Abstract
Objective
To identify the incidence of tuberculosis (TB) in HIV-infected children in a resource-limited setting prior to and after antiretroviral therapy (ART) initiation. A secondary objective was to assess the impact of TB screening by Tuberculin skin testing (TST) and clinical history.
Methods
A retrospective cohort study of 1806 HIV-infected children and adolescents initiating ART from 2003 through July 1, 2006 in Kampala, Uganda. A TB screening program was instituted clinic-wide in January 2006.
Results
311 (17.2%) HIV-infected children had with TB diagnosed among 171 diagnosed pre-ART and among 140 post-ART initiation. During the first 100 days of ART, risk of a new TB diagnosis was 2.7 fold higher compared to the pre-ART period (RR 2.7; 95% CI: 2.1 to 3.5; P<.001). After 100 days of ART, the TB incidence rate decreased below pre-ART levels (RR 0.41; 95% CI: 0.30 to 0.54; P=.002).
After TB screening was instituted in 2006, the proportion of new TB cases diagnosed after starting ART decreased by 70% (95% CI: 51 to 82%; P<.001), abating the early excess risk.
Conclusions
TB is common among African children and adolescents initiating ART in Sub-Saharan Africa. More aggressive screening for active TB prior to starting ART can diminish the rate of TB during immune reconstitution. Future studies are needed to determine optimal screening practice for HIV-infected children.
Keywords: Tuberculosis, HIV, AIDS, children, antiretroviral therapy
BACKGROUND
In high burden countries, children count for more than 20% of tuberculosis (TB) cases (1). After TB exposure, children are at higher risk than adults to develop TB (2,3). HIV subjects are more susceptible to re-activation of latent infection or to new infections (4). Worldwide TB is the most frequent co-infection and the leading cause of mortality and morbidity in HIV-infected individuals (5).
Antiretroviral therapy (ART) has improved the management of pediatric HIV disease(5). However, TB in HIV-infected children remains under-reported due both to the emphasis of TB control programs on smear-positive TB (rare in children) and to difficulties in diagnosing TB in children(5;6). TB diagnosis is primarily based on the history of a TB-contact (adult smear positive patient), symptoms and signs of TB, a positive tuberculin skin test (TST), and/or an abnormal chest radiography (4).
We described the incidence of TB pre- and post-ART in a large urban pediatric HIV-infected cohort starting ART in Uganda. We also studied the effect of pre-ART screening for active TB on the emergence of TB post-ART.
METHODS
Patients and Setting
This was a retrospective cohort study among HIV-infected children and adolescents at the Paediatrics’ Infectious Diseases Clinic (PIDC) of Mulago Hospital at Makerere University in Kampala, Uganda. The PIDC provides HIV care for approximately 5000 children and adolescents and since 2003, the clinic has offered integrated TB-HIV treatment. Charts from all children on ART and anti-TB treatment attending the PIDC were reviewed between January 1, 2003 through July 1, 2006. The clinical outcomes were assessed through January 1, 2008. Starting January 2006, all new patients were screened for active or latent TB infection.
Data were extracted from the electronic clinic records including age, gender, CD4 counts, ART regimen, clinical presentation of TB, date of TB diagnosis and ART initiation. Immunosuppression was defined by WHO criteria by age(7) (see Appendix). Ninety six percent (300/311) of the children were receiving first line ART either zidovudine/lamivudine (AZT/3TC) or stavudine (d4T)/3TC as nucleoside reverse transcriptase inhibitor (NRTI) backbone with a non-nucleoside reverse transcriptase inhibitor (NNRTI), either nevirapine (NVP) or efavirenz (EFV).
Appendix.
WHO Immunologic classification for HIV infection in children
| HIV-associated immunodeficiency | Age-related CD4 values | |||
|---|---|---|---|---|
| <11 months (%CD4+) | 12–35 months (%CD4+) | 36–59 months (%CD4+) | >5 years (absolute number per mm3 or %CD4+) | |
| None or not significant | >35 | >30 | >25 | > 500 |
| Mild | 30–35 | 25–30 | 20–25 | 350–499 |
| Advanced | 25–29 | 20–24 | 15–19 | 200–349 |
| Severe | <25 | <20 | <15 | <200 or <15% |
Written informed consent by a child’s guardian and assent by adolescents was obtained prior to cohort enrollment. All medical record data were prospectively entered into an Oracle database. Institutional Review Board approval was obtained from Makerere University and Baylor College of Medicine.
TB Diagnosis
There was no standardized protocol for TB diagnosis. Diagnostic modalities utilized a combination of clinical history and exam, radiographic imaging, TST, and Ziehl-Neelsen staining for acid-fast bacilli (AFB) of sputum and/or biopsy specimens. Mycobacterial cultures were unavailable. All children diagnosed with active TB received anti-TB therapy according to the Ugandan national guidelines. Screening for TB using TST (1:1000 dilution) and clinical history was implemented as a routine clinic procedure since January 2006. For pulmonary TB, a minimum of 2 or more signs and symptoms merited radiographic evaluation. As well, children with a positive TST (> 5mm at 48 hours) thereafter were evaluated for active TB using chest radiographs and sputum examination. Children without active TB were initiated on isoniazid prophylaxis at 15mg/kg/day for 6 months.
Statistics
SPSS 15.0 (Chicago, IL) was used for statistical analysis. Normally distributed data are presented as mean ± standard deviation (SD), with comparison via the t-test. Non-normally distributed data are presented with median and interquartile range (IQR) with comparisons made via the non-parametric Mann-Whitney U test. Further investigation of univariate significant variables with multivariate Cox proportional hazard regression analysis estimated a hazard ratio (HR) with 95% confidence interval (95% CI). The cumulative exposure time was calculated from the time of clinic registration to ART initiation, TB diagnosis, or end of the study period (July 1, 2008). The TB pre-ART incidence rate was estimated from the number of TB diagnoses versus exposure time pre-ART in person-years. After ART initiation, ART exposure time was calculated to TB diagnosis or the end of the study period.
RESULTS
Subject characteristics
Among 1806 children and adolescents initiating ART, 311 (17.2%) were diagnosed with TB before and/or after starting ART. The average age was 6.4±4.7 years with equal sex representation (50% boys, 50% girls). Advanced HIV was common in those diagnosed with TB with baseline CD4% <15% present in 89% and absolute CD4 counts <200 cells/μL present in 43% (missing CD4 n=21). The median CD4 was 7.8% (IQR: 3.1 to 11.6%) with absolute CD4 260 cells/μL (IQR: 75 to 510 cells/μL) among all children diagnosed with TB. Cohort demographics are presented in Table 1.
Table 1.
Cohort Demographics of HIV-infected children initiated on ART
| Variables | children with TB (n=311) | Children without TB (n=1494) | |
|---|---|---|---|
| Age (years) | mean ± SD | 6.6 ± 4,9 | 9.2±4.5 |
| Female sex | n (%) | 155 (50) | 718 (48) |
| CD4 (% T-cell) | median (IQR) | 7.8 (3.2;11.6) | 8.6 (3.5; 12.7) |
| CD4 T-cell count | median (IQR) | 256 (75;510) | 272 (8 to 516) |
| Clinic registration to ART (weeks) | mean ± SD | 27 ± 25 | N/A |
| Time on HAART (weeks) | median (IQR) | 46 ± 28 | N/A |
SD: standard deviation; IQR: interquartile range
N/A = not available
Tuberculosis pre-ART and post-ART
TB was diagnosed in 171 (9.5%) children pre-ART and 140 children after ART initiation (8.6% of cohort without TB) among the 1806 children who started ART. Pre-ART, the median time from clinic registration to TB diagnosis was 5.5 weeks (IQR: 2.0–19) for those with severe immune suppression (n=137) but 14 weeks (IQR: 4.5–36) among those with non-severe (n=34) immune suppression by WHO age criteria (P=.047). Thus prior to initiation, TB hazard risk was related to the degree of immunosuppression. Cox regression analysis, which controlled for CD4 revealed that every year of age decreased the risk of TB by 6.7% (95% CI: 1.9% to 9.3%; P=.003). In the Pre-ART period, the TB incidence was 10 cases per 100 person-years of clinic time, from the time of clinic registration to developing TB or starting ART.
After ART initiation, the median time from the start of ART to TB diagnosis was 9.9 weeks (IQR: 5.9 to 19.9) for those with severe immune suppression (n=102) and 14.6 weeks (IQR: 8.1 to 33.6) among those with moderate immune suppression (n=38) (P=.074). Baseline CD4 and type of ART prescribed were not associated with risk of TB. Further investigation by Cox regression, which controlled for the ART start year (see below) indicate that each percent increase in CD4% decreased the TB hazard by 4% (HR = 0.96; 95% CI: 0.93 to .999; P = .046). Thus after ART initiation, the majority of TB occurred within the first three months, and there was not a statistical difference in the timing of TB based on the degree of immunosuppression.
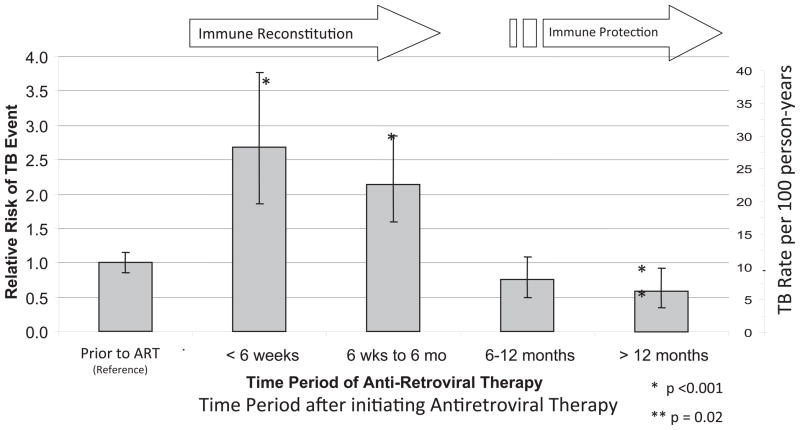
During the first 100 days of ART, the risk of a new TB diagnosis was 2.7 fold higher compared to the pre-ART period (RR = 2.7; 95% CI: 2.1 to 3.5; P<.001). After 100 days of ART, the TB incidence rate decreased below the pre-ART level (RR = 0.41; 95% CI: 0.30 to 0.54; P=.002). The risk of TB remained elevated through 6 months after starting ART (RR = 2.0; 95% CI: 1.6 to 2.6; P=.002) but decreased thereafter (RR = 0.29; 95% CI: 0.21 to 0.41; P=.002) (Figure 1). No baseline demographic differentiated those being diagnosed with TB prior to or after starting ART (P> 0.2). Overall, there appeared to be an excess risk of TB early after ART initiation.
Figure 1.
Incidence of active TB infection in a Ugandan pediatric HIV cohort
Figure 1. Relative Risk is presented with the 95% C.I. of the rate ratio of the Standardized Morbidity Ratio (SMR) with the reference incidence rate being TB diagnosis prior to starting HIV antiretroviral therapy (ART). In the first few months of HIV therapy, the incidence of TB diagnosis is increased compared to the pre-ART period (P<.001). After 6 months of HIV therapy, the incidence of TB decreases with a statistically significant decrease after 12-months of HIV therapy (P=.02). This apparent increase likely is the result of undiagnosed sub-clinical active TB infection. Instituting TB screening pre-ART had a relative reduction by 70% the proportion of TB diagnosed after ART initiation decreasing the SMR to near or slightly below the pre-ART baseline.
Increased TB Screening Policy
Since January 2006, all PIDC patients were screened for TB infection using history and TST. Children with a positive TST thereafter were evaluated for active TB including chest radiograph. Older children (age >6 years) were encouraged to produce sputa for AFB smear. This screening intervention led to a marked reduction in new TB cases after starting ART during 2006 (Table 2). TB screening was associated with a 70% relative risk reduction (RRR) in TB (95% CI: 51 to 82%; P< .001). This TB screening program abated the apparent excess risk of early TB unmasked after ART initiation.
Table 2.
Effect of screening for tuberculosis infection on the subsequent proportion of TB diagnosed during ART
| Time of TB Diagnosis | 2003 – 2005 without TB screening (n=227) | 2006 with TB screening (n=84) | P-value |
|---|---|---|---|
| TB pre-ART initiation | 101 | 70 | |
| TB post-ART initiation | 126 | 14* | |
| Proportion TB Diagnosed on ART | 56% | 17% | < .001 |
through January 1, 2008.
Survival Outcomes
Children with TB had higher mortality than the 1806 child pediatric cohort. As of January 1, 2008, 77% (238/311) HIV-infected children with TB were alive and receiving ongoing care. An additional 5% (15/311) had transferred care to another clinic. Thirty-three children (10.6%) were known to have died at a median of 10 weeks (IQR: 4 to 26 weeks, min 5 days, max 36 months) after starting ART with 62% dying in the first 60 days of ART. There was no mortality difference whether TB was diagnosed prior to (12.6%) or after ART initiation (10.6%). However, TB diagnosed after 6 months of ART only had one (3%) death. Risk of death in children with TB diagnosis was two-fold higher than in other cohort children without TB (5.2%) receiving ART (RR=2.0; 95% CI: 1.1 to 4.0; P=.04). However, this study did not include those who died of TB and never started ART.
Twenty five children with TB (7.7%) were lost to follow up. Four children were lost to follow up within 14 days of starting ART and likely died at home. There were 21 other children lost to follow up after at a median of 10 months (IQR: 6.5 to 15.6 months).
DISCUSSION
In an urban Ugandan pediatric cohort, we report that 9.5% of children were diagnosed with TB prior to initiating ART and an additional 8.3% diagnosed with TB after ART initiation. Without TB screening, there was 2.5 fold excess incidence of new TB diagnosis in the first few months after ART initiation. We believe this excess burden of ART-associated TB that is diagnosed early after ART initiation represents a failure of screening. We demonstrate that with TB screening by history and TST that this excess incidence of active TB disease can be abated.
The diagnosis of pulmonary TB in children is inherently and notoriously difficult, in part, due to the inability of children ≤8 years to generate a productive sputum specimen(8). As the median age in our study was 6.6 years, the diagnostic modalities utilized were predominately non-invasive (80%). An appropriate clinical history and physical examination coupled with an abnormal radiograph and subsequent response to anti-tuberculous therapy is reasonable for a probable TB diagnosis in a resource-limited area where rates of TB are 100-fold higher than North America or Europe. Similarly, in the absence of culture, some AFB-positive biopsy specimens could have been Mycobacterium avium complex (MAC).
The outcomes in this cohort were similar to other published pediatric cohorts in Africa, thus we believe the findings should be generalizable. Specifically, the mortality after starting ART was 10.6% which was similar to the 8–14% mortality reported in two South African cohorts (9,10). In other pediatric cohorts, rates of virologic suppression have been quite variable in children with TB who were initiated on ART. Yotebieng et al reported 90% virologic suppression in a cohort when measuring at a median of 5.5 months (9). Conversely, Reitz et al reported 20% lower incidence of viral suppression in children receiving co-treatment for TB before ART initiation and 42% lower incidence of viral suppression in children who started anti-TB treatment after ART initiation as compared to children without TB diagnosis (94% viral suppression) by 39 weeks. (10). In this TB HIV co-infected cohort, virologic monitoring was not routinely assessed, so we were unable to report the virologic outcome measures. In a separate research cohort drawn from this Ugandan cohort, the overall incidence of viral suppression was 84% at one-year (11).
In a cohort study in Cambodia of HIV-infected children, nearly half of the pre- or post-ART deaths were attributed to TB disease (12). In our study the risk of developing TB pre-ART, but not post-ART, was related to the degree of immune deficiency. During the first 100 days of ART, the risk of a new TB diagnosis was higher compared to the pre-ART period. We also showed that pre-ART screening for active TB was associated with a 70% relative risk reduction in TB post ART.
Our study has some limitation. This was a retrospective study based on chart review. Retrospective analysis encompasses certain inherent biases. The majority of TB diagnoses in this pediatric cohort were based only on clinical and radiographic findings. TB was not confirmed by culture. In Sub-Saharan Africa, we do not believe this is an over-diagnosis. Rennert et al’s autopsy study found that 77% of South Africa HIV-infected children with post-mortem diagnosed TB had not been diagnosed or treated for TB while alive (13).
CONCLUSION
TB is common among African children and adolescents initiating ART in Sub-Saharan Africa. More aggressive screening for active TB prior to starting ART can diminish the rate of TB during immune reconstitution. Future pediatric studies are needed to determine optimal methods of screening for TB in HIV-infected children in resource-limited areas. Pediatric ART programs should include the resources necessary to conduct TB screening and care with integration of TB and HIV services.
Acknowledgments
The authors wish to thank the PIDC-Mulago clinic, the Baylor College of Medicine Children’s Foundation-Uganda, staff, patients, and care givers.
DRB has received support from the NIH L30AI066779; K23AI073192; T32AI055433.
Footnotes
This abstract was presented at the Infectious Diseases Society of American 2007 Annual Meeting in San Diego, CA.
The authors have no conflicts of interest.
References
- 1.Donald PR, Maher D, Qazi S. A research agenda to promote the management of childhood tuberculosis within national tuberculosis programmes. Int J Tuberc Lung Dis. 2007;11(4):370–80. [PubMed] [Google Scholar]
- 2.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8(4):392–402. [PubMed] [Google Scholar]
- 3.Rieder HL. Epidemiology of tuberculosis in children. Annales Nestle. 1997;55:1–9. [Google Scholar]
- 4.Swaminathan S. Tuberculosis in HIV-infected children. Paediatr Respir Rev. 2004;5(3):225–30. doi: 10.1016/j.prrv.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Martinson NA, Moultrie H, van NR, Barry G, Coovadia A, Cotton M, et al. HAART and risk of tuberculosis in HIV-infected South African children: a multi-site retrospective cohort. Int J Tuberc Lung Dis. 2009;13(7):862–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Moyo S, Verver S, Mahomed H, Hawkridge A, Kibel M, Hatherill M, et al. Age-related tuberculosis incidence and severity in children under 5 years of age in Cape Town, South Africa. Int J Tuberc Lung Dis. 2010;14(2):149–54. [PubMed] [Google Scholar]
- 7.WHO. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report. 2007 [cited 2007 Oct 3];Available from: URL: http://www.who.int/hiv/mediacentre/universal_access_progress_report_en.pdf.
- 8.Graham SM, Coulter JB, Gilks CF. Pulmonary disease in HIV-infected African children. Int J Tuberc Lung Dis. 2001;5(1):12–23. [PubMed] [Google Scholar]
- 9.Yotebieng M, Van Rie A, Moultrie H, Cole SR, Adimora A, Behets F, Meyers T. Effect on mortality and virological response of delaying antiretroviral therapy initiation in children receiving tuberculosis treatment. AIDS. 2010;24(9):1341–9. doi: 10.1097/QAD.0b013e328339e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reitz C, Coovadia A, Ko S, Meyers T, Strehlau R, Sherman G, et al. Initial response to protease-inhibitor-based antiretroviral therapy among children less than 2 years of age in South Africa: effect of cotreatment for tuberculosis. J Infect Dis. 2010;201(8):1121–31. doi: 10.1086/651454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamya MR, Mayanja-Kizza H, Kambugu A, Bakeera-Kitaka S, Semitala F, Mwebaze-Songa P, et al. Predictors of long-term viral failure among ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;46(2):187–93. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 12.Raguenaud ME, Isaakidis P, Zachariah R, Te V, Soeung S, Akao K, et al. Excellent outcomes among HIV+ children on ART, but unacceptably high pre-ART mortality and losses to follow-up: a cohort study from Cambodia. BMC Pediatr. 2009;9:54. doi: 10.1186/1471-2431-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rennert WP, Kilner D, Hale M, Stevens G, Stevens W, Crewe-Brown H. Tuberculosis in children dying with HIV-related lung disease: clinical-pathological correlations. Int J Tuberc Lung Dis. 2002;6(9):806–13. [PubMed] [Google Scholar]