Abstract
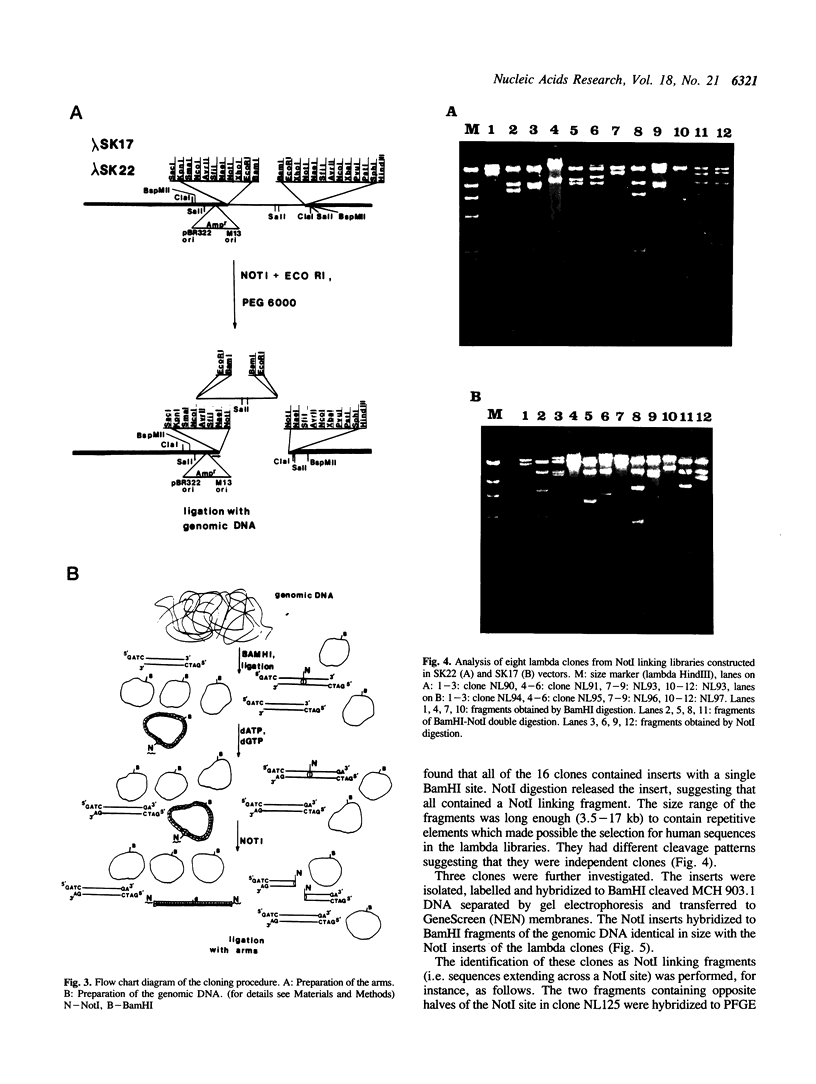
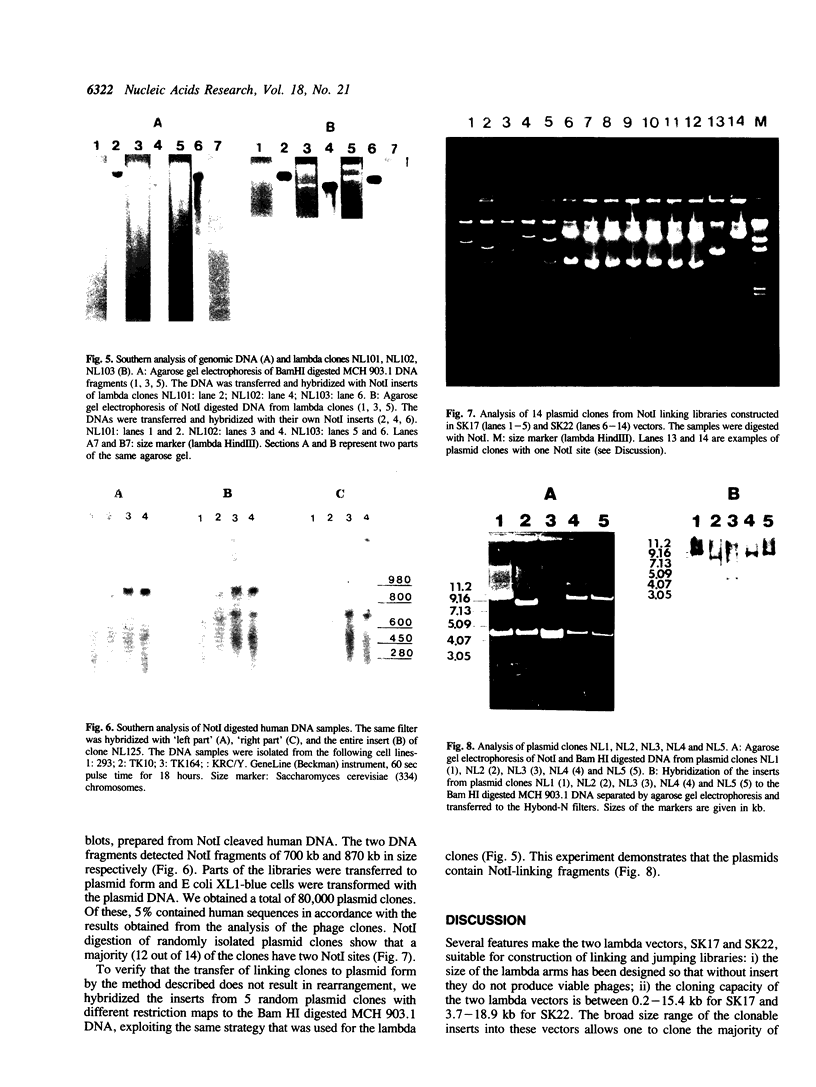
Two new diphasmid vectors (lambda SK17 and SK22) and a novel procedure to construct linking libraries are described. A partial filling-in reaction provides counter-selection against false linking clones in the library, and obviates the need for supF selection. The diphasmid vectors, in combination with the novel selection procedure, have been used to construct a chromosome 3 specific NotI linking library from a human chromosome 3/mouse microcell hybrid cell line. The application of the new vectors and the strong biochemical and biological selections resulted in a library of 60,000 NotI linking clones. As practically all of them are real NotI linking clones (no false recombinants) the library represents approximately 3,000 human recombinants (equal to 10-15 genomic equivalents of chromosome 3). Previously published methods for construction of linking libraries are compared with the procedure described in the present paper. The advantages of the new vectors and the novel protocol are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. C., McKnight T. D., Williams B. G. A simplified and efficient vector-primer cDNA cloning system. Gene. 1984 Nov;31(1-3):79–89. doi: 10.1016/0378-1119(84)90197-5. [DOI] [PubMed] [Google Scholar]
- Bear A., Clayman R. V., Elbers J., Limas C., Wang N., Stone K., Gebhard R., Prigge W., Palmer J. Characterization of two human cell lines (TK-10, TK-164) of renal cell cancer. Cancer Res. 1987 Jul 15;47(14):3856–3862. [PubMed] [Google Scholar]
- Boldog F., Erlandsson R., Klein G., Sumegi J. Long-range restriction enzyme maps of DNF15S2, D3S2, and c-raf1 loci on the short arm of human chromosome 3. Cancer Genet Cytogenet. 1989 Oct 15;42(2):295–306. doi: 10.1016/0165-4608(89)90098-8. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Weissman S. M. Directional cloning of DNA fragments at a large distance from an initial probe: a circularization method. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6812–6816. doi: 10.1073/pnas.81.21.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilee P., van den Broek M., Kuipers-Dijkshoorn N., Kolluri R., Khan P. M., Pearson P. L., Cornelisse C. J. At least four different chromosomal regions are involved in loss of heterozygosity in human breast carcinoma. Genomics. 1989 Oct;5(3):554–560. doi: 10.1016/0888-7543(89)90023-2. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hung M. C., Wensink P. C. Different restriction enzyme-generated sticky DNA ends can be joined in vitro. Nucleic Acids Res. 1984 Feb 24;12(4):1863–1874. doi: 10.1093/nar/12.4.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Sakaki Y. A novel procedure for selective cloning of NotI linking fragments from mammalian genomes. Nucleic Acids Res. 1988 Oct 11;16(19):9177–9184. doi: 10.1093/nar/16.19.9177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G., Erlandsson R., Boldog F., Ingvarsson S., Müller-Brechlin R., Klein G., Sümegi J. Consistent chromosome 3p deletion and loss of heterozygosity in renal cell carcinoma. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1571–1575. doi: 10.1073/pnas.85.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G., Frisch S. Clonal chromosome abnormalities in tumor cells from patients with sporadic renal cell carcinomas. Cancer Res. 1989 Feb 1;49(3):651–659. [PubMed] [Google Scholar]
- Minarovits J., Steinitz M., Boldog F., Imreh S., Wirschubsky Z., Ingvarsson S., Hedenskog M., Minarovits-Kormuta S., Klein G. Differences in c-myc and pvt-1 amplification in SEWA sarcoma sublines selected for adherent or non-adherent growth. Int J Cancer. 1990 Mar 15;45(3):514–520. doi: 10.1002/ijc.2910450324. [DOI] [PubMed] [Google Scholar]
- Naylor S. L., Johnson B. E., Minna J. D., Sakaguchi A. Y. Loss of heterozygosity of chromosome 3p markers in small-cell lung cancer. Nature. 1987 Oct 1;329(6138):451–454. doi: 10.1038/329451a0. [DOI] [PubMed] [Google Scholar]
- Pohl T. M., Zimmer M., MacDonald M. E., Smith B., Bucan M., Poustka A., Volinia S., Searle S., Zehetner G., Wasmuth J. J. Construction of a NotI linking library and isolation of new markers close to the Huntington's disease gene. Nucleic Acids Res. 1988 Oct 11;16(19):9185–9198. doi: 10.1093/nar/16.19.9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealey P. G., Whittaker P. A., Southern E. M. Removal of repeated sequences from hybridisation probes. Nucleic Acids Res. 1985 Mar 25;13(6):1905–1922. doi: 10.1093/nar/13.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seizinger B. R., Rouleau G. A., Ozelius L. J., Lane A. H., Farmer G. E., Lamiell J. M., Haines J., Yuen J. W., Collins D., Majoor-Krakauer D. Von Hippel-Lindau disease maps to the region of chromosome 3 associated with renal cell carcinoma. Nature. 1988 Mar 17;332(6161):268–269. doi: 10.1038/332268a0. [DOI] [PubMed] [Google Scholar]
- Wallace M. R., Fountain J. W., Brereton A. M., Collins F. S. Direct construction of a chromosome-specific NotI linking library from flow-sorted chromosomes. Nucleic Acids Res. 1989 Feb 25;17(4):1665–1677. doi: 10.1093/nar/17.4.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker P. A., Campbell A. J., Southern E. M., Murray N. E. Enhanced recovery and restriction mapping of DNA fragments cloned in a new lambda vector. Nucleic Acids Res. 1988 Jul 25;16(14B):6725–6736. doi: 10.1093/nar/16.14.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H., Maruiwa M., Sugihara S., Kojiro M., Noda S., Eto K. Establishment and characterization of a new human renal cell carcinoma cell line (KRC/Y). In Vitro Cell Dev Biol. 1988 Jan;24(1):9–16. doi: 10.1007/BF02623810. [DOI] [PubMed] [Google Scholar]
- Zabarovsky E. R., Allikmets R. L. An improved technique for the efficient construction of gene libraries by partial filling-in of cohesive ends. Gene. 1986;42(1):119–123. doi: 10.1016/0378-1119(86)90158-7. [DOI] [PubMed] [Google Scholar]
- Zabarovsky E. R., Turina O. V., Kisselev L. L. lambda SK9--a diphasmid for constructing genomic libraries, capable of converting inserts into plasmid and single stranded forms. Nucleic Acids Res. 1989 Jun 12;17(11):4407–4407. doi: 10.1093/nar/17.11.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabarovsky E. R., Turina O. V. Rapid isolation of lambda phage DNA in micro- and macro-variants. Nucleic Acids Res. 1988 Nov 25;16(22):10925–10925. doi: 10.1093/nar/16.22.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabarovsky E. R., Zagorskaya E. G., Kisselev L. L. lambda SK15--a diphasmid for construction expression cDNA libraries at BamHI and EcoRI sites with selection against non-recombinant and false recombinant phages. Nucleic Acids Res. 1989 Jun 12;17(11):4408–4408. doi: 10.1093/nar/17.11.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]