Abstract
Paraoxon (diethyl 4-nitrophenyl phosphate) is an active metabolite of the common insecticide parathion and is acutely toxic due to the inhibition of cholinesterase (ChE) activity in the nervous systems. The inhibition of butyrylcholinesterase (BChE) activity by paraoxon is due to the formation of phosphorylated BChE adduct, and the detection of the phosphorylated BChE adduct in human plasma can serve as an exposure biomarker of organophosphate pesticides and nerve agents. In this study, we developed an immunoaffinity purification and liquid chromatography-mass spectrometry (LC-MS) strategy for identifying phosphorylated BChE in human plasma treated by paraoxon. BChE was captured by biotinylated anti-BChE polyclonal antibodies conjugated to streptavidin magnetic beads. Western blot analysis showed that the antibody was effective to recognize both native and modified BChE with high specificity. Using a purified BChE protein, we initially identified the exact phosphorylation site on the serine residue (S198) with a 108 Da modification by both MS/MS and accurately measured parent ion masses and quantified the extent of phosphorylation on S198 following paraoxon treatment to be >99.9%. Then, the phosphorylated BChE peptide in paraoxon-treated human plasma following immunoaffinity purification was successfully identified based on the accurate measured mass and retention time information initially obtained from the purified BChE protein. Thus, immunoaffinity purification combined with LC-MS represents a viable approach for the detection and quantification of phosphorylated BChE as an exposure biomarker of organophosphates and nerve agents.
Keywords: butyrylcholinesterase, organophosphates, paraoxon, mass spectrometry, immunoaffinity purification
1. Introduction
Organophosphates (OPs) are primarily used as insecticides and chemical warfare agents worldwide [1]. OPs are highly toxic because they rapidly inactivate the cholinesterase (ChE) activity via the phosphorylation of the serine hydroxyl group located in the catalytic triad of the active center by OP agents [2, 3]. Acetylcholinesterase (AChE, EC 3.1.1.7) and butyrylcholinesterase (BChE, EC 3.1.1.8) are serine hydrolases in which the active center contains a serine residue [4, 5]. AChE is primarily responsible for regular nerve function by hydrolyzing the neurotransmitter acetylcholine (ACh), and the inhibition of AChE by OPs results in subsequent build-up of ACh which blocks cholinergic nerve impulses, leading to paralysis, suffocation and death [6]. BChE, also called pseudocholinesterase, catalyzes the hydrolysis of a variety of choline and noncholine esters in the same way as AChE [2, 7]. BChE is known to react more effectively than AChE with a broad range of OP toxicants [3] and thus has been suggested as an effective scavenger of OP toxicants [2]. Higher reactivity with OP compounds and relatively longer residence time (11-day half life in plasma [8]) makes BChE an ideal biomarker for monitoring OP poisoning [9–11].
Over the years, different biomarkers have been used as indicators of OP poisoning such as OP metabolites in blood and urine, decreased ChE activity in serum, free unbound OPs in blood, and OP-ChE adducts, and different analytical methods have been developed to identify them [10–12]. The traditional method uses an enzyme activity assay based on Ellman method [13], and a decrease in enzyme activity serves as an indicator of exposure severity. Although simple and rapid, this method requires information on baseline enzyme levels of individuals before exposure (pre-exposure levels) or a normal population level (in the absence of baseline levels) for comparison with post-exposure levels to obtain meaningful changes in enzyme activity. Since baseline enzyme levels of individuals are usually not available, clinical results using normal population levels as baselines might be inaccurate due to individual variation in enzyme levels. Measurements of OP metabolites by gas chromatography (GC) or GC-MS have been routinely practiced for last several years [14–16], but in some cases the results are confounded by the fact that metabolites might not be derived exclusively from exposure to OPs [12], and are often difficult to be positively identified due to their very short half-life [11]. Alternatively, direct detection of phosphorylated peptide from BChE at the catalytic site after proteolysis represents a promising means for assessing OP poisoning because the protein adduct has long-term presence in blood. Current LC-MS/MS instrumentations offer the potential for effective identification and quantification of the adduct peptide in complex samples.
Despite overall high sensitivity offered by MS technologies, the purification of BChE from complex plasma samples is still necessary due to the low abundance and low stoichiometry of phosphorylation. Recently, procainamide affinity gel purification and LC-MS/MS analysis following digestion has been developed to identify the OP-BChE adduct [11, 17–19]. This method is capable of separating both the unmodified and modified BChE, but is labor intensive and less suitable for high-throughput LC-MS/MS analysis. To overcome this limitation, an immunoaffinity purification method has recently been developed [10, 20] to facilitate the MS detection of the modified BChE. The detection of several OP-BChE adducts with different OP compounds has been demonstrated [10, 20].
In this work, we focused on the detection of phosphorylated BChE from human plasma treated by paraoxon. Paraoxon, a bioactive metabolite of the OP insecticide parathion [21], is used as a model compound for generating phosphorylated BChE adducts. Paraoxon is known as a ChE inhibitor [22] and has been commonly used as a model compound to study OP poisoning because of less toxicity (in rats, 50% lethal dose is 1.8 mg/kg compared to 0.001 mg/kg for O-ethyl S-[2–(diisopropylamino)ethyl] methylphosphonothioate; VX), and less volatility than many other OP compounds [23, 24].
To enable more effective detection of phosphorylated BChE in human plasma, we developed an improved antibody conjugation approach based on streptavidin-biotin interaction, which provided a higher affinity than the previous protein G antibody conjugation [10, 20]. In this approach, a polyclonal antibody for BChE was biotinylated with chromophore-linked biotin and conjugated to streptavidin magnetic beads to form a stable complex. Western blotting experiments confirmed effective purification of both modified and unmodified BChE with high recovery. Moreover, a two-stage MS detection strategy was explored. A BChE recombinant protein treated with paraoxon was used to initially confirm the exact site of phosphorylation occurred at the serine residue (S198) as well as the accurate masses and LC retention times of both the modified and unmodified BChE peptides. The accurate mass and retention time information allowed the detection of phosphorylated BChE peptide in human plasma by accurate mass measurements using high resolution MS instead of relying on MS/MS due to the potential under-sampling issue of low-abundance peptides in complex samples.
2. Experimental methods
2.1. Materials
The ChromaLink biotin labeling kit containing Sulfo-ChromaLink biotin 354 and Streptavidin magnetic beads (STAV) was purchased from SoluLink Bioscience Inc. (SanDiego, CA). The goat polyclonal anti-BChE antibody with epitope mapping near the N-terminal sequence of human BChE (N-15) (catalog# sc-46803) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The human plasma sample was from BioChemed Services Inc. (Winchester, VA). Paraoxon was from Chem Service Inc. (West Chester, PA). Human BChE, rabbit IgG antibody, dithiothreitol, iodoacetamide, urea, formic acid, ammonium bicarbonate (NH4HCO3) were all purchased from Sigma-Aldrich (St. Louis, MO). Modified porcine trypsin (sequencing grade) was from Promega (Madison, WI). All other chemicals were obtained from Sigma (St. Louis, MO) unless otherwise stated and were of analytical research grade.
2.2. Conjugation of antibody to biotin and streptavidin magnetic beads for complex formation (Mag_STAVD/BT_Ab_BChE)
Twenty µg (~100 µl) of the polyclonal anti-BChE antibody were loaded onto a micro Zeba™ desalting spin-column (Product# 89877, Pierce) and buffer-exchange was performed using a modification buffer (pH >8.5) provided by the manufacturer (SoluLink). For biotinylation, 20-fold molar ratio of the ChromaLink biotin to antibody was incubated for 90 min at room temperature. The extent of biotinylation was estimated by UV-absorption maxima at 280/354 nm in Du 640 Spectrophotometer (Beckman Instrument Inc., CA), and the stoichiometry between protein and biotin was calculated using the program calculator provided by the manufacturer.
The streptavidin magnetic beads (10 mg/ml) were suspended by sonication in a bath sonicator for 30 s and 20 µl of the suspension was incubated with 200 µl of blocking solution containing 2% albumin for 30 min at room temperature followed by washing three times with the washing buffer (50 mM Tris-HCl, 150 mM NaCl, 0.05% Tween, pH 8.0). Immediately after washing, 10 µg of biotin conjugated antibody in 120 µl phosphate buffered saline (PBS) was added to the magnetic beads and vortexed for 30 min at room temperature. The antibody coded-magnetic beads (Mag_STAVD/BT_Ab_BChE) were separated with a magnetic device. The solution was aspirated and examined for absorption measurements. The magnetic beads was washed three times with the washing buffer as above and suspended in 100 µl PBS-buffer.
2.3. Organophosphorylated BChE sample preparation
To obtain a model OP-BChE, 100 µl (~100 µg) of commercial BChE in water was mixed with 5 µl of 75 mM paraoxon in acetone (the final acetone concentration in the mixture is less than 5% to avoid protein precipitation). The reaction was allowed to proceed for 72 h at room temperature with gentle agitation. The resulting solution was dialyzed with water in a dialysis cassette with 3500 Da cut-off to remove acetone and unbound paraoxon. The enzyme activity during the reaction was determined according to Ellman’s method [13].
To generate OP-BChE in human plasma, 500 µl of human plasma sample with 1% protease inhibitor cocktail (Roche Applied Science, Germany) was pre-treated with 10 µl (10 µg) of streptavidin magnetic beads for 2 hours to deplete biotin in plasma. The biotin-free plasma was then treated with 9.2 µl of 75 mM paraoxon stock solution for 3 h at 4 °C followed by addition of another 4 µl of paraoxon stock solution. The sample was further incubated at 4 °C for 16 h. Enzyme activities of the samples before and after reaction with paraoxon were measured using Ellman’s method [13].
2.3 Purification of BChE and OP-BChE
For the purification of BChE from standard sample, 10 µg of either untreated or paraoxon treated BChE (OP-BChE) was suspended in 50 µl water and mixed with 20 µl of Mag_STAVD/BT_Ab_BChE complex conjugated with the polyclonal antibody (see above). The mixture was incubated at room temperature for 30 min with gentle shaking. Following separation from reaction solution, magnetic beads were washed with 100 µl of PBS for three times and treated with 50 µl of the elution buffer (70% acetonitrile containing 0.5% acetic acid) for 2 min to release the captured BChE from the complex. The solution was evaporated in a Speed-Vac and either subjected to one-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis (1D SDS-PAGE) or trypsin digestion for LC-MS/MS analysis.
To purify BChE from human plasma, ~100 µl of Mag_STAVD/BT_Ab_BChE complex was mixed with 500 µl of either untreated or paraoxon-treated plasma sample and incubated for 15 h at 4 °C with gentle rotation followed by 1 h incubation at the room temperature. After separating the complex with a magnetic device, the beads were washed once with PBST buffer (PBS buffer with 0.1% Tween 20; pH 7.4) and 500 mM NaCl and then three times with PBST without NaCl. The bound BChE was released with 40 µl of elution buffer, and the process was repeated one more time. The protein concentration of the combined eluate was determined using the BCA protein assay (Pierce, Rockford, IL)
2.4 Western blot analysis
Extracted BChE samples were lyophilized and re-suspended in the SDS-PAGE sample buffer (50 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 1% β-mercaptoethanol, and 0.01% bromophenol blue), and subjected to 1D SDS-PAGE analysis in a 4–12% pre-cast gradient gel (Invitrogen) and electrophoresed at 200 volts for 35 min with MES-SDS running buffer (Invitrogen). The resolved proteins were either silver stained [25] or transferred to PVDF membranes with the aid of a semi-wet transferring system (Bio-Rad) for western blotting. The membrane blots were blocked with a PBS/TBS super-blocking buffer (Thermo Scientific) including 1% polyvinylpyrolidone (PVP) and 2 mM Na3VO4, and immunoblotted with selected polyclonal antibodies. After washing with the washing buffer (50 mM Tris-HCl, 150 mM NaCl, 0.05% Tween, pH 8.0), the membranes were further incubated with the horseradish peroxidase-conjugated secondary antibodies. The membranes were subjected to chemiluminescence detection using a SuperSignal West Femto Chemiluminescent kit (Pierce/Thermo Scientific) and signals were detected by scanning in PDQuest GS-710 on a flatbed scanner.
2.5 Protein digestion
Standard BChE or purified BChE from human plasma were digested following the same protocol. 40 µl aliquots containing about 10 µg of purified BChE were lyophylized and reconstituted in 40 µl of 50 mM NH4HCO3 (pH 7.8). Proteins were denatured with 8 M urea for 30 min and reduced with 10 mM dithiothreitol for 30 min at 60°C, and alkylated with 25 mM iodoacetamide at room temperature in the dark for 1.5 h. The sample was diluted 10 fold with 50 mM NH4HCO3 and 5 µl trypsin freshly prepared in 50 mM NH4HCO3 (0.1 µg/µl) was added to the sample and the digestion was performed at 37 °C for 3 h with gentle shaking. The digested sample was desalted using Omix-C18 tips (Varian Inc.), and peptides from C18 tips were eluted twice, first in 10 µl of 50% acetonitrile, 0.1% acetic acid, and then in 10 µl of 90% acetonitrile, 0.1% acetic acid. The combined eluate was dried in a SpeedVac and reconstituted in 20 µl of 25 mM NH4HCO3 for LC- MS/MS analysis.
2.6 Capillary LC-MS/MS analysis
The peptide samples were analyzed using a custom built automated nano-flow, metal-free nanoLC system [26] with a 40-cm long, 50 µm i.d. capillary column packed (in-house) with 3 µm Jupiter (Phenomenex, Torrance, CA) C18 silica. An uncoated fused silica capillary pulled (5-µm at the capillary tip and 20 µm i.d. at the column junction) was joined to the column using a pico-clear union (both from New Objective, Woburn, MA). 5 µL sample was injected onto the column for each analysis and peptides were separated using a 150 min linear gradient form 0 to 70% of solvent B (mobile phase solvent A contained 0.1 M acetic acid in water and solvent B contained 0.1 M acetic acid in 100% acetonitrile) at a flow rate of 300 nl/min. Mass spectra were acquired using an LTQ-Orbitrap (ThermoScientific, San Jose, CA) that provided a 60,000 resolution for MS survey scans, followed by 10 MS/MS scans of the top 10 abundant parent ions, with a dynamic exclusion time of 30 s used for parent ion selection. The heated capillary was maintained at 200 °C, and the ESI voltage was held at 2.2 kV. MS/MS spectra were searched against the UniProt human database using the X!Tandem [27] algorithm to identify the enzyme using the following parameters: no enzyme rules, carboxamidomethylation of cysteine (57.02 Da) as a fixed modification, and monoethyl-phosphorylation (107.998 Da) and phosphorylation (79.966) on serine, threonine, and tyrosine residues as a variable modification. The maximum allowed mass tolerances for MS parent ions and MS/MS fragments were 5 ppm and 0.5 Da, respectively.
3. Results and discussion
3.1. Immunoaffinity enrichment of BChE
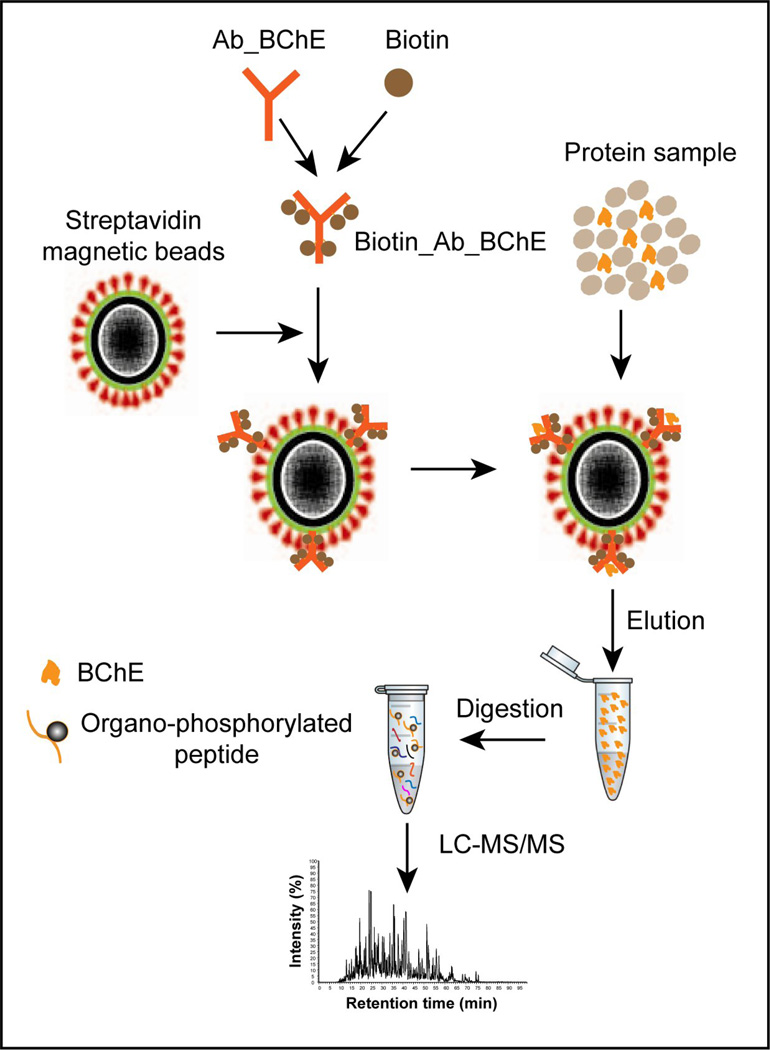
In order to specifically detect the phosphorylated BChE peptide in human plasma, target enrichment is an important step prior to LC-MS/MS analysis. Fig. 1 illustrates the overall strategy for immunoaffinity purification of BChE. Briefly, BChE is captured by streptavidin magnetic beads conjugated with biotin-anti-BChE antibodies, which bind to both the native and modified BChE. The proteins are then eluted for western blot and LC-MS/MS analyses to identify the modified peptide.
Figure 1.
A schematic illustration of the procedure for BChE extraction and LC-MS/MS analysis. Unmodified and paraoxon-modified BChE proteins were extracted using ChromaLink biotin-antibody complex with streptavidin magnetic beads and the extractable BChE was digested with trypsin and analyzed by LC-MS/MS.
To prepare the antibody-coded magnetic beads, the anti-BChE antibody was first labeled with the ChromaLink™ biotin protein labeling kit, which efficiently reacted with protein amine groups through the aromatic water soluble N-hydroxy-sulfosuccinimidyl ester functional group in the reagent. The reagent also contains a UV-traceable linker that allows for simple and direct UV quantification (at 354 nm) of total incorporated biotin on the antibodies. 10 to 20 µg of anti-BChE antibodies were biotinylated through the activated succinimidyl ester in an aqueous solution at pH 7.8. After removal of non-reactive reagents through a micro-desalting spin column, absorption maxima at 354 nm were recorded for biotin and at 280 nm for the presence of antibody (Supplementary Fig. S1A). Based on the absorption intensities and extinction coefficients, the mole ratio of biotin and BChE antibody was calculated as 6. The streptavidin magnetic beads with average size ~800 nm consist of an outer core hydrophilic polymer surface covalently cross-linked to streptavidin and encapsulate a paramagnetic core as designed by the manufacturer (SoluLink). The magnetic beads were used to immobilize biotinylated antibodies. The binding capacity of the streptavidin beads used in this study is approximately 2 nmole of the biotinylated antibodies per mg beads based on the manufacturer information. To evaluate the biotin capture efficiency of streptavidin magnetic beads, the biotinylated antibodies were incubated with calculated amounts of streptavidin magnetic beads and the complex thus formed was pulled aside the micro-tube wall with a magnetic device. Measurement of absorption in the clear supernatant showed disappearance of the absorption peaks at 280 and 354 nm (Supplementary Fig. S1B), indicating a complete complex formation with the antibodies.
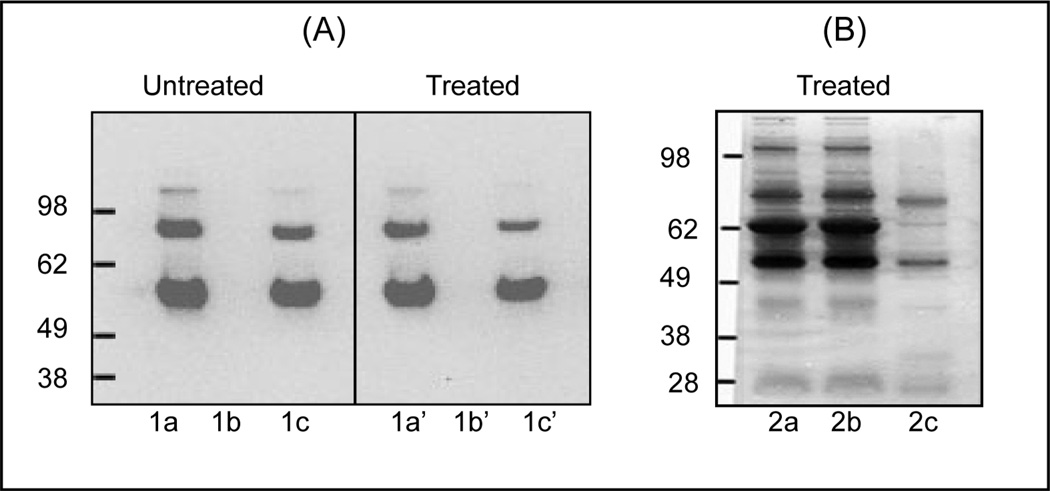
To optimize and assess the efficiency of the enrichment method, the magnetic beads immobilized with antibodies (Mag_STAV/BT_Ab_BChE) was initially incubated with commercially available BChE (paraoxon treated or untreated), and the purified BChE was resolved in a 1D SDS-PAGE gel. After blotting in a PVDF membrane, the blot was reacted with the same anti-BChE antibody and the results were shown in Fig. 2A. Profiles on the unmodified BChE without purification (lane 1a), the supernatant after capturing BChE (lane 1b) and the eluted BChE after capture (lane 1c), and the corresponding panels 1a’, 1b’ and 1c’ for the modified BChE, respectively, clearly demonstrated the ability of magnetic beads to effectively capture both the unmodified and OP-modified BChE and a high recovery of captured BChE following elution.
Figure 2.
Western blot analysis of BChE after immuno-magnetic separation. Un-modified recombinant human BChE was extracted using biotin/streptavidin magnetic beads and reacted with N-terminal polyclonal anti-BChE antibody (N-15) for immunological detection. A, 1a–1c; untreated, 1a’–1c’; treated.1a; BChE (1µg/lane) without extraction, 1b; supernatant after extraction of BChE, and 1c; BChE after releasing from the beads. Panels 1a’, 1b’ and 1c’ represent the results for OP-BChE in the same order as in 1a, 1b and 1c. B, Silver stained 1D gel analysis of BChE extracted from 500 µl human plasma exposed to paraoxon using biotin/streptavidin magnetic beads. Lanes 2a; plasma proteins without BChE extraction (2 µg), 2b; proteins not bound to the magnetic beads, and 2c, proteins bound to the beads. All experiments were run in triplicate for the accuracy and reproducibility of the results.
To extract BChE from human plasma, 500 µl of plasma treated with paraoxon were subjected to immunoaffinity purification and the 1D SDS-PAGE results were shown in Fig. 2B. Following paraoxon treatment, plasma samples were subjected to immunoaffinity purification. As shown in Fig. 2B, silver staining of the gel lanes showed no visible difference in the protein patterns between the non-enriched (control) and unbound fractions (lane 2a and 2b), but only two major bands were detected in the eluted fraction (lane 2c), which corresponded to the plasma BChE. The overall results suggest that the current strategy is applicable to effectively purify both the unmodified as well as OP-modified BChE from biological or clinical samples.
3.2. LC-MS characterization of recombinant human BChE protein
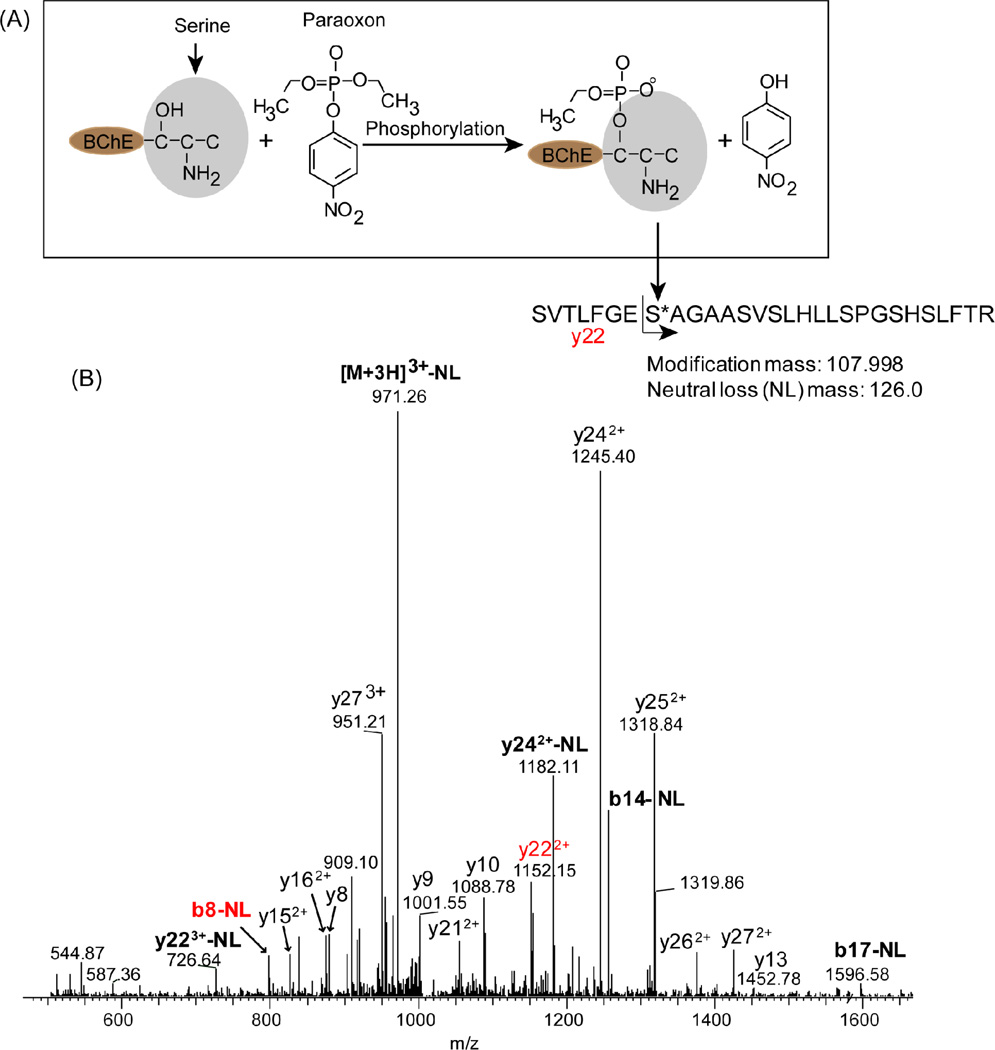
Due to the often encountered challenge in the detection of low-abundance phosphorylated peptides in complex mixture by tandem mass spectrometry, we initially characterized the recombinant BChE with or without paraoxon treatment by LC-MS/MS in order to identify the exact site of paraoxon modification and obtain the accurate mass and LC retention time of the modified peptide. Tryptic digests of both the untreated BChE and OP-BChE were first subjected to LC-MS/MS analysis. Fig. 3A showed the structure and reaction path of BChE and paraoxon after exposure. Fig. 3B showed the confident identification of phosphorylated peptide 191SVTLFGES*AGAASVSLHLLSPGSHSLFTR219 [9] with the site S198 modified with 107.998 Da corresponding to organo-phosphorylation by paraoxon [24]. The organophosphorylation also had a neutral loss mass of 126.0 Da. The specific modification on S198 was clearly validated based on the observation of y22 and b8 fragment containing the modification mass with or without a neutral loss.
Figure 3.
Identification of phosphorylated BChE peptide by tandem mass spectrometry (MS/MS scan mode). A) Covalent binding of paraoxon to BChE. The serine residue 198 at the active site center makes a covalent bond with the phosphate group of paraoxon releasing the 4-hydroxynitrobenzene ring structure. The mass added to BChE by paraoxon is 107.998. B) MS/MS spectrum for triply-charged peptide ions at m/z 1012.85. Product ions corresponding to sequential loss of intact amino acid residues from the C or N terminus are labeled as b- or y-type ions, respectively, whereas peaks arising from neutral loss of hydrated paraoxon (126 amu) are labeled in bold.
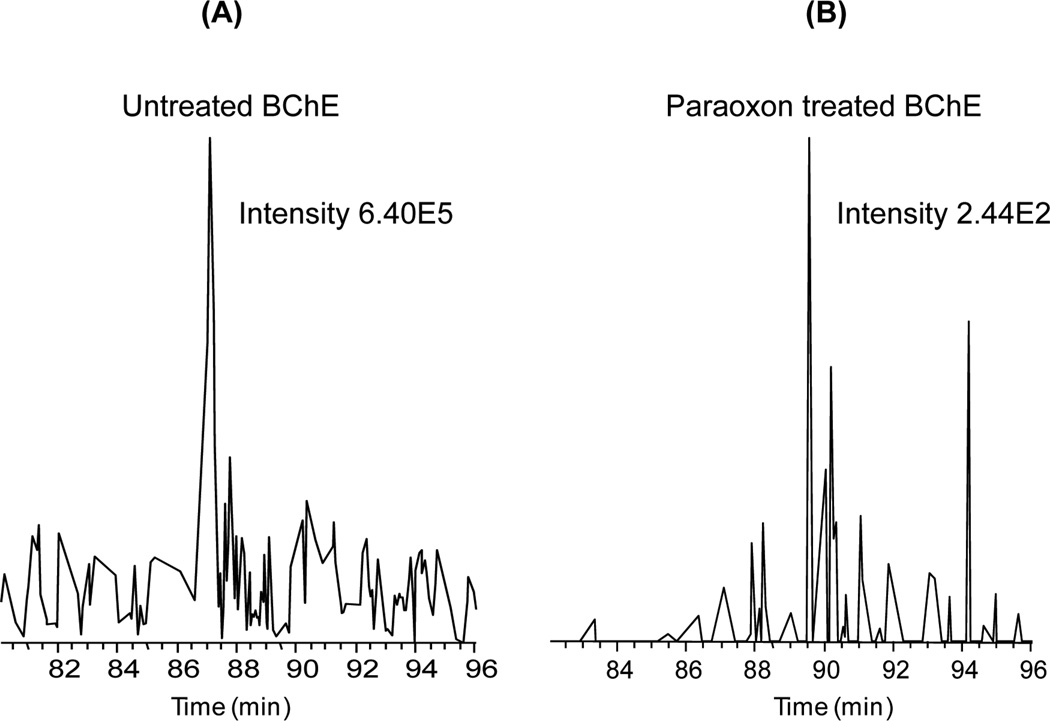
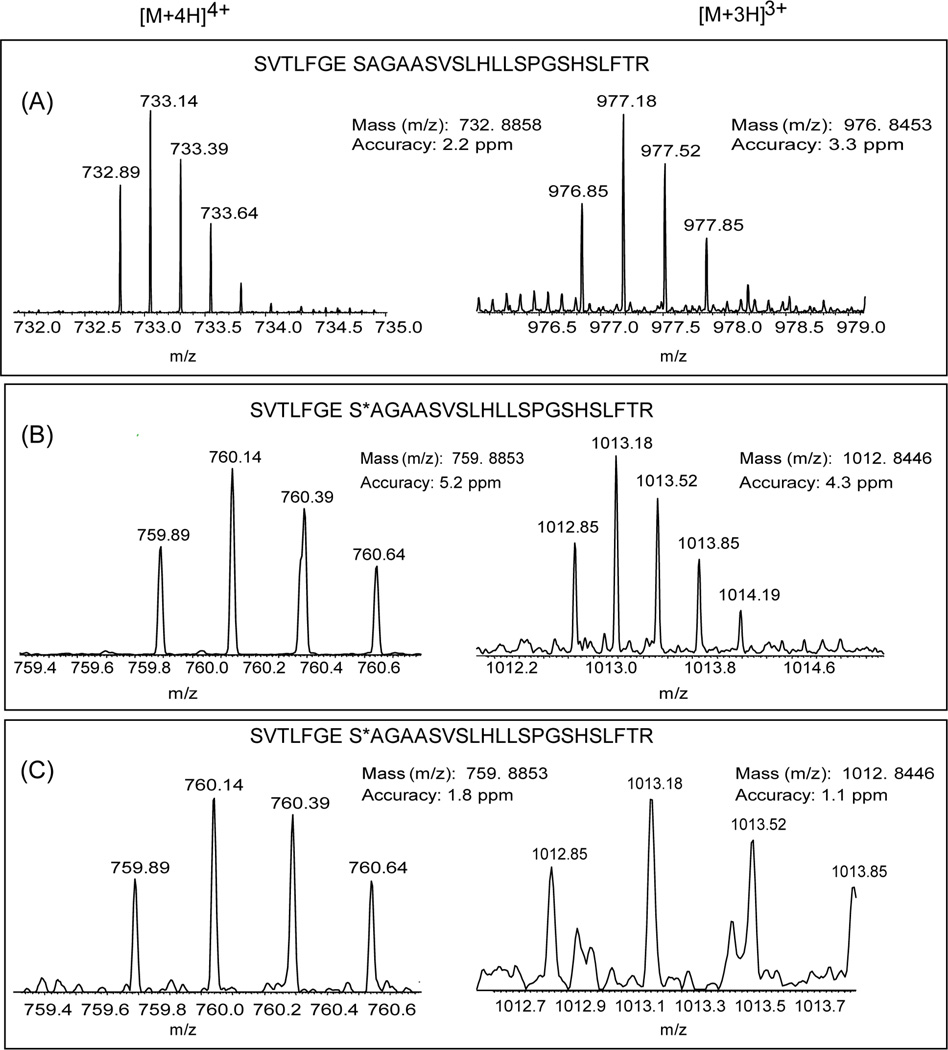
Fig. 4 compares the intensities of extracted ion chromatograms of the unmodified BChE peptide from the untreated and treated BChE samples. The intensity of unmodified BChE peptide was reduced by more than three orders of magnitude following paraoxon treatment, indicating that the treatment led to a nearly complete level of phosphorylation on Ser198, in agreement with the observation of a complete inhibition of enzyme activity. Fig. 5 provided further confirmation of the detection of the phosphorylated peptide as well as the unmodified peptide in 3+ and 4+ charge state forms by accurate mass measurements in untreated and OP-BChE digests where both molecular ions were measured within 5 ppm mass measurement errors. The accurate mass and LC retention time information of these peptides facilitate accurate identification of these peptides by high resolution MS without the need to rely on MS/MS due to potential issues associated with under-sampling.
Figure 4.
Efficiency of paraoxon treatment for phosphorylating BChE. Extracted ion chromatograms of the unmodified peptide SVTLFGESAGAASVSLHLLSPGSHSLFTR originated from untreated (A) and paraoxon-treated (B) BChE. The peak intensity decreased by >1000, suggesting nearly complete phosphorylation of BChE at S198.
Figure 5.
Detection of phosphorylated BChE peptide in recombinant protein and human plasma based on accurate mass measurements. Panels show representative MS spectra of triply (left) and quadruply (right) charged peptide masses. (A) unmodified BCHE peptide from untreated recombinant BChE; (B) modified BCHE peptide from paraoxon-treated recombinant BChE; (C) modified BCHE peptide from paraoxon-treated human plasma (C).
We have also explored whether there are endogenous phosphorylation and other sites of OP-modifications within human BChE. With a sequence coverage of ~88% achieved, our data indicate that human BChE does not contain any sites of endogenous phosphorylation and Ser198 is the only site for OP-modification induced by paraoxon treatment.
3.3. LC-MS detection of phosphorylated BChE in human plasma
To demonstrate the ability for detecting the phosphorylated BChE peptide in human plasma, immunoaffinity purification was applied to the paraoxon treated plasma sample and the final digest was analyzed by high resolution LC-MS and MS/MS. The 3+ and 4+ forms of molecular ions for the S198 phosphorylated peptide were successfully detected from the paraoxon-treated plasma sample as shown in Fig. 4C and the measured masses were exactly identical as those detected in the OP-BCHE recombinant protein (Fig 4B). The LC elution times for the detected phosphorylated peptide from both the recombinant protein and human plasma matched well with both eluted around 120 min within a 150 min separation. The unmodified form of the BChE peptide was not detected in this sample, again indicating a nearly completed phosphorylation following the paraoxon treatment. While the identification of this peptide was observed by a single MS/MS spectrum, the accurate mass measurements of the parent ions provide more confidence of the identification as well as potential quantification of the level of phosphorylation BChE in plasma without the need of MS/MS identification. Since antibody pulls down the whole BChE protein from human plasma, a number of other BChE peptides (supplementary Table) were identified by MS/MS, supporting the confidence of our detection of modified-BChE in human plasma sample.
Taken together, our data provide a detailed characterization of BChE phosphorylation and demonstrate the effective detection and potential quantification of low-abundance OP-modified BChE peptide in complex human plasma by coupling of an effective immunoaffinity purification protocol with high-resolution LC-MS. Our method provides several distinctive features compared to the recently published works [10, 20]. Firstly, the stronger non-covalent interaction of biotin-antibody conjugate with the streptavidin magnetic beads forms the basis for highly selective capture and high recovery of BChE. Secondly, the two-stage MS detection strategy should be a general approach for detecting low-abundance modified peptides by starting the acquisition of information on accurate masses and LC retention times of both modified and unmodified peptides using a recombinant protein or purified protein and then applying the acquired information for matching with the accurate masses and LC retention time of peptides observed in complex samples for the detection and quantification of low-abundance peptides by high resolution LC-MS. Our data clearly demonstrated the advantage of accurate MS measurements combined with the normalized LC-retention time for the detection of low-abundance target peptides in complex samples. Since our approach allows the detection of both the unmodified and modified forms of the BChE peptide, the extent of phosphorylation is quantifiable, especially if appropriate internal standards can be included for LC-MS analyses [10]. Lastly, we utilized trypsin as a more specific enzyme for protein digestion instead of pepsin [10] and chymotrypsin [20]. The full length tryptic peptide of BChE 191SVTLFGES*AGAASVSLHLLSPGSHSLFTR219 was difficult to identify by LC-MS/MS and enzymes such as pepsin and chymotrypsin were used to generate shorter peptides for easier identification. For example, chemotrypsin produced 196GES*AGAASVSLH207 [20] whereas pepsin generated 195FGES*AGAAS203 as modified BChE peptides; however, the non- specific cleavage activities of these enzymes may generate a number of different cleavage products introducing additional variations on quantification. Our work is the first report of confident identification of the phosphorylated BChE tryptic peptide following paraoxon treatment, which can be used as a surrogate peptide for accurate quantification.
4. Conclusions
Quantitative detection of phosphorylated BChE in human plasma as an exposure biomarker of organophosphorous nerve agents is still an analytical challenge. The magnetic bead-based immunoaffinity purification coupled with high resolution LC-MS/MS was demonstrated to be an effective approach for successful detection of phosphorylated BChE target peptide in human plasma treated by paraoxon. The effectiveness of immunoaffinity purification was confirmed by western blot experiments. The exact phosphorylation site in BChE was initially identified with confidence using the recombinant human BChE protein on both MS/MS spectra and accurate mass measurements of the parent ions. Then, the detection of OP-BChE peptide in human plasma was achieved based on the accurately measured mass and LC-retention time information. High resolution LC-MS with accurate mass measurements allows the identification and quantification of low abundance peptides in complex sample without relying on MS/MS spectra, which obviates the potential problem of under-sampling or missing data caused by MS/MS sampling. We anticipate this integrated approach will be extendable to measure other forms of OP-BChE adducts in human plasma, thus serving as a potential general approach for detecting exposure biomarkers of OP pesticides and nerve agents.
Supplementary Material
Acknowledgement
The work was performed at Pacific Northwest National Laboratory (PNNL) located in Richland, Washington, and supported by the Counter ACT Program, Office of the Director, National Institutes of Health (NIH) and the National Institute of Neurological Disorders and Stroke (NINDS), Grant Number U01 NS058161-01. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. A portion of the research was performed at the Environmental Molecular Science Laboratory (EMSL), a national scientific user facility sponsored by the Department of Energy’s Office of Biological and Environmental Research (DOE/BER) and located at Pacific Northwest National Laboratory. PNNL is operated by Battelle for DOE under contract DE-AC05-76RL01830.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singh BK. Nat Rev Microbiol. 2009;7:156–164. doi: 10.1038/nrmicro2050. [DOI] [PubMed] [Google Scholar]
- 2.Lockridge O, Masson P. Neurotoxicology. 2000;21:113–126. [PubMed] [Google Scholar]
- 3.Li H, Schopfer LM, Nachon F, Froment MT, Masson P, Lockridge O. Toxicol Sci. 2007;100:136–145. doi: 10.1093/toxsci/kfm215. [DOI] [PubMed] [Google Scholar]
- 4.Blow DM, Birktoft JJ, Hartley BS. Nature. 1969;221:337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- 5.Nicolet Y, Lockridge O, Masson P, Fontecilla-Camps JC, Nachon F. J Biol Chem. 2003;278:41141–41147. doi: 10.1074/jbc.M210241200. [DOI] [PubMed] [Google Scholar]
- 6.Raushel FM. Nature. 2011;469:310–311. doi: 10.1038/469310a. [DOI] [PubMed] [Google Scholar]
- 7.Lynch TJ, Mattes CE, Singh A, Bradley RM, Brady RO, Dretchen KL. Toxicol Appl Pharmacol. 1997;145:363–371. doi: 10.1006/taap.1997.8187. [DOI] [PubMed] [Google Scholar]
- 8.Ostergaard D, Viby-Mogensen J, Hanel HK, Skovgaard LT. Acta Anaesthesiol Scand. 1988;32:266–269. doi: 10.1111/j.1399-6576.1988.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Tong L, Schopfer LM, Masson P, Lockridge O. Chem Biol Interact. 2008;175:68–72. doi: 10.1016/j.cbi.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 10.Sporty JL, Lemire SW, Jakubowski EM, Renner JA, Evans RA, Williams RF, Schmidt JG, van der Schans MJ, Noort D, Johnson RC. Anal Chem. 2010;82:6593–6600. doi: 10.1021/ac101024z. [DOI] [PubMed] [Google Scholar]
- 11.Tsuge K, Seto Y. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;838:21–30. doi: 10.1016/j.jchromb.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Du D, Lu D, Lin CT, Smith JN, Timchalk C, Liu F, Wang J, Lin Y. Anal Chim Acta. 2011;693:1–3. doi: 10.1016/j.aca.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Ellman GL. Anal Biochem. 1962;3:40–48. doi: 10.1016/0003-2697(62)90042-8. [DOI] [PubMed] [Google Scholar]
- 14.Shih ML, McMonagle JD, Dolzine TW, Gresham VC. J Appl Toxicol. 1994;14:195–199. doi: 10.1002/jat.2550140309. [DOI] [PubMed] [Google Scholar]
- 15.Nakajima T, Sasaki K, Ozawa H, Sekjima Y, Morita H, Fukushima Y, Yanagisawa N. Arch Toxicol. 1998;72:601–603. doi: 10.1007/s002040050549. [DOI] [PubMed] [Google Scholar]
- 16.Barr JR, Driskell WJ, Aston LS, Martinez RA. J Anal Toxicol. 2004;28:372–378. [PubMed] [Google Scholar]
- 17.Fidder A, Hulst AG, Noort D, de Ruiter R, van der Schans MJ, Benschop HP, Langenberg JP. Chem Res Toxicol. 2002;15:582–590. doi: 10.1021/tx0101806. [DOI] [PubMed] [Google Scholar]
- 18.Carol-Visser J, van der Schans M, Fidder A, Hulst AG, van Baar BL, Irth H, Noort D. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;870:91–97. doi: 10.1016/j.jchromb.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Saxena A, Luo C, Doctor BP. Protein Expr Purif. 2008;61:191–196. doi: 10.1016/j.pep.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Marsillach J, Richter RJ, Kim HJ, Stevens RC, MacCoss MJ, Tomazela D, Suzuki SM, Schopfer LM, LOckridge O. Neurotoxicology. 2011 doi: 10.1016/j.neuro.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kousba AA, Sultatos LG, Poet TS, Timchalk C. Toxicol Sci. 2004;80:239–248. doi: 10.1093/toxsci/kfh163. [DOI] [PubMed] [Google Scholar]
- 22.Peter JV, Cherian AM. Anaesth Intensive Care. 2000;28:11–21. doi: 10.1177/0310057X0002800102. [DOI] [PubMed] [Google Scholar]
- 23.de la Pena Mattozzi M, Tehara SK, Hong T, Keasling JD. Appl Environ Microbiol. 2006;72:6699–6076. doi: 10.1128/AEM.00907-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu G, Wang J, Barry R, Petersen C, Timchalk C, Gassman PL, Lin Y. Chemistry. 2008;14:9951–9959. doi: 10.1002/chem.200800412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortz E, Krogh TN, Vorum H, Gorg A. Proteomics. 2001;1:1359–1363. doi: 10.1002/1615-9861(200111)1:11<1359::AID-PROT1359>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 26.Zhao R, Ding SJ, Shen Y, Camp DG, 2nd, Livesay EA, Udseth H, Smith RD. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:663–670. doi: 10.1016/j.jchromb.2008.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craig R, Beavis RC. Bioinformatics. 2004;20:1466–1467. doi: 10.1093/bioinformatics/bth092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.