Abstract
Patients with certain neurological diseases are at increased risk of developing chest infections as well as respiratory failure due to muscular weakness. In particular, patients with certain neuromuscular disorders are at higher risk. These conditions are often associated with sleep disordered breathing. It is important to identify patients at risk of respiratory complications early in the course of their disease, although patients with neuromuscular disorders often present in the acute setting with respiratory involvement. This review of the respiratory complications of neurological disorders, with a particular focus on neuromuscular disorders, explores why this happens and looks at how to recognize, investigate, and manage these patients effectively.
Keywords: respiratory failure, respiratory muscle weakness
Introduction
Many different types of neurological disorders can affect respiratory function (see Table 1). Neuromuscular disorders in particular have a propensity to affect respiratory function adversely and are characterized by either disease of a muscle itself or the nerve(s) supplying that muscle, and this will form the focus of this review, although we will touch upon other respiratory complications of neurological disease. Neuromuscular disorders are commonly encountered in both the acute and outpatient setting, and are typically characterized by progressive muscular impairment. This can lead to loss of mobility and in some instances patients become wheelchair-bound, experiencing significant respiratory muscle weakness, which is a common cause of death from respiratory failure, often associated with aspiration and pneumonia.1 Furthermore, their ability to protect their own airway can become compromised, leading to chest infections. There is also a close association with sleep-disordered breathing which can lead to significant morbidity and precede daytime ventilatory failure.2 Unfortunately, many neurological disorders that affect respiratory function remain incurable, and therefore the broad aim when managing such patients is to help maintain and improve both physical and psychosocial function, including respiratory function.
Table 1.
Neurological disorders affecting respiratory function
| Location | Disease |
|---|---|
| Cerebral cortex | Stroke (aspiration) |
| Neoplasm | |
| Seizures (aspiration) | |
| Brain stem | Multiple sclerosis |
| Basal ganglia | Parkinson’s disease |
| Spinal cord | Multiple sclerosis |
| Trauma | |
| Tetanus | |
| Neoplasm/cord compression | |
| Anterior horn cell | Spinal muscular atrophy* |
| Post-polio syndrome* | |
| Amyotrophic lateral sclerosis* | |
| Toxins (lithium) | |
| Peripheral nerve | Guillain-Barré syndrome* |
| Porphyria* | |
| Critical illness polyneuropathy* | |
| Neuromuscular junction | Myasthenia gravis* |
| Acid maltase deficiency* | |
| Toxins (botulism)* | |
| Drugs, eg, corticosteroids, anticholinesterase inhibitors | |
| Myopathies | Congenital muscular dystrophy* |
| Limb girdle muscular dystrophy* | |
| Myotonic dystrophies* | |
| Duchenne muscular dystrophy* | |
| Polymyositis* | |
| Critical illness myopathy* | |
| Hypokalemia* | |
| Rhabdomyolysis* |
Note:
Neurological disorders likely to cause respiratory muscle weakness.
Some disorders deteriorate acutely within days to weeks, such as Guillain-Barré syndrome and myasthenia gravis. Muscle disorders including Duchenne muscular dystrophy and myotonic dystrophy progress more slowly, whilst amyotrophic lateral sclerosis, an anterior horn cell disease, has a more rapid progression.
Although there are case reports of patients with neuromuscular disorders such as myasthenia gravis presenting as isolated respiratory failure, in most neuromuscular disorders, respiratory failure develops during the course of the disease.3,4 Therefore, when managing patients with neurological disorders and in particular neuromuscular disorders, both in the acute or chronic phase, it is important to be vigilant to the possibility of respiratory muscle involvement.
Materials and methods
We conducted a detailed search of the current literature comprising systematic reviews, using sources including the Cochrane Collaboration alongside a PubMed search for existing clinical reviews and randomized controlled trials of relevance. We also reviewed existing guidelines and consensus opinion, including those of the British Thoracic Society, American Academy of Sleep Medicine, and American Academy of Neurology.
Why do neuromuscular disorders cause respiratory failure?
The diaphragm is the main muscle responsible for respiration and contributes almost 70% to inspiratory tidal volume in a normal individual.5 It is innervated by the phrenic nerve, originating at spinal nerve roots C3 through to C5. External intercostal muscles and accessory muscles (sternocleidomastoid, scalene, trapezius, latissimus dorsi, pectoralis major and minor, and platysma) assist inspiration when there is increased load on the respiratory system, such as during exercise or an asthma attack. Internal intercostal muscles and abdominal muscles largely support exhalation and aid the cough reflex. The bulbar muscles allow the upper airway to remain patent.
Respiratory muscle weakness decreases airflow, causing a gradual decline in vital capacity (the maximum amount of air that can be expelled after maximal inspiration) and patients are unable to inhale deeply. The load on the respiratory system is increased at lower volumes because the lungs do not expand as easily. This eventually affects gas exchange with ventilation-perfusion mismatch ensuing.6 The increased load on the respiratory system and muscle weakness results in patients developing a rapid and shallow breathing pattern.
Early evidence of respiratory system involvement in patients with a neuromuscular disorder is first seen during sleep. During rapid eye movement sleep, the respiratory drive is momentarily decreased and hypotonia of accessory respiratory muscles is seen. Patients with respiratory muscle weakness therefore develop nocturnal O2 desaturation and hypercapnia.1 The degree of nocturnal desaturation correlates to the degree of diaphragmatic weakness.
These factors result in hypoventilation, carbon dioxide (CO2) retention and type 2 respiratory failure.3 Because of a considerable reserve of ventilatory function, respiratory failure is often not seen until respiratory muscle strength has fallen to 25%–30% of normal.5 Because patients with neuromuscular disorders often have a compromised respiratory reserve, they tend to decompensate much more easily when under additional stress. The fact that such patients are more prone to chest infections demonstrates the significant association between neuromuscular disorders and respiratory failure.
Patients with certain neuromuscular disorders, such as spina bifida, poliomyelitis, and some muscular dystrophies, also have an element of kyphoscoliosis, which could be congenital, related to a degenerative condition of the spine, or a result of previous trauma/surgery. This can worsen pulmonary restriction and respiratory impairment caused by an associated neurological condition and therefore lead to earlier and more severe respiratory compromise.
Why do patients with certain neurological disorders develop chest infections?
Pneumonia is a common complication in patients both with acute neurology (eg, cerebrovascular accident) and chronic neurological conditions (eg, multiple sclerosis). It is thought that 20% of deaths in patients with Parkinson’s disease are due to pneumonia, possibly due to an impaired cough and involvement of the muscles of the upper airway.7 Weakness of the facial, oropharyngeal, and laryngeal muscles can result in a compromised swallow and secretion clearance. Weakness of these muscles can also compromise the airway due to physical obstruction, particularly when lying flat.8 The cough reflex is diminished due to weakness of the abdominal muscles, once again increasing the risk of aspiration. This leads to increased risk of developing infections as well as delaying recovery.
Assessing a patient for possible neuromuscular weakness
Acute neuromuscular respiratory disease
If a patient presents with acute weakness, it is important to establish any possible respiratory muscle weakness placing the patient at risk of respiratory failure or bulbar weakness which may lead to aspiration pneumonia. Dyspnea associated with lying flat or whilst immersed in water specifically indicates diaphragmatic weakness. This is an important symptom and reflects a major (70%) contribution of the diaphragm muscles to the inspiratory tidal volume.5
Bulbar dysfunction is associated with a poor swallow, dysarthria, weak mastication, facial weakness, nasal speech, and/or a protruding tongue. It is essential to establish early respiratory involvement in patients with rapid onset weakness involving the bulbar muscles or shoulder girdle5 in order to anticipate the need for mechanical ventilation, such as in patients with Guillain-Barré syndrome.
Chronic neurological disorders and respiratory disease
In patients with chronic neurological disorders it is important to establish nocturnal symptoms:
Is the patient’s sleep often disturbed? If so, what causes them to arouse?
Do they wake up with choking sensations?
Do they wake up with a headache first thing in the morning?
Are they especially tired during the day?
These questions may suggest nocturnal hypoventilation3 which is an early sign of respiratory muscle weakness. It is important to note that patients with neuromuscular disorders may have poor mobility and therefore do not necessarily present with exertional dyspnea.
Signs
The immediate focus should be to observe for signs of respiratory distress (Table 2). Examine the patient for evidence of any spinal abnormalities like kyphosis. Observe for paradoxical abdominal movement (inward abdominal movement on inspiration) which usually suggests considerable weakness of the diaphragm muscle. Cranial nerve and peripheral neurological examination is useful to look specifically for the following signs:
Table 2.
Signs of respiratory distress
| Tachypnea |
| Unable to complete sentences |
| Restless or drowsy |
| Leaning forward |
| Cyanosis |
| Use of accessory muscles including sternocleidomastoid muscles |
| Intercostal recession – indrawing of intercostal spaces during inspiration |
Dysphagia, dysphonia, drooling
Cough after swallowing indicating aspiration
A weak cough.
The single-breath test can be useful in exploring the extent of impairment of a patient’s vital capacity. This test is performed by asking the patient to take a maximal inspiratory breath and begin counting. Normal patients can reach up to 50, but severe impairment of vital capacity is indicated by a single-breath count of <15.8
Investigations
Chest x-ray
A chest radiograph can be helpful not only in identifying associated respiratory problems such as kyphoscoliosis and emphysema, which may be contributing to respiratory compromise, but also precipitating factors for respiratory decompensation, such as respiratory infections (including aspiration pneumonia), pulmonary edema, and atelectasis. A raised hemidiaphragm may be caused by unilateral phrenic nerve palsy which is usually well tolerated. However, a bilateral raised hemidiaphragm in patient with motor neuron disease may need further investigation with a sniff test or phrenic nerve stimulation to establish diaphragmatic weakness/paralysis.9
Arterial blood gases
Arterial blood gas derangement is mainly seen during the latter stages of a neuromuscular disorder with respiratory involvement or when there is an acute respiratory insult. Arterial blood gas measurement helps to confirm hypoxia (PaO2 < 11 kPa) in patients presenting with low SaO2 and to determine type 1 or 2 respiratory failure as a cause of hypoxia (Table 3). A raised pCO2 > 45 mmHg (6 kPa) in the acute or stable patient, especially in the presence of acidosis, requires immediate assessment for invasive or noninvasive ventilation. 10 Metabolic or mixed acidosis with raised lactate points to associated sepsis. In patients with a normal daytime SaO2, elevated serum bicarbonate with a normal pO2 and pCO2 can be suggestive of nocturnal hypoventilation.5
Table 3.
Arterial blood gas patterns in respiratory failure
| pH | PaO2 | PaCO2 | Bicarbonate | |
|---|---|---|---|---|
| Type 1 RF | Normal/high | Low | Normal/low | Normal |
| Acute type 2 RF | Normal/low | Low | High | Normal/rising |
| Acute on chronic type 2 RF | Normal/low | Low | High | Raised |
Abbreviation: RF, respiratory failure.
Swallow assessment
Neuromuscular disorders causing respiratory muscle weakness may also affect the muscles controlling the swallow reflex. A simple bedside swallow test with foods and liquids of different consistencies in the acute setting and subsequent visual swallow assessment using video fluoroscopy in a stable patient by a speech and language therapist helps to identify impaired swallowing. However, swallow assessments by a nontrained health care worker can be harmful. Swallow assessments by trained nurses working out of hours (eg, stroke nurses, night nurse practitioners) can help to avoid long periods without appropriate nutrition.
Respiratory function test
Lung function measurement is useful both in the stable patient with a chronic neuromuscular disorder to diagnose and monitor progression of respiratory muscle weakness and in the acute setting to determine the need for invasive or noninvasive ventilation.
Spirometry
Neuromuscular disorders typically result in a restrictive lung defect on simple spirometry (forced expiratory volume in one second/forced vital capacity ratio > 0.7). The vital capacity should be measured in both the upright and supine positions. A decline in vital capacity by more than 15%–20% in the supine position indicates diaphragmatic weakness.5 Spirometry can be performed at the bedside using simple handheld spirometers in acutely ill patients and in the lung function laboratory in stable patients. Serial bedside spirometry measurement is particularly useful in the acute setting for patients with rapidly progressing neurology, such as Guillain- Barré syndrome, to monitor for deterioration. A value of less than 15 mL/kg should prompt urgent intensive care referral for endotracheal intubation in anticipation of potential respiratory arrest. However, validity of the results is very dependent on technique, and not all patients can understand or generate sufficient effort. Furthermore, spirometry measurement can vary in neuromuscular disorders with variable/intermittent muscle weakness such as myasthenia gravis. Spirometry measurements can therefore be a poor predictor of the need for mechanical ventilation is such patient groups.11 In patients with a stable neuromuscular disorder, no single test can reliably diagnose or predict early respiratory muscle weakness. 12 Therefore, a combination of measures, including vital capacity and mouth pressures, are required.
Mouth pressures
Mouth pressures (inspiratory and expiratory) represent global inspiratory and expiratory respiratory muscle strength.13 Maximum inspiratory pressure reflects the strength of the diaphragm, external intercostal muscles, and accessory muscles, whereas maximum expiratory pressure represents expiratory muscle and cough strength. Mouth pressures with vital capacity should be measured early in all patients with neuromuscular disorders associated with respiratory muscle weakness to establish a baseline. Patients with reduced mouth pressures, maximum inspiratory pressure of <60% predicted (normal value for males > 80 cm H2O, females > 70 cm H2O) required close respiratory follow-up for serial measurements and further investigation including arterial blood gas measurement.5 Mouth pressures can also provide an additional objective measurement to decide on the need for mechanical ventilation8 in the acute setting (Table 5). However, measurement of mouth pressure requires the patient to make an adequate seal around the mouthpiece which may not always be possible in patients who have facial muscle weakness.
Table 5.
Predictors of the need for mechanical ventilation8
| Onset of bulbar symptoms |
| Inability to raise arms/elbow above head or inability to stand in Guillain-Barré syndrome |
| Rapid progression of muscular weakness |
| VC < 15 mL/kg or VC < 1 liter |
| A 50% reduction of VC from admission |
| Maximum inspiratory pressure > −30 cm H2O |
| Maximum expiratory pressure < 40 cm H2O |
Note: Partially based on data from reference 8.
Abbreviation: VC, vital capacity.
Sniff nasal inspiratory pressure
Sniff nasal inspiratory pressure is an additional test for measurement of inspiratory muscle strength and can be used in conjunction with maximum inspiratory pressure. A sniff nasal inspiratory pressure ≥ 70 cm H2O (≥ 60 cm H2O for females) makes significant respiratory muscle weakness unlikely. A combination of tests like sniff nasal inspiratory pressure and maximum inspiratory pressure increases diagnostic precision.14
Sleep studies
Patients with neuromuscular disorders are at increased risk of sleep-disordered breathing which includes disorders such as obstructive sleep apnea and nocturnal hypoventilation.2 Nocturnal desaturations and sleep disturbance usually precede daytime respiratory failure and should be investigated early.2 In patients suspected to have nocturnal hypoventilation, overnight oximetry can be performed. This is a simple investigation which measures oxygen saturation overnight with a pulse oximeter. It can establish underlying hypoventilation and is useful in asymptomatic patients with chronic disease in whom early respiratory failure is suspected. Overnight oximetry parameters helpful for confirmation of nocturnal hypoventilation are SaO2 < 90% for >30% of sleep duration. A prolonged SaO2 desaturation pattern (SaO2 < 90% for continuous five minutes) is characteristic of nocturnal hypoxia due to hypoventilation as opposed to the frequent repetitive desaturations of a saw tooth pattern observed in patients with obstructive sleep apnea. Some patients may develop obstructive sleep apnea prior to developing alveolar hypoventilation.
Polysomnography/polygraphy (multichannel) sleep study with continuous transcutaneous carbon dioxide tension monitoring should be considered when overnight oximetry is indeterminate.15 Respiratory parameters of nasal flow are measured with a nasal pressure transducer, and chest and abdominal excursion is measured with respiratory inductive plethysmography bands. This helps to differentiate between nocturnal hypoxia due to hypoventilation and sleep apnea. These parameters are also able to characterize sleep apnea into a central and obstructive type. Transient increases in transcutaneous CO2 (pCO2 ≥ 50 mmHg for ≥5% of study time) is a definite parameter for identification of nocturnal hypoventilation in patients with neuromuscular disorders.
It is not clear when the most favorable time is to undertake a sleep study during a patient’s disease course. However, there should a low threshold for screening with overnight oximetry in patients with a severe restrictive impairment (vital capacity < 50% of predicted) and/or patients with a history of disturbed sleep, early morning headaches, or daytime fatigue/tiredness/sleepiness.16 There is current interest in the use of symptom-based, self-administered screening questionnaires to identify patients with a neuromuscular disorder and at risk of sleep-disordered breathing who may benefit from polysomnography and intervention with mechanical ventilation, although more research is required in this area.17
The role of sleep studies is therefore to determine the presence of sleep-disordered breathing, and the American Academy of Sleep Medicine suggests that for patients with neuromuscular disorders and sleep-related symptoms, polysomnography is routinely indicated to evaluate symptoms of sleep disorders that are not adequately diagnosed by obtaining a sleep history, assessing sleep hygiene, and reviewing sleep diaries.18
Different patterns may be observed with specific neuromuscular disorders. For example, with amyotrophic lateral sclerosis, nocturnal hypoventilation and oxygen desaturation are the main abnormalities detected on polysomnography, with apnea/hypopneas being less common.10 The main problem is thought to be due to diaphragmatic weakness and hypoventilation rather than obstructive sleep apnea secondary to bulbar events.2 Thirty-six percent of patients with myasthenia gravis have been found to have obstructive sleep apnea in one series,19 with desaturations and sleep-disordered breathing being more common during rapid eye movement sleep.2
Degenerative central nervous system disorders like multiple sclerosis or Parkinson’s disease have been associated with sleep-disordered breathing. Multiple sclerosis affecting the medullary tegmentum can lead to obstructive sleep apnea, whilst Parkinson’s disease has been associated with central apnea and hypoventilation, thought to be secondary to autonomic dysfunction.7
Management of respiratory complications of neurological disorders
Immediate management
Immediate priorities in patients presenting with respiratory failure due to neurological disorders are airway management, oxygenation, and prompt treatment of precipitating factors, such as pneumonia, in addition to assessment for more specialized therapies.
The oxygen concentration (FiO2) required for oxygenation depends on the presence of type 1 or type 2 respiratory failure and the desired target oxygen saturation (Table 4). Arterial blood gases should be repeated following initiation of oxygen or changing FiO2. In patients with type 2 respiratory failure, the conventional wisdom was that they are reliant on a “hypoxic drive” and that high concentrations of oxygen abolished this drive. This, in turn, reduces the ventilatory drive and worsens hypercapnia. However, it is now known that the process is much more complicated and is related to impaired matching of blood and gas flow in the lungs.20 A Venturi mask system should be used to deliver a fixed oxygen concentration with the aim of using the minimum concentration of oxygen possible to achieve a lower target saturation. However, patients with significant hypoxia (SaO2 < 85%) and not at risk of type 2 respiratory failure should be started on high-flow oxygen (eg, 10–15 L/minute with a reservoir bag).20 Patients who are unable to maintain satisfactory oxygen saturation or are acidotic despite maximal medical therapy often require ventilatory support (ie, intubation and mechanical ventilation or noninvasive ventilation).
Table 4.
Oxygen management in respiratory failure, based on 2008 British Thoracic Society guidelines for emergency oxygen use in adult patients20
| Type 1 respiratory failure | Deliver concentration of oxygen that maintains saturations between 94%–98% |
| Type 2 respiratory failure or at risk of type 2 failure | Deliver minimum concentration of oxygen that maintains saturations between 88%–92% |
Note: Reproduced from Thorax, B O’Driscoll, L Howard, AG Davidson, 63, Suppl 6:1–12, 2008 with permission from BMJ Publishing Group Ltd.
Invasive ventilation
Invasive ventilation in the acute setting should be considered in patients who are candidates for treatment in an intensive care unit. Patient wishes, baseline function, comorbidities, and assessment of successful extubation and restoration of a reasonable quality of life need to be considered prior to invasive ventilation. Some of the parameters which are useful in deciding on the need for mechanical ventilation are listed in Table 5. Patients with acute progressive muscular weakness as a result of, eg, Guillain-Barré syndrome, are usually good candidates for intubation, and mechanical ventilation is appropriate. However, the decision to intubate should be semielective because emergency intubation is associated with a higher complication rate, particularly in patients with Guillain-Barré syndrome because of associated autonomic dysfunction and labile blood pressure.8
Acute noninvasive ventilation
The role of nocturnal noninvasive ventilation in patients with a neuromuscular disorder and respiratory failure has increased over the last 20 years, and indications include offering support during an intercurrent chest infection, preventing respiratory decompensation, and providing palliation during end-of-life care.21 It has been shown to prolong survival in patients with hypercapnic respiratory failure.22 Noninvasive ventilation can be initiated acutely to treat respiratory failure, especially type 2 failure, either to avoid endotracheal intubation or when endotracheal intubation is deemed inappropriate.
Long-term ventilation
It is important to take a holistic approach and to identify health care professionals who need to be involved early on. There is a definite role for invasive ventilation via tracheostomy in selected patients with long-term neuromuscular disorders in whom noninvasive ventilation is contraindicated or has failed. This requires very careful planning, and some patients may decline invasive ventilation, with the negative impact on quality of life outweighing the benefits of an extended life.21 Patients may find greater difficulty communicating, and there is a significant psychological impact on patients and carers,23 but survival can be prolonged for many years, with some patients suffering from amyotrophic lateral sclerosis surviving up to 10 years longer.24 A review by Sancho et al,25 based on their experiences at a specialist respiratory care unit, looked at 76 subjects with amyotrophic lateral sclerosis and respiratory failure, of whom 38 refused tracheostomy. Over half were initiated during an acute episode after failure of noninvasive ventilation, and benefits included the ability to clear tracheobronchial secretions, with almost 80% surviving at one year. This is an option that should be discussed with the patient, and the most appropriate time to do this depends on the patient’s clinical progression and wishes.
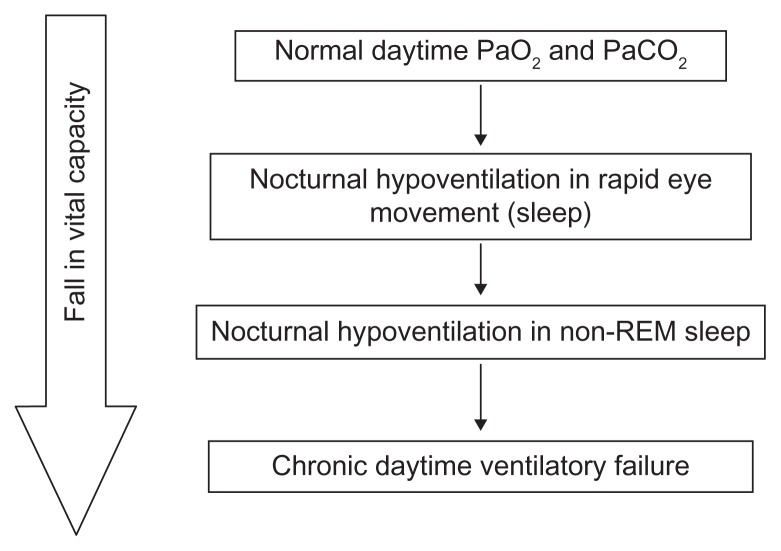
With regards to long-term noninvasive ventilation, although we know how neuromuscular disorders involving the chest progress to respiratory failure as outlined in Figure 1, there remain unanswered questions as to the best moment to commence noninvasive ventilation.26 Ideally, it should be planned and not implemented as an emergency. A study in a group of patients with Duchenne muscular dystrophy trialed noninvasive ventilation before the development of nocturnal hypoventilation or daytime respiratory failure and found that there was no survival benefit or delay in progression and, in fact, it adversely affected quality of life.28 A study initiating noninvasive ventilation for nocturnal hypoventilation, which included patients with neuromuscular disorders, found that it may reduce the frequency of hypercapnic episodes and therefore avoid hospital admissions,27 but that patients have a more pronounced response when they are also experiencing daytime symptoms. A Cochrane review in 2007 concluded that further research is needed to establish the long-term benefits of nocturnal mechanical ventilation, including its impact on survival and hospital admission rates, improvement in hypoventilation symptoms, and a comparison between invasive and noninvasive ventilation.29
Figure 1.
Effect of progressive respiratory muscle weakness on nocturnal and diurnal ventilation.
Note: Reproduced with permission from the American College of Chest Physicians. Simonds A. Recent advances in respiratory care for neuromuscular disease. Chest. 2006;130:1879–1886.21
Abbreviation: REM, rapid eye movement.
The timing of noninvasive ventilation therefore depends very much on the specific indication (Table 6) and the particular setting (acute versus domiciliary). It also very much depends on the underlying disease. For example, in Duchenne muscular dystrophy, there is a predictable disease course which starts with rapid eye movement-related sleep-disordered breathing, followed by nonrapid eye movement and rapid eye movement sleep-disordered breathing and, finally, daytime respiratory failure with a gradual reduction in forced vital capacity through all stages.21 Therefore, it is possible to predict when a patient may need to commence noninvasive ventilation, and it is currently felt that in the presence of nocturnal hypoventilation, daytime respiratory failure is almost inevitable, and so this may be an opportune moment to commence noninvasive ventilation.21
Table 6.
Possible roles for noninvasive ventilation in patients with neuromuscular disorders21
| Acute respiratory failure secondary to chest infection |
| Perioperative support/Peg tube placement |
| Sleep-disordered breathing |
| During pregnancy |
| To treat hypercapnic chronic respiratory failure |
| Palliate symptoms as part of end-of-life care |
The American Academy of Neurology in 2009 produced evidenced-based guidelines on amyotrophic lateral sclerosis which recommends the use of noninvasive ventilation to treat respiratory failure because it probably lengthens survival, slows the rate of decline in forced viral capacity, and improves quality of life.30 It also recommends commencing noninvasive ventilation as soon as there is any evidence of nocturnal hypoventilation or respiratory insufficiency because this improves compliance.30 In patients with amyotrophic lateral sclerosis and bulbar involvement, it is felt that noninvasive ventilation should be trialed but stopped if there is no symptomatic benefit because patients are less likely to be compliant and the survival benefit is reduced in the presence of bulbar involvement.21
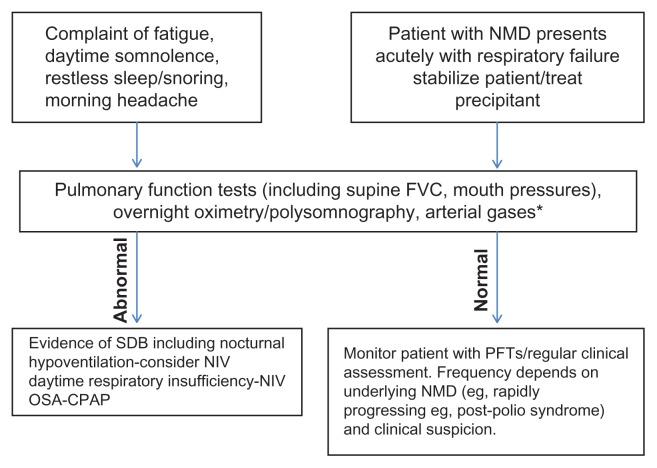
It is evident that there is no set way of managing patients with neuromuscular disorders and respiratory insufficiency, so patients should be evaluated in respiratory centers with appropriate experience. Figure 2 is a suggested pathway, but the specific timing of noninvasive ventilation very much depends on the underlying disease and the specific characteristics of the patient. Some patients may not be candidates for noninvasive ventilation, especially if they are having difficulty with respiratory secretions or have a reduced conscious level.
Figure 2.
Suggested pathway for investigating and managing patients with a neuromuscular disorder and respiratory insufficiency.
Notes: *Depends on oxygen saturations and clinical suspicion of daytime hypercapnoea. Adapted from Upinder K, Dhand K, Dhand. Sleep disorders in neuromuscular diseases. Curr Opin Pulm Med. 2006;12:402–408.
Abbreviations: FVC, forced vital capacity; NMD, neuromuscular disorder; OSA, obstructive sleep apnea; CPAP, continuous positive airways pressure; NIV, noninvasive ventilation; SDB, sleep-disordered breathing.
Other considerations
Patients with pneumonia who are having difficulty clearing their secretions will benefit from chest physiotherapy, both from the hypoxia and comfort points of view, whether the patient is for active management or for palliation only. There may also be a role for cough assist devices in selected patients. Speech and language therapists should be involved to supervise swallow assessment, and dieticians need to be involved to optimize nutrition during an acute insult, whether it is via the nasogastric or oral route. Many patients with neuromuscular disorders may never recover their swallowing ability, and so long-term feeding options such as PEG feeding would need to be considered. Once their acute illness has been treated, further rehabilitation will be required, including physiotherapy and occupational therapy.
Patients known to have a progressive neuromuscular disorder with respiratory muscle involvement should have early discussions about their future wishes regarding ventilation. This is really about quality of life and dignity, and an advanced treatment plan should be formulated whilst the patient can still communicate and has capacity. In advanced disease, palliative care should run side by side with active management, and it should be recognized that maintaining quality of life rather than undertaking aggressive intervention can be in the patient’s best interests.5
Conclusion
Early recognition of respiratory failure in patients with neuromuscular disease is essential for avoiding emergency intubation. In the acute setting, it is paramount that the treating clinician is decisive in order to avoid cardiorespiratory arrest, because deterioration can be rapid and fatal. It is important to recognize exacerbating factors like infection and aspiration. Improvement in understanding of the mechanism(s) of respiratory failure, better diagnostic tests, and provision of earlier ventilation, including home ventilation, has helped to prolong survival of patients with neuromuscular disorders which previously led to early morbidity and mortality.21 This is an area that requires further research to establish clearer practice on when to start noninvasive ventilation and the mode of noninvasive ventilation to be used. There is also a role for tracheostomy ventilation in certain patient groups including amyotrophic lateral sclerosis, but patient selection is crucially important in order to identify those who will benefit most, appreciating the psychological burden it can entail and the significant support and resources required. Finally, it remains important to have open discussions about the patient’s wishes with regard to end-of-life care to avoid treatments that can compromise patient dignity and impact adversely on their quality of life.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Perrin C, Unterborn JN, Ambrosio CD, Hill NS. Pulmonary complications of chronic neuromuscular diseases and their management. Muscle Nerve. 2004;29:5–27. doi: 10.1002/mus.10487. [DOI] [PubMed] [Google Scholar]
- 2.Bourke SC, Gibson GJ. Sleep and breathing in neuromuscular disease. Eur Respir J. 2002;19:1194–1201. doi: 10.1183/09031936.02.01302001a. [DOI] [PubMed] [Google Scholar]
- 3.Kim WH, Kim JH, Kim EK, et al. Myasthenia gravis presenting as isolated respiratory failure: A case report. Korean J Intern Med. 2010;25:101–104. doi: 10.3904/kjim.2010.25.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qureishi AI, Choundry MA, Mohammad Y, et al. Respiratory failure as a first presentation of myasthenia gravis. Med Sci Monit. 2005;10:684–689. [PubMed] [Google Scholar]
- 5.Hutchinson D, Whyte K. Neurosmuscular disease and respiratory failure. Pract Neurol. 2008;8:229–237. doi: 10.1136/pn.2008.152611. [DOI] [PubMed] [Google Scholar]
- 6.Ambrosino N, Carpene N, Gherardi M. Chronic respiratory care for neuromuscular diseases in adults. Eur Respir J. 2009;34:444–451. doi: 10.1183/09031936.00182208. [DOI] [PubMed] [Google Scholar]
- 7.Aboussouan LS. Respiratory disorders in neurologic diseases. Cleve Clin J Med. 2005;72:511–518. doi: 10.3949/ccjm.72.6.511. [DOI] [PubMed] [Google Scholar]
- 8.Mehta S. Neuromuscular disease causing acute respiratory failure. Respir Care. 2006;51:1016–1023. [PubMed] [Google Scholar]
- 9.Qureishi A. Diaphragm paralysis. Semin Respir Crit Care Med. 2009;30:315–320. doi: 10.1055/s-0029-1222445. [DOI] [PubMed] [Google Scholar]
- 10.Upinder K, Dhand K, Dhand R. Sleep disorders in neuromuscular diseases. Curr Opin Pulm Med. 2006;12:402–408. doi: 10.1097/01.mcp.0000245704.92311.1e. [DOI] [PubMed] [Google Scholar]
- 11.Rieder P, Louis M, Jolliet P, Chevrolet JC. The repeated measurement of vital capacity is a poor predictor of the need for mechanical ventilation in myasthenia gravis. Intensive Care Med. 1995;21:663–668. doi: 10.1007/BF01711545. [DOI] [PubMed] [Google Scholar]
- 12.Singh D, Verma R, Garg R, Singh MK, Shukla R, Verma SK. Assessment of respiratory functions by spirometry and phrenic nerve studies in patients of amyotrophic lateral sclerosis. J Neurol Sci. 2011;306:76–81. doi: 10.1016/j.jns.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 13.Polkey M, Green M, Moxham J. Measurement of respiratory muscle strength. Thorax. 1995;50:1131–1135. doi: 10.1136/thx.50.11.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steier J, Kaul S, Seymour J, et al. The value of multiple tests of respiratory muscle strength. Thorax. 2007;62:975–980. doi: 10.1136/thx.2006.072884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [No authors listed] [PubMed] [Google Scholar]
- 16.Lofaso F, Fauroux B, Orlikowski D, Prigent H. Daytime predictors of sleep disordered breathing in neuromuscular patients to better schedule polysomnography. Eur Respir J. 2011;73:231–232. doi: 10.1183/09031936.00122610. [DOI] [PubMed] [Google Scholar]
- 17.Steier J, Jolley CJ, Seymour J, et al. Screening for sleep-disordered breathing in neuromuscular disease using a questionnaire for symptoms associated with diaphragm paralysis. Eur Respir J. 2011;37:400–405. doi: 10.1183/09031936.00036210. [DOI] [PubMed] [Google Scholar]
- 18.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: An update for 2005. American Academy of Sleep Medicine. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 19.Nicolle MW, Rask S, Koopman WJ, George CF, Adams J, Wiebe S. Sleep apnea in patients with myasthenia gravis. Neurology. 2006;67:140–142. doi: 10.1212/01.wnl.0000223515.15691.26. [DOI] [PubMed] [Google Scholar]
- 20.O’Driscoll B, Howard L, Davison AG. Guideline for emergency oxygen use in adult patients. Thorax. 2008;63(Suppl 6):1–12. doi: 10.1136/thx.2008.102947. [DOI] [PubMed] [Google Scholar]
- 21.Simonds A. Recent advances in respiratory care for neuromuscular disease. Chest. 2006;130:1879–1886. doi: 10.1378/chest.130.6.1879. [DOI] [PubMed] [Google Scholar]
- 22.Leger P, Bedicam JM, Cornette A, et al. Nasal intermittent positive pressure ventilation. Long term follow-up in patients with severe chronic respiratory insufficiency. Chest. 1994;105:100–105. doi: 10.1378/chest.105.1.100. [DOI] [PubMed] [Google Scholar]
- 23.Moss AH, Oppenheimer EA, Casey P, et al. Patients with amyotrophic lateral sclerosis receiving long-term mechanical ventilation. Advance care planning and outcomes. Chest. 1996;110:249–255. doi: 10.1378/chest.110.1.249. [DOI] [PubMed] [Google Scholar]
- 24.Turner MR, Parton MJ, Shaw CE, Leigh PN, Al-Chalabi A. Prolonged survival in motor neuron disease: a descriptive study of the King’s Database 1990–2000. J Neurol Neurosurg Psychiatry. 2003;74:995–997. doi: 10.1136/jnnp.74.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sancho J, Servera E, Diaz JL, Banuls P, Marin J. Home tracheotomy mechanical ventilation in patients with amyotrophic lateral sclerosis: causes, complications and 1-year survival. Thorax. 2011;66:948–952. doi: 10.1136/thx.2011.160481. [DOI] [PubMed] [Google Scholar]
- 26.Mellies U, Schwake C, Raqette R, Voit T, Teschler H. Patterns and predictors of sleep disordered breathing in primary myopathies. Thorax. 2002;57:724–728. doi: 10.1136/thorax.57.8.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward S, Chatwin M, Heather S, Simmonds A. Randomised controlled trial of non-invasive ventilation (NIV) for nocturnal hypoventilation in neuromuscular and chest wall disease patients with daytime normocapnia. Thorax. 2005;60:1019–1024. doi: 10.1136/thx.2004.037424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raphael J-C, Chevret S, Chastang C, Bouvet F. Randomised trial of preventive nasal ventilation in Duchenne muscular dystrophy. Lancet. 1994;343:1600–1604. doi: 10.1016/s0140-6736(94)93058-9. [DOI] [PubMed] [Google Scholar]
- 29.Annane D, Chevrolet JC, Chevret S, Orlikowski D, Raphael JC. Nocturnal mechanical ventilation for chronic hypoventilation in patients with neuromuscular and chest wall disorders. Cochrane Database Syst Rev. 2007;2:CD001941. doi: 10.1002/14651858.CD001941.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Miller RG, Jackson CE, Kasarskis EJ, et al. Practice Parameters Update: The care of the patient with amyotrophic lateral sclerosis: Drug, nutritional and respiratory therapies (an evidence-based review): Report of the quality standards committee of the American Academy of Neurology. Neurology. 2009;72:1218–1226. doi: 10.1212/WNL.0b013e3181bc0141. [DOI] [PMC free article] [PubMed] [Google Scholar]