Abstract
Background
The STOP-Bang questionnaire is used to screen patients for obstructive sleep apnoea (OSA). We evaluated the association between STOP-Bang scores and the probability of OSA.
Methods
After Institutional Review Board approval, patients who visited the preoperative clinics for a scheduled inpatient surgery were approached for informed consent. Patients answered STOP questionnaire and underwent either laboratory or portable polysomnography (PSG). PSG recordings were scored manually. The BMI, age, neck circumference, and gender (Bang) were documented. Over 4 yr, 6369 patients were approached and 1312 (20.6%) consented. Of them, 930 completed PSG, and 746 patients with complete data on PSG and STOP-Bang questionnaire were included for data analysis.
Results
The median age of 746 patients was 60 yr, 49% males, BMI 30 kg m−2, and neck circumference 39 cm. OSA was present in 68.4% with 29.9% mild, 20.5% moderate, and 18.0% severe OSA. For a STOP-Bang score of 5, the odds ratio (OR) for moderate/severe and severe OSA was 4.8 and 10.4, respectively. For STOP-Bang 6, the OR for moderate/severe and severe OSA was 6.3 and 11.6, respectively. For STOP-Bang 7 and 8, the OR for moderate/severe and severe OSA was 6.9 and 14.9, respectively. The predicted probabilities for moderate/severe OSA increased from 0.36 to 0.60 as the STOP-Bang score increased from 3 to 7 and 8.
Conclusions
In the surgical population, a STOP-Bang score of 5–8 identified patients with high probability of moderate/severe OSA. The STOP-Bang score can help the healthcare team to stratify patients for unrecognized OSA, practice perioperative precautions, or triage patients for diagnosis and treatment.
Keywords: mass screening, obstructive/ep (epidemiology), polysomnography, prospective studies, questionnaires, sleep apnoea, snoring/di (diagnosis), snoring/ep (epidemiology)
Editor's key points.
The authors investigated the value of STOP-Bang score in predicting obstructive sleep apnoea (OSA) in surgical patients.
Results from 746 patients were analysed.
The odds ratio of moderate-to-severe OSA increased with the increase in the score.
Importantly, the study shows the usefulness of STOP-Bang score in predicting OSA.
Obstructive sleep apnoea (OSA) is a common medical condition affecting 2–26% of the general population1 and can occur in all age groups.2 Studies have shown that even asymptomatic OSA is independently associated with an increased morbidity and mortality.3,4 Patients with OSA were found to have an increase in postoperative complications.5–9 It is, therefore, imperative to have an early diagnosis of OSA. However, it is estimated that 82% of men and 92% of women with moderate-to-severe sleep apnoea have not been diagnosed.10 The use of preoperative screening instruments will help to identify the patients with undiagnosed OSA.11–13
The STOP-Bang questionnaire is a scoring model consisting of eight easily administered questions starting with the acronym STOP-Bang (Appendix) and is scored based on Yes/No answers (score: 1/0). Thus, the scores range from a value of 0 to 8. A score of ≥3 has shown a high sensitivity for detecting OSA: 93% and 100% for moderate and severe OSA, respectively.11
Owing to its high sensitivity at a score of ≥3, the STOP-Bang questionnaire is considered very helpful to rule out patients having moderate and severe OSA.11 However, the specificity at the same cut-off is low: 47% and 37% for moderate and severe OSA, respectively, resulting in fairly high false-positive rates. The objective of this study is to evaluate the predictive probabilities for OSA at different scores on the STOP-Bang questionnaire. We hypothesize that a high STOP-Bang score indicates a high probability of moderate/severe OSA.
Methods
The study was conducted in the preoperative clinics of Toronto Western Hospital and Mount Sinai Hospital, Toronto, Ontario, Canada. Institutional Review Board approvals were obtained from both institutions (MSH: 06-0143-E and 07-0183-E; UHN: 06-0135-AE and 07-0515-AE). Patients aged 18 yr or older, who were ASA I–IV, and were undergoing elective procedures in general surgery, gynaecology, orthopaedics, urology, plastic surgery, ophthalmology, or spinal surgery were included in the screening process and approached for consent by the research assistants for the preoperative polysomnograpy (PSG). Patients who were unwilling or unable to give informed consent or patients who were expected to have abnormal EEG findings (e.g. brain tumour, epilepsy surgery, patients with deep brain stimulator) were excluded.
All the patients were asked to complete the STOP questionnaire.11 Information concerning BMI, age, neck circumference, and gender (Bang) were collected by a research assistant. In the initial 2 yr period of the study, the patients were invited to undergo a laboratory PSG. During the subsequent 2 yr of the study, the patients underwent a portable PSG study at home. The results of the PSG were used to evaluate the various scores of the STOP-Bang questionnaire.
The portable PSG was performed with a level 2 portable sleep device (Embletta X100) which is shown to be a reliable alternative for standard PSG in surgical patients.14 The PSG recordings were performed at the patients’ home. The recording montage consisted of two EEG channels (C3 and C4), electrooculogram (left or right), and chin muscle EMGs. Thoracic and abdominal respiratory effort bands, body position sensors, and pulse oximeter were also used.
The device was attached to patients by a well-trained PSG technician at their home and the overnight recordings were unattended. The patients were advised on how to remove the device which was picked up the next morning from the patients’ home by the same sleep technician. A certified PSG technologist who was blinded to the study information analysed the PSG. The manual scoring was performed using Somnologia Studio 5.0 as the scoring platform. Manual scoring was performed according to the Manual of the American Academy of Sleep Medicine.15
The laboratory PSG was performed overnight and patients went to bed at their usual bedtime. A standard EEG montage consisting of EEG, electrooculogram, submental EMG, and ECG obtained with surface electrodes were used to collect the sleep architectural data. A pulse oximeter measured the oxygen saturation. Additional recordings included the respiratory effort by thoraco-abdominal excursion, respiratory inductive plethysmography, and oronasal airflow.
A certified polysomnographic technologist scored the polysomnographic recordings under the supervision of a sleep physician who assessed and approved the reports. The technologist was blinded to the results of the STOP-Bang questionnaire and other clinical information about the patients. The sleep stages and apnoea–hypopnea index (AHI) were scored according to the American Academy of Sleep Medicine Task Force recommendations.16
The diagnosis of OSA was based on an AHI >5 with fragmented sleep and daytime sleepiness. The severity of OSA with both laboratory and portable PSG was classified based on the AHI values: >5–15 as mild OSA, >15–30 as moderate OSA, and >30 as severe OSA.15,16
Statistical analysis
Statistical analyses were performed using SAS version 9.2. The patient characteristic data are presented with descriptive statistics; median and inter-quartile range were used for non-normally distributed continuous data, and frequency and percentage were used for categorical data. Predicted probabilities for each score at cut-off points of all OSA (AHI>5), moderate/severe OSA (AHI>15), and severe OSA (AHI>30) were calculated using logistic regression, and plotted. The probability and its 95% confidence interval (95% CI) were calculated for each score. The STOP-Bang scores of 7 and 8 were combined due to the small number of patients with either score. A similar strategy was followed with scores 0, 1, and 2.
To assess the performance of the STOP-Bang questionnaire, multiple 2×2 contingency tables were used to calculate sensitivity, specificity, positive predictive values (PPVs), and negative predictive values (NPVs) for each score. The response was dichotomized using all OSA (AHI>5), moderate/severe OSA (AHI>15), and severe OSA (AHI>30) as the cut-offs. The area under the receiver operating curves was calculated using logistic regression to assess the diagnostic ability of the STOP-Bang questionnaire.
Multinomial logistic regression was used to compare the severity of the AHI with the STOP-Bang questionnaire score. For the dependent variable, an AHI ≤5 was classified as non-OSA and was used as the reference. For the independent variable, patients who scored 0, 1, or 2 were grouped as the reference. Odds ratios (ORs) and 95% confidence intervals of each STOP-Bang score group (3, 4, 5, 6, 7, and 8) at different AHI cut-offs were calculated.
Results
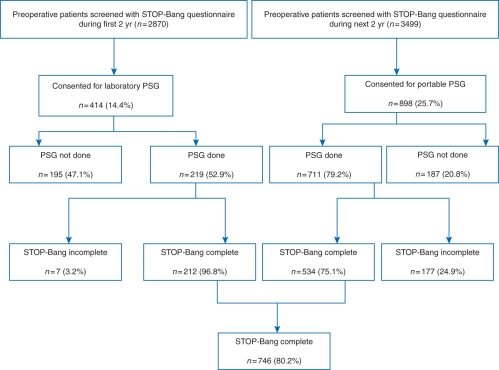
A total of 6369 patients were approached for consent and screened for OSA by the STOP-Bang questionnaire. Of the 2870 patients screened and invited for laboratory PSG, 414 (14.4%) patients gave consent. Of the 3499 patients screened and invited for portable PSG, 898 (25.7%) patients gave consent. Laboratory PSG was completed by 219 patients, and 711 patients completed portable PSG. Of the 930 patients who completed the PSG, 212 patients with a laboratory PSG and 534 patients with a portable PSG answered all of the items in the STOP questionnaire and had complete documentation of BMI, age, gender, and neck circumference. These 746 patients were used for the analysis (Fig. 1).
Fig 1.
Screening flow chart of patients. PSG, polysomnography.
The summary of age, gender, BMI, and neck circumference of the different patient groups is shown in Table 1. The patient characteristics were similar between the 930 patients who underwent a PSG and the 5439 patients who did not undergo a PSG due to the reasons of no consent or no show. The 184 patients, who underwent a PSG but did not complete all the elements of the STOP-Bang questionnaire, were excluded from the analysis set. Patient characteristics other than the neck circumference were similar between the 184 patients excluded from the analysis set and the 746 patients used for the analysis.
Table 1.
Patient characteristics. Data shown as median (inter-quartile range) or number with percentage in parenthesis. *n=1634. †STOP-Bang incomplete due to missing data
| PSG done |
||||
|---|---|---|---|---|
| PSG not done | Total | STOP-Bang complete | STOP-Bang incomplete | |
| n | 5439 | 930 | 746 | 184 |
| Gender [male/female] | 2504/2935 (46/54) | 445/485 (48/52) | 365/381 (49/51) | 80/104 (44/56) |
| Age (yr) | 58 (47–69) | 60 (52–69) | 60 (51–68) | 61 (54–69) |
| Neck circumference (cm) | 38 (35–40)* | 39 (36–42) | 39 (36–42) | —† |
| BMI (kg m−2) | 27 (24–31) | 30 (26–34) | 30 (26–35) | 30 (26–34) |
Of the 746 patients used for analysis, there were 510 (68.4%), 287 (38.5%), and 134 (18.0%) patients who had OSA (AHI>5), moderate/severe OSA (AHI>15), and severe OSA (AHI>30), respectively. The distribution of each of the STOP-Bang scores is detailed in Figure 2. Most patients had a STOP-Bang score of 3 (22.9%) and 4 (22.3%).
Fig 2.
Distribution of patients according to their STOP-Bang score.
The area under the receiver operating curves was 0.65 (95% CI: 0.61–0.70), 0.67 (95% CI: 0.63–0.70), and 0.71 (95% CI: 0.66–0.75) for all OSA, moderate/severe OSA, and severe OSA, respectively. Although the areas under the receiver operating curves do not show perfect discrimination, the confidence intervals do not include 0.5, confirming the diagnostic ability of the STOP-Bang questionnaire. The STOP-Bang questionnaire had the best discrimination with severe OSA.
For a STOP-Bang score of 5, the OR for moderate/severe was 4.8 (95% CI: 2.8–8.0) and for severe OSA was 10.4 (95% CI: 4.5–24.3). For a STOP-Bang score of 6, the OR for moderate/severe was 6.3 (95% CI: 3.4–11.7) and for severe OSA was 11.6 (95% CI: 4.6–28.7). For a STOP-Bang score of 7 and 8, the OR for moderate/severe was 6.9 (95% CI: 3.3–14.3) and for severe OSA was 14.9 (95% CI: 5.6–39.6) (Table 2).
Table 2.
ORs (95% CIs) of different STOP-Bang scores for OSA at different AHI cut-offs. AHI, apnoea–hypopnoea index; OSA, obstructive sleep apnoea; Mod/Sev OSA, moderate/severe OSA
| ORs for OSA at different AHI cut-offs |
|||
|---|---|---|---|
| STOP-Bang score | All OSA (AHI>5) | Mod/Sev OSA (AHI>15) | Severe OSA (AHI>30) |
| Score 3 vs Score 0–2 | 3.01 (1.92–4.70) | 2.59 (1.58–4.27) | 3.56 (1.48–8.58) |
| Score 4 vs Score 0–2 | 3.15 (2.01–4.96) | 3.33 (2.03–5.46) | 5.33 (2.27–12.50) |
| Score 5 vs Score 0–2 | 3.98 (2.38–6.66) | 4.75 (2.81–8.03) | 10.39 (4.45–24.26) |
| Score 6 vs Score 0–2 | 4.52 (2.34–8.74) | 6.29 (3.39–11.66) | 11.55 (4.64–28.71) |
| Score 7 and 8 vs Score 0–2 | 7.04 (2.82–17.55) | 6.88 (3.32–14.25) | 14.86 (5.58–39.56) |
The sensitivity, specificity, PPVs, and NPVs for all OSA, moderate/severe OSA, and severe OSA are summarized in Table 3. As the STOP-Bang score increased from 3 to 8, the sensitivity decreased from 68.4% to 0.4% for moderate/severe OSA patients, and 94.8% to 0% for severe OSA patients. When the STOP-Bang score was 5, the specificity for moderate/severe OSA was 56.1% and for severe OSA was 74.2%.
Table 3.
Predictive parameters of different STOP-Bang score cut-offs. *Percentage out of the 746 patients (n, number of patients in the AHI group who scored the STOP-Bang score indicated or higher). AHI, apnoea–hypopnoea index; PPV, positive predictive value; NPV, negative predictive value
| STOP-Bang score cut-off | n (%)* | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| All OSA (AHI>5) | |||||
| 1 | 504 (67.6) | 98.8 | 2.5 | 68.7 | 50.0 |
| 2 | 488 (65.4) | 95.7 | 17.8 | 71.6 | 65.6 |
| 3 | 429 (57.5) | 84.1 | 40.3 | 75.3 | 54.0 |
| 4 | 306 (41.1) | 60.0 | 60.6 | 76.7 | 41.2 |
| 5 | 185 (24.8) | 36.3 | 79.7 | 79.4 | 36.7 |
| 6 | 90 (12.1) | 17.7 | 91.5 | 81.8 | 34.0 |
| 7 | 36 (4.8) | 7.1 | 97.5 | 85.7 | 32.7 |
| 8 | 4 (0.5) | 0.8 | 98.7 | 57.1 | 31.5 |
| Moderate/severe OSA (AHI>15) | |||||
| 1 | 285 (38.2) | 97.8 | 0.7 | 16.7 | 61.2 |
| 2 | 283 (37.9) | 86.9 | 1.4 | 6.3 | 58.5 |
| 3 | 256 (34.3) | 68.4 | 10.8 | 17.6 | 55.1 |
| 4 | 195 (26.1) | 44.4 | 32.1 | 26.5 | 51.1 |
| 5 | 126 (16.9) | 23.3 | 56.1 | 31.4 | 45.9 |
| 6 | 64 (8.6) | 10.0 | 77.7 | 35.1 | 41.8 |
| 7 | 25 (3.4) | 3.7 | 91.3 | 37.2 | 40.5 |
| 8 | 1 (0.1) | 0.4 | 98.7 | 14.3 | 61.3 |
| Severe OSA (AHI>30) | |||||
| 1 | 134 (18.0) | 100.0 | 2.0 | 18.3 | 100.0 |
| 2 | 134 (18.0) | 100.0 | 10.5 | 19.7 | 100.0 |
| 3 | 127 (17.0) | 94.8 | 27.6 | 22.3 | 96.0 |
| 4 | 105 (14.1) | 78.4 | 52.0 | 26.3 | 91.6 |
| 5 | 75 (10.1) | 56.0 | 74.2 | 32.2 | 88.5 |
| 6 | 38 (5.1) | 28.4 | 88.2 | 34.6 | 84.9 |
| 7 | 16 (2.1) | 11.9 | 95.8 | 38.1 | 83.2 |
| 8 | 0 (0) | 0 | 98.9 | 0 | 81.9 |
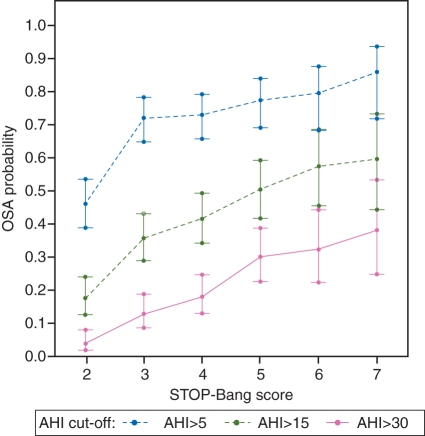
The predicted probabilities of having OSA, moderate/severe OSA, or severe OSA are shown in Table 4. The probabilities of having OSA were greater as the STOP-Bang score increased. This trend was the same across the groups of all OSA, moderate/severe OSA, and severe OSA (Fig. 3). As the STOP-Bang score increased from 0–2 to 7 and 8, the probability of having OSA, moderate/severe OSA, and severe OSA increased from 46% (95% CI: 39–53%) to 86% (95% CI: 72–93%), 18% (95% CI: 13–24%) to 60% (95% CI: 44–73%), and 4% (95% CI: 2–8%) to 38% (95% CI: 29–53%), respectively (Table 4).
Table 4.
Predicted probabilities per score for all OSA, moderate/severe OSA, and severe OSA. CI, confidence interval; AHI, apnoea–hypopnoea index; n, number; Mod/Sev OSA, moderate/severe OSA
| All OSA (AHI>5) |
Mod/Sev OSA (AHI>15) |
Severe OSA (AHI>30) |
||||
|---|---|---|---|---|---|---|
| Score | n | Probability (95% CI) | n | Probability (95% CI) | n | Probability (95% CI) |
| 0–2 | 81 | 0.46 (0.39–0.53) | 31 | 0.18 (0.13–0.24) | 7 | 0.04 (0.02–0.08) |
| 3 | 123 | 0.72 (0.65–0.78) | 61 | 0.36 (0.29–0.43) | 22 | 0.13 (0.09–0.19) |
| 4 | 121 | 0.73 (0.66–0.79) | 69 | 0.42 (0.34–0.49) | 30 | 0.18 (0.13–0.25) |
| 5 | 95 | 0.77 (0.69–0.84) | 62 | 0.50 (0.42–0.59) | 37 | 0.30 (0.23–0.39) |
| 6 | 54 | 0.79 (0.68–0.87) | 39 | 0.57 (0.45–0.69) | 22 | 0.32 (0.22–0.44) |
| 7 and 8 | 36 | 0.86 (0.72–0.93) | 25 | 0.60 (0.44–0.73) | 16 | 0.38 (0.29–0.53) |
Fig 3.
Plot of predicted probabilities for AHI cut-offs of >5, >15, and >30 with the corresponding STOP-Bang score. The vertical bars indicate the 95% confidence intervals. STOP-Bang scores of 0, 1, and 2 are grouped together and are shown as score 2. Scores 7 and 8 are grouped together and is shown as score 7. As the STOP-Bang scores increased, the predicted probabilities were greater. AHI, apnoea–hypopnoea index.
Discussion
The results of the study showed that with an increase in the STOP-Bang score, there was a corresponding increase in the predicted probability, OR, and specificity for having OSA, moderate/severe, and severe OSA. This was accompanied by a progressive decrease in sensitivity. For a STOP-Bang score of 5, the OR for moderate/severe and severe OSA was 4.8 and 10.4, respectively. For STOP-Bang 7 and 8, the OR for moderate/severe and severe OSA was 6.9 and 14.9, respectively. The STOP-Bang questionnaire was initially introduced as a scoring model for the preoperative patients.11 The results from this study further validated the value of STOP-Bang questionnaire as a screening tool in surgical patients. The association between the STOP-Bang score and the probability of OSA would provide the perioperative care team a useful tool to stratify patients for unrecognized OSA and triage patients for diagnosis and treatment.
It is estimated that nearly 80% of men and 93% of women with moderate-to-severe sleep apnoea are undiagnosed,17 which poses a variety of problems for anaesthesiologists. OSA patients are known to have a higher incidence of difficult intubation,18 postoperative complications,19,20 increased intensive care unit admissions,7 and greater duration of hospital stay.21 Memtsoudis and colleagues9 found that OSA was associated with a significantly higher incidence of pulmonary complications. However, no association between postoperative complication and OSA severity was found in obese patients undergoing bariatric surgery.22 This may be due to the fact that most patients with OSA (93%) received perioperative positive airway pressure therapy, and all patients were closely monitored after operation with pulse oximetry on either regular nursing floors or in intensive or intermediate care units.22 Recently, a Canadian publication23 and the American Society of Anesthesiologists guidelines24 both stressed the importance of preoperative diagnosis and perioperative management of OSA patients to avoid postoperative complications.
To identify patients at high risk of OSA is the first step for the perioperative care of OSA patients and prevention of adverse events. Although no test or parameter has been widely accepted as a tool to identify the OSA patients who are particularly at risk for severe postoperative pulmonary adverse events, a recent study does show that patients classified as STOP-Bang high risk had an increased incidence of postoperative complications.25
The STOP-Bang questionnaire is concise and easy to use. It consisted of eight questions with a yes or no answer and has been used as a preoperative screening tool for OSA.12,26–28
Recently, the STOP-Bang questionnaire has been validated in two studies of patients referred to the sleep clinic.29–30 Farney's study showed that the STOP-Bang questionnaire can be used to estimate the probabilities of no, mild, moderate, and severe OSA. The greater the cumulative score of risk factors as reflected by the STOP-Bang model, the greater the probability of severe OSA.29 With any score >4, the probability of having severe OSA increases continuously. With a score of 8, the probability of severe OSA was 81.9%.29 Although our results also showed a similar association between the probabilities of having severe OSA and the score on STOP-Bang, we did not see such a high probability of severe OSA with a higher STOP-Bang score. This may be due to the difference in the study population. Our patients were preoperative patients. The patients in Farney's study were the patients referred to sleep clinic population which have a high prevalence of severe OSA.
Since a STOP-Bang score of ≥3 demonstrated a very high sensitivity and NPV for moderate/severe OSA, this cut-off may be good for a surgical population with high OSA prevalence such as bariatric surgical patients. We would be confident in excluding the possibility of moderate/severe or severe OSA in patients with a STOP-Bang score of 0–2. On the other hand, the patients with a STOP-Bang score of 5–8 have a high specificity to detect moderate and severe OSA. These scores may be useful in the general patient population which has a low OSA prevalence to reduce false-positive rate. It enables identification of those patients most in need of urgent evaluation and to exclude patients from possible harm due to unrecognized sleep apnoea.29 However, further research is needed so that the STOP-Bang can be validated in the different clinical populations.
It is a challenge to establish a practical perioperative care pathway for OSA patients. It is not known whether patients with a STOP-Bang score of 5–8 with co-morbidities having major surgery would benefit from sleep medicine referral, expedited polysomnography (PSG), and continuous positive airway pressure (CPAP) treatment. There have been no studies in the literature to prove that preoperative PSG is of benefit to the surgical patients with suspected OSA. Overnight-attended PSG is an old standard in the diagnosis of OSA, but it is expensive and cumbersome. Often, there is a timeline for patients undergoing surgery. Portable home-based monitoring devices or single channel recording such as nocturnal oximetry might be used as an alternative for the diagnosis of OSA in patients with high probability of moderate-to-severe OSA.31 Thus, a combination of STOP-BANG questionnaire to identify patients at risk of OSA and nocturnal oximetry may allow for a more rapid diagnosis of OSA. Alternatively, in the patients classified as high risk of OSA by the STOP-Bang questionnaire, especially those with a STOP-Bang score of ≥5, practicing perioperative precautions (preparation for possible difficult intubation, using short-acting anaesthesia agents, adequate neuromuscular blocking agent reversal, and use of CPAP after operation) and postoperative monitoring is helpful to prevent adverse outcomes.23,24,32 If patients get earlier treatment for their OSA because of screening in preoperative clinics, there may be long-term health benefits for the patients, besides reducing risk for OSA-related perioperative adverse event. More collaboration between anaesthesiologists, surgeons, and sleep physicians is needed.
There are a few limitations with our study. The study could be criticized because PSG was performed with both the standard PSG in the laboratory and the portable PSG at home. Embletta X-100 is a level 2 diagnostic device for SDB. When installed by a well-trained technician and scored by a certified PSG technologist, parameters measuring sleep-disordered breathing and sleep architecture from Embletta X-100 were comparable with in-laboratory standard PSG.15 Although home monitoring is validated15,33 and all PSG recordings were scored by certified PSG technologists, some inconsistency in the two approaches may exist. Secondly, the study population is surgical patients referred to preoperative clinics. These results may not be applicable to other patient populations. Further validation in the different population, especially the general population, needs to be done. Also, there may be a selection bias involved in the patient recruiting process, the subjects having some OSA-related symptoms might be more motivated to give consent to this study. Finally, like all other screening studies for sleep apnoea, central apnoeas were also not evaluated separately in the report.
In conclusion, the predicted probabilities were greater as the STOP-Bang score increased, showing that patients had a greater probability of having OSA when they scored higher on the STOP-Bang questionnaire. A STOP-Bang score of <3 will allow the healthcare team to rule out patients who do not have OSA. A STOP-Bang score of 5–8 will allow the team to identify patients with increased probability of moderate/severe OSA. The STOP-Bang score can help the healthcare team to stratify patients for unrecognized OSA, practice perioperative precautions, or triage patients for diagnosis and treatment.
Authors' roles
F.C. is the principal investigator. F.C. helped design the study, conduct the study, and write the manuscript and had the overall responsibility for the study. R.S. helped design the study, and write the manuscript. P.L. helped design the study, conduct the study, and write the manuscript. E.S. analysed the data and helped write the manuscript. C.S. helped design the study and supervised sleep studies. Y.S. was responsible for the scoring of PSG.
Registration Site and Number: Mount Sinai Hospital, Toronto, Canada: 06-0143-E and 07-0183-E; Toronto Western Hospital: 06-0135-AE and 07-0515-AE.
Acknowledgements
We acknowledged the help of Santhira Vairavanathan, MBBS, Sazzadul Islam, MSc, Hisham Elsaid, MD, Babak Amirshahi, MD, and Hoda Fazel, MD, for the help in the conduct of the study.
Appendix
STOP-Bang questionnaire11
| 1. Snoring: Do you snore loudly (loud enough to be heard through closed doors)? | |
| Yes | No |
| 2. Tired: Do you often feel tired, fatigued, or sleepy during daytime? | |
| Yes | No |
| 3. Observed: Has anyone observed you stop breathing during your sleep? | |
| Yes | No |
| 4. Blood pressure: Do you have or are you being treated for high blood pressure? | |
| Yes | No |
| 5. BMI: BMI more than 35 kg m−2? | |
| Yes | No |
| 6. Age: Age over 50 yr old? | |
| Yes | No |
| 7. Neck circumference: Neck circumference >40 cm? | |
| Yes | No |
| 8. Gender: Male? | |
| Yes | No |
High risk of OSA: Yes to ≥3 questions.
Low risk of OSA: Yes to <3 questions. Questionnaire reproduced from Chung et al.11 with permission from Wolters Kluwer Health.
Declaration of interest
None declared.
Funding
This work was supported by Physicians Services Incorporated Foundation, University Health Network Foundation, ResMed Foundation, Respironic Foundation and Department of Anesthesia, University Health Network-Mount Sinai Hospital, University of Toronto.
References
- 1.Young T, Hutton R, Finn L, Badr S, Palta M. The gender bias in sleep apnea diagnosis: are women missed because they have different symptoms? Arch Intern Med. 1996;156:2445–51. doi:10.1001/archinte.1996.00440200055007. [PubMed] [Google Scholar]
- 2.Lettieri CJ, Eliasson AH, Andrada T, Khramtsov A, Raphaelson M, Kristo DA. Obstructive sleep apnea syndrome: are we missing an at-risk population? J Clin Sleep Med. 2005;1:381–5. [PubMed] [Google Scholar]
- 3.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen year follow up of the Wisconsin Sleep Cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- 5.Kaw R, Michota F, Jaffer A, Ghamande S, Auckley D, Golish J. Unrecognized sleep apnea in the surgical patient: implications for the perioperative setting. Chest. 2006;129:198–205. doi: 10.1378/chest.129.1.198. doi:10.1378/chest.129.1.198. [DOI] [PubMed] [Google Scholar]
- 6.Liao P, Yegneswaran B, Vairavanathan S, Zilberman P, Chung F. Postoperative complications in patients with obstructive sleep apnea: a retrospective matched cohort study. Can J Anaesth. 2009;56:819–28. doi: 10.1007/s12630-009-9190-y. doi:10.1007/s12630-009-9190-y. [DOI] [PubMed] [Google Scholar]
- 7.Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case–control study. Mayo Clin Proc. 2001;76:897–905. doi: 10.4065/76.9.897. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher SF, Haines KL, Osterlund LG, Mullen M, Downs JB. Postoperative hypoxemia: common, undetected, and unsuspected after bariatric surgery. J Surg Res. 2010;159:622–6. doi: 10.1016/j.jss.2009.09.003. doi:10.1016/j.jss.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Memtsoudis S, Liu SS, Ma Y, et al. Perioperative pulmonary outcomes in patients with sleep apnea after noncardiac surgery. Anesth Analg. 2011;112:113–21. doi: 10.1213/ANE.0b013e3182009abf. doi:10.1213/ANE.0b013e3182009abf. [DOI] [PubMed] [Google Scholar]
- 10.Ancoli-Israel S, Kripke DF, Klauber MR, et al. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–21. doi: 10.1097/ALN.0b013e31816d83e4. doi:10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 12.Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108:822–30. doi: 10.1097/ALN.0b013e31816d91b5. doi:10.1097/ALN.0b013e31816d91b5. [DOI] [PubMed] [Google Scholar]
- 13.Finkel KJ, Searleman AC, Tymkew H, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med. 2009;10:753–8. doi: 10.1016/j.sleep.2008.08.007. doi:10.1016/j.sleep.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Chung F, Liao P, Sun Y, et al. Perioperative practical experiences in using a level 2 portable polysomnography. Sleep Breath. 2010 doi: 10.1007/s11325-010-0340-9. doi:10.1007/s11325-010-0340-9. [DOI] [PubMed] [Google Scholar]
- 15.Iber C, Ancoli-Israel S, Cheeson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events, Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. Available from http://www.aasmnet.org/store/product.aspx?pid=176. (accessed 23 January 2011) [Google Scholar]
- 16.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 17.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 18.Hiremath AS, Hillman DR, James AL, Noffsinger WJ, Platt PR, Singer SL. Relationship between difficult tracheal intubation and obstructive sleep apnoea. Br J Anaesth. 1998;80:606–11. doi: 10.1093/bja/80.5.606. [DOI] [PubMed] [Google Scholar]
- 19.Reeder MK, Goldman MD, Loh L, Muir AD, Casey KR, Lehane JR. Late postoperative nocturnal dips in oxygen saturation in patients undergoing major abdominal vascular surgery. Predictive value of pre-operative overnight pulse oximetry. Anaesthesia. 1992;47:110–5. doi: 10.1111/j.1365-2044.1992.tb02005.x. doi:10.1111/j.1365-2044.1992.tb02005.x. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg J, Wildschiødtz G, Pedersen MH, von Jessen F, Kehlet H. Late postoperative nocturnal episodic hypoxaemia and associated sleep pattern. Br J Anaesth. 1994;72:145–50. doi: 10.1093/bja/72.2.145. doi:10.1093/bja/72.2.145. [DOI] [PubMed] [Google Scholar]
- 21.Ballantyne GH, Svahn J, Capella RF, et al. Predictors of prolonged hospital stay following open and laparoscopic gastric bypass for morbid obesity: body mass index, length of surgery, sleep apnea, asthma, and the metabolic syndrome. Obes Surg. 2004;14:1042–50. doi: 10.1381/0960892041975460. doi:10.1381/0960892041975460. [DOI] [PubMed] [Google Scholar]
- 22.Weingarten TN, Flores AS, McKenzie JA, et al. Obstructive sleep apnoea and perioperative complications in bariatric patients. Br J Anaesth. 2011;106:131–9. doi: 10.1093/bja/aeq290. doi:10.1093/bja/aeq290. [DOI] [PubMed] [Google Scholar]
- 23.Seet E, Chung F. Management of sleep apnea in adults—functional algorithms for the perioperative period. Can J Anaesth. 2010;57:849–64. doi: 10.1007/s12630-010-9344-y. doi:10.1007/s12630-010-9344-y. [DOI] [PubMed] [Google Scholar]
- 24.Gross JB, Bachenberg KL, Benumof JL, et al. American Society of Anesthesiologists Task Force on Perioperative Management. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–93. doi: 10.1097/00000542-200605000-00026. doi:10.1097/00000542-200605000-00026. [DOI] [PubMed] [Google Scholar]
- 25.Vasu TS, Doghramji K, Cavallazzi R, et al. Obstructive sleep apnea syndrome and postoperative complications: clinical use of the STOP-BANG questionnaire. Arch Otolaryngol Head Neck Surg. 2010;136:1020–4. doi: 10.1001/archoto.2010.1020. doi:10.1001/archoto.2010.1020. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran SK, Josephs LA. A meta-analysis of clinical screening tests for obstructive sleep apnea. Anesthesiology. 2009;110:928–39. doi: 10.1097/ALN.0b013e31819c47b6. doi:10.1097/ALN.0b013e31819c47b6. [DOI] [PubMed] [Google Scholar]
- 27.Ong TH, Raudha S, Fook-Chong S, Lew N, Hsu AA. Simplifying STOP-BANG: use of a simple questionnaire to screen for OSA in an Asian population. Sleep Breath. 2010;14:371–6. doi: 10.1007/s11325-010-0350-7. doi:10.1007/s11325-010-0350-7. [DOI] [PubMed] [Google Scholar]
- 28.Gafsou B, Marsac L, Fournier JL, Béloucif S, Baillard C. Validation of the STOP-Bang questionnaire as screening tools for obstructive sleep apnea in patients scheduled for bariatric surgery: 1AP3-5. Eur J Anesthesiol. 2010;27:13. doi:10.1097/00003643-201006121-00039. [Google Scholar]
- 29.Farney RJ, Walker BS, Farney RM, Snow GL, Walker JM. The STOP-Bang equivalent model and prediction of severity of obstructive sleep apnea: relation to polysomnographic measurements of the apnea/hyponea index. J Clin Sleep Med. 2011;7:459–65. doi: 10.5664/JCSM.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva GE, Vana KD, Goodwin JL, Sherrill DL, Quan SF. Identifications of patients with sleep disorderd breathing: comparing the four-variable screening tool, STOP, STOP-Bang and Epworth Sleepiness Score. J Clin Sleep Med. 2011;7:467–72. doi: 10.5664/JCSM.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Medicare and Medicaid Services. Decision memo for sleep testing for obstructive sleep apnea. Available from http://www.cms.hhs.gov/mcd/viewdecisionmemo.asp?id=227. (accessed October 2011)
- 32.Gali B, Whalen FX, Schroeder DR, Gay PC, Plevak DJ. Identification of patients at risk for postoperative respiratory complications using a preoperative obstructive sleep apnea screening tool and postanesthesia care assessment. Anesthesiology. 2009;110:869–77. doi: 10.1097/ALN.0b013e31819b5d70. doi:10.1097/ALN.0b013e31819b5d70. [DOI] [PubMed] [Google Scholar]
- 33.Santos-Silva R, Sartori DE, Truksinas V, et al. Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep. 2009;32:629–36. doi: 10.1093/sleep/32.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]