Abstract
Background
The purpose of this study was to determine whether seizure susceptibility due to antihistamines is provoked in patients with febrile seizures.
Methods
The current descriptive study was carried out from April 2009 to February 2011 in 250 infants and children who visited the Madinah Maternity and Children’s Hospital as a result of febrile convulsions. They were divided into two groups according to administration of antihistamines at the onset of fever.
Results
Detailed clinical manifestations were compared between patients with and without administration of antihistamines. The time from fever detection to seizure onset was significantly shorter in the antihistamine group than that in the nonantihistamine group, and the duration of seizures was significantly longer in the antihistamine group than in the nonantihistamine group. No significant difference was found in time from fever detection to seizure onset or seizure duration between patients who received a first-generation antihistamine and those who received a second-generation antihistamine.
Conclusion
Due to their central nervous system effects, H1 antagonists should not be administered to patients with febrile seizures and epilepsy. Caution should be exercised regarding the use of histamine H1 antagonists in young infants, because these drugs could potentially disturb the anticonvulsive central histaminergic system.
Keywords: antihistamine, nonantihistamine, histamine H1 antagonist, febrile seizures
Introduction
Febrile seizures are the most common convulsive events in childhood, occurring in 2%–5% of children younger than 5 years of age.1 They are age-dependent and are uncommon before 9 months and after 5 years of age. The peak age of onset is approximately 14–18 months. A strong family history of febrile convulsions in siblings and parents suggests a genetic predisposition.2
The pathogenesis of febrile convulsions is not clear even today. Viral infections of the upper airways, exanthema subitum, acute otitis media, infection of the urinary tract, and febrile reactions after vaccination are the most frequent precipitating factors.3
It has been demonstrated that increased histamine levels elevate the seizure threshold and reduce the severity and duration of seizures,4 whereas decreased histamine levels have the opposite effect.5 Of the four histamine receptors, the histamine-1 (H1) and histamine-3 (H3) receptors are suggested to be of importance in decreasing seizure activity. The first-generation H1 receptor antagonists, such as ketotifen and chlorpheniramine, elicit epileptiform activity.6 The H3 receptor antagonists, which block H3 autoreceptor function, are believed to decrease seizure activity by increasing histamine release.7
Antagonists of histamine H1 receptors are commonly classified as first-generation or newer-generation antihistamines on the basis of their often sedating effect at therapeutic doses.7 The latter antihistamines, also known as second-generation or third-generation antihistamines, include astemizole, cetirizine, desloratadine, fexofenadine, loratadine and terfenadine, and were developed as nonsedating alternatives to the first-generation compounds.
Seizures or convulsions have been reported in the literature with some first-generation antihistamines (chlorpheniramine, diphenhydramine, pheniramine, and pyribenzamine) as well as with some of the newer-generation antihistamines (astemizole, cetirizine, fexofenadine, loratadine, and terfenadine).7–9
Churchill and Gammon10 first reported that antihistamines such as diphenhydramine and tripelennamine activated epileptic discharges on electroencephalography and produced clinical manifestations of psychomotor seizures in adult epileptic patients. However, reproducibility is not high in patients suspected of having drug-induced febrile seizures, and other factors, such as fever and infection, can induce febrile seizures. Furthermore, the relationship between febrile seizures and medications has not been actively investigated.11,12 The aim of this study was to determine whether seizure susceptibility due to antihistamines is provoked in patients with febrile seizures.
Materials and methods
The current study was carried out from April 2009 to February 2011 in 283 infants and children who attended the Madinah Maternity and Children’s Hospital as a result of febrile convulsions. For the purposes of our study, the case definition of a febrile convulsion was a convulsive seizure in an infant or child in association with a fever of 38.0°C or higher, but without evidence of any definitive causative illness, such as central nervous system infection, metabolic abnormality, or intoxication. Thus, both simple and complex febrile convulsions were included. Thirty-three patients were excluded due to a history of afebrile seizures, cerebral palsy, or mental retardation. Thus, 250 patients were enrolled in our study. Informed consent was obtained from the parents of children after thorough explanation of the study. A specially designed and validated checklist was used by the attending physicians in the emergency room to record age, gender, family history of febrile seizures, mean maximum temperature, time from detection of fever to seizure onset, underlying disease, medications administered before seizure occurrence, and duration between receiving the last dose of antihistamine and onset of febrile seizure. The duration of convulsions was investigated for chlorpheniramine and dimethindene (first-generation antihistamines) and cetirizine, loratadine, and ketotifen (second-generation antihistamines).
Statistical analysis
Statistical procedures were done using SPSS software for Windows (v 13.0; SPSS Inc, Chicago, IL). Appropriate descriptive and analytic methods were used. The Chi-square test was used for the statistical analysis of percentages. Differences were considered statistically significant at P < 0.05.
Results
We investigated 250 patients (135 boys and 115 girls) of mean age 28.3 ± 18.54 months. Using the study checklist, 84 patients (33.6%) were found to have taken antihistamines at onset of fever. The antihistamines administered were divided into first-generation and second-generation histamine H1 antagonists. No significant difference was observed in the gender ratio, age distribution, frequency of family history of febrile seizures or epilepsy, and degree of fever between the group administered antihistamines and the group not administered antihistamines. The clinical characteristics of the patients are shown in Table 1 and in Figures 1 and 2.
Table 1.
Comparison of characteristics of patients with febrile seizures with and without antihistamine administration
| Variable | Antihistamine group A n = 84 |
Nonantihistamine group n = 166 |
|---|---|---|
| Gender ratio (boys:girls) | 12:10 | 14:11 |
| Age in months (mean ± SD) | 26.8 ± 19.5 | 29.8 ± 17.4 |
| Family history of febrile convulsions, n (%) | 43 (51.2%) | 80 (48.2%) |
| Maximum temperature (°C) | 39.1°C | 39.3°C |
| Time from fever detection to seizure onset, hours (mean ± SD) | 2.99 ± 0.39 | 4.27 ± 1.36* |
| Average seizure duration in minutes (mean ± SD) | 9.0 ± 6.1* | 4.5 ± 4.3 |
Note:
Statistically significant difference from Group A values are expressed as the mean ± SD, numbers, and percentages.
Abbreviation: SD, standard deviation.
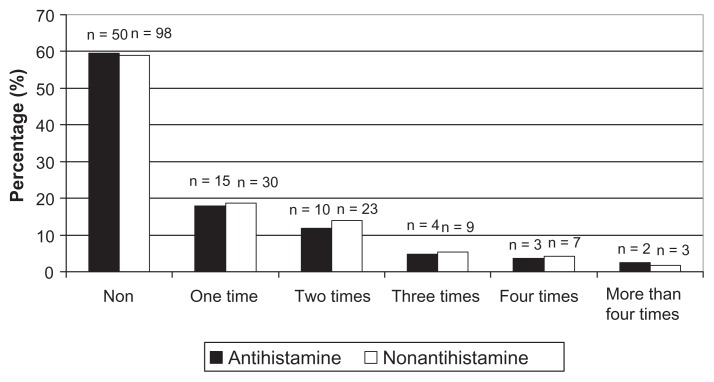
Figure 1.
Distribution of previous febrile seizure frequency of patients with febrile seizures with and without antihistamine administration.
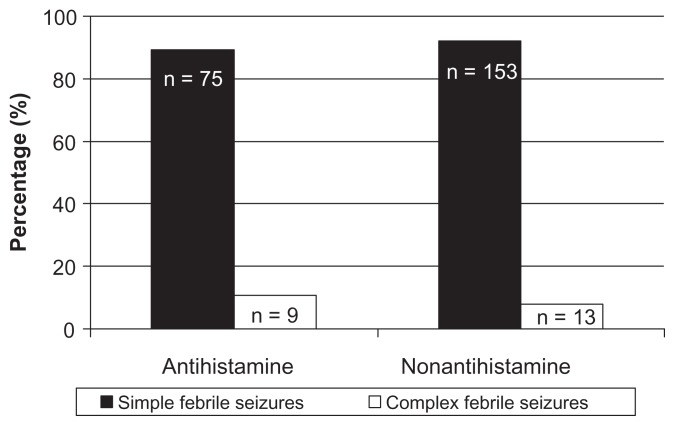
Figure 2.
Distribution of seizure types in patients with febrile seizures with and without antihistamine administration.
The 166 children who did not take antihistamines had an average seizure duration of 4.5 ± 4.3 minutes. The duration was less than 5 minutes in 117 patients (70.5%) and the seizure persisted for 15 minutes or longer in 49 patients (29.5%). The 84 children who took antihistamines had an average seizure duration of 9.0 ± 6.1 minutes. Seizure duration was less than 5 minutes in 58 patients (69.0%), and a seizure persisting for 15 minutes or longer was noted in 26 patients (31%). The 55 patients who took a first-generation antihistamine had an average seizure duration of 9.3 ± 14.2 minutes; the seizure duration was less than 5 minutes in 35 patients (63.3%), and persisted for 15 minutes or longer in 20 patients (36.7%). The 29 children who took a second-generation antihistamine had an average seizure duration of 6.0 ± 6.1 minutes, with a seizure duration of less than 5 minutes in seven patients (79.3%) and for 15 minutes or longer in six patients (20.7%). There was a significant difference in seizure duration between children who did not take an antihistamine and those who took a first-generation antihistamine (P < 0.05). The time from fever detection to seizure onset was significantly shorter in both the antihistamine groups than in the nonantihistamine group (P < 0.05). There was no significant difference in the number of cases of simple and complex febrile seizures between the groups. There was no significant difference in time from fever detection to seizure onset or duration of seizure between the group taking a first-generation antihistamine and that taking a second-generation antihistamine (Tables 2 and 3).
Table 2.
Time and duration characteristics of patients at onset of febrile seizure
| Variable | No drugs Group A |
First-generation antihistamine Group B |
Second-generation antihistamine Group C |
|---|---|---|---|
|
|
|
|
|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Time from fever detection to seizure onset (hours) | 4.27 ± 1.36 | 2. 5 ± 0.79* | 3.01 ± 037* |
| Duration of seizure (minutes) | 4.5 ± 4.3 | 9.3 ± 14.2* | 6.0 ± 6.1 |
Note:
Significant difference from Group A, values are expressed as the mean ± SD.
Abbreviation: SD, standard deviation.
Table 3.
Distribution of seizure characteristics in patients with and without antihistamine administration at onset of febrile seizure
| Variable | No drugs Group A |
First-generation antihistamine Group B |
Second-generation antihistamine Group C |
|---|---|---|---|
|
|
|
|
|
| n (%) | n (%) | n (%) | |
| Patient distribution | 166 (66.4) | 55 (22) | 29 (11.6) |
| Duration 5–15 minutes | 117 (70.5) | 35 (63.3)* | 23 (79.3) |
| From >15 minutes | 49 (29.5) | 20 (36.7)* | 6 (20.7) |
| Simple febrile seizures | 153 (92.2) | 49 (89.1) | 26 (89.7) |
| Complex febrile seizures | 13 (7.8) | 6 (10.9) | 3 (10.3) |
Note:
Significant difference from Group A, values are expressed as numbers and percentages.
Discussion
One of the most interesting findings of this study was the observation that antihistamines were so widely used in the community for mild intercurrent infections of childhood. Although new-generation H1A antagonists with reduced adverse effects have already been introduced into clinical use, utilization of this group of drugs is limited in pediatric patients. Prescription of first-generation H1A antagonists was dominant among the 84 H1A (+) cases, ie, first-generation, n = 55, and new generation, n = 29. First-generation H1A antagonists are still widely used among children in the US as well.11
The main results of this study were that time from fever detection to seizure onset was significantly shorter in both the antihistamine groups compared with the nonantihistamine group.2 Seizure duration was significantly longer in the first-generation antihistamine group than in the nonantihistamine group, and there was no significant difference in time from fever detection to seizure onset or seizure duration between patients given a first-generation antihistamine and those given a second-generation antihistamine.3
The results of the current study are supported by those of another study10 which investigated the use of drugs, including histamine H1 antagonists, in children with febrile seizures. In that study, histamine H1 antagonists were taken by 10 (45.5%) of 22 patients with febrile seizures and 10 (22.7%) of 44 control subjects. The use of histamine H1 antagonists was significantly higher in patients with febrile seizures than in the controls. The histamine H1 antagonists taken by children in that study were carbinoxamine and promethazine, which are available over-the-counter for the common cold. Miyata et al12 reported that clinical doses of histamine H1 antagonists have the potential to modify seizures adversely in children.
The findings of our study are similar to those of two other recent studies13,14 which investigated 14 patients with simple febrile seizures and 35 patients with complex febrile seizures, and reported that time from fever detection to seizure onset was significantly shorter in the antihistamine group than that in the nonantihistamine group, and that seizure duration was significantly longer in the antihistamine group than that in the nonantihistamine group.
The present results demonstrate that administration of antihistamines to patients with febrile seizures shortened the time from fever detection to seizure onset and prolonged the duration of seizures. It is unclear whether interleukin-1β-mediated systemic inflammatory signals to the central nervous system in humans accelerate the turnover of hypothalamic neuronal histamine or how it influences seizure susceptibility. Considering the anticonvulsant and thermoregulation mechanisms controlled by central histaminergic neurons, it should be noted that antihistamine administration may induce some adverse effects, including neuronal excitability and a lower seizure threshold in febrile patients.
Kiviranta et al15 evaluated the possible role of histamine in the pathological mechanism of febrile seizures by measuring histamine concentrations in the cerebrospinal fluid of febrile children with seizures. Febrile children without seizures had a significantly higher histamine concentration than children with febrile seizures, while nonfebrile children with seizures and nonfebrile children without seizures had similar histamine concentrations. The increased susceptibility to seizures during fever may be related to a lack of increase in the histamine level in the cerebrospinal fluid of children with febrile seizures. Central histaminergic neuron systems may be involved in inhibiting seizures associated with febrile illnesses in childhood.
Interleukin-1β, one of the endogenous pyrogens, modulates neural and neuroendocrine activities. This cytokine has been shown to induce thermogenesis after infusion into the central nervous system. Receptors for interleukin-1β have been found in high density in the hippocampus, where they are located on the soma and dendrites of granule cells.16 Interleukin-1 receptors at these sites also colocalize with N-methyl-D-aspartate receptors that mediate the rapid action of glutamate. These receptor areas are crucial sites in the generation of seizures, because granular neurons play a pivotal role in gating the excitatory drive through the hippocampus and in modulating the propagation of seizures.17
Although the molecular mechanisms underlying the onset, generalization, and recurrence of seizures remain largely unknown, functional interactions between interleukin-1β and glutamate are of particular relevance for the convulsant activity of this cytokine. Therefore, interleukin-1 has been suggested to be one of the most important factors causing febrile seizures via its dual role as a pyrogen and convulsant substance. Hypothalamic neuronal histamine may suppress the febrile response induced by interleukin-1β.18
Moreover, an experimental study of central administration of interleukin-1β in rats demonstrated that interleukin-1β may stimulate synthesis and release of hypothalamic histamine at presynaptic terminals by activation of histidine decarboxylase (a histamine-synthesizing enzyme) and thus facilitate the degradation of extracellular histamine by the activation of histamine-N-methyltransferase (a degrading enzyme).18 These results indicate that interleukin-1β activates neural histamine turnover in the hypothalamus. In conclusion, the results of the present study suggest that clinical doses of H1A antagonists have the potential to modify the clinical activity of seizures in children adversely, and that caution needs to be exercised when using these very familiar drugs in this age group.
Acknowledgment
We thank all of our colleagues and the parents who helped to carry out this research.
Footnotes
Disclosure
No benefits in any form have been received or will be received from any commercial party related directly or indirectly to the subject of this article.
References
- 1.Duffner MD. A synopsis of the American Academy of Pediatrics’ practice parameters on the evaluation and treatment of children with febrile seizures. Pediatr Rev. 1999;20:285–287. doi: 10.1542/pir.20-8-285. [DOI] [PubMed] [Google Scholar]
- 2.Kliegman RM, Stanton B, St Geme J, Schor N. Behrman Nelson’s Textbook of Pediatrics. 18th ed. New York: Elsevier; 2007. [Google Scholar]
- 3.Gubser M, Blumberg A, Donati F. Febrile convulsions: assessment of current status. Schweiz Med Wochenschr. 1999;129:649–657. German. [PubMed] [Google Scholar]
- 4.Yawata I, Tanaka K, Nakagawa Y, Watanabe Y, Murashima YL, Nakano K. Role of histaminergic neurons in development of epileptic seizures in EL mice. Brain Res Mol Brain Res. 2004;132:13–17. doi: 10.1016/j.molbrainres.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Yokoyama H, Onodera K, Maeyama K, et al. Histamine levels and clonic convulsions of electrically-induced seizure in mice: the effects of fluoromethylhistidine and metoprine. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:40–45. doi: 10.1007/BF00167568. [DOI] [PubMed] [Google Scholar]
- 6.Fujii Y, Tanaka T, Harada C, Hirai T, Kamei C. Epileptogenic activity induced by histamine H1 antagonists in amygdala-kindled rats. Brain Res. 2003;991:258–261. doi: 10.1016/j.brainres.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Ten Eick AP, Blumer JL, Reed MD. Safety of antihistamines in children. Drug Saf. 2001;24:119–147. doi: 10.2165/00002018-200124020-00003. [DOI] [PubMed] [Google Scholar]
- 8.Blain PG, Lane RJM. Neurological disorders. In: Davies DM, Ferner RE, De Glanville H, editors. Davies’s Textbook of Adverse Drug Reactions. 5th ed. London, UK: Chapman and Hall Medical; 1998. [Google Scholar]
- 9.Murphy K, Delanty N. Drug-induced seizures: general principles in assessment, management and prevention. CNS Drugs. 2000;14:135–146. [Google Scholar]
- 10.Churchill JA, Gammon GD. The effect of antihistaminic drugs on convulsive seizures. J Am Med Assoc. 1949;141:18–21. doi: 10.1001/jama.1949.02910010020004. [DOI] [PubMed] [Google Scholar]
- 11.Haruyama W, Fuchigami T, Noguchi Y, et al. The relationship between drug treatment and the clinical characteristics of febrile seizures. World J Pediatr. 2008;4:202–205. doi: 10.1007/s12519-008-0037-3. [DOI] [PubMed] [Google Scholar]
- 12.Miyata I, Saegusa H, Sakurai M. Seizure-modifying potential of histamine H1 antagonists: A clinical observation. Pediatr Int. 2011;53:706–708. doi: 10.1111/j.1442-200X.2011.03328.x. [DOI] [PubMed] [Google Scholar]
- 13.Takano T, Sakaue Y, Sokoda T, et al. Seizure susceptibility due to antihistamines in febrile seizures. Pediatr Neurol. 2010;42:277–279. doi: 10.1016/j.pediatrneurol.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni R, Valvi C, Kinikar A. Seizure susceptibility due to antihistamines in febrile seizures. Pediatr Neurol. 2010;43:303. doi: 10.1016/j.pediatrneurol.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Kiviranta T, Tuomisto L, Airaksien EM. Histamine in cerebrospinal fluid of children with febrile convulsions. Epilepsia. 1995;36:276–280. doi: 10.1111/j.1528-1157.1995.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 16.Nishiyori A, Minami M, Takami S, Satoh M. Type 2 interleukin-1 receptor mRNA is induced by kainic acid in the rat brain. Brain Res Mol Brain Res. 1997;50:237–245. doi: 10.1016/s0169-328x(97)00195-2. [DOI] [PubMed] [Google Scholar]
- 17.McNamara JO. Cellular and molecular basis of epilepsy. J Neurosci. 1994;14:3413–3425. doi: 10.1523/JNEUROSCI.14-06-03413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiba S, Itateyama E, Oka K, Masaki T, Sakata T, Yoshimatsu H. Hypothalamic neuronal histamine modulates febrile response but not anorexia induced by lipopolysaccharide. Exp Biol Med. 2005;230:334–342. doi: 10.1177/153537020523000507. [DOI] [PubMed] [Google Scholar]