Abstract
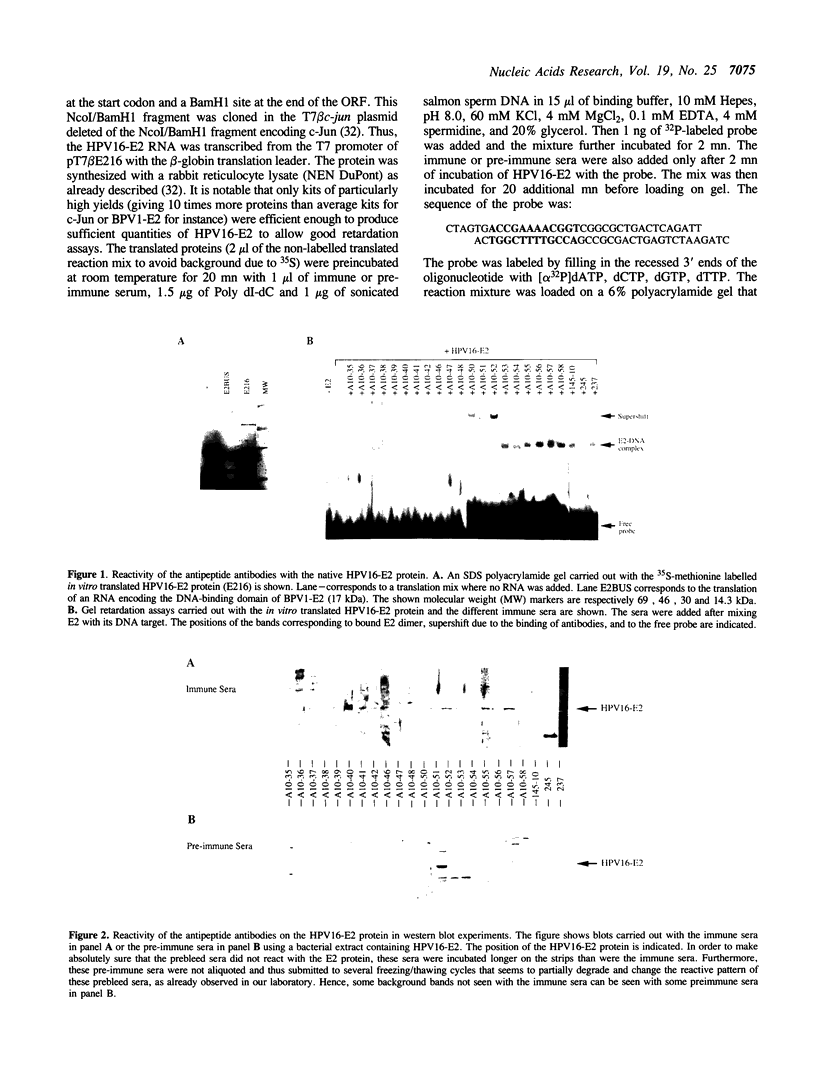
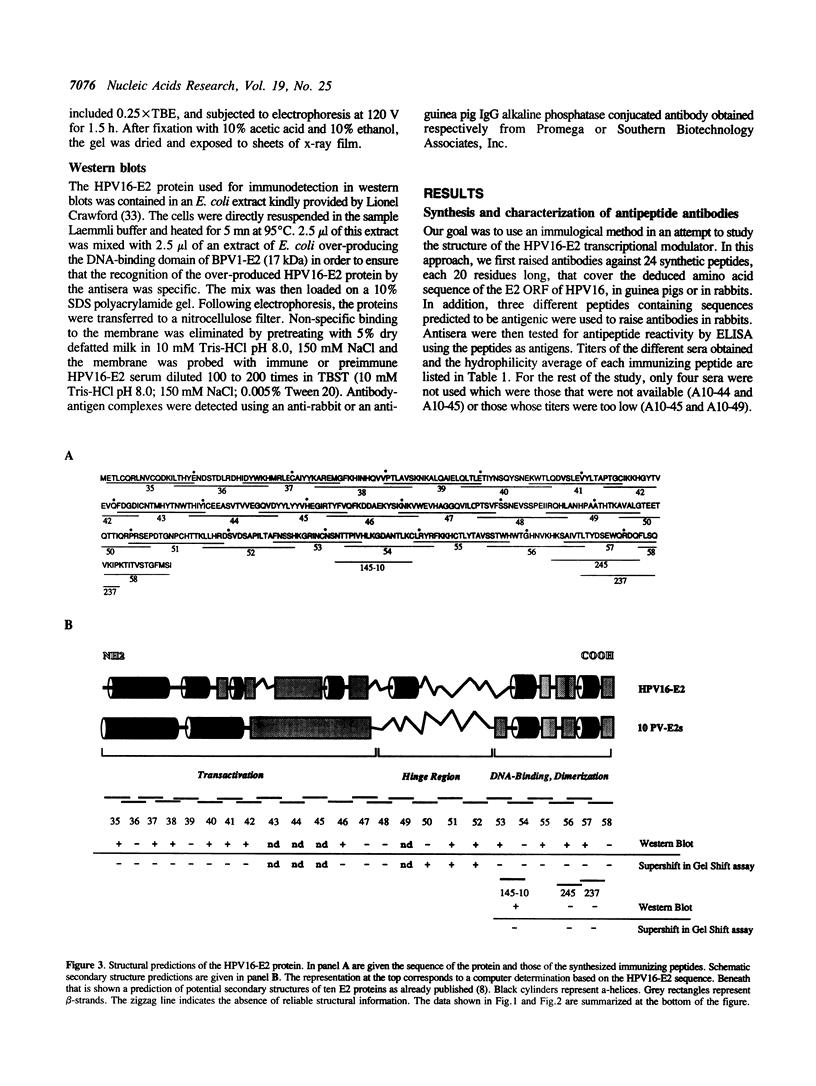
In order to probe the structure of the transcription factor encoded by the E2 Open Reading Frame of papillomaviruses, we raised polyclonal antibodies against a series of synthetic peptides that cover the HPV16-E2 protein. In gel shift experiments with the native form of the protein, we detected supershifts (caused by the binding of antibodies to the E2-DNA complex) with antibodies synthesized against peptides covering a central region 50 residues long in the E2 protein. On the contrary, antibodies raised against peptides from the NH2- and COOH-termini did not give any supershifted band. Western blot experiments showed that several of these non reacting antibodies did however interact with the denatured protein. These results suggest that the central region that connects the NH2-terminal domain responsible for transcriptional activation and the COOH-domain involved in DNA-binding is exposed and maintained in a conformation resembling the peptide, indicating a high mobility region. In contrast, the DNA-binding and transactivation domains were not recognized by the antipeptide antibodies, in line with secondary structure predictions and sequence comparisons indicating that the E2 protein consists of structured and conserved NH2 and COOH-terminal regions separated by a non-conserved and unstructured region. This flexible 'hinge' region may facilitate contacts between E2 dimers at distance in mechanisms of transcriptional activation steps that involve homosynergy or DNA-looping.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berzofsky J. A. Intrinsic and extrinsic factors in protein antigenic structure. Science. 1985 Sep 6;229(4717):932–940. doi: 10.1126/science.2410982. [DOI] [PubMed] [Google Scholar]
- Degrado W. F. Design of peptides and proteins. Adv Protein Chem. 1988;39:51–124. doi: 10.1016/s0065-3233(08)60375-7. [DOI] [PubMed] [Google Scholar]
- Dillner J., Dillner L., Robb J., Willems J., Jones I., Lancaster W., Smith R., Lerner R. A synthetic peptide defines a serologic IgA response to a human papillomavirus-encoded nuclear antigen expressed in virus-carrying cervical neoplasia. Proc Natl Acad Sci U S A. 1989 May;86(10):3838–3841. doi: 10.1073/pnas.86.10.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillner J. Mapping of linear epitopes of human papillomavirus type 16: the E1, E2, E4, E5, E6 and E7 open reading frames. Int J Cancer. 1990 Oct 15;46(4):703–711. doi: 10.1002/ijc.2910460426. [DOI] [PubMed] [Google Scholar]
- Dostatni N., Thierry F., Yaniv M. A dimer of BPV-1 E2 containing a protease resistant core interacts with its DNA target. EMBO J. 1988 Dec 1;7(12):3807–3816. doi: 10.1002/j.1460-2075.1988.tb03265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. D., Kim P. S. Modular structure of transcription factors: implications for gene regulation. Cell. 1991 May 31;65(5):717–719. doi: 10.1016/0092-8674(91)90378-c. [DOI] [PubMed] [Google Scholar]
- Gauthier J. M., Dostatni N., Lusky M., Yaniv M. Two DNA-bound E2 dimers are required for strong transcriptional activation and for cooperation with cellular factors in most cells. New Biol. 1991 May;3(5):498–509. [PubMed] [Google Scholar]
- Geysen H. M., Tainer J. A., Rodda S. J., Mason T. J., Alexander H., Getzoff E. D., Lerner R. A. Chemistry of antibody binding to a protein. Science. 1987 Mar 6;235(4793):1184–1190. doi: 10.1126/science.3823878. [DOI] [PubMed] [Google Scholar]
- Giniger E., Ptashne M. Transcription in yeast activated by a putative amphipathic alpha helix linked to a DNA binding unit. Nature. 1987 Dec 17;330(6149):670–672. doi: 10.1038/330670a0. [DOI] [PubMed] [Google Scholar]
- Giri I., Yaniv M. Structural and mutational analysis of E2 trans-activating proteins of papillomaviruses reveals three distinct functional domains. EMBO J. 1988 Sep;7(9):2823–2829. doi: 10.1002/j.1460-2075.1988.tb03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen T. H., Turek L. P., Mercurio F. M., Cripe T. P., Olson B. J., Anderson R. D., Seidl D., Karin M., Schiller J. Sequence-specific and general transcriptional activation by the bovine papillomavirus-1 E2 trans-activator require an N-terminal amphipathic helix-containing E2 domain. EMBO J. 1988 Dec 20;7(13):4245–4253. doi: 10.1002/j.1460-2075.1988.tb03322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S., Yaniv M. Jun DNA-binding is modulated by mutations between the leucines or by direct interaction of fos with the TGACTCA sequence. New Biol. 1989 Nov;1(2):181–191. [PubMed] [Google Scholar]
- Hope I. A., Struhl K. Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell. 1986 Sep 12;46(6):885–894. doi: 10.1016/0092-8674(86)90070-x. [DOI] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemmerson R. Antigenicity and native structure of globular proteins: low frequency of peptide reactive antibodies. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9180–9184. doi: 10.1073/pnas.84.24.9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Knight J. D., Li R., Botchan M. The activation domain of the bovine papillomavirus E2 protein mediates association of DNA-bound dimers to form DNA loops. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3204–3208. doi: 10.1073/pnas.88.8.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lambert P. F., Spalholz B. A., Howley P. M. A transcriptional repressor encoded by BPV-1 shares a common carboxy-terminal domain with the E2 transactivator. Cell. 1987 Jul 3;50(1):69–78. doi: 10.1016/0092-8674(87)90663-5. [DOI] [PubMed] [Google Scholar]
- Lees E. M., Driessen H. P., Crawford L. V., Clarke A. R. The E2 protein of human papillomavirus type 16. Over-expression and purification of an active transcriptional regulator. Eur J Biochem. 1990 May 31;190(1):85–92. doi: 10.1111/j.1432-1033.1990.tb15549.x. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Zinnecker M., Hamaoka T., Katz D. H. New procedures for preparation and isolation of conjugates of proteins and a synthetic copolymer of D-amino acids and immunochemical characterization of such conjugates. Biochemistry. 1979 Feb 20;18(4):690–693. doi: 10.1021/bi00571a022. [DOI] [PubMed] [Google Scholar]
- Ma J., Ptashne M. A new class of yeast transcriptional activators. Cell. 1987 Oct 9;51(1):113–119. doi: 10.1016/0092-8674(87)90015-8. [DOI] [PubMed] [Google Scholar]
- Ma J., Ptashne M. Deletion analysis of GAL4 defines two transcriptional activating segments. Cell. 1987 Mar 13;48(5):847–853. doi: 10.1016/0092-8674(87)90081-x. [DOI] [PubMed] [Google Scholar]
- McBride A. A., Byrne J. C., Howley P. M. E2 polypeptides encoded by bovine papillomavirus type 1 form dimers through the common carboxyl-terminal domain: transactivation is mediated by the conserved amino-terminal domain. Proc Natl Acad Sci U S A. 1989 Jan;86(2):510–514. doi: 10.1073/pnas.86.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride A. A., Schlegel R., Howley P. M. The carboxy-terminal domain shared by the bovine papillomavirus E2 transactivator and repressor proteins contains a specific DNA binding activity. EMBO J. 1988 Feb;7(2):533–539. doi: 10.1002/j.1460-2075.1988.tb02842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Molla A., Charbit A., Le Guern A., Ryter A., Hofnung M. Antibodies against synthetic peptides and the topology of LamB, an outer membrane protein from Escherichia coli K12. Biochemistry. 1989 Oct 3;28(20):8234–8241. doi: 10.1021/bi00446a040. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Seckler R., Möröy T., Wright J. K., Overath P. Anti-peptide antibodies and proteases as structural probes for the lactose/H+ transporter of Escherichia coli: a loop around amino acid residue 130 faces the cytoplasmic side of the membrane. Biochemistry. 1986 May 6;25(9):2403–2409. doi: 10.1021/bi00357a016. [DOI] [PubMed] [Google Scholar]
- Seedorf K., Krämmer G., Dürst M., Suhai S., Röwekamp W. G. Human papillomavirus type 16 DNA sequence. Virology. 1985 Aug;145(1):181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G., Shinnick T. M., Green N., Lerner R. A. Antibodies that react with predetermined sites on proteins. Science. 1983 Feb 11;219(4585):660–666. doi: 10.1126/science.6186024. [DOI] [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Alexander H., Houghten R. A., Olson A. J., Lerner R. A., Hendrickson W. A. The reactivity of anti-peptide antibodies is a function of the atomic mobility of sites in a protein. Nature. 1984 Nov 8;312(5990):127–134. doi: 10.1038/312127a0. [DOI] [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Paterson Y., Olson A. J., Lerner R. A. The atomic mobility component of protein antigenicity. Annu Rev Immunol. 1985;3:501–535. doi: 10.1146/annurev.iy.03.040185.002441. [DOI] [PubMed] [Google Scholar]
- Van Regenmortel M. H., Daney de Marcillac G. An assessment of prediction methods for locating continuous epitopes in proteins. Immunol Lett. 1988 Feb;17(2):95–107. doi: 10.1016/0165-2478(88)90076-4. [DOI] [PubMed] [Google Scholar]
- Westhof E., Altschuh D., Moras D., Bloomer A. C., Mondragon A., Klug A., Van Regenmortel M. H. Correlation between segmental mobility and the location of antigenic determinants in proteins. Nature. 1984 Sep 13;311(5982):123–126. doi: 10.1038/311123a0. [DOI] [PubMed] [Google Scholar]
- Wilson J. E. The use of monoclonal antibodies and limited proteolysis in elucidation of structure-function relationships in proteins. Methods Biochem Anal. 1991;35:207–250. doi: 10.1002/9780470110560.ch4. [DOI] [PubMed] [Google Scholar]




