Abstract
We previously reported that in the absence of NKT cells, wound closure was accelerated in a murine excisional punch wound model. Here, we explored whether purposefully inhibiting NKT cell activation had similar effects on wound closure and the dermal inflammatory response to injury. We found that prevention of NKT cell activation accelerated wound closure in a dose-responsive manner. If anti-CD1d was administered before wounding, NKT cell infiltration into cutaneous wounds was diminished without quantitative changes in cellular infiltrates. Furthermore, prevention of NKT cell activation transiently enhanced the local production of a subset of chemokines, including MIP-2, MCP-1, MIP-1α, and MIP-1β, and altered the relative expression of CD69 and CXCR2 on the surface of both circulating and wound NKT cells. Taken together, these findings suggest that wounding activates NKT cells via CD1d presentation of glycolipid antigen and help further define a role for NKT cells in the regulation of wound inflammation and closure. Many soluble factors have been targeted as potential wound healing therapies, but their clinical success has been limited. Given our findings, the NKT cell may be an attractive target for wound healing therapies.
Keywords: wound healing, skin, immune cell, injury, cytokines
INTRODUCTION
Despite a number of advances in our understanding and clinical application of soluble growth factors that promote wound closure, delayed or aberrant wound healing remains a costly and often lethal problem. Annually, five to seven million patients in the United States suffer from defects in wound repair, costing billions of dollars in medical expenses [1–3]. We recently reported on a novel role for Natural Killer T (NKT) cells in cutaneous wound repair [4]. In the absence of NKT cells, wound closure was accelerated and there was a transient enhancement in the local production of a subset of chemokines and TGF-β1 accompanied by augmented collagen content[4]. NKT cell-dependent changes in closure and inflammatory mediators occurred early (d 1–3) post-wounding, a time that correlated with NKT cell accumulation within cutaneous wounds. For these findings to be useful therapeutically, one needs a model where NKT cells are present, but their activity attenuated. Instead of targeting the downstream effects of NKT cell activation, we chose to prevent NKT cell activation by interfering with the CD1d-TCR interaction.
NKT cells become activated upon recognition of glycolipid antigen presented in the context ofCD1d molecules found on the surface of antigen presenting cells (APCs) [5]. In certain settings, NKT cells can become activated independent of CD1d, either by direct antigen binding of the invariant TCR or other surface receptors such as the Fcγ receptor [6, 7]. NKT activation is characterized by either pro-inflammatory or immunoregulatory cytokine production dictated by local cytokine signals [8]. NKT cells also up-regulate surface CD69 expression upon antigen recognition via CD1d. While various stimuli can induce CD69 expression in conventional lymphocytes, the only known mechanism for CD69 expression in NKT cells is ligation of the invariant TCR with glycolipid antigen [9, 10]. Numerous studies, including those from our laboratory, have prevented NKT cell activation using an anti-CD1d monoclonal antibody to interfere with NKT cell-APC interactions [11–13]. Such anti-CD1d treatment abrogates the effect of NKT cells as measured by their cytokine production and other downstream consequences, without changing NKT cell quantities in any compartment. The NKT-APC interaction is a one-way communication [14] and does not alter APC function [5, 15–19]. Using anti-CD1d to abrogate NKT cell function relies on a system in which the NKT cell becomes active after antigen presentation with CD1d, and not any of the alternative means of NKT cell activation.
NKT cells are best known for their regulatory functions in such diverse settings as autoimmunity, cancer, and certain infections [13, 20, 21], but they are also known to infiltrate sites of localized inflammation in such organs as the lung or the skin [22–24]. In the case of cutaneous inflammation, like in the early phase of wound healing, the CXC chemokines are classically associated with the inflammatory infiltrate [25]. Although NKT cells are known to respond to several chemokines, they produce and respond to the CXC chemokine, MIP-2, presumably via their surface expression of CXCR2 [26, 27]. The exact mechanisms that direct NKT cell homing and migration into sites of inflammation remains understudied.
Here, we examined whether systemic blockade of NKT cell activation with anti-CD1d mAb influenced cutaneous wound repair in a murine excisional punch wound model. Similar to our previous studies with NKT cell deficient animals [4], prevention of NKT cell activation with anti-CD1d accelerated early wound closure, and this effect was dose-responsive. When anti-CD1d was administered before wounding, NKT cell infiltration into cutaneous wounds was attenuated, while the acceleration in wound closure was enhanced. Furthermore, prevention of NKT cell activation increased the local production of a subset of chemokines, but did not change the quantity or kinetics of neutrophil, macrophage, or T cell infiltrates. Blockade also influenced the relative expression of CD69 and CXCR2 on the surface of circulating NKT cells, correlating with the activated NKT cell phenotype seen within the wound after anti-CD1d treatment. This model therefore confirms that cutaneous injury results in NKT cell activation via CD1d, an event that also prompts NKT cell homing to the site of injury itself.
METHODS
Animals
Eigh- to 12-wk-old male BALB/c mice used in these studies were obtained from Harlan Laboratory (Indianapolis, IN). All animals were housed on a 12-h/12-h light/dark cycle and provided with food and water ad libitum. All mice were treated humanely and in accordance with the guidelines set forth by the Loyola University Institutional Animal Care and Use Committee and the National Institutes of Health.
Excisional Injury Model and Tissue Collection
The excisional punch wound model was used as described previously [4, 28]. Briefly, mice were anesthetized by intraperitoneal injection of 100 mg/kg of ketamine (Abbott Laboratories, North Chicago, IL) and 10 mg/kg of xylazine (Phoenix Pharmaceutical, St. Joseph, MO). The fur on their dorsal surface was then shaved, and six full-thickness dermal excisional wounds were produced on the dorsum of each mouse with a dermal biopsy punch (Acuderm, Ft. Lauderdale, FL). Animal cages were placed on warming pads until full recovery from anesthesia. At 12 h, and d 1, 3, 5, 7, and 10 post-wounding, mice were euthanized, their pelts removed, and the wounds excised with a larger biopsy punch. Some wounds were snap-frozen in liquid nitrogen and stored at −80°C for molecular (protein extraction) analysis, while other wounds were processed immediately for flow cytometry. Animals were weighed prior to wounding and at the time of euthanasia.
In vivo Antibody Administration
Antibodies used for systemic administration included purified (azide-free, low endotoxin) rat anti-mouse CD1d monoclonal antibody (mAb) (clone no. 1B1, eBioscience, Inc., San Diego, CA) and rat IgG2b (eBioscience). All antibodies were delivered intravenously (i.v.) via the tail veins in a final volume of 200 μL as we have described previously [29–31]. For time points beyond 3 d, animals were re-dosed with antibodies every 72 h.
Determination of Wound Closure
Wound closure was determined by digital photography and image analysis as described previously [4, 32, 33]. Briefly, animals were wounded as described above. At 12 h and d 1, 3, 5, 7, and 10 post-injury, groups of animals were euthanized and their pelts removed. After removing all subcutaneous tissue, each wound was placed flat and photographed from a fixed distance of 20 cm with a ruler placed within each photograph. Photoshop 7.0 (Adobe Systems Inc., San Jose, CA) was used to determine the number of pixels in the open wound area with the “magic wand” tool and a tolerance setting of 60. Separate groups of mice were euthanized immediately after wounding to obtain baseline (d 0) wound size. Wound areas at each time point were compared with d 0 wounds (0% closed at time zero) to calculate the percent wound closure.
Measurement of Wound Chemokine Content
A modification of previously published methods was used to extract protein from dermal wounds [34]. Individual wounds were homogenized in ice-cold PBS containing protease inhibitor cocktail (Roche, Mannheim, Germany). Homogenates were then sonicated briefly on ice, centrifuged at 5000 rpm for 10 min, and filtered through a 1.2-μm pore filter. Levels of MIP-2, MIP-1α, MIP-1β, and MCP-1 were determined with commercially available enzyme-linked immunosorbent assay (ELISA) kits (R and D Systems, Minneapolis, MN) according to the manufacturer’s specifications. ELISA plates were read using a SpectraMAX Plus 384 plate reader (Amersham Biosciences, Piscataway, NJ), and analyses of ELISA data were done with SoftMax Pro 3.1.2 (Molecular Devices Corp., Sunnyvale, CA).
Preparation of Dermal Cell Suspensions by Tissue Dispersion
Total wound cell suspensions were prepared as described previously [4]. Animals were wounded as described above. At 12 h, d 1, or d 3 post-wounding, animals were euthanized and their pelts removed. Each pelt was briefly washed in PBS and any remaining subcutaneous tissue including the panniculus carnosus removed by sharp dissection. Three wounds (cephalad, middle, and caudal) were weighed and pooled as one sample. After fine mincing, each sample was placed in a solution containing 1 mg/mL dispase II (Roche), and 10 mg/mL gentamicin sulfate (Mediatech, Inc., Herndon, VA) diluted in a RPMI 1640 (Invitrogen, Carlsbad, CA) solution containing 5% fetal calf serum, penicillin-streptomycin, and glutamine (referred to as 5% complete media throughout). Wound samples were incubated in 15.5 mL of the enzyme solution per gram of tissue on a shaker at 4°C for 16 h. Any remaining tissue was then removed from this solution, weighed, and incubated in an enzyme solution (20 mL/g of tissue) containing hyaluronidase I (1 mg/mL) (Sigma-Aldrich, St. Louis, MO), collagenase type IA (1 mg/mL) (Sigma-Aldrich), DNase I (1.2 mg/mL) (Roche), and gentamicin sulfate (5 mg/mL) (Mediatech) diluted in 5% complete media. Following incubation, this solution was combined with the dispase solution and filtered through a 70-μm nylon cell strainer (BD Biosciences, San Jose, CA) to obtain single cell suspensions. Cells were then washed twice with a solution of 3% heat inactivated fetal bovine serum in sterile PBS before transferring to a solution of 1% bovine serum albumin (Sigma, St. Louis, MO) and 0.1% sodium azide (Sigma) in 1X PBS for cell staining and flow cytometric analysis (this solution will be referred to as “staining buffer” throughout).
Preparation of Splenocytes Cell Suspensions for Flow Cytometric Analysis
Spleen suspensions were prepared as described previously [11]. Briefly, spleens were minced and gently pressed through fine wire mesh. Erythrocytes were lysed with ammonium chloride lysis buffer (Tris-buffered ammonium chloride). Erythrocyte-free splenocytes were resuspended in complete medium consisting of RPMI 1640 (Invitrogen, Grand Island, NY), 5% fetal calf serum (Invitrogen), nonessential amino acids (10 mM) (Sigma-Aldrich), sodium pyruvate (100 mM) (Mediatech), penicillin (10,000 U/mL) (Sigma-Aldrich), streptomycin (10 mg/mL) (Sigma-Aldrich), and glutamine (29.2 mg/mL) (Sigma-Aldrich). Cell viability and numbers were determined by Trypan blue (Invitrogen) exclusion.
Whole Blood Collection, Erythrocyte Lysis, and Serum Separation
Immediately following euthanasia, blood was collected by cardiac puncture. Erythrocytes were lysed and removed using the Easy-Lyse whole blood erythrocyte lysing kit (Leinco Technologies, St. Louis, MO) following the manufacturer’s recommended protocol. Blood samples were re-suspended in staining buffer after lysis. In separate experiments, serum was separated by whole blood using serum separator tubes (Sarstedt, Inc., Newton, NC). Blood was spun down according the manufacturers recommended protocol. Serum was stored at −80°C.
Flow Cytometric Analyses of Wound and Blood Cell Suspensions
All cell suspensions were prepared as described above. Nonspecific staining was blocked with anti-CD16/CD32 (FcBlock) (clone no. 93; eBioscience). After blocking, aliquots of one million cells were stained with either APC-conjugated anti-CD3ε (clone no. 145-2C11, eBioscience), FITC-conjugated anti-CD69 (clone no. H1.2F3, eBioscience), and glycolipid loaded dimeric CD1d: Ig Fusion Protein (Dimer) (BD Pharmingen) or FITC-conjugated anti-CD3ε (clone no. 145-2C11; eBioscience), APC-conjugated anti-CXCR2 (clone no. 242216; R and D Systems), and CD1d dimeric fusion protein. In both cases, the CD1d dimeric fusion protein was counter-stained with a secondary PE-conjugated anti-IgG1 (clone no. A85-1; BD Pharmingen). To identify neutrophils and macrophages, a separate aliquot of one million cells was stained with FITC-conjugated anti-GR-1 (clone no.RB6-8C5; eBioscience) and APC-conjugated anti-F4/80 (clone no. BM8; eBioscience). In separate experiments, splenocytes were stained with APC-conjugated anti-F4/80 (same clone as above), FITC-conjugated anti-CD19 (clone no. MB19-1; eBioscience), and PE-conjugated anti-CD11c (clone no. N418; eBioscience).
RESULTS
Prevention of NKT Cell Activation Accelerated Excisional Punch Wound Closure
In our previous studies with a murine excisional punch wound model and NKT cell deficient mice (Jα281ko or CD1dko) [4], we found that wound closure was significantly accelerated in the absence of NKT cells [4]. Since the acceleration in wound closure seen in NKT cell knockout mice could be attributed to a compensatory developmental response in an immune system that congenitally lacks NKT cells, we asked if the same effect on wound closure occurred in a model where NKT cells were present, but their activation prevented via systemic administration of anti-CD1d mAb. Furthermore, if our initial observation of accelerated wound closure also occurred by preventing NKT cell activation with anti-CD1d, it would suggest that CD1d antigen presentation is required for NKT cell influence at the wound site.
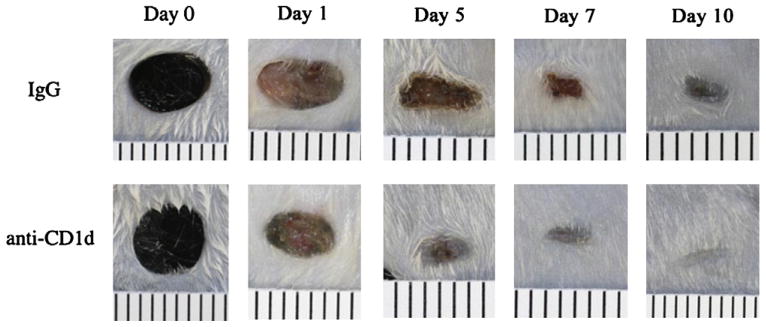
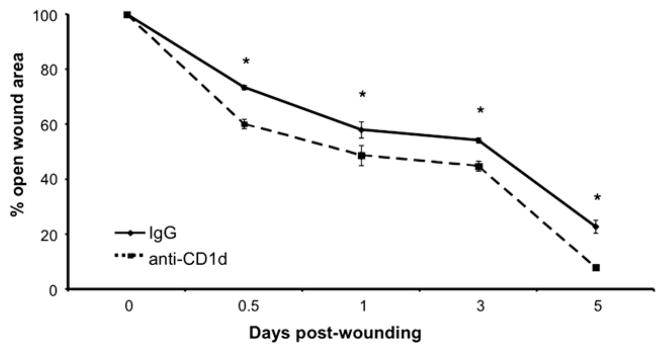
Compared with animals treated with a control rat IgG, those treated with the anti-CD1d mAb immediately following wounding displayed accelerated wound closure through 10 d post-injury (Fig. 1). All wounds were closed by d 12 (data not shown). As early as 12 h post-injury, wounds from animals treated with anti-CD1d mAb had 18.1% less open wound area than wounds from animals treated with the control IgG. Similarly, animals treated with anti-CD1d had wounds with 16.1% and 17.2% less open area than wounds from animals treated with the control antibody at d 1 and 3, respectively (Fig. 2). The greatest difference between the two groups occurred at 5 d post-wounding, when the animals treated with anti-CD1d mAb had wounds with only 8.0% open wound area while wounds from animals treated with the control antibody had 22.8% open area (Fig. 2). Just as with our recent knockout mouse studies [4], transiently preventing NKT cell activation by systemic administration of anti-CD1d mAb resulted in accelerated wound closure.
FIG. 1.
Comparison of wound closure in anti-CD1d versus IgG treated mice. Mice were each given six 3mmdorsal excisional punch wounds followed immediately by either anti-CD1d mAb or control IgG (i.v.). At d 1, 5, 7, and 10 post-injury, pelts were removed and wounds were photographed from a fixed distance. Day 0 wounds are from animals euthanized immediately after wounding. Ruled lines are 1 mm apart. (Color version of figure is available online.)
FIG. 2.
Measurement of wound closure in anti-CD1d versus IgG treated mice. The percent of original open wound area was compared between groups; n = 4 per group. *P < 0.05. Similar results were obtained from three separate experiments.
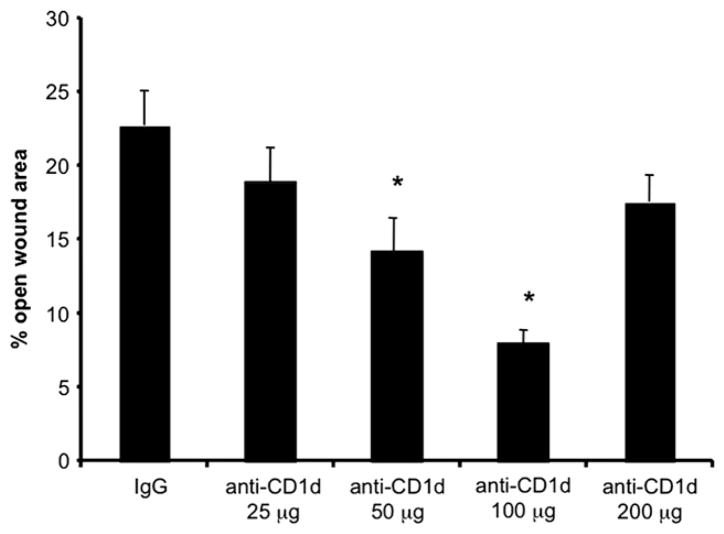
Since use of anti-CD1d in the setting of wound healing was novel, we tested several different doses to determine the optimal dose for accelerating wound closure in the murine system. Because the greatest difference in wound size between IgG and anti-CD1d treated groups in our initial studies occurred at 5 d post-wounding, and since this time point is the traditionally cited end point of epithelialization [25, 35], we chose to examine wound areas of animals treated with varying doses of anti-CD1d (25, 50, 100, and 200 μg) or IgG at 5 d post-injury. We found that the wound closure effect was dose-responsive with a dose of 25 μg anti-CD1d resulting in d 5 wound areas equivalent to the IgG treated animals, while wounds from animals treated with 50 and 100 μg anti-CD1d had wounds with 14.2% and 8.0% open wound areas compared with 22.8% open wound area in IgG treated animals (Fig. 3). A higher dose of 200 μg, however, resulted in wound areas equivalent to those from IgG treated animals (Fig. 3). Hence, anti-CD1d mAb treatment results in a dose-responsive effect on wound closure up to a maximal dose, where the effect is lost or unfavorable, which is a commonly observed phenomenon in pharmacology [36]. Further studies demonstrated why higher antibody doses were deleterious to the animals. One day post-wounding, animals receiving the 200 μg dose had 31.8% fewer circulating B cells (CD19+CD1d+) and 25.6% fewer circulating monocytes (F4/80+CD1d+) compared with animals given IgG. There were no significant changes in the number of B cells, macrophages, or dendritic cells (CD11c+CD1d+) at any of the lower doses (data not shown). Given that 100 μg of anti-CD1d mAb effected the greatest acceleration in wound closure (Fig. 3) without changing circulating APC (B cells, macrophages, and dendritic cells) counts (data not shown), we chose 100 μg to use for subsequent studies.
FIG. 3.
Comparison of wound closure in various dosages of anti-CD1d versus IgG treated mice. Mice were each given six 3 mm dorsal punch excisional wounds followed immediately by either anti-CD1d mAb (at doses indicated in μg) or control IgG (i.v.). At 5 d post-injury, pelts were removed and wounds were photographed. The percent of original open wound area was compared between groups; n = 4 per group. *P < 0.05 compared with the IgG treated group. Similar results were obtained from two separate experiments.
Prevention of NKT Cell Activation Altered Local Inflammatory Signals
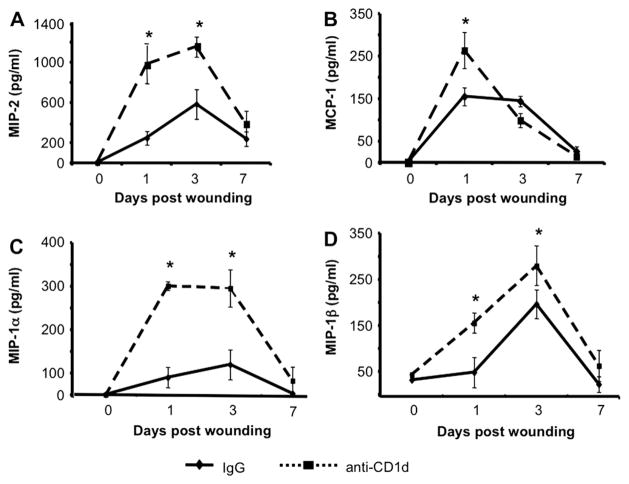
In our previous studies, wounds from NKT cell deficient animals displayed enhanced local production of a subset of neutrophil and monocyte/macrophage chemokines [4]. To determine if these chemokine protein levels differed between wounds from control IgG versus anti-CD1dmAb treated mice, we performed ELISAs for MIP-2, MCP-1, MIP-1α, and MIP-1β on protein extracts from wound homogenates. We found that wounds from anti-CD1d mAb treated mice that were collected early post injury (d 1 and 3) had augmented levels of these chemokines compared with wounds from animals treated with the control IgG (Fig. 4). For example, at 1 d post-wounding, wounds from animals treated with anti-CD1d contain nearly 4-fold more of the neutrophil chemokine, MIP-2, than wounds from animals treated with IgG (Fig. 4A). In examining the monocyte/macrophage chemokines, d 1 wounds from animals given anti-CD1d contained 1.7, 6.3, and 3.2 times more MCP-1, MIP-1α, and MIP-1β, respectively, compared with wounds from mice given control IgG (Fig. 4B–D). MIP-2 and MIP-1α content remained at higher levels in d 3 wounds from animals treated with anti-CD1d compared with those given IgG (Fig. 4A and C). Importantly, the augmentation of this chemokine subset in wounds from animals receiving systemic blockade of NKT cell activation did not persist. MCP-1 and MIP-1β content in wounds from animals treated with either anti-CD1d or IgG reached equivalence by 3 d post-wounding while MIP-2 and MIP-1α content was equivalent in the two groups by d 7 (Fig. 4). It is also important to note that uninjured animals treated with either IgG or anti-CD1d had equivalent local production of all chemokines tested (represented as D 0 data in Fig. 4), indicating that antibody treatment alone did not alter local chemokine production. Therefore, systemic blockade of NKT cell activation resulted in a transient enhancement in the local production of a select subset of selected neutrophil (MIP-2) and monocyte/macrophage (MCP-1, MIP-1α, and MIP-1β) chemokines during the early inflammatory phase of wound repair. Similar observations in the pattern and the kinetics of chemokine elevation were made in previous studies when NKT cells were congenitally absent [4].
FIG. 4.
Wound chemokine content in IgG versus anti-CD1d treated mice. Mice were each given six 3 mm dorsal punch excisional wounds followed immediately by either anti-CD1d mAb or control IgG (i.v.). At 1, 3, and 7 d pos-injury, wounds were excised and homogenized. Chemokine content was determined by ELISA. Skin biopsies from uninjured animals were used as time (d) zero controls. Data are represented as mean pg chemokine per mL of homogenate (1 mL = 1 wound); n = 4 mice per group. *P < 0.05. Similar results were obtained from two separate experiments.
Blockade of NKT Cell Activation Did Not Alter Early Wound Cellular Infiltrates
Since treatment with anti-CD1d mAb enhanced the local production of neutrophil and monocyte chemoattractants, we next chose to evaluate whether infiltration of neutrophils and monocyte/macrophages, as well as T and NKT cells, differed in anti-CD1d versus control Ab-treated mice. In our earlier studies [4], we developed a method of wound digestion and cell suspension preparation followed by flow cytometry for the identification of inflammatory cells as well as infrequent cell types including NKT cells. As in our previously reported studies with NKT cell knockout mice, we did not find differences in neutrophil or macrophage content in wounds from animals treated with anti-CD1d or IgG (Table 1). Additionally, there were no differences in overall T cell or NKT cell content between the two groups (Table 1).
TABLE 1.
Selected Early Wound Infiltrates in IgG versus anti-CD1d Treated Mi
| D 0‡ |
D 1
|
D 3
|
||||
|---|---|---|---|---|---|---|
| IgG | Anti-CD1d | IgG | Anti-CD1d | IgG | Anti-CD1d | |
| CD3+ | 2.81 ± 0.40* | 2.64 ± 0.21§ | 6.59 ± 0.51 | 6.64 ± 0.68§ | 10.58 ± 0.87 | 10.22 ± 0.42§ |
| Dimer+CD3+ | 0.00 ± 0.00† | 0.01 ± 0.01§ | 5.68 ± 0.16 | 5.97 ± 0.25§ | 5.98 ± 0.67 | 5.70 ± 0.59§ |
| F4/80+ | 4.57 ± 0.31 | 4.43 ± 0.84§ | 7.97 ± 0.26 | 8.01 ± 1.37§ | 20.59 ± 0.97 | 20.99 ± 0.81§ |
| GR-1+ | 0.54 ± 0.12 | 0.36 ± 0.14§ | 49.33 ± 1.88 | 50.68 ± 0.88§ | 23.54 ± 1.03 | 24.11 ± 1.18§ |
Values expressed as percentage of live cells ± SEM.
NKT cells expressed as a percentage of lymphocytes ± SEM.
D (day) 0 refers to skin from an uninjured mouse.
No statistical differences (IgG versus anti-CD1d) by Student’s t-test.
Prevention of NKT Cell Activation Does Not Have Off-Target Systemic or Immunologic Consequences
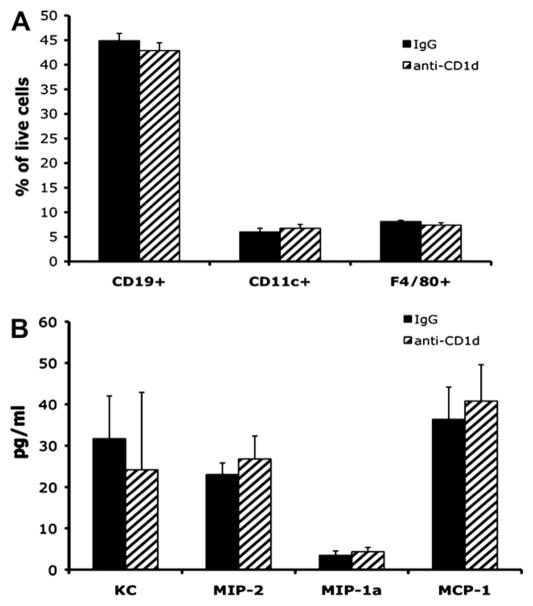
Since CD1d is predominantly found on APCs (macrophages, dendritic cells, and marginal zone B cells), our observation of increased local production of a subset of chemokines after systemic delivery of anti-CD1d mAb might be explained by systemic, rather than local, consequences of this blocking antibody. As mentioned above, high dose (200 μg) anti-CD1d resulted in decreased circulating monocytes and B cells (data not shown). This likely occurs via antibody dependent cytotoxicity. Using flow cytometry, we also measured splenic APCs numbers 24 h after systemic administration of IgG and anti-CD1d (100 μg) (Fig. 5A). Comparing animals given IgG to those given anti-CD1d, there was no difference in the number of splenic F4/80+ (macrophages), CD19+ (B cells), or CD11c+ (dendritic cells) cells (Fig. 5A). The same was true examining circulating APC numbers in whole blood or APCs found in liver homogenates (data not shown). Therefore, the 100 μg dose of anti-CD1dmAb used in these studies did not alter systemic APC numbers. Additionally, the overall health of the animals did not appear to be affected by anti-CD1d administration as their behavior, grooming, and weight did not change compared with animals receiving either IgG or no antibodies at all (data not shown).
FIG. 5.
Systemic immune consequences in IgG versus anti-CD1d treated mice. Uninjured mice were dosed with either IgG or anti-CD1d mAb; 24 h later, the animals were euthanized and splenocytes cell suspensions stained and analyzed by flow cytometry. Results are displayed as the percentage of live cells positive for the cell surface markers indicated (A). In separate experiments, uninjured mice were dosed with either IgG or anti-CD1d mAb; 24 h later, the animals were euthanized and whole blood collected via cardiac puncture. Serum was analyzed for the chemokines indicated by ELISA (B); n = 4 per group.
To determine if the changes in wound chemokine production were the result of increased global or circulating chemokine production, we also measured several serum cytokines and chemokines in unwounded animals 24 h after receiving injections of either anti-CD1d (100 μg) or IgG. Animals receiving anti-CD1d had equivalent serum chemokine levels as those receiving IgG (Fig. 5B). Similarly, the chemokine levels in normal, uninjured skin were equivalent between animals treated with IgG and anti-CD1d (Fig. 4). Hence, the increased local production of chemokines at the wound site was not the by-product of a systemic increase in these same chemokines due to anti-CD1d mAb administration alone.
Pre-Injury Blockade of NKT Cell Activation Reduced the Number of NKT Cells Infiltrating Cutaneous Wounds and Accelerated Wound Closure
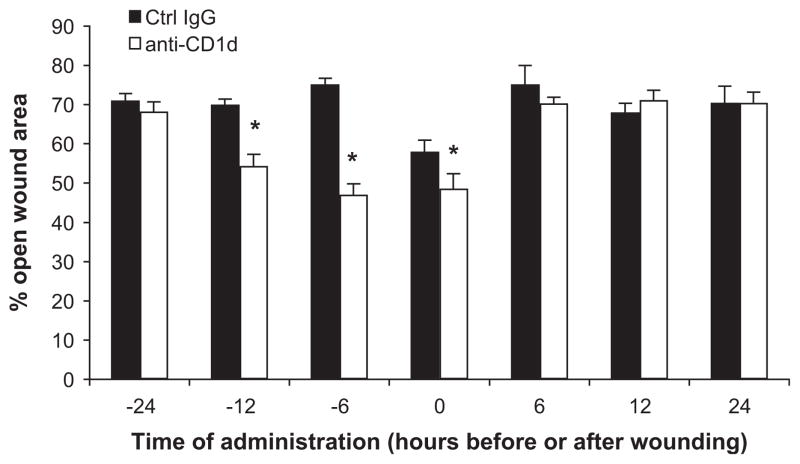
The experiments described thus far involved delivery of anti-CD1d or control IgG concomitantly with the excisional punch wound. Animals were wounded and immediately given these antibodies via tail vein injection such that the injury and CD1d blockade were evolving simultaneously. We next altered the timing of antibody delivery to better define the period during which NKT cells become activated and to pinpoint the optimal intervention time to achieve the greatest acceleration in wound closure. Given the early appearance of NKT cells in cutaneous wounds (12 h), and the short half-life of the antibodies, we examined dosing schedules within 24 h of injury (6, 12, and 24 h before and after the injury). To allow enough time for the cells of interest to infiltrate the wounds and for wound closure to begin occurring, we chose to examine cellular infiltrates and wound closure at 3 d post-wounding. The greatest effect on wound closure occurred when animals received anti-CD1d before rather than after wounding (Fig. 6). Specifically, a 12 h pretreatment resulted in wounds from animals treated with CD1d blocking antibody had 22.3% less open area than wounds from animals treated with the control IgG (Fig. 6). A 6-h pretreatment resulted in the most dramatic differences with wounds from animals receiving the anti-CD1d mAb 37.3% smaller than wounds from animals receiving IgG at the same time (Fig. 6). Antibody delivery immediately following the injury (time 0) resulted in a modest difference with wounds from animals treated with anti-CD1d having 48.7% open wound area, while wounds from animals given IgG had 58.0% open area (Fig. 6). Administration of these antibodies 24 h prior to wounding resulted in no differences in wound size between IgG versus anti-CD1d treated groups as did antibody administration after wounding (Fig. 6).
FIG. 6.
Comparison of pre and post-wounding treatment with IgG or anti-CD1d. Mice were each given six 3 mm dorsal punch excisional wounds. At the times indicated, parallel groups of mice received either anti-CD1d mAb (100 mcg) or control IgG (i.v.). Time 0 groups received antibodies immediately following punch wounding. Three days post-injury, pelts were removed and wounds were photographed. The percent of original open wound area was compared between groups; n = 4 per group. *P < 0.05. Similar results were obtained from two separate experiments.
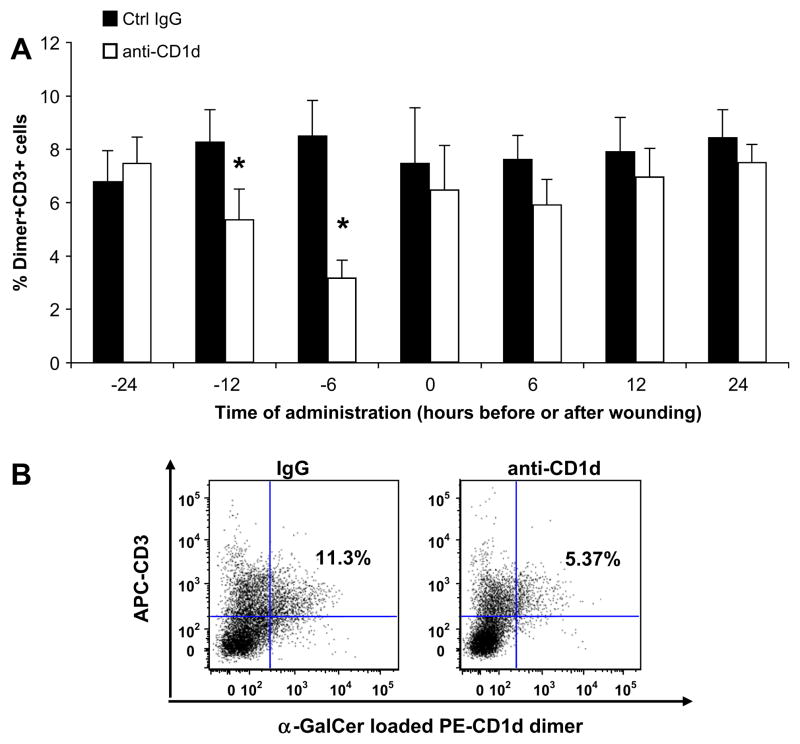
When examining the effect of the various dosing schedules on cellular infiltrates, we noticed a striking relationship between pre-wounding CD1d blockade and wound NKT cell content. Animals receiving anti-CD1d at 12 h before injury had d 3 wounds with 35.0% fewer NKT cells than animals that received the control IgG (Fig. 7). The most dramatic reduction in NKT cell number occurred when animals received anti-CD1d 6 h before injury; these mice had wounds with 62.5% fewer NKT cells than wounds from animals treated with the control IgG antibody at the same time (Fig. 7B). Wounds from animals given IgG or anti-CD1d 24 h before injury demonstrated equivalent NKT cell numbers, as did wounds from animals that received the antibodies after the injury (Fig. 7A). Altering the time of CD1d blockade did not change the neutrophil, macrophage, or overall T cell counts in these wounds (data not shown). Therefore, anti-CD1d administration up to 12 h before, but not after wounding, resulted in smaller wound areas and fewer infiltrating NKT cells at d 3. The greatest difference in both wound area and NKT cell number occurred when anti-CD1d was delivered 6 h prior to wounding. We therefore chose to use the 6-h pretreatment schedule for our next studies. While pretreatment does not translate into a clinically useful wound healing therapy, we pursued this line of investigation to reveal potential mechanisms behind NKT cell recruitment to cutaneous wounds.
FIG. 7.
Wound NKT cell content with varied administration times of IgG or anti-CD1d. Mice were each given six 3 mm dorsal punch excisional wounds. At the times indicated before or after wounding, parallel groups of mice received either anti-CD1d mAb or control IgG (i.v.). Three days post-injury, wounds were excised, and wound cell suspensions were stained and analyzed by flow cytometry. Results are expressed as the percentage of wound lymphocytes that are NKT cells (A), as determined by the number of Dimer+ CD3+ cells (B). The dot plot is representative of the 6 h pretreatment groups (B). n = 4 mice per group. *P < 0.05. Similar results were obtained from three separate experiments. (Color version of figure is available online.)
Activated NKT Cells Are Found in Circulation and at the Wound Site Early After Injury
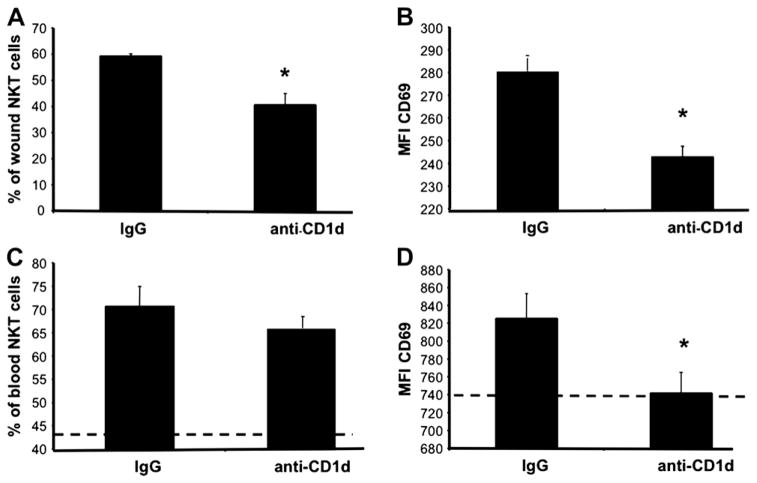
CD69 is part of the natural killer cell gene complex family of cell surface receptors with a C-type lectin binding domain [37]. It is found on the surface of T lymphocytes, including NKT cells, and its expression increases upon triggering the TCR complex with antigen [7, 38]. Our previous work characterized the kinetics of NKT cell infiltration to cutaneous wounds [4]. NKT cells arrived as early as 12 h post-wounding and reached maximal numbers by d 1, comprising approximately 5% of wound cells at that time, and NKT cell content remained at this level through 3 d post-injury [4]. Their role within the wound appeared regulatory in nature since blockade of NKT cell activation (via systemic anti-CD1d mAb) transiently enhanced the local production of a chemokine subset (Fig. 4) and accelerated wound closure (Figs. 1–3). As an additional measure of our anti-CD1d blockade and to support the notion that cutaneous injury causes CD1d mediated ligation of the invariant TCR, we examined wound NKT cell surface expression of CD69 after anti-CD1d treatment. We hypothesized that if antibody delivery indeed prevented NKT cell activation, then CD69 expression would be attenuated. Day one wounds from animals pretreated with anti-CD1d contained an NKT cell population with 34.97% fewer CD69+ cells than animals receiving control IgG (Fig. 8A). Relative expression, or mean fluorescence intensity (MFI), of CD69 was 15.95% less among CD69+ NKT cells in wounds from animals treated with anti-CD1d compared with the CD69+ NKT cell population found in wounds from animals treated with control IgG (Fig. 8B). Therefore, blocking NKT cell activation decreased both the number of CD69+ NKT cells and the relative expression of CD69 on NKT cells found at the wound site, making it unlikely that the alterations in wound closure and local inflammatory mediators seen after anti-CD1d treatment are nonspecific effects of the rat anti-mouse antibody treatment.
FIG. 8.
Day 1 wound and circulating NKT cell activation status in IgG versus anti-CD1d treated mice. Parallel groups of mice received either anti-CD1d mAb or control IgG (i.v.). Six hours later, all mice were given six 3 mm dorsal punch excisional wounds. One day post injury, wounds were excised, and wound cell suspensions were stained and analyzed by flow cytometry (A), (B). Blood was also collected from each animal. After red cell lysis, cells were stained and analyzed by flow cytometry (C), (D). Data is represented as the percentage of NKT cell population that is CD69+ (A), (C) and the mean fluorescence intensity (MFI) of CD69 (B), (D). The dashed lines (C), (D) represent the percentage of CD69+ circulating NKT cells (C), and the MFI of CD69 on circulating NKT cells (D) in uninjured animals; n = 4 mice per group. Similar results were obtained from two separate experiments. *P < 0.05.
Since anti-CD1d administration modulated wound NKT cell CD69 expression, we hypothesized that anti-CD1d therapy would not influence the CD69 expression on NKT cells found in circulation (peripheral blood) at the same time point (1 d post-wounding), as these cells had not yet become activated. While there was no statistical difference in the percentage of CD69+ NKT cells in circulation between IgG and anti-CD1d treated animals (Fig. 8C), there was, however, a modest decrease (11.28% less) in CD69 expression on circulating NKT cells from the anti-CD1d treated animals compared with the IgG treated animals (Fig. 8D). Nevertheless, anti-CD1d treatment reduced CD69 expression to levels found on circulating NKT cells from uninjured animals (Fig. 8D) even though the overall percentage of CD69+ NKT cells was still far greater than that found in uninjured mice (Fig. 8D). Thus, cutaneous injury induced CD69 expression on NKT cells in circulation and those found at the wound site. Moreover, NKT cell activation both at the wound site and in the circulation was modulated by anti-CD1d administration.
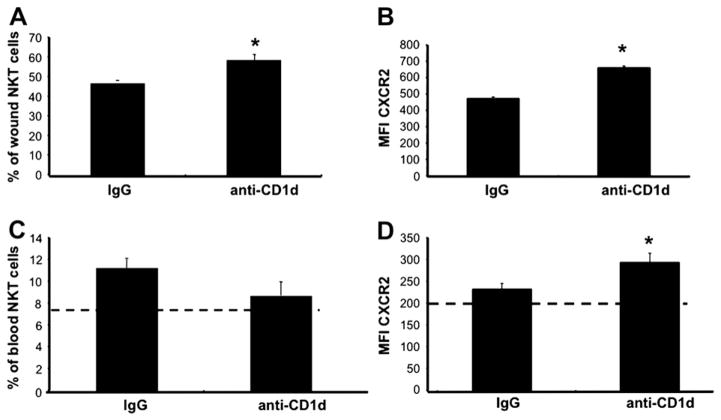
Prevention NKT Cell Activation Altered Their Surface Expression of CXCR2 After Cutaneous Injury
Little is known about NKT cell chemotaxis to sites of cutaneous inflammation, but they are known to both produce and respond to the CXC chemokine MIP-2. NKT cells also express the CXCR2 receptor [26, 27]. We therefore hypothesized that the CXC chemokines classically associated with early wound inflammation would be one of many potential signals attracting circulating NKT cells to cutaneous wounds. Since the CXC receptors are G-protein linked receptors that exhibit desensitization and down-regulation after chemokine binding [39], we first compared the proportion of NKT cells expressing CXCR2 in various compartments (blood and spleen) from uninjured animals to the NKT cell population found in wounds as a baseline measure. A much greater proportion of the NKT cell population in the wound expressed the CXCR2 receptor compared with circulating or splenic NKT cells in uninjured animals (47.78% ± 0.49% versus 7.43% ± 1.13% and 12.71% ± 1.78%, respectively, data not shown). We lacked any means of directly comparing relative expression (MFI) between wound, peripheral blood, and spleen since the cells are obtained and processed by three different methods (enzymatic digestion and tissue dispersion, red cell lysis, and mechanical dissociation with red cell lysis, see the Materials and Methods section).
In contrast to our findings with CD69, pretreatment with anti-CD1d increased the percentage of CXCR2+ NKT cells within the wound by 24.70% compared with the NKT cell population found in wounds from animals treated with the control antibody (Fig. 9). Similarly, the relative expression of CXCR2 is 35.59% greater on wound NKT cells found in animals pretreated with anti-CD1d compared with wound NKT cells found in animals pretreated with IgG (Fig. 9B). Therefore, blockade of NKT cell activation appeared to alter surface expression of CXCR2 on NKT cells found at the wound site.
FIG. 9.
Wound and circulating NKT cell CXCR2 expression. Parallel groups of mice received either anti-CD1d mAb or control IgG (i.v.). Six hours later, all mice were given six 3mmdorsal punch excisional wounds. One day post injury, wounds were excised, and cell suspensions were stained and analyzed by flow cytometry (A), (B). Blood was also collected from each animal. After red cell lysis, cells were stained and analyzed by flow cytometry (C), (D). Data is represented as the percentage of NKT cell population that is CXCR2+ (A), (C) and the mean fluorescence intensity (MFI) of CDXCR2 (B), (D). The dashed lines (C), (D) represent the percentage of CXCR2+ circulating NKT cells (C), and the MFI of CXCR2 on circulating NKT cells (D) in uninjured animals; n = 4 mice per group. Similar results were obtained from two separate experiments. *P < 0.05.
As in our studies with CD69, we then asked if preventing NKT cell activation altered circulating NKT cell CXCR2 expression 1 d after cutaneous injury. Anti-CD1d treatment did not alter the number of circulating CXCR2+ NKT cells (Fig. 9C). Relative expression of CXCR2, however, was 26.32% greater on circulating NKT cells from animals given the anti-CD1d antibody versus those treated with the control antibody (Fig. 9D). Preventing NKT cell activation, therefore, appears to alter the relative expression of the CXCR2 chemokine receptor on NKT cells found both in the wound and in circulation 24 h after cutaneous injury.
DISCUSSION
Following our previous studies with NKT cell-deficient mice, we demonstrated here that excisional cutaneous wounds exhibit accelerated closure when NKT cell activation is prevented by systemic (i.v.) administration of anti-CD1d mAb (Figs. 1 and 2) and that the effects of CD1d were dose-responsive (Fig. 3). This model therefore demonstrates that cutaneous wounding activates NKT cells via CD1d presentation of glycolipid antigen, and not any of the alternative modes of NKT cell activation (FcR engagement or direct binding of antigen to the invariant TCR). Just as when NKT cells are congenitally absent, preventing NKT cell activation leads to augmented local production of a key subset of neutrophil and monocyte macrophage chemokines (Fig. 4). Despite enhanced wound content of MIP-2, MCP-1, MIP-1α, and MIP-1β, inflammatory cell infiltrates in the wounds of IgG and anti-CD1d treated animals remained unchanged (Fig. 4 and Table 1). While somewhat surprising, these observations confirmed our previous observations of alterations in the local inflammatory response and acceleration of wound closure in mice in which NKT cells are functionally impaired or congenitally absent (CD1dko or Jα281ko) [4]. In other words, our earlier observations in knockout animals were not due to the compensatory response of an immune system lacking a regulatory element.
Increased levels of chemokines without a corresponding increase in inflammatory cell number may seem contradictory. This contradiction can be resolved when one considers that these chemoattractant molecules reach a level of saturation, and a healing wound may already be at or near the threshold for recruitment [39, 40]. Aside from leukocyte recruitment, the chemokines have a variety of alternative functions. In a healing wound, these include epithelialization, angiogenesis, and fibrosis [39, 41–43]. For example, the CXC chemokines such as MIP-2 have been associated with angiogenesis and re-epithelialization [42, 44], while the CC chemokines, MCP-1 and MIP-1α, contribute to extracellular matrix deposition and remodeling [45–47]. These alternative chemokine functions are consistent with accelerated wound closure, which we also observed when NKT cells are absent [4] or their activation prevented (Figs. 1 and 2).
We also identified the optimal dose and timing of anti-CD1d in a mouse system. A dose of 100 μg proved to be most effective as the higher dose, 200 μg, resulted in wound areas equivalent to those seen in animals given control IgG (Fig. 3). This higher dose also reduced the numbers of macrophages and B cells likely via an antibody-dependent cell-mediated cytotoxicity. Macrophages are essential for wound repair, so a reduction in their numbers might counterbalance any benefit gained from preventing NKT cell activation on wound closure [25, 48]. The schedule to achieve the greatest effect on wound area proved to be a 6-h pretreatment with anti-CD1d (Fig. 6). This probably reflects the very early timing of NKT cell activation and the interval needed for sufficient CD1d blockade at site(s) of NKT cell activation.
Interestingly, pretreatment also dramatically decreased wound NKT cell content (Fig. 7), indicating that ligation of the invariant TCR may influence NKT cell recruitment to sites of cutaneous injury. These findings were supported by the appearance of activated (CD69+) NKT cells both in the circulation and in wounds themselves early (24 h) after injury (Fig. 8). The appearance of activated (CD69+) NKT cells in circulation early after injury was unexpected since we hypothesized that the wound itself was the site of activation. This finding may imply that cutaneous injury revealed self glycolipid systemically or at sites distant to the skin. In addition, we believe that NKT cell activation is not singular in time or place. Cutaneous injury also resulted in elevated expression of CXCR2 on both wound and circulating NKT cells. CXCR2 expression remained high in animals treated with anti-CD1d, but was diminished in animals receiving the control IgG (Fig. 9), suggesting that ligation of invariant TCR may influence CXCR2 expression level or recycling. This is the first demonstration of such a phenomenon in NKT cells. These data suggest that an activation step via CD1d presentation of glycolipid antigen is required for NKT cells influence at the wound site, and that the events surrounding NKT cell activation are linked to their homing to sites of cutaneous injury. This information could not be gleaned from our earlier work with knockout models [4].
Blockade of NKT cell activation also reduced the numbers of NKT cells that infiltrated cutaneous wounds. In a murine model of renal ischemia-reperfusion injury, Li and colleagues similarly found that pretreatment with anti-CD1d likewise dramatically reduced the numbers of NKT cells that infiltrated the re-perfused kidney at 24 h, and protected the renal parenchyma from inflammatory damage [12]. Just as in our model of cutaneous injury, blockade of NKT cell activation with anti-CD1d reduced the number of infiltrating NKT cells and improved the overall post-injury outcome. It is unclear whether the salutary effects of preventing NKT cell activation in both settings result from simply a diminished local quantity of NKT cells alone, absent NKT cell function locally, or reduced NKT cell function systemically.
Regardless of the exact mechanism that benefits wound closure when NKT cell activation is blocked with anti-CD1d, it appears that NKT cell activation may assist NKT cell recruitment/homing to sites of inflammation. CXCR2 expression on circulating and wound NKT cells also displayed some degree of plasticity in response to blockade of NKT cell activation. Aside from CXCR2, NKT cells also express various receptors for inflammation-related chemokines, including CXCR3, CXCR4, CXCR6, CCR1, CCR2, and CCR5 [27, 49]. Others have described how NKT cell subsets exhibit differential expression of chemokine receptors [27, 50]. Furthermore, T cell phenotype (IL-4 versus IFN-γ producing) also influences chemokine receptor expression patterns. For example, it has been reported that CCR4 is expressed by almost all IL-4 producing CD4+ T cells, while CXCR3 is expressed by IFN-γ producing cells [51]. While many of these studies involve human lymphocyte subsets, it is intriguing that phenotype determines chemokine receptor expression profile since NKT cell phenotype is determined at the time of activation. Because we were able to modulate NKT cell CXCR2 expression patterns by blocking NKT cell activation, we favor the hypothesis that triggering the invariant TCR modulates the NKT cell’s homing machinery.
The site of NKT cell activation in this excisional punch wound model remains unknown. Qin and colleagues first suggested that only a small subpopulation of NKT cells express CXCR2 [52]. As Faunce and colleagues have shown, MIP-2 recruited NKT cells to the spleen, where they became activated [26]. In other words, chemokine recruitment preceded activation. Perhaps, ligation of the invariant TCR favors a net down-regulation of surface CXCR2. Therefore, when NKT cell activation is prevented, the NKT cell continues to recycle its CXCR2 receptors, resulting in greater CXCR2 expression at 1 d post-wounding (Fig. 8). Given the augmented local production of MIP-2 that also occurs with anti-CD1d administration, one might expect the higher ligand quantities to eventually result in less relative expression of the receptor at later time points because of the receptor desensitization and down-regulation [39, 40, 53]. Our laboratory is currently investigating this possibility as well as the connection between NKT cell activation and chemokine receptor expression.
We also found that CD69 expression by NKT cells in circulation was decreased by pretreatment with anti-CD1d. One potential explanation for these findings is that some subset of NKT cells encounters their endogenous glycolipid antigen prior to entering the wound site. NKT cell activation may not be singular in time or place. Since preventing NKT cell activation via anti-CD1d diminished expression of CD69 on circulating NKT cells, this suggests that cutaneous injury results in a systemic, rather than a purely local, revelation of glycolipid antigen and subsequent invariant TCR ligation. Although the exact mechanisms behind NKT cell regulation of wound inflammation requires further investigation, the studies presented here demonstrate the powerful therapeutic potential of anti-CD1d in achieving accelerated wound closure. In the clinical setting, achieving faster wound closure greatly benefits patients since it quells the nutritional and metabolic demands of the patient and ends an opportunity for infections. The last 30 y have seen abundant laboratory and clinical testing of the various soluble cytokines and growth factors important in wound repair in an attempt at accelerating wound closure [33, 54–56]. Among these, only one (platelet-derived growth factor) has been approved by the Food and Drug Administration (FDA) as a wound healing therapy [57]. Directing therapy at any single cytokine or growth factor may not overcome evolutionary redundancy and, alternatively, might grossly alter the microenvironment, thereby negating any potential benefit and even possibly driving the process toward pathologic or excessive healing. Instead, targeting a cell type such as the NKT cell rather than a soluble factor might enable a subtler, multi-faceted therapy, since the NKT cell functions by interacting with multiple cell types and secreting a myriad of soluble factors. Hence, a cell-based approach such as targeting NKT cell activation with anti-CD1d might advance an opportunity to more precisely alter the wound microenvironment in a manner that ultimately improves wound healing.
Acknowledgments
The authors are grateful to Ms. Patricia Simms for her assistance with flow cytometry. The studies presented here were supported by NIH R01 AI56108, NIH R21 AI073987, NIH T32 GM08750, and the Ralph and Marion C. Falk Medical Research Trust.
Footnotes
SUPPLEMENTARY DATA
Supplementary data associated with this article can be found in the online version, at doi: 10.1016/j.jss. 2010.03.030.
References
- 1.Petrie NC, Yao F, Eriksson E. Gene therapy in wound healing. Surg Clin N Am. 2003;83:597. doi: 10.1016/S0039-6109(02)00194-9. [DOI] [PubMed] [Google Scholar]
- 2.Frykberg RG, Armstrong DG, Giurini J, et al. Diabetic foot disorders: A clinical practice guideline. American college of foot and ankle surgeons. J Foot Ankle Surg. 2000;39:S1. [PubMed] [Google Scholar]
- 3.Harrington C, Zagari MJ, Corea J, et al. A cost analysis of diabetic lower-extremity ulcers. Diabetes Care. 2000;23:1333. doi: 10.2337/diacare.23.9.1333. [DOI] [PubMed] [Google Scholar]
- 4.Schneider D, Tulley JM, Palmer JL, et al. NKT cells participate in cutaneous wound repair. J Surg Res. 2009 doi: 10.1016/j.jss.2009.09.030. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendelac A, Lantz O, Quimby ME, et al. CD1 recognition by mouse NK+ T lymphocytes. Science. 1995;268:863. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 6.Kim HY, Kim S, Chung DH. Fc γRIII engagement provides activating signals to NKT cells in antibody-induced joint inflammation. J Clin Invest. 2006;116:2484. doi: 10.1172/JCI27219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinjo Y, Tupin E, Wu D, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 8.Godfrey DI, Konenberg M. Going both ways: Immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson MT, Johansson C, Olivares-Villagomez D, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. PNAS. 2003;100:10913. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moretta A, Poggi A, Pende D, et al. CD69-mediated pathway of lymphocyte activation: Anti-CD69 monoclonal antibodies trigger the cytolytic activity of different lymphoid effector cells with the exception of cytolytic T lymphocytes expressing T cell receptor α/β. J Exp Med. 1991;174:1393. doi: 10.1084/jem.174.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faunce DE, Gamelli RL, Kovacs EJ. A role for CD1d-NKTcells in injury-associated T cell supression. J Leukoc Biol. 2003;73:747. doi: 10.1189/jlb.1102540. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Huang L, Sung SJ, et al. NKT cell activation mediates neutrophil IFN-γ production and renal ischemia-reperfusion injury. J Immunol. 2007;178:5899. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- 13.Schneider DF, Glenn CH, Faunce DE. Innate lymphocyte subsets and their immunoregulatory roles in burn injury and sepsis. J Burn Care Res. 2007;28:365. doi: 10.1097/BCR.0B013E318053D40B. [DOI] [PubMed] [Google Scholar]
- 14.Joyce S. CD1d and natural T cells: How their properties jump-start the immune system. Cell Molec Life Sci. 2001;58:442. doi: 10.1007/PL00000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieuwenhuis EES, Matsumoto T, Exley MA, et al. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med. 2002;8:588. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- 16.Sonoda KH, Stein-Streilein J. CD1d on antigen-transporting APC and splenic marginal zone B cells promotes NKT cell-dependent tolerance. Eur J Immunol. 2002;32:848. doi: 10.1002/1521-4141(200203)32:3<848::AID-IMMU848>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 17.Porcelli SA, Modlin RL. The CD1 system: Antigen-presenting molecules for T cell recognition of lipids and glycolipids. Ann Rev Immunol. 1999;17:297. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 18.Exley MA, Garcia J, Balk SP, et al. Requirements for CD1d recognition by human invariant Vα24+ CD4− CD8− T cells. J Exp Med. 1997;186:108. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brossay L, Kronenberg M. Highly conserved antigen-presenting function of CD1d molecules. Immunogenetics. 1994;50:146. doi: 10.1007/s002510050590. [DOI] [PubMed] [Google Scholar]
- 20.Skold M, Behar SM. Role of CD1d-restricted NKT cells in microbial immunity. Infect Immun. 2003;71:5447. doi: 10.1128/IAI.71.10.5447-5455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein-Streilein J. Invariant NKT cells as initiators, licensors, and facilitators of the adaptive immune response. J Exp Med. 2003;198:1779. doi: 10.1084/jem.20031946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nickoloff BJ, Bonish BD, Huang BB, et al. Characterization of a T cell line bearing natural killer receptors and capable of creating psoriasis in a SCID mouse model system. J Dermatol Sci. 2000;24:212. doi: 10.1016/s0923-1811(00)00120-1. [DOI] [PubMed] [Google Scholar]
- 23.Hwang SJ, Kim S, Park WS, et al. IL-4 secreting NKT cells prevent hypersensitivity pneumonitis by suppressing IFN-γ producing neutrophils. J Immunol. 2006;177:5258. doi: 10.4049/jimmunol.177.8.5258. [DOI] [PubMed] [Google Scholar]
- 24.Kosaka H, Yoshimoto T, Fujimoto J, et al. Interferon-γ is a therpeutic target molecule for prevention of postoperative adhesion formation. Nat Med. 2008;14:437. doi: 10.1038/nm1733. [DOI] [PubMed] [Google Scholar]
- 25.Singer AJ, Clark RAF. Cutaneous wound healing. N Eng J Med. 1999;341:738. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 26.Faunce DE, Sonoda KH, Stein-Streilein J. MIP-2 recruits NKT cells to the spleen during tolerance induction. J Immunol. 2001;166:313. doi: 10.4049/jimmunol.166.1.313. [DOI] [PubMed] [Google Scholar]
- 27.Kim CH, Johnston B, Butcher EC. Trafficking machinery of NKT cells: Shared and differential chemokine receptor expression among Va24+VB11+ NKT cell subsets with distinct cytokine-producing capacity. Blood. 2002;100:11. doi: 10.1182/blood-2001-12-0196. [DOI] [PubMed] [Google Scholar]
- 28.Dovi JV, He L-K, DiPietro LA. Accelerated wound closure in neutrophil-delpeted mice. J Leukoc Biol. 2003;73:448. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 29.Tulley JM, Palmer JL, Gamelli RL, et al. Prevention of Injury-induced supression of T-cell immunity by the CD1d/NKT cell-specific ligand α-galactosylceramide. Shock. 2008;29:269. doi: 10.1097/shk.0b013e31811ff60c. [DOI] [PubMed] [Google Scholar]
- 30.Faunce DE, Palmer JL, Paskowicz KK, et al. CD1d-restricted NKT cells contribute ot the age-associated decline of T cell immunity. J Immunol. 2005;175:3102. doi: 10.4049/jimmunol.175.5.3102. [DOI] [PubMed] [Google Scholar]
- 31.Palmer JL, Tulley JM, Kovacs EJ, et al. Injury-induced supression of effector T cell immunity requires CD1d-positive APCs and CD1d-restricted NKT cells. J Immunol. 2006;177:92. doi: 10.4049/jimmunol.177.1.92. [DOI] [PubMed] [Google Scholar]
- 32.Greenhalgh DG. Models of wound healing. J Burn Care Rehabil. 2005;26:293. doi: 10.1097/01.bcr.0000169885.66639.b5. [DOI] [PubMed] [Google Scholar]
- 33.Jackson CJ, Xue W, Thompson P, et al. Activated protein C prevents inflammation yet stimulates angiogenesis to promote cutaneous wound healing. Wound Rep Regen. 2005;13:284. doi: 10.1111/j.1067-1927.2005.00130311.x. [DOI] [PubMed] [Google Scholar]
- 34.DiPietro LA, Polverini PJ, Rahbe SM, et al. Modulation of JE/MCP-1 expression in dermal wound repair. Am J Pathol. 1995;146:868. [PMC free article] [PubMed] [Google Scholar]
- 35.Krawczyk WS. A pattern of epidermal cell migration during wound healing. J Cell Biol. 1971;49:247. doi: 10.1083/jcb.49.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruffolo RR. Review important concepts of receptor theory. J Auton Pharmacol. 1982;2:277. doi: 10.1111/j.1474-8673.1982.tb00520.x. [DOI] [PubMed] [Google Scholar]
- 37.Testi R, D’Ambrosio D, DeMaria R, et al. The CD69 receptor: A multipurpose cell surface trigger for hematopoietic cells. Immunol Today. 1994;15:479. doi: 10.1016/0167-5699(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 38.Testi R, Phillips JH, Lanier LL. Leu23 induction as an early marker of functional CD3/T cell antigen receptor triggering: Requirement for receptor crosslinking, prolonged elevation of intracellular [Ca++] and activation of PKC. J Immunol. 1989;142:1854. [PubMed] [Google Scholar]
- 39.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513. [PubMed] [Google Scholar]
- 40.Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032. [PubMed] [Google Scholar]
- 41.Yamamoto T, Eckes B, Mauch C, et al. Monocyte chemoattractant protein-1 enhances gene expression and synthesis of matrix metalloproteinase-1 in human fibroblasts by an autocrine IL-1a loop. J Immunol. 2000;164:6174. doi: 10.4049/jimmunol.164.12.6174. [DOI] [PubMed] [Google Scholar]
- 42.Kulke R, Bornscheuer E, Schluter C, et al. The CXC receptor 2 is overexpressed in psoriatic epidermis. J Invest Dermatol. 1998;110:90. doi: 10.1046/j.1523-1747.1998.00074.x. [DOI] [PubMed] [Google Scholar]
- 43.Goede V, Brogelli L, Ziche M, et al. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer. 1999;19:2085. doi: 10.1002/(sici)1097-0215(19990827)82:5<765::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 44.Devalaraja RM, Nanney LB, Du J, et al. Delayed wound healing in CXCR2 knock out mice. J Invest Dermatol. 2000;115:234. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gharaee-Kermani M, Denholm EM, Phan SH. Costimulation of fibroblast collagen and transforming growth factor B1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem. 1996;271:17779. doi: 10.1074/jbc.271.30.17779. [DOI] [PubMed] [Google Scholar]
- 46.Gibran NS, Ferguson M, Heimbach DM, et al. Monocyte chemoattractant protein-1 mRNA expression in the human burn wound. J Surg Res. 1997;70:1. doi: 10.1006/jsre.1997.5017. [DOI] [PubMed] [Google Scholar]
- 47.Johnatty RN, Taub DD, Reeder SP, et al. Cytokine and chemokine regulation of proMMP-9 and TIMP-1 production by human peripheral blood lymphocytes. J Immunol. 1997;158:2327. [PubMed] [Google Scholar]
- 48.Leibovich SJ, Ross R. The role of the macrophage in wound repair. Am J Pathol. 1975;78:71. [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas SY, Runhua H, Boyson JE, et al. CD1d-restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. J Immunol. 2003;171:2571. doi: 10.4049/jimmunol.171.5.2571. [DOI] [PubMed] [Google Scholar]
- 50.Kim CH, Butcher EC, Johnston B. Distinct subsets of human Va24-invariant NKT cells: Cytokine responses and chemokine receptor expression. Trends Immunol. 2002;23:516. doi: 10.1016/s1471-4906(02)02323-2. [DOI] [PubMed] [Google Scholar]
- 51.Kim CH, Rott L, Kunkel EJ, et al. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin S, LaRossa G, Campbell JJ, et al. Expression of monocyte chemoattractant protein-1 and interleukin-8 receptors on subsets of T cells: Correlation with transendothelial chemotactic potential. Eur J Immunol. 1996;26:640. doi: 10.1002/eji.1830260320. [DOI] [PubMed] [Google Scholar]
- 53.Belperio JA, Keane MP, Arenberg DA, et al. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1. [PubMed] [Google Scholar]
- 54.Mooney DP, O’Reilly M, Gamelli RL. Tumor necrosis factor and wound healing. Annal Surg. 1990;211:124. doi: 10.1097/00000658-199002000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mills SJ, Ashworth JJ, Gilliver SC, et al. The sex steroid precursor DHEA accelerates cutaneous wound healing via the estrogen receptors. J Invest Dermatol. 2005;125:1053. doi: 10.1111/j.0022-202X.2005.23926.x. [DOI] [PubMed] [Google Scholar]
- 56.Rumalla VK, Borah GL. Cytokines, growth factors, and plastic surgery. Plastic Reconstruct Surg. 2001;108:719. doi: 10.1097/00006534-200109010-00019. [DOI] [PubMed] [Google Scholar]
- 57.Greenhalgh DG, Sprugel KH, Murray MJ, et al. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990;6:1235. [PMC free article] [PubMed] [Google Scholar]