Abstract
Preclinical research and learning theory suggest that a longer duration of varenicline treatment prior to the target quit date (TQD) should reduce smoking rates before cessation and improve abstinence outcomes. A double-blind RCT tested this hypothesis among 60 smokers randomized to either Extended (4 weeks of pre-TQD varenicline) or standard run-in (3 weeks of placebo, 1 week of pre-TQD varenicline); everyone received 11 weeks of post-TQD varenicline and brief counseling. During the pre-quit run-in, the reduction in smoking rates was greater among the Extended group (42% vs. 24%, p<0.01) and this effect was greater among women (57% vs. 26%, p=0.001). Continuous abstinence during the final four weeks of treatment was enhanced among women in the Extended group (67% vs. 35%). While these data suggest that extending pre-quit varenicline reduces smoking during the pre-quit period and may further enhance cessation rates, confirmatory evidence is needed from larger clinical trials.
Trial Registration: www.clinicaltrials.gov identifier: NCT00835900
Keywords: smoking cessation, varenicline, reinforcement, extinction
Introduction
Cigarette smoking remains the single largest preventable cause of death in the United States.(1) Despite the fact that the vast majority of the 45 million smokers in the U.S. today want to stop smoking, most are unable to do so easily with currently available therapies for treating nicotine addiction.(1)
The 2008 update on smoking cessation from the Public Health Service(2) added varenicline to the limited list of first line pharmacotherapies for smoking cessation, and trials published since that time have continued to support the use of varenicline as producing outcomes comparable to or better than any other cessation agent.(3) Nevertheless, long-term abstinence rates remain disappointing; by the end of 12-weeks of treatment with varenicline, fewer than 50% of smokers in clinical trials remain abstinent, and abstinence rates generally drop to around 25% within the first year after quitting.(3) However, there is good reason to believe a better understanding of the mechanisms by which varenicline works can lead to improved outcomes.(4, 5)
Varenicline binds to the alpha-4 beta-2 receptor subunit of nicotinic acetylcholine receptors (nAChR), exerting effects as both a partial nicotine agonist, by stimulating dopamine release, and as an antagonist, by blocking the binding of nicotine to this site. Preclinical studies suggest that varenicline is also a full agonist at the alpha-7 nAChR subunit.(6) In two clinical trials, post-cessation smoking urges, cravings, and satisfaction with cigarettes during lapses were robustly reduced with varenicline.(7, 8)
Though post-quit cessation mechanisms are certainly important, it is also important to examine the effects of medication prior to actual cessation.(2, 9, 10) This is especially important for an agent like varenicline that is typically administered for a week prior to quitting and is believed to work, in part, by reducing the reinforcing or desirable effects of smoking. From a learning perspective, when the favorable consequences are removed, the behavior decreases in frequency, or is extinguished. In the case of smoking and varenicline, we follow the preclinical work of Coe and Rollema in hypothesizing that varenicline reduces the reinforcing effects of smoking, thereby promoting the extinction of smoking behavior.(11, 12)
Building on the pioneering work of Rose and colleagues (13), a small literature is developing on extending pre-treatment pharmacotherpy. A meta-analysis of 4 studies using nicotine patch therapy prior to quitting concluded that pre-treatment patch use resulted in a doubling of 6 week and 6 month quit rates.(14) Consistent with an extinction framework, the 3 studies that assessed this parameter noted reductions in cigarettes smoked per day. Similarly, we (Hawk, Mahoney, Ashare, Rhodes,Oliver, Cummings, & Fickling, unpublished data) recently observed that four weeks of pre-quit bupropion reduced smoking rate and ratings of how good cigarettes tasted during the pre-quit period, relative to bupropion treatment that began one week prior to cessation. Extended pre-quit bupropion also improved time to first lapse and approximately doubled short-term cessation.
This paper presents data from a randomized clinical trial designed to test whether extending the duration of pre-quit varenicline from one week (standard run-in) to four weeks (extended run-in) would lead to greater pre-quit reductions in smoking rate, expired-air breath CO, and favorable ratings of cigarette taste and smoking satisfaction. We also conducted exploratory analyses of smoking abstinence measured 3 months after the target quit date. In addition, given that participant sex often moderates the behavioral pharmacology of nicotine and smoking (15, 16), as well as cessation (17, 18), we explored the moderating role of sex in the effects of pre-quit varenicline duration.
Results
Participant disposition
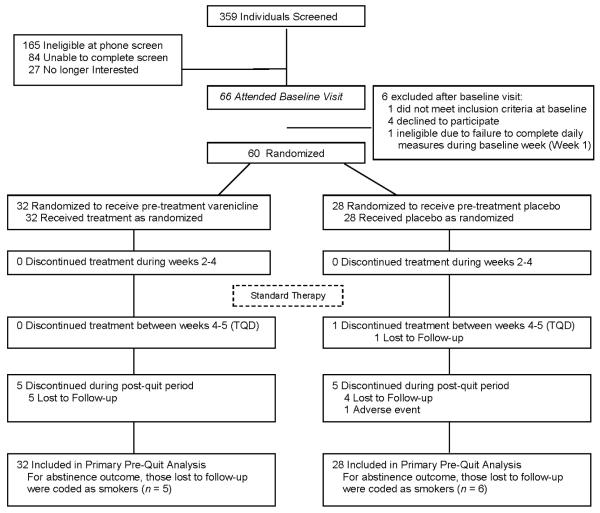
A total of 60 participants were eligible, randomly assigned, and included in primary analysis (see Figure 1 for participant flow). Table 1 provides demographic information and baseline smoking characteristics for all Run-In Group × Sex conditions. There were no statistically significant main effects or interactions for any baseline characteristic. On average, participants were 48 years old, reported smoking 21 cigarettes per day, and were moderately nicotine dependent (mean FTND = 5.2).
Figure 1.
Disposition of participants
Table 1. Selected demographic and tobacco use characteristics at baseline.
| Run-In Group |
|||||||
|---|---|---|---|---|---|---|---|
| Extended | Standard | p-value | |||||
| Male | Female | Male | Female | Run-In Group |
Sex | GxS | |
| n | 14 | 18 | 11 | 17 | -- | -- | 0.73 |
| Age, years | 46.3 (10.7) | 48.8 (7.5) | 49.0 (7.5) | 48.7 (11.7) | 0.61 | 0.67 | 0.57 |
| Non-white, n (%) | 1 (7%) | 2 (11%) | 1 (9%) | 4 (24%) | 0.34 | 0.30 | -- |
| Income, (% below 40K) | 50% | 33% | 18% | 41% | 0.50 | 0.93 | -- |
| Education, (% greater than high school education) |
21% | 39% | 28% | 53% | 0.35 | 0.09 | -- |
| Weight (kg) | 85.6 (19.4) | 76.4 (17.6) | 85.9 (10.8) | 81.0 (18.1) | 0.59 | 0.12 | 0.63 |
| Years Smoking | 27.1 (11.5) | 26.8 (10.8) | 25.3 (10.4) | 28.2 (13.8) | 0.94 | 0.68 | 0.60 |
| Baseline CPD | 21.4 (5.6) | 21.4 (5.7) | 23.6 (7.2) | 19.4 (3.4) | 0.94 | 0.15 | 0.15 |
| FTND | 5.4 (2.2) | 5.4 (1.8) | 5.4 (2.7) | 4.5 (1.6) | 0.37 | 0.45 | 0.43 |
| ≥1 Prior Quit, n (%) | 11 (79%) | 16 (89%) | 10 (91%) | 15 (88%) | 0.58 | 0.61 | -- |
Note: Except where indicated, all the values represent mean and standard deviation (SD). GxS= Run-In Group (G) x Sex (S) interaction; FTND=Fagerstrom Test for Nicotine Dependence; CPD=cigarettes per day
Pre-quit changes in smoking
Cigarettes smoked per day
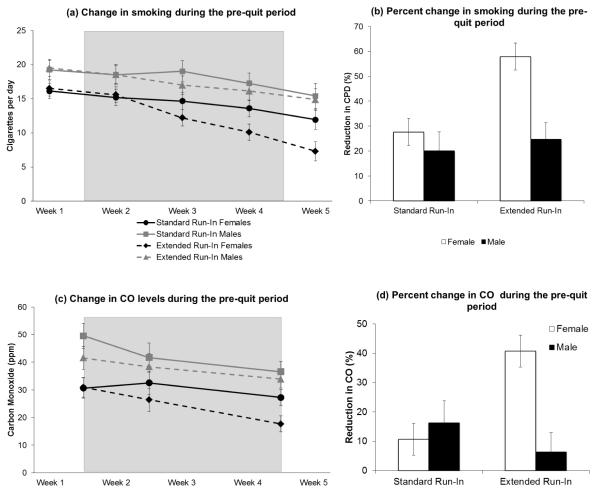
Figure 2a presents the mean (SE) cigarettes per day for each Run-In Group × Sex condition across the 5-week pre-quit period. The predicted Run-In Group × Time interaction was significant, p = 0.004. The reduction in CPD from week 2 to week 5 was greater for the Extended Run-In group (mean difference = 6.3, p = 0.0001) compared to the Standard Run-In group (mean difference = 3.2, p = 0.0003). Results also suggested that the Group × Time interaction differed by sex, p = 0.029. Women in the Extended Run-In group showed a greater reduction in CPD compared to those in the Standard Run-In group (mean difference = 4.9 CPD), p = 0.003. There was no treatment group effect for men (mean difference = 0.78 CPD, F < 1). The same pattern was evident for percent reduction in CPD during the pre-quit period (Figure 2b), Run-In Group × Sex p = 0.045. Women in the Extended Run-In Group exhibited a greater reduction in smoking than that observed for each of the other Run-In × Sex groups, all pairwise Fs > 11.7, all ps < 0.01, all of which showed comparable reductions across the pre-quit period, pairwise Fs < 1, ps > 0.6.
Figure 2.
(a) Unadjusted mean and standard error (SE) cigarettes per day for all Run-in Group × Sex conditions during the five-week pre-quit period. Note. Shaded region reflects the 3-week drug manipulation phase. Baseline (week 1) was used as a covariate in repeated measures ANOVA. (b) Mean (SE) percent reduction in CPD during the pre-quit phase (Final Week Pre-TQD; Week 5 vs. Baseline Week; Week 1) in all Run-in Group × Sex conditions, (c) Mean (SE) expired-air CO measurements for all Run-in Group × Sex conditions during the five-week pre-quit period. Analyses included Baseline (end of week 1) and Drug Manipulation Phase (end of weeks 2 and 4). During the 3-week Drug Manipulation Phase, the Extended Run-in Group received varenicline, while the Standard Run-in Group received placebo, (d) Mean (SE) percent reduction in CO during the pre-quit phase (end of week 4 vs. end of week 1) in all Run-in Group × Sex conditions.
Carbon monoxide (CO)
Figure 2c presents the mean (SE) CO levels for each Run-In Group × Sex condition across the pre-quit period. The predicted Run-In Group × Time interaction was not significant for CO, p = 0.22. However, the Run-In Group × Sex interaction was significant, p = 0.005. During the three-week run-in manipulation period, women in the Extended Run-In group had reduced CO levels compared to women in the Standard Run-In Group (mean difference = 8.1 ppm, p = 0.002); there was no Run-In group effect for males (mean difference = 3.3 ppm, p = 0.28). Percent CO reduction during the pre-quit period (Figure 2d) exhibited a similar pattern, with a significant Run-In Group × Sex interaction, p = 0.015: women in the Extended Run-In Group showed a greater percent reduction in CO than that observed for each of the other Run-In × Sex groups, all pairwise F’s > 8.7, all p’s < 0.01, all of which showed comparable CO reductions to one another, pairwise F’s < 1.
Pre-quit changes in craving, withdrawal, and cigarette effects
Craving and withdrawal
There were no significant effects involving run-in group for self-reported morning craving and withdrawal during the pre-quit period, all F’s <1.6, p’s > 0.21.
Cigarette effects
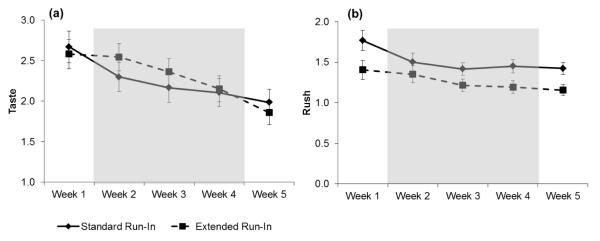
There was a marginal Run-In group ×Time interaction for CEQ satisfaction, p = 0.08, which appeared driven by reductions in the single “How good did it taste?” item. Therefore, we examined the cigarette taste item separately. The Run-In Group × Time interaction was significant, p = 0.032. As predicted, the Extended Run-In group reported greater reductions in how good their cigarettes tasted, p = 0.0001, than did the Standard Run-In group, p = 0.08 (See Figure 3a).
Figure 3.
Unadjusted means (SE) for single CEQ item “Did it taste good?” (panel a) and CEQ rush scale (panel b) in reference to the first cigarette of the day by Run-in Group during the five-week pre-quit period. Note. Shaded region reflects the 3-week drug manipulation phase. Baseline (week 1) was used as a covariate in repeated measures ANOVA.
For the CEQ “rush” scale (lightheadedness and headrush items; the scale is often described as aversion for non-smokers), there was a significant Run-In Group × Time interaction, p = 0.009. Simple main effects tests revealed that the Extended Run-In group reported significant decreases in the rush provided by the first cigarette of the day across the pre-quit period, p = 0.004; the Standard Run-In group showed no change in this rush, F < 1 (See Figure 3b).
For the remaining CEQ subscales, there were no significant changes over time, group differences, or interactions, all F’s < 1.9, p’s > 0.14.
Abstinence
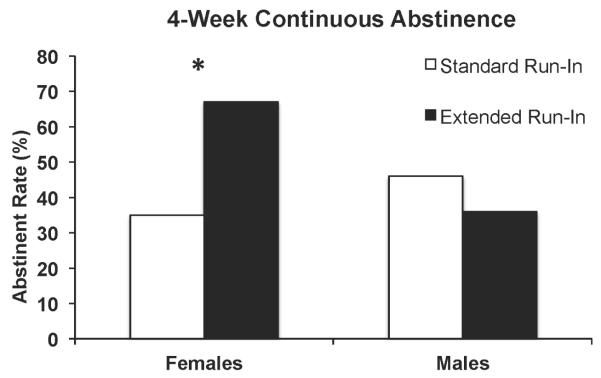
Although continuous abstinence during the final four weeks of treatment was higher among the Extended Run-In group (53%) than the Standard Run-In group (40%), OR = 1.8, the Run-In group effect was not significant, Wald χ2 < 1. However, the treatment effect tended to be moderated by sex, Run-In Group × Sex Wald χ2 (1) = 3.0, p = 0.08, OR = 5.50, 95% CI, 0.65 - 46.5. Follow-up tests revealed that, among females, continuous abstinence rates were higher for the Extended Run-in group (67%) compared to Standard Run-in group (35%) (OR = 4.3, 95% CI, 0.978-18.65, p = 0.05). In contrast, there were no differences in continuous abstinence rates between the Extended (36%) and Standard Run-in (46%) groups among males (OR = 0.60, 95% CI, 0.114-3.17, p = 0.5) (see Figure 4).
Figure 4.
CO-verified 4-week continuous abstinence (not even a puff during the final four weeks of treatment; post-quit weeks 8 through 11) for all Run-in Group × Sex conditions.
Note. * p = 0.05.
Medication adherence
Overall, medication adherence was excellent with means above 96% at each clinic visit. There were no Run-In group differences in adherence, all ps > 0.21. Although males tended to be less compliant during Weeks 2-4 compared to females, this was only a 2% absolute difference, p = 0.05 and did not vary by Run-In group, F < 1. There were no sex differences in adherence at subsequent time points, all ps > 0.19.
Evaluation of treatment blind
Whereas only 39% of participants taking placebo during this period believed they were taking varenicline, 75% of those actually taking varenicline believed they were taking varenicline, p=.008. This effect was virtually identical for males (36% and 71%) and females (41% and 78%).
Adverse Events
Table 2 reports presents a systematic assessment of adverse events by group during both the 3-week drug manipulation phase (Weeks 2-4) and in the subsequent 3-week period during which all participants were taking varenicline (Weeks 5-7). During Weeks 2-4, nausea, constipation, bloating, and indigestion were significantly more common among the Extended Run-In group compared to the Standard Run-In group, but none of these side effects reliably differentiated the run-in groups in Weeks 5-7. Relative to the Extended Run-In group, the standard run-in group tended to report more problems with dry mouth during Weeks 2-4 (when they were not receiving active medication) and increases in headache and skin problems during Weeks 5-7. No other side effects reliably differentiated the two run-in groups in either 3-week period. The majority of all side effects were rated as ‘mild’.
Table 2.
Self-reported side effects by run-in group, separately for Weeks 2-4 and Weeks 5-7.
| Weeks 2-4 |
Weeks 5-7 |
|||||
|---|---|---|---|---|---|---|
| Extended | Standard | p-value | Extended | Standard | p-value | |
| Nausea | 56% | 21% | 0.006 | 38% | 18% | 0.11 |
| Constipation | 25% | 4% | 0.02 | 22% | 11% | 0.27 |
| Bloating | 25% | 7% | 0.06 | 22% | 15% | 0.49 |
| Indigestion | 19% | 4% | 0.07 | 12% | 11% | 0.87 |
| Dry Mouth | 16% | 36% | 0.07 | 12% | 26% | 0.19 |
| Headache | 28% | 21% | 0.55 | 13% | 37% | 0.03 |
| Skin Problems | 3% | 7% | 0.48 | 0% | 11% | 0.05 |
| Insomnia | 34% | 21% | 0.27 | 34% | 15% | 0.09 |
| Flatulence | 22% | 14% | 0.44 | 28% | 30% | 0.90 |
| Vomiting | 9% | 7% | 0.76 | 3% | 4% | 0.90 |
| Decreased Appetite | 25% | 18% | 0.50 | 16% | 19% | 0.77 |
| Taste Problems | 25% | 11% | 0.15 | 9% | 15% | 0.52 |
| Abnormal Dreams | 34% | 21% | 0.27 | 34% | 26% | 0.48 |
| Forgetfulness | 12% | 14% | 0.84 | 12% | 15% | 0.80 |
| Fatigue | 22% | 18% | 0.70 | 25% | 26% | 0.94 |
| Muscle Aches | 19% | 11% | 0.38 | 12% | 4% | 0.23 |
| Dizziness | 9% | 11% | 0.86 | 9% | 7% | 0.79 |
| Ringing in Ears | 16% | 11% | 0.58 | 12% | 15% | 0.80 |
| Mood | 12% | 7% | 0.49 | 12% | 15% | 0.80 |
| Suicidal Thoughts | 0% | 0% | - | 0% | 4% | 0.27 |
Note. For each side effect, values represent % of participants reporting an increase from baseline. Weeks 2-4 were the drug manipulation phase, during which the extended run-in group received varenicline and the standard run-in group received placebo. Weeks 5-7 included the TQD visit and the two-week follow-up visit and represent the first three weeks during which the standard run-in group received varenicline.
Given that sex moderated Run-In group effects on multiple outcomes, we examined the highest-frequency side effect, nausea, separately in males and females. Paralleling the data for CPD and CO, Run-In group differences were evident among women, but not men. During the drug manipulation phase, nausea was more common among women in the Extended Run-In group (78%) compared to women in the Standard Run-In group (24%), p=.001. This difference tended to persist during Weeks 5-7, 50% v. 24%, p=0.11. There effect of Run-In group on the incidence of nausea among men was not statistically significant, (29% v. 18% for Weeks 2-4 and 21% vs. 10% for Weeks 5-7), p’s = 0.55 and 0.46, respectively.
During the 3-week study manipulation phase, no participant discontinued treatment. One Standard Run-In participant discontinued pharmacotherapy during the final pre-TQD week. During the interval when all participants were receiving standard varenicline therapy, 5 participants in each arm discontinued use of medication, including one participant in the standard therapy arm who reported feelings of hostility and irritability 3 weeks after being started on varenicline. Medication was stopped immediately and all symptoms resolved within several days. No serious adverse events were observed.
Discussion
The present study evaluated the hypothesis that extending the pre-quit run-in period for varenicline from 1 to 4 weeks would alter smoking behavior and subjective effects in a manner consistent with the theorized reduction-of-reinforcement mechanism (12, 19). Consistent with our primary hypothesis, the Extended Run-In group exhibited greater pre-quit reductions in smoking rate, as well as greater decreases in the taste and buzz from the first cigarette of the day, compared to the Standard Run-In group. Although the pattern of pre-quit expired-air CO was not as clear, CO-verified continuous abstinence (an exploratory outcome) during the final four weeks of the three-month post-quit period were encouraging. The odds ratio for quitting with extended pre-cessation varenicline was 1.8, relative to the standard run-in of one week. The preliminary outcome data are particularly notable when one considers that the “control” condition in the present study is at least as effective as any other front-line cessation strategy (2) and produced a 3-month abstinence rate of 40% in the present study.
The results of another recently published clinical trial with a nearly identical research design (20) also found that pre-quit reductions of CPD, CO, and cigarette enjoyment, and 3-month abstinence rates were enhanced with four weeks of pre-quit varenicline compared to one week. Important limitations of that study, including missing pre-quit data from 15-30% of participants on each key pre-quit measure and the absence of bioverification of 3-month abstinence, are addressed in the present study. For example, the daily PDA-based assessment method employed in the present study minimized retrospective biases (21) and resulted in complete time series data for every participant, ruling out possible selection biases or differences due to attrition. More generally, the replication of key results across both studies bolsters confidence in suggesting that increasing pre-quit duration of varenicline treatment is a strong candidate for further study in larger trials with longer follow-up periods.
Together with data from other research examining pre-quit strategies to enhance tobacco cessation (14, 20, 22, 23), the present findings can be integrated within a broader reinforcement and extinction framework (24). For extinction to occur, people must continue smoking in order to learn that the reinforcing effects are attenuated. Extinction is maximized when numerous “trials” are conducted over a long period of time and across a range of contexts (25-29). Though there are promising data with as little as 2 weeks of pre-quit NRT therapy, the pre-quit CPD data (Figure 2) suggests that the effect or pre-quit treatment grows over the three-week drug manipulation phase (see also (20)). Indeed, the results of one study of smokers who were not trying to quit suggest that the decline in smoking seen with varenicline may continue gradually over a period of weeks or even months (30, 31). Future work might consider whether pre-quit therapy might optimally be combined with a flexible quit date(31) in order to begin a quit date only once a critical reduction in smoking behavior - some studies suggest 50%(10, 20) – has occurred.
The above discussion implicitly assumes that varenicline is reducing the positively reinforcing aspects of smoking – eliminating the positive consequences that follow smoking. However, the marked increase in nausea prompted by extended pre-quit varenicline raises the possibility that the changes in subjective effects and smoking behavior develop because smoking at one’s normal rate during varenicline treatment is aversive. Nausea is the most common side effect with varenicline and is also a relatively common reason for discontinuation of varenicline treatment (32). However, in both the present study and in Hajek et al.(20), extended pre-quit varenicline increased nausea prior to cessation without leading to discontinuation. Perhaps nausea that develops with standard varenicline therapy, typically around and shortly after cessation, is more readily attributed to varenicline, increasing the likelihood of stopping medication; conversely, nausea that occurs in the pre-quit period with an extended run-in may be most proximally associated with smoking, leading to reductions in smoking rate. Tests of competing positive reinforcement (reductions in smoking due to reduced reward from smoking) versus negative reinforcement (e.g., reductions in smoking in an attempt to limit nausea) mechanisms will require adequate sampling of both processes over time in a large sample of smokers. Such data could provide valuable data regarding causal processes that may aid in setting a quit date or suggest a target in the development of new therapies.
No treatment helps everyone, and it is important to consider potential moderators of treatment (33). In the present work, the effects of extended-pre-quit were consistently, albeit unexpectedly, moderated by gender across behavioral, biochemical, and subjective measures. Among women, extended pre-quit varenicline prompted greater reductions in self-reported smoking rate and biochemical evidence of smoking exposure (expired-air CO) prior to the TQD and greater nausea pre-TQD, and it doubled rates of bio-verified abstinence at 3-month follow-up. Among males, none of these effects were statistically significant. However, consistent with data from larger clinical trials of varenicline which report equivalent abstinence rates for men and women (7, 8), we observed no gender differences among those receiving standard treatment, and abstinence rates were solidly within the range typically observed with standard varenicline treatment.
These data raise the possibility that gender specifically moderates pre-quit processes that are the focus of extended run-in period. The results of the largest existing trial of extended pre-quit treatment are broadly consistent with this hypothesis. Specifically, the beneficial effects of a pre-quit regimen of transdermal nicotine and denicotinized cigarettes on short-term abstinence were driven primarily by women.(34)
It is important to consider whether the ‘sex’ differences observed here are really reflective of a more proximal variable, such as differences in drug concentration. Indeed, steady-state varenicline levels are predictive of cessation outcome (35). Although the lack of robust sex differences in weight or medication adherence fail to support the hypothesis that varenicline concentrations were markedly different in the present study, future work should include direct measures over time (see also 36).
Still, we are not suggesting that extending pre-quit treatment is ineffective among men. The results of one recent study raise the possibility that, on average, the effects of pre-quit varenicline may emerge more slowly among men (37). More generally, rather than propose that sex is the critical factor to examine, we suggest that future large-scale studies of extended pre-quit treatment explicitly consider individual differences in a range of parameters, including baseline smoking rate(38), nicotine metabolism(33), and varenicline concentration(35).
In summary, the present data demonstrate that extended use of varenicline during the weeks leading up to a quit attempt reduces smoking behavior and subjective effects of smoking during the pre-quit period. The data are consistent with an extinction model of the mechanism of varenicline. The outcome data, though exploratory, suggest that extending the duration of pre-quit varenicline improves short-term abstinence rates above the already notable rates obtained with standard varenicline dosing, at least for a subset of smokers. The combination of a strong theoretical foundation, straightforward treatment modification, and encouraging data on both process and outcome suggest that Phase III trials of extended run-in varenicline therapy for smoking cessation are warranted.
Methods
Study Design
This study used a 2-group balanced randomized, double-blind, placebo-controlled parallel-group design. Groups are identified on the basis of the run-in period, i.e., the duration of varenicline treatment that occurs prior to the target quit date (TQD). The standard run-in group received three weeks of placebo, followed by standard dosing: 12 weeks of varenicline, including 1 week pre-TQD and 11-weeks post-TQD. The extended run-in group received 4 weeks of varenicline pre-TQD, then continued with standard (11 weeks) post-quit treatment. Both groups received brief cognitive-behavioral counseling. All visits took place at Roswell Park Cancer Institute (RPCI). This study was conducted in accordance with the ethical principles of the Declaration of Helsinki (39) and all procedures were approved by the Institutional Review Board of the Roswell Park Cancer Institute. The trial was registered with www.clinicaltrials.gov [NCT00835900].
Participants
Adult smokers were recruited via ads in newspapers, television promotion, and through web posting and email. Inclusion criteria included: age 18-65 years, smoking at least 10 CPD for the past year, and willingness to refrain from additional treatments for smoking cessation during the study period. Exclusion criteria included: serious medical condition(s) (e.g., diabetes, renal impairment, uncontrolled hypertension); depression requiring treatment in the past year; history of panic disorder, psychosis, or bipolar disorder; a history of alcohol or drug abuse in the past year; use of tobacco products other than cigarettes; current use of other cessation pharmacotherapies; and pregnant/planned pregnancy. Participant disposition is summarized in Figure 1 while demographics and smoking characteristics are presented in Table 1.
Study Procedures
Randomization
A study statistician provided the research pharmacist with a randomization scheme designating small-block (2:2) randomization within sex. Remaining study personnel and participants were blinded to group membership, but participants were asked to guess their treatment condition at the end of the three-week drug manipulation phase, a week prior to the TQD.
Interventions
Pfizer provided all varenicline and identical appearing placebo for the trial. Participants were dispensed an initial 1-week supply of study medication (either varenicline or placebo administered orally) at the randomization visit (end of Week 1) and instructed on use (0.5 mg daily × 3 days, 0.5 mg twice daily × 4 days, then 1.0 mg twice daily, beginning on day 8). One week prior to TQD, participants assigned to the placebo arm were switched over to varenicline in a blinded fashion with standard dose titration during the initial week of use by using 0.5 mg tablets during the transition phase. During both titration weeks, multiple pill bottles were provided, along with explicit instructions for their use. At each clinic visit, participants returned any unused pills and were dispensed only enough medication to last until the next visit plus two additional doses.
Brief (~15 min) behavioral counseling was provided at each contact. Until the end of the drug manipulation phase, participants were instructed to smoke as usual to allow their bodies to get used to the medication, per Rose et al.(13) At one week prior to TQD, participants were also encouraged to sign up for the Pfizer Get-Quit program (http://www.chantix.com/support-plan.aspx).
Clinic visits
Each clinic visit included a review of adverse events, pill counts, assessment of vital signs, and expired breath CO measurement. Participants completed weekly self-report measures (e.g., craving, withdrawal, side effects checklist), returned any unused medication, and received new supplies of study medication and brief behavioral counseling. Through Week 7, personal digital assistant (PDA) data (e.g., CPD and cravings/withdrawal) were down-loaded at each visit (reactivity to PDA-presented smoking and neutral cues during Weeks 1-5 are considered in a separate manuscript). Participants were compensated up to g$434 for attending visits and completion of study measures.
Daily Assessments
Daily assessments of smoking patterns and smoking satisfaction began 1-week prior to the randomization visit and continued throughout the pre-quit period and the first two weeks of the post-TQD period. For these assessments, participants were trained individually to use Palm Tungsten E2 PDAs and completed these ecological momentary assessments (EMA) before and after the first cigarette of the day, logged cigarettes smoked, and responded to four alarms per day that assessed craving, withdrawal and reactivity to smoking and neutral cues.
The present manuscript focuses on the morning assessment. Prior to smoking the first cigarette of the day, participants reported previous day CPD, craving, mood, and tobacco withdrawal. Craving, assessed with 4-item Craving Questionnaire(40), was rated on a 5-point scale from 1 (strongly disagree) to 5 (strongly agree). Mood items (not reported here) consisted of a single positive mood item and a single negative mood item rated on a 5-point scale from 1 (strongly disagree) to 5 (strongly agree). Withdrawal symptoms were assessed using 8 items from the Minnesota Nicotine Withdrawal Scale (MNWS)(41) rated on a 5-point scale from 1 (none) to 5 (severe). Within 15 minutes after smoking the first cigarette of the day, participants completed a modified version of the Cigarette Effects Scale (CEQ)(42, 43). Ten items were rated on a 5-point scale from 1 (not at all) to 5 (extremely).
In studies like the present, it is critical to accurately capture change over days and weeks. EMA assessments offer important advantages over retrospective recall at each visit or traditional paper diaries (44). To enhance compliance, participants were remunerated to encourage high rates of EMA completion (for a maximum weekly payment of $66); on average participants completed the morning assessment on 33 of the 35 mornings during the pre-quit period.
Adverse events
Adverse events were monitored throughout the trial with a structured checklist and open-ended queries at each visit. An independent Data Safety and Monitoring Board provided review and oversight, but did not issue any actions or directives. .
Primary outcomes
The primary outcome measure was the number of cigarettes smoked per day during the pre-quit period, as recorded daily via EMA (see description above). Participant error with the PDA led to invalid data in 2 cases (5 weeks for one participant; 1 week for another) that were replaced with self-report data based on Time Line Follow Back (TLFB) interviews conducted at each visit. Problems with remaining EMA data were rare, with only 1.1% of EMA data excluded due to isolated inconsistencies with: a) the rest of the EMA time series, b) EMA cigarette logs, and/or c) TLFB. Expired-breath carbon monoxide (CO) was examined during the pre-quit phase to biologically verify changes in self-reported CPD. Based on previous work suggesting that varenicline may reduce satisfaction from smoking(45), pre-quit changes in the CEQ satisfaction scale was also a co-primary outcome.
Secondary outcomes. Because the trial was not powered (see below) to detect effects
on dichotomous cessation outcomes, analyses of cessation outcomes are considered to be exploratory. To parallel most clinical trials of varenicline(3), we focused on CO-verified (<11 parts per million) continuous abstinence (not even a puff) during the final four weeks of treatment (i.e., post-quit weeks 8 through 11). Participants lost to follow-up were coded as smokers.
Sample size
Prior published work with NRT (14) and preliminary work with bupropion (Hawk et al., unpublished data) suggested the effect of extended pretreatment on our primary outcome, reduction in pre-quit smoking rate was ~d=.7 (50% v. 25%, with SD=35%). A sample size of 30 participants per group was chosen to provide power of .8, with two-tailed alpha=.05, to detect such an effect.
Statistical Analysis
Pre-quit changes in cigarettes per day and cigarette satisfaction were analyzed in 2 Run-In Group × 2 Sex × 4 Time (Weeks 2, 3, 4, and 5) ANOVAs, using the Huynh-Feldt (H-F) correction for violations of sphericity(46). Parallel analyses examined secondary pre-quit measures (craving, withdrawal, and remaining subjective effects of smoking scales [psychological reward, craving reduction, aversion, and respiratory sensations]). The baseline (Week 1) for each variable was included as a covariate. A parallel analysis of pre-quit changes in CO targeted CO obtained at the end of Weeks 2 and 4, with baseline CO (end of Week 1) as a covariate. To further characterize pre-quit changes in smoking patterns, we analyzed percent reduction in pre-quit CPD [(week 1 – week 5)/week 1 × 100] and CO level [(end of week 1 – end of week 4)/end of week 1 × 100] in parallel ANOVAs.
During the post-quit period, an intent-to-treat approach was utilized with participants lost to follow-up or missing CO-verification coded as smoking (see Figure 1). Abstinence measures were analyzed with logistic regression models that examined the separate and combined effects of run-in group and sex. Odds ratios and 95% confidence intervals (CIs) are reported, and all significance tests were 2-tailed.
Group differences in study blinding at the end of the 3-week drug manipulation phase, as well as percent mediation adherence and the frequency of increases in adverse events, relative to baseline (end of Week 1), were analyzed by χ2. Because both run-in groups received varenicline but started medication at different times, side-effect data and percent adherence were examined separately for the three-week drug manipulation phase (maximum report at Weeks 2, 4), when only the Extended group was taking varenicline, and the subsequent three-week period (max at Weeks 5, 7), which were the first three weeks of varenicline for the Standard run-in group.
Acknowledgments
Author Contributions: Dr. Hawk had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drs. Hawk, Mahoney, Tiffany, and Cummings.
Acquisition of data: Drs. Hawk, Mahoney, Tiffany, and Ashare. Ms. Rhodes, Ms. Lohnes, Ms. Gass and Mr. Schlienz also contributed to the acquisition of data.
Analysis and interpretation of data: Drs. Hawk, Mahoney, and Ashare.
Drafting of the manuscript: Drs. Hawk, Mahoney, and Ashare.
Critical revision of the manuscript for important intellectual content: Drs. Hawk, Mahoney, Tiffany, Ashare and Cummings; Ms. Rhodes, Ms. Lohnes, Ms. Gass and Mr. Schlienz. Statistical analysis: Drs. Hawk and Ashare.
Obtained funding: Drs. Mahoney and Hawk.
Funding/Support: This research was funded, in part, by a 2008 Global Research Award for Nicotine Dependence (GRAND) awarded to MCM and by NIDA R21 DA019653 to STT. GRAND is an independent investigator initiated research program sponsored by Pfizer, Inc. Preparation of the report was supported in part by NIH U01 DA020830 to LWH.
Role of the Sponsor: Pfizer Inc. had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Acknowledgment: The authors appreciate the oversight of randomization by Andrew Hyland, Ph.D.
Footnotes
Disclosures
Financial Disclosures: Dr. Mahoney has served on the speaker’s bureau for Pfizer and as the medical director for the NYS Smokers Quit Line. Dr. Cummings reports receiving consulting fees from Pfizer for work on smoking cessation; consulting fees as an expert witness for plaintiff’s attorneys suing tobacco companies; and receiving research contracts from Nabi Biopharmaceutical. Dr. Hawk, Dr. Ashare, Ms. Lohnes, Mr. Schlienz, Ms. Rhodes, Dr. Tiffany, and Ms. Gass have no financial disclosures to report.
References
- (1).Center for Disease Control . MMWR Morb Mort Wkly Rep. Vol. 57. 2008. Cigarette smoking among adults - United States, 2007; pp. 1221–6. [PubMed] [Google Scholar]
- (2).AFiore MC, Jaen CR, Baker TB, et al. Clinical Practice Guideline. U.S. Department of Health and Human Service. Public Health Service; Rockville, MD: 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- (3).Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2011;2:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- (4).Lerman C, et al. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6:746–62. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- (5).Baker TB, et al. New methods for tobacco dependence treatment research. nn Behav Med. 2011:1–16. doi: 10.1007/s12160-010-9252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–5. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- (7).Gonzales D, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- (8).Jorenby DE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- (9).Rose JE, Levin ED. Inter-relationships between conditioned and primary reinforcement in the maintenance of cigarette smoking. British Journal of Addiciton. 1991;86:605–9. doi: 10.1111/j.1360-0443.1991.tb01816.x. [DOI] [PubMed] [Google Scholar]
- (10).Cummings KM, Mahoney MC. Strategies for smoking cessation: what is new and what works? Expert Rev Respir Med. 2008;2:201–13. doi: 10.1586/17476348.2.2.201. [DOI] [PubMed] [Google Scholar]
- (11).O’Connor EC, Parker D, Rollema H, Mead AN. The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology (Berl) 2010;208:365–76. doi: 10.1007/s00213-009-1739-5. [DOI] [PubMed] [Google Scholar]
- (12).Rollema H, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–94. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- (13).Rose JE, Behm FM, Westman EC. Nicotine-mecamylamine treatment for smoking cessation: The role of pre-cessation therapy. Experimental & Clinical Psychopharmacology. 1998;6:331–43. doi: 10.1037//1064-1297.6.3.331. [DOI] [PubMed] [Google Scholar]
- (14).Shiffman S, Ferguson SG. Nicotine patch therapy prior to quitting smoking: a meta-analysis. Addiction. 2008;103:557–63. doi: 10.1111/j.1360-0443.2008.02138.x. [DOI] [PubMed] [Google Scholar]
- (15).Benowitz NL, Hatsukami D. Gender differences in the pharmacology of nicotine addiction. Addiction Biology. 1998;3:383–404. doi: 10.1080/13556219871930. [DOI] [PubMed] [Google Scholar]
- (16).Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine & Tobacco Research. 1999;1:301–15. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- (17).Cepeda-Benito A, Reynoso JT, Erath S. Meta-Analysis of the Efficacy of Nicotine Replacement Therapy for Smoking Cessation: Differences Between Men and Women. J Consult Clin Psychol. 2004;72:712–22. doi: 10.1037/0022-006X.72.4.712. [DOI] [PubMed] [Google Scholar]
- (18).Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10:1245–50. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- (19).Coe JW, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–7. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- (20).Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji AR. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med. 2011;171:770–7. doi: 10.1001/archinternmed.2011.138. [DOI] [PubMed] [Google Scholar]
- (21).Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- (22).Rose JE, Behm FM, Westman EC, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res. 2006;8:89–101. doi: 10.1080/14622200500431866. [DOI] [PubMed] [Google Scholar]
- (23).Rose JE. Nicotine preloading: the importance of a pre-cessation reduction in smoking behavior. Psychopharmacology (Berl) 2011:1–2. doi: 10.1007/s00213-011-2350-0. [DOI] [PubMed] [Google Scholar]
- (24).Benowitz NL. Nicotine Addiction. N Engl J Med. 2010;362:2295–303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–60. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- (26).Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–94. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- (27).Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–86. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- (28).Conklin CA. Environments as cues to smoke: implications for human extinction-based research and treatment. Exp Clin Psychopharmacol. 2006;14:12–9. doi: 10.1037/1064-1297.14.1.12. [DOI] [PubMed] [Google Scholar]
- (29).Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–67. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- (30).Poling J, Rounsaville B, Gonsai K, Severino K, Sofuoglu M. The safety and efficacy of varenicline in cocaine using smokers maintained on methadone: a pilot study. The American Journal on Addictions. 2010;19:401–8. doi: 10.1111/j.1521-0391.2010.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hughes JR, Russ CI, Arteaga CE, Rennard SI. Efficacy of a flexible quit date versus an a priori quit date approach to smoking cessation: A cross-study analysis. Addict Behav. 2011 doi: 10.1016/j.addbeh.2011.08.001. [DOI] [PubMed] [Google Scholar]
- (32).Swan GE, et al. Varenicline for smoking cessation: nausea severity and variation in nicotinic receptor genes. Pharmacogenomics J. 2011 doi: 10.1038/tpj.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Patterson F, et al. Toward Personalized Therapy for Smoking Cessation: A Randomized Placebo-controlled Trial of Bupropion. Clin Pharmacol Ther. 2008 doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- (34).Becker KM, Rose JE, Albino AP. A randomized trial of nicotine replacement therapy in combination with reduced-nicotine cigarettes for smoking cessation. Nicotine Tob Res. 2008;10:1139–48. doi: 10.1080/14622200802123294. [DOI] [PubMed] [Google Scholar]
- (35).Ravva P, Gastonguay MR, French JL, Tensfeldt TG, Faessel HM. Quantitative assessment of exposure-response relationships for the efficacy and tolerability of varenicline for smoking cessation. Clin Pharmacol Ther. 2010;87:336–44. doi: 10.1038/clpt.2009.282. [DOI] [PubMed] [Google Scholar]
- (36).Faessel HM, Obach RS, Rollema H, Ravva P, Williams KE, Burstein AH. A review of the clinical pharmacokinetics and pharmacodynamics of varenicline for smoking cessation. Clin Pharmacokinet. 2010;49:799–816. doi: 10.2165/11537850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- (37).Poling J, Rounsaville B, Gonsai K, Severino K, Sofuoglu M. The safety and efficacy of varenicline in cocaine using smokers maintained on methadone: a pilot study. Am J Addict. 2010;19:401–8. doi: 10.1111/j.1521-0391.2010.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Hughes JR, Rennard SI, Fingar JR, Talbot SK, Callas PW, Fagerstrom KO. Efficacy of Varenicline to Prompt Quit Attempts in Smokers Not Currently Trying to Quit: A Randomized Placebo-Controlled Trial. Nicotine Tob Res. 2011 doi: 10.1093/ntr/ntr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).World Medical Association web site [Accessed March 14 2011];Declaration of Helsinki ethical principles for medical research involving human subjects. < http://www.wma.net/e/policy/b3.htm.<.
- (40).Carter BL, Tiffany ST. The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Experimental & Clinical Psychopharmacology. 2001;9:183–90. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- (41).Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- (42).Westman E, Levin E, Rose J. Smoking while wearing the nicotine patch: Is smoking satisfying or harmful. Clin Res. 1992;40:871A. [Google Scholar]
- (43).Rose JE, Behm FM. Extinguishing the rewarding value of smoke cues: Pharmacological and behavioral treatments. Nicotine & Tobacco Research. 2004;6:523–32. doi: 10.1080/14622200410001696501. [DOI] [PubMed] [Google Scholar]
- (44).Stone AA, Shiffman S, Atienza A. The science of real-time data capture: Self-reports in health research. Oxford University Press; USA: 2007. [Google Scholar]
- (45).Lee JH, Jones PG, Bybee K, O’Keefe JH. A longer course of varenicline therapy improves smoking cessation rates. Preventive cardiology. 2008;11:210–4. doi: 10.1111/j.1751-7141.2008.00003.x. [DOI] [PubMed] [Google Scholar]
- (46).Huynh H, Feldt LS. Estimation of the Box correction for degrees of freedom from sample data in randomized block and split-plot designs. Journal of Educational and Behavioral Statistics. 1976;1:69. [Google Scholar]