N. A. et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 25, 5086–5096 (2007). [DOI] [PubMed] [Google Scholar]
- CDC. Flu Vaccine Effectiveness: Questions and Answers for Health Professionals. (Centers for Disease Control and Prevention, Atlanta, GA, 2011).
- Nelson K. S., Janssen J. M., Troy S. B. & Maldonado Y. Intradermal fractional dose inactivated polio vaccine: A review of the literature. Vaccine (2011). [DOI] [PubMed] [Google Scholar]
- Brown D., Fooks A. R. & Schweiger M. Using intradermal rabies vaccine to boost immunity in people with low rabies antibody levels. Adv Prev Med 2011, 601789 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommareddy S. et al. Dissolvable microneedle patches for the delivery of cell-culture-derived influenza vaccine antigens. J Pharm Sci (2011). [DOI] [PubMed] [Google Scholar]
- Kenney R. T., Frech S. A., Muenz L. R., Villar C. P. & Glenn G. M. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med 351, 2295–2301 (2004). [DOI] [PubMed] [Google Scholar]
- Van Damme P. et al. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine 27, 454–459 (2009). [DOI] [PubMed] [Google Scholar]
- Naito S. et al. Antigen-loaded dissolving microneedle array as a novel tool for percutaneous vaccination. Vaccine (2011). [DOI] [PubMed] [Google Scholar]
- Simon J. K. et al. Safety, tolerability, and immunogenicity of inactivated trivalent seasonal influenza vaccine administered with a needle-free disposable-syringe jet injector. Vaccine 29, 9544–9550 (2011). [DOI] [PubMed] [Google Scholar]
- Kim Y. C., Quan F. S., Compans R. W., Kang S. M. & Prausnitz M. R. Formulation of microneedles coated with influenza virus-like particle vaccine. AAPS PharmSciTech 11, 1193–1201 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W. Hemagglutination-inhibition: rapid assay for neuraminic acid-containing viruses. J Virol 14, 1307–1309 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skountzou I., Quan F. S., Jacob J., Compans R. W. & Kang S. M. Transcutaneous immunization with inactivated influenza virus induces protective immune responses. Vaccine 24, 6110–6119 (2006). [DOI] [PubMed] [Google Scholar]
- Reed L. J.a.M. H. A simple method of estimating fifty percent endpoints. The American Journal of Hygiene 27, 493–497 (1938). [Google Scholar]
- Skountzou I. et al. Incorporation of glycosylphosphatidylinositol-anchored granulocyte- macrophage colony-stimulating factor or CD40 ligand enhances immunogenicity of chimeric simian immunodeficiency virus-like particles. J Virol 81, 1083–1094 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO/CDS/CSR/NCS. WHO Manual of Animal Influenza Diagnosis and Surveillance. Department of Communicable Disease Surveillance and Response (2002). [Google Scholar]
- Crotty S., Aubert R. D., Glidewell J. & Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods 286, 111–122 (2004). [DOI] [PubMed] [Google Scholar]