Abstract
Lasers are a relatively recent addition to laryngeal surgery. Since their invention, laser use and applications have expanded rapidly. In this paper, we discuss the benefits and disadvantages of lasers for different procedures, as well as ways to overcome commonly faced clinical problems. The use of lasers in surgery has offered a time- and cost-efficient alternative to cold surgical techniques, and has been employed in the treatment of numerous laryngeal pathologies, including stenoses, recurrent respiratory papillomatosis, leukoplakia, nodules, malignant laryngeal disease, and polypoid degeneration (Reinke’s edema). However, lasers can incur adjacent tissue damage and vocal fold scarring. These problems can be minimized through understanding the mechanisms by which lasers function and correctly manipulating the parameters under a surgeon’s control. By varying fluence, power density, and pulsation, tissue damage can be decreased and lasers can be used with greater confidence. The various types of lasers and their applications to the treatment of specific pathologies are reviewed with the intention of helping surgeons select the best tool for a given procedure. Recent applications of lasers to treat benign laryngeal lesions and severe laryngomalacia demonstrate that additional research must be conducted in order to realize the full potential of this surgical tool.
Keywords: Laser, laryngeal surgery, phonosurgery
History
Shortly following the invention of the ruby laser in 1960 by Theodore Maiman (1), the laser was incorporated into the medical community, with an emphasis placed on its potential capability to remove cancerous lesions (2). Otolaryngologists were among the first to make use of the tool (3). Early lasers caused significant tissue damage, and achieving adequate wavelength for tissue absorption was a struggle for many years (2). When Patel invented the CO2 laser, it quickly gained popularity among the medical community. Because water easily absorbs the 10,600 nm wavelength of the CO2, biological tissue responds well to it (2,4). In addition, the laser had promise in surgery due to its continuous wave, high output power, and means of focusing its beam on a small area (4). In 1967, Jako and Polyani used the first CO2 laser on cadaveric larynges (5). Following Bredemeier’s development of an endoscopic delivery system in 1968 (6), Jako used the CO2 laser in a canine model, the first in vivo laryngeal laser experiment (5,7). It was Bredemeier’s invention of the micromanipulator to deliver CO2 energy through a microscope that signaled the beginning of the current laryngeal laser surgery era (8). In the 1980s, different lasers were produced and found to have characteristic benefits and drawbacks, creating specific uses for each (2).
The Physics of Lasers
In order to apply the appropriate laser effectively to each clinical situation, it is important to understand the physics behind laser function. Lasers are useful in surgical procedures due to their high intensity. This power is generated through light amplification, which is achieved by pumping a large group of atoms in the optical cavity of the laser (9). The optical cavity is comprised of two mirrors and the space between them. As the atoms return to the ground state, the energy released moves along the optical cavity and stimulates atoms that are in the excited state. This results in an increase in, or amplification of, photons. As photons reflect off the mirrors, the energy of the light increases and a portion can be emitted along the axis of the optical cavity (10).
Three properties of the light produced by lasers are essential to their function: monochromaticity, coherence, and directionality. Monochromaticity means that the light has only one wavelength. Coherence means that the light waves are in phase and traveling in one direction. Directionality means that the laser beam is very concentrated, as opposed to other forms of light which are diffuse and consequently less intense. Together, these properties influence the controllable parameters of a surgical laser.
Power, given in watts (W), measures the rate at which energy is transmitted by the laser beam. The beam is focused by altering the spot size, the radius of the aperture that transmits 86% of the beam energy (11). Beam energy can be found by calculating dosage, measured in joules (J), by time, measured in seconds (s). Related to power is power density, measured in units of watts per square centimeter (W/cm2). Power density, also called irradiance or spot brightness, determines the rate at which tissue is removed at the surgical site (10). Fluence is a key parameter and one that must be understood if minimal damage is to be incurred on tissues adjacent to the incision site. It combines previously mentioned parameters of power density and dosage and is measured in units of joules per square centimeter (J/cm2). Fluence increases with wattage, such that fifty watts delivered over one second would have a greater fluence than one watt delivered over fifty seconds. One may think that a lower power would reduce damage to adjacent tissue; however, using a higher power for a shorter time allows for greater precision and decreased damage to adjacent tissue.
The types of lasers are characterized by two parameters that cannot be manipulated by the user: laser medium and target chromophore. The laser medium, which can be solid, liquid, or gas, specifies the laser wavelength. Solid and liquid mediums are optically energized using a flashing lamp or another laser, while gas lasers are electrically energized using a current (10). Target chromophore describes the substance that absorbs the laser beam. This is often hemoglobin or water. A summary of laser wavelengths and target chromophores can be seen in table 1.
The aforementioned parameters specific to each laser as well as the parameters under the control of the surgeon affect the laser-tissue interaction, which is characterized by the conversion of laser energy into heat. This heat can then cut, vaporize, or coagulate the affected tissue. The efficiency by which the energy is transferred to the tissue is dependent upon the wavelength of the laser. When laser energy is delivered to tissue, it can be reflected, transmitted, scattered, or absorbed (10). If the light is reflected by or transmitted through the tissue, no heating occurs. Scattering increases the amount of tissue over which the laser energy is distributed, thereby decreasing the intensity of the interaction.
When performing laser surgery, it is necessary to consider the amount of thermal damage to the target and surrounding tissues. Tissue damage is dependent upon the tissue absorption coefficient, the wavelength of the laser, power density, and the length of time over which the energy is delivered (9). An additional property which can affect the severity of tissue damage is thermal relaxation time, the time required for tissue to lose 50% of its heat through diffusion (12). One can decrease tissue damage by allowing heated tissue to cool during a procedure, which can be accomplished through the use of a pulsed laser.
Cold Surgery vs. Laser Surgery
Although good surgical outcomes can be obtained from cold surgery, laser surgery, or a combination of the two, lasers possess some physiological and technical advantages. Besides being convenient and exact (13,14), lasers offer surgeons an opportunity for unobstructed vision of the operation field (14) with minimal tissue manipulation and a longer working distance (15). Decreased risk of postoperative bleeding, increased sterility (14,16), minimal surrounding tissue damage (14), and better intraoperative hemostasis (16) are among the potential benefits of laser surgery. Although fewer complications, side-effects, and better postoperative voice quality (17) have been reported, this is largely technique-dependent. Lasers can be more cost-effective than cold surgeries when managing laryngeal tumors, as they afford briefer hospital stays and shorter wound recovery periods (17), particularly for patients with laryngeal cancer.
However, laser surgery requires a greater number of personnel in order to ensure effectiveness and safety, thereby increasing procedural costs. Installation of laser technology in hospitals and offices is also more expensive than cold surgery equipment (18). As is the case with most medical equipment, the value of laser equipment depreciates as technological advances are made. The constant need to purchase new developments makes maintaining safe and valuable laser surgery systems even more costly. Even when hospitals update equipment regularly, lasers cannot be used on patients until the surgeon is familiar with them (2). This adds to the cost and inconvenience of laser surgery. Implementing microelectrodes during cold surgery produces similar tissue healing, oncological resection, post-operative voice quality, and complications as laser surgery (19). Although microelectrodes have led to decreased bleeding (19,20), time, and cost compared to previous cold surgery techniques and some laser surgery (19), it has not eliminated the occasional need for preoperative tracheotomy for open laryngeal procedures, whereas tracheotomy is rarely required prior to laser surgery.
With the emergence of the Pulsed Dye Laser (PDL) and Potassium Titanyl Phosphate (KTP) laser, new applications of lasers in laryngeal surgery continue to evolve. These lasers have been shown not only to perform angiolysis but also to affect connective tissue and the extracellular matrix. The ability to eradicate some vascular lesions (figures 1 and 2) while preserving overlying epithelium was first applied to management of respiratory papillomatosis with the PDL (21). More recently, the PDL and KTP laser have been used to treat vocal fold cancers by attacking the blood supply as a theoretical application of the angiogenesis of cancer (22). The PDL is also used empirically to manage some cases of Reinke’s edema that formerly would require cold knife surgical intervention (22).
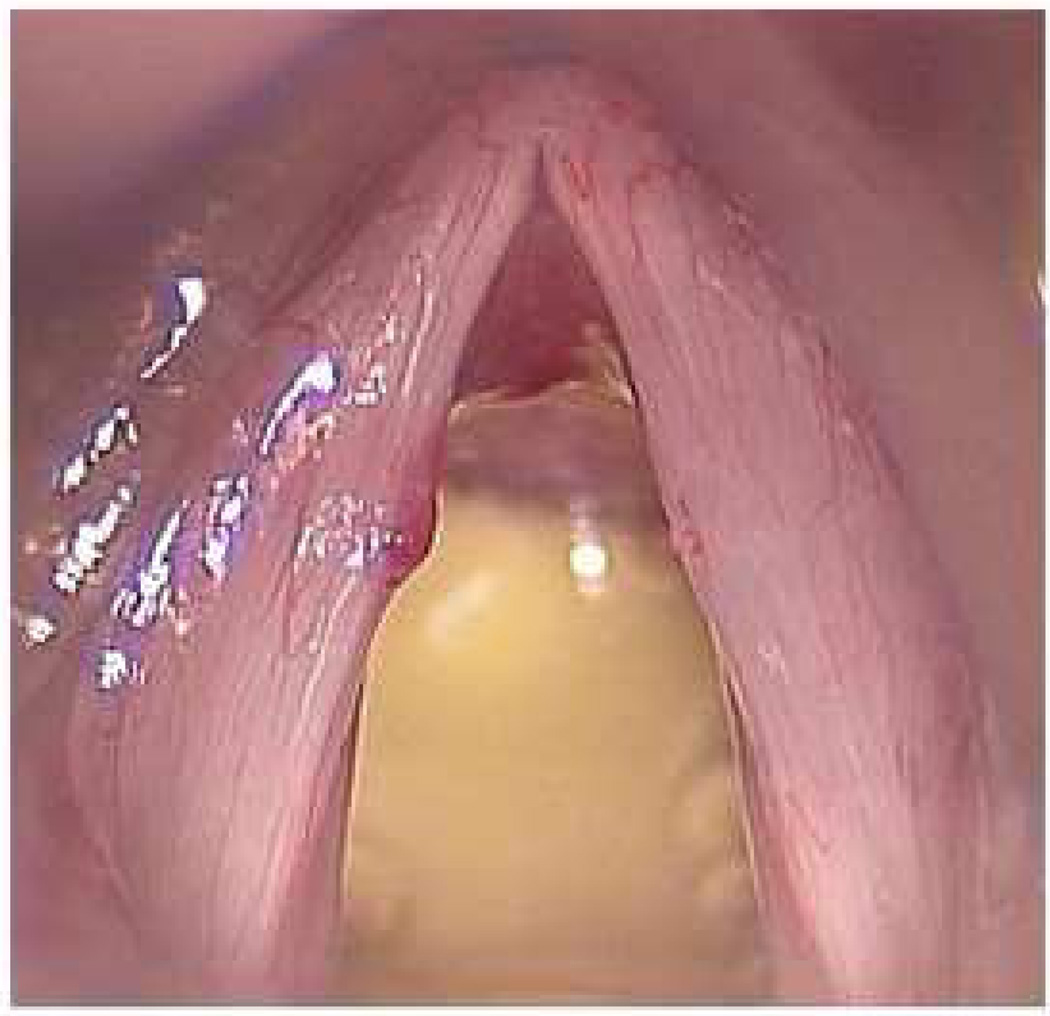
Figure 1.
a) Preoperative hemorrhagic nodule amendable to treatment with photoangiolytic laser. b) Intraoperative image after ablation. Images courtesy of Charles Ford, MD.
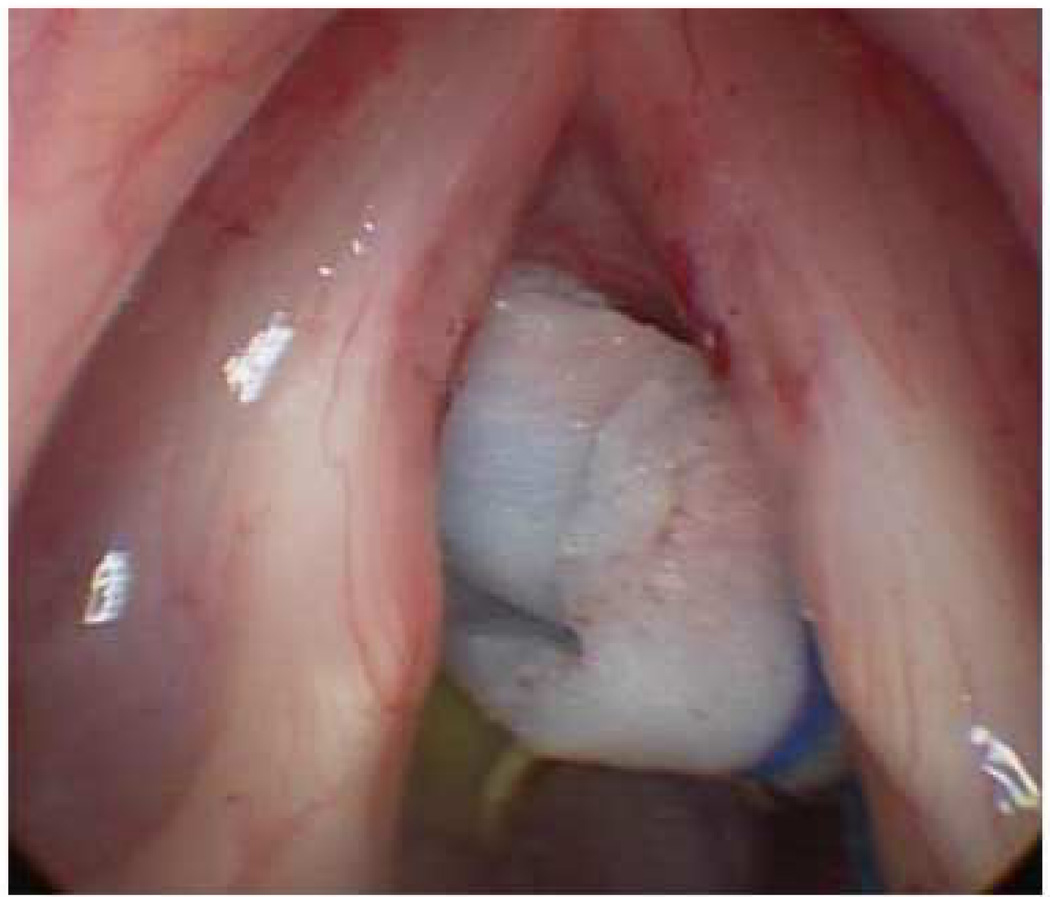
Figure 2.
a) Preoperative microlaryngoscopic image of vocal fold capillary ectasia. b) Intraoperative image after ablation with pulsed dye laser (PDL). Images courtesy of Charles Ford, MD.
Certain surgical techniques possess specific advantages and disadvantages compared to lasers. Microflap surgery, for example, prevents clear visualization of the surgical plane and can result in hydrodissection of the normal basement membrane from the superficial lamina propria (23). However, unlike some laser procedures, microflap surgery does not usually require post-operative intubation (24), decreasing hospital stays and health care costs.
Comparing Types of Lasers
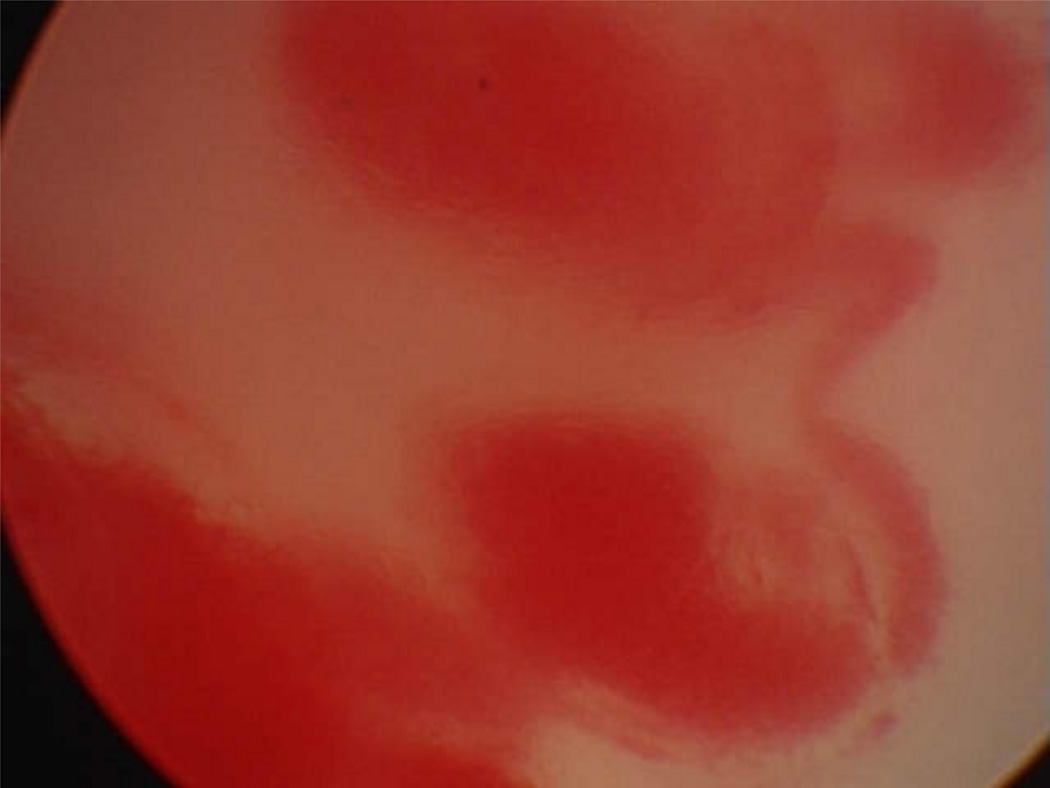
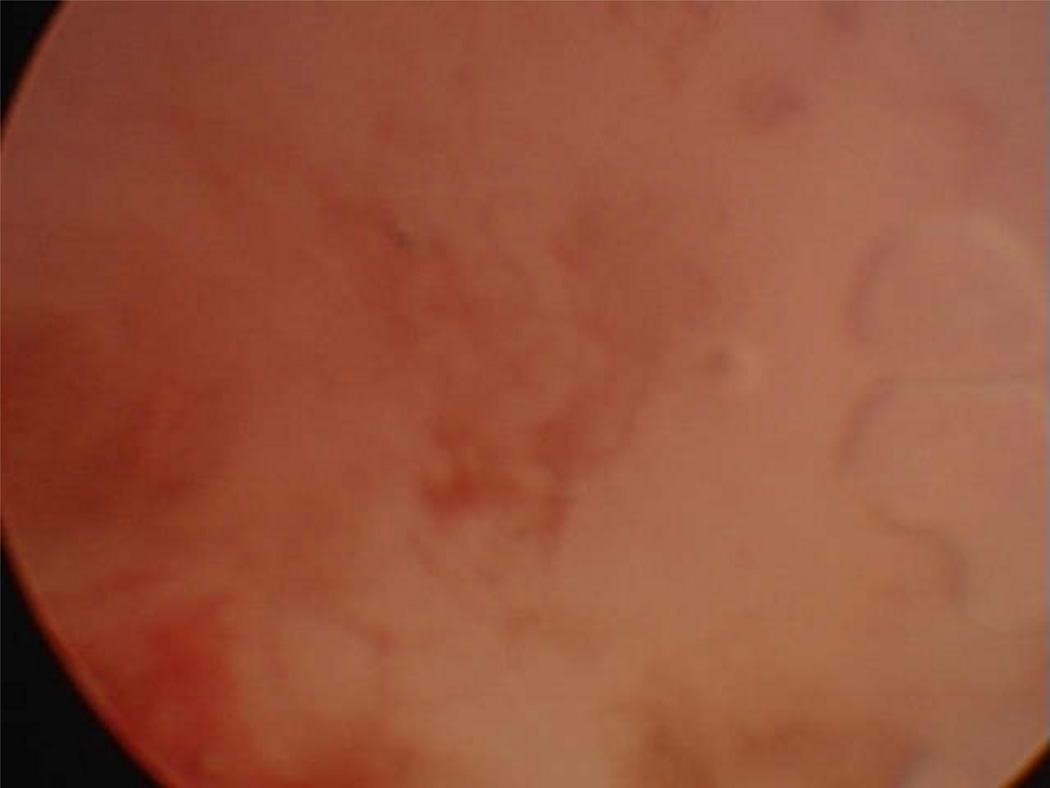
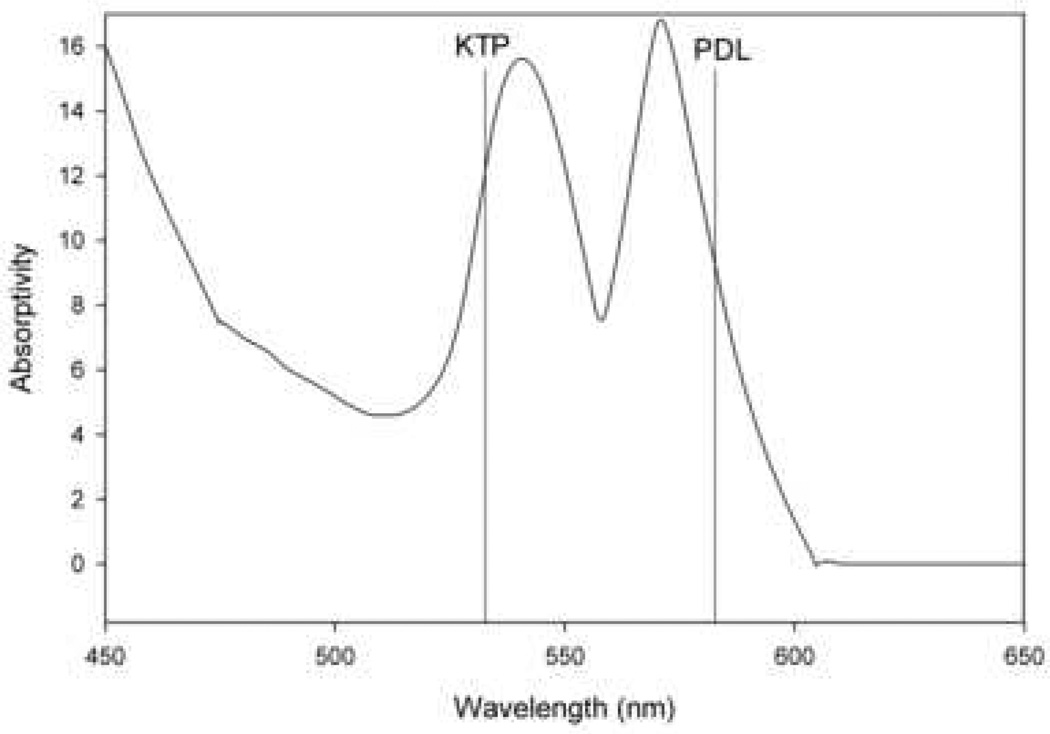
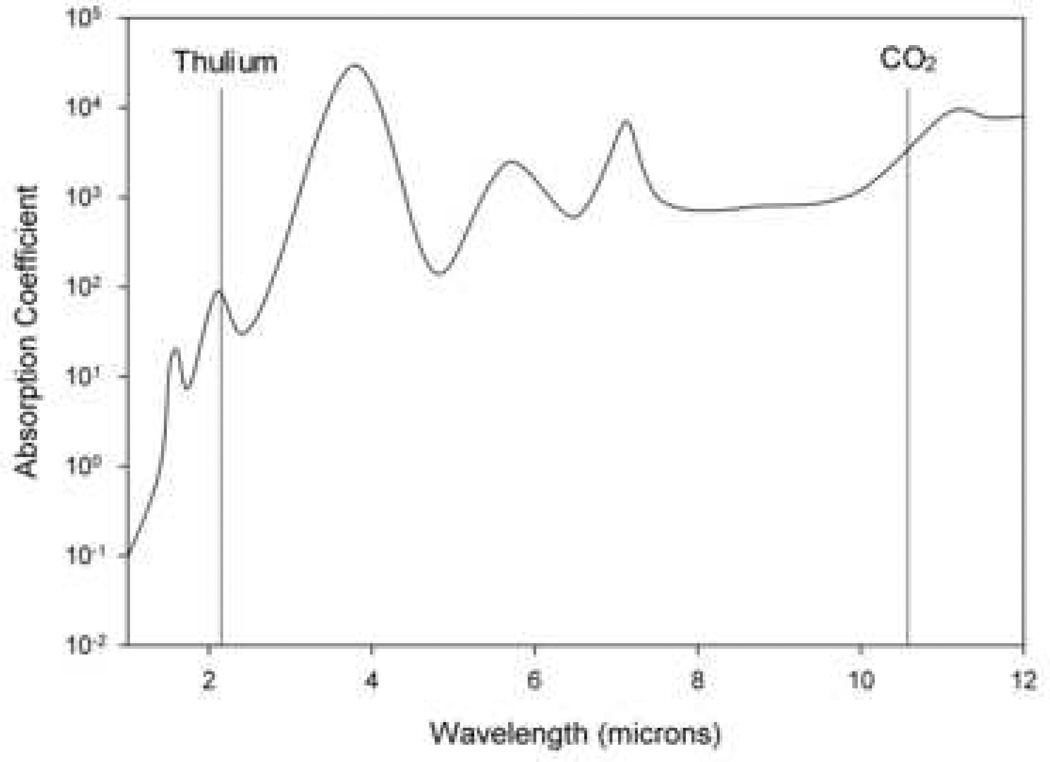
Two broad types categorize all lasers: photoangiolytic and cutting/ablating lasers, which differ in their selectivity. Photoangiolytic lasers, such as KTP and PDL, selectively target hemoglobin (figure 3), while cutting/ablating lasers, such as the CO2 and Thulium (RevoLix Jr. 2micron) are absorbed largely by water (figure 4).
Figure 3.
Absorption curve for hemoglobin, which is targeted by the pulsed dye laser (PDL) and potassium-titanyl-phosphate (KTP) laser. Information used when making this figure was obtained from Zijlstra et al., 1991 (66).
Figure 4.
Absorption curve for water, which is targeted by the CO2 and thulium lasers. Information used when making this figure was obtained from Burns et al., 2008 (56).
Lasers are used to treat pathologies such as stenoses, recurrent respiratory papillomatosis (RRP), leukoplakia, nodules, malignant laryngeal disease, polypoid degeneration (Reinke’s edema), and granulomas for which antireflux treatment is ineffective (15). In the past, only continuous and pulsed CO2 lasers were available (12). For flat lesions (leukoplakia, papillomas, or verrucous carcinoma), the continuous laser was preferred due to its coagulative and hemostatic properties. The pulsed laser avoided heat build-up more effectively, incurring less damage on surrounding tissue, and so was more useful for incisions (12).
Technology has expanded our options in laser surgery, and choosing the appropriate laser for a given procedure should be done on a case-by-case basis. In choosing a laser, one must strike a balance between tissue efficacy and thermal damage. For this reason, many studies have been done to compare lasers and the damage they cause to tissues (25).
Since 1972, CO2 lasers have been the primary lasers used for pathologies such as vocal cord keratosis, nodules, polyps, papillomas, carcinoma in situ, and T1 cancer because they offer minimal surrounding tissue damage (26). Polanyi first tested the CO2 laser in human cadaver larynges, and found that it produced discrete wounds (6). Developments such as an endoscopic delivery system led to Jako’s use of the tool in canine larynges (5). Because CO2 lasers can be delivered to the larynx through a microscope, removing small tumors is accurate and efficient (25). CO2 lasers can be more focused than PDL, KTP, or thulium lasers; however, these other lasers allow for fiber optic transmission, advancing their use to both the office and the operating room (27–29). Fiber optic transmission allows the surgeon to use either contact or non-contact mode when delivering laser energy to the tissue. Because fiber optic transmission is not possible with a traditional CO2 laser, it can only be used in areas that can be aligned directly, and use is restricted to the operating room (18). Thus a key distinction between these types of lasers is that fiber optic transmission lasers can be used for office-based procedures that do not require general anesthesia such as papilloma treatment, whereas procedures using CO2 and other non-fiber optic transmission lasers require general anesthesia. PDL and KTP offer better hemostatic effects than the CO2 (2). The fact that PDL and KTP lasers (and all office-based photoangiolytic lasers) are available for in-office use increases their popularity a great deal, as office-based procedures offer decreased cost and procedure length. Rees et al. (30) calculated that $5000 were saved per case when moving a procedure from the operating room to the office.
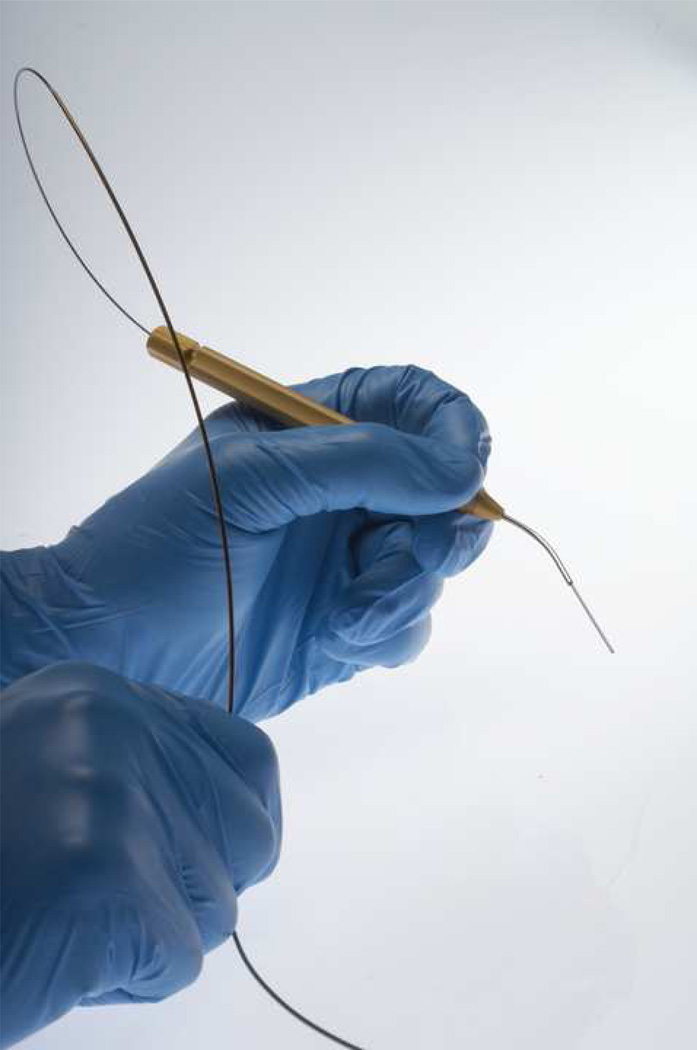
The recent invention of the flexible CO2 laser delivery system (figure 5) has expanded its use to the office as well. This new flexible fiber system overcomes many limits of rigid CO2 lasers, such as the need for general anesthesia and difficulty exposing all areas of the head and neck (31). The flexible system’s greater control over laser angulation potentially results in decreased thermal damage to surrounding tissue (32). The flexible fiber may decrease blood loss and offer better tissue coagulation. The gas flow from the distal tip and through the fiber’s hollow core minimizes the smoke plume and bleeding. Wang et al. claim that benefits of the new system include rapid wound healing and reduced inflammation (31). In addition, Zeitels et al. (32) describe much smoother cut edges with the photonic bandgap fiber than with traditional CO2 lasers. However, as with all newer lasers, the benefits of this new delivery system depend on surgical technique. More use in the clinical and experimental settings is necessary to determine the merits of the CO2, KTP, and PDL laser systems.
Figure 5.
OmniGuide BeamPath™ - L fiber and hand piece. Image courtesy of OmniGuide.
The CO2 laser is a popular tool for treating early glottic cancer as it combines cutting ability with some degree of coagulation depending on the degree of focus. Ledda et al. (33) showed that the CO2 laser can be used effectively to treat early glottic cancer because of the good oncologic results, the reduced surgical trauma, speedy recovery period, and preservation of voice quality. The best form of treatment for early glottic carcinoma is still a topic of debate (34). In cases involving the anterior commissure, Steiner et al. (34) advocate the use of laser microsurgery, as other treatment methods such as radiotherapy are associated with inferior local control rates, longer procedure and healing time, and higher risk of complication. However, endoscopic resection using a CO2 laser is not sufficient for transglottic tumors exhibiting lateral submucosal extension, as the cancer is likely to spread into adjacent visceral spaces. In these cases, open neck surgery and radiotherapy should be considered as alternative treatment options (35).
Despite its drawbacks, the CO2 laser is still the most commonly used laser for vocal pathologies (18,25). Having been available for the longest time, doctors are familiar with its use, capabilities, and limitations. More recently, the thulium laser has been used effectively for cancer treatment. An office-based laser that simulates the properties of the CO2 laser (25), it has the advantage of improved coagulation and more versatile delivery by virtue of its flexible fiber and the option of use in the contact mode.
Recent additions to the CO2 laser have increased its applicability and efficacy. The AcuSpot scanning micromanipulator creates the smallest possible beam diameter available to date (250 µm for a local length of 200 mm), and allows the beam to sweep surfaces quickly (36–38). The AcuBlade scanner software modification changes the “shaving” circular motion, making it possible for the beam to cut the target tissue in a straight or curved incision line in multiple possible lengths and penetration depths. SuperPulse and UltraPulse CO2 laser waves can be used to reduce thermal damage and coagulation of surrounding tissue as they allow tissue to cool between pulses (36,37,39). Tissue damage can be further reduced by combining the waves with AcuBlade software. SuperPulse’s cone shape and its higher peak power with less energy means that it results in more thermal damage than UltraPulse. Though AcuBlade was designed for SuperPulse and continuous modes, it is now used with UltraPulse mode as well. Procedures that use these pulse modes, although accurate, create incisions that exhibit indentation, unlike the regular incisions that are possible with microscissors. The AcuBlade offers a regular incision as well as hemostasis that are not possible with cold surgery (40).
Photoangiolysis of the microvasculature of the superficial lamina propria (SLP) has been found to be a good technique for treating papillomatosis, dysplasia, and microvascular angiomata (29). Besides having fiber optic transmission, the photoangiolytic KTP and PDL are precise and offer minimal surrounding tissue damage. They are useful for incision, coagulation, and ablation with substantial preservation of overlying epithelium (2,22). Anderson et al. developed the concept of selective photothermolysis in the treatment of dermatologic vascular malformations which led to the 585 nm PDL (41). Bower et al. and McMillan et al. performed pilot studies using the PDL (42,43), followed by Zeitels and Anderson (44–46). Although originally used in conjunction with cold microflap epithelial resection, it became evident that this tool would be most useful if used independently in the treatment of papillomatosis and dysplasia. The short pulse width makes it difficult to quantify the energy being delivered and can result in breaching of the microcirculation vessel walls. Extravasated blood in the surrounding tissue can then absorb the laser, decreasing procedure efficacy (18). The pulsed KTP laser is a more recent addition to vocal fold surgery than the PDL and several advantages of it have been reported (29,47–49). Originally used in continuous mode, it was found to be most useful in treating subglottic hemangiomas, vocal fold ectasias, and varices (18). The KTP laser has a wavelength of 532 nm, which hemoglobin absorbs more strongly than the 585 nm wavelength of the PDL (47,48). The wider pulse width of the KTP is its principle advantage, as it is able to spread laser energy over a longer time period, thus offering slower heating and more even coagulation (47–49). The PDL is used effectively to treat RRP (48), keratosis, leukoplakia (47), and other lesions, but its short pulse width can lead to vessel wall rupture before coagulation is complete (47–49). Other stated advantages of the pulsed KTP laser are its adjustable fiber size and durable solid state design, which lessen the likelihood of expensive repairs sometimes required of the PDL (29). However, some recent studies (22,50,51) advocate the use of PDL over KTP. When leukoplakias in the nonphonatory regions of the vocal folds are treated with the PDL, the basement membrane and SLP are undisturbed, which is not the case with the KTP (51). The PDL also creates a cleavage plane between the SLP and basement membrane (22), though the mechanism by which this occurs is unknown. Treatment of Reinke’s edema and recurrent granulomas that are unresponsive to anti-reflux treatment are among the uses of the PDL (50). In treating dysplasia, the PDL was demonstrated to be more effective than the KTP (22). As the PDL is able to penetrate deeper than the KTP, it is also the preferred laser for thick lesions (50). As with all lasers, efficacy is highly dependent upon operative technique and the skills of the surgeon.
Laser Precautions and Safety
Despite the notable benefits, laser surgery is not without disadvantages. Laser heat can increase scarring and cause damage to adjacent tissue (16). With lasers, there is potential for endotracheal explosion, facial burns, mucosal burns, vocal fold webs, stenoses, and glottic incompetence (52). In addition to recognizing when to use certain types and settings of lasers, surgeons should recognize when lasers should not be used. Xu et al. (17) showed that although lasers are preferred for malignant lesions, microsurgery is preferred for the treatment of pre-cancerous lesions. According to Ossoff et al. (2) and Reinisch (16), determining whether or not laser surgery is the best option depends heavily on the surgeon’s experience, skills, and interpretation of the lesion. The surgeon must consider the anatomical location of the lesion and the relevant anatomy of the patient. Certain anatomical features are unfavorable for direct laryngoscopic exposure. For example, prominent fragile teeth, unfavorable Malampati rating, mandible size and obtuse thyro-mental angle compromise exposure and make use of the CO2 laser in the operating room more dangerous and less effective. (16). Sataloff et al. (8) claim that incisions on the superior vocal fold surface prior to removal of Reinke’s edema should be done with cold knife surgery rather than laser surgery, as laser surgery is slower.
To avoid many heat-related problems caused by lasers, the entire operating team should be educated about potential complications and safety precautions (51). With microlaryngeal laser surgery, there are opposing needs for both airway access and ventilation (14). For this reason, good communication, especially between the surgeon and anesthesiologist, and plans with different alternatives available are crucial to ensure safe and effective procedures (14). CO2 lasers have more immediate and intense effects than other lasers, so it is especially important to be informed and careful when using this instrument. The most dramatic laser complication, endotracheal tube fire (8,14,53) can be avoided by paying attention to safety and delivery, as well as using laser-resistant endotracheal tubes (8,14). It has been proven that red rubber tubes present less danger than plastic tubes (50,54). Wrapping the tubes in saline-coated gauze pads has been presented as an option (8,14,52), but maintaining the moisture of these can be difficult, and if allowed to dry, gauze pads present an even greater fire hazard (54). Other options to reduce the risk of fire include limiting the oxygen content in the anesthetic gas to 30%, not using a tube, wrapping the tube in reflective metal (52), using a metal tube, and jet ventilating with a needle or metal tube (8,14,52).
Laser surgery is a complicated procedure, and surgeons should understand the relation between power density and time of laser use (known as the radiant exposure concept), as well as the concept of lateral thermal spread (8), the most common laser-related complication (8,10,14,55). Lateral thermal spread refers to the increased thermal damage to adjacent tissue with increased laser-tissue contact (8). In order to prevent thermal damage, surgeons should choose the shortest possible time pulse and highest possible power that will accomplish the procedure (8,10). Using a higher pulse power for a shorter period of time results in less tissue damage than using a lower power for a longer period of time. To avoid using higher powers than necessary and making incisions that cut tissue too deeply, surgeons should start by pulsing at high powers when vaporizing and gradually work their way up from low powers when incising at fine spots (10). Additionally, spacing out laser impact, even for a continuous incision, decreases thermal damage by allowing time for the tissue to cool between impacts (8,10,56). This is referred to as the “skip technique,” and is beneficial because laser bursts immediately next to each other create the risk of thermal overlap and increased tissue damage (8).
Lasing over areas already charred can cause significant tissue damage and scarring, as it can increase the heat administered from 100 °C on the first impact to 1500 °C when going over the same area. This effect can be avoided by choosing the best spot-size and power setting for each surgical task (10). Wiping lased tissues with wet sponges between lasing can also decrease this risk (10,15). Vessel wall rupture and bleeding can be minimized by defocusing the CO2 laser or by starting at a distance and slowly approaching the target when using the PDL laser (50).
Future
Laser surgery has thus far offered a method of treating early and medial stage laryngeal cancer (57). In the future, it may be beneficial to be able to apply lasers to the treatment of advanced cancer. When CO2 laser supraglottoplasty was found to be an effective treatment for severe laryngomalacia (58), it offered decreased costs, complications, and morbidity when compared to previous procedures, which included tracheotomies and microdissection. There is much incentive to investigate areas such as this where lasers could potentially replace the more cumbersome and costly procedures currently in effect. Zeitels et al. (28) found that the thulium laser is a useful tool for extensive endolaryngeal resections, offering improved hemostasis and the option of cutting tangentially. However, further studies must be done to minimize tissue damage by determining optimal power settings and cutting parameters and developing ways to decrease thermal damage.
The optimal treatment of vocal fold vascular lesions has shifted back and forth from cold surgical techniques to different kinds of laser treatments since Baker suggested direct laryngoscopic excision of these lesions in 1962 (59). Continuing to study recent advances in both techniques and comparing their merits and downfalls should help ensure that the most effective surgical option is applied in each case.
In the past, lasers proved to be less effective than microsurgery at treating most benign lesions. However, in recent years, the CO2 laser has been used to excise benign laryngeal lesions precisely and with minimal bleeding. One application was the treatment of adult laryngeal hemangiomas (60). Adult supraglottic hemangiomas were destroyed with little damage to the adjacent tissue using the CO2 laser. Because many still believe this use of lasers is inadvisable, more work towards general expansion of laser use to such procedures would be beneficial. Lucioni et al. (60) believe staged CO2 laser surgeries are the best option for extended laryngeal cavernous hemangiomas involving the hypopharynx. Adequate spacing should be used between procedural stages to allow for tissue recovery. Currently, temporary tracheotomy is necessary prior to laser excision of extended laryngeal hemangiomas. Decreasing the need for dangerous, invasive procedures of this sort is a major motivation to continue studying laser usage in laryngology. This might well be an area where the thulium laser will prove beneficial because of its superior hemostatic ability and the versatility of its delivery fiber placement. However, the improved hemostasis and control may be offset by the possibility of increased damage to the vocal folds (61). Although studies have demonstrated that lasers produce similar results to cold surgery techniques (62,63), others (64) claim that these comparisons are misleading as they do not compare lasers with the most recent cold surgery techniques. Thus, in order to evaluate the efficacy of new laser surgery applications, more accurate comparisons must be made.
Treatment of early glottic cancer is still a topic of debate. Recently, selectively treating early cancerous lesions with photoangiolytic lasers was found to be a safe and effective procedure. As early glottic cancer seldom metastasizes, the photoangiolytic laser’s direct nonionizing radiation of the neoplastic blood supply effectively removed the early tumors. This technique also results in better phonatory mucosal wave vibration, thereby improving vocal function. Although found to be efficient, larger-scale studies are needed to determine whether angiolytic lasers are the best treatment option (65). A benefit of pulsed angiolytic lasers is the ability to concentrate energy specifically in the cancerous region, which is not possible with radiotherapy. Therefore, this approach may be used preferentially, particularly for early glottic cancer. Current cancer treatment options such as radiotherapy and chemotherapy are often used in conjunction with other treatments. Pulsed angiolytic laser surgery presents a treatment option which can be used by itself, thus decreasing costs and treatment duration. However, before angiolytic laser surgery can be applied to more advanced cancer and other diseases, it is necessary for surgeons to become familiar with the procedures, beginning with small lesions in easily accessible locations (65).
In the treatment of laryngeal cancer, laser surgery has already demonstrated reliable tumor removal with fewer complications than open surgery in managing many glottic and supraglottic lesions. Ferlito et al. (57) suggest future use of lasers in photodynamic therapy to selectively activate photosensitive chemicals to destroy tumors. Laser surgery could also be used in conjunction with conventional microsurgery in order to take advantage of the benefits of each. Lasers have already demonstrated superiority in a variety of applications, and future research should offer promising solutions to problems inadequately addressed with current treatment options.
Acknowledgments
This research was supported by NIH grant number R01 DC008850 from the National Institute on Deafness and Other Communication Disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Maiman TH. Stimulated optical radiation in ruby. Nature. 1960;187:493. [Google Scholar]
- 2.Ossoff RH, Coleman JA, Courey MS, Duncavage JA, Werkhaven JA, Reinisch L. Clinical applications of lasers in otolaryngology--head and neck surgery. Lasers Surg Med. 1994;15(3):217–248. doi: 10.1002/lsm.1900150302. Review. [DOI] [PubMed] [Google Scholar]
- 3.Duncavage JA, Ossoff RH. Laser application in the tracheobronchial tree. Otolaryngol Clin North Am. 1990 Feb;23(1):67–75. Review. [PubMed] [Google Scholar]
- 4.Ossoff RH, Matar SA. The advantages of laser treatment of tumors of the larynx. Oncology (Williston Park) 1988 Sep;2(9):58–61. 64–65. [PubMed] [Google Scholar]
- 5.Jako GJ. Laser surgery of the vocal cords. An experimental study with carbon dioxide lasers on dogs. Laryngoscope. 1972 Dec;82(12):2204–2216. doi: 10.1288/00005537-197212000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Polanyi TG, Bredemeier HC, Davis TW. A CO2 laser for surgical research. Med Biol Eng. 1970;8:541–548. doi: 10.1007/BF02478228. [DOI] [PubMed] [Google Scholar]
- 7.Shapshay SM. Jako: “Laser surgery of the vocal cords; an experimental study with carbon dioxide lasers on dogs.” (Laryngoscope 1972; 82:2204–2216) Laryngoscope. 1996 Aug;106(8):935–938. doi: 10.1097/00005537-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Sataloff RT, Spiegel JR, Hawkshaw M, Jones A. Laser surgery of the larynx: the case for caution. Ear Nose Throat J. 1992 Nov;71(11):593–595. [PubMed] [Google Scholar]
- 9.Fuller TA. Surgical Lasers: A Clinical Guide. New York: Macmillan; 1987. pp. 1–17. [Google Scholar]
- 10.Absten GT. Physics of light and lasers. Obstet Gynecol Clin North Am. 1991 Sep;18(3):407–427. [PubMed] [Google Scholar]
- 11.Polanyi TG. Physics of surgery with lasers. Clinics in Chest Medicine. 1985 Jun;6(2):179–202. [PubMed] [Google Scholar]
- 12.Benninger MS. Microdissection or microspot CO2 laser for limited vocal fold benign lesions: a prospective randomized trial. Laryngoscope. 2000 Feb;110(2 Pt 2 Suppl 92):1–17. doi: 10.1097/00005537-200002001-00001. [DOI] [PubMed] [Google Scholar]
- 13.Haug MH, Møller P, Olofsson J. Laser surgery in otorhinolaryngology: a 10-year experience. J Otolaryngol. 1993 Feb;22(1):42–45. [PubMed] [Google Scholar]
- 14.Hermens JM, Bennett MJ, Hirshman CA. Anesthesia for laser surgery. Anesth Analg. 1983 Feb;62(2):218–229. [PubMed] [Google Scholar]
- 15.Koufman JA, Rees CJ, Frazier WD, Kilpatrick LA, Wright SC, Halum SL, Postma GN. Office-based laryngeal laser surgery: a review of 443 cases using three wavelengths. Otolaryngol Head Neck Surg. 2007 Jul;137(1):146–151. doi: 10.1016/j.otohns.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 16.Reinisch L, Ossoff RH. Laser applications in otolaryngology. Otolaryngol Clin North Am. 1996 Dec;29(6):891–892. [PubMed] [Google Scholar]
- 17.Xu W, Han D, Hou L, Zhang L, Yu Z, Huang Z. Voice function following CO2 laser microsurgery for precancerous and early-stage glottic carcinoma. Acta Oto-Laryngologica. 2007;127:6, 637–641. doi: 10.1080/00016480600987776. [DOI] [PubMed] [Google Scholar]
- 18.Zeitels SM, Burns JA. Laser applications in laryngology: past, present, and future. Otolaryngol Clin North Am. 2006 Feb;39(1):159–172. doi: 10.1016/j.otc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Basterra J, Frías S, Alba JR, Pérez A, Zapater E. Comparative study of acute tissue damage induced by the CO2 laser versus microelectrodes in cordectomies. Otolaryngol Head Neck Surg. 2006 Dec;135(6):933–936. doi: 10.1016/j.otohns.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Basterra J, Zapater E, Moreno R, Hernández R. Electrosurgical endoscopic cordectomy with microdissection electrodes: a comparative study with CO2 laser. J Laryngol Otol. 2006 Aug;120(8):661–664. doi: 10.1017/S0022215106001368. [DOI] [PubMed] [Google Scholar]
- 21.McMillan K, Shapshay SM, McGilligan JA, Wang Z, Rebeiz EE. A 585-nanometer pulsed dye laser treatment of laryngeal papillomas: preliminary report. Laryngoscope. 1998 Jul;108(7):968–972. doi: 10.1097/00005537-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Franco RA, Jr, Zeitels SM, Farinelli WA, Faquin W, Anderson RR. 585-nm pulsed dye laser treatment of glottal dysplasia. Ann Otol Rhinol Laryngol. 2003 Sep;112(9 Pt 1):751–758. doi: 10.1177/000348940311200902. [DOI] [PubMed] [Google Scholar]
- 23.Nerurkar N, Narkar N, Joshi A, Kalel K, Bradoo R. Vocal outcomes following subepithelial infiltration technique in microflap surgery: a review of 30 cases. J Laryngol Otol. 2007;121:768–771. doi: 10.1017/S002221510700744X. [DOI] [PubMed] [Google Scholar]
- 24.Ketcham A, Smith J, Lee F, Halstead L, White D. Clinical course following endoscopic repair of type 1 laryngeal clefts. Intl J Pediatr Otorhinolaryngol. 2008;72:1261–1267. doi: 10.1016/j.ijporl.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Kothari P, Dhillon R. Key developments in otolaryngology. Practitioner. 2006 Feb;250(1679):57–58. 60, 62 passim. [PubMed] [Google Scholar]
- 26.Strong MS, Jako GJ. Laser surgery in the larynx. Early clinical experience with continuous CO2 laser. Ann Otol Rhinol Laryngol. 1972 Dec;81(6):791–798. doi: 10.1177/000348947208100606. [DOI] [PubMed] [Google Scholar]
- 27.Crockett DM, Reynolds BN. Laryngeal laser surgery. Otolaryngol Clin North Am. 1990 Feb;23(1):49–66. Review. [PubMed] [Google Scholar]
- 28.Zeitels SM, Burns JA, Akst LM, Hillman RE, Broadhurst MS, Anderson RR. Office-based and microlaryngeal applications of a fiber-based thulium laser. Ann Otol Rhinol Laryngol. 2006 Dec;115(12):891–896. doi: 10.1177/000348940611501206. [DOI] [PubMed] [Google Scholar]
- 29.Zeitels SM, Burns JA. Office-based laryngeal laser surgery with local anesthesia. Curr Opin Otolaryngol Head Neck Surg. 2007 Jun;15(3):141–147. doi: 10.1097/MOO.0b013e3281574582. Review. [DOI] [PubMed] [Google Scholar]
- 30.Rees CJ, Postma GN, Koufman JA. Cost savings of unsedated office-based laser surgery for laryngeal papillomas. Ann Otol Rhinol Laryngol. 2007 Jan;116(1):45–48. doi: 10.1177/000348940711600108. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Devaiah AK, Feng L, Dasai U, Shapira G, Weisberg O, Torres DS, Shapshay SM. Fiber-guided CO2 laser surgery in an animal model. Photomed Laser Surg. 2006 Oct;24(5):646–650. doi: 10.1089/pho.2006.24.646. [DOI] [PubMed] [Google Scholar]
- 32.Zeitels SM, Kobler JB, Heaton JT, Faquin W. Carbon dioxide laser fiber for laryngeal cancer surgery. Ann Otol Rhinol Laryngol. 2006 Jul;115(7):535–541. doi: 10.1177/000348940611500708. [DOI] [PubMed] [Google Scholar]
- 33.Ledda GP, Grover N, Pundir V, Masala E, Puxeddu R. Functional outcomes after CO2 laser treatment of early glottic carcinoma. Laryngoscope. 2006 Jun;116(6):1007–1011. doi: 10.1097/01.MLG.0000217557.45491.BD. [DOI] [PubMed] [Google Scholar]
- 34.Steiner W, Ambrosch P, Rödel RM, Kron M. Impact of anterior commissure involvement on local control of early glottic carcinoma treated by laser microresection. Laryngoscope. 2004 Aug;114(8):1485–1491. doi: 10.1097/00005537-200408000-00031. [DOI] [PubMed] [Google Scholar]
- 35.Peretti G, Piazza C, Bolzoni A, Mensi MC, Rossini M, Parrinello G, Shapshay SM, Antonelli AR. Analysis of recurrences in 322 Tis, T1, or T2 glottic carcinomas treated by carbon dioxide laser. Ann Otol Rhinol Laryngol. 2004 Nov;113(11):853–858. doi: 10.1177/000348940411301101. [DOI] [PubMed] [Google Scholar]
- 36.Remacle M, Lawson G, Watelet JB. Carbon dioxide laser microsurgery of benign vocal fold lesions: indications, techniques, and results in 251 patients. Ann Otol Rhinol Laryngol. 1999;108:156–164. doi: 10.1177/000348949910800210. [DOI] [PubMed] [Google Scholar]
- 37.Remacle M, Lawson G, Degols JC, Evrard I, Jamart J. Microsurgery of sulcus vergeture with carbon dioxide laser and injectable collagen. Ann Otol Rhinol Laryngol. 2000;109:141–148. doi: 10.1177/000348940010900206. [DOI] [PubMed] [Google Scholar]
- 38.Keilmann A, Biermann G, Hormann K. CO2 laser versus conventional microlaryngoscopy in benign changes of the vocal cords. Laryngorhinootologie. 1997;76:484–489. doi: 10.1055/s-2007-997465. [DOI] [PubMed] [Google Scholar]
- 39.Wilder-Smith P, Dang J, Kurosaki T. Investigating the range of surgical effects on soft tissue produced by a carbon dioxide laser. J Am Dent Assoc. 1997;128:583–588. doi: 10.14219/jada.archive.1997.0257. [DOI] [PubMed] [Google Scholar]
- 40.Remacle M, Lawson G, Nollevaux MC, Delos M. Current state of scanning micromanipulator applications with the carbon dioxide laser. Ann Otol Rhinol Laryngol. 2008 Apr;117(4):239–244. doi: 10.1177/000348940811700401. [DOI] [PubMed] [Google Scholar]
- 41.Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983 Apr 29;200(4596):524–527. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
- 42.Bower CM, Flock S, Waner M. Flash pump dye laser treatment of laryngeal papillomas. Ann Otol Rhinol Laryngol. 1998;107:1001–1005. doi: 10.1177/000348949810701201. [DOI] [PubMed] [Google Scholar]
- 43.McMillan K, Shapshay SM, McGilligan JA. A 585-nanometer pulsed dye laser treatment of laryngeal papillomas: preliminary report. Laryngoscope. 1998;108:968–972. doi: 10.1097/00005537-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Anderson RR, Jaenicke KF, Parrish JA. Mechanisms of selective vascular changes caused by dye lasers. Lasers Surg Med. 1983;3:211–215. doi: 10.1002/lsm.1900030303. [DOI] [PubMed] [Google Scholar]
- 45.Zeitels SM, Healy GB. Laryngology and phonosurgery. N Engl J Med. 2003;349:882–892. doi: 10.1056/NEJMra035148. [DOI] [PubMed] [Google Scholar]
- 46.Zeitels SM. Premalignant epithelium and microinvasive cancer of the vocal fold: the evolution of phonomicrosurgical management. Laryngoscope. 1995;105 Suppl 67:1–51. doi: 10.1288/00005537-199503001-00001. [DOI] [PubMed] [Google Scholar]
- 47.Zeitels SM, Burns JA. Office-based laryngeal laser surgery with the 532-nm pulsed-potassium-titanyl-phosphate laser. Curr Opin Otolaryngol Head Neck Surg. 2007 Dec;15(6):394–400. doi: 10.1097/MOO.0b013e3282f1fbb2. Review. [DOI] [PubMed] [Google Scholar]
- 48.Burns JA, Zeitels SM, Akst LM, Broadhurst MS, Hillman RE, Anderson R. 532 nm pulsed potassium-titanyl-phosphate laser treatment of laryngeal papillomatosis under general anesthesia. Laryngoscope. 2007 Aug;117(8):1500–1504. doi: 10.1097/MLG.0b013e318064e869. [DOI] [PubMed] [Google Scholar]
- 49.Broadhurst MS, Akst LM, Burns JA, Kobler JB, Heaton JT, Anderson RR, Zeitels SM. Effects of 532 nm pulsed-KTP laser parameters on vessel ablation in the avian chorioallantoic membrane: implications for vocal fold mucosa. Laryngoscope. 2007 Feb;117(2):220–225. doi: 10.1097/mlg.0b013e31802b5c1c. [DOI] [PubMed] [Google Scholar]
- 50.Franco JRA., Jr In-office laryngeal surgery with the 585-nm pulsed dye laser. Curr Opin Otolaryngol Head Neck Surg. 2007 Dec;15(6):387–393. doi: 10.1097/MOO.0b013e3282f19ef2. [DOI] [PubMed] [Google Scholar]
- 51.Ayala C, Selig M, Faquin W, Franco RA., Jr Ultrastructural evaluation of 585-nm pulsed-dye laser-treated dysplasia. J Voice. 2007;21:119–126. doi: 10.1016/j.jvoice.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 52.Ossoff RH. Laser safety in otolaryngology--head and neck surgery: anesthetic and educational considerations for laryngeal surgery. Laryngoscope. 1989 Aug;99(8 Pt 2 Suppl 48):1–26. Review. [PubMed] [Google Scholar]
- 53.Sesterhenn AM, Dünne AA, Braulke D, Lippert BM, Folz BJ, Werner JA. Value of endotracheal tube safety in laryngeal laser surgery. Lasers Surg Med. 2003;32(5):384–390. doi: 10.1002/lsm.10174. [DOI] [PubMed] [Google Scholar]
- 54.Strong MS, Jako GJ, Vaughan CW, Healy GB, Polanyi T. The use of CO2 laser in otolaryngology: a progress report. Trans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol. 1976 Sep–Oct;82(5):595–602. [PubMed] [Google Scholar]
- 55.Arch-Tirado E, Verduzco-Mendoza A, Taboada-Picazo V, Mota-Rojas D, Alonso-Spilsbury MD, Alfaro-Rodríguez A. Analysis of Normal and Denerved Laryngeal Vocalization in Guinea Pigs (Cavia porcellus) J Voice. 2007 Nov 13; doi: 10.1016/j.jvoice.2007.03.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 56.Burns JA, Kobler JB, Heaton JT, Anderson RR, Zeitels SM. Predicting Clinical Efficacy of Photoangiolytic and Cutting/Ablating Lasers using the Chick Chorioallantoic Membrane Model: Implications for Endoscopic Voice Surgery. Laryngoscope. 2008 Mar 18; doi: 10.1097/MLG.0b013e31816902bb. [DOI] [PubMed] [Google Scholar]
- 57.Ferlito A, Buckley JG, Ossoff R, Rinaldo A, Weir N. The Future of Laryngology. Acta Oto-Laryngologica. 2001;121:7, 859–867. doi: 10.1080/00016480152602348. [DOI] [PubMed] [Google Scholar]
- 58.Lee KS, Chen BN, Yang CC, Chen YC. CO2 laser supraglottoplasty for severe laryngomalacia: a study of symptomatic improvement. Int J Pediatr Otorhinolaryngol. 2007 Jun;71(6):889–895. doi: 10.1016/j.ijporl.2007.02.010. Epub 2007 Apr 9. [DOI] [PubMed] [Google Scholar]
- 59.Baker DC. Laryngeal problems in singers. Laryngoscope. 1962;72:902–908. doi: 10.1288/00005537-196207000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Lucioni M, Marioni G, Della Libera D, Rizzotto G. Adult laryngeal hemangioma CO2 laser excision. A single institution 3-year experience (Vittorio Veneto 2001–2003) Acta Otolaryngol. 2006 Jun;126(6):621–626. doi: 10.1080/00016480500452517. [DOI] [PubMed] [Google Scholar]
- 61.Sulica L, Behrman A. Management of benign vocal fold lesions: a survey of current opinion and practice. Ann Otol Rhinol Laryngol. 2003;112:827–833. doi: 10.1177/000348940311201001. [DOI] [PubMed] [Google Scholar]
- 62.Mahieu HF, Patel P, Annyas AA, Van der Laan T. Carbon dioxide laser vaporization in early glottic carcinoma. Arch Otolaryngol Head Neck Surg. 1994;120:383–387. doi: 10.1001/archotol.1994.01880280011002. [DOI] [PubMed] [Google Scholar]
- 63.Rogerson AR, Clark KF, Bandi SR, Bane B. Voice and healing after vocal fold epithelium removal by CO2 laser vs. microlaryngeal stripping. Otolaryngol Head Neck Surg. 1996 Oct;115(4):352–359. doi: 10.1016/S0194-5998(96)70050-5. [DOI] [PubMed] [Google Scholar]
- 64.Zeitels SM, Hillman RE, Desloge R, Mauri M, Doyle PB. Phonomicrosurgery in singers and performing artists: treatment outcomes, management theories, and future directions. Ann Otol Rhinol Laryngol Suppl. 2002 Dec;190:21–40. doi: 10.1177/0003489402111s1203. [DOI] [PubMed] [Google Scholar]
- 65.Zeitels SM, Burns JA, Lopez-Guerra G, Anderson RR, Hillman RE. Photoangiolytic laser treatment of early glottic cancer: a new management strategy. Ann Otol Rhinol Laryngol Suppl. 2008 Jul;199:3–24. doi: 10.1177/00034894081170s701. [DOI] [PubMed] [Google Scholar]
- 66.Zijlstra WG, Buursma A, Meeuwsen-van der Roest WP. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin Chem. 1991;37(9):1633–1638. [PubMed] [Google Scholar]