Abstract
Consumption of arsenic-contaminated drinking water is associated with numerous cancers and dermal and vascular diseases. Arsenic is also a potent nervous system toxicant and epidemiological studies indicate that intellectual functions in children are compromised following early developmental exposure. This study was designed to examine the effects of arsenic on a broad range of age-specific behaviors including basic sensory-motor responses in neonates, locomotor activity and grip strength in juveniles, and operant measures of learning and attention in adults. Pregnant C57BL6/J mice consumed drinking water containing 0, 8, 25, or 80 ppm sodium arsenite from the fourth day of gestation until birth. Arsenic produced a range of behavioral impairments in male and female offspring at each of the test ages. The most striking effects of arsenic were on the development of gait and other motor responses including acoustic startle, righting reflexes, and forelimb grip. These results suggest that developmental arsenic exposure can produce other behavioral impairments in children in addition to cognitive impairment.
Keywords: arsenic, behavior, C57BL6/J, development, learning, motor, prenatal exposure
1. Introduction
Arsenic is a common and widely-distributed element in crustal rocks and soils. Water is the most important distribution medium and both terrestrial and water-borne inorganic arsenic is comprised of pentavalent (As5+) and trivalent states (As3+). Human activities, especially coal-burning and metal smelting, can increase local water and atmospheric concentrations of arsenic thereby increasing the prevalence of arsenic-induced diseases in nearby communities (Bhattacharya et al. 2002; Watanabe et al. 2003). An unfortunate example of anthropogenic exposure involved the drilling of thousands of tube wells throughout South Asia during the second half of the twentieth century with the intention of reducing diseases associated with the consumption of contaminated surface waters (Chakraborti et al. 2003). Many of these tube wells terminated in sediments rich in geologic arsenic (Mukherjee & Bhattacharya, 2001).
Chronic exposure to inorganic arsenic from sources such as contaminated drinking water can damage tissue throughout the body and is associated with a wide range of human disease including skin hyperpigmentation and keratosis, various cancers (bladder, lung, kidney, liver, skin), COPD, vascular conditions such as Blackfoot disease, atherosclerosis, hypertension, and diabetes (Kapaj et al. 2006; Brown and Zeise, 2004; Eisler, 1988). Inorganic arsenic also targets the human nervous system producing peripheral neuropathies and behavioral changes such as lowered intelligence scores on standardized tests that are indicative of central nervous system deficits (Calderon et al., 2001; Rocha-Amador et al., 2007; Rosado et al., 2007; Tsai et al., 2003; von Ehrenstein et al. 2007; Wang et al., 2007; Wasserman et al., 2004; Wright et al., 2006).
Although developmental exposure to arsenic can produce cognitive deficits in humans, most of the neurobehavioral studies with animal models have focused on locomotor activity (Bardullas et al. 2009; Rodriguez et al. 2002; Rodriguez et al. 2001; Schultz et al. 2002; Itoh et al. 1990; Pryor et al. 1983). However, when comparing six animal studies that report such changes, no two studies used the same dose range or replicated even a single dose if issues of route of administration and exposure duration are considered. Three of these studies observed hypoactivity in rats as dose increased (Schultz et al. 2002; Rodriguez et al. 2001; Pryor et al. 1983), one study noted hyperactivity in rats (Rodriguez et al. 2002), while two studies reported dose-specific hyper- or hypoactivity in mice (Bardullas et al. 2009; Itoh et al. 1990).
One way to interpret these conflicting results is to focus on mouse rather than rat studies since tissue disposition in mice appears to better model that of humans (Vahter, 1999). Rat hemoglobin binds the metabolite, dimethylarsinic acid, and alters the rate of excretion (Lu et al. 2007). Furthermore, developmental studies are perhaps more useful than acute exposures during adulthood since arsenic’s effects on human cognition have been observed primarily in children. For example, Martinez-Finley et al. (2009) found that exposure throughout the gestational and lactational periods to low level arsenic in maternal drinking water increased indices of anxiety in mouse offspring during a novel object exploration task. Exposed offspring also performed worse than controls on a radial arm maze task. During an earlier study, a similar arsenic exposure paradigm increased the escape latency during an active avoidance task and increased periods of immobility during a forced-swim task (Martinez et al. 2008). In both experiments there were changes in hippocampal glucocorticoid receptors suggesting that arsenic disrupted the development of the hypothalamic-pituitary-adrenal axis. Colomina et al. (1996) examined a range of simple functions in younger mouse offspring following a briefer gestational exposure and noted few behavioral deficits with the exception an increase in pivoting, a type of abnormal gait behavior.
Arsenic exposure could produce behavioral changes through a direct effect on the developing brain since arsenic freely crosses the mammalian placenta and blood-brain barrier (Lindgren et al. 1984; Willhite and Ferm, 1984). One of the most common fetal malformations in exposed mice is exencephaly (Nemec et al. 1998; Vahter and Norin, 1980). Early in gestation, arsenic selectively accumulates in the neuroepithelium (Lindgren et al. 1984) and compared to other valence forms, As3+ is retained in brain tissue for longer periods of time (Vahter and Norin, 1980). Embryonic exposure has been shown to produce neural tube defects, increase neuronal apoptosis, disrupt neural outgrowth, and reduce overall head size in both mouse and zebrafish models (Chaineau et al. 1990; Li et al. 2009). Administration of a single, high dose of arsenic increases fetal mouse morbidity and mortality in the absence of obvious maternal toxicity (Baxley et al. 1981) while exposure to lower levels of arsenic throughout the gestational period is associated with dose-related loss of pregnancy and increased neonatal death. Viable offspring do not show signs of gross toxicity but there is early evidence of neurobehavioral toxicity such as reduced open field locomotion in neonatal rats (Chattopadhyay et al. 2002).
The current study examined the neurobehavioral effects of developmental arsenic exposure in one the most common inbred mouse strains, the C57BL6/J. A wide range of age-appropriate behaviors were examined in both male and female offspring, including the acquisition of basic sensory-motor responses in neonates, locomotor activity in juveniles and adults, and performance during two operant conditioning schedules in adults. The final series of locomotor trials were preceded by an injection of amphetamine or nicotine since both of these drugs have a well-known bell-shaped dose-response effect on rodent locomotion and arsenic exposure has been shown to affect dopamine and acetylcholine esterase content in the rat brain (Itoh et al. 1990; Nagaraja and Desiraju, 1993; Nagaraja and Desiraju, 1994; Rodriguez et al. 2001, 2003). The concurrent examination of a wide range of behaviors was designed to determine if cognitive deficits in mice such as impaired operant schedule acquisition or altered responses to schedule transitions would emerge at the same doses as the more frequently-studied locomotor changes.
The trivalent, rather than the pentavalent form was chosen for this study since others have found that it results in higher total arsenic tissue levels in both developing and adult rats and mice (Cui et al. 2004; Jin et al. 2006). The dose range was based on previous work in this laboratory that found that drinking water concentrations up to 80 ppm are tolerated by pregnant dams and do not produce maternal toxicity or weight loss (Markowski et al. 2010).
2. Materials and Methods
2.1 Breeding and Arsenic Exposure
Adult male and female C57BL6/J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were allowed to acclimate to the University of Southern Maine vivarium quarters for at least one week before breeding. Females were then housed with stud males and examined each morning (0830) for the presence of a sperm plug. The sperm-positive day was regarded as gestation day (GD) 0. Sperm-positive mice were placed individually into polycarbonate shoebox cages and assigned to an exposure condition according to a randomized block design. Maternal body weights were recorded daily during the gestational period. Beginning on GD4, pregnant mice were given deionized (Millipore) drinking water containing 0, 8, 25, or 80 ppm sodium arsenite. Exposure continued until GD18 or the day of parturition (postnatal day 0 or PND0), whichever came first.
During the breeding and exposure procedures, adult mice were fed standard pellet chow (Teklad Global 18% Protein Rodent Diet). The Teklad Global 2018 Rodent Diet contained approximately 0.15 mg/kg total arsenic, which is typical of grain-based, vegetarian rodent diets. Housing rooms were on a 12h light/12h dark cycle in a barrier facility with an ambient temperature of 20 ± 1°C and 40–60% humidity. All animal procedures complied with approved institutional animal care protocols and were in accordance with NIH guidelines (Institute of Laboratory Animal Resources 1996). Animal care and welfare were supervised by a veterinarian and an AALAS-certified Registered Laboratory Animal Technologist.
2.2 Offspring and Developmental Milestones
Cages were inspected each morning for the presence of litters. Litters were culled to six pups on PND1 using a randomized selection procedure. Three male and three female pups were kept from each litter whenever possible. Litter size, sex distribution, pup bodyweights, crown-rump lengths, and anogenital distances were recorded on PND1 and every other day thereafter. Litters with fewer than six pups were recorded, included in the litter size calculations, and then removed from the study. The ages at which both pinna detached, the upper and lower incisors erupted, and both eyes opened were also determined. The age of puberty was determined by vaginal opening or descent of the testes. After weaning on PND21, offspring were housed with same-sex littermates.
2.3 Functional Observation Battery
A functional observation battery (FOB) was used to examine homecage, reflexive, and sensorimotor behaviors in young mice. The FOB consists of a sequence of 16 individual tests arranged from the least to the most interactive or intrusive to reduce interference between procedures. Observations in the homecage were collected first. Pups were then placed in an arena for observation of spontaneous activities followed by an evaluation of responses to a variety of sensory stimuli. The surface of the arena was warmed with a heating pad until PND14, at which time pups had a full fur coat and were more active. See Table 1 for a representation of the score key used by the two trained observers who collected the measures. The observers were blind to the exposure history of the animals. Additional information on the design of the FOB for young mice can be found in our earlier paper (Rice et al. 2007). The FOB was conducted every other day from PND1-21. Every pup in each litter was examined with the FOB.
Table 1.
Score Key for the Functional Observation Battery for Young Mice
| Ob.ID # |
Behaviors / Observations | Categories | Score |
|---|---|---|---|
| 1 | Posture in cage prior to removing / handling animals | Resting or asleep in nest with other pups and/or dam | 0 |
| Feeding (either nursing or solid food) | 1 | ||
| Stationary with no contact with dam or other pups | 2 | ||
| Moving around cage | 3 | ||
| Hyperactive, excessive movement, jumping, rearing | 4 | ||
| 2 | Behavior while removing from cage | Sits quietly and is easily grasped | 0 |
| Runs around cage, hard to grab, may or may not vocalize | 1 | ||
| Freeze response (with or without vocalization) | 2 | ||
| Aggressive, tail and/or throat rattle, may attempt to bite | 3 | ||
| 3 | Color of mucous membranes/eyes/skin: if pup's eyes are not open, note color of skin only | Pink | 0 |
| Pale | 1 | ||
| Bright, deep red flush | 2 | ||
| The remaining observations are collected outside of the homecage in the test arena | |||
| 4 | Touch response: touch rump with cotton end of swab | No reaction | 0 |
| Turns toward site | 1 | ||
| Walks forward, away from stimulus | 2 | ||
| 5 | Righting reflex: roll mouse on either side and determine amount of time to return to a sternal or standing position with stopwatch. One minute time limit for each side. | Rights when turned on both sides | 0 |
| Attempts but does not right from left side | 1 | ||
| Attempts but does not right from right side | 2 | ||
| Attempts but does not right from either side | 3 | ||
| Does not attempt to right | 4 | ||
| 6 | Tail pinch: grasp tail 5 mm from base with forceps | No response | 0 |
| Sluggish turn | 1 | ||
| Vocalization only | 2 | ||
| Rapid turn toward stimulus with or without vocalization | 3 | ||
| Jumps or walks forward with or without vocalization | 4 | ||
| Jumps, rolls and/or walks forward with vocalization. May raise hindquarters. Exaggerated response. | 5 | ||
| 7 | Death | No death | 0 |
| Found dead in cage | 1 | ||
| Euthanized. Record reason. | 2 | ||
| 8 | Palpebral closure | Both eyes wide open | 0 |
| Eye(s) partially closed (including recently unsealed eyes) | 1 | ||
| Eyelids swollen or drooping | 2 | ||
| Eyes closed (including sealed eyes) | 3 | ||
| The following observations are collected only after eyes have opened/unsealed | |||
| 9 | Noise response: with mouse free to move about, snap K-9 clicker behind the head. | No reaction. Does not orient to sound. | 0 |
| Startles but does not orient toward sound | 1 | ||
| Startles and orients toward sound | 2 | ||
| Orients to sound without startle | 3 | ||
| 10 | Approach response: move cotton end of swab to within 3 cm of snout and hold for 4-s. | No reaction | 0 |
| Approaches slowly and sniffs or grabs object | 1 | ||
| Freezes | 2 | ||
| Pulls away slightly | 3 | ||
| Jumps or turns away to avoid object | 4 | ||
| 11 | Palpebral reflex: touch medial canthus with stick end of swab. Movement must be slow and must not stimulate corneal or menace reflex. | Eyes are sealed | 0 |
| Eyelids are partially opened | 1 | ||
| Eyelids blink | 2 | ||
| Eyelids do not blink | 3 | ||
| The following behaviors should be monitored continuously during handling | |||
| 12 | Response to handling | Alert and calm | 0 |
| Animal is limp | 1 | ||
| Attempts to escape, may vocalize or bite | 2 | ||
| 13 | Aberrant movements / Bizarre behavior | None | 0 |
| Spatial disorientation (walking or stumbling into objects) | 1 | ||
| Side-to-side rocking | 2 | ||
| Body tremor/vibration | 3 | ||
| Other (record specific observations) | 4 | ||
| 14 | Gait | Too young to walk | 0 |
| Normal | 1 | ||
| Abnormal gait (including waddling, high stepping, etc). Record specific observations. | 2 | ||
| Incapacity, unable to walk | 3 | ||
| 15 | Locomotor activity | Resting / asleep | 0 |
| No spontaneous movement, responds only when stimulated | 1 | ||
| Casual scratching, grooming, slow spatial orientation | 2 | ||
| Vigorous scratching, grooming, moderate spatial orientation (including normal, age-appropriate pup movement) | 3 | ||
| Ballistic movements: sharp, rapid, and/or darting | 4 | ||
| 16 | Rearing activity | None (including if pup is too young to walk) | 0 |
| Rears on hindlimbs with use of tail | 1 | ||
| Falls over backward or to the side when rearing | 2 | ||
Most of the FOB measures were nominal scales, with the assigned scores for defined behaviors being arbitrary, such that averaging the scores provided no meaningful indication of effects. Three of the measures were ordinal (responses during cage removal, acoustic startle response, open field movement) with a higher mean value indicating a greater degree of motor activity and/or sensory response.
2.4 Grip Strength
Forelimb grip strength was evaluated using a meter equipped with a digital sensor (Columbus Instruments, Columbus, OH). Grip tests began after both eyes had opened. Each animal was held by the scruff and the base of the tail until they grasped the pull-bar with both forepaws. The scruff was then released and the tail was pulled steadily away from the bar until the animal released both forepaws. Every pup in each litter was examined on PND15, 17, 19, and 21. Two male-female pairs were examined on the day of puberty onset and PND60 (see Table 2 for behavioral test assignments). On a given test day each animal performed three trials, which were later averaged.
Table 2.
Distribution of Behavior Test Assignments for Each Litter
| Offspring per litter |
Neonatal Measures |
Juvenile Measures |
Adult Measures |
|---|---|---|---|
| Male-female pair #1 | FOB, developmental milestones, PND15–21 grip | Puberty grip & Rotarod | PND60 grip, activity, Go/No Go operant |
| Male-female pair #2 | FOB, developmental milestones, PND15–21 grip | Puberty grip & activity | PND60 grip, Rotarod, Random Ratio operant |
| Male-female pair #3 | FOB, developmental milestones, PND15–21 grip | n/a | Nicotine and amphetamine activity at 18-months |
2.5 Coordination and Balance
Fine motor coordination and balance were assessed with an automated rotarod (Rotamex-5, Columbus Instruments). On the day of puberty, one male and one female from each litter were selected (see Table 2) and given two training trials with the rod moving at a fixed speed of 4rpm for 60 s or until the animal fell. Animals were placed on the rod and after finding their balance, a trial was started. Animals were positioned so that they had to make a forward walking motion to remain on the rod. Each trial was separated by at least 5 min. The day after the training session, the same animals received 4 trials where the rod accelerated from 4 to 40 rpm over 360 s. Rotation was accelerated in 0.1 steps. The four trials were averaged before statistical analysis. The latency to fall, maximum speed, and total run time variables were examined. On PND60, a naïve male and female littermate received the same training and acceleration test procedure.
2.6 Activity
On the day of puberty, locomotor activity in a novel environment was tested in a standard mouse operant chamber (18cm w × 18cm d × 30cm h; Coulbourn Instruments, Allentown, PA). One male and one female were selected from each litter (see Table 2) and placed individually in the chamber for a 2 h session that began at least 2 h after the onset of the dark cycle in the vivarium. The number of movement episodes was recorded by an overhead infrared activity monitor (model H24-61, Coulbourn Instruments). The field of view of the activity monitor was calibrated to the interior dimensions of the operant cage and recorded movement at any elevation in the cage, including rearing or leaning against the cage wall. A movement episode was defined as contiguous motor output with inter-event intervals of less than 400 ms. Prior to analysis, the number of movement episodes in the 2 h activity session was grouped into eight consecutive 15-min time blocks. On PND60, a second male-female pair of littermates from each litter was tested in the operant chamber.
2.7 Drug Challenges
At approximately 18-months, the remaining male-female pairs of littermates that had not yet been examined in the activity chambers were tested for 5 consecutive daily sessions (see Table 2). Each session was preceded by an intraperitoneal injection of nicotine (0.0, 0.4, 0.8, 1.6, or 3.2 mg/kg BW), administered in a randomized, counterbalanced order. A one-week washout period followed the nicotine challenges. The same animals were then assigned a second series of activity sessions preceded by an intraperitoneal injection of D-amphetamine (0.0, 0.5, 1.5, 3.0, or 6.0 mg/kg BW), administered in a randomized, counterbalanced order. The nicotine (N5260, Sigma-Aldrich, St. Louis, MO) and D-amphetamine (A5880, Sigma-Aldrich) were dissolved in sterile physiological saline. Injection volumes ranged from 0.08 to 0.18 ml depending on the weight of the animal.
2.8 Lever Press Training and Random-Ratio Schedule
Operant behavior in adult mice was tested in commercial chambers that were controlled by Graphic State software, ver. 3.01, for Windows XP. Each chamber contained a single response lever on the middle of one chamber wall and a food bin centered on the opposite wall. A multicolored LED display was positioned directly above the lever and served as the discriminative stimulus that a response would be reinforced. An overhead houselight was illuminated during sessions. Single food pellets (20 mg, Bio-Serv, Frenchtown, NJ) were automatically delivered into the food bin to reinforce correct responses.
A continuous reinforcement schedule was used to train naïve mice to press the lever. During training sessions, the cuelights above the lever were illuminated and a lever press produced an audible click from the food dispenser, a 3 s illumination of the food hopper, and delivery of a single food pellet. Training sessions lasted for 12 h or until a subject earned 60 food pellets. Subjects were considered to have learned the lever press response when they had completed a session with collection of 60 food pellets.
After subjects acquired the lever press response, all of the animals that had been assigned to activity testing during puberty were tested for 10 sessions under a RR1 reinforcement schedule where one lever press resulted in delivery of one pellet (P = 1.0). Subjects were then assigned to 10 sessions each of RR2, RR5, and RR10 where the delivery of reinforcement was intermittent. For instance, during the RR5 schedule, one out of every five presses on average (P = 0.2) was reinforced. Each RR session lasted for 30 min or until a subject earned 60 food pellets. Sessions were run 5 days a week during the dark phase of the subjects’ 12 h light / 12 h dark cycle. The following variables were examined: start latency, number of earned food pellets, total lever presses, overall response rate, lever run rate, postreinforcement pause, and session duration.
2.9 Go/No Go Schedule
The subjects that were assigned to activity testing at 2-months were used in the Go/No Go procedure. To receive reinforcement during Go/No Go, animals had to remain vigilant for transient visual stimuli that indicated that a “go trial” was in effect. Lever pressing during a go trial was reinforced with food. Different visual stimuli marked the “no go trials”, where subjects were reinforced for inhibiting their responses and lever pressing was punished with time-outs and correction trials. Accurate performance during the Go/No Go schedule required both attention and response inhibition.
The current procedure consisted of 26 sessions where 80% of the trials were go trials and the remaining 20% were no go trials. The two types of trials were presented in randomized order and separated by intertrial intervals of 12, 18, 24, or 30 sec. Go trials lasted for 5 sec and during the 1st 3 sec, the cue lights above the response lever were illuminated. If the subject pressed the lever during the 5 sec go trial, it was reinforced. During the 5 sec no go trials, the overhead houselight was illuminated but the cue lights were not. If the subject inhibited lever pressing during the no go trials, it was reinforced. If the subject failed to inhibit its lever pressing, the 5 sec no go trial was restarted. During the intertrial intervals the houselight was turned off and the chamber was dark.
The frequencies of five types of responses were collected from each session. Two of these responses, hit and correct rejection, reflected accurate performance that was reinforced with food. A hit was a lever press during a go trial and a correct rejection was lever press inhibition during a no go trial. Three other responses; miss, false alarm, and intertrial interval responses were errors that did not produce food. Misses were derived from go trials where an animal failed to respond and false alarms were responses during the no go trials. Prior to analysis, response rates (lever presses/minute) for each of the five responses were calculated.
2.10 Statistical Methods
All repeated measurements including maternal and offspring bodyweight, anogenital distance, crown-rump length, grip strength, rotarod, locomotor activity, and operant behavior were analyzed with repeated-measures analysis of variance (ANOVA) with PROC GLM SAS version 9.1 (SAS Institute Inc., Cary, NC). Prior to analysis, developmental data from individual pups were averaged with their same-sex littermates at each observation day. The length of gestation, total pups on PND1, sex distribution, neonatal mortality, and developmental milestone days were examined with one-way ANOVA.
For the nominal FOB endpoints, the percent male and female pups per litter that performed various behavioral categories of interest were examined with repeated measures ANOVA. In most cases, the behavioral categories were those that represented a normal developmental progression, although abnormal behaviors were also compared.
For all endpoints derived from the offspring, the litter served as the statistical unit of analysis, with the arsenic exposure level as a between-litter factor and sex and postnatal day as within-litter factors. For the nicotine and amphetamine drug challenge procedures, the drug dose served as an additional within-litter factor. For the operant behavior procedures, daily sessions served as a within-litter factor. In cases where the sex factor was not significant, data from the male and female pup were averaged and the litter means were analyzed. Newman-Keuls tests were used to make pairwise comparisons. A p ≤ 0.05 was considered statistically significant.
3. Results
3.1 Gestation and Parturition
Gestational exposure to inorganic arsenic did not affect maternal bodyweight gain, the length of gestation (see Table 3), or the number of male or female pups (see Table 4). Neonatal mortality on PND1 was low and was not affected by exposure.
Table 3.
Mean ± SEM Gestation Length and Maternal Bodyweights for all Females that Delivered a Litter
| Exposure Group |
Gesta- tion (days) |
GD 0 |
GD 1 |
GD 2 |
GD 3 |
GD 4 |
GD 5 |
GD 6 |
GD 7 |
GD 8 |
GD 9 |
GD 10 |
GD 11 |
GD 12 |
GD 13 |
GD 14 |
GD 15 |
GD 16 |
GD 17 |
GD 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 ppm (n = 13) |
18.8, 0.12 |
20.4, 0.35 |
20.3, 0.36 |
20.7, 0.34 |
21.1, 0.37 |
21.5, 0.35 |
21.8, 0.32 |
22.2, 0.29 |
22.5, 0.26 |
23.1, 0.26 |
24.1, 0.28 |
25.0, 0.29 |
26.2, 0.33 |
27.4, 0.36 |
29.0, 0.39 |
30.7, 0.40 |
32.2, 0.46 |
33.7, 0.54 |
35.4, 0.59 |
35.9, 0.77 |
| 8 ppm (n = 13) |
19.1, 0.18 |
22.3, 1.07 |
22.0, 1.09 |
22.2, 1.09 |
22.5, 1.13 |
22.7, 1.07 |
22.9, 1.05 |
23.2, 1.0 |
23.6, 0.92 |
23.8, 0.91 |
24.6, 0.93 |
25.9, 0.99 |
26.7, 0.93 |
28.2, 1.01 |
29.9, 1.07 |
31.4, 1.16 |
33.4, 1.28 |
35.0, 1.23 |
37.3, 1.36 |
37.9, 1.51 |
| 25 ppm (n = 12) |
18.9, 0.15 |
21.6, 0.98 |
21.3, 0.94 |
21.6, 0.98 |
21.8, 0.96 |
22.0, 0.95 |
22.2, 0.96 |
22.6, 0.93 |
23.2, 0.91 |
23.5, 0.91 |
24.4, 0.94 |
25.4, 0.88 |
26.7, 1.00 |
28.1, 0.94 |
29.5, 1.05 |
31.3, 1.05 |
33.4, 1.04 |
34.9, 1.21 |
37.1, 1.23 |
38.5, 1.35 |
| 80 ppm (n = 12) |
18.9, 0.08 |
22.1, 0.94 |
22.1, 0.95 |
22.3, 0.98 |
22.7, 1.01 |
22.9, 1.00 |
22.4, 0.96 |
22.3, 0.93 |
22.5, 0.92 |
23.0, 0.91 |
23.7, 0.87 |
24.6, 0.84 |
25.7, 0.95 |
26.7, 0.95 |
28.2, 1.07 |
29.9, 1.03 |
32.09, 1.13 |
33.6, 1.24 |
35.6, 1.30 |
36.6, 1.59 |
Table 4.
Mean ± SEM Number of Pups per Litter and Emergence of Developmental Milestones During the Neonatal Period
| Exposure-by-sex Group |
Number of pups on PND0 |
Pinnae Detachment (PND) |
Incisor Eruption (PND) |
Eyes Open (PND) |
Puberty Onset (PND) |
|---|---|---|---|---|---|
| 0 ppm male (n = 11) | 3.7, 0.26 | 3.4, 0.13 | 11.3, 0.12 | 13.4, 0.18 | 24.2, 0.25 |
| 0 ppm female (n = 11) | 3.3, 0.27 | 3.5, 0.14 | 11.2, 0.16 | 13.5, 0.16 | 29.1, 0.34 |
| 8 ppm male (n = 10) | 3.9, 0.42 | 3.6, 0.17 | 11.3, 0.19 | 13.6, 0.18 | 24.2, 0.37 |
| 8 ppm female (n = 11) | 4.1, 0.44 | 3.7, 0.17 | 11.3, 0.15 | 13.8, 0.22 | 28.7, 0.64 |
| 25 ppm male (n = 9) | 4.5, 0.46 | 3.8, 0.23 | 11.5, 0.19 | 13.6, 0.23 | 24.6, 0.24 |
| 25 ppm female (n = 11) | 3.5, 0.34 | 3.7, 0.18 | 11.2, 0.18 | 13.7, 0.18 | 29.0, 0.42 |
| 80 ppm male (n = 10) | 2.9, 0.40 | 3.8, 0.27 | 11.7, 0.28 | 13.9, 0.31 | 25.0, 0.49 |
| 80 ppm female (n = 9) | 3.3, 0.47 | 4.1, 0.29 | 11.5, 0.26 | 13.9, 0.29 | 29.7, 0.51 |
3.2 Offspring Body Measurements and Milestones
Arsenic exposure did not affect offspring bodyweight gain, anogenital distance, crown-rump length, or the age that the pinna detached, incisors erupted, eyes opened, or puberty onset (see Table 4). As expected, there were significant main effects of postnatal day, main effects of sex, and sex-by-PND interactions for each of the repeated measures.
3.3 Functional Observation Battery
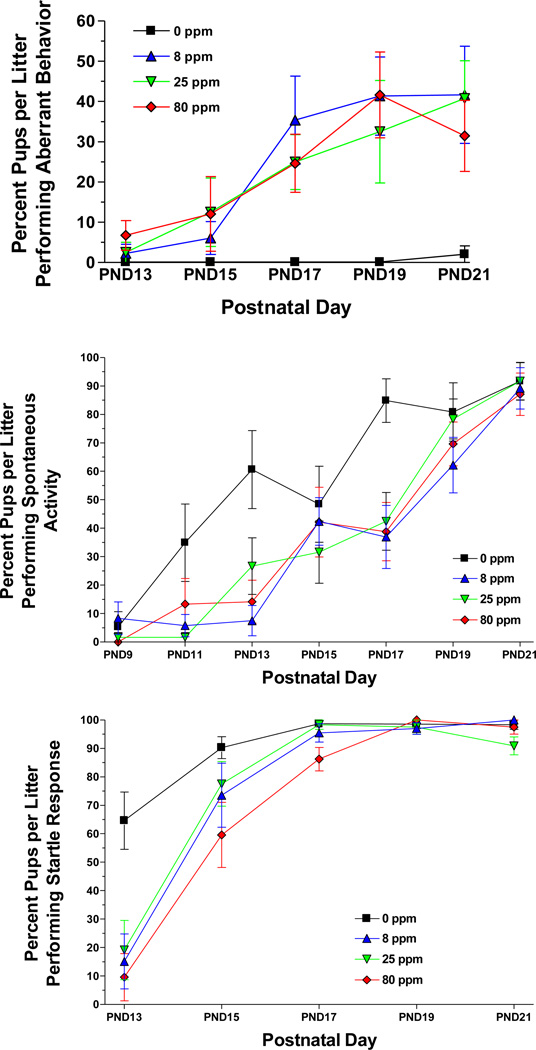
Arsenic affected most of the endpoints that required a coordinated locomotor response. The most striking effects were on gait during the PND13-21 period, where there was a significantly higher incidence of aberrant behaviors [F(3,35)=6.98, P=0.0008]. There were significant main effects of arsenic on gait abnormalities [F(3,35)=9.87, P<0.0001]. There were also main effects of PND [aberrant: F(10,350)=14.23, P<0.000; gait: F(6,210)=21.07, P<0.0001] and exposure-by-PND interactions [aberrant: F(30,350)=1.96, P=0.02; gait: F(18,210)=1.91, P=0.02; Figure 1] for these endpoints. No aberrant behaviors were noted in the control animals throughout the observation period and there was a low incidence in the arsenic groups from PND1-15. However from PND17-21, all of the exposure groups performed significantly more aberrant behaviors compared to controls. Three types of behaviors were prevalent in the arsenic groups: an intermittent whole body shudder or spasm, an intense grooming of the rear paws where the pup would vigorously pull a hindlimb forward and hold it against the ventral surface of the body, or an unusual dorsoflexion of the back with the head held high. Pups would often lose balance and wobble or fall to the side after striking this posture. This last behavior was observed only in exposed female pups. Post hoc comparisons of the exposure-by-PND interaction indicated that the 0 ppm group performed significantly fewer aberrant behaviors than each of the arsenic groups on PND19 and 21 (see Table 5). On PND17, the 0 ppm group performed fewer aberrant behaviors than the 8 ppm group.
Figure 1.
Top panel: Mean ± SEM percent pups per litter performing aberrant behaviors during the open field assessment portion of the FOB. Sample size: 0 ppm n = 8 litters, 8 ppm n = 11, 25 ppm n = 10, 80 ppm n = 10. Middle panel: Pups per litter producing locomotion during the open field assessment portion of the FOB. Sample size: 0 ppm n = 8 litters, 8 ppm n = 11, 25 ppm n = 10, 80 ppm n = 10. Bottom panel: Pups per litter producing an active startle response following an acoustic stimulus during the sensory response portion of the FOB. Sample size: 0 ppm n = 10 litters, 8 ppm n = 11, 25 ppm n = 10, 80 ppm n = 10. In each case, data were averaged across sex within each litter.
Table 5.
Summary of Significant Effects Compared to Controls Across the Test Period
| Arsenic Exposure Group |
Effects in Neonates | Effects in Juveniles |
Effects in Adults |
|---|---|---|---|
| 8 ppm |
|
|
|
| 25 ppm |
|
|
|
| 80 ppm |
|
|
|
Perhaps because of the gait difficulties, arsenic exposed pups showed significantly less spontaneous activity in the observation arena. There were significant main effects of exposure [F(3,35)=4.05, P=0.01] and PND [F(6,210)=56.47, P<0.0001] on activity. There were also sex-by-exposure [F(3,35)=2.98, P=0.04] and PND-by-exposure [F(18,210)=1.92, P=0.02; Figure 1] interactions on spontaneous activity. Post hoc tests of the PND-by-exposure interaction indicated that the 0 ppm group was significantly more active than each of the arsenic groups on PND13 and PND17.
Two other motor responses were affected by prenatal arsenic exposure. For the startle response there was a significant main effect of PND [F(4,148)=93.58, P<0.0001] and an exposure-by-PND interaction [F(12,148)=3.62, P=0.002; Figure 1]. Post hoc tests indicated that: The 0 ppm group startled more than each of the arsenic groups on PND13. The 80 ppm group startled less than each of the other groups on PND17. The 25 ppm group startled less than each of the other groups on PND21. For the righting reflex there were significant main effects of arsenic [F(3,35)=3.40, P=0.03], sex [F(3135)=17.60, P=0.0002], PND [F(4,140)=333.36, P<0.0001], and a sex-by-PND interaction [F(4,140)=3.20, P=0.04]. A post hoc test of the main effect of arsenic indicated that the 0 ppm group successfully completed more righting responses than each of the arsenic exposure groups.
3.4 Forelimb Grip Strength
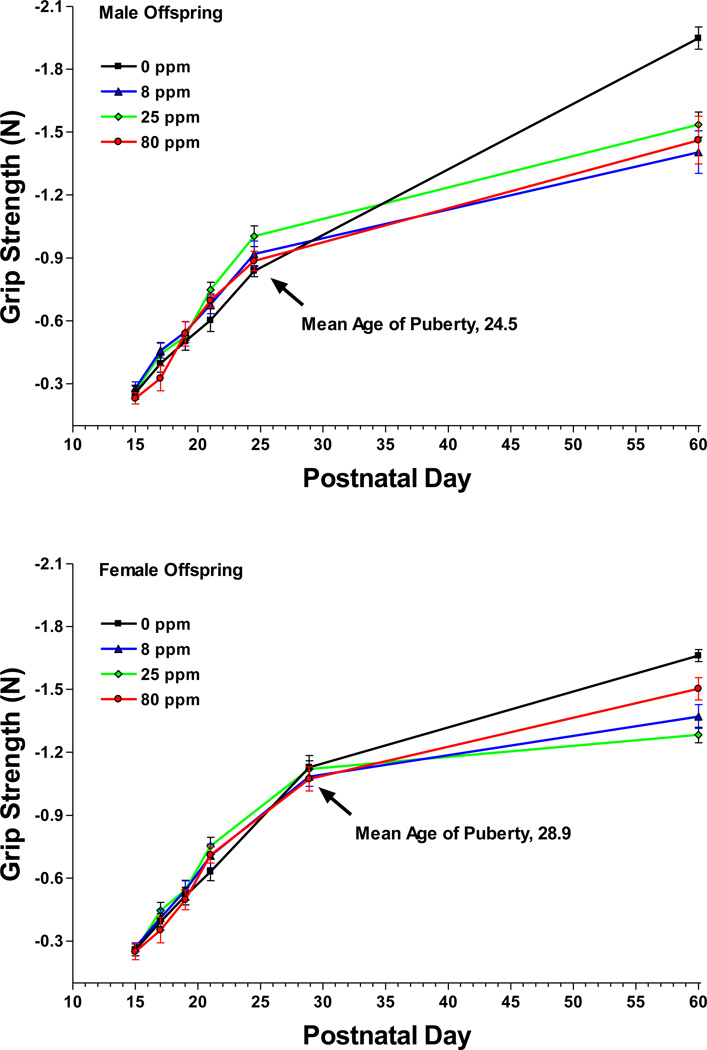
As expected, forelimb grip strength increased significantly over the first 60 days [F(5,125)=11252.4, P<0.0001]. There were also significant main effects of exposure [F(3,25)=591.67, P<0.0001] and sex [F(1,25)=11317.7, P<0.0001]. There were numerous interactions [P<0.0001 for sex-by-exposure, age-by-exposure, and sex-by-age] as well as a higher-order sex-by-age-by-exposure interaction [F(15,125)=547.13, P<0.0001; Figure 2]. To further examine this last interaction, the male and female exposure groups were compared at each of the postnatal test ages. On PND60, the control male and female groups pulled with significantly more force than each of the respective male and female arsenic groups. The 80 ppm female group pulled with more force than the 25 ppm group.
Figure 2.
Mean ± SEM forelimb grip strength force in newtons (N) at six postnatal ages for male offspring (top panel) and female offspring (bottom panel). The puberty age measurements were collected on the day of puberty onset for each juvenile animal. Puberty ages were then averaged to generate the X-axis coordinate. Sample size: 0 ppm n = 7 litters, 8 ppm n = 10, 25 ppm n = 6, 80 ppm n = 6.
3.5 Rotarod Motor Coordination
There was a significant main effect of sex [F(1,23)=10.97, P=0.003] and a sex-by-PND-by-exposure interaction [F(3,23)=3.67, P=0.03] on the terminal speed variable. There were similar effects on the total run time variable (sex: [F(1,22)=10.78, P=0,003]; sex-by-age-by-exposure: [F(3,22)=3.55, P=0.03]). However, when each age group was examined separately there were only main effects of sex (terminal speed at puberty: [F(1,37)=9.26, P=0.004]; terminal speed at adulthood: [F(1,26)=8.31, P=0.008]). There were no arsenic exposure effects within each age group.
3.6 Activity
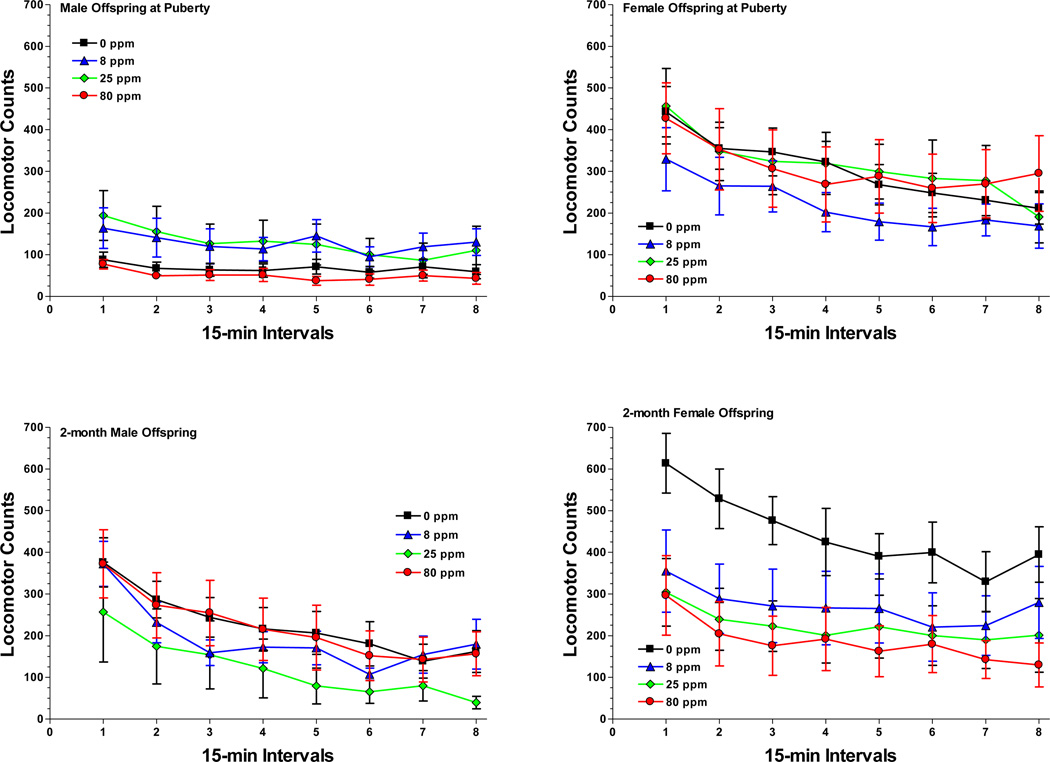
Activity in the puberty-aged animals was compared to that of their young adult (2-months) littermates but the activity of the oldest animals (18-months) was examined separately because these animals were tested during consecutive sessions preceded by nicotine and amphetamine drug challenges. In the puberty-aged and young adult animals, exploratory activity in the operant chambers was significantly affected by time block [F(7,175)=53.82, P<0.0001] as the animals habituated and produced less movement as the 2 h tests proceeded. There was also a significant main effect of sex [F(1,25)=26.59, P<0.0001], an age-by-time block interaction [F(7,175)=5.22, P=0.002], and a sex-by-age-by-time block interaction [F(7,175)=4.67, P=0.002; Figure 3]. To probe the sex-by-age-by-time block interaction, the activity counts were averaged across exposure groups and compared. The puberty-aged male group was significantly less active than all other groups during the first five time blocks. By the 6th time block, the adult male group’s activity had decreased and was no longer different from the puberty males but both male groups were significantly less active than both female groups during the 6th time block. The puberty males remained significantly less active than the two female groups during time block 7 and 8.
Figure 3.
Mean ± SEM activity counts measured in the operant chambers during the 2 h test periods for the male offspring (left panels) and female offspring (right panels) on the day of puberty onset (top panels) and in adulthood (bottom panels). Although there were significant effects of sex and age, prenatal arsenic did not affect locomotor activity in the chambers. Sample size: 0 ppm n = 8 litters, 8 ppm n = 8, 25 ppm n = 6, 80 ppm n = 7.
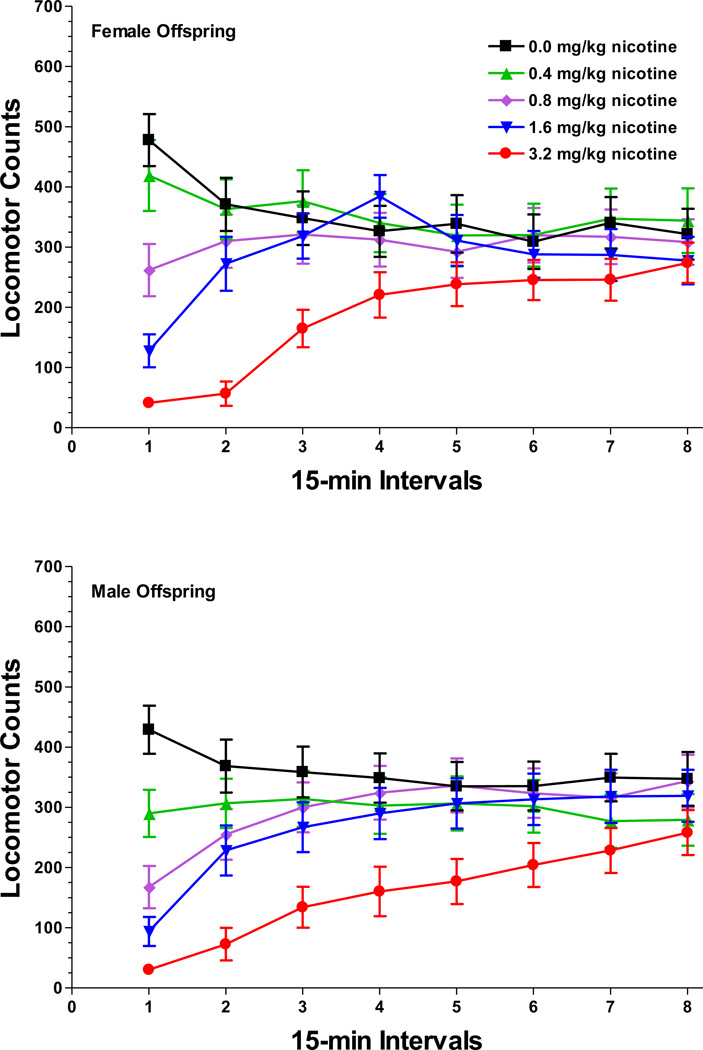
In the 18-month animals there were main effects of nicotine dose [F(4,76)=16.22, P<0.0001], time block [F(7,133)=12.57, P<0.0001], a dose-by-time block interaction [F(28,532)=19.90, P<0.0001], and a sex-by-dose-by-time block interaction [F(28,532)=1.86, P=0.04; Figure 4]. Post hoc tests indicated that, in both sexes, nicotine produced a dose-related suppression of locomotor activity that subsided by the 5th time block. However, nicotine administration did not differentially affect the arsenic exposure groups.
Figure 4.
Mean ± SEM activity counts in 18-month old female offspring (top panel) and male offspring (bottom panel) following pretreatment with nicotine. Although there were significant effects of nicotine dose, sex, and time, prenatal arsenic exposure did not interact with nicotine treatment. Within each nicotine dose group, data were averaged across prenatal arsenic conditions for this figure. Sample size: 0 ppm n = 5 litters, 8 ppm n = 8, 25 ppm n = 5, 80 ppm n = 5.
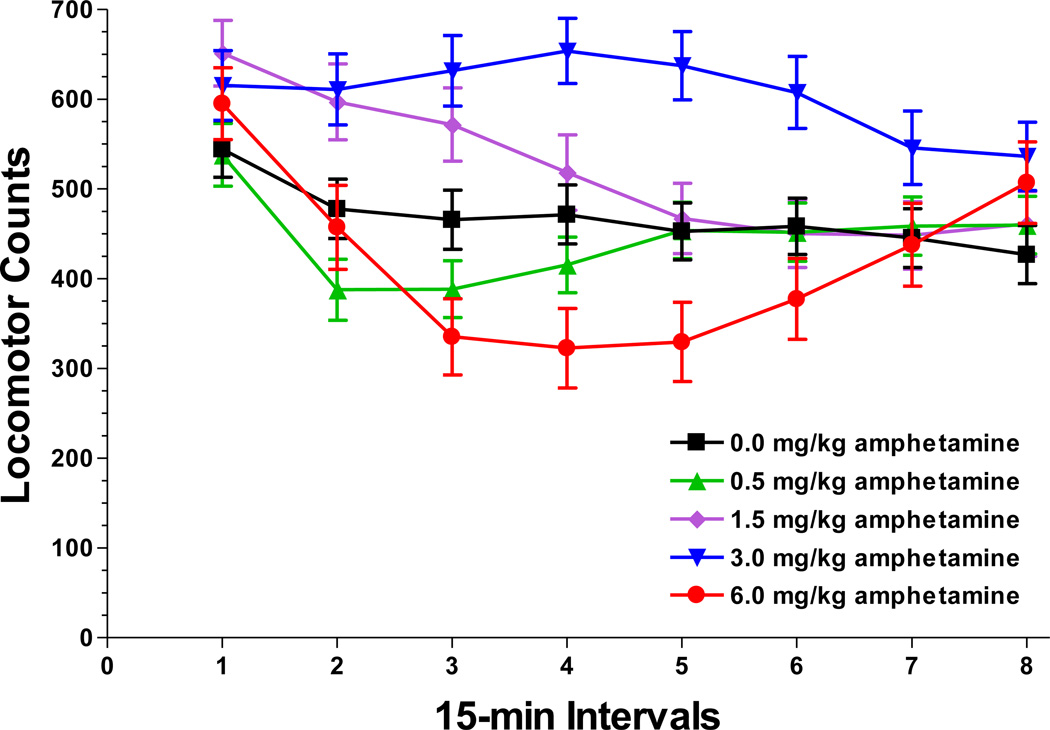
After the nicotine challenges, litters were examined a third time following acute doses of amphetamine. There were main effects of amphetamine dose [F(4,64)=13.15, P<0.0001], time block [F(7,112)=38.94, P<0.0001], and sex [F(1,16)=7.08, P=0.02]. There were also sex-by-time block [F(7,112)=4.95, P=0.002] and dose-by-time block [F(28,448)=13.04, P<0.0001; Figure 5] interactions but amphetamine did not differentially affect the arsenic exposure groups. Amphetamine produced a biphasic response with increased activity produced by the 3.0 mg/kg dose during time blocks 2–6 and the 1.5 mg/kg dose during blocks 2–3, while the 6.0 mg/kg dose suppressed activity during the time blocks 3–4.
Figure 5.
Mean ± SEM activity counts in 18-month old offspring (averaged across sex) following pretreatment with amphetamine. Although there were significant effects of amphetamine dose and time, prenatal arsenic exposure did not interact with amphetamine treatment. Within each amphetamine dose group, data were averaged across prenatal arsenic conditions for this figure. Sample size: 0 ppm n = 5 litters, 8 ppm n = 5, 25 ppm n = 5, 80 ppm n = 5.
3.7 Random Ratio Operant Behavior
There were significant main effects of sex, the RR value, and session (P≤0.002 for each) on five of the variables (number of earned food pellets, total lever presses, overall response rate, lever run rate, postreinforcement pause). There were also significant RR-by-session interactions (P≤0.007) for these variables. For the total lever presses, overall response rate, and postreinforcement pause variables there were significant sex-by-RR interactions (P≤0.01).
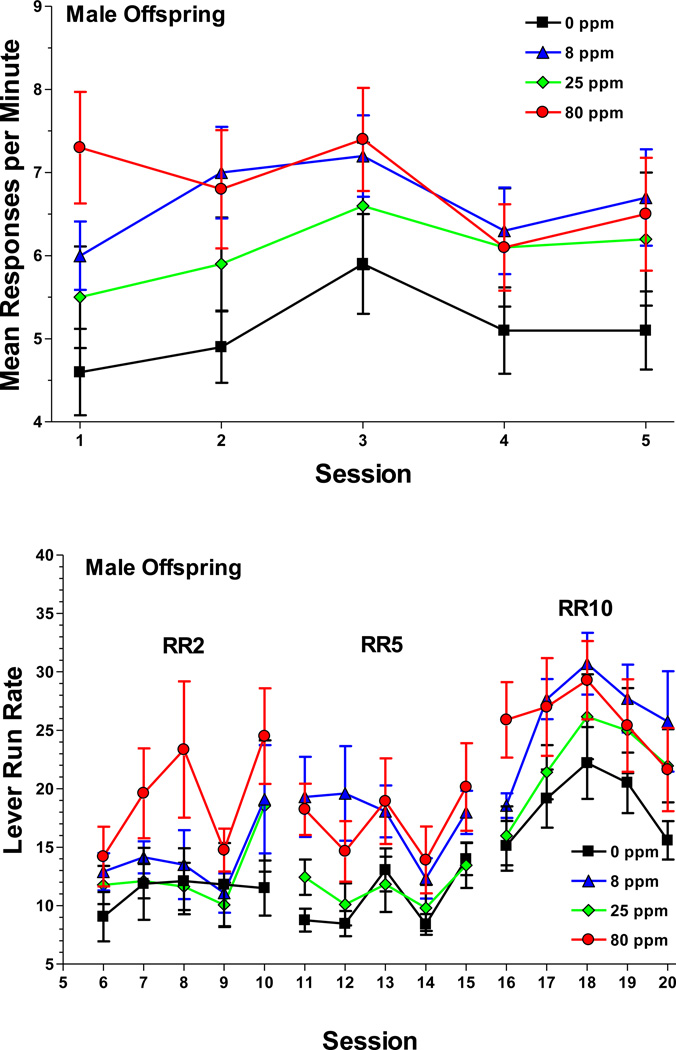
Exposure to arsenic impacted RR behavior in the following fashion: For the response rate, there was a sex-by-session-by-exposure [F(12,144)=2.18, P=0.03; Figure 6] interaction. To examine this interaction further, data from sessions 1 to 5 were averaged across the four RR values and the male and female exposure groups were compared within each of the sessions. Post hoc analysis indicated that during the first session, the 80 ppm male group responded at a higher rate than the control males. During the second session, the 8 ppm males responded at a higher rate than controls. None of the female exposure groups differed. There were also higher-order sex-by-RR-by-session-by-exposure interactions for the lever run rate [F(24,288)=1.89, P=0.03; Figure 6] and earned food [F(36,432)=1.75, P=0.01] variables. For the lever run rate, the male 8 and 80 ppm groups responded at higher rates than the male control group. Post hoc tests indicated that these differences reached statistical significance during session 11, 12, and 16, which were times of schedule transition i.e. the RR value had just increased. Because of the higher run rates, the male 8 ppm and 80 ppm groups earned significantly more food pellets than the control group during these sessions. The lever run rates and earned food pellets did not differ among the female exposure groups. There was no arsenic-related variance for the start latency and session duration variables.
Figure 6.
Mean ± SEM overall response rate (top panel) and lever run rate (bottom panel) for male offspring during the random ratio (RR) operant procedure. For the overall response rate, data were averaged across the RR value. The 80 ppm group responded at a higher rate than the control during session 1 and the 8 ppm group responded at a higher rate than the control during session 2. For the lever run rate, the 8 and 80 ppm groups responded at higher rates than the control during session 11, 12, and 16. Sample size: 0 ppm n = 11 litters, 8 ppm n = 10, 25 ppm n = 9, 80 ppm n = 10.
3.8 Go/No Go Operant Behavior
Prenatal exposure to arsenic did not affect the hit rate during go trials or the correct rejection rate during no go trials. Males generated more hits than females (main effect of sex: [F(1,35)=21.61, P<0.0001]), while females generated more correct rejections (main effect of sex: [F(1,35)=18.24, P<0.0001]). Both males and females performed the same number of hits during the first 10 sessions, with males showing greater improvement after the 10th session (sex-by-session interaction: [F(25,875)=5.61, P<0.0001]). In general, performance tended to improve with time as indicated by main effects of session (hits: [F(25,875)=7.11, P<0.0001]; correct rejections: [F(25,875)=20.83, P<0.0001]).
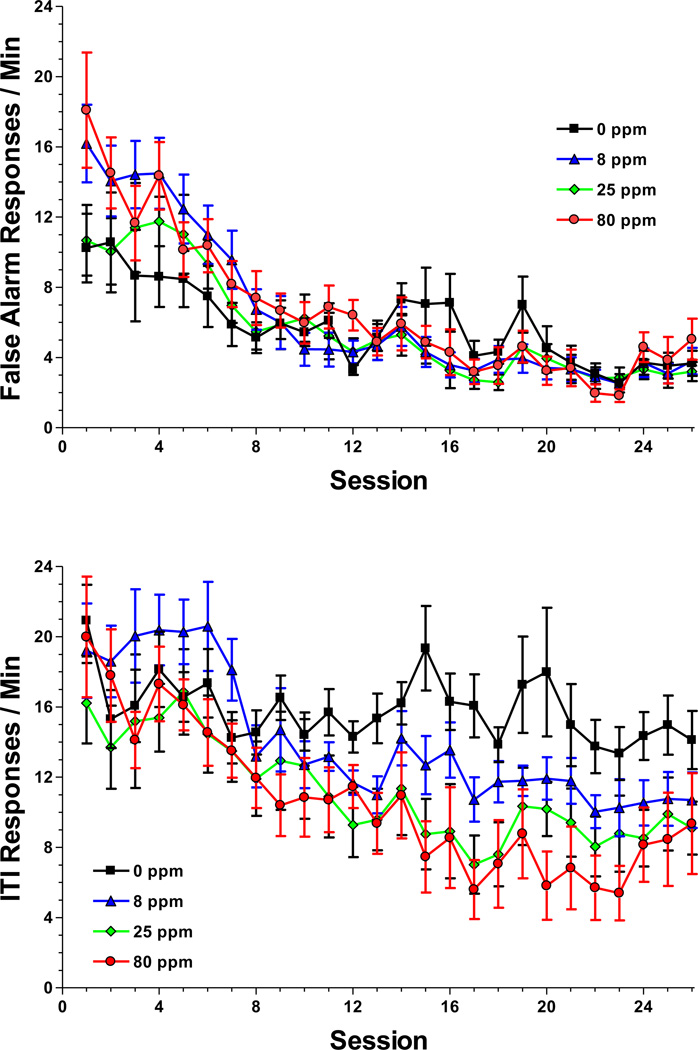
Males performed more false alarms than females (main effect of sex: [F(1,35)=32.78, P<0.0001]) primarily during the first 8 sessions after which their rate fell and approached that of the females (sex-by-session interaction: [F(25,875)=2.81, P=0.007]). However, the false alarm rate was also affected by prenatal exposure to arsenic (session-by-exposure interaction: [F(75,875)=1.93, P=0.01]; Figure 7) with the 8 ppm animals committing more false alarms than controls during the first 4 sessions. There was a similar effect of sex on the number of responses during the intertrial interval (sex-by-session interaction: [F(25,875)=4.81, P=0.0001]). However, there was also a main effect of exposure [F(3,35)=4.61, P=0.008] and a session-by-exposure interaction [F(75,875)=1.80, P=0.006; Figure 7]. Post hoc tests of the interaction indicated that the 0 ppm animals responded at a higher rate during the ITI than exposed animals from session 12 to 24.
Figure 7.
Mean ± SEM false alarm rate (top panel) and intertrial interval response rate (bottom panel) during the Go / No Go operant procedure. For the false alarm rate, the 8 ppm group committed more errors than the control during the first 4 sessions. For the intertrial interval rate, the control group responded at a higher rate than exposed groups from session 12 to 24. Data were averaged across sex for each variable. Sample size: 0 ppm n = 11 litters, 8 ppm n = 10, 25 ppm n = 9, 80 ppm n = 9.
4. Discussion
The current study found that gestational exposure to sodium arsenite in the maternal drinking water produced a number of persistent sensory-motor changes in C57BL6/J mouse offspring. The most striking deficits were noted in juvenile males and females who displayed gait-related impairments. Many of the motor impairments subsided with advancing age, although arsenic-exposed offspring had significantly less forelimb grip strength as adults compared to control offspring. In adulthood, the exposed offspring did not show global impairments during the operant procedures but there were several persistent response rate differences and some indication of altered attention, primarily in the exposed males.
The arsenic doses used in the current study did not produce any obvious maternal toxicity nor did they affect any general measures of growth in the offspring. Although we did not track maternal food and water consumption, the dams were weighed daily (Table 3) and there were no exposure-related differences. Our laboratory had used a similar range of doses in a previous study of the tissue disposition of arsenic in C57BL6/J mice and did not observe exposure-related effects on bodyweight in that study either (Markowski et al. 2010). In the earlier study, there was a significant increase in total arsenic in the PND1 brain, kidney, liver, and blood following gestational exposure to 80 ppm in the maternal drinking water. There was a trend towards higher tissue levels following 10, 20, or 40 ppm but these differences were not significant, even though significant behavioral changes following 8 or 25 ppm were observed in the current study. In the disposition study, tissue levels were no longer higher than controls by PND21, an age where we continued to observe behavioral deficits in the current study. In the current study, even the lowest dose of 8 ppm produced a range of deficits including aberrant behaviors from PND17-21, less motor activity on PND 13 and 17, less acoustic startle on PND13, less grip strength in PND60 males and females, altered response rates during the random ratio operant procedure, and a higher number of false alarms during the Go/No Go procedure.
The abnormal gait behaviors observed in the exposed offspring during the PND13-21 period were similar to descriptions of arsenic-induced peripheral neuropathy in the clinical literature. Adults that have been acutely exposed to high doses of arsenic by accident or during attempted suicide, experience muscle aches in the calf and toes and a severe burning pain in the soles of the feet 7–14 days after ingestion. Sore feet are often accompanied by abnormal gait patterns including steppage and waddling (Jenkins, 1966; Le Quesne and McLeod, 1977). An epidemiological examination of residents who were exposed to arsenic dust blown from a pesticide plant located in the center of a town in Georgia had prominent grip strength, hand-eye coordination, and finger tapping deficits that were interpreted as indicative of peripheral nerve dysfunction (Gerr et al. 2000). Since the pesticide plant manufactured and packaged arsenic-containing compounds from 1925 to 1985, it is quite likely that some lifetime residents were exposed during gestation. Although it was beyond the scope of this study, it would be interesting to identify and compare the lifetime exposure group to residents with briefer exposures limited to adulthood. Consequently, it seems reasonable to conclude that the current arsenic-exposed mouse offspring were experiencing peripheral neuropathy several weeks after the end of the prenatal exposure, during a period when the maturing corticospinal tracts should provide enough lower limb control to permit weight-bearing, four-limbed gait (Whelan, 2003). These effects were transient since gait behavior appeared to recover after PND21 in all of our animals and rotarod behavior was normal at puberty and in adulthood. A reduction of forelimb grip strength was observed in all of the male and female arsenic groups in adulthood, which suggests that prenatal arsenic produced some permanent motor changes. In hindsight, it might have been more informative to measure hindlimb grip strength as well, since the clinical literature indicates that arsenic-induced peripheral neuropathy targets the lower limbs more than the upper.
It was surprising that there was absolutely no effect of gestational arsenic on the puberty, 2-month, and 18-month activity tests. Increased open field locomotion was previously observed in rats that were exposed to 36.7 ppm arsenic in the maternal drinking water from GD15 to adulthood (Rodriguez et al. 2002). Besides the species difference, Rodriguez et al. (2002) used an apparatus with a larger open field and they measured a specific increase in vertical but not horizontal movement. Perhaps the relatively small operant chambers used in the present experiment did not provide enough space to differentiate the exposure groups. This seems unlikely though because the chambers did detect significant sex, age, and amphetamine dose differences. The longer exposure period in the Rodriguez et al. (2002) study also produced a much higher mean total arsenic content in the brain compared to our laboratory’s mid-range dose of 40 ppm (4.40 µg/g measured at 4 months vs. 0.0065 µg/g measured on PND21) (Markowski et al. 2010).
The amphetamine drug challenges produced the expected bell-shaped dose-response relationship in the 18-month animals but amphetamine did not differentiate the arsenic groups. The mid-range 1.5 and 3.0 mg/kg doses increased locomotion, an effect that peaked at 45–60 min post-injection. The 6.0 mg/kg dose significantly decreased locomotion during this same interval. These results are similar to those reported by Yates et al. (2007) who observed a locomotor increase in C57BL6/J mice that peaked at 40 min following 2.0 mg/kg, while 6.0 mg/kg reduced locomotion from 40 to 60 min post-injection. The amphetamine data suggest that prenatal arsenic, at the doses and during the exposure period used in the current study, did not produce a lasting functional effect on the striatal dopamine system. The higher doses of nicotine suppressed locomotion for the 1st hour of the activity test in accordance with previous studies in the C57BL6/J mouse (Lopez et al. 2003) but again, the drug challenges did not differentiate the arsenic groups. It should be noted that amphetamine and nicotine were administered only to the 18-month animals. The younger animals were not treated to avoid potential carry-over effects to the operant procedures scheduled for these animals. Perhaps the younger animals would have responded differently to the amphetamine or nicotine challenges.
During the RR operant testing, a sex-specific difference that has previously been noted in rats, was replicated in mice where males responded at higher rates than females (Heinsbroek et al. 1987a,b; Markowski et al. 2000; Markowski et al. 2002). Prenatal exposure to arsenic actually increased the RR response rate in the 8- and 80 ppm males during those sessions where the schedule transitioned to a higher response requirement. One interpretation of this effect is that prenatal arsenic exposure improved the ability of male mice to alter their behavior following a subtle change of the reinforcement contingency. Although it is difficult to explain this effect on a mechanistic level, it is probably not due to nonspecific motor activation since the activity tests in the operant chambers immediately before lever press training did not reveal any differences. And, although it was a sex-specific effect, prenatal arsenic did not appear to demasculinize the male nervous system since arsenic increased rather than decreased response rates.
Developmental exposure to a mixture of lightly-chlorinated PCBs has been shown to increase operant responding during differential reinforcement of high rates (DRH) schedules in male rats (Sable et al. 2006). Although there are important differences between the DRH and RR schedules, successful performance on both requires high rates of rsponding with little need for the inhibitory control. Sable et al. (2006) suggested that the increased DRH response rates could be a consequence of a PCB-induced depletion of catecholamines in the frontal cortex. However, it seems unlikely that the increased RR response rates in the current study were due to a pervasive effect of arsenic on catecholamine systems in the forebrain because amphetamine did not produce a differential effect on locomotor activity.
Developmental exposure to aluminum has also been shown to increase response rates during progressive ratio and DRH schedules, which suggests that aluminum can enhance food motivation in mice (Golub and Germann 1998). Perhaps prenatal arsenic exposure increased food motivation in male mice in the current study. In adult mice, arsenic exposure alters the levels and turnover of hypothalamic monoamines that regulate food intake (Mejia et al. 1997; Delgado et al. 2000; Itoh et al. 1990) and impairs glucose tolerance (Paul et al. 2007; Paul et al. 2011). Epidemiological studies have linked both aluminum and arsenic with altered glucose utilization and metabolic diseases such as Type II diabetes (Navas-Acien et al. 2006; Serdar et al. 2009).
The Go/No Go procedure did not reveal an exposure-related difference in the hit rate or correct rejection rate but the 8 ppm animals did commit more false alarm responses than controls. This effect was transient though, occurring only during the 1st four sessions. Indeed, as training progressed the exposed animals showed greater evidence of schedule-control since their response rates during the unreinforced intertrial intervals declined and were significantly lower than the controls by session 13. Consequently, although arsenic may have produced a transient effect on response inhibition, there was no effect on measures of sustained attention in the Go/No Go procedure.
In summary, prenatal exposure to sodium arsenite produced a number of behavioral changes in exposed offspring. Two of these effects, transient gait impairments in animals and a more persistent reduction of forelimb grip strength in adult animals, could be due to a common underlying effect on the peripheral motor system. However, there were three prominent effects on operant behavior that are more indicative of CNS changes: increased response rates and subsequent earned food reinforcement during RR, a transient increase of false alarm responses, and a more persistent reduction of unreinforced intertrial interval responses during Go/No Go. Collectively, the operant effects do not suggest that prenatal arsenic impaired schedule acquisition but might have instead disrupted inhibitory control and/or increased the motivation to perform in food-reinforced tasks. There was no clear dose-response relationship in the current study. All of the doses, from 8 to 80 ppm in the maternal drinking water, were associated with significant behavioral changes.
It is well-established that the Wechsler and related tests of intelligence can detect cognitive deficits in children who are exposed to arsenic-contaminated drinking water. If the current animal effects are indicative of other potential effects in humans, then arsenic exposure during development could also be producing subtle motor deficits that have gone unrecognized.
Highlights.
-
➢
Pregnant C57BL6/J mice consumed drinking water containing 0, 8, 25, or 80 ppm sodium arsenite.
-
➢
The following endpoints were examined in their offspring: growth, sensory-motor behaviors, grip strength, learning, and attention.
-
➢
Each arsenic dose produced behavioral impairments.
-
➢
Motor behaviors were the most sensitive to prenatal arsenic.
Acknowledgement
This research was funded by grant 1R15ES015351-01 from the NIEHS to V. Markowski.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
They authors declare that they have no actual or potential conflicts of interest including any financial, personal or other relationships with other people or organizations that inappropriately influenced, could have influenced, or be perceived to influence, their work.
References
- Baxley MN, Hood RD, Vedel GC, Harrison WP, Szczech GM. Prenatal toxicity of orally administered sodium arsenite in mice. B Environ Contam Tox. 1981;26:749–756. doi: 10.1007/BF01622166. [DOI] [PubMed] [Google Scholar]
- Bardullas U, Limón-Pacheco JH, Giordano M, Carrizales L, Mendoza-Trejo MS, Rodríguez VM. Chronic low-level arsenic exposure causes gender-specific alterations in locomotor activity, dopaminergic systems, and thioredoxin expression in mice. Toxicol Appl Pharm. 2009;239:169–177. doi: 10.1016/j.taap.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P, Jacks G, Frisbie S, Smith E, Naidu R, Sarkar B. Arsenic in the environment: a global perspective. In: Sarkar B, editor. Heavy metals in the environment. New York: Marcel Dekker Inc; 2002. pp. 147–215. [Google Scholar]
- Brown J, Zeise L. Public health goal for arsenic in drinking wate. Drinking Water Public Health Goals, Pesticide and Environmental Toxicology Section, Office of Environmental Health Hazard Assessment, California Environmental Protection Agency. 2004 [Google Scholar]
- Calderon J, Navarro ME, Jimenez-Capedeville ME, Santos-Diaz MA, Golden A, Rodriguez-Leyva I, et al. Exposure to arsenic and lead and neuropsychological development in Mexican children. Environ Res Section A. 2001;85:69–76. doi: 10.1006/enrs.2000.4106. [DOI] [PubMed] [Google Scholar]
- Chaineau E, Binet S, Pol D, Chatellier G, Meininger V. Embryotoxic effects of sodium arsenite and sodium arsenate on mouse embryos in culture. Teratol. 1990;41:105–112. doi: 10.1002/tera.1420410111. [DOI] [PubMed] [Google Scholar]
- Chakraborti D, Mukherjee SC, Pati S, Sengupta MK, Rahman MM, Chowdhury UK, et al. Arsenic groundwater contamination in Middle Ganga Plain, Bihar, India: A future danger? Environ Health Persp. 2003;111:1194–1201. doi: 10.1289/ehp.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Bhaumik S, Purayastha M, Basu S, Chaudhuri AN, Gupta SD. Apoptosis and necrosis in developing brain cells due to arsenic toxicity and protection with antioxidants. Toxicol Lett. 2002;136:65–76. doi: 10.1016/s0378-4274(02)00282-5. [DOI] [PubMed] [Google Scholar]
- Colomina MT, Albina ML, Domingo JL, Corbella J. Influence of maternal restraint stress on arsenic-induced pre- and postnatal alterations in mice. Psychobiol. 1996;24:227–234. [Google Scholar]
- Cui X, Kobayashi Y, Hayakawa T, Hirano S. Arsenic speciation in bile and urine following exposure to inorganic and organic arsenics in rats. Toxicol Sci. 2004;82:478–487. doi: 10.1093/toxsci/kfh265. [DOI] [PubMed] [Google Scholar]
- Delgado JM, Dufour L, Grimaldo JI, Carrizales L, Rodriguez VM, Jimenez-Capdeville ME. Effects of arsenite on central monoamines and plasmatic levels of adrenocorticotropic hormone (ACTH) in mice. Toxicol Lett. 2000;117:61–67. doi: 10.1016/s0378-4274(00)00240-x. [DOI] [PubMed] [Google Scholar]
- Eisler R. Arsenic hazards to fish, wildlife and invertebrates: a synoptic review. U.S. Fish Wildl Serv Biol Rep. 85(1.12) [Google Scholar]
- Gerr F, Letz R, Ryan PB, Green RC. Neurological effects of environmental exposure to arsenic in dust and soil among humans. Neurotoxicology. 2000;21:475–488. [PubMed] [Google Scholar]
- Golub MS, Germann SL. Aluminum effects on operant performance and food motivation of mice. Neurotoxicol Teratol. 1998;20:421–427. doi: 10.1016/s0892-0362(97)00133-5. [DOI] [PubMed] [Google Scholar]
- Heinsbroek RBW, van Haaren F, Zantvoord F, van de Poll NE. Effects of pentobarbital and progesterone on random ratio responding in male and female rats. Psychopharmacol. 1987;93:178–181. doi: 10.1007/BF00179930. [DOI] [PubMed] [Google Scholar]
- Heinsbroek RBW, van Haaren F, Zantvoord F, van de Poll NE. Sex differences in response rates during random ratio acquisition: effects of gonadectomy. Physiol Behav. 1987;39:269–272. doi: 10.1016/0031-9384(87)90020-5. [DOI] [PubMed] [Google Scholar]
- Itoh T, Zhang YF, Murai S, Saito H, Nagahama H, Miyate H, et al. The effect of arsenic trioxide on brain monoamine metabolism and locomotor activity of mice. Toxicol Lett. 1990;54:345–353. doi: 10.1016/0378-4274(90)90202-w. [DOI] [PubMed] [Google Scholar]
- Jenkins RB. Inorganic arsenic and the nervous system. Brain, A Journal of Neurology. 1966;89:479–498. doi: 10.1093/brain/89.3.479. [DOI] [PubMed] [Google Scholar]
- Jin Y, Xi S, Li X, Lu C, Li G, Xu Y, et al. Arsenic speciation transported through the placenta from mother mice to their newborn pups. Environ Res. 2006;101:349–355. doi: 10.1016/j.envres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Kapaj S, Peterson H, Liber K, Bhattacharya P. Human health effects From chronic arsenic poisoning–a review. J Environ Sci Heal A. 2006;41:2399–2428. doi: 10.1080/10934520600873571. [DOI] [PubMed] [Google Scholar]
- Le Quesne PM, McLeod JG. Peripheral neuropathy following a single exposure to arsenic. J Neurol Sci. 1997;32:437–451. doi: 10.1016/0022-510x(77)90025-9. [DOI] [PubMed] [Google Scholar]
- Li D, Lu C, Wang J, Hu W, Cao Z, Sun D, et al. Developmental mechanisms of arsenite toxicity in zebrafish (Danio rerio) embryos. Aquat Toxicol. 2009;91:229–237. doi: 10.1016/j.aquatox.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Lindgren A, Danielsson BRG, Dencker L, Vahter M. Embryotoxicity of arsenite and arsenate: distribution in pregnant mice and monkeys and effects on embryonic cell in vitro. Acta Pharmacol et Toxicol. 1984;54:311–320. doi: 10.1111/j.1600-0773.1984.tb01936.x. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Simpson DD, White NM, Randall CL. Age- and sex-related differences in alcohol and nicotine effects in C57BL/6J mice. Addict Biol. 2003;8:419–427. doi: 10.1080/13556210310001648176. [DOI] [PubMed] [Google Scholar]
- Lu M, Wang H, Li XF, Arnold LL, Cohen SM, Le XC. Binding of dimethylarsinous acid to cys-13alpha of rat hemoglobin is responsible for retention of arsenic in rat blood. Chem Res Toxicol. 2007;20:27–37. doi: 10.1021/tx060195+. [DOI] [PubMed] [Google Scholar]
- Markowski VP, Cox C, Preston R, Weiss B. Effects of age and gender but not prenatal cocaine on random ratio and delayed spatial alternation responding in rats. Neurotoxicol Teratol. 2000;22:421–428. doi: 10.1016/s0892-0362(99)00080-x. [DOI] [PubMed] [Google Scholar]
- Markowski VP, Cox C, Preston R, Weiss B. Impaired cued delayed alternation behavior in adult rat offspring following prenatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on gestation day 15. Neurotoxicol Teratol. 2002;24:209–218. doi: 10.1016/s0892-0362(02)00186-1. [DOI] [PubMed] [Google Scholar]
- Markowski VP, Currie D, Reeve EA, Thompson D, Wise JP., Sr Tissue-specific and dose-related accumulation of arsenic in mouse offspring following maternal consumption of arsenic-contaminated water. Basic Clin Pharmacol Toxicol. 2010;108:326–332. doi: 10.1111/j.1742-7843.2010.00660.x. [DOI] [PubMed] [Google Scholar]
- Martinez EJ, Kolb BL, Bell A, Savage DD, Allan AM. Moderate perinatal arsenic exposure alters neuroendocrine markers associated with depression and increases depressive-like behaviors in adult mouse offspring. Neurotoxicology. 2008;29:647–655. doi: 10.1016/j.neuro.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Finley EJ, Ali A-MS, Allan AM. Learning deficits in C57BL/6J mice following perinatal arsenic exposure: consequence of lower corticosterone receptor levels? Pharmacol Biochem Behav. 2009;94:271–277. doi: 10.1016/j.pbb.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia JJ, Diaz-Barriga F, Calderon J, Rios C, Jimenez-Capdeville ME. Effects of lead-arsenic combined exposure on central monoaminergic systems. Neurotoxicol Teratol. 1997;19:489–497. doi: 10.1016/s0892-0362(97)00066-4. [DOI] [PubMed] [Google Scholar]
- Mukherjee AB, Bhattacharya P. Arsenic in groundwater in the Bengal Delta Plain: slow poisoning in Bangladesh. Environ Rev. 2001;9:189–220. [Google Scholar]
- Nagaraja TN, Desiraju T. Effects of operant learning and brain acetylcholine esterase activity in rats following chronic inorganic arsenic intake. Human Exp Toxicol. 1994;13:353–356. doi: 10.1177/096032719401300511. [DOI] [PubMed] [Google Scholar]
- Nagaraja TN, Desiraju T. Regional alterations in the levels of brain biogenic amines, glutamate, GABA, and GAD activity due to chronic consumption of inorganic arsenic in developing and adult rats. Bull Environ Contam Toxicol. 1993;50:100–107. doi: 10.1007/BF00196547. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E. Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiologic evidence. Environ Health Persp. 2006;114:641–648. doi: 10.1289/ehp.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemec MD, Holsen JF, Farr CH, Hood RD. Developmental toxicity assessment of arsenic acid in mice and rabbits. Reprod Toxicol. 1998;12:647–658. doi: 10.1016/s0890-6238(98)00053-7. [DOI] [PubMed] [Google Scholar]
- Paul DS, Hernández-Zavala A, Walton FS, Adair BM, Dědina J, Matoušek T, et al. Examination of the effects of arsenic on glucose homeostasis in cell culture and animal studies: development of a mouse model for arsenic-induced diabetes. Toxicol Appl Pharm. 2007;222:305–314. doi: 10.1016/j.taap.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul DS, Walton FS, Saunders RJ, Stýblo M. Characterization of the impaired glucose homeostasis produced in C57BL/6 mice by chronic exposure to arsenic and high-fat diet. Environ Health Persp. 2011;119:1104–1109. doi: 10.1289/ehp.1003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor GT, Uyeno ET, Tilson HA, Mitchell CL. Assessment of chemicals using a battery of neurobehavioral tests: a comparative study. Neurobehav Toxicol Teratol. 1983;5:91–117. [PubMed] [Google Scholar]
- Rice DC, Reeve EA, Herlihy A, Zoeller RT, Thompson WD, Markowski VP. Developmental delays and locomotor activity in the C57BL6/J mouse following neonatal exposure to the fully-brominated PBDE, decabromodiphenyl ether. Neurotoxicol Teratol. 2007;29:511–520. doi: 10.1016/j.ntt.2007.03.061. [DOI] [PubMed] [Google Scholar]
- Rocha-Amador D, Navarro ME, Carrizales L, Morales R, Calderon J. Decreased intelligence in children and exposure to fluoride and arsenic in drinking water. Cad Saude Publica Rio de Janeiro. 2007;23:S579–S587. doi: 10.1590/s0102-311x2007001600018. [DOI] [PubMed] [Google Scholar]
- Rodriguez VM, Carrizales L, Jimenez-Capdeville ME, Dufour L, Giordano M. The effects of sodium arsenite on behavioral parameters in the rat. Brain Res Bull. 2001;55:301–308. doi: 10.1016/s0361-9230(01)00477-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez VM, Carrizales L, Mendoza MS, Fajardo OR, Giordano M. Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicol Teratol. 2002;24:743–750. doi: 10.1016/s0892-0362(02)00313-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez VM, Jimenez-Capdeville ME, Giordano M. The effects of arsenic exposure on the nervous system. Toxicol Lett. 2003;145:1–18. doi: 10.1016/s0378-4274(03)00262-5. [DOI] [PubMed] [Google Scholar]
- Rosado JL, Ronquillo D, Kordas K, Rojas O, Alatorre J, Lopez P, et al. Arsenic exposure and cognitive performance in Mexican schoolchildren. Environ Health Persp. 2007;115:1371–1375. doi: 10.1289/ehp.9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sable HJK, Powers BE, Wang VC, Widholm JJ, Schantz SL. Alterations in DRH and DRL performance in rats developmentally exposed to an environmental PCB mixture. Neurotoxicol Teratol. 2006;28:548–556. doi: 10.1016/j.ntt.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Schultz H, Nagymajtenyi L, Institoris L, Papp A, Siroki O. A study on behavioral, neurotoxicological, and immunocytological effects of subchronic arsenic treatment in rats. J Environ Sci Heal A. 2002;65:1181–1193. doi: 10.1080/152873902760125390. [DOI] [PubMed] [Google Scholar]
- Serdar MA, Bakir F, Haşimi A, Çelik T, Akin O, Kenar L, et al. Trace and toxic element patterns in nonsmoker patients with noninsulin-dependent diabetes mellitus, impaired glucose tolerance, and fasting glucose. Int J Diab Dev Ctries. 2009;29:35–40. doi: 10.4103/0973-3930.50713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Chou HY, The HW, Chen CM, Chen CJ. The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology. 2003;24:747–753. doi: 10.1016/S0161-813X(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Vahter M. Methylation of inorganic arsenic in different mammalian species and population groups. Sci Prog. 1999;82:69–88. doi: 10.1177/003685049908200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M, Norin H. Metabolism of 74As-labeled trivalent and pentavalent inorganic arsenic in mice. Environ Res. 1980;21:446–457. doi: 10.1016/0013-9351(80)90049-3. [DOI] [PubMed] [Google Scholar]
- Wang SX, Wang ZH, Cheng XT, Li J, Sang ZP, Zhang XD, et al. Arsenic and fluoride exposure in drinking water: children's IQ and growth in Shanyin County, Shanxi Province, China. Environ Health Persp. 2007;115:643–647. doi: 10.1289/ehp.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, Geen AV, et al. Water arsenic exposure and children's intellectual function in Araihazar, Bangladesh. Environ Health Persp. 2004;112:1329–1333. doi: 10.1289/ehp.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe C, Inaoka T, Matsui T, Ishigaki K, Murayama N, Ohtsuka R. Effects of arsenic on younger generations. J Environ Sci Heal A. 2003;A38:129–139. doi: 10.1081/ese-120016885. [DOI] [PubMed] [Google Scholar]
- Whelan PJ. Developmental aspects of spinal locomotor function: insights from using the in vitro mouse spinal cord preparation. J Physiol. 2003;553:695–706. doi: 10.1113/jphysiol.2003.046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willhite CC, Ferm VH. Prenatal and developmental toxicology of arsenicals. Adv Exp Med and Bio. 1984;177:205–228. doi: 10.1007/978-1-4684-4790-3_9. [DOI] [PubMed] [Google Scholar]
- Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology. 2006;27:210–216. doi: 10.1016/j.neuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- von Ehrenstein OS, Poddar S, Yuan Y, Mazumder DG, Eskenazi B, Basu A, et al. Children's intellectual function in relation to arsenic exposure. Epidemiology. 2007;18:44–51. doi: 10.1097/01.ede.0000248900.65613.a9. [DOI] [PubMed] [Google Scholar]
- Yates JW, Meij JTA, Sullivan JR, Richtand NM, Yu L. Bimodal effect of amphetamine on motor behaviors in C57BL/6 mice. Neurosci Lett. 2007;427:66–70. doi: 10.1016/j.neulet.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]