Abstract
Background
Colorectal cancer is one of the most frequent and lethal cancers. The aim of this study was to analyze the costs relating to treatment of colorectal cancer between Xelox and Folfox-4 at a regional level according to the clinical experience at an Italian hospital in Lombardy.
Methods
A cost analysis was carried out regarding resource consumption by patients suffering from colorectal cancer based on data collected over a 12-month period between 2010 and 2011. The analysis involved 40 patients who attended the Department of Medical Oncology and Hematology at Carlo Poma Hospital to undergo adjuvant therapy for colorectal cancer. A chart was created for each patient containing their medical history, their pharmacological therapy indicating the number and duration of chemotherapy cycles, dose in mg administered for each cycle, number of day hospital visits for each cycle, number of days spent in hospital to position the central vein catheter, type of infusion pump used, any subsequent supportive therapy, and any side effects and outpatient visits connected with side effects.
Results
The cost analysis shows the savings involved in using Xelox for a single cycle of treatment, ie, approximately €1414.00 per patient (53% compared with Folfox-4). For each single cycle of treatment, the savings generated by using capecitabine compared with 5-FU can be attributed mostly to the fact that oral administration of chemotherapy requires fewer resources and does not require use of a central vein catheter (approximately 70% of overall cost) which amply compensates for the higher cost of capecitabine compared with 5-FU-LV. Sensibility analysis confirms the results of the base-case scenario.
Conclusion
The results of our study indicate that infusion via a central vein catheter represents a significant cost, and that substitution with an oral therapy, even when associated with drugs administered intravenously, represents a consistent saving of hospital resources.
Keywords: colorectal cancer, capecitabine, oxaliplatin, 5-fluorouracil, Folfox-4, cost analysis, economic evaluation
Introduction
Colorectal cancer is one of the most common lethal cancers. In western countries, it is estimated that approximately 8% of all cancer deaths are caused by colorectal cancer, and standardized rates place Italy at a medium to low level for incidence (29.46 cases/100,000 per year) and mortality (12.32 deaths/100,000 per year) compared with the European population as a whole.1,2 Approximately 40%–60% of patients survive for 3–5 years after diagnosis, depending on where the cancer is located.3 Treatment of colorectal cancer includes surgery, chemotherapy, and radiotherapy, or a combination of these.4 Chemotherapy is the standard of care in the treatment of colorectal cancer, but the efficacy of the different schedules of chemotherapy varies depending on the combination of drugs administered. 5-Fluorouracil (5-FU) by infusion or pills has proven to be highly effective in the treatment of early or advanced colorectal cancer. In combination with folinic acid, it has increased the clinical response, and when associated with irinotecan or oxaliplatin, it has increased survival rates in patients.5,6 The guidelines suggest that 5-FU should preferably be administered by infusion.4,7 Capecitabine administered orally as a single therapy has also proved to be highly effective in the first-line treatment of metastatic colorectal cancer, showing lower toxicity levels and a notable reduction in side effects such as diarrhea, stomatitis, nausea, and neutropenia.8 Its efficacy, tolerability, and ease of administration make it suitable for use in colorectal cancer, particularly in older patients who are more exposed to the difficulties and inconvenience of frequent hospital visits to receive drugs intravenously which are often associated with a high risk of infection. These issues were an integral part of the 12-month evaluation using capecitabine + oxaliplatin (Xelox) and 5-FU + oxaliplatin (Folfox-4) in the treatment of colorectal cancer. Capecitabine is a new oral fluoropyrimidine carbamate selectively activated to 5-FU in tumor cells. It passes through the intestinal mucosal membrane intact and is subsequently activated by a cascade of three enzymes, resulting in the preferential release of 5-FU at the tumor site. Randomized clinical studies have supported the indications of capecitabine as monotherapy and in combination with other chemotherapy drugs for patients suffering from metastatic colorectal cancer as an alternative to 5-FU administered by infusion.7,9 Analyses carried out within the Italian health care system10,11 have demonstrated that differences in the methods of administering the two drugs, (by infusion or orally), and their toxicity profiles affect patient quality of life. In a situation where best practice regarding resources should be used with the aim of safeguarding health, evaluation of health technology is of crucial importance. In addition, we must also take into account clinical considerations and organizational and economic aspects, without forgetting the point of view of the patient, which extends the parameters used in analysis of the health environment to include the social context. From this perspective, pharmacological treatments must be examined within the context of the diagnostic, therapeutic, and care management program, given that the characteristics of the drugs modify the way in which treatment will be administered, which impacts organizational and economic aspects. The aim of this study was to compare costs relating to treatment between Xelox and Folfox-4 at a regional level, according to clinical experience at an Italian hospital in Lombardy.
Materials and methods
This was a retrospective study of 40 patients who underwent surgery for stage III colorectal cancer followed by adjuvant chemotherapy using Xelox or Folfox-4. The Xelox group comprised 13 men and seven women of mean age 58 (38–69) years. Fourteen patients had a performance status of 0 and six patients had a performance status of 1. The Folfox-4 group comprised 10 women and 10 men of mean age 59 (42–69) years. Sixteen patients had a performance status of 0 and four patients had a performance status of 1. Given that this was a very recent study, data on recurrence rate and relapse-free survival are not yet available. An analysis was carried out regarding resource consumption for patients suffering from colorectal cancer based on data collected over a 12-month period between 2010 and 2011. The analysis involved 40 patients who attended the Department of Medical Oncology and Hematology at Carlo Poma Hospital to undergo adjuvant therapy for colorectal cancer. A chart was created for each patient containing their medical history, pharmacological therapy, indicating the number and duration of the chemotherapy cycles, dose in mg administered for each cycle, number of day hospital visits for each cycle, number of days spent in hospital to position the central vein catheter, type of infusion pump used, any eventual supportive therapy, and any side effects and outpatient visits connected to side effects.
Consumption of resources
Using patient medical charts, the unit cost of each single drug and number of cycles administered, and their duration, the dose in mg for each cycle was calculated, yielding the cost per mg for each single drug used in the different cycles (Tables 1 and 2). The total cost of the different cycles administered was then estimated, to which the cost of administration was added, including the number of day hospital visits. The eventual use of a central vein catheter, complications arising from the central vein catheter, supportive therapy, diagnostic investigations, and control visits were counted and added to the total. Adverse effects relating to chemotherapy for both groups of treatment were taken on aggregate. Values attributed to the resources consumed were supplied by the Carlo Poma Hospital Administration Department. Information on standard therapeutic courses of treatment was collected, in particular on procedures relating to administration of chemotherapy, supportive therapy, and programmed control visits. Eventual day hospital visits were analyzed to exclude diagnosis-related group (DRG, inpatient tariff for hospital reimbursement) costs from the cost analysis to avoid costs being counted twice and to support further the results of the comparison study.
Table 1.
Unit costs of the resources considered in the analysis
| Treatment | Unit cost (per mg) |
|---|---|
| 5-FU | €0.0020 |
| LV | €0.0930 |
| Capecitabine | €0.0056 |
| Oxaliplatin | €3.7100 |
| Day hospital | €424.00 |
| Ambulatory visits (first/control visits) | €22.51 |
| Hospital admissions | €2203.00 |
| Day-surgery for CVC | €219.00 |
| Central venous catheter Portacath | €21.38 |
| Central venous catheter Groshon | €359.50 |
| Disposable baxter infusion elastomeric pump | €11.61 |
| ECG | €16.92 |
| Kit for CVC positioning | €10.80 |
Abbreviations: LV, Leucovorin; CVC, Central Vein Catheter.
Table 2.
Mean cycles and doses per patient in both arms
| XELOX | FOLFOX-4 | |
|---|---|---|
| Average cycles carried out | 7 | 10.75 |
| Cycle duration (days) | 21 | 14 |
| Average overall dose (mg) per cycle | ||
| Capecitabine | 3187.50 | |
| Oxaliplatin | 214.75 | |
| 5-FU | 3500.50 | |
| LV | 345.50 | |
| Oxaliplatin | 148.95 | |
Results
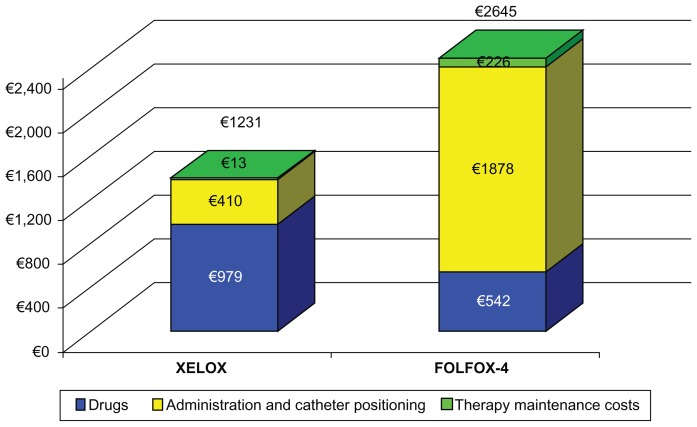
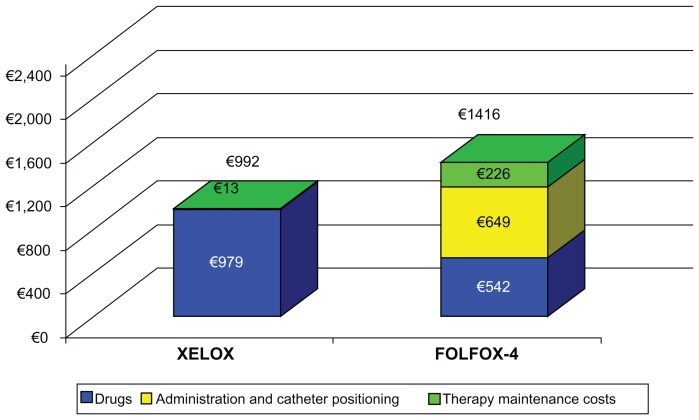
Table 3 shows the average cost per patient per cycle and overall duration of therapy for both treatment arms, highlighting the role of the resources involved. Calculations for this table were based on DRG tariffs, where available. The cost of all eventual equipment utilized and installation costs were added. Table 3 clearly shows the savings involved in using Xelox for a single cycle of treatment, which were approximately €1414.00 per patient (53% compared with Folfox-4). For each single cycle of treatment, the savings generated using capecitabine compared with 5-FU can mostly be attributed to the fact that oral administration of chemotherapy requires fewer resources and does not require use of a central vein catheter (approximately 70% of the overall cost) which amply compensates for the higher cost of capecitabine compared with 5-FU-LV. Figure 1 shows that the initial higher cost of the drug is offset by the savings resulting from less use of implants, catheters, and general maintenance of therapy. In order to strengthen our analysis, we hypothetically excluded DRG costs from the cost analysis. Once again, Xelox proved to be more advantageous, with a cost reduction of 30% compared with Folfox-4 (Figure 2).
Table 3.
Mean cost per patient by treatment and type of cost
| XELOX | FOLFOX-4 | |
|---|---|---|
| Drugs | ||
| 5-FU | – | €6.68 |
| LV | – | €30.57 |
| Capecitabine | €247.10 | – |
| Oxaliplatin | €731.93 | €504.31 |
| Total | €979.03 | €541.56 |
| Administration and catheter positioning | ||
| Positioning CVC | – | €648.89 |
| DRG and ambulatory visits | €410.01 | €1,229.03 |
| Total | €410.01 | €1,877.93 |
| Therapy maintenance costs | ||
| Extra visits | – | €79.93 |
| Support therapy | €13.06 | €126.01 |
| CVC complications | – | €19.98 |
| Total | €13.06 | €225.91 |
| Total per cycle | €1,402.10 | €2,645.40 |
| Difference | €1,243.31 | |
| Total per complete treatment | €9,814.68 | €28,438.08 |
| Difference | €18,623.40 | |
Figure 1.
Mean cost per patient by treatment and type of cost.
Figure 2.
Sensibility analysis excluding diagnosis-related group costs.
Discussion
This analysis clearly indicates that clinical choices made in oncology based on the efficacy of therapy have economic repercussions and that the appropriateness of treatment must necessarily take this aspect into account considering, above all, the efficacy and safety of the treatments analyzed. The availability of oral therapy represents a valid alternative in the medical treatment of colorectal cancer, which has already been demonstrated in terms of efficacy and safety but poorly quantified in economic terms in the international or Italian literature. This retrospective study assessed the health costs generated by patients who underwent two chemotherapy regimes, highlighting how administration costs can account for over 70% of costs involved in adjuvant chemotherapy.
From an economic point of view, the use of capecitabine means that medical staff spend less time administering therapy, which results in more efficient use of the day hospital which, according to information obtained from DRG costs, has a different cost in every region (from €345 to €44). However, Lombardy uses a lower tariff than other regions. Administration by infusion using a central vein catheter involves substantial costs, whereas replacing it with oral therapy combined with drugs administered intravenously results in savings in health resources for the hospital which translates into a reduction in the time that the medical staff spend administering therapy and more efficient use of the day hospital. From the point of view of patient quality of life, Xelox requires fewer visits and takes less time to administer than Folfox-4. This improves patient quality of life because it reduces the number of hospital visits. Oxaliplatin is administered as a 2-hour infusion on both schedules, but Folfox-4 also requires insertion of a central vein catheter (for a 2-day infusion of 5-FU) which can be upsetting for patients because it has a physical and a psychological impact and can lead to complications such as infections, bleeding, pneumothorax, and venous thromboembolism.
Our study is somewhat limited given that it was carried out in a single hospital with a relatively small number of patients. Therefore, these results cannot be considered representative of current clinical practice, even though the therapies and methods used reflect a certain standard in many oncological departments. It should also be noted that, in our experience, a central vein catheter has not resulted in infections or other complications related to central vein catheterization, which have been amply described in the literature. In centers with less experience, these complications could have a greater impact and could therefore lead to additional costs. However, taking the necessary precautions, use of capecitabine instead of 5-FU in the Xelox schedule has led to a reduction in hospital visits and adverse events, and a general improvement in patient quality of life, together with a reduction in the global cost of treatment compared with Folfox-4.
However, our results are in line with those of other European studies carried out in metastatic patients. An Italian study compared the costs for patients involved in a clinical study comparing capecitabine with 5-FU in single therapy, estimating a saving of €823 per patient over 6 months of treatment, with a cost driver represented by the cost of chemotherapy in the capecitabine arm and by infusion in the 5-FU arm.10,12 A recent retrospective study carried out in France estimated a saving of €2000–€7200, depending on the 5-FU chemotherapy regime.13 A recent report by the English Health Technology Assessment estimated a saving per patient for capecitabine compared with a modified de Gramont regime carried out in hospital of €5100.14 In conclusion, the results of our study indicate that drug infusion through a central vein catheter represents significant costs and that substitution with an oral therapy, even when associated with drugs administered intravenously, represents a consistent saving of resources for the hospital. It is our opinion that further studies should be carried out, with the aim of establishing this type of analysis as a guide for doctors and hospital administrators in their choices of health strategies.
Footnotes
Disclosure
The study was financially supported by Roche SpA Italy.
References
- 1.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18(3):581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 2.Rosso S, Spitale A, Balzi D, Franceschi S, Zanetti R. Estimate of cancer incidence in Italian regions, 2001. Epidemiol Prev. 2004;28(4–5):247–257. [PubMed] [Google Scholar]
- 3.Zanetti R, Falcini F, Simonato L, Vercelli M. Survival of cancer patients in Italy in the nineties: the importance of population based data. Epidemiol Prev. 2001;25(3 Suppl):1–8. [PubMed] [Google Scholar]
- 4.Barni S, Venturini M, Beretta GD, et al. Agreement between oncology guidelines and clinical practice in Italy: the ‘right’ program. A project of the Italian Association of Medical Oncology (AIOM) Ann Oncol. 2007;18( Suppl 6):179–184. doi: 10.1093/annonc/mdm252. [DOI] [PubMed] [Google Scholar]
- 5.Saltz LB, Cox JV, Blanke C, et al. for the Irinotrecan Study Group. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000;343(13):905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 6.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 7.Schmoll HJ, Cartwright T, Tabernero J, et al. Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: a planned safety analysis in 1,864 patients. J Clin Oncol. 2007;25(1):102–109. doi: 10.1200/JCO.2006.08.1075. [DOI] [PubMed] [Google Scholar]
- 8.Feliu J, Escudero P, Llosa F, et al. Capecitabine as first-line treatment for patients older than 70 years with metastatic colorectal cancer: an Oncopaz Cooperative Group study. J Clin Oncol. 2005;23(13):3104–3111. doi: 10.1200/JCO.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy J, Clarke S, Díaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26(12):2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 10.Giuliani G, Lucioni C, Mazzi S, De Carli C, Jamieson C. Economic evaluation of oral capecitabine vs intravenous 5-fluorouracil plus leucovorin (Mayo regimen) in the treatment of advanced colorectal cancer [Valutazione di convenienza economica comparata tra un farmaco orale (capecitabina) e una terapia parenterale a base di 5-FU (regime Mayo) nel trattamento del carcinoma del colon-retto metastizzato] PharmacoEconomics – Italian Research Articles. 2002;4(1):31–38. [Italian.] [Google Scholar]
- 11.Di Costanzo F, Ravasio R, Sobrero A, et al. Capecitabine versus bolus fluorouracil plus leucovorin (folinic acid) as adjuvant chemotherapy for patients with Dukes’ C colon cancer : economic evaluation in an Italian NHS setting. Clin Drug Investig. 2008;28(10):645–655. doi: 10.2165/00044011-200828100-00005. [DOI] [PubMed] [Google Scholar]
- 12.Lopatriello S, Amoroso D, Alabiso O, et al. The CAP-CR study: direct medical costs in Italian metastatic colorectal cancer patients on first-line infusional 5-fluorouracil or oral capecitabine. Eur J Cancer. 2008;44(17):2615–2622. doi: 10.1016/j.ejca.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Limat S, Bracco-Nolin CH, Legat-Fagnoni C, et al. Economic impact of simplified de Gramont regimen in first-line therapy in metastatic colorectal cancer. Eur J Health Econ. 2006;7(2):107–113. doi: 10.1007/s10198-006-0338-1. [DOI] [PubMed] [Google Scholar]
- 14.Ward S, Kaltenthaler E, Cowan J, Brewer N. Clinical and cost- effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation. Health Technol Assess. 2003;7(32):1–93. doi: 10.3310/hta7320. [DOI] [PubMed] [Google Scholar]