SUMMARY
A new test for utricular function has recently been introduced and validated, namely the ocular vestibular-evoked myogenic potential (oVEMP), which refers to the myogenic potentials recorded by surface EMG electrodes beneath both eyes in response to bone conducted vibration (BCV) of the head or air conducted sound (ACS). The oVEMP test differs from another vestibular-evoked myogenic potential recorded by surface EMG electrodes over the sternocleidomastoid muscles in that the cervical vestibular-evoked myogenic potential (cVEMP) due to saccular activation is measured. oVEMP is a reliable clinical test that relies on extensive physiological evidence from studies on guinea pigs, and in particular on recording the vestibular primary afferent responses to BCV, demonstrating that the same BCV causes similar eye movements in both guinea pigs and humans. This review briefly integrates the most recent physiological and behavioural evidence that substantiates the clinical use of oVEMP.
KEY WORDS: oVEMP, Utricular, Saccular, Otoliths, Bone conduction, Sound, Vibration
RIASSUNTO
Recentemente è stato introdotto e validato in letteratura un nuovo test per lo studio della funzione otolitica ed in particolar modo della macula utricolare. Questo test è il potenziale evocato vestibolare miogenico oculare (oVEMPs) e corrisponde ad un potenziale miogenico registrato da elettrodi posti al di sotto degli occhi del soggetto in esame in risposta ad un stimolo condotto per via ossea oppure ad uno stimolo condotto per via aerea. Questo nuovo test si differenzia da un altro potenziale evocato vestibolare registrabile direttamente dal muscolo sternocleidomastoideo. Grazie a tale nuova modalità di registrazione è stato possibile differenziare le metodiche denominando la nuova entità come potenziale evocato vestibolare oculare (ocular VEMPs) mentre il precedente vestibolare è stato ridefinito come potenziale evocato vestibolare cervicale (cervical VEMPs). Il nuovo potenziale è un efficace strumento di valutazione del nervo vestibolare superiore a differenza del potenziale vestibolare cervicale che si conferma idoneo nella valutazione della funzione del nervo vestibolare inferiore. Entrambi i test possono essere condotti con uno stimolo acustico ed uno stimolo vibratorio. La review che segue si propone di riassumere e confermare le solide basi neurali che sostengono e confermano l'utilizzo della nuova metodica in ambito clinico.
Introduction
Otolith receptors respond to linear acceleration, but for the clinician a major challenge has been how to measure linear acceleration in a simple and safe manner to test otolith function. Large, expensive, complex and potentially dangerous devices such as sleds, centrifuges or tilt chairs have been used to deliver controlled linear accelerations, and the usual measures have been either eye movements or perception. Current evaluation of dynamic otolith function has used either eccentric displacement mode (unilateral stimulation generated; i.e., by translating the subject's chair radially from the vertical rotation axis with a constant angular velocity) or tilt mode (bilateral stimulation generated by full head-and-body tilt about the nasooccipital axis). In spite of the considerable data obtained, in reality these methods are clinically unsuitable.
One potential solution is through the use of a brief bone conducted vibration stimulus (7 msec, short tone burst). Such a stimulus is capable, delivered at Fz (the skull location of the midline of the forehead at the hairline) in generating linear acceleration at the mastoids, and as such is an effective otolith stimulus 1 or bone conducted vibration (BCV) stimulation of the head. Measures with linear accelerometers on the mastoids show that when subjects receive a bone conducted stimulus (e.g. from a gentle tap on the forehead with a tendon hammer or a modest 500 H z vibration from a Bruel and Kjaer 4810 minishaker, Naerum, Denmark 1), there is a series of rapidly changing linear accelerations at each mastoid 1. In humans, there is evidence from neurophysiological studies in guinea pigs that these stimuli act, or that otolithic receptors respond, to changing linear accelerations (jerk) at very low stimulus intensities at levels that are close to the ABR thresholds (all stimulus intensities were referred to the animal's own auditory brainstem response threshold to BCV clicks; the maximum intensity used was within the animal's physiological range and was normally around 70 dB above the BCV threshold), initiating action potentials in otolithic afferent neurons 2 3. Such neurons show pronounced tuning for rapid accelerations, namely changes in linear acceleration (jerk).
In particular, the neurophysiological evidence consists of extracellular recordings of single primary otolithic vestibular neurons in the vestibular nerve of guinea pigs in response to BCV. The recorded neurons were identified as vestibular by their location in the vestibular portion of the VII nerve, and by the fact that they show prolonged activation to maintained linear acceleration stimulation at different tilt positions. However, not all otolithic neurons are activated by BCV, and recordings from vestibular afferent neurons in guinea pigs have shown that one class of otolith neurons – otolith irregular neurons – are sensitively and selectively activated by linear (jerk) accelerations 2 3. Semicircular canal neurons do not respond to such linear acceleration stimuli at the low intensities used 2.
Otolith irregular neurons are selectively activated by sound and vibration
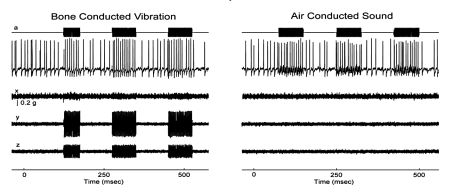
"Activation" consists of an increase in the firing rate locked to stimulus presentation (Fig. 1).. Usually neurons have a regular spontaneous firing rate and neurons from semicircular canals, whether regular or irregular, are not activated at low vibration intensities (up to 2 g peak to peak). Other evidence shows that irregular otolith neurons are highly specialized neurons and originate from type I (calyx) receptors at the striola of the utricular and saccular maculae 4 5. Irregular otolithic afferents respond well to changes in linear acceleration. The BCV stimulus normally used (500 H z BCV) consists of 500 changes in linear acceleration per second. This is apparently the optimum stimulus, and neurons are activated at very low stimulus intensities (close to the ABR thresholds). Figure 1 shows that as the intensity is increased neurons show a very strong increase in firing. Thus, otolith irregular neurons have a low threshold and very sensitive response to BCV (Fig. 2). Many of these irregular otolith neurons can also be activated by 500 H z air-conducted sound (ACS), although the intensity for activation by ACS must be high (about 120-130 dB SPL).
Fig. 1.
Response of an irregular otolith neuron during stimulation by bone conducted vibration and air-conducted sound. The top trace (a) shows the command voltage indicating when the stimulus is on. The second trace shows the recording of action potentials in this single neuron. The three bottom traces (x, y, z) show the triaxial accelerometer recording of the stimulus. The left panel is an example of BCV stimulation, while the right panel shows an example of ACS stimulation of the same neuron. Note the scale of stimulus intensity in g at the left margin between traces x and y. (Reproduced with permission from Curthoys et al. 3).
Fig. 2.
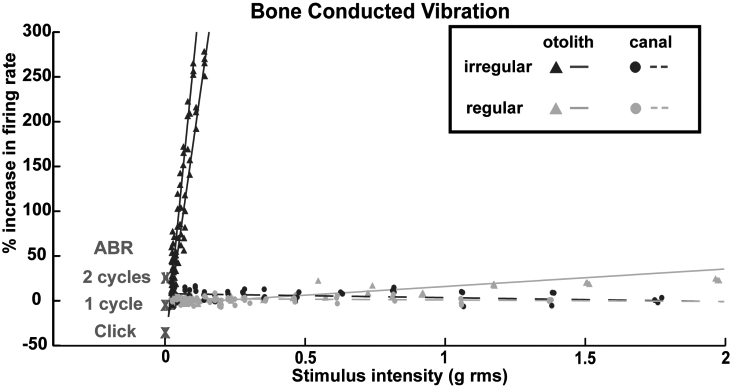
Examples of sensitivity plots of neurons to BCV. Each point shows the percentage increase in firing rate from baseline firing rate during a single stimulus presentation. The stimulus intensity is calculated in g, root mean square of three axes as recorded by the accelerometer for BCV and dB SPL for ACS. Each line shows the linear best-fit of the responses for one neuron. The X show the mean ABR thresholds with 95% confidence intervals for click, one- and two-cycle tone stimuli. Canal neurons do not respond, whereas otolith irregular neurons show a steep increase in firing for very small increases in stimulus intensity. (Reproduced with permission from Curthoys et al. 3).
Many of the activated neurons are utricular as they are located in the dorsal superior vestibular nerve where afferents from the utricular macula travel 3. At low stimulus intensities, BCV and ACS are relatively specific stimuli for otolith irregular neurons. Despite the fact that ACS and BCV have very different modes of transduction, both BCV and ACS can activate the same irregular otolith neuron.
Given that 500 H z BCV activates irregular otolith neurons, one interesting aspect is the behavioural responses produced by such stimulation. Suzuki et al. 6 showed that specific electrical stimulation of the utricular nerve by high frequency electrical stimulation in cats caused a characteristic pattern of eye movements, namely roll of both eyes away from the stimulated side, with both horizontal and vertical components. They also showed that these eye movements are achieved predominantly by activation of the contralateral inferior oblique (IO), inferior rectus (IR), ipsilateral superior oblique (SO) and superior rectus (SR).
Stimulation of recording of eye movements
In light of the evidence that BCV causes increased firing of utricular afferents in guinea pigs, we reasoned that BCV should elicit eye movements in guinea pigs analogous to those reported by Suzuki et al. in cats in response to high frequency electrical stimulation of the utricular nerve. In our laboratory, we tested this hypothesis by using binocular miniature 3D scleral search coils in awake alert guinea pigs to measure the eye movement response to 500 H z BCV stimulation (Fig. 3). As predicted, the BCV stimulus elicited stimulus-locked movements of both eyes which, allowing for the lateral placement of the eyes in guinea pigs, are in analogous directions to those reported by Suzuki et al. for utricular stimulation in cats. BCVevoked eye movements in guinea pigs were vestibular since they were abolished by bilateral labyrinthectomy. Moreover, hair cell loss caused by treatment with bilateral intratympanic gentamicin reduced the amplitude of BCVevoked eye movements but had little effect on the cochlea as demonstrated by a lack of change in the auditory brainstem response (ABR) 7.
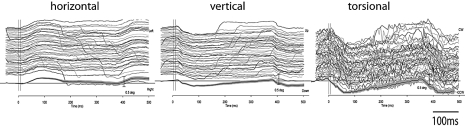
Fig. 3.
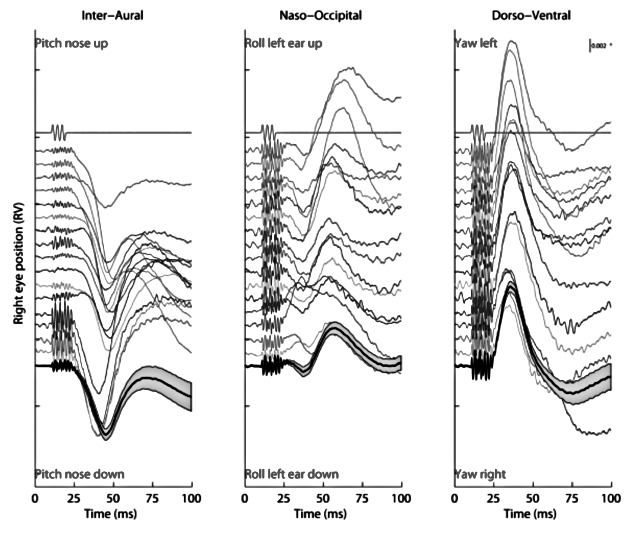
Eye movement response to 10 consecutive 500 Hz BCV tone bursts from a single trial in one animal. The first line indicates the command voltage, and defines stimulus onset and duration. Each subsequent line shows a component of the response to a single tone burst of the left and the right eye as broken down into three axes. (unpublished data: see 24 for full details of methods).
Extending this in humans, we reasoned that 500 H z BCV in a mastoid in healthy human subjects should activate human otolithic receptors, and thereby elicit small eye movements analogous to those in cats and guinea pigs. Using high precision high speed video measures of eye movement at 100 msec bursts of 500 H z mastoid vibration in healthy humans (without semicicircular canal dehiscences), we found small but reliable eye movements locked to the stimulus in the majority of healthy subjects tested 8. These movements had torsional, horizontal and vertical components (Fig. 4) as had been found in cats and guinea pigs.
Fig. 4.
3D eye movements recorded from the right eye in one subject during repeated trials of right mastoid stimulation at 500 Hz with a B71 bone vibrator. The traces show the responses to two successive stimuli. The response is highly repeatable. The eye moves horizontally and torsionally away from the side of stimulation during unilateral mastoid stimulation and vertically downward. The bottom recording is the mean and 95% confidence interval across the individual records. (unpublished data, see 7 for full details of methods)
Therefore, we suggest that the eye movements induced by bone conducted vibration are primarily of utricular origin because anatomical and physiological evidence suggests that the sacculo-ocular pathway is weak and polysynaptic.
These vibration-induced eye movements in human subjects are very small (usually < 0.5°) and of little clinical utility. They are also strongly dependent on the exact location and force of the stimulator on the mastoid. However, it is possible to measure the myogenic precursors to actual eye movement to assess utricular function; such a myogenic measure is clinically practical and is now routinely used in many clinics worldwide 9-13. In particular, myogenic potentials are measured by surface EMG electrodes just beneath the eyes as the subject looks up.
Method of stimulation and recording oVEMPs to bone conducted vibration and air conducted sound
The bone conducted vibration stimulus is usually delivered to the junction of the midline of the forehead and the hairline (a skull location called Fz). The stimulus is either a light tap to Fz with a tendon hammer or a modest vibration delivered by a Bruel and Kjaer minishaker 4810 1. In response to BCV from a tendon hammer or a minishaker, the surface EMG electrodes record a stimulus-locked ocular vestibular-evoked myogenic potential (oVEMP). The first negative (excitatory) component of the oVEMP at a latency of about 10 ms is called n10. This n10 component most likely indicates the myogenic potentials of IO and IR, since the magnitude of n10 increases as the subject looks up and brings these muscles close to the electrodes. The location of the BCV stimulus at Fz is unique because it ensures equal and simultaneous stimulation of both labyrinths. However, a weaker bone conduction stimulator such as a standard clinical bone oscillator (e.g. Radioear B-71) at Fz is ineffective since the magnitude of the linear acceleration generated at the mastoids by such a weak stimulator are not great enough to activate otolith afferents in most individuals 14. Attempting to give equal stimulation to both ears by a standard clinical bone oscillator (such as a B-71) placed on each mastoid successively is not clinically practical since small changes in the location, direction or force of B-71 on the mastoid cause substantial changes in the applied linear accelerations. As a result, the measured n10 response would result in an artificial AR.
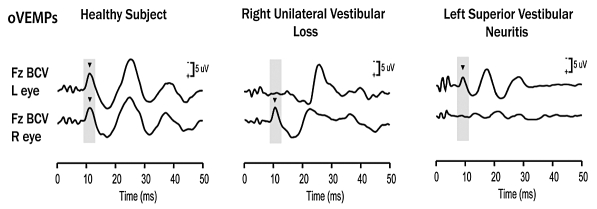
For stimuli delivered to the midline, both mastoids receive approximately equal linear acceleration stimulation, and in a healthy subject approximately equal amplitude oVEMP n10s are recorded beneath both eyes (Fig. 5) 14 15. If a patient with a complete unilateral vestibular loss receives such a stimulus, the oVEMP n10 beneath the contralateral eye is reduced or absent 14 16 17. The oVEMP n10 is a crossed otolith- ocular response 14. Loss of function of the superior vestibular nerve (in which all utricular afferents lie) reduces or abolishes the contralateral oVEMP n10 18 19, whereas loss of the inferior vestibular nerve has no detectable effects on the contralateral oVEMP n10 20. These results provide support to the conclusion that oVEMP n10 predominantly reflects contralateral utricular function.
Fig. 5.
oVEMP responses for a healthy subject (left panel), a patient with right unilateral vestibular loss (middle panel), and a patient with left superior vestibular neuritis (right panel). The yellow bars mark the time of healthy oVEMP and n10 responses, respectively. The healthy subject displays n10 responses of similar magnitude beneath both eyes. The patient with right unilateral vestibular loss has normal n10 responses beneath the right (ipsilesional) eye, but no n10 responses beneath the left (contralesional) eye. These results are consistent with complete loss of otolithic function on the left side. The patient with left superior vestibular neuritis shows no oVEMPs beneath the right (contralesional) eye, but a normal oVEMP response beneath the left (ipsilesional) eye. This is consistent with oVEMPs being a predominantly utricular response. (Modified from Iwasaki et al. 2008, with permission 14).
Stimulation by air-conducted sound also elicits n10 responses beneath the contralateral eye. However, the sound intensities needed are large, while the n10 potentials to ACS are typically small 21.
The question then arises as to whether oVEMP n10 is really vestibular. Considering that n10 is absent in patients without vestibular function, and present in patients who are totally deaf but still have vestibular function, the answer would seem to be 'yes' 1. The oVEMP n10 is also useful for identifying other conditions. In patients with superior canal dehiscence, the amplitude of the oVEMP n10 contralateral to the affected ear in response to Fz BCV or air conducted sound (ACS) is enhanced in terms of amplitude 22. A very large n10 (> 10 μV baseline to peak) to Fz BCV is indicative of dehiscence. Additionally, in patients with early Ménière's disease tested at attack, the contralateral oVEMP n10 is enhanced compared to measures in the same patients at quiescence 23. We speculate that this enhancement by Ménière's disease attack could be due to mechanical changes in the labyrinth that enhance the sensitive response of utricular receptors to BCV stimulation. It has recently been documented that a patient with Lermoyez syndrome showed exactly the opposite, namely that when measured at attack the amplitude of the contralateral n10 decreased 24.
The reader is also referred to a recent clinical review of the clinical use of both cervical and ocular VEMPs 25. This was also based upon the neural and behavioural data reviewed herein.
Conclusions
The oVEMP n10 has only recently been introduced, but has gained wide acceptance. There are over 50 publications on oVEMP in the scientific and clinical literature since it was introduced in 2005. It has been in use for 3 years at Sydney, Tokyo, Zurich and Cassino, and well over 3000 patients have been tested without any adverse effects. The oVEMP to BCV is a clinically practical way of measuring utricular function simply, safely and quickly, and as overviewed herein relies on strong scientific foundations.
Acknowledgements
The authors are grateful for the support of National Health and Medical Research Council of Australia, and the Garnett Passe and Rodney Williams Memorial Foundations.
Abbreviations
- AR
asymmetry ratio for the relative size of the n10 of the oVEMPs for both eyes;
- ACS
air conducted sound;
- BCV
bone conducted vibration;
- EMG
electromyogram;
- Fz
location on the forehead in the midline at the hairline;
- Fz BCV
bone conducted vibration delivered to Fz;
- B-71
standard clinical bone conduction oscillator (Radioear B71);
- 4810
Bruel and Kjaer Mini-shaker;
- cVEMP
cervical vestibular evoked myogenic potentials;
- oVEMP
ocular vestibular evoked myogenic potentials;
- n10
initial negative potential in the oVEMP response at latency of around 10 ms;
- TH
tendon hammer (or reflex hammer);
- μV
microvolt.
References
- 1.Iwasaki S, Smulders YE, Burgess AM, et al. Ocular vestibular evoked myogenic potentials to bone conducted vibration of the midline forehead at Fz in healthy subjects. Clin Neurophysiol. 2008;119:2135–2147. doi: 10.1016/j.clinph.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 2.Curthoys IS, Kim J, McPhedran SK, et al. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res. 2006;175:256–267. doi: 10.1007/s00221-006-0544-1. [DOI] [PubMed] [Google Scholar]
- 3.Curthoys IS, Vulovic V. Vestibular primary afferent responses to sound and vibration in the guinea pig. Exp Brain Res. 2011;210:347–352. doi: 10.1007/s00221-010-2499-5. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg JM, Desmadryl G, Baird RA, et al. The vestibular nerve of the chinchilla IV. Discharge properties of utricular afferents. J Neurophysiol. 1990;63:781–790. doi: 10.1152/jn.1990.63.4.781. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res. 2000;130:277–297. doi: 10.1007/s002210050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki JI, Tokumasu K, Goto K. Eye movements from single utricular nerve stimulation in the cat. Acta Otolaryngol. 1969;68:350–362. doi: 10.3109/00016486909121573. [DOI] [PubMed] [Google Scholar]
- 7.Vulovic V, Curthoys IS. Bone conducted vibration activates the vestibulo-ocular reflex in the guinea pig. Brain Res Bull. 2011;86:74–81. doi: 10.1016/j.brainresbull.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Cornell ED, Burgess AM, MacDougall HG, et al. Vertical and horizontal eye movement responses to unilateral and bilateral bone conducted vibration to the mastoid. J Vestib Res. 2009;19:1–7. doi: 10.3233/VES-2009-0338. [DOI] [PubMed] [Google Scholar]
- 9.Rosengren SM, Todd NPM, Colebatch JG. Vestibular-evoked extraocular potentials produced by stimulation with boneconducted sound. Clin. Neurophysiol. 2005;116:1938–1948. doi: 10.1016/j.clinph.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Todd NPM, Rosengren SM, Aw ST, et al. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air- and bone-conducted sound. Clin Neurophysiol. 2007;118:381–390. doi: 10.1016/j.clinph.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Curthoys IS, Manzari L, Smulders YE, et al. A review of the scientific basis and practical application of a new test of utricular function – ocular vestibular-evoked myogenic potentials to bone-conducted vibration. Acta Otorhinolaryngol Ital. 2009;29:179–186. [PMC free article] [PubMed] [Google Scholar]
- 12.Curthoys IS, et al. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin. Neurophysiol. 2010;121:132–144. doi: 10.1016/j.clinph.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 13.Rosengren SM, Welgampola MS, Colebatch JG. Vestibular evoked myogenic potentials: past, present and future. Clin. Neurophysiol. 2010;121:636–651. doi: 10.1016/j.clinph.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki S, McGarvie LA, Halmagyi GM, et al. Head taps evoke a crossed vestibulo-ocular reflex. Neurology. 2007;68:1227–1229. doi: 10.1212/01.wnl.0000259064.80564.21. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki S, Smulders YE, Burgess AM, et al. Ocular vestibular evoked myogenic potentials in response to bone conducted vibration of the midline forehead at Fz - a new indicator of unilateral otolithic loss. Audiol Neurotol. 2008;13:396–404. doi: 10.1159/000148203. [DOI] [PubMed] [Google Scholar]
- 16.Manzari L, Burgess AM, Curthoys IS. Effect of bone-conducted vibration of the midline forehead (Fz) in unilateral vestibular loss (uVL). Evidence for a new indicator of unilateral otolithic function. Acta Otorhinolaryngol Ital. 2010;30:175–181. [PMC free article] [PubMed] [Google Scholar]
- 17.Iwasaki S, Murofushi T, Chihara Y, et al. Ocular vestibular evoked myogenic potentials to bone-conducted vibration in vestibular schwannomas. Otol Neurotol. 2010;31:147–152. doi: 10.1097/MAO.0b013e3181c0e670. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki S, Chihara Y, Smulders YE, et al. The role of the superior vestibular nerve in generating ocular vestibularevoked myogenic potentials to bone conducted vibration at Fz. Clin Neurophysiol. 2009;120:588–593. doi: 10.1016/j.clinph.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 19.Manzari L, Tedesco AR, Burgess AM, et al. Ocular vestibular- evoked myogenic potentials to bone conducted vibration in superior vestibular neuritis show utricular function. Otolaryngol. Head Neck Surg. 2010;143:274–280. doi: 10.1016/j.otohns.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Manzari L, Burgess AM, Curthoys IS. Ocular and cervical vestibular-evoked myogenic potentials to bone conducted vibration in patients with probable inferior vestibular neuritis. 2011 doi: 10.1017/S0022215112000692. [in submission] [DOI] [PubMed] [Google Scholar]
- 21.Curthoys IS, Iwasaki S, Chihara Y, et al. The ocular vestibular- evoked myogenic potential to air-conducted sound; probable superior vestibular nerve origin. Clin Neurophysiol. 2011;122:611–616. doi: 10.1016/j.clinph.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Manzari L, Burgess AM, MacDougall HG, et al. Enhanced otolithic function in semicircular canal dehiscence. Acta Otolaryngol. 2011;131:107–112. doi: 10.3109/00016489.2010.507780. [DOI] [PubMed] [Google Scholar]
- 23.Manzari L, Tedesco AR, Burgess AM, et al. Ocular and cervical vestibular-evoked myogenic potentials to bone conducted vibration in Ménière's disease during quiescence vs during acute attacks. Clin Neurophysiol. 2010;21:1092–1101. doi: 10.1016/j.clinph.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Manzari L, Burgess AM, Curthoys IS. Vestibular function in Lermoyez syndrome at attack. Eur Arch Otorhinolaryngol. 2012;269:685–691. doi: 10.1007/s00405-011-1657-0. [DOI] [PubMed] [Google Scholar]
- 25.Eleftheriadou A, Koudounarakis E. Vestibular-evoked myogenic potentials eliciting: an overview. Eur Arch Otorhinolaryngol. 2011;268:331–339. doi: 10.1007/s00405-010-1408-7. [DOI] [PubMed] [Google Scholar]