Abstract
Many women experience bothersome vasomotor and vaginal symptoms during the menopausal transition. Decreasing levels of estrogens during menopause are also associated with reduced bone density and an increased risk of osteoporosis. Combined estrogen/progestin therapy (hormone therapy) effectively treats menopausal symptoms and prevents bone loss, but has been associated with some safety and tolerability concerns. A novel menopausal therapy is the tissue selective estrogen complex, which pairs a selective estrogen receptor modulator with one or more estrogens. In preclinical studies, the tissue selective estrogen complex partnering bazedoxifene (BZA) with conjugated estrogens (CE) antagonized stimulation of breast and endometrial tissue, reduced vasomotor instability, and preserved bone mass in rat and mouse models. The specific attributes seen with BZA/CE were different from those observed with other selective estrogen receptor modulator/estrogen pairings. BZA/CE has undergone clinical evaluation in the Phase III Selective estrogens, Menopause, And Response to Therapy (SMART) trials in postmenopausal women with an intact uterus. Of the various doses of BZA/CE evaluated, BZA 20 mg/CE 0.45 mg and 0.625 mg were associated with a low incidence of endometrial hyperplasia (<1%) similar to placebo, and showed significant improvements in hot flushes and vulvar/vaginal symptoms and increases in bone mineral density. BZA 20 mg/CE 0.45 mg and 0.625 mg were associated with a low incidence of breast-related adverse events and demonstrated no difference from placebo in age-related changes in mammographic breast density. Both BZA/ CE doses showed a favorable tolerability profile, with no increases in uterine bleeding or breast tenderness, and had positive effects on metabolic parameters and quality of life. BZA/CE may be a promising alternative to hormone therapy for the treatment of menopausal symptoms and prevention of osteoporosis in nonhysterectomized postmenopausal women.
Keywords: tissue selective estrogen complex, bazedoxifene, conjugated estrogens, menopause, osteoporosis, vasomotor symptoms
Introduction
Postmenopausal women may experience a variety of symptoms associated with a decline in the level of estrogens.1 Vasomotor symptoms (VMS), such as hot flushes, are reported by 60%–85% of women during menopause.2 Vulvar/vaginal atrophy (VVA; symptoms of which may include vaginal dryness, irritation, soreness, or dyspareunia, and increases in urinary frequency, urgency, or incontinence) is reported by 50% of postmenopausal women.3 The menopausal transition may also accelerate the rate of bone loss, leading to susceptibility to postmenopausal osteoporosis and associated fractures.4–6 In addition to the burden of the reduced quality of life (QoL) imposed by menopausal symptoms, the financial costs of menopausal treatments, lost productivity, and increased health care expenses present a significant economic burden.7
Many menopausal therapies are designed to treat individual symptoms.5 Topical estrogens or estradiol preparations such as creams, pessaries, tablets, and vaginal rings have been shown to be effective for the treatment of VVA.8 Tibolone has been shown to effectively treat VMS and VVA.9 Bisphosphonates, selective estrogen receptor modulators (SERMs), parathyroid hormone, estrogens, and calcitonin are approved pharmacologic agents for the prevention and/ or treatment of postmenopausal osteoporosis.10
The most common treatments for postmenopausal women who experience moderate-to-severe VMS along with other menopausal symptoms are estrogens and combined estrogen/ progestin or estradiol/progestin therapy (hormone therapy [HT]).11 HT options include conjugated estrogens (CE)/ medroxyprogesterone acetate (MPA), sequential estrogens/ trimegestone, drospirenone/estradiol, estradiol/norethindrone, and transdermal estradiol/norethindrone acetate.5 In addition to effectively treating VMS and VVA, estrogen monotherapy and HT have also shown efficacy in preventing postmenopausal osteoporosis and are considered comprehensive treatment options.11 Although the addition of progestins to estrogens provides protection against endometrial stimulation for postmenopausal women with a uterus,12,13 HT has been associated with some safety and tolerability concerns, including cardiovascular risk, breast stimulation, and irregular vaginal bleeding.11,14,15
A novel therapy currently under investigation for the treatment of menopausal symptoms and the prevention of osteoporosis is the tissue selective estrogen complex (TSEC), which pairs a SERM with one or more estrogens.16 SERMs have estrogen receptor (ER) agonist or antagonist activities depending on the target tissue.17,18 The goal of TSEC is to combine the established efficacy of estrogens on VMS, VVA, and bone with the protective effects of a SERM on the uterus and breast.16 The first TSEC in clinical development partners bazedoxifene (BZA) with CE. BZA is a SERM that has demonstrated efficacy in the treatment and prevention of osteoporosis with a favorable endometrial/breast safety profile in postmenopausal women. This review provides an overview of the key preclinical findings for BZA/CE and describes the results from the Phase III clinical trials of BZA/CE in postmenopausal women with an intact uterus. The emerging role of TSEC in the current treatment paradigm for postmenopausal women is also discussed.
Preclinical studies with BZA/CE
In vitro studies
BZA/CE was developed with the goal of achieving an optimal balance of ER agonist and antagonist activities. Berrodin evaluated BZA/CE, raloxifene (RLX)/CE, and lasofoxifene (LAS)/CE in a multiplex biochemical assay.19 In this assay, BZA interacted with some cofactor peptides and not others, while LAS and RLX inhibited all of the peptides to which they were exposed. These results suggest that when combined with CE, BZA is more likely to exhibit tissue-selective effects than LAS or RLX.19 In a cell-based system of Michigan Cancer Foundation-7 (MCF-7) breast cancer cells, the global gene expression pattern associated with BZA/CE differed from that with RLX/CE or LAS/CE. The expression profile of BZA/CE was more similar to that with CE alone; in contrast, RLX/CE and LAS/CE had profiles that were similar to the SERM alone.19 In another study, the proliferation of MCF-7 cells was not induced by BZA/CE, although some proliferation was observed with RLX/CE and LAS/CE.20 These findings support a distinct activity profile for BZA/CE among other TSEC pairings in displaying agonist activity in some tissues and antagonist activity in others.19
In vivo studies
The effects of BZA, RLX, and LAS (all 3 mg/kg) combined with estradiol (1 μg/kg) on breast and uterine tissues have been evaluated in ovariectomized (OVX) mice.21 In this model, BZA, RLX, and LAS prevented estradiol-induced increases in uterine wet weight (P < 0.05 vs estradiol alone). BZA was a more effective antagonist of estradiol-induced increases in uterine wet weight than RLX or LAS (P < 0.05 vs estradiol/RLX or estradiol/LAS). In addition, treatment with BZA and RLX, but not LAS, reduced the estradiol-induced mammary gland end bud proliferation and all three SERMs significantly reduced the estradiol-induced expression of GPR105 and INDO mRNA markers of uterine and breast stimulation, respectively (P < 0.05 vs estradiol alone for both markers).21
In a study of mature OVX rats treated with BZA/CE daily for 6 weeks,16 the combination of BZA 3.0 mg/kg with CE 0.5, 1.0, or 2.5 mg/kg prevented CE-induced increases in uterine wet weight. Treatment with BZA 0.1, 0.3, 1.0, or 3.0 mg/kg/CE 2.5 mg/kg and BZA 3.0 mg/kg/CE 0.5, 1.0, 2.5, or 5.0 mg/kg resulted in higher proximal tibia total bone mineral density (BMD) than with vehicle control (P < 0.01). CE 10.0 mg/kg significantly reduced tail skin temperature versus vehicle control in the morphine-addicted rat model of vasomotor instability. Addition of BZA 0.3, 3.0, or 10.0 mg/kg to CE 10.0 mg/kg maintained this response (P < 0.05 vs vehicle control). BZA 3.0 mg/kg/CE 0.5, 1.0, 2.5, or 5.0 mg/kg was also associated with significant decreases in serum total cholesterol compared with vehicle control (P < 0.01).16
The endometrial and breast effects of BZA/CE were further evaluated in OVX sexually immature mice.22 The minimal doses of BZA, RLX, and LAS that prevented CE-induced increases in uterine wet weight were determined to be BZA 2 mg/kg, RLX 10 mg/kg, and LAS 2 mg/kg. At these doses, the combinations of BZA and RLX with CE 3 mg/kg were more effective than LAS 2 mg/kg/CE 3 mg/kg at preventing CE-induced increases in uterine wet weight (P < 0.05). BZA was a better antagonist of CE-induced breast stimulation than RLX or LAS (P < 0.05), as measured by mammary gland amphiregulin mRNA expression. In an analysis of mammary gland whole mounts using these same SERM/CE doses, BZA/CE reduced the number of ductal branch points, a measure of ductal tree complexity, to a level similar to that with vehicle control and significantly lower than RLX/CE or LAS/CE (P < 0.05).22
The skeletal effects of BZA/CE were evaluated in OVX rats treated daily for 1 year with BZA 0.3 mg/kg, CE 2.5 mg/kg, or BZA 0.1, 0.3, or 1.0 mg/kg/CE 2.5 mg/kg.23 BMD of the lumbar spine and right proximal femur was significantly increased at 1 year compared with OVX control for all doses of BZA/CE (P < 0.05). Similarly, trabecular BMD of the proximal tibia metaphysis was significantly increased at 1 year with all doses of BZA/CE compared with OVX control (P < 0.05). Histomorphometry evaluation showed that all doses of BZA/CE prevented the OVX-induced changes in static and dynamic parameters of the cortical compartment of the tibia and cancellous compartment of the L1 and L2 vertebrae. BZA 0.1, 0.3, and 1.0 mg/kg/CE 2.5 mg/kg reduced uterine wet weights in a dose-dependent manner. BZA 0.1, 0.3, and 1.0 mg/kg/CE 2.5 mg/kg were associated with significant decreases in serum total cholesterol compared with OVX control (P < 0.05).
Overall, preclinical studies have shown that BZA/CE effectively prevents stimulation of uterine and breast tissue in a variety of in vitro and in vivo models. BZA/CE has also been shown to preserve bone mass, reduce vasomotor effects, and reduce serum total cholesterol in OVX rats. These findings in preclinical models suggest that BZA/CE has the potential to effectively treat menopausal symptoms, prevent bone loss, and have favorable effects on the lipid profile while maintaining breast and endometrial safety in nonhysterectomized postmenopausal women. BZA/CE has been subsequently evaluated in a series of Phase III clinical trials.
Clinical studies with BZA/CE
The efficacy and safety of BZA/CE have been evaluated in multicenter, randomized, double-blind, placebo- and active-controlled, Phase III studies referred to as the Selective estrogens, Menopause, And Response to Therapy (SMART) trials (Table 1). These studies were conducted in postmenopausal women with an intact uterus, and assessed the efficacy, safety, and tolerability of BZA/CE in treating menopausal symptoms and preventing osteoporosis. Published reports of the Phase III studies of BZA/CE are described in this review.
Table 1.
Study designs for Phase III clinical trials of BZA/CE
| SMART-124–27 | SMART-228 | SMART-329 | |
|---|---|---|---|
| Duration | 2 years | 12 weeks | 12 weeks |
| Design | Randomized, double-blind, placebo-controlled, Phase III study | ||
| Treatments | BZA 10, 20, or 40 mg/CE 0.45 or 0.625 mg RLX 60 mg Placebo |
BZA 20 mg/CE 0.45 or 0.625 mg Placebo |
BZA 20 mg/CE 0.45 or 0.625 mg BZA 20 mg Placebo |
| Enrolled | Healthy, postmenopausal women with an intact uterus | ||
| Aged 40–75 years | Aged 40–65 years ≥7 moderate-to-severe hot flushes per day (or ≥50 per week) |
Aged 40–65 years ≥1 moderate-to-severe symptom of VV A |
|
| Primary endpoints | Incidence of endometrial hyperplasia | Frequency/severity of hot flushes | Four coprimary measures of VV A (proportion of vaginal superficial cells, proportion of parabasal cells, vaginal pH, most bothersome symptom) |
| Secondary endpoints | BMD, BTM, frequency/severity of hot flushes, measures of VV A, sleep, QoL | Sleep, QoL | Sexual function, satisfaction with treatment, QoL |
Abbreviations: BZA, bazedoxifene; CE, conjugated estrogens; SMART, Selective estrogens, Menopause, And Response to Therapy; RLX, raloxifene; VV A, vulvar vaginal atrophy; BMD, bone mineral density; BTM, bone turnover markers; QoL, quality of life.
The 2-year SMART-1 trial (n = 3544) was conducted to evaluate the incidence of endometrial hyperplasia at 1 year in generally healthy women aged 40 to 75 years.24–27 Other endpoints included the effects of BZA/CE on BMD, bone turnover markers, VMS, VVA, safety, metabolic parameters, uterine bleeding, and QoL. The treatments evaluated in this trial were BZA 10, 20, and 40 mg/CE 0.45 and 0.625 mg, RLX 60 mg, and placebo.
The SMART-2 trial (n = 332)28 evaluated the change from baseline in frequency and severity of hot flushes at 12 weeks in women aged 40 to 65 years who reported at least seven moderate-to-severe hot flushes per day (or ≥50 per week) at baseline. The SMART-3 trial (n = 664)29 evaluated changes in four measures of VVA (the proportion of vaginal superficial cells, the proportion of parabasal cells, vaginal pH, and severity of the most bothersome VVA symptom) in women aged 40 to 65 years who had at least one moderate-to-severe symptom of VVA at baseline. Secondary endpoints included effects on sleep (SMART-2 only), QoL, and safety. The treatments evaluated in these trials were BZA 20 mg/CE 0.45 and 0.625 mg, BZA 20 mg (SMART-3 only), and placebo.
Efficacy on menopausal symptoms
Vasomotor effects
The efficacy of BZA/CE on VMS was evaluated by measuring the frequency and severity of hot flushes in response to treatment. In the SMART-1 trial, vasomotor effects were assessed in a subset of women from the overall study population who had at least seven moderate-to-severe hot flushes per day (or ≥50 per week) at baseline (n = 216).24 At Week 12, treatment with all doses of BZA/CE significantly reduced the mean daily number of moderate and severe hot flushes from baseline (range, −51.7% to −85.7%) compared with placebo (−17.1%; P < 0.05). The mean change in hot flush severity from baseline to Week 12 was reduced for all doses of BZA/CE compared with placebo. These reductions were significant (P < 0.001) for BZA 10 and 20 mg/CE 0.45 and 0.625 mg. The significant treatment effects on hot flush frequency and severity with BZA 10 and 20 mg/CE 0.45 and 0.625 mg were maintained through 2 years.24
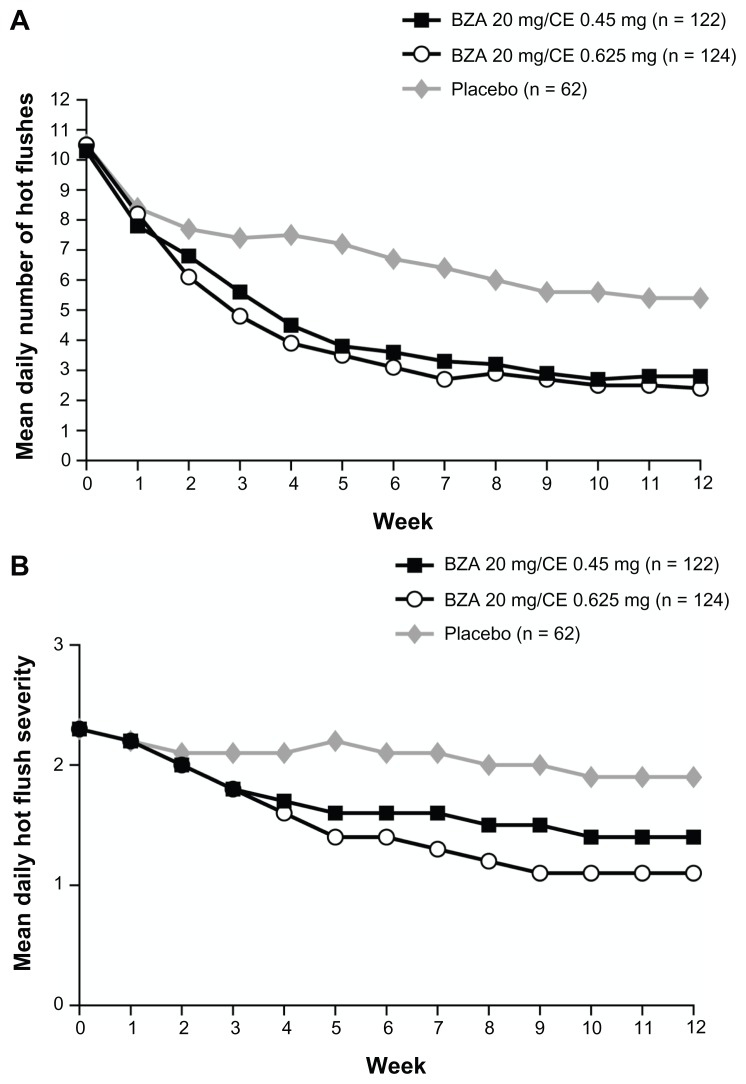
The SMART-2 trial evaluated the efficacy of VMS on a larger population of symptomatic women (n = 332). At Week 12,28 treatment with BZA 20 mg/CE 0.45 and 0.625 mg significantly reduced the number of hot flushes from baseline by 74% and 80%, respectively, compared to 51% with placebo (P < 0.001; Figure 1). The mean daily hot flush severity was also significantly reduced for BZA 20 mg/CE 0.45 and 0.625 mg compared with placebo at Week 12 (P < 0.001). With BZA 20 mg/CE 0.45 and 0.625 mg, 61% and 73% of women, respectively, had a 75% or greater decrease in the mean number of hot flushes than those treated with placebo (27%; P < 0.001) at Week 12.28
Figure 1.
The mean daily number (A) and severity (B) of moderate-to-severe hot flushes over 12 weeks in the SMART-2 trial.
Copyright © 2009, Wolters Kluwer Health. Reprinted with permission from Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause. 2009;16(6):1116–1124.
Abbreviations: SMART, Selective estrogens, Menopause, And Response to Therapy; BZA, bazedoxifene; CE, conjugated estrogens.
Effects on VV A
The efficacy of BZA/CE in treating VVA symptoms was evaluated by changes in the degree of maturation of the vaginal epithelium, measured by the proportion of superficial, intermediate, and parabasal cells on a vaginal smear.24 In the SMART-1 trial, VVA was assessed in a subset of women from the overall study population who had no more than 5% superficial cells at baseline (n = 1867).24 At Month 24, BZA 10 and 20 mg/CE 0.45 and 0.625 mg were associated with a significant increase in superficial cells compared with placebo (P < 0.001 for BZA 10 mg/CE 0.45 and 0.625 mg; P < 0.01 for BZA 20 mg/CE 0.625 mg). There was a significant increase in intermediate cells and a significant decrease in parabasal cells with BZA 10 and 20 mg/CE 0.45 and 0.625 mg compared with placebo (P < 0.001 for all). BZA 10 mg/CE 0.625 mg and BZA 20 mg/CE 0.45 and 0.625 mg were also associated with a lower incidence of dyspareunia (defined as pain during sexual intercourse) than placebo (P < 0.05) during Weeks 9 to 12.24
Similar to the subset within the SMART-1 trial, the SMART-3 trial enrolled women with at least one moderate-to- severe VVA symptom. BZA 20 mg/CE 0.45 and 0.625 mg significantly increased superficial cells (P ≤ 0.001), decreased parabasal cells (P ≤ 0.001), and increased intermediate cells (P < 0.01 for BZA 20 mg/CE 0.45 mg; P ≤ 0.001 for BZA 20 mg/CE 0.625 mg) compared with placebo at 12 weeks.29 Mean vaginal pH significantly decreased from baseline to Week 12 for both doses of BZA/CE (P < 0.001). With BZA 20 mg/CE 0.625 mg, but not BZA 20 mg/CE 0.45 mg, severity scores for the vaginal symptom originally identified by each woman as the most bothersome were significantly improved at Week 12 compared with placebo (P = 0.048). At Week 12, 81% of women treated with BZA 20 mg/CE 0.625 mg and 78% of women treated with BZA 20 mg/CE 0.45 mg had responded to treatment (defined as vaginal superficial cells >5%, vaginal pH <5, and/or improvement from baseline in most bothersome vaginal symptom) compared with 66% of women given placebo (P = 0.005 and P = 0.027, respectively).29
Bone effects
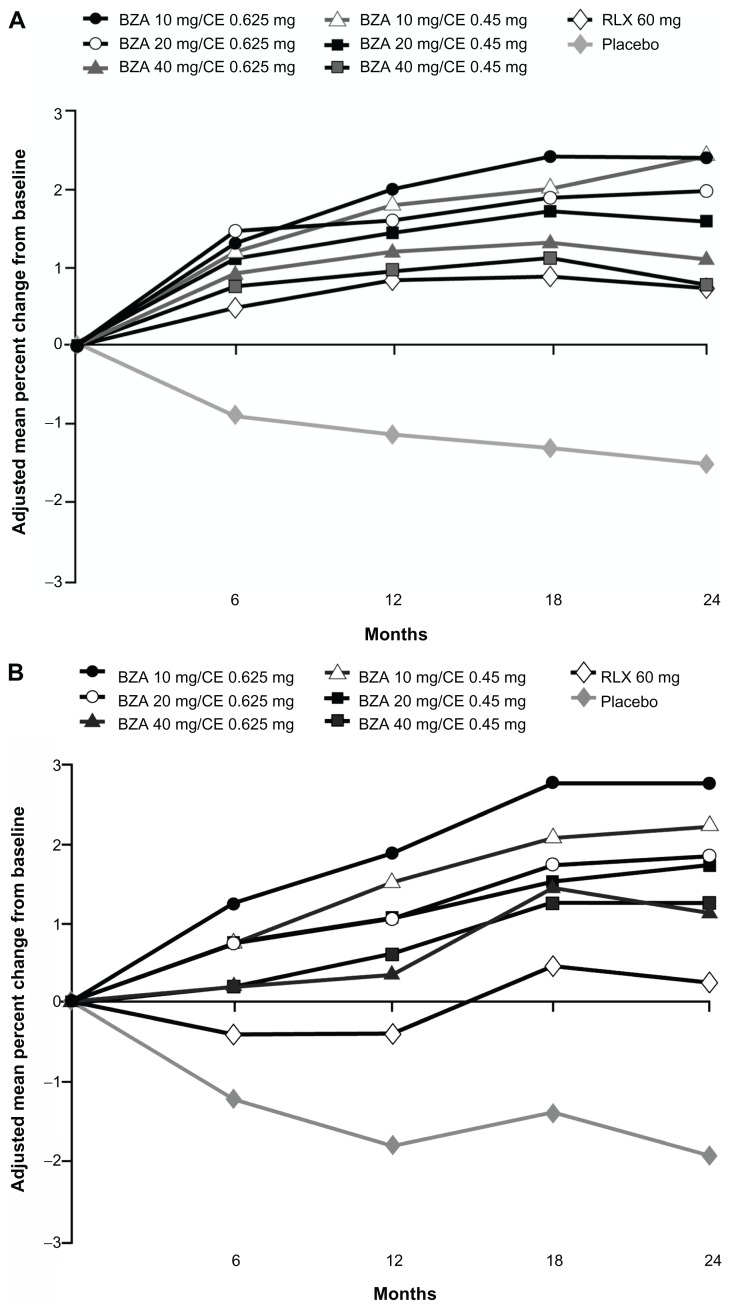
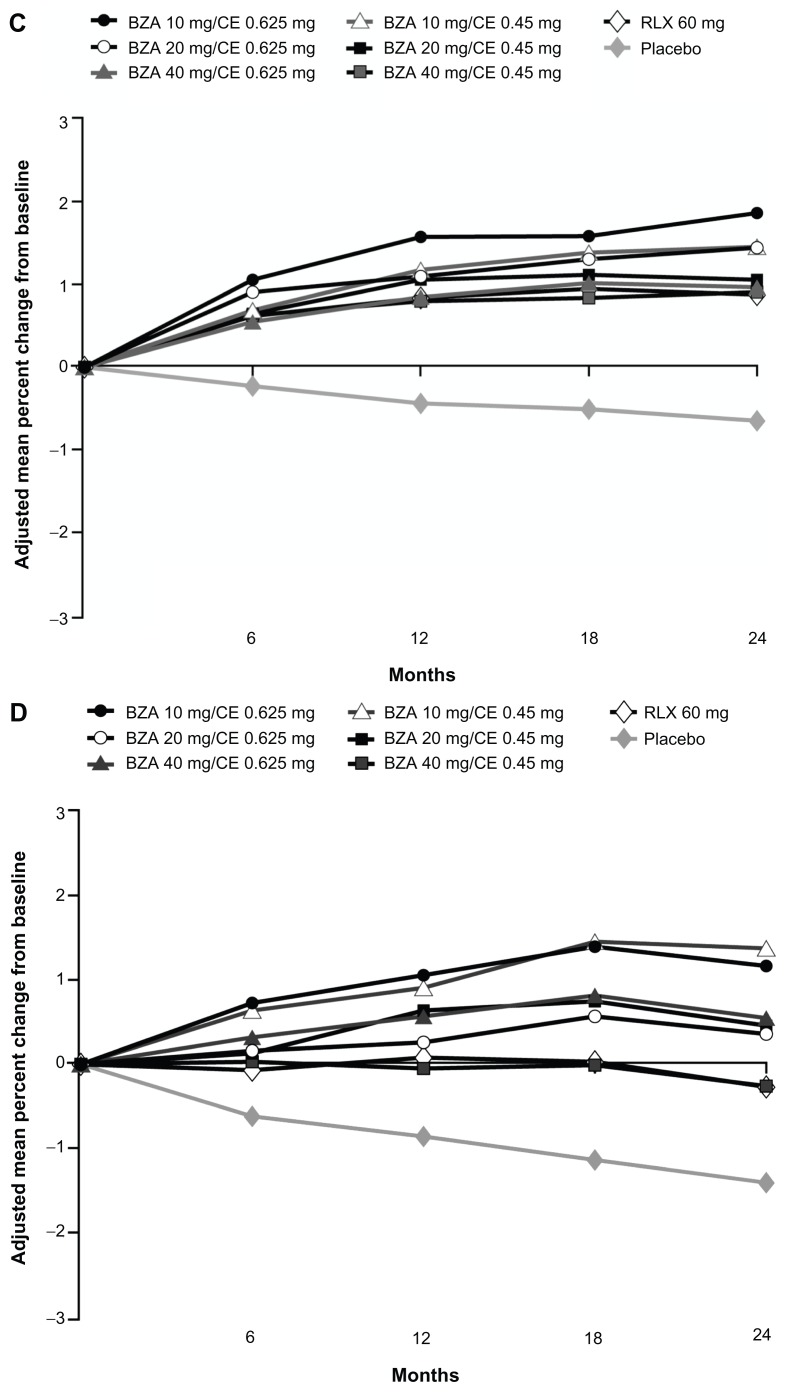
The efficacy of BZA/CE in preventing bone loss was evaluated by changes in BMD as measured by dual-energy X-ray absorptiometry; changes in bone turnover markers (surrogate markers of bone formation and bone resorption) were also assessed. Although there has been some variability associated with bone turnover marker measurements, these markers are widely used to evaluate bone metabolism and treatment response to bone-active therapies.30 Effects on BMD were evaluated in two substudies within the SMART-1 trial, in women who were between 1 and 5 years since menopause (YSM) and in those who were greater than 5 YSM. All doses of BZA/CE significantly increased lumbar spine and total hip BMD compared with placebo at 2 years in the SMART-1 trial (P < 0.001 for lumbar spine for all women, P < 0.01 for total hip in women 1–5 YSM, and P < 0.001 for total hip in women >5 YSM; Figure 2).26
Figure 2.
Adjusted mean percent change in BMD from baseline over 2 years in the SMART-1 trial.
Copyright © 2009, Elsevier. Reprinted with permission from Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045–1052.
Notes: Lumbar spine BMD was evaluated for women >5 YSM (A), or between 1 and 5 YSM (B); total hip BMD was evaluated for women >5 YSM (C), or between 1 and 5 YSM (D).
Abbreviations: BMD, bone mineral density; SMART, Selective estrogens, Menopause And Response to Therapy; YSM, years since menopause; BZA, bazedoxifene; CE, conjugated estrogens; RLX, raloxifene.
Changes from baseline in C-telopeptide and osteocalcin were measured in women who were between 1 and 5 YSM. At 2 years, the median percent change in levels of C-telopeptide (range, −42.72% to −53.40%) and osteocalcin (range, −19.78% to −28.05%) with all doses of BZA/CE were significantly reduced compared with placebo (−13.81% and 3.08%, respectively; P < 0.001 for all).26
Safety
Endometrial safety
In the SMART-1 trial, endometrial safety was assessed by the incidence of endometrial hyperplasia, which has been used as a surrogate marker for endometrial cancer.27 In the SMART-1 trial, endometrial biopsy results showed one case of endometrial hyperplasia (0.32%) with BZA 20 mg/CE 0.625 mg and no cases with BZA 20 mg/CE 0.45 mg or BZA 40 mg/CE 0.45 or 0.625 mg at 1 year. These hyperplasia rates were not significantly different from placebo or RLX 60 mg. With BZA 10 mg/CE 0.45 and 0.625 mg, there were three cases (0.94%) and 13 cases (3.81%) of endometrial hyperplasia, respectively. The 3.81% rate of endometrial hyperplasia with BZA 10 mg/CE 0.625 mg was determined to be unacceptable per study criteria. 27 As a result, further investigations of BZA/CE have focused on doses of BZA 20 mg/CE 0.45 and 0.625 mg.
Breast safety
High mammographic breast density has been shown to be a moderate independent risk factor for breast cancer.31,32 The effects of BZA/CE on age-related changes in mammographic breast density were evaluated in an ancillary study of the SMART-1 trial. Findings showed that the change in baseline breast density was similar for BZA 20 mg/CE 0.45 mg (−0.39%), BZA 20 mg/CE 0.625 mg (−0.05%), RLX 60 mg (−0.23%), and placebo (−0.42%) over 2 years of treatment.33 The incidence of breast cancer in the SMART-1 trial was low and similar for BZA 20 mg/CE 0.45 mg (n = 1), BZA 20 mg/CE 0.625 mg (n = 0), and placebo (n = 1) over 2 years.34 There was no significant difference in the incidence of abnormal mammograms for BZA 20 mg/CE 0.45 mg (4.4%), BZA 20 mg/CE 0.625 mg (4.2%), and placebo (2.6%).35,34 In a pooled analysis of data from the SMART-1, SMART-2, and SMART-3 trials, the incidence of breast-related adverse events (AEs) was low (<0.3%) and similar for BZA 20 mg/CE 0.45 mg and placebo (BZA 20 mg/CE 0.625 mg was not included in this analysis).35 There were no reports of fibrocystic breast disease or breast mass with BZA 20 mg/CE 0.45 mg or placebo in these three trials.35 Together, these results suggest that BZA/CE has a neutral effect on the breast.
General safety and tolerability
Among all the SMART trials, the overall incidence of treatment-emergent AEs was similar among groups.24,28,29 There were no significant differences between the BZA/CE and placebo groups in the percentage of women reporting breast pain in the SMART-1, SMART-2, and SMART-3 trials.24,28,29 In the SMART-1 trial, BZA 20 mg/CE 0.45 and 0.625 mg were associated with low rates of bleeding/spotting and high rates of amenorrhea over consecutive 4-week cycles (>83% during Cycles 1–13 and >93% during Cycles 10–13 during Year 1), with no significant differences compared with placebo.25
Venous thromboembolic events
In the SMART-1 trial, the incidence of venous thromboembolic events (VTEs) was 0.75 per 1000 woman-years for the combined BZA/CE treatment groups compared with 1.56 per 1000 woman-years for the placebo group (relative risk, 0.48; 95% confidence interval, 0.05–4.66). These VTEs included one report of deep vein thrombosis in each of the BZA 10 mg/CE 0.625 mg and BZA 40 mg/CE 0.625 mg groups and one case of deep vein thrombosis in the placebo group, as well as one case of pulmonary embolism with BZA 40 mg/CE 0.625 mg.24 No VTEs were reported in the SMART-2 or SMART-3 trials, but these studies were of 12-week duration.28,29
Lipid and coagulation parameters
Lipids
Women may experience adverse changes to the metabolic profile during the menopausal transition, including increases in total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, and lipoprotein (a) and decreases in high-density lipoprotein (HDL) cholesterol.36 The effects of BZA/CE on lipid parameters were evaluated in the SMART-1 trial. At Month 24, BZA 20 mg/CE 0.45 and 0.625 mg were associated with decreases from baseline in total cholesterol (P < 0.05 vs placebo for BZA 20 mg/ CE 0.45 mg) and LDL cholesterol (P < 0.01 vs placebo for both). HDL cholesterol increased from baseline (P < 0.01 vs placebo) with BZA 20 mg/CE 0.45 and 0.625 mg, as did triglyceride (P < 0.01), HDL-2 cholesterol (P < 0.001), and apolipoprotein A1 (P < 0.001) levels compared with placebo. BZA 20 mg/CE 0.45 and 0.625 also showed significant decreases from baseline in apolipoprotein B (P < 0.05) and lipoprotein (a) (P < 0.01) versus placebo.24
At Week 12 of the SMART-2 trial, both doses of BZA/CE were associated with significant decreases from baseline compared with placebo in total and LDL cholesterol. HDL cholesterol decreased from baseline over 12 weeks with placebo, but was maintained at baseline levels with BZA/ CE. Triglyceride levels significantly increased from baseline with BZA 20 mg/CE 0.45 and 0.625 mg and with placebo. The increase in triglycerides was clinically important for more women in the placebo group than in either BZA/CE group (P = 0.04).28
Both doses of BZA/CE were associated with significant decreases in LDL cholesterol (P < 0.01) at Week 12 in the SMART-3 trial. BZA 20 mg/CE 0.625 mg showed a small increase in triglyceride levels that was statistically significant compared with placebo (P < 0.05).29
Coagulation parameters
Changes in coagulation factors have been shown to occur during the menopausal transition, such as increases in levels of the coagulation proteins factor VII and fibrinogen.36 The effects of BZA/CE on coagulation parameters were evaluated in the SMART-1 trial. At Month 24, there were significant decreases from baseline in levels of fibrinogen (P < 0.001), protein S activity (P < 0.01), and antithrombin III activity (P < 0.05 and P < 0.01) for BZA 20 mg/CE 0.45 and 0.625 mg compared with placebo.24 Neither dose of BZA/CE affected partial thromboplastin time, prothrombin time, or D-dimer levels. There were small decreases from baseline with BZA 20 mg/CE 0.45 and 0.625 mg in plasminogen activator inhibitor-1 activity (P < 0.05 for both doses) and plasminogen activator inhibitor-1 antigen levels (P < 0.05 for BZA 20 mg/CE 0.625 mg). Plasminogen activity was significantly increased from baseline with BZA 20 mg/CE 0.45 and 0.625 mg (P < 0.001).24
Sleep, QoL, and satisfaction
During menopause, many women experience bothersome symptoms such as VMS, mood disturbances, or sexual dysfunction, which may negatively impact QoL.7 Sleep disruptions, including increased time to fall asleep, night-time awakenings, and daytime tiredness, are also common among postmenopausal women.37 The effects of BZA/CE on sleep parameters were evaluated in the SMART-1 trial using daily diaries and in the SMART-2 trial using the Medical Outcomes Study sleep scale. Impact on QoL measures was assessed in each SMART trial using the Menopause-specific Quality Of Life (MENQOL) questionnaire. In the SMART-3 trial, effects on sexual function were assessed using the Arizona Sexual Experiences scale. Satisfaction with treatment was evaluated in the SMART-2 and SMART-3 trials as measured by the Menopause Symptoms Treatment Satisfaction Questionnaire.
Sleep
In the SMART-1 trial, women treated with BZA 20 mg/ CE 0.45 and 0.625 mg had significant reductions in mean minutes to fall asleep (−11.6 and −10.9 minutes, respectively), increases in mean minutes slept (22.6 and 19.7 minutes, respectively), and increases in quality of sleep score (0.29 and 0.27, respectively) compared with placebo (−5.6 minutes, 10.1 minutes, and 0.13, respectively; P < 0.05) at 13 weeks.38 In a subset of women who had at least seven hot flushes per day at baseline (n = 81), BZA 20 mg/CE 0.45 and 0.625 mg improved mean minutes slept and quality of sleep score, but did not affect time to fall asleep (this analysis was not powered to show statistical significance).38 In the SMART-2 trial, BZA 20 mg/CE 0.45 and 0.625 mg significantly improved time to fall asleep, sleep disturbance, sleep adequacy, and overall sleep problems indexes I and II compared with placebo (P < 0.001 for all) at 12 weeks in the SMART-2 trial as assessed on the Medical Outcomes Study sleep scale.39
QoL
At Week 12 in the SMART-1 trial, BZA 20 mg/CE 0.45 and 0.625 mg significantly improved total (−0.7 and −0.8, respectively) and vasomotor function (−1.2 and −1.5, respectively) MENQOL scores compared with placebo (−0.5 for both domains; P < 0.001).40 Similar results were observed in the SMART-2 trial in which BZA 20 mg/CE 0.45 and 0.625 mg significantly improved total (−1.6 and −1.9, respectively) and vasomotor function (−3.3 and −3.8, respectively) MENQOL scores compared with placebo (−1.0 and −1.6, respectively; P < 0.001).39,40 In the SMART-3 trial, women treated with BZA 20 mg/CE 0.45 and 0.625 mg reported significant improvements in ease of lubrication on the Arizona Sexual Experiences scale (−0.82 and −0.85, respectively) compared with placebo (−0.50; P < 0.05). BZA 20 mg/CE 0.45 and 0.625 mg were also associated with significant improvements from baseline in the total (−1.09 and −1.18, respectively), vasomotor (−1.33 and −1.67, respectively), and sexual (−1.95 and −1.91, respectively) function compared with placebo (−0.67, −0.51, and −1.24, respectively; P < 0.001 for all).41
Treatment satisfaction
In the SMART-2 trial, Menopause Symptoms Treatment Satisfaction Questionnaire results showed that 73.5% of women treated with BZA 20 mg/CE 0.45 mg and 78.2% of women treated with BZA 20 mg/CE 0.625 mg were satisfied with treatment compared with 44.4% of women given placebo (P < 0.001). In particular, women treated with BZA 20 mg/CE 0.45 and 0.625 mg showed significantly higher satisfaction than those given placebo in the categories of ability to control hot flushes during the day (P < 0.001) and during the night (P < 0.001), effect on quality of sleep (P < 0.001), and effect on mood and emotions (P < 0.05).39 Similarly, in the SMART-3 trial, 62.6% of women treated with BZA 20 mg/CE 0.45 mg and 69.4% of women treated with BZA 20 mg/CE 0.625 mg were satisfied with treatment compared with 47.5% of women given placebo (P < 0.05 and P < 0.001, respectively). Significantly greater proportions of women treated with BZA 20 mg/CE 0.45 and 0.625 mg were satisfied with their ability to control hot flushes during the day (P < 0.001) and during the night (P < 0.001), effect on mood or emotions (P < 0.05), and effect on quality of sleep (P < 0.001) compared with placebo at 12 weeks.41
Conclusion
The TSEC is a novel menopausal therapy that has been developed to blend the uterine- and breast-protective effects of a SERM in women with a uterus with the established benefits of estrogens on VMS, VVA, and bone. TSECs, such as BZA/ CE, may be an appropriate alternative to HT for women with concerns about the potential safety risks of HT (combined estrogen/progestin or estradiol/progestin therapy) such as cardiovascular risk and breast stimulation.11,14,15 Tolerability concerns include breast pain and irregular vaginal bleeding.11
Preclinical studies showed that BZA/CE has favorable vasomotor effects and prevents bone loss in OVX rats while minimizing stimulation of uterine and breast tissue in a variety of in vitro and in vivo models. Furthermore, the tissue-selective effects of BZA/CE were distinct from those of RLX/CE or LAS/CE. BZA was shown to be a stronger antagonist of CE-induced breast cancer cell proliferation and mammary gland end bud formation than RLX or LAS. BZA and RLX were more effective in inhibiting CE-induced increases in uterine wet weight than was LAS.
In Phase III clinical studies, BZA 20 mg/CE 0.45 and 0.625 mg were shown to significantly reduce the frequency/ severity of hot flushes and improve VVA measures in symptomatic postmenopausal women. These BZA/CE doses also significantly increased BMD at the lumbar spine and total hip while preventing endometrial stimulation, as shown by low rates of endometrial hyperplasia similar to those with placebo.
Results of the SMART trials showed that treatment with BZA 20 mg/CE 0.45 and 0.625 mg maintained breast safety, with low rates of breast-related AEs and no changes in breast density. In contrast, HT use has been reported to increase mammographic breast density,42–44 and long-term estrogen plus progestin therapy has been associated with an increased risk of breast cancer.11 Study findings also showed that BZA/ CE has a favorable tolerability profile, with incidences of bleeding/spotting and breast tenderness that were low and similar compared with placebo. These results contrast those reported for HT, which has been associated with increased rates of bleeding/spotting as well as breast pain/tenderness compared with placebo.45 BZA/CE also showed positive effects on coagulation factors and improvements in lipid parameters in the SMART trials, including decreases in total and LDL cholesterol and increases in HDL cholesterol. These effects on blood lipids with BZA/CE have been shown to be similar to those observed with CE alone and with CE/MPA.46
BZA/CE has demonstrated significant improvements in sleep parameters, including time to fall asleep and overall sleep scores. Studies of HT have also shown improvements in sleep, although the effects were relatively small.47–50 BZA/CE also showed significant improvements in total and vasomotor function in all three SMART trials; in comparison, the effects of HT on QoL parameters have been mixed.47,48,51–53
Overall, BZA/CE has been shown to be generally safe and well tolerated over 2 years of treatment in postmenopausal women and is associated with high rates of treatment satisfaction. Collectively, these data suggest that BZA/CE is a promising option for the comprehensive treatment of menopausal symptoms and prevention of postmenopausal osteoporosis. Further studies are needed to determine the long-term efficacy and safety of BZA/CE in postmenopausal women.
Acknowledgments
Medical writing support for this manuscript was provided by Katie Gersh, PhD, of MedErgy, and was funded by Pfizer Inc. The authors retained full editorial control over the content of the article.
Footnotes
Disclosure
BSK and SM are employees of Pfizer Inc.
References
- 1.Burger HG. The endocrinology of the menopause. Maturitas. 1996;23(2):129–136. doi: 10.1016/0378-5122(95)00969-8. [DOI] [PubMed] [Google Scholar]
- 2.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nation. Am J Public Health. 2006;96(7):1226–1235. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85(1):87–94. doi: 10.4065/mcp.2009.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riggs BL, Khosla S, Melton LJ., III A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13(5):763–773. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 5.Lewis V. Undertreatment of menopausal symptoms and novel options for comprehensive management. Curr Med Res Opin. 2009;25(11):2689–2698. doi: 10.1185/03007990903240519. [DOI] [PubMed] [Google Scholar]
- 6.National Osteoporosis Foundation. Fast Facts on Osteoporosis. 2010. [Accessed November 17, 2011]. Available from: http://www.nof.org/node/40.
- 7.Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes. 2005;3:47. doi: 10.1186/1477-7525-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;4:CD001500. doi: 10.1002/14651858.CD001500.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Swanson SG, Drosman S, Helmond FA, Stathopoulos VM. Tibolone for the treatment of moderate to severe vasomotor symptoms and genital atrophy in postmenopausal women: a multicenter, randomized, double-blind, placebo-controlled study. Menopause. 2006;13(6):917–925. doi: 10.1097/01.gme.0000247016.41007.c9. [DOI] [PubMed] [Google Scholar]
- 10.North American Menopause Society. Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17(1):25–54. doi: 10.1097/gme.0b013e3181c617e6. [DOI] [PubMed] [Google Scholar]
- 11.North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17(2):242–255. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- 12.Voigt LF, Weiss NS, Chu J, Daling JR, McKnight B, van Belle G. Progestagen supplementation of exogenous oestrogens and risk of endometrial cancer. Lancet. 1991;338(8762):274–277. doi: 10.1016/0140-6736(91)90417-n. [DOI] [PubMed] [Google Scholar]
- 13.Weiderpass E, Adami HO, Baron JA, et al. Risk of endometrial cancer following estrogen replacement with and without progestins. J Natl Cancer Inst. 1999;91(13):1131–1137. doi: 10.1093/jnci/91.13.1131. [DOI] [PubMed] [Google Scholar]
- 14.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 15.Rolnick SJ, Kopher RA, DeFor TA, Kelley ME. Hormone use and patient concerns after the findings of the Women’s Health Initiative. Menopause. 2005;12(4):399–404. doi: 10.1097/01.GME.0000148644.55486.36. [DOI] [PubMed] [Google Scholar]
- 16.Kharode Y, Bodine PV, Miller CP, Lyttle CR, Komm BS. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149(12):6084–6091. doi: 10.1210/en.2008-0817. [DOI] [PubMed] [Google Scholar]
- 17.Komm BS, Lyttle CR. Developing a SERM: stringent preclinical selection criteria leading to an acceptable candidate (WAY-140424) for clinical evaluation. Ann N Y Acad Sci. 2001;949:317–326. doi: 10.1111/j.1749-6632.2001.tb04039.x. [DOI] [PubMed] [Google Scholar]
- 18.Komm BS, Kharode YP, Bodine PV, Harris HA, Miller CP, Lyttle CR. Bazedoxifene acetate: a selective estrogen receptor modulator with improved selectivity. Endocrinology. 2005;146(9):3999–4008. doi: 10.1210/en.2005-0030. [DOI] [PubMed] [Google Scholar]
- 19.Berrodin TJ, Chang KC, Komm BS, Freedman LP, Nagpal S. Differential biochemical and cellular actions of premarin estrogens: distinct pharmacology of bazedoxifene-conjugated estrogens combination. Mol Endocrinol. 2009;23:75–85. doi: 10.1210/me.2008-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang KCN, Wang Y, Bodine PV, Nagpal S, Komm BS. Gene expression profiling studies of three SERMs and their conjugated estrogen combinations in human breast cancer cells: insights into the unique antagonistic effects of bazedoxifene on conjugated estrogens. J Steroid Biochem Mol Biol. 2010;118(1–2):117–124. doi: 10.1016/j.jsbmb.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Crabtree JS, Peano BJ, Zhang X, Komm BS, Winneker RC, Harris HA. Activity of three selective estrogen receptor modulators on hormonedependent responses in the mouse uterus and mammary gland. Mol Cell Endocrinol. 2008;287(1–2):40–46. doi: 10.1016/j.mce.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Peano BJ, Crabtree JS, Komm BS, Winneker RC, Harris HA. Effects of various selective estrogen receptor modulators with or without conjugated estrogens on mouse mammary gland. Endocrinology. 2009;150(4):1897–1903. doi: 10.1210/en.2008-1210. [DOI] [PubMed] [Google Scholar]
- 23.Komm BS, Vlasseros F, Samadfam R, Chouinard L, Smith SY. Skeletal effects of bazedoxifene paired with conjugated estrogens in ovariectomized rats. Bone. 2011;49(3):376–386. doi: 10.1016/j.bone.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 24.Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/ conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril. 2009;92(3):1025–1038. doi: 10.1016/j.fertnstert.2009.03.113. [DOI] [PubMed] [Google Scholar]
- 25.Archer DF, Lewis V, Carr BR, Olivier S, Pickar JH. Bazedoxifene/ conjugated estrogens (BZA/CE): incidence of uterine bleeding in postmenopausal women. Fertil Steril. 2009;92(3):1039–1044. doi: 10.1016/j.fertnstert.2009.05.093. [DOI] [PubMed] [Google Scholar]
- 26.Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92(3):1045–1052. doi: 10.1016/j.fertnstert.2009.02.093. [DOI] [PubMed] [Google Scholar]
- 27.Pickar JH, Yeh I-T, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/ conjugated estrogens as a menopausal therapy. Fertil Steril. 2009;92(3):1018–1024. doi: 10.1016/j.fertnstert.2009.05.094. [DOI] [PubMed] [Google Scholar]
- 28.Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause. 2009;16(6):1116–1124. doi: 10.1097/gme.0b013e3181a7df0d. [DOI] [PubMed] [Google Scholar]
- 29.Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause. 2010;17(2):281–289. doi: 10.1097/GME.0b013e3181b7c65f. [DOI] [PubMed] [Google Scholar]
- 30.Dreyer P, Vieira JG. Bone turnover assessment: a good surrogate marker? Arq Bras Endocrinol Metabol. 2010;54(2):99–105. doi: 10.1590/s0004-27302010000200003. [DOI] [PubMed] [Google Scholar]
- 31.Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92(13):1081–1087. doi: 10.1093/jnci/92.13.1081. [DOI] [PubMed] [Google Scholar]
- 32.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 33.Harvey JA, Pinkerton JV, Baracat EC, Shi H, Mirkin S, Chines AA. Evaluation of changes in mammographic breast density associated with bazedoxifene/conjugated estrogens in postmenopausal women. Endocr Rev. 2011;32(3):P1–P79. [Google Scholar]
- 34.Pinkerton JA, Constantine GD, Komm BS, Yu H, Pickar JH. Breast effects of bazedoxifene/conjugated estrogens in a randomized, controlled trial of postmenopausal women. Poster presented at: The 19th Annual Meeting of the North American Menopause Society; September 24–27, 2008; Lake Buena Vista, FL, USA. 2008. Abstract P-46. [Google Scholar]
- 35.Pinkerton JV, Taylor H, Pan K, Chines A, Mirkin S. Breast parameters with bazedoxifene/conjugated estrogens in randomized, controlled trials of postmenopausal women. Menopause. 2010;17(6):1221–1222. [Google Scholar]
- 36.Spencer CP, Godsland IF, Stevenson JC. Is there a menopausal metabolic syndrome? Gynecol Endocrinol. 1997;11(5):341–355. doi: 10.3109/09513599709152559. [DOI] [PubMed] [Google Scholar]
- 37.Kronenberg F. Hot flashes: phenomenology, quality of life, and search for treatment options. Exp Gerontol. 1994;29(3–4):319–336. doi: 10.1016/0531-5565(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 38.Pinkerton JV, Chines AA, Racketa J, Mirkin S. Bazedoxifene/conjugated estrogens (BZA/CE): effect on sleep parameters in postmenopausal women. Menopause. 2010;17(6):1237–1238. [Google Scholar]
- 39.Utian W, Yu H, Bobula J, Mirkin S, Olivier S, Pickar JH. Bazedoxifene/ conjugated estrogens and quality of life in postmenopausal women. Maturitas. 2009;63(4):329–335. doi: 10.1016/j.maturitas.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Pinkerton JV, Chines AA, Racketa J, Mirkin S. Menopause-related quality of life and satisfaction in postmenopausal women treated with bazedoxifene/conjugated estrogens (BZA/CE) Menopause. 2010;17(6):1219. [Google Scholar]
- 41.Bachmann G, Bobula J, Mirkin S. Effects of bazedoxifene/conjugated estrogens on quality of life in postmenopausal women with symptoms of vulvar/vaginal atrophy. Climacteric. 2010;13(2):132–140. doi: 10.3109/13697130903305627. [DOI] [PubMed] [Google Scholar]
- 42.Topal NB, Ayhan S, Topal U, Bilgin T. Effects of hormone replacement therapy regimens on mammographic breast density: the role of progestins. J Obstet Gynaecol Res. 2006;32(3):305–308. doi: 10.1111/j.1447-0756.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 43.Martin LJ, Minkin S, Boyd NF. Hormone therapy, mammographic density, and breast cancer risk. Maturitas. 2009;64(1):20–26. doi: 10.1016/j.maturitas.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Freedman M, San Martin J, O’Gorman J, et al. Digitized mammography: a clinical trial of postmenopausal women randomly assigned to receive raloxifene, estrogen, or placebo. J Natl Cancer Inst. 2001;93(1):51–56. doi: 10.1093/jnci/93.1.51. [DOI] [PubMed] [Google Scholar]
- 45.Barnabei VM, Cochrane BB, Aragaki AK, et al. Menopausal symptoms and treatment-related effects of estrogen and progestin in the Women’s Health Initiative. Obstet Gynecol. 2005;105:1063–1073. doi: 10.1097/01.AOG.0000158120.47542.18. [DOI] [PubMed] [Google Scholar]
- 46.Lobo RA, Bush T, Carr BR, Pickar JH. Effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate on plasma lipids and lipoproteins, coagulation factors, and carbohydrate metabolism. Fertil Steril. 2001;76(1):13–24. doi: 10.1016/s0015-0282(01)01829-5. [DOI] [PubMed] [Google Scholar]
- 47.Hays J, Ockene JK, Brunner RL, et al. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348(19):1839–1854. doi: 10.1056/NEJMoa030311. [DOI] [PubMed] [Google Scholar]
- 48.Brunner RL, Gass M, Aragaki A, et al. Effects of conjugated equine estrogen on health-related quality of life in postmenopausal women with hysterectomy: results from the Women’s Health Initiative Randomized Clinical Trial. Arch Intern Med. 2005;165(17):1976–1986. doi: 10.1001/archinte.165.17.1976. [DOI] [PubMed] [Google Scholar]
- 49.Polo-Kantola P, Erkkola R, Helenius H, Irjala K, Polo O. When does estrogen replacement therapy improve sleep quality? Am J Obstet Gynecol. 1998;178(5):1002–1009. doi: 10.1016/s0002-9378(98)70539-3. [DOI] [PubMed] [Google Scholar]
- 50.Barnabei VM, Grady D, Stovall DW, et al. Menopausal symptoms in older women and the effects of treatment with hormone therapy. Obstet Gynecol. 2002;100(6):1209–1218. doi: 10.1016/s0029-7844(02)02369-4. [DOI] [PubMed] [Google Scholar]
- 51.Gelfand MM, Moreau M, Ayotte NJ, Hilditch JR, Wong BA, Lau CY. Clinical assessment and quality of life of postmenopausal women treated with a new intermittent progestogen combination hormone replacement therapy: a placebo-controlled study. Menopause. 2003;10(1):29–36. doi: 10.1097/00042192-200310010-00006. [DOI] [PubMed] [Google Scholar]
- 52.Hlatky MA, Boothroyd D, Vittinghoff E, Sharp P, Whooley MA. Quality-of-life and depressive symptoms in postmenopausal women after receiving hormone therapy: results from the Heart and Estrogen/ Progestin Replacement Study (HERS) trial. JAMA. 2002;287(5):591–597. doi: 10.1001/jama.287.5.591. [DOI] [PubMed] [Google Scholar]
- 53.Haines CJ, Yim SF, Chung TK, et al. A prospective, randomized, placebo-controlled study of the dose effect of oral oestradiol on menopausal symptoms, psychological well being, and quality of life in postmenopausal Chinese women. Maturitas. 2003;44(3):207–214. doi: 10.1016/s0378-5122(02)00340-7. [DOI] [PubMed] [Google Scholar]