Abstract
Objectives
We compared the pharmacokinetics of lopinavir (LPV) and ritonavir (RTV) between female and males.
Methods
This two-step, multicenter, pharmacokinetic study enrolled HIV-infected adults on lopinavir/ritonavir (LPV/r) capsules (400/100mg BID) plus 1 or more NRTIs. All subjects underwent 12 hour pharmacokinetic sampling. The PK sampling was repeated in subjects receiving the LPV/r tablet formulation.
Results
Step 1 enrolled 37 women and 40 men; step 2 included 42 subjects from step 1 plus 35 new participants (39 women and 38 men). LPV pharmacokinetics in females and males were not significantly different with either formulation. Females had significantly higher median RTV AUC0–12h with both the soft gel capsule and tablet formulations (SGC:5395 vs. 4119 ng*hr/ml, p=0.026; tablet 5310 vs. 3941 ng*hr/ml, p=0.012), higher median Cmax (SGC:802 vs. 635 ng/mL, p=0.032; tablet: 773 vs. 570 ng/ml, p=0.006)) and lower median CL/F (SGC:18.54 vs. 24.31 L/hour, p=0.026; tablet: 18.83 vs. 25.37 L/hour, p=0.012). RTV CL/F was slower in females after weight adjustment with both formulations.
Conclusion
The pharmacokinetics of LPV in the SGC and tablet formulations are comparable in HIV infected subjects. Females had higher RTV AUC0–12h and lower CL/F with both formulations. The mechanism of the sex difference in RTV CL/F warrants elucidation.
Keywords: HIV infection, lopinavir/ritonavir, pharmacokinetics, sex differences
INTRODUCTION
Currently, approximately half of all people living with HIV infection worldwide are women.1 Most information about the safety, tolerability, and efficacy of antiretroviral drugs in current use has been obtained from studies of predominantly male subjects. There is growing awareness that the under representation of women in clinical trials, in particular phase 1 studies, may lead to an incomplete understanding of the optimal dosing of antiretroviral drugs in women. Antiretroviral drugs have dose-limiting adverse reactions; therefore, defining those populations with increased or decreased clearance of antiretroviral drugs could lead to improved safety and effectiveness. Sex differences in the pharmacokinetics and clinical manifestations of several antiretroviral drugs have been reported.2–7 Several of these reports suggest that females achieve higher plasma antiretroviral drug concentrations than males do on the same doses of these antiretroviral agents, although this is not a uniform finding. The clinical significance of the differences observed is not fully apparent. In one study, women had higher saquinavir concentrations than men, and the higher concentrations were associated with a greater percentage of women who had undetectable levels of HIV-1 RNA.7 The objective of our study was to investigate prospectively sex differences in the pharmacokinetics of lopinavir and ritonavir in HIV-infected females and males receiving LPV/r as part of their antiretroviral regimen. LPV/r was chosen as the study drug because it is widely used to treat both antiretroviral naïve and treatment-experienced HIV patients.
METHODS
Population and Treatment
This was a two-step, prospective, non-randomized, multicenter intensive pharmacokinetic study whose primary objective was to compare the area under the plasma concentration time curve (AUC) of LPV (both the soft gel capsule and the melt extrusion tablet) between HIV-infected females and males. Pre-planned secondary objectives included an assessment of use of tenofovir disoproxil fumarate [TDF] and LPV and RTV pharmacokinetic characteristics. Eligible study participants included females and males age 18 years and over receiving the soft gel capsule (SGC) formulation of LPV/ r (400/100mg twice daily) in combination with 1 or more nucleoside/tide antiretroviral agents for treatment of HIV infection for ≥ 2 weeks prior to screening. Major exclusion criteria were pregnancy, concomitant use of a second active protease inhibitor, a non-nucleoside reverse transcriptase inhibitor or concomitant use of medications known to interact with LPV or RTV. Enrollment of subjects was balanced with respect to sex and race/ethnicity. Race/ethnicity was categorized as White Non-Hispanic, Black Non-Hispanic, Hispanic and Other (Asian/Pacific Islander). Laboratory inclusion criteria included a hemoglobin level > 9.4g/dl, serum creatinine < 1.5mg/dl and ALT/AST < 1.5 times upper limit of normal (ULN). In addition, alkaline phosphatase, total bilirubin, albumin and prothrombin time was required to be less than 1.5 times ULN within 30 days prior to study entry.
During the course of this study, a new formulation of LPV/r, the melt extrusion tablet (LPV, 400 mg; RTV, 100 mg), was approved for use by the FDA, and the manufacturer (Abbott Laboratories, Chicago, IL) announced plans to phase out availability of the SGC. Following completion of enrollment and all pharmacokinetic evaluations of the SGC, the protocol was amended to compare the pharmacokinetic parameters of the new tablet formulation of LPV/r with the SGC. At the time of introduction of the tablet LPV/r formulation the only comparative pharmacokinetic data between the two formulations were in healthy volunteers. All participants from step one were offered enrollment into step 2. Additional subjects were recruited to step 2 using identical eligibility criteria for step 1 with the addition of the requirement to be receiving the melt extrusion tablet formulation of LPV/r. All study evaluations of the pharmacokinetics of the LPV/r tablet were conducted exactly the same as for the SGC.
Pharmacokinetic Evaluations
Blood samples were obtained pre-dose and 1, 2, 3, 4, 5, 6, 8, 10 and 12 hours post dose following an observed morning dose of LPV/r, and a standardized breakfast on the day of the pharmacokinetic evaluations. All subjects were required to keep an LPV/r medication diary in the 48 hours immediately prior to the corresponding pharmacokinetic study day, and 100% compliance during this period was required to proceed with the pharmacokinetic evaluations. LPV and RTV concentrations were quantified by validated high-pressure liquid chromatography (HPLC). The assay was linear in the range of 20ng/mL to 20,000ng/mL with a lower limit of quantification (LLOQ) of 20ng/mL using 0.200mL of human plasma. Inter- and intraday accuracy and precision were within ±20% at the LLOQ and ±15% at all other concentrations. Steady-state AUC over the 12-hour (AUC0–12h) dosing interval was determined by non-compartmental methods employing the log-linear trapezoidal rule (WinNonLin v 5.0.1, Pharsight Corporation, Mountain View, CA).8 The steady-state maximum plasma concentration (Cmax), the minimum plasma concentration (Cmin) and 12-hour post dose concentration (C12h) were obtained directly from the data. Apparent oral clearance (CL/F) was obtained from the formula: dose/AUC0–12h and was adjusted for body weight (CLw/F).
Statistical Analyses
Sex differences in LPV and RTV pharmacokinetic parameters were compared using the Wilcoxon rank-sum test while the differences among the racial groups was evaluated using the Kruskal-Wallis test. The effects of TDF on the PK parameters were evaluated by comparing subjects on TDF and subjects not on TDF using the Wilcoxon rank-sum test. The planned sample size was 78 (39 males and 39 females), which had 80% power to detect a 30% difference in LPV AUC between females and males. This difference was chosen to represent a potentially clinically significant difference. All conclusions of statistical tests were made on 2-tailed tests at a 0.05 level of significance.
We also performed a relative bioavailability study on the two LPV/r formulations at steady state. Comparative bioavailability of the tablet formulation relative to the soft-gel formulation was assessed in PK parameters AUC0–12, Cmax, and C12h using 90% confidence intervals (CI). Analyses of the LPV and RTV pharmacokinetic parameters were performed after logarithmic transformation. The corresponding 90% CIs for the geometric mean ratios were then obtained by back transformation. The decision rule of bioequivalence was based on the 80–125% rule proposed by the Food and Drug Administration (FDA; US Food and Drug Administration: Bioavailability and bioequivalence studies for orally administered drug products – general considerations, FDA biopharmaceutics guidance, March 2003 Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070246.pdf. Therefore, bioequivalence was declared if the 90% CIs for the geometric mean ratios of the pharmacokinetic parameters for the tablet formulation relative to the soft-gel formulation were contained within the range of 0.80 and 1.25.
RESULTS
Step 1 began accrual in October 2005 and closed to enrollment in March 2006. Twenty-nine sites from 26 domestic AIDS Clinical Trials Units participated in the study providing geographic diversity in enrollment from within the United States. Step 2 began accrual in October 2006 and completed follow-up in July 2007.
Step 1 enrolled a total of 79 subjects (39 females and 40 males). Pharmacokinetic evaluations from 77 patients (37 females and 40 males) were included in the analysis. Two females were excluded because of incorrect timing of a pharmacokinetic blood sample and a missed scheduled pharmacokinetic blood sample, respectively. Two additional subjects (1 female and 1 male) were initially ineligible because of incorrect timing of a pharmacokinetic sample and violation of the standardized breakfast requirement, respectively. However, these subjects later underwent a repeat pharmacokinetic session and those results were included in the analysis. Step 2 enrolled 79 subjects, 40 females and 39 males. Two subjects (1 female and 1 male) did not provide PK samples and were excluded from the analyses. Of the 77 subjects included in the Step 2 analyses, 22 females and 20 males were enrolled in both Step 1 and Step 2. Demographic and clinical characteristics are shown in Table 1. The median age was 42 years in step 1 and 46 years in step 2, with a higher proportion of older women than men only in step 1. The median BMI in Black and Hispanic females was higher than their male counterparts and the rest of the study population. Female and male groups were balanced with respect to race/ethnicity.
Table 1.
Demographics and Baseline Characteristics
| Step 1 SG Capsule | Step 2 Tablet | |||||
|---|---|---|---|---|---|---|
| Total N=77 |
Male N=40 |
Female N=37 |
Total N=77 |
Male N=38 |
Female N=39 |
|
| Median Age (years) | 42 | 41 | 46 | 46 | 47 | 46 |
| 18 – 29 | 4 (5%) | 3 (8%) | 1 (3%) | 4 (5%) | 3 (8%) | 1 (3%) |
| 30 – 39 | 21 (27%) | 12 (30%) | 9 (24%) | 14 (18%) | 4 (11%) | 10 (26%) |
| 40 – 49 | 28 (36%) | 14 (35%) | 14 (38%) | 31 (40%) | 16 (42%) | 15 (38%) |
| 50 – 59 | 19 (25%) | 8 (20%) | 11 (30%) | 23 (30%) | 11 (29%) | 12 (31%) |
| ≥ 60 | 5 (7%) | 3 (8%) | 2 (6%) | 5 (7%) | 4 (10%) | 1 (3%) |
| Race/Ethnicity | ||||||
| White Non-Hispanic | 24 (31%) | 11 (28%) | 13 (35%) | 22 (29%) | 11 (29%) | 11 (28%) |
| Black Non-Hispanic | 24 (31%) | 13 (33%) | 11 (30%) | 31 (40%) | 16 (42%) | 15 (38%) |
| Hispanic | 23 (30%) | 12 (30%) | 11 (30%) | 22 (29%) | 11 (29%) | 11 (28%) |
| Other | 6 (8%) | 4 (10%) | 2 (5%) | 2 (2%) | 0 (0%) | 2 (6%) |
| Median (Range) Weight (kg) | ||||||
| White Non-Hispanic | 79 (61, 134) | 62 (57, 102) | 74 (61, 106) | 57 (48, 92) | ||
| Black Non-Hispanic | 78 (64, 152) | 83 (61, 129) | 79 (64, 168) | 72 (53, 125) | ||
| Hispanic | 80 (63, 92) | 64 (51, 134) | 73 (63, 96) | 68 (54,125) | ||
| Other | 70 (61, 113) | 81 (78, 83) | NA | 64 (51, 76) | ||
| Median (Range) BMI (kg/m2) | ||||||
| White Non-Hispanic | 26 (21, 38) | 26 (18, 36) | 24 (19, 32) | 21 (15, 35) | ||
| Black Non-Hispanic | 25 (20, 42) | 34 (27, 46) | 25 (20, 46) | 30 (23, 48) | ||
| Hispanic | 21 (21, 32) | 31 (21, 45) | 26 (23, 33) | 27 (21, 47) | ||
| Other | 25 (19, 36) | 30 (29, 31) | NA | 25 (21, 28) | ||
| Median (Range) IBW (kg) | ||||||
| White Non-Hispanic | 73 (62, 82) | 55 (44, 73) | 60 (52, 73) | 44 (39, 52) | ||
| Black Non-Hispanic | 74 (64, 85) | 54 (50, 65) | 62 (53, 85) | 46 (34, 55) | ||
| Hispanic | 66 (55, 76) | 49 (38, 64) | 57 (50, 68) | 46 (39, 47) | ||
| Other | 71 (55, 75) | 57 (57, 57) | NA | 44 (38, 50) | ||
| Median CD4 cells/mm^3 | 506 | 432 | 576 | 500 | 540 | 465 |
| Median CD8 cells/mm^3 | 876 | 871 | 954 | 794 | 917 | 697 |
| HIV-1 RNA (copies/mL) | ||||||
| < 400 | 70 (91%) | 36 (90%) | 34 (92%) | 72 (94%) | 37 (97%) | 35 (90%) |
| ≥ 400 | 7 (9%) | 4 (10%) | 3 (8%) | 5 (6%) | 1 (3%) | 4 (10%) |
| Use of tenofovir | ||||||
BMI= body mass index; IBW= ideal body weight
Median trajectory plots showed that LPV concentrations with the SGC and the tablet were slightly higher in females compared to males at all time points (Figure 1a). However, there was no statistically significant difference in LPV AUC0–12h between females and males with either formulation (Table 2). Further, there were no significant between sex group differences in the other LPV pharmacokinetic parameters with either formulation.
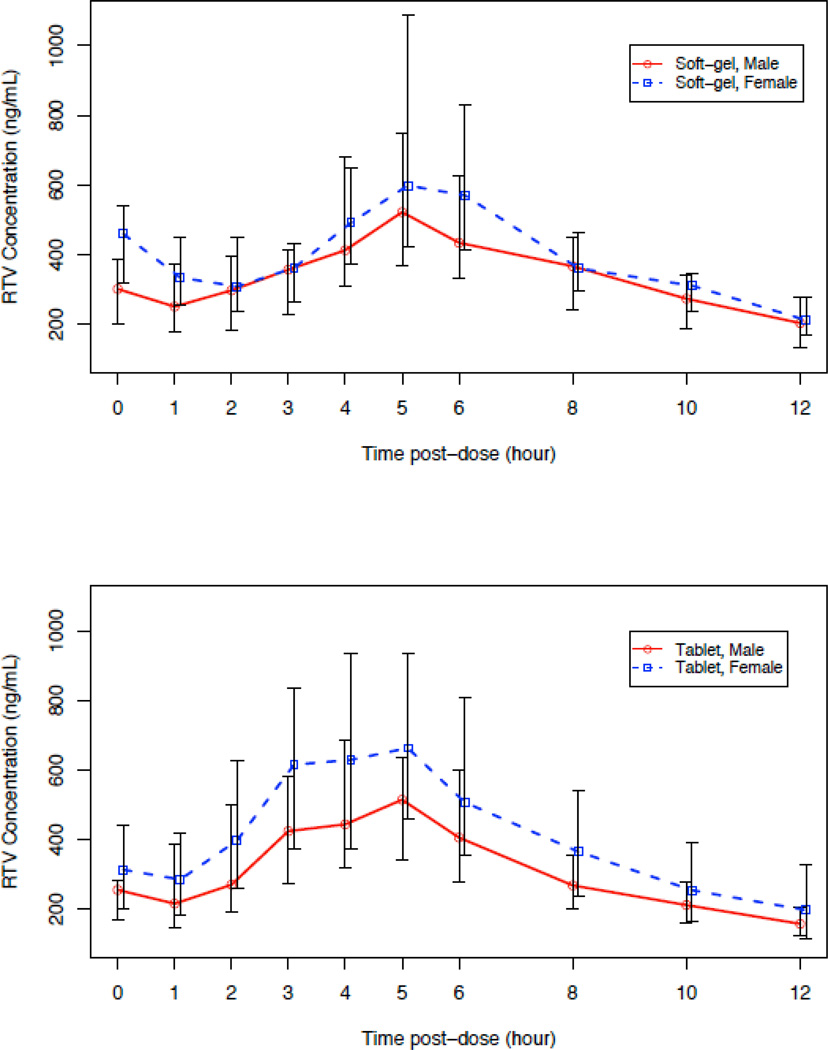
Figure 1.
Median Trajectory Plots of LPV and RTV concentrations over 12 hours.
Table 2.
| A. LPV PK Parameters by sex and drug formulation | |||||||
|---|---|---|---|---|---|---|---|
| LPV PK Parameter |
Sex | Soft Gel Capsule N=(40 males, 37 females) |
Tablet N=(38 males, 39 females) |
||||
| Median | IQR (Q1, Q3) | p-value1 | Median | IQR (Q1, Q3) | p-value1 | ||
| AUC0–12h (ng*hr/mL) | M | 76657 | (59482, 97636) | 0.092 | 81801 | (54670, 93391) | 0.174 |
| F | 91535 | (75051, 97489) | 88909 | (69675, 112954) | |||
| C12 (ng/mL) | M | 4831 | (3178, 6023) | 0.164 | 4041 | (2682, 5378) | 0.113 |
| F | 5413 | (3602, 6614) | 4579 | (2973, 7860) | |||
| Cmax (ng/mL) | M | 9043 | (7015, 10936) | 0.111 | 9327 | (7268, 10711) | 0.118 |
| F | 10129 | (8902, 11596) | 10219 | (8658, 12580) | |||
| CL/F (L/hour) | M | 5.22 | (4.10, 6.73) | 0.092 | 4.89 | (4.28, 7.32) | 0.174 |
| F | 4.37 | (4.10, 5.33) | 4.50 | (3.54, 5.74) | |||
| CLw/F (L/hr/kg) | M | 0.059 | (0.05, 0.09) | 0.351 | 0.064 | (0.05, 0.10) | 0.558 |
| F | 0.061 | (0.04, 0.08) | 0.060 | (0.04, 0.09) | |||
| B. RTV PK parameters by sex and drug formulation | |||||||
|---|---|---|---|---|---|---|---|
| RTV Parameter |
Sex | Soft Gel Capsule N=(40 males, 37 females) |
Tablet N=(38 males, 39 females)) |
||||
| Median | IQR (Q1, Q3) | p-value1 | Median | IQR (Q1, Q3) | p-value1 | ||
| AUC0–12h (ng*hr/mL) | M | 4119 | (3025, 5581) | 0.026 | 3941 | (2912, 4852) | 0.012 |
| F | 5395 | (3824, 6379) | 5310 | (3440, 7192) | |||
| C12 (ng/mL) | M | 203 | (131, 282) | 0.325 | 157 | (121, 208) | 0.100 |
| F | 211 | (167, 276) | 197 | (110, 338) | |||
| Cmax (ng/mL) | M | 635 | (410, 898) | 0.032 | 570 | (421, 745) | 0.006 |
| F | 802 | (492, 1233) | 773 | (587, 1038) | |||
| CL/F (L/hour) | M | 24.31 | (17.92, 33.06) | 0.026 | 25.37 | (20.61, 34.35) | 0.012 |
| F | 18.54 | (15.68, 26.15) | 18.83 | (13.90, 29.07) | |||
| CLw/F (L/hr/kg) | M | 0.305 | (0.229, 0.408) | 0.057 | 0.325 | (0.240, 0.460) | 0.094 |
| F | 0.255 | (0.180, 0.366) | 0.277 | (0.182, 0.412) | |||
p-values of male and female difference using Wilcoxon rank-sum test.
Median trajectory plots showed that RTV concentrations were higher in females compared to males at all time points with both formulations (Figure 1b). In contrast to the results with LPV, females had statistically significant higher median RTV AUC0–12 (SGC: 5395 vs. 4119 ng*hr/ml, p=0.026; tablet 5310 vs 3941 ng*hr/ml p=0.012). Additionally, females had a significantly higher median Cmax and lower median CL/F. After adjustment for body weight, the between sex-group difference in CL/F was only marginally significant (SCG: p=0.057; tablet: p=0.094). In a secondary analysis we evaluated, for each sex, whether the LPV AUC0–12h was dependent on RTV AUC0–12h after adjusting for potential sex differences. The correlation of LPV AUC0–12h with RTV AUC0–12h was statistically significant for both males and females (Spearman’s ρ = 0.89 (tablet) and 0.87 (SGC), p<0.001 for males and ρ = 0.87 (tablet) and 0.61 (SGC), p<0.001 for females). These results suggest that females and males with higher RTV AUCs are expected to have a higher LPV AUC. However, in the overall study population with either formulation, despite the higher RTV AUC0–12h observed among women, the sex difference in LPV AUC did not reach statistical significance.
There were no apparent differences in LPV and RTV pharmacokinetic parameters among the different racial groups (female and males combined) with either drug formulation. Further, a 2-way ANOVA did not show any interaction effects in the LPV and RTV PK parameters between race and sex with the SGC; however, for the tablet formulation, LPV Tmax was significantly later in white non-Hispanic females (p=0.019) and Hispanic females (p=0.033) compared to their male counterparts, whereas RTV Tmax was significantly later in White non-Hispanic females (p=0.018) compared to White non-Hispanic males (p=0.018).
We examined the effect of TDF on LPV and RTV pharmacokinetic parameters for both formulations. Median LPV (RTV) AUC0–12h, C12 and Cmin of the tablet formulation in subjects who were on TDF (n=53) were 0% (13%), 3% (24%), and 4% (16%) lower, respectively, compared to those who were not on TDF (n=23), but these reductions were not statistically significant. Similar trend of changes were shown for the SGC formulation. Within the subgroup of participants who were on TDF and the tablet formulation, median RTV pharmacokinetic parameters AUC0–12h and Cmax were significantly higher in females (23%, p=0.021 and 33%, p=0.007, respectively) while median RTV CL/F was significantly lower in females (19%, p=0.021). After adjustment for weight, RTV CL/F was not significantly different between females and males.
A total of 42 subjects (22 females and 20 males) had evaluable AUC0–12h, Cmax, and C12 parameters for both step 1 and step 2. Table 3 presents relative bioavailability (as geometric mean ratios) and 90% CIs for the within subject changes in LPV and RTV AUC0–12h, Cmax, and C12 for females and males combined and separately. In the analyses with all subjects combined, the 90% CIs for all the LPV PK parameters were well within the FDA bioequivalence acceptance range and the two formulations were considered to be bioequivalent in LPV pharmacokinetics. However, when men and women were considered separately, the upper bound of the 90% CI for the LPV C12 slightly exceeded 1.25 for both women (1.285) and men (1.282). The two formulations in the RTV AUC0–12h and Cmax were considered to be bioequivalent when both males and females were combined. However, the 90% CIs for RTV C12 did not fall within the bioequivalence acceptance range. When men and women were examined separately, only the 90% CI for RTV C12 was not within the bioequivalence acceptance range in men. In women, however, none of the RTV pharmacokinetic parameters of interest (ie., AUC0–12h, Cmax and C12) had their 90% CIs within the bioequivalence acceptance range with lower bounds of 0.755, 0.717 and 0.649, respectively. When sex was considered as a covariate in the bioequivalence evaluations the results were largely consistent, although in this analysis the 90% CI for C12 of either LPV or RTV was not within the bioequivalence acceptance range.
Table 3.
Relative bioavailability and 90% confidence intervals for LPV and RTV comparing soft-gel capsules (reference) to tablet (test).
| PK parameter | males and female combined (N = 42) |
males and females separately (N = 20 males, 22 females) |
All data (model including SEX as a covariate, N=42) |
||
|---|---|---|---|---|---|
| GMR (90% CI) | GMR (90% CI) | Estimated mean (90% CI) |
|||
| LPV | AUC0–12h (ng*hr/mL) | 0.987 (0.904, 1.077) | M | 0.977 (0.880, 1.085) | 0.977 (0.859, 1.112) |
| F | 0.996 (0.861, 1.151) | ||||
| Cmax (ng/mL) | 0.969 (0.898, 1.046) | M | 0.952 (0.861, 1.053) | 0.952 (0.851, 1.065) | |
| F | 0.985 (0.872, 1.111) | ||||
| C12 (ng/mL) | 1.014 (0.863, 1.191) | M | 1.000 (0.780, 1.282) | 1.000 (0.790, 1.267) | |
| F | 1.026 (0.819, 1.285) | ||||
| RTV | AUC0–12h (ng*hr/mL) | 0.944 (0.835, 1.068) | M | 0.962 (0.834, 1.108) | 0.962 (0.802, 1.153) |
| F | 0.929 (0.755, 1.143) | ||||
| Cmax (ng/mL) | 0.937 (0.822, 1.068) | M | 0.991 (0.848, 1.159) | 0.991 (0.819, 1.200) | |
| F | 0.890 (0.717, 1.103) | ||||
| C12 (ng/mL) | 0.884 (0.687, 1.138) | M | 0.913 (0.578, 1.441) | 0.913 (0.630, 1.322) | |
| F | 0.859 (0.649, 1.136) | ||||
DISCUSSION
We found no statistically or clinically significant difference in the pharmacokinetics of LPV between females and males with either the SGC or tablet formulation of LPV/r. However, we did identify a statistically significant difference in the pharmacokinetics of RTV between females and males with both the SCG and tablet formulations. The median RTV AUC0–12h was 31% higher in females compared with males on the SCG formulation and 35% higher with the tablet. These increased concentrations in females arose from a lower median RTV CL/F in females compared with males, which was 24% lower for the SGC and 26% lower for the tablet. There was a statistically significant correlation between the AUC0–12 values of RTV and LPV for both females and males.
There was no statistically significant relationship between race and pharmacokinetics of LPV or RTV. Our results are consistent with the findings of prior investigations examining race and LPV pharmacokinetics.9 An analysis of single dose pharmacokinetic studies in healthy volunteers (n=194) showed a marginally significantly lower LPV AUC (−14%, p=0.053) and Cmax (−14%, p=0.02) among Blacks (n=20) without adjustment for weight or sex.12 More recently, a large therapeutic drug monitoring study of LPV found no effect of race on LPV trough concentrations after controlling for weight.10 We observed a non-significant trend towards decreased LPV AUC0–12h in patients treated with TDF. Our study was not designed a priori with statistical power to detect the effect of use of TDF on LPV AUC. The data from previous studies regarding the effect of TDF co-administration on LPV AUC are conflicting. While two small studies11–12 concluded that co-administration resulted in a decreased LPV AUC, a third study showed no effect.13 The data from prospective clinical trials indicate that combination therapy with LPV/r and TDF is associated with sustained virologic suppression over 48–96 weeks of treatment.14
To our knowledge, this is the first prospective pharmacokinetic study of LPV/r specifically designed and powered to detect sex differences. Much of the early literature on sex differences in antiretroviral pharmacokinetics arose from retrospective reviews of therapeutic drug monitoring databases, studies conducted in HIV-uninfected individuals, studies based on random drug concentrations, or not designed to have adequate statistical power to detect sex differences. Collectively, the results from our study suggest that there are not likely to be clinically significant differences in LPV pharmacokinetics between females and males. Prior retrospective studies of LPV have reported conflicting results. Whereas one large study did not find any effect of sex on pharmacokinetic parameters,9, another showed that plasma LPV concentration-ratios were significantly higher in females compared to males, and this difference was explained by lower weight in females.5 It is possible that the higher average weight observed among the women in our study [median body weight 77.6 kg (range 50.6 to 134 kg)] precluded us from identifying a relationship between lower weight and female sex and LPV pharmacokinetics. Our findings of no sex difference in LPV pharmacokinetics are consistent with two large population pharmacokinetic studies with a combined total of 1181 in HIV-infected persons, 27% female, receiving the tablet LPV/r formulation that also found no sex difference.15–16 Sex differences in pharmacokinetics have been reported for the protease inhibitors saquinavir7 and indinavir6. Our results also demonstrate a sex difference for RTV, with females having a 31% higher AUC0–12h with the SGC formulation and a 35% higher AUC0–12h with the tablet. These results are consistent with those from a recently reported small pharmacokinetic sub-study within the Gender Race and Antiretroviral Experience (GRACE) trial, which reported a 20% higher AUC for darunavir and a 70% higher AUC for RTV among women compared to men.17
The mechanism of the lower RTV CL/F in females is unknown. There are reports of sex differences in drug metabolizing enzymes, drug transporters and factors affecting drug distribution.18–20 A study of midazolam as a selective probe for intestinal CYP3A activity demonstrated a faster systemic and oral clearance in women than in men.21 In contrast, the apparent oral clearance of verapamil, a mixed CYP3A and P-glycoprotein substrate (like LPV/r), was found to be slower in women than in men.22–23 These findings might indicate that the basis for the sex difference in RTV concentrations arises as a consequence of P-glycoprotein expression or function. It is also of interest that higher RTV concentrations among women taking the tablet formulation occurred among the subset of subjects who were also taking TDF. A drug-drug interaction between TDF and the protease inhibitor atazanavir has been described, but in this interaction TDF lowers the plasma concentrations of atazanavir whether it is given with RTV or not. These data suggest that TDF has the ability to interact with protease inhibitors, but the mechanism(s) of these interactions is/are unknown.
Our study design allowed us the opportunity to examine the bioequivalence of the soft gel capsule and tablet formulations of LPV and RTV in the group as a whole and by sex, however we acknowledge that the study was not originally designed as a bioequivalence study. We found the SGC and tablet formulations met the FDA definition of bioequivalence for the pharmacokinetic characteristics of LPV. Bioequivalence was also shown for RTV AUC0–12h and Cmax; however the lower bound of the 90% confidence interval for RTV C12 was 0.69, lower than the 0.80 threshold. The only other assessment of the bioequivalence of the SGC and tablet formulations of LPV and RTV has been performed in healthy volunteers.24 That study demonstrated the SGC and tablet formulations were bioequivalent for LPV and RTV AUC. The Cmax for both LPV and RTV did not meet bioequivalence with the point estimates of 1.24 and 1.35 for LPV and RTV, respectively, and upper bounds of the 90% confidence intervals greater than 1.25. While the formulations were bioequivalent for LPV and RTV, the point estimates for AUC, were 1.18 and 1.19, respectively, indicating consistently higher mean LPV and RTV concentrations occurred with the tablet formulation. It has been suggested this may be the result of a higher bioavailability of the tablet formulation although it may also be a consequence of the single dose design of the healthy volunteer study. In the present study in HIV-infected persons, a pattern of consistently higher mean LPV and RTV concentrations with the tablet formulation was not observed. Possible explanations include differences in the standardized meals used between the two studies, the absence of a food effect for the tablet formulation or subtle differences in pharmacokinetics between healthy volunteers and HIV-infected persons.
Our study had some important limitations. First, we selected a patient population who were already stable on LPV/r. This limited our ability to observe a relationship between drug concentrations and side effects as subjects who developed toxicity early who may have discontinued the drug. A more appropriate way to evaluate the toxicity relationship would be to conduct the study in treatment naïve patients initiating therapy. Our study was also not powered to examine bioequivalence by sex. Our pharmacokinetic study was conducted under rigorous conditions. Patients receiving concomitant medications that might be used in clinical practice, and which were known to affect the pharmacokinetics of LPV or RTV, were excluded. This setting afforded us the best chance to isolate the effects of sex on pharmacokinetics, but may limit the generalizability of the results.
In conclusion, we found no statistically significant differences in the pharmacokinetics of LPV between males and females. We did find the median RTV AUC0–12h was higher in females compared with males with both formulations (31% higher with SGC and 35% higher with tablet) and that this higher AUC0–12h did not arise simply because of a body weight difference in females vs. males as the weight adjusted oral clearance of RTV was slower in females than males. The higher median RTV concentrations in women on the tablet formulation were most evident in the subset receiving TDF. The potential impact of these differences in clinical practice are not fully understood as the higher RTV AUC0–12h in this study did not impact overall LPV exposure. However, the sex differences in RTV concentrations might have a significant influence in settings where RTV is used to boost other protease inhibitors or in the magnitude and perhaps clinical significance of RTV drug-drug interactions. These issues warrant careful evaluation, as do studies to elucidate the mechanism of the lower RTV oral clearance in HIV-infected females.
ACKNOWLEDGEMENTS
We would like to thank all the ACTG sites nationwide (listed below) that participated in this study. We would like to acknowledge the contributions of Lane Bushman, Michelle Ray, Tom Delahunty, Sarah Nelson and Brian Robbins of the Antiviral Pharmacology Laboratory at the University of Colorado Health Sciences Center and University of Nebraska Medical Center for performing the analysis of lopinavir and ritonavir concentrations. We thank Richard Rode and Juki Ng for their helpful comments. Most especially, we are very grateful to the study participants.
FUNDING
Support for this study was provided by the National Institutes of Allergy and Infectious Diseases, AIDS Clinical Trials Group (NIAID AI-38858) and Abbott Laboratories. Dr Currier is supported by UOI AI 069424 and K24 AI 56933 and Dr Fletcher is supported by UOI AI68636. Support for this study to the SDAC/Harvard School of Public Health was provided by the NIAID AI-68634.
Janet Forcht, RN and Judith Aberg, MD- NYU/NYC HHC at Bellevue (A0401) CTU Grant #AI069532 – 01, GCRC Grant # M01-RR00096
Maricela Gonzalez -UCLA School of Medicine (A0601) CTU Grant #AI069424
Angela Grbic and Eric Daar- Harbor-UCLA Medical Center (A0603) CTU Grant #AI069424
Sharon Riddler, MD, MPH, and Carol Oriss, BSN, RN- University of Pittsburgh (A1001) CTU Grant #U01 AI 69494-01
Princy N. Kumar, MD and Karyn Hawkins, RN- Georgetown University (A1008) ACTU Grant #1U01AI069494, Sub Award #0002268
Jane Reid, RNc, MS, ANP and Carol Greisberger, RN- University of Rochester (A1101) CTU Grant #AI69411, GCRC Grant #5-MO1 RR00044
Kim Whitely and Ann Conrad- MetroHealth Medical Center (A2503) CTU Grant #1U01AI069501, GCRC Grant #M01RR000080
Beverly E. Sha, MD and Janice Fritsche, MS, APRN, BC- Rush University Medical Center (A2702) CTU Grant #1 U01 AI069471
Oluwatoyin Adeyemi, MD and Joanne Despotes, RN- CORE Center, Chicago (A2705) CTU Grant #5 UO1 AI025915-18
Kristine Patterson, MD and Susan Pedersen, RN- University of North Carolina (A3201) CTU Grant #AI69423-01, GCRC Grant #RR00046, CFAR Grant #AI50410
Ilene Wiggins, RN and Charles Flexner, MD- Johns Hopkins School of Medicine (A0201) CTU grant #AI-69465 and GCRC Grant #RR-00052
Vicki Bailey, RN and Huso Erdem- Vanderbilt University (A3652) CTU Grant #AI069439
Edward Seefried and Dee Pacheco- University of California, San Diego (A0701) CTU Grant # AI 69432
Sondra Middleton, PA-C and Gwendolyn Costantini, FNP- Beth Israel Medical Center (A2851) CTU Grant #AI46370
Lorna Nagamine, RN and Debra Ogata-Arakaki, RN- University of Hawaii at Manoa (A5201) CTU Grant # AI34853
Columbia University (A7802)
Jane Norris PA-C, and Sandra Valle PA-C- Stanford University (A0501) CTU Grant # AI 69556-01
Connie A. Funk, RN, MPH and Luis Mendez, BS- University of Southern California (A1201) CTU Grant # 1UO1AI069428-01
Shelia Dunaway, MD and Ann Collier, MD- University of Washington, Harborview (A1401) CTU Grant #AI 069434
Mark Rodriguez, RN, BSN and Michael K. Klebert, RN-CS, MSN, ANP- Washington University in St. Louis (A2101) CTU Grant #AI069495
Indiana University (A2601)
Graham Ray and Monica Carten- University of Colorado Health Sci Ctr (A6101) CTU Grant #1U01AI069450, GCRC Grant #RR00051
University of Pennsylvania (A6201)
Paul R. Skolnik, MD and Betsy Adams, RN- Boston Medical Center (A0104) CTU Grant #1 U01 AI069472-01
Susan L. Koletar, MD and Kathy Watson, RN- Ohio State University (A2301) CTU Grant #AI069474, GCRC Grant #Grant M01-RR00034
Pamposh Kaul, MD and Jenifer Baer, RN- University of Cincinnati (A2401) CTU Grant # AI-069513
Jorge L Santana Bagur, MD and Santiago Marrero de León, MD- University of Puerto Rico (A5401) CTU Grant #1 U01 AI069415-01
University of Texas, Galveston (A3601)
Leslie Thompson RN, BSN and Margaret A. Fischl, MD- University of Miami (A0901)
Footnotes
Disclosures: A.E.H is an employee of Abbott Laboratories. All other authors: none to declare.
REFERENCES
- 1. [Accessed March 29, 2010]; JC1700_Epi_Update_2009_en.pdf. Available at http://data.unaids.org/pub/EpiReport/2009/2009.
- 2.Anderson PL, Kakuda TN, Kawle S, Fletcher CV. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS. 2003;17(15):2159–2168. doi: 10.1097/00002030-200310170-00003. [DOI] [PubMed] [Google Scholar]
- 3.Bersoff-Matcha SJ, Miller WC, Aberg JA, van Der Horst C, Hamrick HJ, Jr, Powderly WG, Mundy LM. Sex differences in nevirapine rash. Clin Infect Dis. 2001;32(1):124–129. doi: 10.1086/317536. Epub 2000 Dec 13. [DOI] [PubMed] [Google Scholar]
- 4.Burger DM, van der Heiden I, La Porte CJL, van er Ende ME, Groeneveld P, Richter C, Koopmans PP, et al. Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor: efavirenz: the effect of gender, age, race, and CYP2B6 polymorphism. Br J Clin Pharmacol. 2006 Feb;61(2):148–154. doi: 10.1111/j.1365-2125.2005.02536.x. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Leur MR, Burger DM, La Porte CJ, Koopmans PP. A retrospective TDM database analysis of interpatient variability in the pharmacokinetics of lopinavir in HIV-infected adults. Ther Drug Monit. 2006 Oct;28(5):650–653. doi: 10.1097/01.ftd.0000245681.12092.d6. [DOI] [PubMed] [Google Scholar]
- 6.Burger DM, Siebers MC, Hugen PW, Aamoutse RE, Hekster YA, Koopmans PP. Pharmacokinetic variability caused by gender: do women have higher indinavir exposure than men? J Acquir Immune Defic Syndr. 2002;29(1):101–102. doi: 10.1097/00126334-200201010-00014. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher CV, Jiang H, Brundage RC, Acosta EP, Haubrich R, Katzenstein D, Gulick RM. Sex-based differences in saquinavir pharmacology and virologic response in AIDS Clinical Trials Group Study 359. J Infect Dis. 2004;189(7):1176–1184. doi: 10.1086/382754. Epub 2004 Mar 16. [DOI] [PubMed] [Google Scholar]
- 8.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York: Marcel Dekker, Inc; 1982. [Google Scholar]
- 9.Bertz R, Lam W, Hsu A, Granneman GR, Sun E. Effects of Gender, Race, Age and Weight on the Pharmacokinetics of Lopinavir (Lopinavir/Ritonavir) in Healthy Adult Subjects™ after Single-Dose Kaletra. Second International Workshop on Clinical Pharmacology of HIV therapy; Noordwijk, Netherland. 2001. Abstract 3.12. [Google Scholar]
- 10.van der Leur MR, Burger DM, la Porte CJL, Koopmans PP. A retrospective TDM database analysis of interpatient variability in the pharmacokinetics of lopinavir in HIV-infected adults. Ther Drug Monit. 2006;28(5):650–653. doi: 10.1097/01.ftd.0000245681.12092.d6. [DOI] [PubMed] [Google Scholar]
- 11.Breilh DH, Rouzes A, Djabarouti S, Pellegrin I, Xuereb F, Coupet AC. Pharmacokinetic drug interaction of lopinavir/ritonavir in combination with tenofovir in experienced HIV positive patients. Forty-fourth Interscience Conference on Antimicrobials and Chemotherapy; Washington DC, USA. 2004. Abstract A-445. [Google Scholar]
- 12.Ofotokun I, Chuck SK, Binongo JN, Palau M, Lennox JL, Acosta EP. Lopinavir/Ritonavir Pharmacokinetic Profile: Impact of Sex and Other Covariates Following a Change From Twice-Daily to Once-Daily Therapy. J Clin Pharmacol. 2007;47(8):970–977. doi: 10.1177/0091270007302564. Epub 2007 Jul 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kearney BP, Mathias A, Mittan A, Sayre J, Ebrahimi R, Cheng AK. Pharmacokinetics and safety of tenofovir disoproxil fumarate on coadministration with lopinavir/ritonavir. J Acquir Immune Defic Syndr. 2006;43(3):278–283. doi: 10.1097/01.qai.0000243103.03265.2b. [DOI] [PubMed] [Google Scholar]
- 14.Johnson MA, Gathe JC, Podzamczer D, et al. A Once-Daily Lopinavir/Ritonavir-Based Regimen Provides Noninferior Antiviral Activity Compared With a Twice-Daily Regimen. J Acquir Immune Defic Syndr. 2006;43(2):153–160. doi: 10.1097/01.qai.0000242449.67155.1a. [DOI] [PubMed] [Google Scholar]
- 15.Klein C, Ng J, Diderichsen PM, de Silva B, Bernstein B, Awni W. HIV 9. Glasgow, United Kingdom; 2008. Nov 9–13, Population pharmacokinetic/pharmacodynamic analyses of lopinavir and ritonavir in subjects receiving the tablet formulation. Abstract P245. [Google Scholar]
- 16.Ng J, Noertersheuser PA, Mensing S, Awni WA, Klein CE. Population pharmacokinetic analysis of lopinavir and ritonavir in HIV-1-treatment experienced subjects receiving the lopinavir/ritonavir tablet formulation. 12th European AIDS Conference; November 11–14, 2009; Cologne, Germany. Abstract PE4.1/4. [Google Scholar]
- 17.Sekar V, Ryan R, Schaible D, Mazikewich A, Mrus J. Pharmacokinetic profile of darunavir (DRV) co-administered with low dose ritonavir in treatment experienced women and men: 4 week analysis in a substudy of the GRACE trial. 9th International Workshop on Clinical Pharmacology of HIV Therapy (IWCPHIV); April 7–9, 2008; New Orleans, LA. [Google Scholar]
- 18.Gleiter CH, Gundert-Remy U. Gender differences in pharmacokinetics: Eur J Drug Metab Pharmacokinet. 1996;21(2):123–128. doi: 10.1007/BF03190260. [DOI] [PubMed] [Google Scholar]
- 19.Harris RZ, Benet LZ, Schwartz JB. Gender effects in pharmacokinetics and pharmacodynamics. Drugs. 1995;50(2):222–239. doi: 10.2165/00003495-199550020-00003. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka E. Gender-related differences in pharmacokinetics and their clinical significance. J Clin Pharm Ther. 1999;24(5):339–346. doi: 10.1046/j.1365-2710.1999.00246.x. [DOI] [PubMed] [Google Scholar]
- 21.Gorski JC, Jones DR, Haehner-Daniels BD, Hamman MA, O’Mara EM, Jr, Hall SD. The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther. 1998;64:133–143. doi: 10.1016/S0009-9236(98)90146-1. [DOI] [PubMed] [Google Scholar]
- 22.Kates RE, Keefe DL, Schwartz J, Harapat S, Kirsten EB, Harrison DC. Verapamil disposition kinetics in chronic atrial fibrillation. Clin Pharmacol Ther. 1981;30:44–51. doi: 10.1038/clpt.1981.125. [DOI] [PubMed] [Google Scholar]
- 23.Krecic-Shepard ME, Barnas CR, Slimko J, Jones MP, Schwartz JB. Gender-specific effects on verapamil pharmacokinetics and pharmacodynamics in humans. J Clin Pharmacol. 2000;40:219–230. doi: 10.1177/00912700022008883. [DOI] [PubMed] [Google Scholar]
- 24.Klein CE, Chiu YL, Awni WA, Zhu T, Heuser RS, Doan T, Breitenbach J, Morris JB, Brun SC, Hanna GJ. The tablet formulation of lopinavir/ritonavir provides similar bioavailability to the soft gelatin capsule formulation with less pharmacokinetic variability and diminished food effect. J Acquir Immune Defic Syndr. 2007;44:401–410. doi: 10.1097/QAI.0b013e31803133c5. [DOI] [PubMed] [Google Scholar]