Abstract
The goal of regenerative medicine is to restore form and function to damaged and aging tissues. Adult stem cells, present in tissues such as skeletal muscle, comprise a reservoir of cells with a remarkable capacity to proliferate and repair tissue damage. Muscle stem cells, known as satellite cells, reside in a quiescent state in an anatomically distinct compartment, or niche, ensheathed between the membrane of the myofiber and the basal lamina. Recently, procedures for isolating satellite cells were developed and experiments testing their function upon transplantation into muscles revealed an extraordinary potential to contribute to muscle fibers and access and replenish the satellite cell compartment. However, these properties are rapidly lost once satellite cells are plated in culture. Accordingly, elucidating the role of extrinsic factors in controlling muscle stem cell fate, in particular self-renewal, is critical. Through careful design of bioengineered culture platforms, analysis of specific proteins presented to stem cells is possible. Critical to the success of the approach is single cell analysis, as more rapidly proliferating progenitors may mask the behavior of stem cells that proliferate slowly. Bioengineering approaches provide a potent means of gaining insight into the role of extrinsic factors in the stem cell microenvironment on stem cell function and the mechanisms that control their diverse fates. Ultimately, the multidisciplinary approach presented here will lead to novel therapeutic strategies for degenerative diseases.
The adult stem cell and its niche
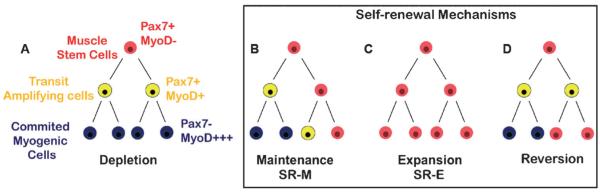
Adult stem cells are identified as cells with the ability to produce more stem cells of the same type (self-renew) and to give rise to a defined set of mature differentiated progeny to maintain or repair their host tissue.1-3 In theory, stem cells can self-renew via two different mechanisms (Fig. 1):4 (i) It has been suggested that to maintain homeostasis, stem cells use asymmetric cell division whereby each stem cell generates one stem cell and one differentiated cell, resulting in a constant number of stem cells (self-renewal of maintenance, SR-M) (ii) Conversely, by symmetric division, or self-renewal of expansion (SR-E), each stem cell gives rise to two daughter stem cells resulting in an increase in stem cell numbers. This strategy permits stem cells to expand in number to create and reconstitute the stem cell pool during development and acute injury. Tissue damage leading to an influx of signals is thought to cause a loss of contact of the stem cell with its surrounding environment resulting in a loss of quiescence and induction of proliferation that is accompanied by differentiation and loss of stemness. Therefore, although stem cells can divide asymmetrically under steady state conditions, they must retain the capacity to divide symmetrically to restore stem cell pools depleted by injury or disease.5
Fig. 1. Simplified models of muscle stem cell progression towards diverse fates.
Freshly isolated muscle stem cells (Pax7+MyoD−) can adopt divergent cell fates. (A) They can undergo symmetric divisions, giving rise to two daughter committed cells (blue), leading to a depletion of the stem cell pool; (B) they can undergo asymmetric divisions, giving rise to one daughter stem cell (red) and one daughter transit amplifying cell (yellow), maintaining a constant stem cell pool but expanding the committed progeny (blue) (self-renewal of maintenance, SR-M); (C) they can undergo symmetric divisions giving rise to two daughter stem cells, thus expanding stem cell number (self-renewal of expansion, SR-E); or (D) they can undergo reversion, from a amplifying progenitor cell to a quiescent muscle stem cell. Pax7 expression is present from the time of isolation, and persists until committed cells begin differentiating. MyoD expression is not detected until the committed cell stage, but persists even after terminal differentiation.
In the adult, stem cells reside within instructive, tissue-specific microenvironments, or “niches”, that physically localize them, protect them and regulate many of their functions. In niches, stem cells are surrounded by other cells and are exposed to a complex mixture of cell membrane components, soluble and insoluble extracellular molecules. First described in Drosophila melanogaster and C. elegans ovary and testis,6-10 niches have now been identified in many mammalian tissues such as bone marrow, skin, intestine, brain, testis and skeletal muscle.11-21 The key function of stem cell niches appears to be the regulation of stem cell activity by maintaining a constant level of slowly dividing stem cells during homeostasis and by balancing quiescence and activation in order to proliferate and expand their numbers in response to injury. Every time a stem cell exits the niche, a process that is induced by signals from outside the niche such as inflammatory cytokines, it must be replenished through self-renewal and generate daughter stem cells that differentiate.22,23
The “niche” is a concept originally coined by Schofield,24 based on a synthesis of in vivo genetic evidence and in vitro cell culture observations showing that both appropriate environmental and hematopoietic cell types are essential for the maintenance of normal hematopoiesis. Schofield ascertained that the microenvironment was so crucial that no hematopoietic cell was intrinsically a stem cell. Seminal studies of Drosophila germ stem cells first verified the concept of the niche, providing evidence that short-range signals in the vicinity of stem cells, including cell–cell junctions, extracellular matrix components and soluble signals, were key to their function.6-10,25 Elegant studies in mammalian skin identified the hair follicle outer root sheath (ORS) bulge, as a differentiation- and growth-restricted environment, and to date this is one of the best-defined niches in mammals.13,26 Potten first described a feature shared by many stem cells, slow cell cycling in steady-state conditions leading to label retention (Labeling Retaining Cells, LRC).27-29 Label retention results because stem cells are generally quiescent unless removed from their physiological microenvironment whereupon they rapidly proliferate, give rise to progenitors, and fully differentiate in vitro. Importantly, these properties are retained following serial transplantation of stem cells into other animals. Intestinal crypts comprise the niche for intestinal stem cells,15,30,31 neural stem cells reside in the subventricular zone in the brain,17,32 and muscle stem cells known as satellite cells are found localized beneath the muscle fiber basal lamina.19-21 Functional evidence is accumulating that factors extrinsic to stem cells in these niche compartments, or microenvironments, play a critical role in regulating self-renewal.33
The most striking evidence for the protective function of niches is the behavioral and functional changes that occur when stem cells are artificially removed from their niche. For example, hematopoietic stem cells, the best characterized adult stem cell to date, when plated in culture divide rapidly, differentiate, and lose their stem cell properties; niche components provided by stroma are required to slow the cell cycle and maintain stem cell function.34,35 The niche prevents stem cells from differentiating and stem cell progeny must be evicted or excluded from the niche to be exposed to differentiating stimuli and become committed. Localized proteins, including cellular transmembrane proteins, secreted soluble morphogens or insoluble extracellular matrix proteins, direct asymmetric cell division leading to the production of one self-renewing stem cell, which remains in contact with the niche, and one differentiated daughter cell, which has lost niche contact. Polarity essential to such an asymmetric division can be achieved by mitotic spindle orientation, which if perpendicular to the niche, leads to two daughter cells that are different because they are exposed to different microenvironmental cues, as shown for germ cells,25,36 neural stem cells37 and recently in skeletal muscle.38,39 Moreover, in response to tissue injury, stem cells must also divide symmetrically to give rise to two stem cell daughters in order to expand the stem cell pool. Thus, the localization and regulation of cellular and molecular components of the niche are critical to maintaining the stem cell pool throughout the lifetime of an organism. This process must be tightly orchestrated, as loss of stem cells ultimately leads to impaired regenerative capacity and over-production can lead to cancer. Remarkably, recent evidence suggests that stem cells are not the only cells capable of self-renewal, but “potential stem cells”, normally not self-renewing such as progenitors, can reacquire self-renewal properties when put in contact with the niche, both in Drosophila40,41 and in mammals.42,43 Together, these findings strongly implicate the niche as the key regulator in establishing stem cell function, and suggest that self-renewal is not an intrinsic function restricted to stem cell populations, as it can be induced in other cell types by the “dominant niche”. Thus, a better understanding of the regulatory proteins within niches is essential to unveil mechanisms that regulate adult stem cell function.
Regulation of satellite cell self-renewal by the niche
Adult muscle stem cells, identified as satellite cells, are considered to be largely responsible for postnatal muscle growth, regeneration and hypertrophy (reviewed in21). Satellite cells were first identified and defined anatomically by electron microscopy as mononucleated cells localized in vivo underneath the basal lamina of skeletal myofibers.19 Thus, a role for an anatomically well-defined niche in mammals is probably most apparent for satellite cells, as these muscle stem cells are compartmentalized and separated from other cells, intercalated between the basal lamina and the sarcolemma of muscle fibers. In their niche, satellite cells are mitotically quiescent, but they become activated to divide and differentiate in response to muscle growth or repair.
A molecular hierarchy of myogenic transcription factors with a role in adult muscle stem cell activation has emerged based on studies of gene deletions in mice and studies of single myofibers in culture (reviewed in44). Pax7, a paired box transcription factor, marks quiescent satellite cells in all muscles, and its absence in knockout mice results in a paucity of satellite cells and early death.45 Pax3 marks satellite cells in a subset of muscles, which does not include the limb. Upon satellite cell activation in response to injury, satellite cells express bHLH transcription factors, MyoD and Myf5, begin proliferating and down-regulate Pax genes before differentiating into myotubes.20,46 Those cells that maintain Pax expression are thought to be capable of homing to and reconstituting the satellite cell compartment.47 Moreover, it has been postulated that activated satellite cells that co-express Pax7 and Myf5 or MyoD may not be irreversibly committed to differentiation, but could revert to a quiescent satellite cell state (reviewed in ref. 48). Although muscle stem cell activation and differentiation has been well described, we are only beginning to understand the processes that allow satellite cells to maintain their quiescence49 and self-renewal capacity.38,39,50,51
Several recent studies have focused on elucidating pathways that regulate satellite cell quiescence, asymmetric and symmetric division. Studies of cultured satellite cells have shown that asymmetric division behavior is regulated, in part, by the cytoplasmic cell fate determinant Numb, a Notch1 antagonist, which is asymmetrically segregated to one pole of a dividing satellite cell.52,53 Asymmetric cell divisions are also evident by a selective template-DNA strand segregation that occurs during mitosis of satellite cells on muscle fibers in vivo and in culture.53,54 Although, these results suggest that this asymmetric division behavior is independent of the niche, exposure to extrinsic cues likely also plays a role.5
The well-defined anatomical position of satellite cells suggests that reciprocal interactions with the adjacent myofiber on one side and with the basal lamina on the other are crucial to satellite cell function. The complex microenvironment surrounding satellite cells can be regarded as a “niche,” a milieu that physically tethers stem cells, protects them, and regulates their behavior.21 Microenvironmental signals with a potential role in satellite cell activation have been identified. For example, Notch signaling has been shown to play an important role in satellite cell activation, proliferation and differentiation.52 Similarly, the Wnt family of regulators also plays an important role.55,56 M-cadherin, N-cadherin, integrins, and dystrophin are among the proteins that are localized to the membranes of the satellite cell compartment.21,44 Further, Nitric Oxide (NO) and Hepatocyte Growth Factor (HGF) have been shown to be capable of direct and very rapid induction of satellite cell activation in vivo and in vitro.57,58 Although several soluble factors in the microenvironment have been linked to muscle stem cell function,21,44 the role of membrane-bound molecular components and the extracellular matrix (ECM) that comprise the satellite cell niche in maintaining quiescence and preventing activation, and in regulating symmetric or asymmetric divisions remains poorly understood. This is due, in part, to a lack of suitable model systems for studying stem cell-niche interactions.
Bioengineering approaches to investigate self-renewal of single stem cells in culture
Model systems that allow rigorous analysis of components of the tissue-specific niche that adult stem cells occupy and that regulates its functions are of great advantage to the study of factors that regulate the delicate balance between self-renewal and differentiation. Bioengineering approaches offer many advantages for elucidating the molecular nature of the soluble and insoluble factors that control stem cell behavior.59 Here we describe a hydrogel microarray culture platform that was developed to dissect key molecular mechanisms that govern muscle stem cell fate choices.60,61 The approach described below is predicated on the importance of single cell studies.
No matter how many phenotypic markers are used to purify cells by flow cytometry, all adult stem cells, including satellite cells, can only be isolated with limited purity. The resulting inevitable cell mixture leads to heterogeneous behavior and is a major limitation of population-based (“bulk”) in vitro analyses. Accordingly, read-outs targeted at defining stem cell behavior may be skewed by the behavior of more rapidly growing progenitors, underscoring the need for clonal analyses. Although cells can be assayed as clones using conventional tissue culture formats, this is both cumbersome and highly ineffcient. For these reasons methods to interrogate large numbers of individual, spatially confined stem cells, such as hydrogel microwell arrays, are required (Fig. 2). This platform enables the study of thedynamic, clonal behavior of single stem cells. Microwells are particularly important in the case of highly migratory cell types such as MuSCs, where maintaining accurate lineage relationship is contingent on the cells remaining confined within a particular field of view. Moreover, statistically robust data sets can be acquired, as the effects of specific proteins on hundreds of single stem cells can be analyzed simultaneously and the effects of different proteins ascertained in parallel and directly compared.
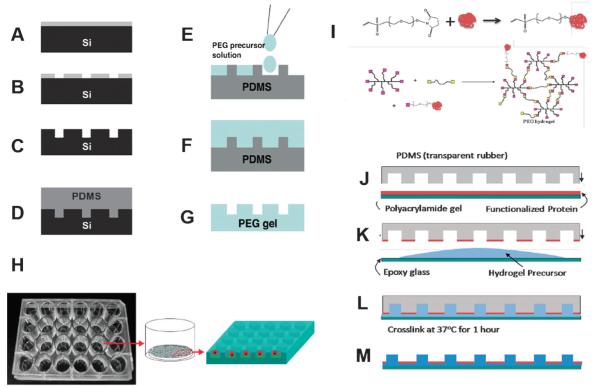
Fig. 2. Single cell analysis of stem cells is enabled by bioengineered hydrogel microwell arrays.
(A–D) Scheme of PDMS microwell replica generation. (E–G) Production of PEG microwell arrays. (H) Scheme of a hydrogel microwell array in a single well of a 24 well plate. (I) Protein functionalization. Vinyl sulfone is attached to protein through reaction with lysine residues. Functionalized protein can then be polymerized into the network. (J–M) Microcontacting printing and production of hydrogel microwell arrays. (J) Microcontact printing of protein onto PDMS stamp; (K) stamp with protein adsorbed on posts is placed on a glass slide with a drop of PEG precursor; (L) Hydrogel is polymerized at 37 °C for 1 h. (M) Stamp is removed leaving behind 24 bottom functionalized hydrogel arrays per slide.
Standard microfabrication techniques using PDMS as a replica to topographically structure hydrogels containing arrays of microwells that enable the spatial segregation of large numbers of single stem cells can be employed (Fig. 2). Specifically, the first step in the fabrication is to make a silicon wafer with the desired pattern etched into the substrate. This is achieved using standard photolithography, where a layer of photoresist is spun onto the wafer and then exposed to UV light through a mask containing the desired features (Fig. 2A). The regions exposed to UV light become soluble in the developing solvent and can be rinsed away (Fig. 2B). The wafer with photoresist is then exposed to an etching solution that etches away exposed regions of the silicon crystal. Once the desired depth has been achieved, the wafer is removed from the etching solution and the photoresist is removed using a plasma cleaning procedure (Fig. 2C). This silicon master can be used to make a replica in PDMS (transparent rubber). For this purpose, a fluorocarbon layer is deposited onto the surface to prevent the rubber from sticking to the silicon features. The PDMS replica is made by pouring liquid thermocurable PDMS onto the wafer and then polymerizing and peeling the PDMS away from the wafer (Fig. 2D). This elastomeric template can be used to cast biocompatible, hydrophilic and inert polymer precursors such as poly(ethylene glycol) (PEG) hydrogels using conventional covalent crosslinking chemistry.
Hydrogels are an advantageous class of biomaterials for cell culture, as they are inert to protein adsorption and mimic natural tissue in that they are soft and highly hydrated (up to 98% water), thus allowing diffusion of nutrients and presentation of proteins in a more physiological state than conventional plastic dishes. Hydrogels consist of long polymer chains that are cross-linked together to form a mesh network as illustrated in Fig. 2. The network is typically very hydrophilic and therefore when the gel is placed in excess solvent (for example water), the network swells, stretching the chains and increasing the water content of the materials. While some PEG hydrogels require chemical or UV cross-linking to form the polymer network, others can self assemble via a highly selective Michael-type addition reaction of vinyl sulfone (VS) groups at the PEG termini and thiols (–SH) from PEG-dithiols. Production of PEG hydrogels containing arrays of microwells to spatially segregate single stem cells is feasible using a PDMS replica as a master. Briefly, PEG precursor solution is placed on a PDMS stamp containing an inverted replica of the microwell design (Fig. 2E). After polymerization (Fig. 2F), PEG hydrogels are peeled from the PDMS master revealing the microwell design (Fig. 2G). By culturing single cells in microwells, experiments can be designed to dissect the impact of extrinsic factors that comprise the stem cell niche.
The microwell platform described above can be readily used to test the influence of soluble factors on populations of single stem cells. However, considering the in vivo localization of stem cells in “niches”, it is likely that both extracellular matrix(ECM) and cell-cell interactions are also crucial for maintenance of stem cell function. Previous studies by others have shown that many ECM and cell–cell interactions require that the signaling protein be physically tethered, as opposed to being simply soluble in media.62-65 In order to test the effects on stem cells of signaling by tethered factors, methods to both covalently tether and spatially pattern proteins onto a hydrogel matrix have been developed.60,66 This technological advance is critical because it provides a way to explore the relevance of stem cell-niche interactions that were previously difficult to study in vitro. Tethering proteins to hydrogels can be achieved by attaching a chemical moiety to the protein of interest and subsequently crosslinking it into the hydrogel network (Fig. 2I). Bulk functionalization of the hydrogel is straightforward; functionalized protein is simply mixed with the precursor solution and polymerized into the matrix. However, when microwells are bulk functionalized, cells can migrate over the entire surface of the gel, rather than remaining confined within the microwells, making it difficult to follow their fate. This experimental challenge is readily overcome through the use of “reactive microcontact printing,” which is a method to specifically functionalize only the bottom of the microwells (Fig. 2J–M). In this method, functionalized protein is microcontact printed onto the posts of the PDMS stamp (Fig. 2J) and then the hydrogel is polymerized against the PDMS (Fig. 2K–L), transferring both the topographic pattern and protein pattern onto the hydrogel (Fig. 2M). Printed ECMs also serve to restrict the migration of highly migratory cells such as MuSCs to a particular area. The bioengineering platform described above allows researchers to define single proteins or combinations of proteins capable of inducing stem cell self-renewal and expansion.
Methods to elucidate mechanisms of muscle stem cell self-renewal
Satellite cell self-renewal is essential to the maintenance of the muscle stem cell pool. Elucidation of molecular mechanisms regulating muscle stem cell self-renewal is enabled by methods to visualize and quantify asymmetric and symmetric division events in culture. Hydrogel microwell arrays in conjunction with long-term time-lapse microscopy and retrospective analysis of gene expression permits correlations of muscle stem cell division history and phenotypic analysis to generate cell fate maps that reveal evidence of asymmetric and symmetric division events. To derive the relationship of cells to one another within a clonal population (i.e. within a single microwell) the genealogic division history of single cells cultured in microwells can be tracked using computer-assisted analysis of time-lapse videos so that the division history of each cell within a clone contained within a microwell at the end of the experiment is known. Subsequently, correlating retrospective staining for each cell within a microwell enables establishment of cell fate maps. The power of continuous long-term single cell analysis to provide clear answers to long-standing biological questions is highlighted by work borne from the Schroeder Laboratory.67-72
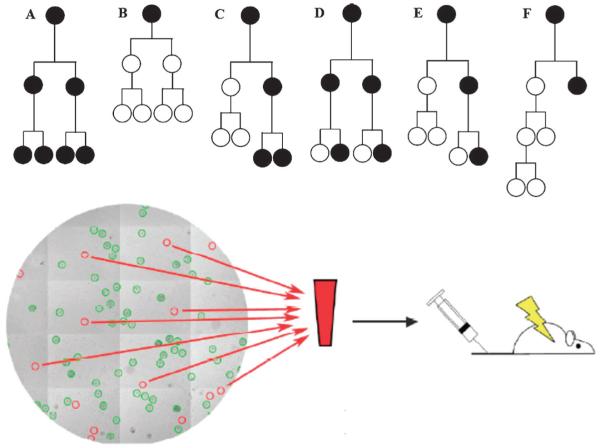
A particular strength of the satellite cell field is the availability of molecular markers that define each stage of the myogenic progression toward differentiation (see Fig. 1). For example, Pax7 and MyoD have been shown by other groups to define divergent fates of muscle stem cells20,46 and serve as indicators of stem cell maintenance. For example: (1) Pax7, a diagnostic marker of adult satellite cells is present at the earliest single cell stage and persist for the duration of the experiment. (2) MyoD, a marker of the committed muscle cell, appears once cells exit the satellite cell stage and become determined myogenic progenitors. Therefore the presence of Pax7 “only” in individual cells will be a hallmark of the muscle stem cell phenotype. The phenotypic analysis of clonal cell populations allows assessment of whether a given protein maintains or expands the number of Pax7+MyoD− cells. However, it will not reveal what type of division events occurred. For example, several division fates that would be missed using phenotypic analysis alone, but are readily resolved using a combination of cell fate mapping and phenotypic analysis can be envisioned using this culture approach (Fig. 3; top). While these examples are not exhaustive, they demonstrate the importance of uncovering the relationship of each cell to other cells within a clone in order to elucidate mechanisms of self-renewal. Importantly, the in vivo relevance of culture findings must be confirmed with a functional assay (Fig. 3; bottom). The single cell analyses of cell division and differentiation behavior in bioengineered niches are most informative if performed in conjunction with in vivo transplantation assays to determine the role of specific niche proteins in regulating muscle stem cell fate and function (see for example60,61).
Fig. 3. In vivo validation of cell fates predicted from culture studies.
(Top) Cell fate map predictions. Examples of genealogical trees and phenotypic analysis indicating Pax7+MyoD− muscle stem cells (black circles) and Pax7+MyoD+ transit amplifying progenitor cells (white circles). To distinguish the phenotypic outcomes displayed in C and D or E and F cell fate mapping is required, while A and B could be differentiated using phenotypic analysis alone. (Bottom) Validation of cell fate using in vivo functional assays. Single stem cells are spatially segregated in hydrogel microwell arrays and followed by timelapse microscopy. Microwells containing cells that display specific behaviors can be harvested by micromanipulation and used in functional assays in mice to validate their predicted fate.
Substrate rigidity regulates muscle stem cell self-renewal
Recent studies applying the single cell approach described above demonstrated that substrate rigidity profoundly impacts muscle stem cell self-renewal.61 The profound impact of substrate biophysical properties on cell fate was documented in early work by the Bissell Laboratory in the course of their studies, which showed the profound impact of the extracellular matrix on mammary gland differentiation.73,74 PEG hydrogels not only allow for regulation of the identity and presentation of biochemical cues presented to stem cells, but they also allow the user to modulate the stiffness, or biophysical cues of the culture substrate. Therefore, single MuSCs spatially segregated in arrays of hydrogel microwells of variable stiffness were analyzed by time-lapse microscopy. The microwell platform is designed to perform high throughput experiments; however, automation of time-lapse video analysis is a prerequisite in order to use the platform for screening applications, as analysis is a major bottleneck. Towards this goal, an automated computer algorithm called the ‘Baxter Algorithm’ was developed specifically for this purpose (Fig. 4).61 The software automatically finds the trajectories of the cells, their outlines in all frames and the lineage trees representing the mother-daughter relationships between them. Critical to the derivation of the lineage relationships was the maintenance of cells within a single field of view. As MuSCs are a highly migratory cell type, this critical element was achieved through the use of microwells that were microcontact printed with ‘islands’ of ECM protein. The generated tracking data could then be processed using analysis and visualization tools in the software, to give information about proliferation, death, cell morphology, cell migration and other parameters of interest. We also created a user interface for manual editing of cell trajectories, which enabled us to produce 100% accurate cell trajectories much faster than they could have been created manually. In vitro analysis demonstrated that MuSCs cultured on soft hydrogel substrates that mimic the in vivo mechanical properties exhibited improved survival and decreased differentiation, when compared to MuSC cultured on tissue culture plastic, which is five orders of magnitude more rigid than muscle tissue.
Fig. 4. The Baxter Algorithm.

The Baxter Algorithm provides analysis of time-lapse videos allowing the user to rapidly assess the effect of biochemical and biomechanical parameters on single cell behavior during clonal outgrowth while maintaining lineage relationships. Here we show a representative example of a microwell containing a clone borne from a single MuSC after 69 h of culture (right) in which the Baxter Algorithm established the genealogical relationships between each cell automatically by analyzing time-lapse sequences (left).
To test whether these in vitro observations translated to improved in vivo functionality of cultured MuSCs, functional assays in mice were performed.75 MuSCs were isolated from animals expressing GFP and Luciferase by flow cytometry, cultured for one week on culture substrates of increasing stiffness and subsequently 100 cultured MuSCs were transplanted into the irradiated hindlimbs of recipient mice. After one month, animals were analyzed for bioluminescence to determine engraftment. Strikingly, it was determined that MuSCs cultured on compliant substrates (2 or 12 kPa) promoted higher and more engraftment than those cultured on stiff matrices. Most importantly, it was noted that the culture condition that consistently promoted the most engraftment events was hydrogel that mimicked the elastic modulus of endogenous skeletal muscle tissue. This data constituted the first example of a culture condition that resulted in a population of MuSCs that are competent to regenerate skeletal muscle tissue with high efficiency using low numbers of transplanted cells.61
To determine whether hydrogel culture could permit the self-renewal of MuSCs in culture, the following experimental strategy was utilized: Single MuSCs were spatially segregated in hydrogel microwell arrays with pliant (12 kPa) or stiff (~106 kPa) bottoms and a tiled image of the entire array was acquired immediately after plating the cells to determine those wells that contained a single cell (see Fig. 3; bottom). After ~2–3 days a second tiled image was acquired and those wells that contained two cells (doublets) that arose from a single cell were identified. If a MuSC underwent division and was still able to engraft into regenerating skeletal muscle, this would indicate that at least one of the cells had retained ‘stemness’ and that a self-renewal event had occurred (symmetric or asymmetric division). As such, GFP/Luciferase expressing MuSC doublets were collected and pooled by micromanipulation (10 total cells) and transplanted into the irradiated hindlimb of recipient mice and assayed for contribution to skeletal muscle regeneration after one month. Notably, engraftment of MuSC doublets derived on stiff culture substrates was never observed, but ~25% of animals transplanted with doublets from pliant substrates contained donor derived skeletal muscle fibers—demonstrating the ability of these cells to regenerate muscle tissue. This data demonstrated for the first time that mechanical properties regulate the ability of MuSCs to self-renew in culture. In addition, this work concluded that the regenerative potential of MuSCs cultured on plastic is rapidly lost (within 2–3 days, a single division). It further demonstrates the potential of bioengineering approaches to provide mechanistic insights into the regulation of stem cell self-renewal when combined with in vivo assays to validate function.
Conclusions
Single cell analyses are revolutionizing our understanding of the factors that impact stem cell fate and function. Such studies are critically important as the behavior of individual cells is masked at the population level, because rapidly dividing cells outgrow slow dividing cells and important information is lost. The bioengineered microwell platform for designing artificial niches described here presents numerous exciting possibilities for probing as yet unanswered questions by enabling single cell analyses on a large scale. Single biomechanical and biochemical parameters can be altered to investigate the influence of ‘niche components’ on stem cell fate and function. Further, while cell fate can be followed in genealogical studies employing C. elegans and Drosophila, such opportunities have been challenging for mammalian cell studies. Single cell time-lapse microscopy of cell division behavior and subsequent analysis using the Baxter Algorithm now enables such studies. Changes in cell division behavior can be acquired in an automated fashion with a low error rate in a manner previously not possible. Thus, using this approach, hypotheses regarding the roles of asymmetric and symmetric self-renewal on fate can be directly tested for specific stem cells under well-defined conditions. Finally, by transplanting low numbers of cells in vivo after culture, the influence of culture parameters can be tested in the most rigorous test of stem cell function, reconstitution of the stem cell pool in an animal. Insights gained as a result of the interdisciplinary scientific approach described herein promise to provide novel therapeutics for regenerative medicine based on regulating stem cells within their native niche or ex vivo for cell based therapies. We hope that this article will inspire stem cell biologist to probe old questions in new ways, shedding light on unresolved questions.
Insight, innovation and integration.
Adult stem cells, including muscle stem cells (MuSCs), reside within a complex three-dimensional microenvironment that dynamically exerts influence over cell fate. Efforts to elucidate the mechanisms underlying MuSC self-renewal have been hindered by the inability to culture this cell type immediately after isolation without loss of stemness. Using an innovative bioengineered hydrogel culture platform together with functional assays in mice, we recently demonstrated that substrate rigidity, a property of the native stem cell niche, is a potent modulator of MuSC self-renewal. This work was enabled by evaluation of the clonal behavior of single MuSCs in culture over time, which required integrating our biomaterials approach together with a novel computer algorithm that rapidly analyzes timelapse microscopy videos in an automated manner. It is fitting to write about the influence of rigidity on stem cell fate in an issue of Integrative Biology dedicated to Mina Bissell as she pioneered studies highlighting the effect of substrate biophysical properties (e.g. 3D Matrigel) on cell fate.
Acknowledgements
This work was financially supported by PHS CA09151, CIRM TG2-01159, and NIH grant 1K99AR061465 (P.M.G.); NSF and NIH Training Grant 2 T32 HD007249 (K.H.); John och Karin Engbloms Stipendiefond (K.E.G.M.) and NIH grants HL096113, HL100397, AG020961, and AG009521, JDRF grant 34-2008-623, MDA grant 4320, CIRM grant RT1-01001, LLS grant TRP 6025-09, Fulbright Specialist Grant from the CIES, Mayent/Rothschild Fellowship and the Baxter Foundation (H.M.B.). As this review is of a focused nature, we offer apology to those whose related work might not have been cited.
Footnotes
Published as part of an iBiology themed issue entitled “From Single Cells to Biology” Editors: Dr Mina Bissell, Distinguished Scientist, and Prof Luke Lee.
References
- 1.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116(6):769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 2.Osawa M, et al. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273(5272):242–5. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 3.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287(5457):1427–30. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 4.Krosl JK, Faubert A, Sauvageau G. Molecular basis for stem-cell self-renewal. The hematology journal: the official journal of the European Haematology Association/EHA. 2004;5(Suppl 3):S118–21. doi: 10.1038/sj.thj.6200436. [DOI] [PubMed] [Google Scholar]
- 5.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441(7097):1068–74. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 6.Lam N, Chesney MA, Kimble J. Wnt signaling and CEH-22/tinman/Nkx2.5 specify a stem cell niche in C. elegans. Curr. Biol. 2006;16(3):287–95. doi: 10.1016/j.cub.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiger AA, et al. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294(5551):2542–5. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 8.Tran J, Brenner TJ, DiNardo S. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature. 2000;407(6805):754–7. doi: 10.1038/35037613. [DOI] [PubMed] [Google Scholar]
- 9.Kiger AA, White-Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407(6805):750–4. doi: 10.1038/35037606. [DOI] [PubMed] [Google Scholar]
- 10.Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290(5490):328–30. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 11.Gupta P, et al. Structurally specific heparan sulfates support primitive human hematopoiesis by formation of a multimolecular stem cell niche. Blood. 1998;92(12):4641–51. [PubMed] [Google Scholar]
- 12.Reya T, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 13.Tumbar T, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303(5656):359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimura EK, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416(6883):854–60. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 15.Gordon JI, Schmidt GH, Roth KA. Studies of intestinal stem cells using normal, chimeric, and transgenic mice. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1992;6(12):3039–50. doi: 10.1096/fasebj.6.12.1521737. [DOI] [PubMed] [Google Scholar]
- 16.Kim KM, Shibata D. Methylation reveals a niche: stem cell succession in human colon crypts. Oncogene. 2002;21(35):5441–9. doi: 10.1038/sj.onc.1205604. [DOI] [PubMed] [Google Scholar]
- 17.Doetsch F. A niche for adult neural stem cells. Curr. Opin. Genet. Dev. 2003;13(5):543–50. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317(5845):1722–6. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 19.Mauro A. Satellite cell of skeletal muscle fibers. J. Cell Biol. 1961;9:493–5. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins CA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122(2):289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15(12):666–73. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Laterveer L, et al. Interleukin-8 induces rapid mobilization of hematopoietic stem cells with radioprotective capacity and long-term myelolymphoid repopulating ability. Blood. 1995;85(8):2269–75. [PubMed] [Google Scholar]
- 23.Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88(3):287–98. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- 24.Schofield R. The stem cell system. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 1983;37(8):375–80. [PubMed] [Google Scholar]
- 25.Yamashita YM, et al. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315(5811):518–21. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanpain C, et al. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118(5):635–48. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Potten CS. Early and late incorporation of tritiated thymidine into skin cells and the presence of a long-lived G 0 -specific precursor pool. J. Cell Biol. 1971;51(3):855–61. doi: 10.1083/jcb.51.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potten CS, Kovacs L, Hamilton E. Continuous labelling studies on mouse skin and intestine. Cell and tissue kinetics. 1974;7(3):271–83. doi: 10.1111/j.1365-2184.1974.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 29.Bickenbach JR. Identification and behavior of label-retaining cells in oral mucosa and skin. J. Dent. Res. 1981;60(Spec No C):1611–20. doi: 10.1177/002203458106000311011. [DOI] [PubMed] [Google Scholar]
- 30.de Lau W, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476(7360):293–7. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 31.Leedham SJ, et al. Intestinal stem cells. J. Cell. Mol. Med. 2005;9(1):11–24. doi: 10.1111/j.1582-4934.2005.tb00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan SH, et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One. 2011;6(3):e17540. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311(5769):1880–5. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 35.Dykstra B, et al. High-resolution video monitoring of hematopoietic stem cells cultured in single-cell arrays identifies new features of self-renewal. Proc. Natl. Acad. Sci. U. S. A. 2006;103(21):8185–90. doi: 10.1073/pnas.0602548103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamashita YM, Fuller MT, Jones DL. Signaling in stem cell niches: lessons from the Drosophila germline. J. Cell Sci. 2005;118(Pt 4):665–72. doi: 10.1242/jcs.01680. [DOI] [PubMed] [Google Scholar]
- 37.Kaltschmidt JA, et al. Rotation and asymmetry of the mitotic spindle direct asymmetric cell division in the developing central nervous system. Nat. Cell Biol. 2000;2(1):7–12. doi: 10.1038/71323. [DOI] [PubMed] [Google Scholar]
- 38.Le Grand F, et al. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4(6):535–47. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuang S, et al. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129(5):999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428(6982):564–9. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- 41.Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304(5675):1331–4. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- 42.Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev. Cell. 2007;12(2):195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Simon A, Frisen J. From stem cell to progenitor and back again. Cell. 2007;128(5):825–6. doi: 10.1016/j.cell.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 44.Holterman CE, Rudnicki MA. Molecular regulation of satellite cell function. Semin. Cell Dev. Biol. 2005;16(4-#x2013;5):575–84. doi: 10.1016/j.semcdb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Seale P, et al. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102(6):777–86. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 46.Zammit PS, et al. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biol. 2004;166(3):347–57. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J. Histochem. Cytochem. 2006;54(11):1177–91. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- 48.Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr. Opin. Genet. Dev. 2006;16(5):525–32. doi: 10.1016/j.gde.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Shea KL, et al. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell. 2010;6(2):117–29. doi: 10.1016/j.stem.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palacios D, et al. TNF/p38alpha/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell. 2010;7(4):455–69. doi: 10.1016/j.stem.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cosgrove BD, et al. A home away from home: challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation. 2009;78(2-3):185–94. doi: 10.1016/j.diff.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell. 2002;3(3):397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 53.Shinin V, et al. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat. Cell Biol. 2006;8(7):677–87. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- 54.Collins CA, et al. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25(4):885–94. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- 55.Brack AS, et al. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2(1):50–9. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Brack AS, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317(5839):807–10. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 57.Allen RE, et al. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J. Cell. Physiol. 1995;165(2):307–12. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- 58.Anderson JE, Wozniak AC. Satellite cell activation on fibers: modeling events in vivo–an invited review. Can. J. Physiol. Pharmacol. 2004;82(5):300–10. doi: 10.1139/y04-020. [DOI] [PubMed] [Google Scholar]
- 59.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462(7272):433–41. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lutolf MP, et al. Perturbation of single hematopoietic stem cell fates in artificial niches. Integr. Biol. 2009;1(1):59–69. doi: 10.1039/b815718a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gilbert PM, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329(5995):1078–81. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Massia SP, Hubbell JA. An RGD spacing of 440 nm is sufficient for integrin alpha V beta 3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. J. Cell Biol. 1991;114(5):1089–100. doi: 10.1083/jcb.114.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kouvroukoglou S, et al. Endothelial cell migration on surfaces modified with immobilized adhesive peptides. Biomaterials. 2000;21(17):1725–33. doi: 10.1016/s0142-9612(99)00205-7. [DOI] [PubMed] [Google Scholar]
- 64.Alberti K, et al. Functional immobilization of signaling proteins enables control of stem cell fate. Nat. Methods. 2008;5(7):645–50. doi: 10.1038/nmeth.1222. [DOI] [PubMed] [Google Scholar]
- 65.Fan VH, et al. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25(5):1241–51. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- 66.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 67.Rieger MA, Schroeder T. Exploring hematopoiesis at single cell resolution. Cells Tissues Organs. 2008;188(1-2):139–49. doi: 10.1159/000114540. [DOI] [PubMed] [Google Scholar]
- 68.Schroeder T. Tracking hematopoiesis at the single cell level. Ann. N. Y. Acad. Sci. 2005;1044:201–9. doi: 10.1196/annals.1349.025. [DOI] [PubMed] [Google Scholar]
- 69.Schroeder T. Imaging stem-cell-driven regeneration in mammals. Nature. 2008;453(7193):345–51. doi: 10.1038/nature07043. [DOI] [PubMed] [Google Scholar]
- 70.Schroeder T. The electronic crystal ball: predicting cell fate from time-lapse data. Nat. Methods. 2010;7(3):190–1. doi: 10.1038/nmeth0310-190. [DOI] [PubMed] [Google Scholar]
- 71.Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457(7231):896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 72.Rieger MA, et al. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325(5937):217–8. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- 73.Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc. Natl. Acad. Sci. U. S. A. 1994;91(26):12378–82. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petersen OW, et al. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 1992;89(19):9064–8. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sacco A, et al. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456(7221):502–6. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]